Supplemental Digital Content is available in the text.
Abstract
Background:
Unconventional perfusion flaps offer multiple potential advantages compared with traditional flaps. Although there are numerous experimental articles on unconventional perfusion flaps, the multiple animal species involved, the myriad vascular constructions used, and the frequently conflicting data reported make synthesis of this information challenging. The main aim of this study was to perform a systematic review and meta-analysis of the literature on the experimental use of unconventional perfusion flaps, to identify the best experimental models proposed and to estimate their global survival rate.
Methods:
The authors performed a systematic review and meta-analysis of all articles written in English, French, Italian, Spanish, and Portuguese on the experimental use of unconventional perfusion flaps and indexed to PubMed from 1981 until February 1, 2017.
Results:
A total of 68 studies were found, corresponding to 86 optimized experimental models and 1073 unconventional perfusion flaps. The overall unconventional perfusion flap survival rate was 90.8 percent (95 percent CI, 86.9 to 93.6 percent; p < 0.001). The estimated proportion of experimental unconventional perfusion flaps presenting complete survival or nearly complete survival was 74.4 percent (95 percent CI, 62.1 to 83.7 percent; p < 0.001). The most commonly reported animal species in the literature were the rabbit (57.1 percent), the rat (26.4 percent), and the dog (14.3 percent). No significant differences were found in survival rates among these species, or among the diverse vascular patterns used.
Conclusion:
These data do not differ significantly from those reported regarding the use of unconventional perfusion flaps in human medicine, suggesting that rabbit, rat, and canine experimental unconventional perfusion flap models may adequately mimic the clinical application of unconventional perfusion flaps.
Unconventional perfusion flaps have been used increasingly in the clinical setting for the past four decades, offering multiple advantages relative to traditional flaps.1 These flaps comprise arterialized venous flaps and venous flaps. Arterialized venous flaps receive an arterial inflow at one end of their venous system, and drain their blood through another portion of their venous system to either a vein or an artery.1 Venous flaps receive venous blood through one end of their venous system and drain their blood into a venous outflow.1 Unconventional perfusion flaps present various potential advantages compared with conventional flaps.1,2 Being composite blocks of tissues perfused solely through their venous system, their dissection is relatively simple, expeditious, and not associated with major donor-site morbidity. Moreover, these flaps are intrinsically thin and pliable. These last features are potentially highly advantageous for reconstructing shallow defects, particularly in mobile regions where the integument is thin.1,3–6 Finally, as they are usually tailored around the superficial venous system, which is often visible through the skin, their harvesting precludes the need for ancillary preoperative tests. Consequently, unconventional perfusion flaps are particularly useful for emergent reconstruction, as occurs in trauma cases.1,7
Although there are several clinical and experimental articles on unconventional perfusion flaps, the multiple animal species involved, the myriad vascular constructions used, and the frequently conflicting data reported make synthesis of this information challenging.1,3–6,8 Thus, the main aim of this study was to perform a systematic review and meta-analysis of the literature on the experimental use of unconventional perfusion flaps, in order to identify the best experimental models proposed and to estimate their global survival rate. Secondarily, this study aimed at estimating the unconventional perfusion flaps survival rate for each animal species and vascular patterns used in these optimized experimental models.
Methods
On February 1, 2017, the authors searched the PubMed database concerning experimental animal models of unconventional perfusion flaps (Fig. 1). The following terms were used: “arterialized venous flap,” “arterialised venous flap,” “unconventional flap,” “unconventional perfusion flap,” “nonconventional flap,” “nonconventional perfusion flap,” “venous flap,” and “venous perfusion flap.” These search terms were combined with the Boolean operators ‘‘OR” and “AND.”
Fig. 1.
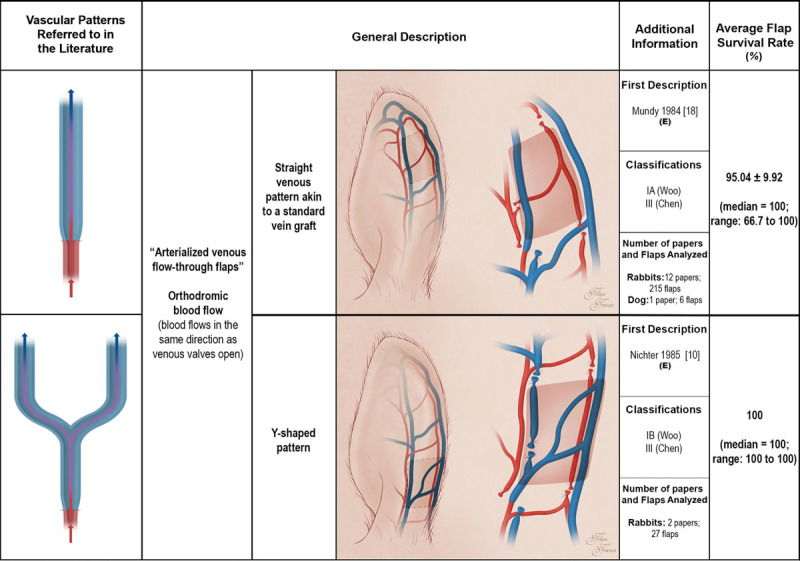
Schematic representation of arterialized venous flaps with an orthodromic blood perfusion performed in experimental models according to the literature. Arterialized venous flaps receive arterial blood through one or more of their veins. Arterialized venous flap venous drainage occurs through one or more veins to neighboring veins and/or arteries. Red areas represent arterial blood flow. Blue and purple regions denote venous and mixed arterial and venous blood, respectively. The arrows specify the direction of blood flow. The curved lines inside the vessels illustrate venous valves. First description: in cases where the first description of the type of unconventional pattern was not performed in the experimental setting (E), the description in the clinical setting is also indicated. Classifications: The classifications used were those proposed by Woo et al. (Woo SH, Kim KC, Lee GJ, et al. A retrospective analysis of 154 arterialized venous flaps for hand reconstruction: An 11-year experience. Plast Reconstr Surg. 2007;119:1823–1838) and by Chen et al. (Chen HC, Tang YB, Noordhoff MS. Four types of venous flaps for wound coverage: A clinical appraisal. J Trauma 1991;31:1286–1293). The drawings are not to scale.
Inclusion Criteria
All articles reporting experimental animal studies, written in English, Spanish, Portuguese, French, or Italian and describing flap survival and/or necrosis qualitatively and/or quantitatively, were selected.
Exclusion Criteria
The following articles/experimental models were excluded from the analysis:
Studies written in languages different from those mentioned above.
Articles referring exclusively to human or dissection studies.
Studies/experimental models addressing solely histologic features or pharmacologic and/or genetic manipulation of flaps with no information regarding quantitative or qualitative evaluation of flap necrosis or survival.
Studies/experimental models whose flap vascular network included total blood flow reversal at the expense of one major artery without apparent potential clinical benefit.
Studies/experimental models addressing revascularization of the extremities by reverse circulation.
Articles/experimental models describing exclusively prefabricated flaps with arteriovenous fistulas, because these flaps tend to behave in a manner similar to that of conventional perfusion flaps.9,10
In each article included for analysis, individuals whose vascular anastomoses presented thrombosis were excluded from the analysis. The title and abstract of all identified studies were examined independently by three reviewers (D.C., D.T., and T.C.). In cases where suitability of a given study for inclusion in the review was not clear, the entire article was obtained and evaluated for appropriateness. Furthermore, the references contained in the retrieved articles were scanned by the three independent reviewers, to obtain further articles that were missed in the first-round search. All articles were acquired in their full-text version and read independently by the three reviewers.
For each study, the following variables were recorded: year of publication, nationality, animal species and strains used, and experimental animal sex. When a study reported more than one vascular construction, the one (or ones) with a better flap survival rate (p < 0.05) was (or were) chosen as optimized experimental models. These models were used as individual units for the sake of subsequent statistical analysis.
For each experimental model, the following parameters were assessed: number of animals used; anatomical region of the unconventional perfusion flap donor site; vascular pattern used to perfuse the flap1 [when considering rabbit ears, the authors took into consideration that the largest veins (central vein, anterior marginal vein, and posterior marginal vein] are devoid of venous valves11,12)]; vascularization of the recipient wound bed (well-vascularized if the flap was placed over viable muscle, fascia, fat, synovial tissue, epitenon or granulation tissue; and nonvascularized, if the flap was placed over bone, cartilage, or prosthetic material); application of an impermeable barrier between the flap and the recipient bed (to prevent diffusion of oxygen and nutrients and/or neoangiogenesis from the wound to the deep aspect of the flap); resort to flap delay procedures; flap composition; flap innervation; flap survival rate; and percentage of flaps that presented complete survival (defined as 100 percent survival area or superficial necrosis with self-healing of the flap’s surface at its latest evaluation), nearly complete survival (considered 85 to 100 percent flap survival or unspecified inconsequential “marginal necrosis”), significant necrosis (>15 percent flap necrosis), and complete necrosis (100 percent flap necrosis or “nonsurviving flaps”). If in doubt, a higher necrosis category was considered for each experimental model. The numeric values of necrosis considered were always those reported on the last day of the experiments described in each individual article.
The presence and nature of complications were also recorded (epidermolysis, flap congestion, and venous insufficiency by themselves were not considered complications, as they were reportedly present in the first few days after surgery according to most authors).13 If the damage to the skin extended deeper than the epidermis, flap necrosis was considered to be present, as described above.
When estimating effect sizes for the entire population, the authors included only studies with at least three animals, to minimize publication bias. Whenever individual animal data were present in articles, they were used for individual data meta-analysis.14
The data were retrieved from the available literature, each parameter at a time, from each article in turn. Finally, the data were inserted into an Excel database (Microsoft Corp., Redmond, Wash.). When discrepancies were found in data retrieval, the articles were reanalyzed by the three reviewers independently.
Statistical Analysis
Quantitative variables were expressed as average ± SD. Qualitative variables were expressed as percentages. IBM SPSS Version 21.0 software (IBM Corp., Armonk, N.Y.) was used for descriptive and inferential statistical analysis.
Analysis of variance and t tests were used to compare averages in normally distributed data.15 Kruskal-Wallis and Mann-Whitney tests were used to compare median values in nonnormally distributed data. Proportions were analyzed with the chi-square test or Fisher’s exact test. Dichotomous variables were compared with the binomial test. Association between numerical variables was assessed using the Pearson correlation coefficient.
Comprehensive Meta-Analysis 2.0 software (Biostat, Englewood, N.J.) was used to estimate population summary. Study heterogeneity for each parameter was assessed using the Cochran Q test, I2, and τ2 statistics.16 A two-tail value of p < 0.05 was used.
RESULTS
Sixty-eight studies on the use of unconventional perfusion flaps in the experimental setting were identified. (See Figure, Supplemental Digital Content 1, which shows the data collection from the literature. A total of 68 studies were retrieved from the literature, corresponding to data from 1073 individual unconventional perfusion flaps, http://links.lww.com/PRS/D414.) Overall, this corresponded to a total of 1073 flaps, and 86 optimized experimental models of unconventional perfusion flaps. [See Table, Supplemental Digital Content 2, which shows the summary of the studies on unconventional perfusion flaps in experimental animal models identified in the systematic review and included in the meta-analysis.12,17,20,41,43,49-111 For each study, the optimized experimental animal model is identified, along with its characteristics. These experimental models were those that presented better flap survival rates in each published article (p < 0.05). n, number of flaps in each optimized experimental model; n/a, information not available; Min., minimum; Max., maximum. Categorical flap survival: CN, complete necrosis; CS, complete survival; NCS, nearly complete survival; SN, significant necrosis. Strain: BC, Big Chinchilla rabbit; F, Fischer rat; J, Japanese white rabbit; L, Lewis rat; M, mongrel dog; NZ, New Zealand white rabbit; SD, Sprague-Dawley rat; W, Wistar rat; Y, Yorkshire pig. Gender: F, female; M, male; M + F, both males and females. Flap donor site: A/G, abdomen and/or groin; D, dorsum; E, ear; F, forelimb; H, hindlimb; T/A, thorax and abdomen. Flap vascular pattern: 1, type Ia arterialized venous flap according to the Woo classification; 2, type Ib arterialized venous flap according to the Woo classification; 3, type IIa arterialized venous flap according to the Woo classification; 4, type IIb arterialized venous flap according to the Woo classification; 5, type III arterialized venous flap according to the Woo classification; 6, pedicled arterialized venous flap; 7, type I venous flap according to the Chen classification; 8, type IIa venous flap according to the Chen classification; 9, type IIb venous flap according to the Chen classification; 10, sliding venous flap. Wound bed blood supply: B/C, bone or cartilage; IB, impermeable barrier underneath the flap; SE, skeletonized ear; WV, well vascularized. Flap composition: B/C, includes bone and/or cartilage (other than skin with its appendages and subcutaneous tissue); FC, fasciocutaneous (skin with its appendages and subcutaneous tissue); MFC, myofasciocutaneous (skin with its appendages, subcutaneous tissue and muscle/portion of muscle), http://links.lww.com/PRS/D415.]
Studies publication spanned from 1981 to 2017. Half of the identified articles were published until 1994, and three-quarters of these were published until 2003. Studies were performed in 20 different countries12,17,20,41,43,49-111 (cited in Table, Supplemental Digital Content 2, http://links.lww.com/PRS/D415). The most commonly used animal species was the rabbit (57.1 percent), followed by the rat (26.4 percent), the dog (14.3 percent), and the pig (2.2 percent). Murine models were not reported in the literature.
On average, each optimized experimental model was developed based on the dissection of 12.14 ± 8.01 unconventional perfusion flaps (median, 10; range, two to 40). Regarding animal sex, most studies were conducted on male animals (26.4 percent), or using both male and female animals (15.4 percent). Only 3.3 percent of studies reported experiments conducted exclusively on female animals. However, the majority of the studies did not specify the sex of experimental animals (54.9 percent). Concerning anatomical location, most unconventional perfusion flap models were performed in the ear (36.3 percent). In decreasing order of frequency, the next most common anatomical regions used were the abdomen and/or groin (25.3 percent), the thorax and the abdomen (18.7 percent), the hindlimb (13.2 percent), the forelimb (4.4 percent), and the dorsum (2.2 percent). The most common anatomical regions used in each species to produce unconventional perfusion flaps were as follows: the ear in the rabbit (63.5 percent), the abdomen and/or groin in the rat (58.3 percent), and the hindlimb in the dog (69.2 percent) and in the pig (100 percent). Multiple vascular patterns were reported in the literature (Figs. 1 through 5).
Fig. 5.
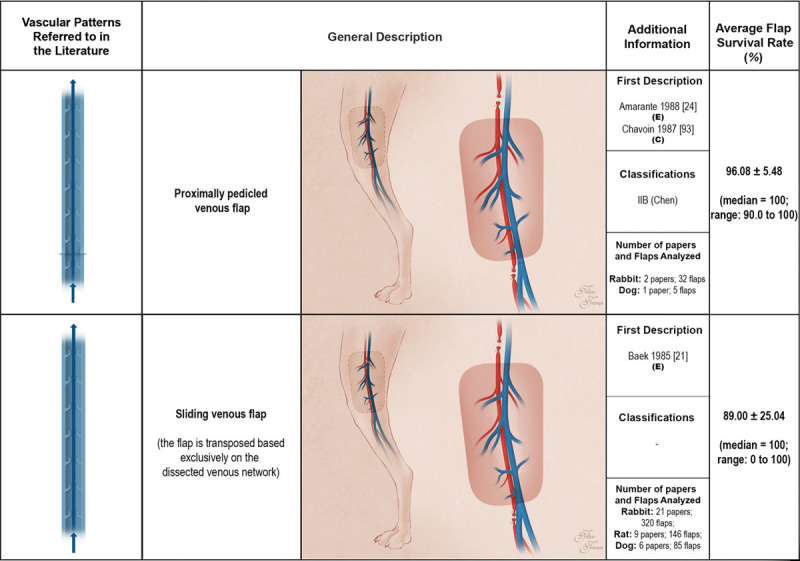
Schematic representation of the proximally pedicled venous flap and of the sliding venous flap performed in experimental models according to the literature. These flaps receive venous blood through one or more of their veins. Venous flap venous drainage occurs through one or more veins to neighboring veins. Red areas represent arterial blood flow. Blue and purple regions denote venous and mixed arterial and venous blood, respectively. The arrows specify the direction of blood flow. The curved lines inside the vessels illustrate venous valves. First description: in cases where the first description of the type of unconventional pattern was not performed in the experimental setting (E), the description in the clinical setting (C) is also indicated. Classifications: The classifications used were those proposed by Woo et al. (Woo SH, Kim KC, Lee GJ, et al. A retrospective analysis of 154 arterialized venous flaps for hand reconstruction: An 11-year experience. Plast Reconstr Surg. 2007;119:1823–1838) and by Chen et al. (Chen HC, Tang YB, Noordhoff MS. Four types of venous flaps for wound coverage: A clinical appraisal. J Trauma 1991;31:1286–1293). The drawings are not to scale.
Fig. 2.
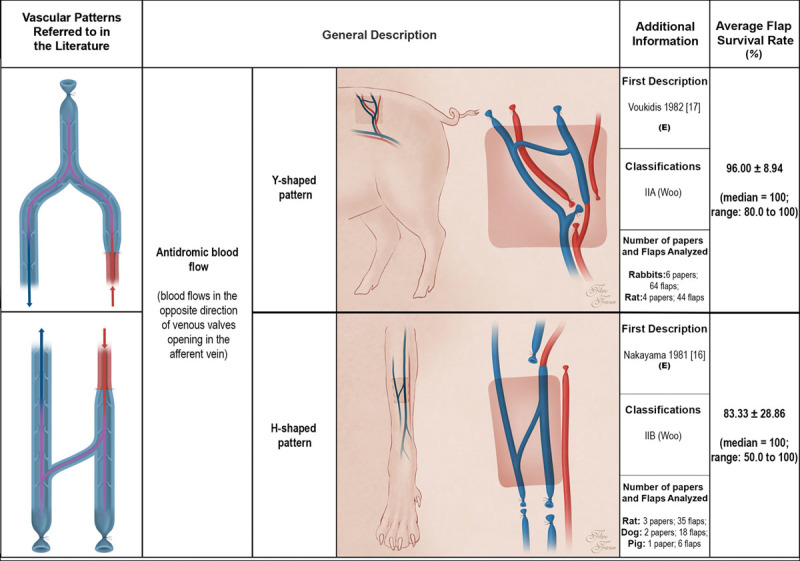
Schematic representation of arterialized venous flaps with an antidromic blood flow performed in experimental models according to the literature. Arterialized venous flaps receive arterial blood through one or more of their veins. Arterialized venous flap venous drainage occurs through one or more veins to neighboring veins and/or arteries. Red areas represent arterial blood flow. Blue and purple regions denote venous and mixed arterial and venous blood, respectively. The arrows specify the direction of blood flow. The curved lines inside the vessels illustrate venous valves. First description: in cases where the first description of the type of unconventional pattern was not performed in the experimental setting (E), the description in the clinical setting is also indicated. Classifications: The classifications used were those proposed by Woo et al. (Woo SH, Kim KC, Lee GJ, et al. A retrospective analysis of 154 arterialized venous flaps for hand reconstruction: An 11-year experience. Plast Reconstr Surg. 2007;119:1823–1838) and by Chen et al. (Chen HC, Tang YB, Noordhoff MS. Four types of venous flaps for wound coverage: A clinical appraisal. J Trauma 1991;31:1286–1293). The drawings are not to scale.
Fig. 3.
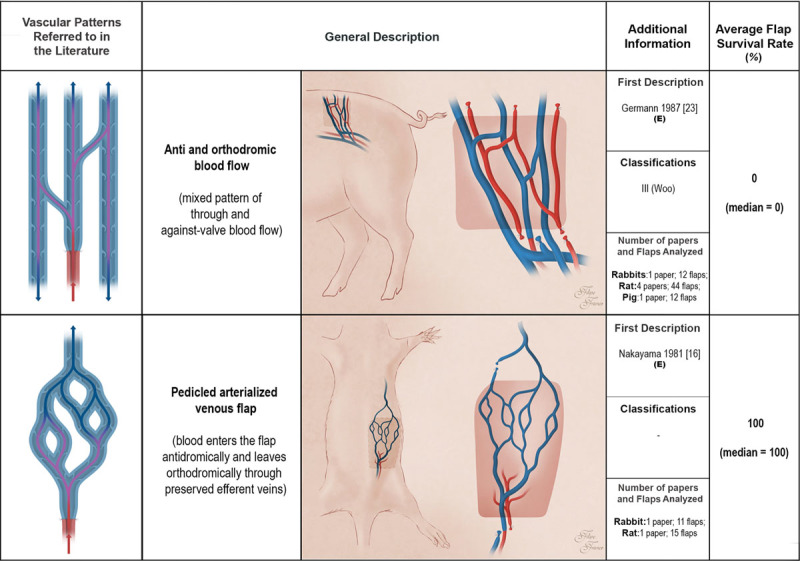
Schematic representation of arterialized venous flaps with an antidromic and an orthodromic blood perfusion, and pedicled arterialized venous flaps performed in experimental models according to the literature. Arterialized venous flaps receive arterial blood through one or more of their veins. Arterialized venous flap venous drainage occurs through one or more veins to neighboring veins and/or arteries. Red areas represent arterial blood flow. Blue and purple regions denote venous and mixed arterial and venous blood, respectively. The arrows specify the direction of blood flow. The curved lines inside the vessels illustrate venous valves. First description: in cases where the first description of the type of unconventional pattern was not performed in the experimental setting (E), the description in the clinical setting is also indicated. Classifications: The classifications used were those proposed by Woo et al. (Woo SH, Kim KC, Lee GJ, et al. A retrospective analysis of 154 arterialized venous flaps for hand reconstruction: An 11-year experience. Plast Reconstr Surg. 2007;119:1823–1838) and by Chen et al. (Chen HC, Tang YB, Noordhoff MS. Four types of venous flaps for wound coverage: A clinical appraisal. J Trauma 1991;31:1286–1293). The drawings are not to scale.
Fig. 4.
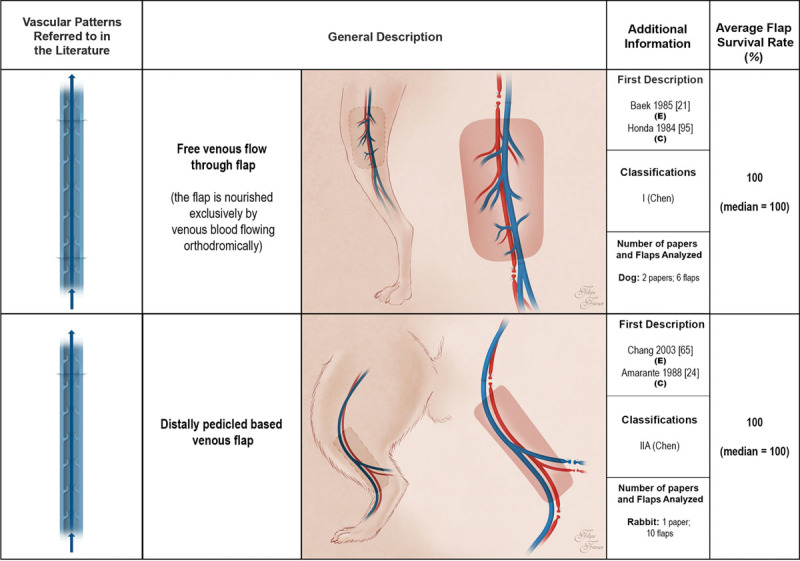
Schematic representation of the free venous flow-through flap and the distally pedicled venous flap performed in experimental models according to the literature. These flaps receive venous blood through one or more of their veins. Drainage of venous flaps occurs through one or more veins to neighboring veins. Red areas represent arterial blood flow. Blue and purple regions denote venous and mixed arterial and venous blood, respectively. The arrows specify the direction of blood flow. The curved lines inside the vessels illustrate venous valves. First description: in cases where the first description of the type of unconventional pattern was not performed in the experimental setting (E), the description in the clinical setting (C) is also indicated. Classifications: The classifications used were those proposed by Woo et al. (Woo SH, Kim KC, Lee GJ, et al. A retrospective analysis of 154 arterialized venous flaps for hand reconstruction: An 11-year experience. Plast Reconstr Surg. 2007;119:1823–1838) and by Chen et al. (Chen HC, Tang YB, Noordhoff MS. Four types of venous flaps for wound coverage: A clinical appraisal. J Trauma 1991;31:1286–1293). The drawings are not to scale.
The most common vascular patterns were, in decreasing order of frequency, sliding venous flaps (40.7 percent), type IA arterialized venous flaps (20.9 percent), type IIA arterialized venous flaps (8.8 percent), pedicled arterialized venous flaps (6.6 percent), and both type IIB arterialized venous flaps and proximally pedicled venous flaps (5.5 percent each). Infrequent vascular patterns included type IB arterialized venous flaps (4.4 percent), type III arterialized venous flaps (3.3 percent), free venous flow-through (2.2 percent), and distally based venous flaps (1.1 percent).
In the majority of cases (54.7 percent), flaps were placed over well-perfused wound beds. In 18.6 percent of cases, flaps were placed over bare bone or cartilage. (See Figure, Supplemental Digital Content 3, which shows a star plot illustrating unconventional perfusion flap models’ features in different animal species. AVF, arterialized venous flap; VF, venous flap; UPF, unconventional perfusion flap, http://links.lww.com/PRS/D416.) In 15.6 percent of the models, an impermeable barrier was placed under the unconventional perfusion flap to prevent flap nutrition or gas exchanges from the wound bed. When rabbits were used, unconventional perfusion flaps were frequently based on skeletonized ears or ear segments. In these cases (11.6 percent of all experimental models), the flap was also completely dependent on its own vascular pedicle, not being able to depend on a vascularized wound bed. In 18.7 percent of cases, some sort of surgical delay procedure was performed before flap elevation to increase flap survival.
Concerning flap composition, most unconventional perfusion flaps were fasciocutaneous (85.7 percent; p < 0.001). Flaps included bone and/or cartilage in 13.2 percent of cases. There was only one study reporting a myofasciocutaneous flap, corresponding to 1.1 percent of all optimized experimental models.17 In almost all cases, unconventional perfusion flaps were noninnervated (91.2 percent; p < 0.001).
Meta-analysis of experimental unconventional perfusion flaps using a random effects model estimated an overall flap survival rate of 90.8 percent (95 percent CI, 86.9 to 93.6 percent; p < 0.001). [See Figure, Supplemental Digital Content 4, which shows a forest plot of all studies reporting unconventional perfusion flap survival rates. Meta-analysis of experimental unconventional perfusion flaps using a random effects model estimated an overall flap survival rate of 90.8 percent (95 percent CI, 86.9 to 93.6 percent; p < 0.001), http://links.lww.com/PRS/D417.] Study heterogeneity assessment for this parameter was as follows: Cochran Q = 134.98; p < 0.001; I2 47.40; and τ2 = 1.24. The funnel plot of the studies used to produce this estimate suggested there was evidence of publication bias regarding this parameter, which was further supported by the Egger test (p < 0.001).18,19 [See Figure, Supplemental Digital Content 5, which shows a funnel plot of the studies used to estimate the survival rate of the experimental unconventional perfusion flaps. This graphic suggests there is publication bias. This was confirmed by the application of the Egger test (p < 0.001). Study heterogeneity assessment for this parameter was as follows: Cochran Q = 134.98; p < 0.001; I2 = 47.40; and τ2 = 1.24, http://links.lww.com/PRS/D418.]
The estimated proportion of experimental unconventional perfusion flaps presenting complete survival or nearly complete survival was 74.4 percent (95 percent CI, 62.1 to 83.7 percent; p < 0.001). [See Figure, Supplemental Digital Content 6, which shows a forest plot of all studies describing the proportion of unconventional perfusion flaps presenting complete survival or nearly complete survival. The estimated proportion of experimental unconventional perfusion flaps presenting complete survival or nearly complete survival was 74.4 percent (95 percent CI, 62.1 to 83.7 percent; p < 0.001), http://links.lww.com/PRS/D419.] Evaluation of study heterogeneity regarding this variable was as follows: Cochran Q = 162.77; p < 0.001; I2 = 71.74; and τ2 = 2.58. The funnel plot regarding the estimation of this parameter suggested the presence of publication bias. [See Figure, Supplemental Digital Content 7, which shows a funnel plot of the studies used to estimate proportion of experimental unconventional perfusion flaps that presented complete survival or nearly complete survival. This graphic might suggest there is publication bias. However, the Egger test failed to confirm this assumption (p = 0.342). Evaluation of study heterogeneity regarding this variable was as follows: Cochran Q = 162.77; p < 0.001; I2 = 71.74; and τ2 = 2.58, http://links.lww.com/PRS/D420.] However, the Egger test failed to support this assumption (p = 0.342).
In all animal species except the pig, most flaps presented complete or nearly complete survival. [See Figure, Supplemental Digital Content 8, which shows bar charts illustrating the survival of the most common types of unconventional perfusion flaps according to animal species and vascular pattern used. AVF, arterialized venous flap; UPF, unconventional perfusion flap. There were no statistically significant differences between the different types of unconventional perfusion flaps (p < 0.05), http://links.lww.com/PRS/D421.] In the pig, there was only one study using a type III arterialized venous fasciocutaneous flap. In this model, all flaps suffered complete necrosis.20 No significant differences were found between unconventional perfusion flap necrosis rates among the other animal species. In the same way, no significant differences were found between survival rates of the different vascular patterns (see Figure, Supplemental Digital Content 8, http://links.lww.com/PRS/D421). Similarly, no differences were found in unconventional perfusion flap survival rates regarding sex; anatomical location where the flap was produced; wound bed blood supply, including the placement or not of an impermeable barrier underneath the flap; resort to surgical delay procedures; and unconventional perfusion flap histologic composition and/or innervation. All articles addressing the clinical features of unconventional perfusion flaps described flap congestion, edema, venous engorgement, blister formation, and/or epidermolysis as constant findings in the first days after surgery.
DISCUSSION
As far as the authors could determine, this article is the first systematic review and meta-analysis on the experimental use of unconventional perfusion flaps. Using a random effects model, the authors estimated the unconventional perfusion flap survival rate to be 90.8 percent (see Figure, Supplemental Digital Content 4, http://links.lww.com/PRS/D417). Moreover, using a similar methodology, the estimated proportion of unconventional perfusion flaps that survive completely or nearly completely was 74.4 percent (see Figure, Supplemental Digital Content 6, http://links.lww.com/PRS/D419). These data indicate that, according to the available literature, the majority of unconventional perfusion flaps performed in the experimental setting survived, although a significant fraction of these flaps presented a variable degree of necrosis.
Interestingly, the estimated overall unconventional perfusion flap survival rate in the experimental setting (90.8 percent) was similar to that reported by the authors in a previous meta-analysis addressing the clinical application of unconventional perfusion flaps (89. percent).1 In contrast, the estimated proportion of unconventional perfusion flaps presenting complete or nearly complete survival was 74.4 percent in the experimental setting, compared with 92.0 percent in the clinical context.1 However, in both meta-analyses, the majority of unconventional perfusion flaps presented complete or nearly complete survival. The differences observed may be partially explained by the different vascular patterns used in the experimental and clinical contexts. In the experimental setting, the most common vascular constructs were, in decreasing order of frequency, sliding venous flaps (40.7 percent), type IA arterialized venous flaps (20.9 percent), and type IIA arterialized venous flaps (8.8 percent). In the clinical context, the patterns most frequently reported were type IA arterialized venous flaps (33.5 percent), type IV arterialized venous arterial flaps (14.8 percent), and type I venous flap (12.5 percent).1,21
Contrary to what could be expected, no differences were found in unconventional perfusion flap survival rates regarding vascular pattern, sex, anatomical location, wound bed blood supply (including the placement or not of an impermeable barrier underneath the flap), resort to surgical delay procedures, and unconventional perfusion flap histologic composition and/or innervation. This may be attributable to either inherent meta-analysis limitations (i.e., publication bias, as negative or neutral results are less likely to be published and thus to be included in studies such as this one) or the lack of biological association.22–25 Therefore, further experimental studies addressing these issues are warranted.
The animal species most commonly used to produce unconventional perfusion flaps was the rabbit (57.1 percent), followed by the rat (26.4 percent), the dog (14.3 percent), and the pig (2.2 percent). Mice were not used for this purpose. This contrasts with the majority of the literature on experimental flap surgery, which indicates that the rat is the most widely used animal model.26 This is certainly because rabbits and rats are easy to obtain and keep, relatively inexpensive, and sufficiently large for microvascular procedures to be performed.26–28 Although dogs and pigs have larger vessels, they are more expensive to obtain and to maintain. In addition, the use of these animal species has been submitted to increasingly stringent control by ethical committees and animal welfare bodies.29–31 Noteworthily, there were no significant differences in unconventional perfusion flap survival rates in the most commonly used animal species (i.e., rabbit, rat, and dog) (see Figure, Supplemental Digital Content 8, http://links.lww.com/PRS/D421).
Concerning flap composition, the majority of unconventional perfusion flaps were fasciocutaneous (85.7 percent). Flaps included bone and/or cartilage in 13.2 percent of cases. It was possible to identify a single study reporting myofasciocutaneous flaps, corresponding to 1.1 percent of all experimental models.17 This adds strength to the widely held belief that unconventional perfusion is most adequately suited to perfuse tissues with low metabolic needs, such as those of the integument, cartilage, and/or bone.1,3–6,8
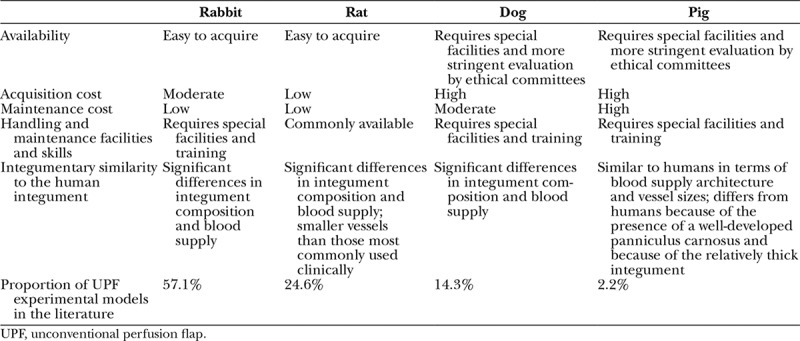
The authors feel that care must be used when extrapolating the results of this meta-analysis for the clinical setting, because there are important differences between the unconventional perfusion flaps performed experimentally and those performed in humans (Table 1). Moreover, the blood supply to the integument of various experimental animals has been shown to vary substantially from that reported in humans.32,33 For example, Taylor and Minabe32 and Taylor and Pan32 have shown that in loose-skinned animals, such as the rabbit, the rat, or the dog, there is a preponderance of the direct cutaneous vessels, compared with the dominance of the musculocutaneous vessels in humans and pigs. Furthermore, experimental animals, particularly those with loosely draped skin, possess a layer of smooth muscle in the deep aspect of the integument known as panniculus carnosus, which is associated with vascular plexuses of its own.27,34 In humans, this layer is virtually absent in the majority of the body, being represented mostly by the platysma and the palmaris longus muscles. In pigs, the panniculus carnosus layer is present in most of the integument. However, it is firmly adherent to overlying skin and to the underlying muscle fascia, making pig skin apparently a more suitable model for comparison with the human integument.35,36 Despite all these data, the only study conducted on the pig hindlimb to produce a type III arterialized venous fasciocutaneous flap revealed complete necrosis of all flaps.20 This may be explained by the greater thickness of the pig’s integument relative to that of the other experimental animal species and even humans in the usual locations where these flaps are harvested in this latter species.1,35 In fact, according to most authors, unconventional perfusion flaps depend, at least initially, on gas exchanges in the vicinity of the venous system of the flap, which could help explain why thin flaps present the best results in the clinical setting.1,7,37,38
Table 1.
Comparison of the Different Animal Species Used for Producing Experimental Unconventional Perfusion Flaps
Preclinical meta-analyses such as this one have become increasing frequent in recent years, as they provide a systematic and reproducible way to thoroughly identify, assess, and critically evaluate available evidence on a specific experimental subject.14 Moreover, meta-analyses of animal studies allow a quantitative estimate with maximal statistical power, precision, and generalizability to be obtained, avoiding unnecessary repetition of experiments, and thus minimizing resource waste and especially laboratory animal use and suffering.39 This is particularly useful when evidence is apparently contradictory, as is the case with unconventional perfusion flaps.40
Clinically, several pressing questions remain to be addressed to increase the efficacy of these flaps. For example, several authors have reported a higher rate of necrosis and subsequent need for another flap when there is prior bacterial contamination or colonization of the wound bed.1,38 Nevertheless, to the best of the authors’ knowledge, there are no studies on the susceptibility of unconventional perfusion flaps to different bacteria, in diverse concentrations, associated or not with foreign bodies. Furthermore, the choice of the best vascular architecture to increase the survival of unconventional perfusion flaps clinically according to the anatomical region being reconstructed, the size of the defect, and/or the composition of the flap itself would benefit from a firmer grasp of the underlying physiologic mechanisms. Although several authors have proposed algorithms based on their clinical experience and/or on the revision of clinical series, experimental data to systematically and unequivocally tackle these issues are strikingly lacking.1,2,7,38,41–44
This article presents in a systematic fashion the available information on the experimental application of unconventional perfusion flaps. This, in turn, may aid researchers in conducting studies aimed at answering several of the yet lingering questions regarding the clinical application of these flaps.
Limitations
This study may be affected by several types of bias, as occurs in all meta-analyses, particularly retrospective meta-analyses, such as this one.45,46 One of the problems of including unconventional perfusion flaps performed in different animal species using multiple vascular patterns is that there is a variable degree of inherent heterogeneity. In fact, this heterogeneity was confirmed for both population estimates using the Cochran Q test (p < 0.001). The authors tried to partially circumvent this problem by using random effects models for estimating population parameters.16
Another major potential caveat of this study was the effect of publication bias. The latter bias reflects the observation that positive results are more likely to be published compared with neutral or negative ones. The Egger test supported the presence of this type of bias for the estimate of overall unconventional perfusion flap survival but failed to support it in the estimation of the proportion of unconventional perfusion flaps whose survival was complete or nearly complete. It is widely accepted that the most efficacious way to downplay the effect of publication bias is to perform a systematic and comprehensive review of the literature, as was performed in this study.45,46 In addition, the authors have strictly adhered to the widely accepted Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist for systematic reviews and meta-analyses to minimize the risk of committing methodologic mistakes.47,48
Finally, the authors believe that although this article has the significant merit of providing a synthesis of the available literature regarding the use of experimental unconventional perfusion flaps, it contributes only modestly to understanding the mechanisms underlying the survival or necrosis of these flaps, making further studies in this field warranted. Ideally, a large-animal study in primates could help to elucidate more perfectly the mechanisms of unconventional perfusion flap perfusion, viability, and overall survival in humans. However, such a study would be logistically vexing and expensive to conduct.
CONCLUSIONS
According to the present data, the majority of unconventional perfusion flaps performed in the experimental setting survive (90.8 percent; 95 percent CI, 86.9 to 93.6 percent; p < 0.001). Furthermore, survival is complete or nearly complete in an estimated 74.4 percent of cases (95 percent CI, 62.1 percent to 83.7 percent; p < 0.001). Although the most common vascular patterns reported in the literature were sliding venous flaps (40.7 percent) and type IA arterialized venous flaps (20.9 percent), statistical scrutiny failed to establish the superiority of a given vascular pattern between different studies. There were no significant differences in unconventional perfusion flap survival rates in the most commonly used animal species (i.e., rabbit, rat, and dog). These data suggest that the rabbit, rat, and canine experimental unconventional perfusion flap models can adequately mimic the clinical application of unconventional perfusion flaps.
ACKNOWLEDGMENTS
Diogo Casal received a grant from the Programme for Advanced Medical Education sponsored by Fundação Calouste Gulbenkian, Fundação Champalimaud, Ministério da Saúde, and Fundação para a Ciência e Tecnologia, Portugal. The authors are very grateful to Filipe Franco for producing all of the drawings in this article.
Supplementary Material
Footnotes
Disclosure: The authors have no financial or commercial interest to declare in relation to the content of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the text; simply type the URL address into any Web browser to access this content. Clickable links to the material are provided in the HTML text of this article on the Journal’s website (www.PRSJournal.com).
REFERENCES
- 1.Casal D, Cunha T, Pais D, et al. Systematic review and meta-analysis of unconventional perfusion flaps in clinical practice. Plast Reconstr Surg. 2016;138:459–479. [DOI] [PubMed] [Google Scholar]
- 2.Casal D, Carvalho S, Pais D, et al. Casal D. Unconventional perfusion flaps. In: Flap Surgery. 2017:Berlin, Germany: AvidScience; 2–41. [Google Scholar]
- 3.Goldschlager R, Rozen WM, Ting JW, Leong J. The nomenclature of venous flow-through flaps: Updated classification and review of the literature. Microsurgery 2012;32:497–501. [DOI] [PubMed] [Google Scholar]
- 4.Yan H, Brooks D, Ladner R, Jackson WD, Gao W, Angel MF. Arterialized venous flaps: A review of the literature. Microsurgery 2010;30:472–478. [DOI] [PubMed] [Google Scholar]
- 5.Yan H, Zhang F, Akdemir O, et al. Clinical applications of venous flaps in the reconstruction of hands and fingers. Arch Orthop Trauma Surg. 2011;131:65–74. [DOI] [PubMed] [Google Scholar]
- 6.Jabir S, Frew Q, El-Muttardi N, Dziewulski P. A systematic review of the applications of free tissue transfer in burns. Burns 2014;40:1059–1070. [DOI] [PubMed] [Google Scholar]
- 7.Wharton R, Creasy H, Bain C, James M, Fox A. Venous flaps for coverage of traumatic soft tissue defects of the hand: A systematic review. J Hand Surg Eur Vol.2017;42:817–822. [DOI] [PubMed] [Google Scholar]
- 8.Weng W, Zhang F, Zhao B, et al. The complicated role of venous drainage on the survival of arterialized venous flaps. Oncotarget 2017;8:16414–16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo L, Pribaz JJ. Clinical flap prefabrication. Plast Reconstr Surg. 2009;124(Suppl):e340–e350. [DOI] [PubMed] [Google Scholar]
- 10.Abbase EA, Shenaq SM, Spira M, el-Falaky MH. Prefabricated flaps: Experimental and clinical review. Plast Reconstr Surg. 1995;96:1218–1225. [PubMed] [Google Scholar]
- 11.Haines P, Nichter LS, Morgan RF, Horowitz JH, Edgerton MT. A digit replantation model. Microsurgery 1985;6:70–72. [DOI] [PubMed] [Google Scholar]
- 12.Nichter LS, Haines PC. Arterialized venous perfusion of composite tissue. Am J Surg. 1985;150:191–196. [DOI] [PubMed] [Google Scholar]
- 13.Werner B. Epidermolysis. In: Stedman’s Medical Dictionary, 2000:27th ed Philadelphia: Lippincott Williams & Wilkins; 604. [Google Scholar]
- 14.Korevaar DA, Hooft L, ter Riet G. Systematic reviews and meta-analyses of preclinical studies: Publication bias in laboratory animal experiments. Lab Anim. 2011;45:225–230. [DOI] [PubMed] [Google Scholar]
- 15.Ghasemi A, Zahediasl S. Normality tests for statistical analysis: A guide for non-statisticians. Int J Endocrinol Metab. 2012;10:486–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Identifying and quantifying heterogeneity. In: Introduction to Meta-analysis. 2009:New York: Wiley; 107–125. [Google Scholar]
- 17.Fukui A, Tamai S, Maeda M, Inada Y, Mii Y, Mine T. The pedicled venous flap: An experimental study. Br J Plast Surg. 1993;46:116–121. [DOI] [PubMed] [Google Scholar]
- 18.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Germann GK, Eriksson E, Russell RC, Mody N. Effect of arteriovenous flow reversal on blood flow and metabolism in a skin flap. Plast Reconstr Surg. 1987;79:375–380. [DOI] [PubMed] [Google Scholar]
- 21.Chen HC, Tang YB, Noordhoff MS. Four types of venous flaps for wound coverage: A clinical appraisal. J Trauma 1991;31:1286–1293. [DOI] [PubMed] [Google Scholar]
- 22.Freshwater MF. Reviewing systematic reviews and analyzing meta-analyses. J Plast Reconstr Aesthet Surg. 2014;67:291–293. [DOI] [PubMed] [Google Scholar]
- 23.Lerner F, Hamblen JL. Methodology and reporting of systematic reviews and meta-analyses. Br J Psychiatry 2013;202:75–76. [DOI] [PubMed] [Google Scholar]
- 24.Biondi-Zoccai G, Lotrionte M, Landoni G, Modena MG. The rough guide to systematic reviews and meta-analyses. HSR Proc Intensive Care Cardiovasc Anesth. 2011;3:161–173. [PMC free article] [PubMed] [Google Scholar]
- 25.Simunovic N, Sprague S, Bhandari M. Methodological issues in systematic reviews and meta-analyses of observational studies in orthopaedic research. J Bone Joint Surg Am. 2009;91(Suppl 3):87–94. [DOI] [PubMed] [Google Scholar]
- 26.Dunn RM, Mancoll J. Flap models in the rat: A review and reappraisal. Plast Reconstr Surg. 1992;90:319–328. [PubMed] [Google Scholar]
- 27.Casal D, Pais D, Iria I, et al. A model of free tissue transfer: The rat epigastric free flap. J Vis Exp. 2017;119:doi: 10.3791/55281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mapara M, Thomas BS, Bhat KM. Rabbit as an animal model for experimental research. Dent Res J (Isfahan) 2012;9:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swindle MM, Smith AC, Laber-Laird K, Dungan L. Swine in biomedical research: Management and models. ILAR J. 1994;36:1–5. [Google Scholar]
- 30.Tanaka H, Kobayashi E. Education and research using experimental pigs in a medical school. J Artif Organs 2006;9:136–143. [DOI] [PubMed] [Google Scholar]
- 31.Hasiwa N, Bailey J, Clausing P, et al. Critical evaluation of the use of dogs in biomedical research and testing in Europe. ALTEX 2011;28:326–340. [DOI] [PubMed] [Google Scholar]
- 32.Taylor GI, Minabe T. The angiosomes of the mammals and other vertebrates. Plast Reconstr Surg. 1992;89:181–215. [DOI] [PubMed] [Google Scholar]
- 33.Taylor GI, Pan WR. Dodwell P. The angiosome concept. In: The Angiosome Concept and Tissue Transfer. 2014:Vol. 1 St. Louis: Quality Medical; 354–395. [Google Scholar]
- 34.Pearl RM, Johnson D. The vascular supply to the skin: An anatomical and physiological reappraisal. Part II. Ann Plast Surg. 1983;11:196–205. [DOI] [PubMed] [Google Scholar]
- 35.Rose EH, Vistnes LM, Ksander GA. The panniculus carnosus in the domestic pig. Plast Reconstr Surg. 1977;59:94–97. [DOI] [PubMed] [Google Scholar]
- 36.Kerrigan CL, Zelt RG, Thomson JG, Diano E. The pig as an experimental animal in plastic surgery research for the study of skin flaps, myocutaneous flaps and fasciocutaneous flaps. Lab Anim Sci. 1986;36:408–412. [PubMed] [Google Scholar]
- 37.Kovács AF. Comparison of two types of arterialized venous forearm flaps for oral reconstruction and proposal of a reliable procedure. J Craniomaxillofac Surg. 1998;26:249–254. [DOI] [PubMed] [Google Scholar]
- 38.Woo SH, Kim KC, Lee GJ, et al. A retrospective analysis of 154 arterialized venous flaps for hand reconstruction: An 11-year experience. Plast Reconstr Surg. 2007;119:1823–1838. [DOI] [PubMed] [Google Scholar]
- 39.Nordmann AJ, Kasenda B, Briel M. Meta-analyses: What they can and cannot do. Swiss Med Wkly. 2012;142:w13518. [DOI] [PubMed] [Google Scholar]
- 40.Mueller KF, Briel M, Strech D, et al. Dissemination bias in systematic reviews of animal research: A systematic review. PLoS One 2014;9:e116016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weng W, Zhang F, Zhao B, et al. The complicated role of venous drainage on the survival of arterialized venous flaps. Oncotarget 2017;8:16414–16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niimi Y, Mori S, Takeuchi M. A new procedure for wrapped-negative pressure wound therapy for congestion after arterialized venous flap surgery. Clin Med Insights Case Rep. 2017;10:1179547617747279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borumandi F, Higgins JP, Buerger H, et al. Arterialized venous bone flaps: An experimental investigation. Sci Rep. 2016;6:31970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garlick JW, Goodwin IA, Wolter K, Agarwal JP. Arterialized venous flow-through flaps in the reconstruction of digital defects: Case series and review of the literature. Hand (N Y) 2015;10:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Borenstein M. Publication bias. In: Introduction to Meta-analysis. 2009:New York: Wiley; 277–292. [Google Scholar]
- 46.Sterne JA, Egger M, Mother D. Higgins JP, Green S. Addressing reporting biases. In: Cochrane Handbook for Systematic Review of Interventions. 2009:New York: Wiley; 297–333. [Google Scholar]
- 47.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One 2013;8:e83138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakayama Y, Soeda S, Kasai Y. Flaps nourished by arterial inflow through the venous system: An experimental investigation. Plast Reconstr Surg. 1981;67:328–334. [DOI] [PubMed] [Google Scholar]
- 50.Voukidis T. An axial-pattern flap based on the arterialised venous network: An experimental study in rats. Br J Plast Surg. 1982;35:524–529. [DOI] [PubMed] [Google Scholar]
- 51.Mundy JC, Panje WR. Creation of free flaps by arterialization of the venous system. Arch Otolaryngol. 1984;110:221–223. [DOI] [PubMed] [Google Scholar]
- 52.Ji SY, Chia SL, Cheng HH. Free transplantation of venous network pattern skin flap: An experimental study in rabbits. Microsurgery 1984;5:151–159. [DOI] [PubMed] [Google Scholar]
- 53.Beehary S, Hoang P, Foucher G. Arterialization of venous flaps (in French). Ann Chir Plast Esthet. 1985;30:95–97. [PubMed] [Google Scholar]
- 54.Baek SM, Weinberg H, Song Y, Park CG, Biller HF. Experimental studies in the survival of venous island flaps without arterial inflow. Plast Reconstr Surg. 1985;75:88–95. [DOI] [PubMed] [Google Scholar]
- 55.Thatte RL, Thatte MR. A study of the saphenous venous island flap in the dog without arterial inflow using a non-biological conduit across a part of the length of the vein. Br J Plast Surg. 1987;40:11–15. [DOI] [PubMed] [Google Scholar]
- 56.Amarante J, Costa H, Reis J, Soares R. Venous skin flaps: An experimental study and report of two clinical distal island flaps. Br J Plast Surg. 1988;41:132–137. [DOI] [PubMed] [Google Scholar]
- 57.Fukui A, Inada Y, Tamai S, Mizumoto S, Yajima H, Sempuku T. Skin graft including subcutaneous vein: Experimental study and clinical applications. J Reconstr Microsurg. 1988;4:223–231. [DOI] [PubMed] [Google Scholar]
- 58.Sasa M, Xian WQ, Breidenbach W, Tsai TM, Shibata M, Firrell J. Survival and blood flow evaluation of canine venous flaps. Plast Reconstr Surg. 1988;82:319–327. [DOI] [PubMed] [Google Scholar]
- 59.Inada Y, Fukui A, Tamai S, Masuhara K. Experimental studies of skin flaps with subcutaneous veins. J Reconstr Microsurg. 1989;5:249–261. [DOI] [PubMed] [Google Scholar]
- 60.Yuen QM, Leung PC. Some factors affecting the survival of venous flaps: An experimental study. Microsurgery 1991;12:60–64. [DOI] [PubMed] [Google Scholar]
- 61.Noreldin AA, Fukuta K, Jackson IT. Role of perivenous areolar tissue in the viability of venous flaps: An experimental study on the inferior epigastric venous flap of the rat. Br J Plast Surg. 1992;45:18–22. [DOI] [PubMed] [Google Scholar]
- 62.Takato T, Zuker RM, Turley CB. Viability and versatility of arterialized venous perfusion flaps and prefabricated flaps: An experimental study in rabbits. J Reconstr Microsurg. 1992;8:111–119. [DOI] [PubMed] [Google Scholar]
- 63.Chow SP, Chen DZ, Gu YD. A comparison of arterial and venous flaps. J Hand Surg Br. 1992;17:359–364. [DOI] [PubMed] [Google Scholar]
- 64.Angel MF, Knight KR, Dvir E, Mellow CG, Morrison WA, O’Brien BM. Biochemical analysis of the venous flap in the dog. J Surg Res. 1992;53:24–29. [DOI] [PubMed] [Google Scholar]
- 65.Inada Y, Hirai T, Fukui A, Omokawa S, Mii Y, Tamai S. An experimental study of the flow-through venous flap: Investigation of the width and area of survival with one flow-through vein preserved. J Reconstr Microsurg. 1992;8:297–302. [DOI] [PubMed] [Google Scholar]
- 66.Inada Y, Fukui A, Tamai S, Mizumoto S. The arterialised venous flap: Experimental studies and a clinical case. Br J Plast Surg. 1993;46:61–67. [DOI] [PubMed] [Google Scholar]
- 67.Matsushita K, Firrell JC, Ogden L, Tsai TM. Blood flow and tissue survival in the rabbit venous flap. Plast Reconstr Surg. 1993;91:127–135; discussion 136137. [PubMed] [Google Scholar]
- 68.Gençosmanoğlu R, Ulgen O, Yaman C, Songür E, Akin Y, Cağdas A. Mechanisms of viability in rabbit flank venous flaps. Ann Plast Surg. 1993;30:60–66. [PubMed] [Google Scholar]
- 69.Ueda K, Harada T, Nagasaka S, Oba S, Inoue T, Harashina T. An experimental study of delay of flow-through venous flaps. Br J Plast Surg. 1993;46:56–60. [DOI] [PubMed] [Google Scholar]
- 70.Takato T, Komuro Y, Yonehara H, Zuker RM. Prefabricated venous flaps: An experimental study in rabbits. Br J Plast Surg. 1993;46:122–126. [DOI] [PubMed] [Google Scholar]
- 71.Thatte M, Healy C, McGrouther D. Laser Doppler and microvascular pulsed Doppler studies of the physiology of venous flaps. Eur J Plast Surg. 1993;16:134–138. [Google Scholar]
- 72.Lenoble E, Lavau L, Foucher G, Voisin MC, Goutallier D. Influence of the anatomy of the pedicle on the survival of venous vascularized flaps: Experimental study on the rat (in French). Ann Chir Plast Esthet. 1993;38:612–620. [PubMed] [Google Scholar]
- 73.Smith RJ, Fukuta K, Wheatley M, Jackson IT. Role of perivenous areolar tissue and recipient bed in the viability of venous flaps in the rabbit ear model. Br J Plast Surg. 1994;47:10–14. [DOI] [PubMed] [Google Scholar]
- 74.Dvir E, Hickey MJ, Hurley JV, Morrison WA. A histological and carbon perfusion study of cephalic and saphenous venous flaps in the dog. Br J Plast Surg. 1994;47:263–267. [DOI] [PubMed] [Google Scholar]
- 75.Suzuki Y, Suzuki K, Ishikawa K. Direct monitoring of the microcirculation in experimental venous flaps with afferent arteriovenous fistulas. Br J Plast Surg. 1994;47:554–559. [DOI] [PubMed] [Google Scholar]
- 76.Wolff KD, Telzrow T, Rudolph KH, Franke J, Wartenberg E. Isotope perfusion and infrared thermography of arterialised, venous flow-through and pedicled venous flaps. Br J Plast Surg. 1995;48:61–70. [DOI] [PubMed] [Google Scholar]
- 77.Byun JS, Constantinescu MA, Lee WP, May JW., Jr Effects of delay procedures on vasculature and survival of arterialized venous flaps: An experimental study in rabbits. Plast Reconstr Surg. 1995;96:1650–1659. [DOI] [PubMed] [Google Scholar]
- 78.Xiu ZF, Chen ZJ. The microcirculation and survival of experimental flow-through venous flaps. Br J Plast Surg. 1996;49:41–45. [DOI] [PubMed] [Google Scholar]
- 79.Pittet B, Chang P, Cederna P, Cohen MB, Blair WF, Cram AE. The role of neovascularization in the survival of an arterialized venous flap. Plast Reconstr Surg. 1996;97:621–629. [DOI] [PubMed] [Google Scholar]
- 80.Adamo C, Rubino C. Venous flaps and perivenous areolar tissue: An experimental study in rats. J Reconstr Microsurg. 1996;12:179–181. [DOI] [PubMed] [Google Scholar]
- 81.Xiu Z, Chen Z. The effect of glutathione, superoxide dismutase and adenosine triphosphate on venous flap survival. Eur J Plast Surg. 1996;19:170–173. [Google Scholar]
- 82.Miles DA, Crosby NL, Clapson JB. The role of the venous system in the abdominal flap of the rat. Plast Reconstr Surg. 1997;99:2030–2033. [DOI] [PubMed] [Google Scholar]
- 83.Yilmaz M, Menderes A, Vayvada H, Karaca C, Barutçu A. Effects of the number of pedicles on perfusion and survival of venous flaps: An experimental study in rabbits. Ann Plast Surg. 1997;39:278–286. [DOI] [PubMed] [Google Scholar]
- 84.Fukui A, Inada Y, Murata K, Ueda Y, Tamai S. A method for prevention of arterialized venous flap necrosis. J Reconstr Microsurg. 1998;14:67–74. [DOI] [PubMed] [Google Scholar]
- 85.Woo SH, Kim SE, Lee TH, Jeong JH, Seul JH. Effects of blood flow and venous network on the survival of the arterialized venous flap. Plast Reconstr Surg. 1998;101:1280–1289. [DOI] [PubMed] [Google Scholar]
- 86.Yuan R, Shan Y, Zhu S. Circulating mechanism of the “pure” venous flap: Direct observation of microcirculation. J Reconstr Microsurg. 1998;14:147–152. [DOI] [PubMed] [Google Scholar]
- 87.Atabey A, Gezer S, Vayvada H, et al. Ischemia/reperfusion injury in flow-through venous flaps. Ann Plast Surg. 1998;40:612–616. [DOI] [PubMed] [Google Scholar]
- 88.Cho BC, Lee MS, Lee JH, Byun JS, Baik BS. The effects of surgical and chemical delay procedures on the survival of arterialized venous flaps in rabbits. Plast Reconstr Surg. 1998;102:1134–1143. [DOI] [PubMed] [Google Scholar]
- 89.Mutaf M, Tasaki Y, Fujii T. Expansion of venous flaps: An experimental study in rats. Br J Plast Surg. 1998;51:393–401. [DOI] [PubMed] [Google Scholar]
- 90.Yilmaz M, Menderes A. Investigation of metabolism and perfusion in arterialized venous replantation: Experimental study in rabbits. Ann Plast Surg. 1999;43:67–73. [DOI] [PubMed] [Google Scholar]
- 91.Murata K, Tamai S, Inada Y, Fukui A, Miyamoto S. Transfer of a pedicled venous flap containing perivenous areolar tissue and nerve: An experimental study. Br J Plast Surg. 1999;52:223–229. [DOI] [PubMed] [Google Scholar]
- 92.Yücel A, Bayramiçli M. Effects of hyperbaric oxygen treatment and heparin on the survival of unipedicled venous flaps: An experimental study in rats. Ann Plast Surg. 2000;44:295–303. [DOI] [PubMed] [Google Scholar]
- 93.Tang YB, Simchon S, Chen HC. Microcirculation of a venous flap: An experimental study with microspheres in rabbits. Scand J Plast Reconstr Surg Hand Surg. 2000;34:207–212. [DOI] [PubMed] [Google Scholar]
- 94.Wungcharoen B, Pradidarcheep W, Santidhananon Y, Chongchet V. Pre-arterialisation of the arterialised venous flap: An experimental study in the rat. Br J Plast Surg. 2001;54:621–630. [DOI] [PubMed] [Google Scholar]
- 95.Saray A, Can B, Sevin K. Effects of methylprednisolone on the viability of experimental flow-through venous flaps. J Reconstr Microsurg. 2002;18:615–622. [DOI] [PubMed] [Google Scholar]
- 96.Chang SM, Gu YD, Li JF. Comparison of different managements of large superficial veins in distally based fasciocutaneous flaps with a veno-neuro-adipofascial pedicle: An experimental study using a rabbit model. Microsurgery 2003;23:555–560. [DOI] [PubMed] [Google Scholar]
- 97.Coruh A, Abaci K, Gunay GK. Effect of topical nitroglycerine on the survival of ischemic flow-through venous flaps in rabbits. J Reconstr Microsurg. 2004;20:261–266. [DOI] [PubMed] [Google Scholar]
- 98.Lin CH, Wei FC, Mardini S, Ma SF. Microcirculation study of rabbit ear arterial and venous flow-through flaps using a window chamber model. J Trauma 2004;56:894–900. [DOI] [PubMed] [Google Scholar]
- 99.Başer NT, Silistreli OK, Sişman N, Oztan Y. Effects of surgical or chemical delaying procedures on the survival of proximal pedicled venous island flaps: An experimental study in rats. Scand J Plast Reconstr Surg Hand Surg. 2005;39:197–203. [DOI] [PubMed] [Google Scholar]
- 100.Zhang F, Brooks D, Chen W, Mustain W, Chen MB, Lineaweaver WC. Improvement of venous flap survival by application of vascular endothelial growth factor in a rat model. Ann Plast Surg. 2006;56:670–673. [DOI] [PubMed] [Google Scholar]
- 101.Ozyazgan I, Tuncer A, Yazici C, Günay GK. Reactive oxygen species in experimental ischemic flow-through venous flaps and effects of antioxidants on reactive oxygen species and flap survival. Ann Plast Surg. 2007;58:661–666. [DOI] [PubMed] [Google Scholar]
- 102.Ozyazgan I, Ozköse M, Başkol G. Nitric oxide in flow-through venous flaps and effects of L-arginine and nitro-L-arginine methyl ester (L-NAME) on nitric oxide and flap survival in rabbits. Ann Plast Surg. 2007;59:550–557. [DOI] [PubMed] [Google Scholar]
- 103.Pittet B, Quinodoz P, Alizadeh N, Schlaudraff KU, Mahajan AL. Optimizing the arterialized venous flap. Plast Reconstr Surg. 2008;122:1681–1689. [DOI] [PubMed] [Google Scholar]
- 104.Tan MP, Lim AY, Zhu Q. A novel rabbit model for the evaluation of retrograde flow venous flaps. Microsurgery 2009;29:226–231. [DOI] [PubMed] [Google Scholar]
- 105.Yan H, Brooks D, Jackson WD, Angel MF, Akdemir O, Zhang F. Improvement of prearterialized venous flap survival with delay procedure in rats. J Reconstr Microsurg. 2010;26:193–200. [DOI] [PubMed] [Google Scholar]
- 106.Lalković M, Kozarski J, Panajotović L, et al. The new experimental design of arterialized venous flap on the rabbit ear model. Acta Veterinaria 2010;60:633–640. [Google Scholar]
- 107.Iglesias M, Fonseca-Lazcano JA, Moran MA, Butron P, Díaz-Morales M. Revascularization of arterialized venous flaps through a total retrograde reverse blood flow: Randomized experimental trial of viability. Plast Reconstr Surg Glob Open 2013;1:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yan H, Kolkin J, Zhao B, et al. The effect of hemodynamic remodeling on the survival of arterialized venous flaps. PLoS One 2013;8:e79608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yan H, He Z, Li Z, et al. Large prefabricated skin flaps based on the venous system in rabbits: A preliminary study. Plast Reconstr Surg. 2013;132:372e–380e. [DOI] [PubMed] [Google Scholar]
- 110.Ceylan R, Kaya B, Çaydere M, Terzioğlu A, Aslan G. Comparison of ischaemic preconditioning with surgical delay technique to increase the viability of single pedicle island venous flaps: An experimental study. J Plast Surg Hand Surg. 2014;48:368–374. [DOI] [PubMed] [Google Scholar]
- 111.Lalković M, Kozarski J, Panajotović L, et al. Surface enlargement of a new arterialised venous flap by the surgical delay method. Vojnosanit Pregl. 2014;71:547–553. [DOI] [PubMed] [Google Scholar]


