Supplemental Digital Content is available in the text.
Keywords: hypertension, natriuretic peptides, sodium-glucose transporter 2 inhibitors, type 2 diabetes mellitus
Abstract
Background:
The risk of cardiovascular disease and mortality in salt-sensitive patients with diabetes mellitus and uncontrolled nocturnal hypertension is high. The SACRA (Sodium-Glucose Cotransporter 2 [SGLT2] Inhibitor and Angiotensin Receptor Blocker [ARB] Combination Therapy in Patients With Diabetes and Uncontrolled Nocturnal Hypertension) study investigated changes in blood pressure (BP) with empagliflozin plus existing antihypertensive therapy.
Methods:
This multicenter, double-blind, parallel study was conducted in Japan. Adult patients with type 2 diabetes mellitus and uncontrolled nocturnal hypertension receiving stable antihypertensive therapy including angiotensin receptor blockers were randomized to 12 weeks’ treatment with empagliflozin 10 mg once daily or placebo. Clinic BP was measured at baseline and weeks 4, 8, and 12; 24-hour ambulatory BP monitoring was performed at baseline and week 12; and morning home BP was determined for 5 days before each visit. The primary efficacy end point was change from baseline in nighttime BP (ambulatory BP monitoring).
Results:
One hundred thirty-two nonobese, older patients with well-controlled blood glucose were randomized (mean age 70 years, mean body mass index 26 kg/m2). Empagliflozin, but not placebo, significantly reduced nighttime systolic BP versus baseline (–6.3 mm Hg; P=0.004); between-group difference in change from baseline was –4.3 mm Hg (P=0.159). Reductions in daytime, 24-hour, morning home, and clinic systolic BP at 12 weeks with empagliflozin were significantly greater than with placebo (–9.5, –7.7, –7.5, and –8.6 mm Hg, respectively; all P≤0.002). Between-group differences in body weight and glycosylated hemoglobin reductions were significant, but small (–1.3 kg and –0.33%; both P<0.001). At 4 weeks, N-terminal pro-B-type natriuretic peptide levels were reduced to a greater extent in the empagliflozin versus placebo group (–12.1%; P=0.013); atrial natriuretic peptide levels decreased with empagliflozin versus placebo at weeks 4 and 12 (–8.2% [P=0.008] and –9.7% [P=0.019]). Changes in antihypertensive medication during the study did not differ significantly between groups.
Conclusions:
Nonseverely obese older diabetes patients with uncontrolled nocturnal hypertension showed significant BP reductions without marked reductions in glucose with the addition of empagliflozin to existing antihypertensive and antidiabetic therapy. Use of sodium-glucose cotransporter 2 inhibitors in specific groups (eg, those with nocturnal hypertension, diabetes, and high salt sensitivity) could help reduce the risk of heart failure and cardiovascular mortality.
Clinical Trial Registration:
URL: https://www.clinicaltrials.gov. Unique identifier: NCT03050229.
Clinical Perspective.
What Is New?
Patients with type 2 diabetes mellitus and hypertension are at increased risk of developing cardiovascular disease, particularly when nighttime blood pressure is elevated.
This is the first time that a sodium-glucose cotransporter 2 inhibitor has been studied in patients with type 2 diabetes mellitus and uncontrolled nocturnal hypertension in a randomized, placebo-controlled clinical trial.
The addition of empagliflozin to existing antihypertensive therapy was associated with significant reductions in a variety of blood pressure measures, including ambulatory and home blood pressure.
What Are the Clinical Implications?
There is a growing body of data supporting the important benefits of sodium-glucose cotransporter 2 inhibition in patients with type 2 diabetes mellitus and hypertension.
The reductions in blood pressure that occurred after the addition of empagliflozin therapy were of a clinically relevant magnitude that would be expected to reduce cardiovascular risk, and occurred in patients already receiving antihypertensive therapy (including angiotensin receptor blockers).
Editorial, see p 2110
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are oral hypoglycemic agents that act primarily by increasing the urinary elimination of glucose.1 In addition to reducing blood glucose, treatment with the SGLT2 inhibitor empagliflozin has also been associated with weight loss and reductions in blood pressure (BP).2–5 In the EMPA-REG OUTCOME trial (BI 10773 [Empagliflozin] Cardiovascular Blood Pressure Trial in Type 2 Diabetes Mellitus Patients), treatment of patients with type 2 diabetes mellitus (T2DM) and high cardiovascular risk with empagliflozin versus placebo significantly reduced cardiovascular and all-cause mortality, heart failure–related hospitalizations, and progression of diabetic nephropathy.6–8 Although clinic BP was reduced by empagliflozin, the contribution of decreases in 24-hour BP, including nocturnal and morning time periods, to the observed benefit on cardiovascular outcomes could not be determined. In particular, nocturnal hypertension, or the nondipper pattern of nighttime BP, is a strong predictor of cardiovascular disease including nonischemic heart failure, both in patients with hypertension and in the general population.9–11
The risk of developing cardiovascular disease is also increased in T2DM patients.12 The cardiovascular disease and mortality risk are particularly high in T2DM patients with nocturnal hypertension.13–15 This highlights the importance of nighttime BP as a cardiovascular and mortality risk factor in patients with T2DM, and suggests that nighttime BP could be an important therapeutic target in these patients.
The SACRA study (SGLT2 inhibitor and Angiotensin Receptor Blocker [ARB] Combination Therapy in Patients With Diabetes and Uncontrolled Nocturnal Hypertension) used ambulatory BP monitoring (ABPM) to investigate the effects of adding empagliflozin to existing antihypertensive therapy on nighttime BP in T2DM patients and uncontrolled nocturnal hypertension.
Methods
The SACRA study (NCT03050229) was a multicenter, randomized, placebo-controlled, double-blind, 2-arm, parallel group trial conducted in Japan. The study protocol was approved by the ethics committee of Jichi Medical University School of Medicine. The study was conducted in accordance with the principles of the Declaration of Helsinki, International on Harmonization Tripartite Guideline for Good Clinical Practice. All patients provided written informed consent before enrollment into the study.
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Participants
Eligible patients were aged ≥20 years; had T2DM (glycosylated hemoglobin [HbA1c] ≥6% and <10%), seated clinic systolic BP (SBP) 130 to 159 mm Hg or diastolic BP (DBP) 80 to 99 mm Hg, and nocturnal hypertension (SBP ≥115 mm Hg at 2, 3, and 4 a.m. during sleep 5 days before randomization, measured using home BP monitoring [HEM-7080-IC; Omron Healthcare Co Ltd, Kyoto, Japan]); were receiving stable antidiabetic treatment with or without antidiabetics for ≥8 weeks before baseline; and were receiving stable antihypertensive treatment that included an ARB for ≥8 weeks before baseline. Key exclusion criteria included history of diabetic ketoacidosis or diabetic coma, renal and liver damage, and cardiovascular disease events (within 3 months before randomization; Table I in the online-only Data Supplement).
Randomized Treatment
After an 8-week run-in period, eligible patients were randomized (1:1) to receive empagliflozin 10 mg or matching placebo once daily for 12 weeks. Randomization was performed using a computer-generated, pseudorandom sequence and was stratified by nighttime SBP, age, sex, and study center. Antihypertensive and antidiabetic medication could be changed at the discretion of the investigator.
Assessments
Study visits were scheduled at baseline, week 4, week 8, and week 12. Morning home BP monitoring using a validated BP monitoring device (HEM-7080-IC) was performed for 5 consecutive days before each study visit. Clinic BP was measured at each study visit. Twenty-four-hour ABPM was performed at baseline and week 12, after administration of study medication, using methods described previously.16 In brief, an automatic oscillometric method ABPM device (TM2431; A&D Co, Tokyo, Japan) was used to record BP and pulse rate every 30 minutes for 24 hours. Twenty-four-hour BP was defined as the average of all readings over a 24-hour period. Nighttime BP was calculated as the average of BP values recorded over the period from when the patient went to bed until the patient got up; values over the rest of the day were used to calculate daytime BP. Investigators were unaware of glucose results during the entire treatment period. Safety was assessed based on laboratory findings, and the occurrence of adverse events and side effects documented during study visits.
Outcomes
The primary efficacy end point was change from baseline in nighttime SBP and DBP measured using ABPM. Key secondary end points were change from baseline in mean 24-hour SBP/DBP and daytime BP at week 12. Other secondary efficacy end points were change from baseline to week 12 in morning home BP, clinic BP, HbA1c, and body weight. Additional end points were low-density lipoprotein and high-density lipoprotein cholesterol levels, NT-proBNP (N-terminal pro B-type natriuretic peptide) levels, atrial natriuretic peptide levels, magnesium levels, estimated glomerular filtration rate (eGFR: men, 194×serum creatinine-1.094×age-0.287; women, 194× serum creatinine -1.094×age-0.287×0.739),17 urinary albumin creatinine ratio, and laboratory findings.
Statistical Analysis
Given that there are no existing published data on the effects of empagliflozin in combination with an ARB on nighttime BP in Japanese patients with T2DM and nocturnal hypertension, expected changes in BP during treatment for inclusion in the sample size calculation were taken from another trial of empagliflozin in patients with diabetes mellitus and hypertension.18 Adjusted mean differences versus placebo in the change from baseline in mean 24-hour SBP were –4.18 mm Hg with empagliflozin 10 mg and –5.04 mm Hg with empagliflozin 25 mg. It was assumed that reductions in nighttime BP would be greater than those in mean 24-hour SBP, and therefore a between-group difference (empagliflozin versus placebo) was assumed for the mean change in the mean nighttime SBP of –5 mm Hg (with a SD of 10 mm Hg in each group). To detect differences between the 2 groups with 80% power, 63 patients in each group would be required (126 in total). Assuming that 20% of recruited patients would not meet the nighttime BP entry criteria and another 10% would be withdrawn during the treatment period, the enrollment target was 176 patients.
Patients who were noncompliant with the Ethical Guidelines for Clinical Research were excluded from both efficacy and safety analyses. The full analysis set included all enrolled participants who received their assigned therapy at least once after randomization and who had made 24-hour ABPM recordings at least once. The safety analysis set included all randomized participants who had received the assigned therapy at least once during the study period. Efficacy analyses for BP values were conducted in the full analysis set, and safety was determined in the safety analysis set.
Intergroup comparisons were tested with a t test for continuous variables, and Pearson χ2 test or Fisher exact test was used for dichotomous data. Mixed-effects model repeated measures analysis was used to compare the changes in nighttime BP, daytime BP, mean 24-hour ABPM BP, clinic BP, and HbA1c from baseline to week 12. Mixed-effects model repeated measures included the randomized study group, time point (0, 4, 8, and 12 weeks), interaction between the study group and time points as fixed effects, and age and sex as covariates. We used compound symmetry as the covariance structure.
Data were analyzed using SAS version 9.4 (SAS Institute, Cary, NC) at the Jichi Medical University Center of Global Home and Ambulatory BP Analysis, Shimotsuke, Japan. A 2-sided test was used; P values of <0.05 were considered statistically significant.
Results
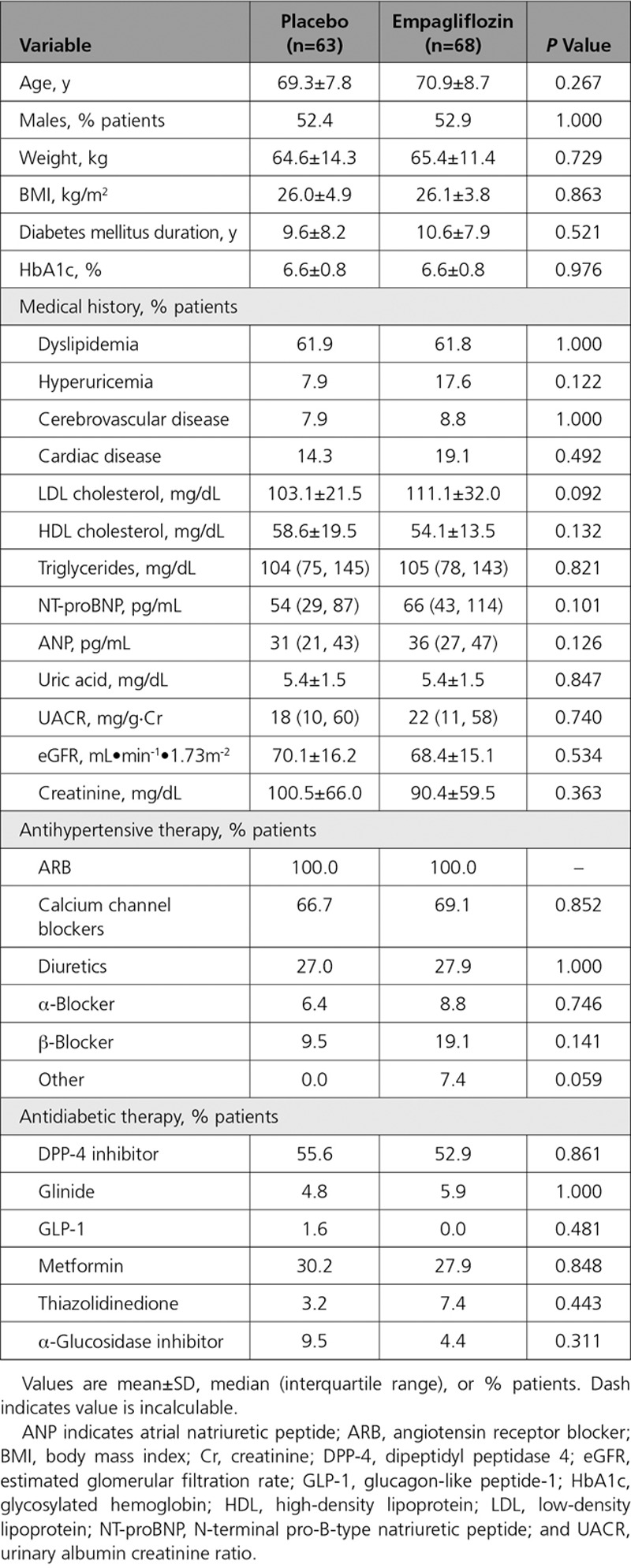
A total of 174 patients with diabetes mellitus and uncontrolled nocturnal hypertension were screened between January and September 2017, of whom 132 met the inclusion criteria and were randomized (Figure 1). One patient in the placebo group withdrew because of protocol violation. Therefore, 131 patients were included in the full analysis set (68 in the empagliflozin group and 63 in the placebo group). There was an antihypertensive drug dosage increase in only 1 patient during the trial (in the placebo group). Baseline demographics and characteristics were well matched between the treatment groups (Table 1), apart from a higher baseline pulse rate in the placebo versus empagliflozin group (70.7±9.5 versus 67.1±8.3 beats/min; P=0.026).
Figure 1.

Study flow chart.
Table.
Patient Demographic and Clinical Characteristics at Baseline
Blood Pressure
There was a significant reduction from baseline to week 12 in nighttime SBP with empagliflozin (–6.3 mm Hg; P=0.004) but not with placebo (–2.0 mm Hg; P=0.367); between-group difference was –4.3 (P=0.159; Figure 2). Reductions from baseline in daytime SBP and 24-hour SBP were also statistically significant in the empagliflozin group but not in the placebo group, with statistically significant between-group differences in the change from baseline (both P<0.001; Figure 2). Changes in nighttime, daytime, and 24-hour DBP showed a similar pattern, all being significantly reduced from baseline by empagliflozin and not placebo, and significant between-group differences in the reduction from baseline for daytime and 24-hour DBP (Figure 2).
Figure 2.
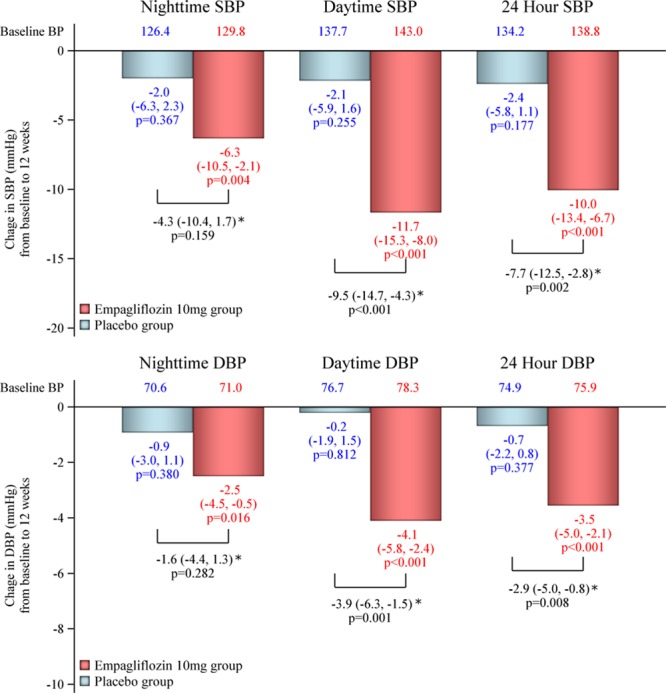
Changes from baseline in nighttime, daytime, and 24-hour systolic (SBP) and diastolic (DBP) blood pressure (BP). Bars and values represent the change (mean and 95% CI) from baseline using the mixed-effects model with repeated measures adjusted for age and sex. *Between-group difference in the change from baseline.
Reductions from baseline in clinic and morning home SBP were also statistically significant in the empagliflozin group, with statistically significant between-group differences in the change from baseline (both P<0.001; Figure 3).
Figure 3.

Changes from baseline in clinic and morning home BPs. A, clinic systolic BP (SBP) and diastolic BP (DBP); B, morning home SBP and DBP. Points and bar represent the least-square mean and standard error using the mixed-effects model with repeated measures adjusted for age and sex. P values are for the between-group difference change from baseline.
Other End Points
After 12 weeks, HbA1c and body weight decreased from baseline to a significantly greater extent in patients receiving empagliflozin compared with placebo (both P<0.001), but numeric differences between the groups were small (–1.3 kg and –0.33%, respectively; Figure 4A and 4B). Significant reductions in uric acid and eGFR with empagliflozin versus placebo were evident after 4 weeks’ treatment (Figure 4C and 4D).
Figure 4.

Change from baseline in glycosylated hemoglobin (HbA1c), body weight, uric acid, and estimated glomerular filtration rate (eGFR). HbA1c (A), body weight (B), uric acid (C), and eGFR (D). Points and bar represent the least-square mean and standard error using the mixed-effects model with repeated measures adjusted for age and sex. P values are for the between-group difference change from baseline.
At the 4-week follow-up, levels of NT-proBNP had reduced to a significantly greater extent in the empagliflozin versus placebo group (P=0.013), but at the 12-week follow-up, the between-group difference was no longer statistically significant (P=0.361; Figure I in the online-only Data Supplement). Significantly greater reductions in atrial natriuretic peptide at both 4 and 12 weeks (Figure II in the online-only Data Supplement) and urinary albumin creatinine ratio at 12 weeks (Figure III in the online-only Data Supplement) were seen with empagliflozin versus placebo.
Pulse rates for each BP measurement were not significantly different between the empagliflozin and placebo groups (Figure IV in the online-only Data Supplement). In addition, the proportion of patients who changed antihypertensive medication during the study did not differ significantly between treatment groups. Lipid levels were also similar in the empagliflozin and placebo groups throughout the study (Table II in the online-only Data Supplement).
Safety
Empagliflozin was well tolerated; no serious adverse events occurred during treatment, and there were no new safety signals. There were 4 adverse events in 4 patients in the placebo group and 14 events in 8 patients in the empagliflozin group. Of these, 7 events in the empagliflozin group (1 each of thirst, polyuria, lumbago, genital itching, fatigue, nausea, and constipation) and 1 in the placebo group (heartburn) were considered to be related to treatment. There were no episodes of hypotension, dehydration, urinary tract infection, or acute kidney injury.
Discussion
The SACRA study is the first randomized, double-blind, placebo-controlled clinical trial of an SGLT2 inhibitor in patients with T2DM and uncontrolled nocturnal hypertension despite ARB-based antihypertensive therapy. The addition of empagliflozin was associated with significant reductions from baseline in nighttime, clinic, 24-hour ambulatory, and morning home BP. Reductions in the majority of BP parameters were significantly better with empagliflozin versus placebo. The only parameter that did not reach statistical significance at 12 weeks with empagliflozin versus placebo was the reduction in nighttime BP. However, the numeric difference between the two treatments (–4.3 mm Hg) and the reduction from baseline with empagliflozin (–6.3 mm Hg) were of a magnitude that is likely to have important clinical implications given that a 5–mm Hg reduction in mean nighttime SBP has been independently associated with a 20% reduction in cardiovascular risk.19
The home BP-lowering effects of empagliflozin in this high-risk population were also clinically meaningful because home BP-guided management of hypertension is the best approach in clinical practice.20 In the large, Japan-wide home BP registry-based HONEST study (Home Blood Pressure Measurement With Olmesartan-Naive Patients to Establish Standard Target Blood Pressure), on-treatment home BP was more closely associated with cardiovascular event risk than clinic BP.21 Morning home SBP after 12 weeks’ treatment with empagliflozin in the current study was 126.6 mm Hg, close to the 124 mm Hg that was found to be the morning SBP associated with minimum cardiovascular risk in the HONEST study.21 In addition, reductions in morning home SBP (13.3 mm Hg) and clinic SBP (9.9 mm Hg) in the SACRA study were of a clinically relevant magnitude given that data from a meta-analysis showed that a 10 mm Hg reduction in clinic SBP reduced the risk of major cardiovascular events by 20%, coronary heart disease by 27%, stroke by 27%, heart failure by 28%, and all-cause mortality by 13%.22 Based on available evidence, the cardiovascular risk associated with a ≥10–mm Hg reduction in home SBP would be greater than that achieved with a similar reduction in clinic SBP. This morning home BP reduction is particularly relevant in patients of Asian ethnicity who have been shown to have an exaggerated morning BP surge and morning hypertension compared with Western populations, and are at increased stroke risk.23–25
Good reductions in daytime and 24-hour ambulatory BP were seen during empagliflozin therapy. This is notable given the elderly nature of the study population (mean age approximately 70 years). Consistent with the EMPA-REG BP trial results, we also noted slightly greater reductions in daytime compared with nighttime BP during empagliflozin therapy. Compared with the same empagliflozin dosage in the EMPA-REG BP trial (10 mg/d),18 reductions in 24-hour BP compared with placebo were greater in our study. Empagliflozin reduced 24-hour SBP by 7.7 mm Hg versus placebo compared with 3.44 mm Hg in the EMPA-REG BP trial; corresponding reductions in 24-hour DBP in the 2 trials were 2.9 and 1.36 mm Hg.18 Reasons for these differences are not clear. The majority of patients in the EMPA-REG BP trial (>90%) were receiving concomitant antihypertensives, as was the case in our study. The rate of use of angiotensin-converting enzyme inhibitors in the EMPA-REG BP trial was >75%, but there was no information on ARB use,18 whereas all patients in the current study were using an ARB. Nevertheless, reductions in BP with empagliflozin were found to be independent of concomitant antihypertensive use (number and type of agents) in an analysis of EMPA-REG BP data.26 Reductions in 24-hour BP in the current study were also greater than those from a meta-analysis of all randomized, double-blind, placebo-controlled trials investigating the effects of SGLT2 inhibitors on 24-hour ambulatory BP (–3.76 and –1.83 mm Hg for 24-hour SBP and DBP, respectively).27
Differences between trials in the reported BP reductions could be attributable to differences in the patient populations. Participants in the SACRA study were elderly (mean age approximately 70 years), had good glycemic control (mean HbA1c <7.0%), and were selected based on the presence of nocturnal hypertension only. In addition, patient ethnicities differed between studies. All patients in the SACRA study were from Japan, compared with predominantly white populations in the previous trials. Japanese and Asian patients have a genetic predisposition to salt sensitivity and tend to have higher dietary salt intake.20,28,29 Despite these differences in patient characteristics, the beneficial effects of empagliflozin on cardiovascular outcomes and all-cause mortality in Asian participants in the EMPA-REG OUTCOME trial were even greater and consistent with those in the overall patient population.30
Nocturnal hypertension is a clinical phenotype of salt sensitivity, with higher blood pressures required to secrete sodium from the body. This increases stress on the left ventricle and the cerebral circulation, resulting in greater end-organ damage. It has been suggested that the BP-lowering effects of SGLT2 inhibitors could be a result of their effects on osmotic diuresis and mild natriuresis,27,31 with a resulting reduction in preload.32 Osmotic diuresis might contribute to early decreases in BP during SGLT2 inhibitor therapy, with natriuresis playing a role in longer-term BP reductions.33 Significant reductions in the tissue sodium content of the skin have also been reported after 6 weeks’ therapy with dapagliflozin.32 It has been suggested that greater reductions in daytime versus nighttime BP during SGLT2 inhibitor therapy may, at least in part, be a result of the glucose-dependent nature of glycemic control with these agents.27 Specifically, diuresis might be higher during daytime periods of higher glucose levels as a result of higher food and fluid intake. Conversely, smaller reductions in BP at nighttime with empagliflozin could be attributable to lower nocturnal urine production as a result of circadian rhythms in renal function. Circadian variations in sympathetic tone are another potential mechanism for the observed differences in diurnal and nighttime BP regulation by SGLT2 inhibitors. Additional research is needed to more clearly elucidate the specific mechanism of BP reduction with SGLT2 inhibitors, particularly in patients with cardiovascular high risk.
In addition to glucose-lowering and antihypertensive effects, treatment with empagliflozin was also associated with significant reductions in body weight and uric acid compared with placebo, consistent with the EMPA-REG OUTCOME study.6–8 Even though baseline levels of NT-proBNP and atrial natriuretic peptide were within the normal range, these parameters were significantly reduced in patients receiving empagliflozin. Reductions in natriuretic peptides, even within the normal range, can have a clinically relevant beneficial effect on cardiovascular risk and mortality.34 In addition, the urinary albumin creatinine ratio decreased to a greater extent in the empagliflozin group than in the placebo group. Furthermore, the pattern of these effects seen for empagliflozin in this study, which enrolled an elderly population with slightly higher body mass index and good glycemic control, including the reductions in natriuretic peptides, without marked reduction in glucose, suggested that empagliflozin may be a useful therapeutic option in patients with heart failure who have similar characteristics.
The eGFR reduction seen in empagliflozin recipients in this study was not progressive. In previous clinical studies, acute, reversible declines in eGFR with commensurate increases in serum creatinine have been observed with SGLT2 inhibitors, consistent with the hemodynamic effect of these agents. The initial decline in eGFR observed with SGLT2 inhibitors is likely related to afferent arteriolar vasoconstriction through a tubuloglomerular feedback mechanism.35 Reduction of uric acid could be explained by the stimulating effects that SGLT2 inhibition has on excretion of uric acid by GLUT9 isoform 2 on the apical membrane of the proximal tubule and possible inhibition of uric acid reabsorption in the collecting ducts.36
Study Limitations
The most important limitation of our study is likely to be the lack of study power. Sample size calculations were based on a 5–mm Hg difference in nighttime BP between the treatment and placebo group, whereas the actual difference was 4.3 mm Hg. Lower-than-expected study power is the most likely explanation for the lack of statistical significance in the difference between empagliflozin and placebo in mean change in nighttime BP from baseline. In addition, the findings are only applicable to populations similar to those enrolled in the study, and need to be confirmed in patients of different ethnicities.
Conclusions
The SACRA study results add to the growing body of evidence suggesting that SGLT2 inhibitors offer a number of important benefits in T2DM and hypertension, and are therefore a valuable therapeutic option in these patients. Use of SGLT2 inhibitors to lower 24-hour BP in specific groups, such as those with nocturnal hypertension, diabetes mellitus, and high salt sensitivity, could help to reduce the risk of heart failure and cardiovascular mortality in these patients.
Appendix
SACRA study collaborators: Physicians: Satoshi Hoshide, Jichi Medical University School of Medicine, Tochigi, Japan; Mitsuyoshi Yamamoto, Kotake Town Hospital, Fukuoka, Japan; Kazuo Eguchi, International University of Health and Welfare Hospital, Tochigi, Japan; Atsushi Mizuno, St. Luke’s International Hospital, Tokyo, Japan; Shigeru Nakano, Shiga Clinic, Ishikawa, Japan; Yuta Kemi, Ueno Village Clinic, Gumma, Japan; Research coordinators: Chie Iwashita, Jichi Medical University School of Medicine, Tochigi, Japan; Kyouichi Wada, Satt Co, Ltd, Tokyo, Japan; Azusa Kaneko, Satt Co, Ltd, Tokyo, Japan; Medical writer: Nicola Ryan, independent medical writer.
Acknowledgments
We acknowledge all the patients, physicians (Satoshi Hoshide, Mitsuyoshi Yamamoto, Kazuo Eguchi, Atsushi Mizuno, Shigeru Nakano, and Yuta Kemi), and medical staff who supported this study. We also thank Chie Iwashita for the coordination and data management of this study. The independent study control center was managed, and all data were collected, by a contract research organization, Satt Co Ltd (Tokyo, Japan). We thank Kyouichi Wada and Azusa Kaneko for their effort. Medical writing assistance was provided by Nicola Ryan, independent medical writer.
Sources of Funding
This study was funded by the Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance. Empagliflozin 10 mg and placebo were provided by Boehringer Ingelheim.
Disclosures
Dr Kario has received research grants from Tanabe Mitsubishi Pharma Corp. Dr Okada has received scholarship funding from Daiichi Sankyo Co and honoraria from Sanofi K.K. The other authors report no conflicts.
Supplementary Material
Footnotes
Sources of Funding, see page 2096
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.118.037076.
Contributor Information
Satoshi Hoshide, Jichi Medical University School of Medicine, Tochigi, Japan.
Mitsuyoshi Yamamoto, Kotake Town Hospital, Fukuoka, Japan.
Kazuo Eguchi, International University of Health and Welfare Hospital, Tochigi, Japan.
Atsushi Mizuno, St. Luke’s International Hospital, Tokyo, Japan.
Shigeru Nakano, Shiga Clinic, Ishikawa, Japan.
Yuta Kemi, Ueno Village Clinic, Gumma, Japan.
Chie Iwashita, Jichi Medical University School of Medicine, Tochigi, Japan.
Kyouichi Wada, Satt Co, Ltd, Tokyo, Japan.
Azusa Kaneko, Satt Co, Ltd, Tokyo, Japan.
Collaborators: SACRA study collaborators:, Physicians:, Satoshi Hoshide, Mitsuyoshi Yamamoto, Kazuo Eguchi, Atsushi Mizuno, Shigeru Nakano, Yuta Kemi, Research coordinators:, Chie Iwashita, Kyouichi Wada, Azusa Kaneko, Medical writer:, and Nicola Ryan
References
- 1.Grempler R, Thomas L, Eckhardt M, Himmelsbach F, Sauer A, Sharp DE, Bakker RA, Mark M, Klein T, Eickelmann P. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab. 2012;14:83–90. doi: 10.1111/j.1463-1326.2011.01517.x. doi: 10.1111/j.1463-1326.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- 2.Häring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Broedl UC, Woerle HJ EMPA-REG MET Trial Investigators. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37:1650–1659. doi: 10.2337/dc13-2105. doi: 10.2337/dc13-2105. [DOI] [PubMed] [Google Scholar]
- 3.Häring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Woerle HJ, Broedl UC EMPA-REG METSU Trial Investigators. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396–3404. doi: 10.2337/dc12-2673. doi: 10.2337/dc12-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovacs CS, Seshiah V, Swallow R, Jones R, Rattunde H, Woerle HJ, Broedl UC EMPA-REG PIO™ trial investigators. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16:147–158. doi: 10.1111/dom.12188. doi: 10.1111/dom.12188. [DOI] [PubMed] [Google Scholar]
- 5.Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, Broedl UC EMPA-REG MONO trial investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208–219. doi: 10.1016/S2213-8587(13)70084-6. doi: 10.1016/S2213-8587(13)70084-6. [DOI] [PubMed] [Google Scholar]
- 6.Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE EMPA-REG OUTCOME® trial investigators. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016;37:1526–1534. doi: 10.1093/eurheartj/ehv728. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 8.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 9.Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Kuznetsova T, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Wang J, Sandoya E, O’Brien E, Staessen JA International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO) investigators. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219–1229. doi: 10.1016/S0140-6736(07)61538-4. doi: 10.1016/S0140-6736(07)61538-4. [DOI] [PubMed] [Google Scholar]
- 10.Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension. 2011;57:3–10. doi: 10.1161/HYPERTENSIONAHA.109.133900. doi: 10.1161/HYPERTENSIONAHA.109.133900. [DOI] [PubMed] [Google Scholar]
- 11.Ingelsson E, Björklund-Bodegård K, Lind L, Arnlöv J, Sundström J. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA. 2006;295:2859–2866. doi: 10.1001/jama.295.24.2859. doi: 10.1001/jama.295.24.2859. [DOI] [PubMed] [Google Scholar]
- 12.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Astrup AS, Nielsen FS, Rossing P, Ali S, Kastrup J, Smidt UM, Parving HH. Predictors of mortality in patients with type 2 diabetes with or without diabetic nephropathy: a follow-up study. J Hypertens. 2007;25:2479–2485. doi: 10.1097/HJH.0b013e3282f06428. doi: 10.1097/HJH.0b013e3282f06428. [DOI] [PubMed] [Google Scholar]
- 14.Bouhanick B, Bongard V, Amar J, Bousquel S, Chamontin B. Prognostic value of nocturnal blood pressure and reverse-dipping status on the occurrence of cardiovascular events in hypertensive diabetic patients. Diabetes Metab. 2008;34(6 p)(t 1):560–567. doi: 10.1016/j.diabet.2008.05.005. doi: 10.1016/j.diabet.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Wijkman M, Länne T, Engvall J, Lindström T, Ostgren CJ, Nystrom FH. Masked nocturnal hypertension–a novel marker of risk in type 2 diabetes. Diabetologia. 2009;52:1258–1264. doi: 10.1007/s00125-009-1369-9. doi: 10.1007/s00125-009-1369-9. [DOI] [PubMed] [Google Scholar]
- 16.Mizuno H, Hoshide S, Fukutomi M, Kario K. Differing effects of aliskiren/amlodipine combination and high-dose amlodipine monotherapy on ambulatory blood pressure and target organ protection. J Clin Hypertens (Greenwich) 2016;18:70–78. doi: 10.1111/jch.12618. doi: 10.1111/jch.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 18.Tikkanen I, Narko K, Zeller C, Green A, Salsali A, Broedl UC, Woerle HJ EMPA-REG BP Investigators. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–428. doi: 10.2337/dc14-1096. doi: 10.2337/dc14-1096. [DOI] [PubMed] [Google Scholar]
- 19.Hermida RC, Ayala DE, Mojón A, Fernández JR. Sleep-time blood pressure as a therapeutic target for cardiovascular risk reduction in type 2 diabetes. Am J Hypertens. 2012;25:325–334. doi: 10.1038/ajh.2011.231. doi: 10.1038/ajh.2011.231. [DOI] [PubMed] [Google Scholar]
- 20.Kario K. Evidence and perspectives on the 24-hour management of hypertension: hemodynamic biomarker-initiated ‘anticipation medicine’ for zero cardiovascular event. Prog Cardiovasc Dis. 2016;59:262–281. doi: 10.1016/j.pcad.2016.04.001. doi: 10.1016/j.pcad.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Kario K, Saito I, Kushiro T, Teramukai S, Ishikawa Y, Mori Y, Kobayashi F, Shimada K. Home blood pressure and cardiovascular outcomes in patients during antihypertensive therapy: primary results of HONEST, a large-scale prospective, real-world observational study. Hypertension. 2014;64:989–996. doi: 10.1161/HYPERTENSIONAHA.114.04262. doi: 10.1161/HYPERTENSIONAHA.114.04262. [DOI] [PubMed] [Google Scholar]
- 22.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 23.Hoshide S, Kario K, de la Sierra A, Bilo G, Schillaci G, Banegas JR, Gorostidi M, Segura J, Lombardi C, Omboni S, Ruilope L, Mancia G, Parati G. Ethnic differences in the degree of morning blood pressure surge and in its determinants between Japanese and European hypertensive subjects: data from the ARTEMIS study. Hypertension. 2015;66:750–756. doi: 10.1161/HYPERTENSIONAHA.115.05958. doi: 10.1161/HYPERTENSIONAHA.115.05958. [DOI] [PubMed] [Google Scholar]
- 24.Kario K, Bhatt DL, Brar S, Bakris GL. Differences in dynamic blood pressure variability between Japanese and American treatment-resistant hypertensive populations. Circ J. 2017;81:948–957. doi: 10.1253/circj.CJ-16-1237. doi: 10.1253/circj.CJ-16-1237. [DOI] [PubMed] [Google Scholar]
- 25.Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. doi: 10.1161/01.cir.0000056521.67546.aa. doi: 10.1161/01.CIR.0000056521.67546.AA. [DOI] [PubMed] [Google Scholar]
- 26.Mancia G, Cannon CP, Tikkanen I, Zeller C, Ley L, Woerle HJ, Broedl UC, Johansen OE. Impact of empagliflozin on blood pressure in patients with type 2 diabetes mellitus and hypertension by background antihypertensive medication. Hypertension. 2016;68:1355–1364. doi: 10.1161/HYPERTENSIONAHA.116.07703. doi: 10.1161/HYPERTENSIONAHA.116.07703. [DOI] [PubMed] [Google Scholar]
- 27.Baker WL, Buckley LF, Kelly MS, Bucheit JD, Parod ED, Brown R, Carbone S, Abbate A, Dixon DL. Effects of sodium-glucose cotransporter 2 inhibitors on 24-hour ambulatory blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6:e005686. doi: 10.1161/JAHA.117.005686. doi: 10.1161/JAHA.117.005686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katsuya T, Ishikawa K, Sugimoto K, Rakugi H, Ogihara T. Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens Res. 2003;26:521–525. doi: 10.1291/hypres.26.521. doi: 10.1291/hypres.26.521. [DOI] [PubMed] [Google Scholar]
- 29.Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, Engell RE, Lim SS, Danaei G, Mozaffarian D. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013;3:e003733. doi: 10.1136/bmjopen-2013-003733. doi: 10.1136/bmjopen-2013-003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ EMPA-REG OUTCOME® Investigators. Empagliflozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: results from EMPA-REG OUTCOME®. Circ J. 2017;81:227–234. doi: 10.1253/circj.CJ-16-1148. doi: 10.1253/circj.CJ-16-1148. [DOI] [PubMed] [Google Scholar]
- 31.Majewski C, Bakris GL. Blood pressure reduction: an added benefit of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes. Diabetes Care. 2015;38:429–430. doi: 10.2337/dc14-1596. doi: 10.2337/dc14-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karg MV, Bosch A, Kannenkeril D, Striepe K, Ott C, Schneider MP, Boemke-Zelch F, Linz P, Nagel AM, Titze J, Uder M, Schmieder RE. SGLT-2-inhibition with dapagliflozin reduces tissue sodium content: a randomised controlled trial. Cardiovasc Diabetol. 2018;17:5. doi: 10.1186/s12933-017-0654-z. doi: 10.1186/s12933-017-0654-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawasoe S, Maruguchi Y, Kajiya S, Uenomachi H, Miyata M, Kawasoe M, Kubozono T, Ohishi M. Mechanism of the blood pressure-lowering effect of sodium-glucose cotransporter 2 inhibitors in obese patients with type 2 diabetes. BMC Pharmacol Toxicol. 2017;18:23. doi: 10.1186/s40360-017-0125-x. doi: 10.1186/s40360-017-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 35.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 36.Lytvyn Y, Škrtić M, Yang GK, Yip PM, Perkins BA, Cherney DZ. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol. 2015;308:F77–F83. doi: 10.1152/ajprenal.00555.2014. doi: 10.1152/ajprenal.00555.2014. [DOI] [PubMed] [Google Scholar]


