INTRODUCTION:
Treatment options are limited for people infected with hepatitis C virus (HCV) with decompensated liver disease. The C-SALT study assessed elbasvir (EBR) plus grazoprevir (GZR) in individuals with HCV genotype 1 infection and Child-Pugh class B (CP-B) cirrhosis.
METHODS:
In this 12-week, phase 2, nonrandomized, open-label study (NCT02115321; Protocol MK-5172-059), participants with CP-B cirrhosis received EBR 50 mg plus GZR 50 mg once daily, and a control group of noncirrhotic participants received EBR 50 mg plus GZR 100 mg once daily. The primary endpoint was sustained virologic response 12 weeks after the end of therapy.
RESULTS:
Sustained virologic response at 12 weeks after the end of therapy was achieved by 27/30 (90.0%) CP-B participants and 10/10 (100.0%) noncirrhotic participants. Two participants relapsed, and one died during follow-up after having undetectable HCV RNA at the end of treatment. Most CP-B participants had stable or improved model for end-stage liver disease and Child-Pugh scores at follow-up week 12 compared with baseline. There was no significant difference in drug exposure between groups, despite the differing GZR dose. Adverse events occurring in >10% of participants were fatigue (CP-B: 30.0%; noncirrhotic: 30.0%), arthralgia (16.7%; 20.0%), nausea (10.0%; 20.0%), and headache (10.0%; 50.0%). No serious treatment-related adverse events or hepatic events of clinical interest occurred.
CONCLUSIONS:
EBR 50 mg plus GZR 50 mg once daily for 12 weeks was highly effective and well tolerated in a traditionally hard-to-treat population.
TRANSLATIONAL IMPACT:
Although EBR plus reduced-dose GZR is not available for people with CP-B cirrhosis, these results complement phase 2/3 trial data and real-world experience with EBR/GZR.
INTRODUCTION
Direct-acting antiviral agents (DAAs) have revolutionized the treatment of chronic hepatitis C virus (HCV) infection; however, for individuals with decompensated liver disease (Child-Pugh [CP] class B [CP-B] or class C [CP-C], defined by a CP score ≥ 7), treatment options are limited (1). Given that the number of HCV-infected people with liver decompensation is projected to rise (2) and that viral eradication in these individuals is associated with substantial long-term benefits (3,4), effective treatment options for this population remain a priority. Clinical trial data (5–7), supported by real-world observational evidence (8–10) and retrospective analyses (11,12), suggest that all-oral DAA regimens are efficacious in individuals with HCV and decompensated cirrhosis. In the United States, treatment guidelines for people with genotype (GT) 1 infection and decompensated cirrhosis recommend sofosbuvir plus ledipasvir, velpatasvir, or daclatasvir, either with ribavirin for 12 weeks or without ribavirin for 24 weeks for individuals ineligible for ribavirin therapy, or for 24 weeks with ribavirin for those who have failed a nonstructural protein 5A (NS5A) inhibitor– or sofosbuvir-containing regimen (13).
The combination of elbasvir (EBR), a once-daily NS5A inhibitor (14), and grazoprevir (GZR), a once-daily nonstructural protein 3/4A (NS3/4A) protease inhibitor (15), has demonstrated high efficacy and favorable tolerability in phase 2 and 3 clinical trials (16–20). This DAA combination is approved in the United States, Europe, and other countries worldwide for the treatment of HCV GT1 and GT4 infection, including in people with compensated cirrhosis (21–23). Recent “real-world” studies have affirmed the efficacy and safety of this regimen in large databases (24). The purpose of the C-SALT study was to assess the efficacy, safety, and pharmacokinetics (PK) of EBR plus GZR (EBR/GZR) in participants with HCV infection and CP-B cirrhosis.
METHODS
Study design
This phase 2, nonrandomized open-label study was conducted at 9 centers in the United States between May 2014 and April 2015 (NCT02115321; Protocol MK-5172-059). The study was conducted in accordance with principles of Good Clinical Practice and approved by the appropriate institutional review boards and regulatory agencies. All participants provided written informed consent. The study protocol and list of institutional ethics committees are provided in the supplementary text (see Text, Supplementary Digital Content 1, http://links.lww.com/CTG/A5). All authors had access to the study data and reviewed and approved the final manuscript.
The study was designed to be conducted in 3 parts. Part A evaluated EBR 50 mg once daily (q.d.) plus GZR 50 mg q.d. for 12 weeks in participants with HCV GT1 infection and CP-B cirrhosis. The 50-mg dose was selected for participants with CP-B cirrhosis based on the impact of cirrhosis and HCV infection on steady-state GZR concentrations as determined by results from phase 1 and 2 studies (22). A cohort of noncirrhotic participants with HCV GT1 infection were also enrolled in part A for the purposes of PK analyses. In part A, this regimen showed acceptable safety and efficacy; however, because the development program for EBR/GZR was focused on the fixed-dose combination tablet containing EBR 50 mg/GZR 100 mg, the study was terminated upon completion of part A.
Participants with CP-B cirrhosis received EBR 50 mg q.d. plus GZR 50 mg q.d. administered as separate entities for 12 weeks, without regard to food intake. EBR (one 50-mg tablet) and GZR (two 25-mg tablets) were supplied by the study sponsor. Noncirrhotic participants enrolled in the PK cohort received EBR 50 mg q.d. plus GZR 100 mg q.d. for 12 weeks. Dose modifications were not permitted.
Participants
Male or female participants aged ≥18 years with chronic HCV infection were enrolled. Key inclusion criteria were HCV RNA ≥ 10,000 IU/mL at screening and documented chronic HCV GT1 infection. Participants enrolled in the primary analysis population were required to have CP-B cirrhosis with a CP score of 7–9, and no liver transplant anticipated for the next 36 weeks. CP score was assigned based on laboratory data obtained at the screening visit. Cirrhosis was defined by liver biopsy with a METAVIR fibrosis score of F4; FibroScan >12.5 kPa performed within 12 months of day 1; or FibroSure (FibroTest) performed during screening with a score of >0.75 and an aspartate aminotransferase (AST):platelet ratio index of >2. Noncirrhotic participants enrolled in the PK cohort were required to have a liver biopsy performed within 24 months showing absence of cirrhosis; FibroScan ≤ 9.5 kPa performed within 12 months or a FibroSure (FibroTest) score of ≤0.48 and AST:platelet ratio index ≤1 during screening. Participants with hepatitis B virus or human immunodeficiency virus co-infection, previous treatment with DAA therapy for HCV infection, or evidence of hepatocellular carcinoma were excluded.
Assessments and analyses
The primary end point was the proportion of participants achieving a sustained virologic response at 12 weeks after the end of study therapy (SVR12), defined as HCV RNA <15 IU/mL (COBAS AmpliPrep/COBAS TaqMan HCV Test version 2.0; Roche, Branchburg, NJ; lower limit of quantitation = 15 IU/mL, lower limit of detection = 15 IU/mL). Efficacy is presented for the intention-to-treat population, which includes all participants who received ≥1 dose of study medication.
Additional analyses included resistance-associated substitutions (RASs), PK evaluation, change in model for end-stage liver disease (MELD) score and CP score from baseline to follow-up weeks (FWs) 12 and 24, and evaluation of safety (including adverse events [AEs] and events of clinical interest [ECI]). ECIs were defined as overdose; first instance of alanine aminotransferase (ALT) or AST >500 IU/L; first instance of ALT or AST >3× baseline and >100 IU/L; first instance of bilirubin >2× baseline and total bilirubin >3.0 mg/dL; first instance of increase in international normalized ratio >2× baseline and international normalized ratio >2.3; and first instance of alkaline phosphatase >3× the upper limit of normal.
RASs were assessed for all participants at baseline and in those with virologic failure. HCV RNA was amplified using reverse transcription polymerase chain reaction followed by population sequencing of the NS5A gene on an ABI Sequencer (Applied Biosystems, Inc., Foster City, CA) from samples with RNA levels of ≥1,000 IU/mL. The limit of minority variant detection in the population was approximately >20% of the viral population. Any variant at position 155 or 168 was reported, and the following specific variants in the NS3 region were selected for reporting: V36A/G/L/M/I, T54A/C/G/S, V55A/I, Y56H, Q80K/R, V107I, S122A/G/R, I132V, R155G/T/W, A156S/T/V/F/G, V158I, I/V170A/F/T/V, and M175L. The following variants in the NS5A region were selected for reporting: M28T/A, Q30E/H/R/G/K/L/D, L31M/V/F, H58D, and Y93C/H/N.
For PK evaluation, plasma samples were collected in a subset of CP-B and noncirrhotic participants at treatment week (TW) 4. Blood was collected predose and 0.5, 1, 2, 3, 4, 6, 8, 12, 16, and 24 hours after dosing. PK variables assessed included EBR and GZR concentrations 2, 4, and 24 hours after dosing, maximum concentration, and area under the curve between 0 and 24 hours.
Because the study was terminated after completion of part A, no hypothesis testing was performed. The Clopper-Pearson method was used to construct 95% confidence intervals for SVR12.
RESULTS
Participant demographics
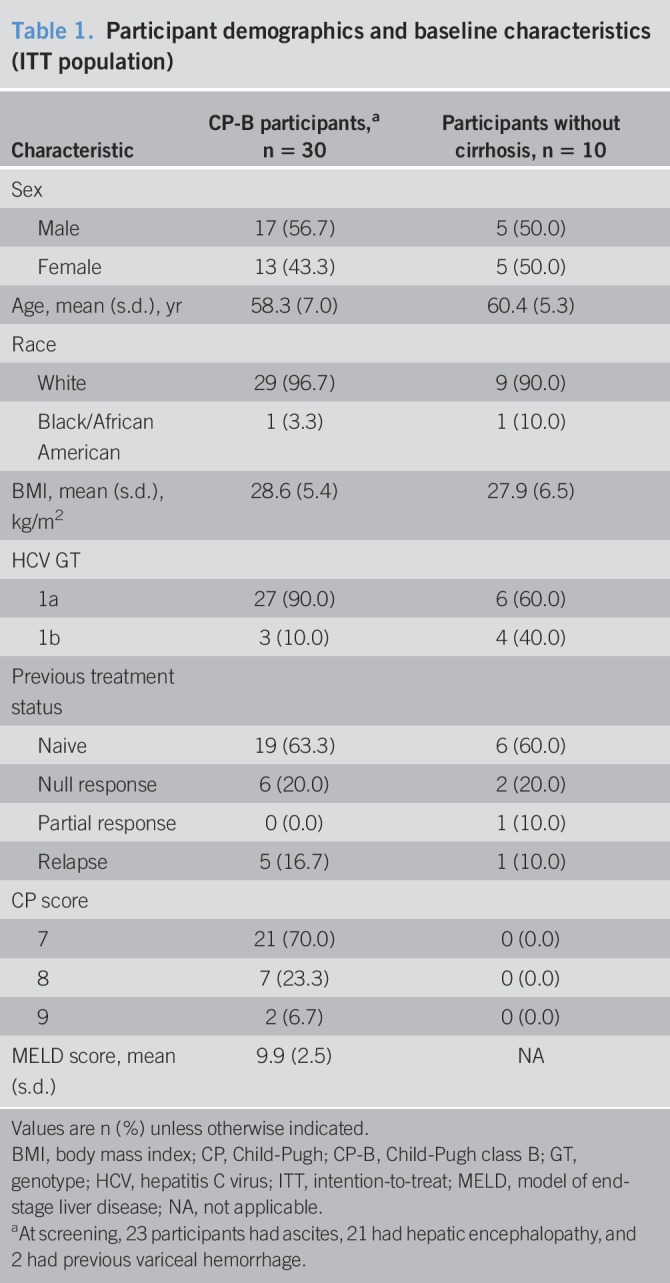
Thirty participants with HCV GT1 infection and CP-B cirrhosis and 10 noncirrhotic participants with HCV GT1 infection were enrolled in part A (Table 1). Most participants (≥90%) were white, with a mean age of 58–60 years, and ≥60% were treatment-naive. The majority of CP-B participants (70%) had a CP score of 7 at baseline. Baseline mean MELD scores were 9.9 for CP-B participants. At screening, 23 participants reported a history of ascites, 21 reported a history of hepatic encephalopathy, and 2 reported previous variceal hemorrhage.
Table 1.
Participant demographics and baseline characteristics (ITT population)
Efficacy
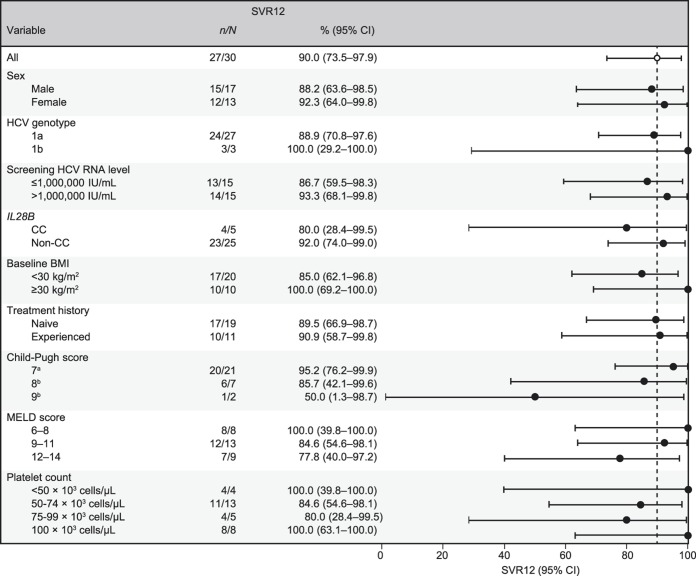
SVR12 was achieved by 27/30 (90.0%) participants with CP-B cirrhosis and 10/10 (100.0%) noncirrhotic participants (Figure 1). Of the 3 participants who failed to attain SVR12, 2 experienced relapse and 1 died at FW4 after achieving undetectable HCV RNA at TW12. No treatment failures were observed after FW12.
Figure 1.
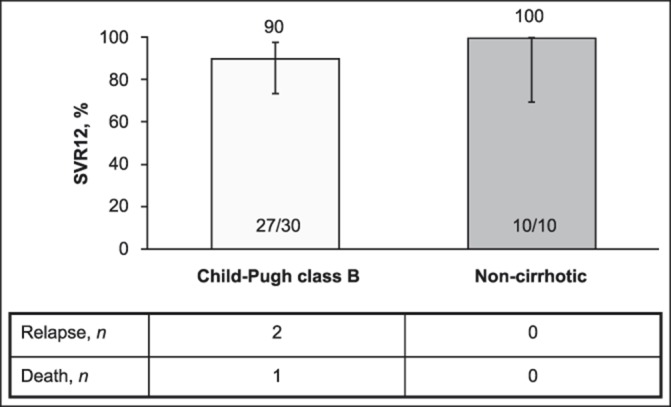
SVR12. SVR, sustained virologic response, SVR12, sustained virologic response at 12 weeks after the end of therapy.
Subgroup analyses suggest a tendency toward lower SVR12 in participants with CP-B cirrhosis scores of 8 or 9 compared with those with CP-B scores of 7, and in those with MELD scores of 12–14 vs those with scores ≤11; however, these data are based on small participant numbers (Figure 2). Low platelet count did not appear to predict response, with SVR12 achieved by all 4 participants with baseline platelet counts <50 × 103 cells/μL. Analysis of SVR12 across other subgroups was also compromised by small sample sizes. SVR12 was achieved by 89.5% (17/19) of treatment-naive participants and 90.0% (10/11) of treatment-experienced participants. Variables such as sex, baseline viral load, and body mass index also did not predict SVR12.
Figure 2.
SVR12 subgroup analyses. BMI, body mass index; CI, confidence interval; CP, Child-Pugh; FW, follow-up week; HCV, hepatitis C virus; MELD, model of end-stage liver disease; SVR12, sustained virologic response at 12 weeks after the end of therapy. aOne participant (CP score 7) died of progressive liver failure at FW4. bOne participant with a CP score of 8 and 1 participant with a CP score of 9 experienced relapse.
Resistance analyses
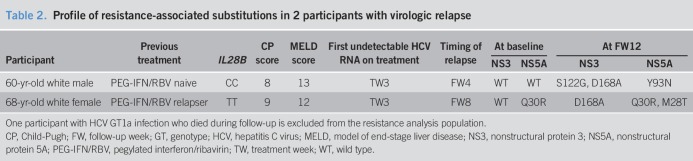
The resistance analysis population comprised 39 participants, including the 37 who achieved SVR12 (CP-B, n = 27; noncirrhotic, n = 10) and the 2 CP-B participants who experienced virologic failure. The participant who died during follow-up was excluded from the resistance analysis.
Seven participants with GT1b infection were assessed for the presence of baseline RASs: NS3 RASs were not detected in any participant, and 1 had an NS5A 31M RAS. All 7 participants achieved SVR12.
Thirty-two participants with GT1a infection were included in the resistance analysis population. NS3 and NS5A RASs that confer >5× resistance to EBR or GZR were present in 0/32 (0%) and 4/32 (12.5%) participants, respectively. SVR12 was achieved by 30/32 (93.8%) participants with no NS3 RAS at baseline, 27/28 (96.4%) participants with no NS5A RAS, and 3/4 (75.0%) participants with NS5A RASs. When considering all RASs (not only those that confer a >5× resistance increase to EBR or GZR), NS3 RASs were detected in 21/32 (65.6%) participants with GT1a infection, all of whom achieved SVR12 (Figure 3). NS5A RASs were detected in 8/32 (25.0%) participants with GT1a infection, 7 of whom achieved SVR and 1 of whom relapsed. Both CP-B participants who relapsed had GT1a infection (Table 2).
Figure 3.
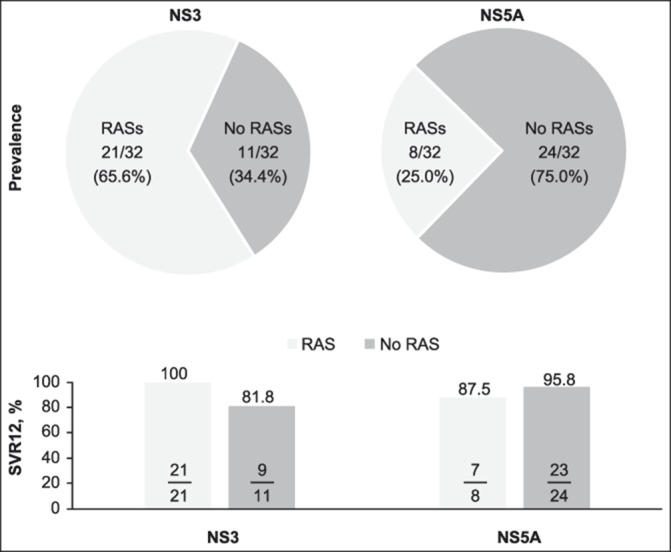
Prevalence and impact of NS3 and NS5A RASs in participants with HCV GT1a infection, including both participants with Child-Pugh class B cirrhosis and those without cirrhosis. GT, genotype; HCV, hepatitis C virus; NS3, nonstructural protein 3; NS5a, nonstructural protein 5A; RAS, resistance-associated substitution; SVR12, sustained virologic response at 12 weeks after the end of therapy.
Table 2.
Profile of resistance-associated substitutions in 2 participants with virologic relapse
Pharmacokinetics
There was no significant difference in EBR or GZR exposure between the CP-B participants who received EBR 50 mg plus GZR 50 mg and the noncirrhotic participants who received EBR 50 mg plus GZR 100 mg (see Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/CTG/A5). GZR exposure was numerically slightly higher in participants with CP-B cirrhosis receiving the 50-mg dose compared with noncirrhotic participants receiving the 100-mg dose. EBR (50 mg) exposure was similar in both populations. Of the 2 participants who relapsed, one had low GZR and EBR plasma concentrations 4 hours after dosing and low GZR trough concentrations, whereas the other had low EBR trough concentrations.
Change in MELD and CP scores
At FW12, 12/29 (41.4%) CP-B participants experienced an improvement in MELD score (1-point improvement, n = 5; 2-point, n = 4; 3-point, n = 3), 11 (37.9%) had no change and 6 (20.7%) had a worsening in MELD score (1-point, n = 5; 6-point, n = 1). Improvements in MELD scores were primarily attributable to decreasing bilirubin levels. Similarly, at FW12, 20/29 (69.0%) CP-B participants had improvements in CP score compared with baseline (1-point improvement, n = 15; 2-point, n = 4; 3-point, n = 1), 7 participants had no change, and 2 participants had a 1-point worsening. The participant who died during follow-up had MELD scores of 11 at baseline and at end of treatment, and a baseline CP score of 7 (no CP score was available at the end of treatment for this participant). Further description of change in MELD and CP scores at FW24 is provided in the Supplementary Text and in Supplementary Figure 1 (see Supplementary Digital Content 1, http://links.lww.com/CTG/A5).
Safety
Overall, 34/40 (85.0%) participants reported 1 or more AEs during the treatment or follow-up (Table 3), of which 14/40 (35.0%) were recorded by the investigator as treatment related. No serious treatment-related AEs occurred. One participant experienced an overdose of study medication during treatment, which, per protocol, was classified as an ECI. No hepatic ECIs occurred during the study. One participant died during the follow-up period. This participant had a baseline CP score of 7 and presented at TW9 with fever, and was subsequently diagnosed with culture-positive (Streptococcus viridans) spontaneous bacterial peritonitis (a non–treatment-related serious AE that resolved with ceftriaxone) and endocarditis (based on transthoracic echocardiography). After completing 12 weeks of treatment, his condition worsened, with subsequent cerebral infarction, oliguric renal failure, and progressive hepatic failure at FW4. At screening, this participant's medical history included anemia, ascites, esophageal varices, portal vein thrombosis, and splenomegaly-associated thrombocytopenia. The episodes of spontaneous bacterial peritonitis were not associated with increases in ALT, AST, or total bilirubin, and the participant did not experience a late ALT/AST elevation event while receiving study medication. HCV RNA levels at FW4 were not obtained. This participant had MELD scores of 11 at baseline and at end-of-treatment. The death was assessed by the investigator as unrelated to treatment.
Table 3.
Summary of AEs during treatment and follow-up (all participants as treated)
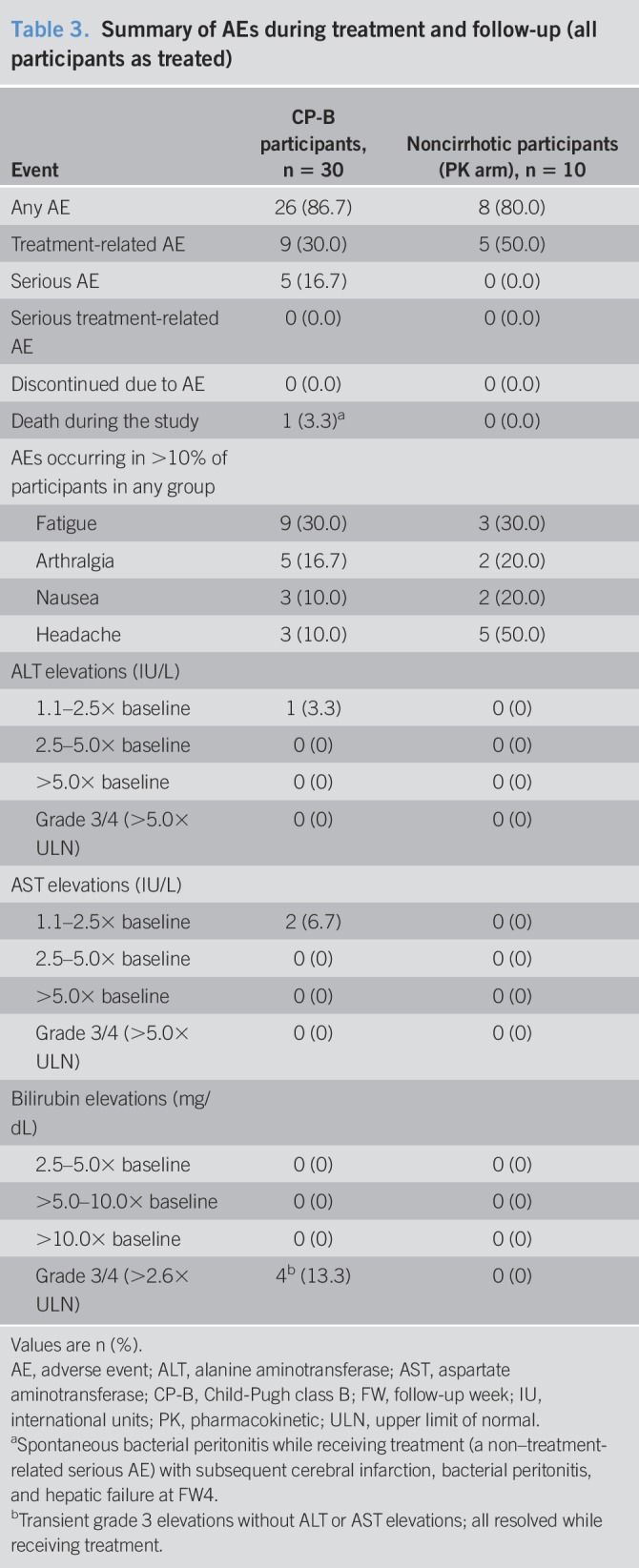
AEs occurring in >10% of participants in either group were fatigue (CP-B: 30.0%; noncirrhotic: 30.0%), arthralgia (16.7%; 20.0%), nausea (10.0%; 20.0%), and headache (10.0%; 50.0%; Table 3). No participants discontinued treatment because of an AE. One CP-B participant had a grade 1 ALT increase, and 2 participants had a grade 1 AST increase. There were no ALT or AST increases >2.5× baseline or >5× the upper limit of normal. Four participants had grade 3 bilirubin increases 2.6–5.0× the upper limit of normal that were mild and transient and resolved with ongoing therapy. There were no grade 4 bilirubin increases.
DISCUSSION
In this phase 2, open-label study, high rates of SVR12 (90%) were achieved in participants with HCV GT1 infection and CP-B decompensated cirrhosis receiving EBR 50 mg plus GZR 50 mg for 12 weeks. This regimen was well tolerated, with no evidence of hepatotoxicity. MELD scores and CP scores remained stable or were reduced in most participants, with only a minority showing a deterioration in liver health status or increased risk of mortality. Of the 30 participants with CP-B cirrhosis, 27 achieved SVR12 and 2 experienced relapse. Both participants who experienced relapse had RASs in both NS3 and NS5A regions at the time of failure.
The approved EBR/GZR fixed-dose combination contains a 100-mg dose of GZR, in contrast to the 50-mg dose administered to the CP-B participants in the present study. The lower dose of GZR was selected for this study based on the previous phase 1 studies showing elevated GZR plasma concentrations in non–HCV-infected individuals with hepatic impairment compared with those with normal hepatic function. In the phase 1 clinical trial, GZR concentrations were 1.7-fold, 5-fold, and 12-fold higher in people with mild, moderate, and severe hepatic impairment, respectively, compared with those with normal hepatic function (22). Furthermore, GZR steady-state area-under-the-curve values are projected to be 1.65-fold higher in HCV-infected individuals with compensated cirrhosis compared with HCV-infected noncirrhotic individuals (22). Given that a GZR dose of 100 mg is known to be well tolerated in HCV-infected individuals with CP-A cirrhosis and without cirrhosis, a starting GZR dose of 50 mg was considered appropriate for the participants with CP-B cirrhosis in this study. Data from the present study showed no statistically significant difference in GZR exposure in CP-B participants receiving GZR 50 mg compared with noncirrhotic participants receiving GZR 100 mg. In consideration of these data, exposure for dose assessments between the 2 groups would suggest that CP-B participants have higher drug exposures than noncirrhotic participants, which is consistent with the observations from previous studies (22). Elevated GZR concentrations in individuals with compensated cirrhosis may also contribute to the high efficacy seen in the EBR/GZR phase 2/3 clinical development program, with several studies reporting SVR rates of 94%–100% in participants with compensated cirrhosis receiving the clinically approved EBR 50 mg/GZR 100 mg FDC for 12 weeks (16–18,20). However, because of the elevated GZR concentrations seen in participants with moderate or severe (CP-B or -C) hepatic impairment, and the consequent increased risk of ALT elevations, the FDC of EBR 50 mg/GZR 100 mg is contraindicated in this population (22). Similarly, other regimens containing HCV protease inhibitors are also listed as not recommended or contraindicated in this population (25–28). An FDC tablet containing EBR plus the lower GZR 50-mg dose is not clinically available, and EBR and GZR are also not available clinically as separate entities.
SVR12 was achieved by 90% of CP-B participants in the current study, which is consistent with rates of 83%–100% reported from other recent open-label clinical trials of DAAs in participants with decompensated liver disease (5–7). In the phase 2 SOLAR-2 study, the combination of sofosbuvir 400 mg and ledipasvir 90 mg plus ribavirin 600–1,200 mg daily for 12 weeks produced SVR12 in 22/26 (84.6%) CP-B participants who had not undergone transplant (SVR was 87% in those with GT1 infection and 67% in those with GT4 infection) (6). A 24-week regimen slightly increased efficacy, with 24/25 (96.0%) participants achieving SVR12 (96% with GT1 and 100% with GT4 infection) (6). In the phase 3 ALLY-1 study, 30/32 (93.8%) CP-B participants achieved SVR12 after treatment with sofosbuvir 400 mg and daclatasvir 60 mg plus ribavirin 600–1,000 mg (7). In the phase 3 ASTRAL-4 study, 90 HCV-infected CP-B participants received 400 mg sofosbuvir plus 100 mg velpatasvir for 12 weeks, and 75 (83.3%) achieved SVR12 (88% of participants with GT1, 50% with GT3, and 100% with GT2 or GT4 infection) (5). The addition of ribavirin to this 12-week treatment regimen slightly improved efficacy (82/87 [94.3%] of participants achieved SVR12), but a longer treatment duration of 24 weeks had no noticeable effect (77/90 [85.6%] achieved SVR12) (5). Although the number of participants in these clinical studies is small, the efficacy of DAAs in treating HCV-infected people with decompensated liver disease is supported by real-world evidence encompassing several thousand individuals (8,9,12).
Based on the overall size of the trial population, as well as the small number of RASs in the 2 participants with virologic failure, limited conclusions can be drawn from this study regarding the impact of NS3 and NS5A RASs on SVR12. One participant who relapsed had baseline NS5A RASs, and both participants who relapsed had lower drug concentrations, which potentially may have contributed to the development of treatment-emergent RASs.
The safety profile of EBR plus GZR in CP-B participants in the current study is comparable to the safety profile in participants without cirrhosis and those with CP-A cirrhosis as assessed during the clinical development program (16–20). One participant experienced decompensation and death during follow-up with repeated episodes of spontaneous bacterial peritonitis, but had no increase in AST, ALT, or total bilirubin while receiving treatment. This decompensation event was considered unrelated to study medication. No treatment-related serious AEs or hepatic ECIs were reported during this study.
Individuals with decompensated liver cirrhosis frequently receive medications for comorbidities, and therefore, the potential for drug–drug interactions should be evaluated when considering treatment options for this population. EBR and GZR are substrates of cytochrome P450 (CYP) 3A and P-glycoprotein (P-gp) (22). Co-administration of EBR/GZR with moderate or strong inducers of CYP3A may therefore decrease EBR and GZR plasma concentrations, leading to reduced therapeutic effect, whereas co-administration of EBR/GZR with strong CYP3A inhibitors may increase EBR or GZR concentrations. Some studies have suggested that the magnitude of drug interactions that arise through reversible inhibition of CYP3A may be reduced or even eliminated in people with advanced cirrhosis because of decreased hepatic drug uptake and reduced hepatic enzyme expression (29). In the present study, concomitant use of strong CYP3A/P-gp inhibitors, such as clarithromycin and itraconazole, strong/moderate CYP3A/P-gp inducers, such as rifampin and carbamazepine, and organic anion transporting polypeptide inhibitors, such as cyclosporine, was not permitted.
In conclusion, the treatment regimen of EBR 50 mg plus GZR 50 mg administered for 12 weeks was highly effective and well tolerated in a traditionally hard-to-treat population with few currently approved treatment options. Although this regimen of EBR and reduced-dose GZR is not available for people with CP-B cirrhosis, the results of this trial complement phase 2/3 trial data and real-world experience with EBR/GZR.
CONFLICTS OF INTEREST
Guarantor of the article: Ira M. Jacobson, MD.
Specific author contributions: I.M.J. provided study concept and design, acquisition of data, initial drafting of the manuscript, and critical revision of the manuscript for important intellectual content. F.P., R.F.-M., G.T.E., and E.C.V. performed the acquisition of data. S.B. and P.H. provided analysis and interpretation of data and statistical analysis. L.C. performed analysis and interpretation of data. M.R. provided study concept and design. E.D.C. and H.P. provided study concept and design and initial drafting of the manuscript. All authors have approved the final draft submitted.
Financial support: Funding for this research was provided by Merck Sharp & Dohme Corp. (MSD), a subsidiary of Merck & Co., Inc., Kenilworth, NJ. Medical writing support was provided by Tim Ibbotson, PhD, of ApotheCom, Yardley, Pennsylvania. This assistance was funded by MSD.
Potential competing interests: I.M.J. is a consultant for AbbVie, Assembly Biosciences, Bristol-Myers Squibb, Gilead, Intercept, Janssen, Merck & Co., Inc., Novo Nordisk, Springbank, and Trek; has received research funding from Assembly Biosciences, Bristol-Myers Squibb, Enanta, Gilead, and Merck & Co., Inc.; and has conducted speaking and teaching activities for AbbVie, Gilead, Intercept, and Merck. F.P. is on the speakers' bureau for and has received grants from Merck, Gilead, and AbbVie. R.F.-M. has received research grants from Merck. E.C.V. is an advisor for Gilead. S.B. and L.C. are employed by Merck & Co., Inc., Kenilworth, NJ. P.H., M.R., and H.P. are employed by and own stock in Merck & Co., Inc., Kenilworth, NJ. E.D.C. was formerly employed by and owns stock in Merck & Co., Inc., Kenilworth, NJ, and is currently employed by and owns stock in Bristol-Myers Squibb. G.T.E. discloses no conflicts.
Study Highlights.
WHAT IS KNOWN
✓ EBR/GZR is approved for the treatment of HCV GT 1/4 infection.
✓ EBR/GZR is not approved for use in people with Child-Pugh B cirrhosis.
WHAT IS NEW HERE
✓ This study assessed EBR plus low-dose GZR in participants with Child-Pugh B cirrhosis.
✓ EBR 50 mg plus GZR 50 mg was effective and well tolerated in this study.
TRANSLATIONAL IMPACT
✓ Although EBR plus reduced-dose GZR is not available for people with CP-B cirrhosis, these results complement phase 2/3 trial data and real-world experience with EBR/GZR.
Supplementary Material
ACKNOWLEDGEMENTS
We extend our gratitude to the patients, their families, investigators, and site personnel who participated in this study. The primary investigators in the C-SALT study were Saleh Alqahtani, Michael R. Charlton, Raymond Chung, Gregory T. Everson, Roberto J. Firpi-Morell, Ira M. Jacobson, Paul Y. Kwo, Fred Poordad, Andrew H. Talal, and Elizabeth C. Verna.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A5
Preliminary data from the C-SALT study were reported at The International Liver Congress, sponsored by the European Association for the Study of the Liver (EASL); April 22–26, 2015; Vienna, Austria.
REFERENCES
- 1.Mücke MM, Mücke VT, Lange CM, et al. Special populations: treating hepatitis C in patients with decompensated cirrhosis and/or advanced renal impairment. Liver Int 2017;37(Suppl 1):19–25. [DOI] [PubMed] [Google Scholar]
- 2.Davis GL, Alter MJ, El-Serag H, et al. Aging of hepatitis C virus (HCV)-infected persons in the United States: A multiple cohort model of HCV prevalence and disease progression. Gastroenterology 2010;138:513–21.e6. [DOI] [PubMed] [Google Scholar]
- 3.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012;308:2584–93. [DOI] [PubMed] [Google Scholar]
- 4.Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol 2011;9:509–16.e1. [DOI] [PubMed] [Google Scholar]
- 5.Curry MP, O'Leary JG, Bzowej N, et al. For the ASTRAL-4 Investigators. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med 2015;373:2618–28. [DOI] [PubMed] [Google Scholar]
- 6.Manns M, Samuel D, Gane EJ, et al. ; for the SOLAR-2 Investigators. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis 2016;16:685–97. [DOI] [PubMed] [Google Scholar]
- 7.Poordad F, Schiff ER, Vierling JM, et al. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology 2016;63:1493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster GR, Irving WL, Cheung MC, et al. ; on behalf of HCV research, UK. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol 2016;64:1224–31. [DOI] [PubMed] [Google Scholar]
- 9.Backus LI, Belperio PS, Shahoumian TA, et al. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology 2016;64:405–14. [DOI] [PubMed] [Google Scholar]
- 10.Modi AA, Nazario H, Trotter JF, et al. Safety and efficacy of simeprevir plus sofosbuvir with or without ribavirin in patients with decompensated genotype 1 hepatitis C cirrhosis. Liver Transpl 2016;22:281–6. [DOI] [PubMed] [Google Scholar]
- 11.Aqel BA, Pungpapong S, Leise M, et al. Multicenter experience using simeprevir and sofosbuvir with or without ribavirin to treat hepatitis C genotype 1 in patients with cirrhosis. Hepatology 2015;62:1004–12. [DOI] [PubMed] [Google Scholar]
- 12.Ioannou GN, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology 2016;151:457–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.HCVguidelines.org. Recommendations for testing, managing, and treating hepatitis C. (https://www.hcvguidelines.org/sites/default/files/full-guidance-pdf/HCVGuidance_September_21_2017_e.pdf). Accessed November 6, 2017.
- 14.Coburn CA, Meinke PT, Chang W, et al. Discovery of MK-8742: An HCV NS5a inhibitor with broad genotype activity. ChemMedChem 2013;8:1930–40. [DOI] [PubMed] [Google Scholar]
- 15.Harper S, McCauley JA, Rudd MT, et al. Discovery of MK-5172, a macrocyclic hepatitis C virus NS3/4a protease inhibitor. ACS Med Chem Lett 2012;3:332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rockstroh JK, Nelson M, Katlama C, et al. Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): A non-randomised, open-label trial. Lancet HIV 2015;2:e319–e327. [DOI] [PubMed] [Google Scholar]
- 17.Roth D, Nelson DR, Bruchfeld A, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): A combination phase 3 study. Lancet 2015;386:1537–45. [DOI] [PubMed] [Google Scholar]
- 18.Zeuzem S, et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic HCV genotype 1, 4, or 6 infection: A randomized trial. Ann Intern Med 2015;163:1–13. [DOI] [PubMed] [Google Scholar]
- 19.Dore GJ, Altice F, Litwin AH, et al. ; on behalf of the C-EDGE CO-STAR Study Group. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med 2016;165:625–34. [DOI] [PubMed] [Google Scholar]
- 20.Kwo P, Gane EJ, Peng CY, et al. Effectiveness of elbasvir and grazoprevir combination, with or without ribavirin, for treatment-experienced patients with chronic hepatitis C infection. Gastroenterology 2017;152:164–75.e4. [DOI] [PubMed] [Google Scholar]
- 21.CATIE. Zepatier for hepatitis C approved in Canada. (http://www.catie.ca/en/catienews/2016-01-29/zepatier-hepatitis-C-approved-canada). Accessed November 6, 2017.
- 22.Zepatier [prescribing information]. Kenilworth, NJ: Merck & Co., Inc., 2018. [Google Scholar]
- 23.European Medicines Agency. EPAR summary for the public. Zepatier: elbasvir/grazoprevir. (http://wwweEma.europa.eu/docs/en_gb/document_library/EPAR_-_Summary_For_The_Public/Human/004126/WC500211238.Pdf). Accessed November 6, 2017.
- 24.Kramer JR, Puenpatom A, Erikson K, et al. Real world experience with elbasvir/grazoprevir in the Veterans Affairs Healthcare System [abstract]. J Hepatol 2017;66(Suppl):S54–5. [Google Scholar]
- 25.Olysio [prescribing information]. Latina, Italy: Janssen-Cilag SpA, 2017. [Google Scholar]
- 26.Viekira Pak [prescribing information]. North Chicago, IL: AbbVie, 2016. [Google Scholar]
- 27.Vosevi [prescribing information]. Foster City, CA: Gilead Sciences, 2017. [Google Scholar]
- 28.Mavyret [prescribing information]. North Chicago, IL: AbbVie, 2017. [Google Scholar]
- 29.Palatini P, De Martin S. Pharmacokinetic drug interactions in liver disease: An update. World J Gastroenterol 2016;22:1260–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.