Supplemental Digital Content is available in the text.
Keywords: 3-hydroxybutyrate, echocardiography, heart failure, ketone bodies, metabolism, positron-emission tomography
Abstract
Background:
Myocardial utilization of 3-hydroxybutyrate (3-OHB) is increased in patients with heart failure and reduced ejection fraction (HFrEF). However, the cardiovascular effects of increased circulating plasma-3-OHB levels in these patients are unknown. Consequently, the authors’ aim was to modulate circulating 3-OHB levels in HFrEF patients and evaluate: (1) changes in cardiac output (CO); (2) a potential dose-response relationship between 3-OHB levels and CO; (3) the impact on myocardial external energy efficiency (MEE) and oxygen consumption (MVO2); and (4) whether the cardiovascular response differed between HFrEF patients and age-matched volunteers.
Methods:
Study 1: 16 chronic HFrEF patients (left ventricular ejection fraction: 37±3%) were randomized in a crossover design to 3-hour of 3-OHB or placebo infusion. Patients were monitored invasively with a Swan-Ganz catheter and with echocardiography. Study 2: In a dose-response study, 8 HFrEF patients were examined at increasing 3-OHB infusion rates. Study 3 to 4: 10 HFrEF patients and 10 age-matched volunteers were randomized in a crossover design to 3-hour 3-OHB or placebo infusion. MEE and MVO2 were evaluated using 11C-acetate positron emission tomography.
Results:
3-OHB infusion increased circulating levels of plasma 3-OHB from 0.4±0.3 to 3.3±0.4 mM (P<0.001). CO rose by 2.0±0.2 L/min (P<0.001) because of an increase in stroke volume of 20±2 mL (P<0.001) and heart rate of 7±2 beats per minute (bpm) (P<0.001). Left ventricular ejection fraction increased 8±1% (P<0.001) numerically. There was a dose-response relationship with a significant CO increase of 0.3 L/min already at plasma-3-OHB levels of 0.7 mM (P<0.001). 3-OHB increased MVO2 without altering MEE. The response to 3-OHB infusion in terms of MEE and CO did not differ between HFrEF patents and age-matched volunteers.
Conclusions:
3-OHB has beneficial hemodynamic effects in HFrEF patients without impairing MEE. These beneficial effects are detectable in the physiological concentration range of circulating 3-OHB levels. The hemodynamic effects of 3-OHB were observed in both HFrEF patients and age-matched volunteers. 3-OHB may potentially constitute a novel treatment principle in HFrEF patients.
Clinical Perspective.
What Is New?
In patients with heart failure and reduced ejection fraction, infusion of the ketone body 3-hydroxybutyrate increased cardiac output by 2 L/min (40%) with an absolute improvement in left ventricular ejection fraction (8%). The observed effects were accompanied by vasodilation with a resultant stable systemic and pulmonary blood pressure.
The hemodynamic effects of ketones were dose-dependent and detectable in the physiological concentration range.
The coupling between myocardial oxygen consumption and cardiac work did not deteriorate despite increased cardiac output.
The hemodynamic effects of 3-hydroxybutyrate were observed in both heart failure and reduced ejection fraction patients and age-matched volunteers.
What Are the Clinical Implications?
The current study is the first in man to demonstrate that modulation of circulating ketone levels may represent a novel treatment principle in patients with heart failure.
The ketone bodies 3-hydroxybutyrate (3-OHB) and acetoacetate are able to maintain ATP generation in vital organs, including heart and brain, during periods of low carbohydrate availability.1 Ketone bodies have distinct beneficial properties since they provide more energy per 2-carbon moiety metabolized than glucose, and require less oxygen for ATP generation than fatty acids. In addition, they minimize mitochondrial uncoupling and oxidative stress.2–4
Studies on myocardial tissue from patients with severe heart failure (HF) suggest increased myocardial utilization of 3-OHB in these patients.5 At present, it is unknown whether this metabolic shift is beneficial, maladaptive, or merely epiphenomenal. Because the energetic properties of 3-OHB seem favorable, it has been suggested that increased myocardial ketone body oxidation may mitigate HF disease progression.6
3-OHB increases myocardial blood flow (MBF) in healthy volunteers by 75%, suggesting the presence of profound hemodynamic effects. Notably, it has been hypothesized that the reduction in HF hospitalizations observed in patients treated with the glucose-lowering drugs sodium-glucose cotransporter 2 inhibitors7 may be attributable, in part, to an associated increase in circulating 3-OHB levels.4,8,9
To date, the hemodynamic effects caused by modulation of circulating 3-OHB levels in patients with HF are unknown. We hypothesized that short-term 3-OHB infusion has beneficial hemodynamic effects in stable patients with chronic HF and reduced ejection fraction (HFrEF). Hence, the aims of the present study of HFrEF patients were therefore to: (1) evaluate the effect of 3-OHB infusion on cardiac output (CO) and myocardial contractile function; (2) investigate a dose-response relationship between 3-OHB infusion rate and CO changes; (3) address whether 3-OHB improves myocardial external efficiency (MEE), defined as the ratio between stroke work and myocardial oxygen consumption (MVO2), and whether 3-OHB affects MBF measured by 11C-acetate positron emission tomography (PET); and (4) compare the response to 3-OHB infusion in HFrEF patients and age-matched volunteers.
Materials and methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure, per se. However, on request, anonymized data may be made available to other researchers after appropriate approval from the local ethics committee.
Patients and Age-Matched Volunteers Without Heart Failure
We studied 24 HFrEF patients and 10 age-matched volunteers. For HFrEF patients, the inclusion criteria were (a) left ventricular ejection fraction (LVEF) ≤40% and optimal, stable HFrEF treatment according to guidelines; (b) New York Heart Association (NYHA) class II-III; and (c) ability to give informed consent. Exclusion criteria were uncorrected cardiac valvular disease, signs or symptoms of myocardial infarction within the previous 3 months, and treatment for diabetes or HbA1c level >53 mmol/mol. Age-matched volunteers had normal LVEF (defined as >50%), ECG, physical examination, systolic blood pressure <160 mm Hg, and blood samples (including NT-proBNP [N-terminal pro-B-type natriuretic peptide], cholesterol, creatinine, transaminases, electrolytes, and blood cell count) and were not allowed to have any cardiac symptoms (angina, dyspnea, palpitations and edema). Treatment with statins, antihypertensive, and antiplatelet medication was allowed. All patients were recruited from the outpatient HF clinic at Aarhus University Hospital, Denmark, and age-matched volunteers by advertisements.
Design
Four studies addressed each of the aims. In all studies, the participants fasted overnight and avoided strenuous exercise for 48 hours prior to the investigations. At 7:15 am, venous catheters were inserted into participants’ cubital veins. The local pharmacy prepared the dissolved sodium (Na)-3-OHB at a 7.5% concentration. Placebo and 3-OHB were provided in red bags for concealment. Potassium chloride (KCl) was added to a concentration of 60 mM. To minimize endogenous production of 3-OHB during prolonged fasting throughout the study and to mimic a fed postprandial condition, a low-dose insulinemic euglycemic clamp (0.3 IE insulin · kg-1 · h-1) was administered to all participants. Glucose (20% solution, 60 mM KCl) was infused to maintain euglycemia.
Study 1: 16 HFrEF patients were investigated once for 6 hours and received either 3-OHB infusion (0.18 g · kg-1 · h-1; Na-3-OHB, Gold Biotechnology Inc., USA [racemic mixture 50/50 D and L]) or placebo (isotonic saline) at an equivalent volume for 3 hours in a randomized crossover design. The participants were blinded to the order of the infusion and were continuously monitored with pulmonary artery catheter, noninvasive blood pressure measurements, ECG, repeated blood sampling, and echocardiography. They received an oral load of 60 mmol KCl when randomized to initiate with 3-OHB and 20 mmol when randomized to initiate with placebo.
Study 2: After Study 1 was completed, 8 additional HFrEF patients were enrolled consecutively in the dose-response study. These participants were studied once for 6 hours and received placebo infusion (isotonic saline 1.2 mL · kg-1 · h-1) followed by 3-OHB 0.045 g · kg-1 · h-1 with coadministration of saline 0.6 mL · kg-1 · h-1 and finally 3-OHB 0.09 g · kg-1 · h-1. Each dosage level lasted 2 hours and equivalent volumes were infused. Participants were monitored as described in Study 1 and received 30 mmol KCl orally.
Study 3: 10 of the HFrEF patients enrolled in Study 1 were consecutively requested to participate in additional 11C-acetate PET examinations on a separate day. These subjects then received either 3-OHB infusion or placebo in a randomized single-blinded crossover design, as well as KCl administered in the same way as in Study 1. 11C-acetate PET was conducted to evaluate MEE, MVO2, and MBF.
Study 4: 10 age-matched subjects underwent interventions and PET examinations similar to those performed in Study 3 (Figure 1).
Figure 1.
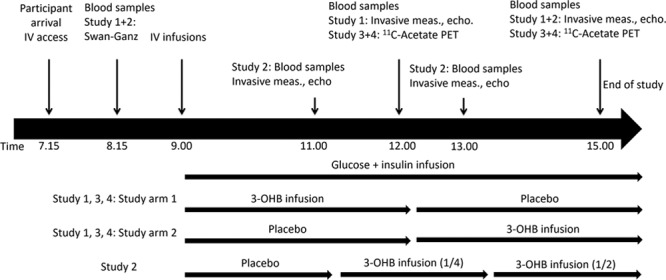
Study flowchart. The figure depicts the flow in each study. End point measurements lasted approximately 15 (Studies 1 and 2) to 30 (Studies 3 and 4) minutes. During measurements, the infusions were continued. The subsequent study arm was postponed accordingly to ensure either 3 (Studies 1, 3, and 4) or 2 hours (Study 2) for each infusion. In Study 4, placebo infusion was followed by 25% (1/4) of the 3-OHB dosage in Study 1 and then increased to 50% (1/2). Blood was sampled, and invasive hemodynamic measures were recorded repeatedly throughout the studies. 3-OHB indicates 3-betahydroxybutyrate; echo, echocardiography; IV, intravenous; meas, measurements; and PET, positron emission tomography.
Pulmonary Arterial Catheterization (Studies 1 and 2)
Central venous pressure (CVP), mean pulmonary arterial pressure (mPAP), and mean pulmonary capillary wedge pressure (PCWP) were measured at end-expiration. Mixed venous saturation (SVO2) was measured continuously, and cardiac output (CO) was assessed by thermodilution technique and averaged over 3 measurements. Heart rate (HR) and blood pressure were measured noninvasively. Stroke volume (SV) (SV=CO/HR), mean arterial pressure (MAP), and systemic (SVR=[MAP-CVP]/CO) and pulmonary (PVR=[mPAP-PCWP]/CO) vascular resistance were calculated. All hemodynamic measurements were performed with the patient in the supine position.
Echocardiography (Studies 1 and 2)
End diastolic and systolic volumes were measured by Simpson biplane methods to assess LVEF. Numeric global longitudinal strain (GLS), mitral inflow velocities (E and A), and mitral plane velocities in the lateral mitral annulus (e’ and s’) were measured. Velocity time integral of the left ventricular outflow track was assessed. A GE Vivid E9 (GE Healthcare, USA) was used for echocardiographic acquisition and EchoPAC 13 (GE-Vingmed Ultrasound, Norway) for analysis. All measures were averaged over 3 heart beats, and the assessor was blinded to allocation and study.10
Positron Emission Tomography (Studies 3 and 4)
A total of 400 MBq 11C-acetate was injected, followed by list-mode PET recording for 27 minutes on a Biograph TruePoint TrueV 64 PET/computed tomography scanner (Siemens, Germany). Blood pressure and HR were measured at 1, 5, 10, and 20 minutes after injection. The examination was performed to obtain the global clearance rate (k2) and to calculate MVO2 as previously described:11
 |
Dynamic data sets were analyzed to estimate SV by use of aQuant Research.12 HR and MAP were measured noninvasively. Left ventricular mass and CO were obtained from the PET dataset, and MEE was calculated as:13
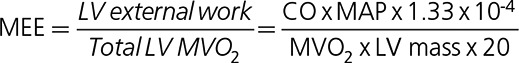 |
MBF (mL · g-1 · min-1) was estimated by measuring k1 and correcting for rate pressure product as previously described.14 The assessor of the PET examinations was blinded to treatment allocation and group.
Blood Samples
Blood samples were drawn before and during the examinations. Glucose, electrolytes, lactate, and pH were analyzed using YSI STAT 2100 (YSI Inc., Netherlands) immediately after sampling. All other samples were stored at −20°C and analyzed in batch. Plasma (P)-3-OHB was measured using hydrophilic interaction liquid chromatography tandem mass spectrometry. Insulin was measured with ELISA (Mercodia, Sweden) and free fatty acids (FFA) quantified by in vitro enzymatic colorimetric method assay (Trichem, Denmark). Brain natriuretic peptide (BNP) was analyzed by chemiluminescent microparticle immunoassay on an Abbott Architect i2000SR (Abbott, Germany), as described by the manufacturer, and catecholamines (adrenaline and noradrenaline) by an in-house time-resolved high-performance liquid chromatography method. High sensitive cardiac troponin T was analyzed by an immunoassay kit according to the manufacture (Cobas; Roche Diagnostics GmbH, Germany).
End Points
The primary end point for Studies 1 and 2 was CO measured by thermodilution technique. Secondary end points were SV, SVO2, PCWP, PVR, SVR, HR, MAP, mPAP, CVP, LVEF, GLS, catecholamines, P-potassium, pH, and P-lactate levels.
In Studies 3 and 4, the primary end point was MEE by 11C-acetate PET. Secondary end points were MVO2, MBF, CO and SV, catecholamines, P-potassium, pH, and P-lactate levels.
In all studies, the end point measurements were compared at the end of each intervention period.
Power Calculation and Statistics
The coefficient of variation for CO is 4%.15 Hence, we expected to be able to detect relative differences of 3% (Study 1, N=16) and 4% (Study 2, N=8) with a power of 80% and an alpha of 5%. At our institution, coefficient of variation for MEE assessed by 11C-acetate PET is approximately 9%.13 Therefore, we expected to be able to detect relative differences of 9% in each study group and of 6% for both groups combined (Studies 3 and 4).
Data were investigated for normal distribution by qq-plots and transformed when appropriate. Unpaired t tests were used for comparison between groups (baseline characteristics). Changes in data are presented as mean±SEM and between-group differences as mean±SD, if not stated otherwise. When appropriate, 2-way repeated measures ANOVA (Study 1: terms were intervention and intervention sequence), 1-way repeated measures ANOVA (Study 2), and 3-way repeated measures ANOVA tests were applied (Studies 3 and 4: terms were intervention, group [age-matched versus HFrEF], and intervention sequence). Pairwise comparisons were performed if the overall ANOVA analysis demonstrated significant difference. P values <0.05 were considered statistically significant. All data analyses were performed using STATA version 14 (StataCorp, USA).
Ethics
All patients gave informed consent, and the local Ethics Committee of the Central Denmark Region and the Danish Data Protection Agency approved the study. The study is registered at clinicaltrials.gov (NCT03073356).
Results
Patients and Age-Matched Volunteers
Thirty-three stable chronic HFrEF patients were screened. Two withdrew consent and 7 had LVEF >40%; hence, 24 were enrolled. Twelve age-matched volunteers were screened, of whom 1 withdrew consent and another was excluded because of asymptomatic left bundle-branch block (Figure I in the online-only Data Supplement).
Characteristics
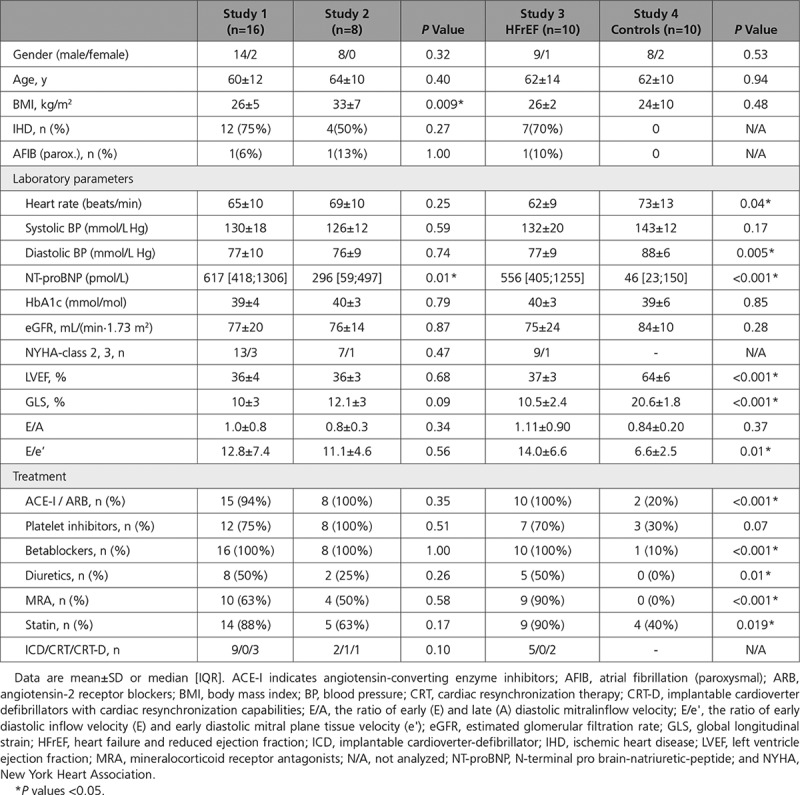
HFrEF patients enrolled in Study 1 had lower body mass index and higher NT-proBNP levels than those enrolled in Study 2, but were otherwise comparable on baseline variables. As scheduled, the age-matched volunteers enrolled in Study 4 had normal LVEF and received no treatment for HFrEF, as opposed to those enrolled in Study 3 (Table 1). All participants were white.
Table 1.
Baseline Characteristics
Study 1: Effect of 3-OHB Infusion on Hemodynamics and Echocardiographic Measurements in HFrEF Patients
Compared with placebo, 3-OHB infusion (0.18 g · kg-1 · h-1) increased circulating P-3-OHB levels from 0.4±0.3 mM to 3.3±0.4 mM after 3 hours (P<0.001; Table 2, Figure 2A). The increment was associated with an increase in CO of 2.0±0.3 L/min (P<0.001) ie, approximately 40% (Figure 2B, Figure II in the online-only Data Supplement), and with an increase in SVO2 from 72±3 to 79±4% (P<0.001; Table 3). The changes in CO were caused by increases in SV of 20±2 mL (P<0.001) and HR of 7±2 bpm (P<0.001), however, differences in CO between study arms were already present within the first 30 minutes (0.9 L/min, P=0.01) and preceded the changes in HR within the same period (P=0.99). We observed no significant changes in MAP or mPAP, but a minor decrease in CVP and PCWP (approximately 1 mm Hg; P=0.05 and P=0.03, respectively). Thus, SVR decreased by 30% (P<0.001) and PVR by 21% (P=0.003) (Figure III in the online-only Data Supplement). The administered volumes did not differ between study arms (Table 2). However, the increase in CO was most pronounced in those who were randomized to placebo followed by 3-OHB infusion (interaction between randomization sequence and treatment P=0.03).
Table 2.
Blood Sample Measurements and Quantities of Infused Volumes (Studies 1 and 2)
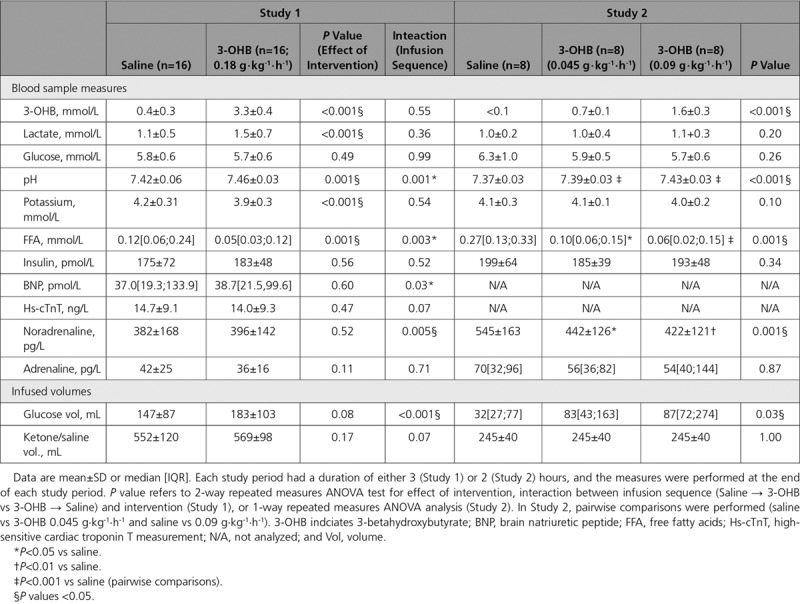
Figure 2.
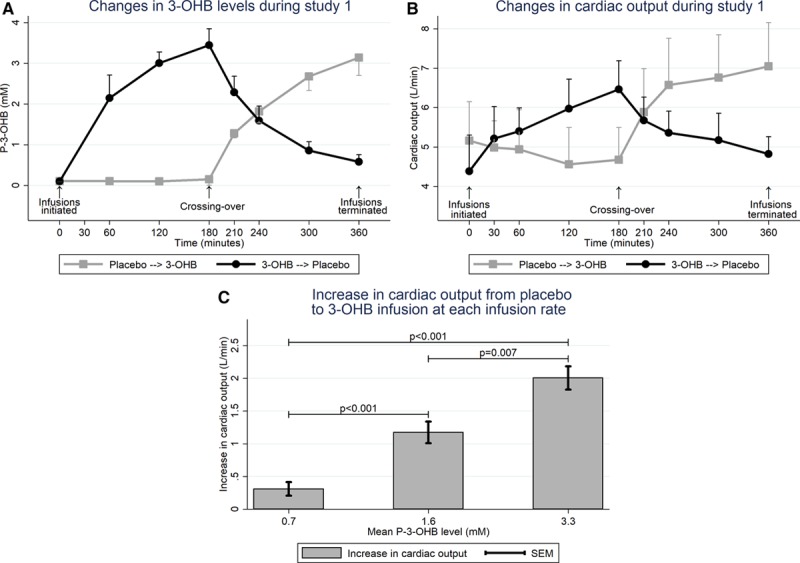
Circulating P-3-OHB levels (Study 1) and changes in cardiac output in HFrEF patients (Studies 1 and 2). Mean with bars indicating standard deviation (A and B) or SEM (C). A, P-3-OHB levels were low until 3-OHB infusion was initiated and decreased after 3-OHB was substituted with placebo (n=16). B, Cardiac output increased from placebo to 3-OHB infusion and decreased when 3-OHB infusion was terminated (n=16). C, CO was assessed in Study 2 (n=8) at a low infusion rate (0.045 g · kg-1 · h-1, mean P-3-OHB: 0.7 mM) and an intermediate infusion rate (0.09 g · kg-1 · h-1, mean P-3-OHB: 1.6 mM; paired analysis). These results were compared with those obtained in Study 1 (high infusion rate [0.18 g · kg-1 · h-1], mean P-3-OHB: 3.3 mM, n=16; unpaired data) and demonstrated a dose-response association. 3-OHB indicates 3-betahydroxybutyrate; and CO, cardiac output.
Table 3.
Hemodynamic Measures and Echocardiographic Findings in the Invasive Studies (Studies 1 and 2)
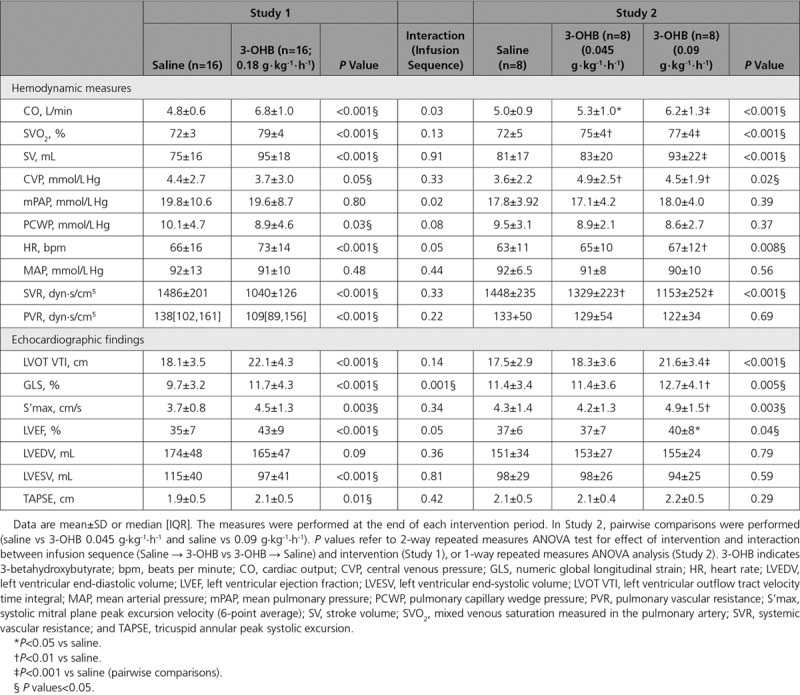
Infusion of 3-OHB increased systolic left ventricular echocardiographic variables: left ventricular outflow track velocity time integral (4.0±0.6 cm; P<0.001), GLS (2.0±0.3% [absolute improvement]; P<0.001), S’max (0.79±0.22 cm/sec; P=0.003) and LVEF (8±1% [absolute increase]; P<0.001) compared with placebo (Table 3). We observed no association between LVEF at baseline and changes in either CO (P=0.72) or SV (P=0.49). There were no changes in end diastolic volumes (P=0.09), whereas end systolic volumes decreased significantly by 17±3 mL (P<0.001). The increase in GLS was most pronounced in those who were randomized to receive placebo followed by 3-OHB (P<0.001), whereas there was no interaction between randomization sequence and treatment in other echocardiographic measures (Table 3).
Study 2: Dose-Response Effect of 3-OHB Infusion in HFrEF Patients
In the dose-response study, circulating P-3-OHB increased from <0.1 mM to 0.7±0.1 mM (P<0.001) at an infusion rate of 0.045 g · kg-1 · h-1, and to 1.6±0.3 mM at an infusion rate of 0.09 g · kg-1 · h-1, P-3-OHB (P<0.01; Table 2). The increase in P-3-OHB was associated with a significant, gradual increase in CO (Table 3, Figure IV in the online-only Data Supplement). The maximal increase in CO (1.2±0.1 L/min; P<0.001) was lower than the increase from placebo to 3-OHB infusion in Study 1 (P=0.007), where a higher infusion rate was applied (0.18 g · kg-1 · min-1) (Figure 2C). The changes in regard to SV, HR and SVO2 were similar to those observed in Study 1, whereas CVP increased approximately 1 mm Hg (P=0.02).
In line with Study 1, we observed a gradual increase in left ventricular outflow track velocity time integral, GLS, LVEF and S’max during 3-OHB infusion. The increments were not statistically significant until the infusion rate was increased to 0.09 g · kg-1 · h-1 (Table 3).
Studies 3 and 4: Effect of 3-OHB Infusion on MEE in HFrEF Patients and Age-Matched Volunteers
Circulating P-3-OHB concentrations increased from 0.4±0.3 to 3.4±0.6 mM (P<0.001; Table I in the online-only Data Supplement) after infusion of 3-OHB, with no difference between HFrEF patients and control subjects (test for interaction P=0.43). There was no difference between the administered 3-OHB and placebo volumes, whereas slightly more glucose was administered during 3-OHB infusion (P=0.04; Table I in the online-only Data Supplement). As opposed to Study 1, there was no interaction between infusion sequence and CO (P=0.40).
In Study 3 (HFrEF patients), 3-OHB infusion increased LV external work by 38% (P=0.002) because of an increase in SV of 20±3 mL (P=0.001) and an increase in HR of 8±2 bpm (P=0.003) compared with placebo (Figure V in the online-only Data Supplement). We observed a proportional increase in MVO2. Hence, MEE did not change (P=0.87; Table 4, Figure 3).
Table 4.
11C-acetate-PET Examination Results
Figure 3.
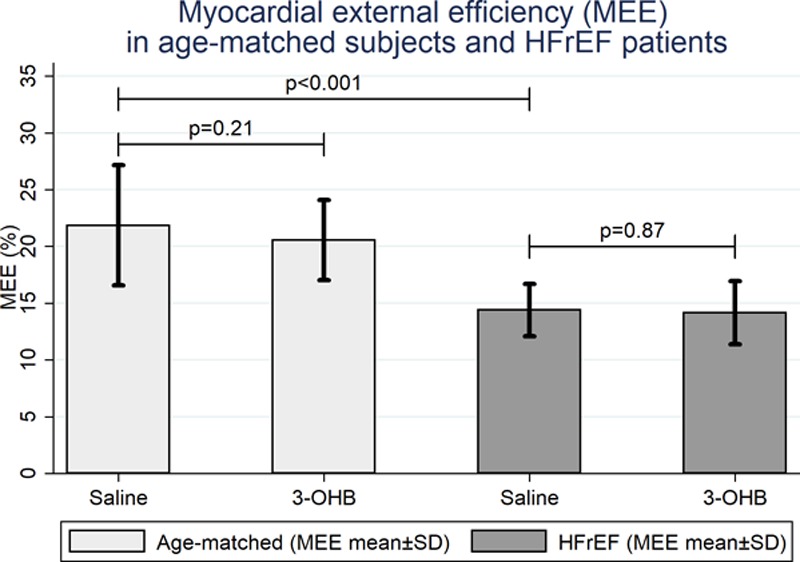
Changes in myocardial external efficiency in Studies 3 and 4. MEE was significantly lower in HFrEF patients (n=8) than in age-matched subjects (n=8) (P<0.001), but 3-OHB did not affect MEE in either study group. 3-OHB indicates 3-betahydroxybutyrate; and MEE, myocardial external efficiency.
MEE was higher in age-matched volunteers (Study 4) than in HFrEF patients (22±5% versus 14±2%; P<0.001). As in HFrEF patients, CO, SV, left ventricular external work, and MVO2 increased during 3-OHB compared with placebo infusion in age-matched subjects. Consequently, MEE was unaffected by 3-OHB infusion (P=0.21; Table 4, Figure 3), and we observed no difference between HFrEF patients and age-matched volunteers (P=0.42, test for interaction). MBF increased in both study groups during 3-OHB infusion. The increment was slightly higher in age-matched volunteers than in HFrEF patients (P=0.03, test for interaction). Infusion sequence did not interact with MEE (P=0.11) or any other PET-derived findings (Table 4).
Relative changes in key measurements for Study 1 and MEE for Studies 3 and 4 are summarized in Figure 4. Examples of time-activity curves during the PET examinations are demonstrated in Figure VI in the online-only Data Supplement.
Figure 4.
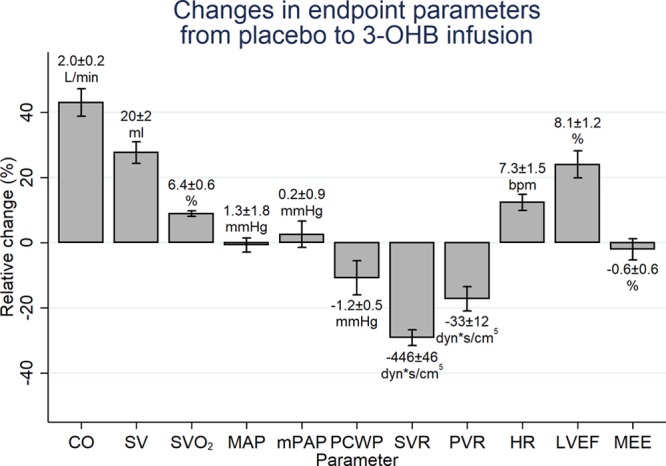
Changes in end point parameters from placebo to 3-OHB infusion. Mean relative change with SEM and the corresponding mean absolute change±SEM listed above or below each bar, respectively. Bpm indicates beats per minute; CO, cardiac output; HR, heart rate; LVEF, left ventricular ejection fraction (Study 1, n=16); MAP, mean arterial pressure; MEE, myocardial external efficiency (Studies 3 and 4, n=20); mPAP, mean pulmonary pressure; PVR, pulmonary vascular resistance; SV, stroke volume; SVO2, mixed venous saturation measured in the pulmonary artery; and SVR, systemic vascular resistance.
Studies 1 Through 4: Lactate, pH, Potassium, and Hormones
In Study 1, circulating P-lactate concentrations and pH increased, whereas P-potassium concentrations decreased during 3-OHB infusion despite potassium substitution (Table 2, Figure VII in the online-only Data Supplement). In Study 2, pH increased slightly during 3-OHB infusion, whereas P-lactate and P-potassium concentrations were stable (Table 2, Figure VIII in the online-only Data Supplement). In the PET studies, the observed changes were similar to those observed in Study 1 (Table I and Figure IX in the online-only Data Supplement). Circulating BNP, high sensitive cardiac troponinT, noradrenalin, and insulin levels did not change during 3-OHB infusion, whereas FFA levels were slightly lower during 3-OHB than during placebo infusion (Table 2, Table I in the online-only Data Supplement). Adrenaline decreased in the healthy volunteers during 3-OHB infusion (P=0.003). In Studies 3 and 4, insulin sensitivity assessed by glucose infusion rate during the last 30 minutes of each infusion periods did not differ between 3-OHB and placebo infusion (P=0.11, Figure IXb and Table I in the online-only Data Supplement). Glucose infusion rate was not analyzed in Studies 1 and 2, as additional glucose was infused to measure CO by thermodilution. For those parameters affected by treatment in Study 1, there was an interaction between infusion sequence and pH and FFA levels (Table 2, Figures VIIB and VIIF in the online-only Data Supplement). Similarly, for those parameters affected by treatment in Studies 3 and 4, infusion sequence interacted with pH, potassium, and FFA levels (Table I and Figures IXC, IXG, and IXH in the online-only Data Supplement).
Safety
All participants completed the study program. One HFrEF patient (LVEF: 23%, NYHA 3) experienced asymptomatic, sustained ventricular tachycardia episodes (frequency: 100 bpm) during both study arms. These episodes occurred 2 hours after 3-OHB infusion had been initiated and continued for approximately 1 hour into the placebo infusion arm. The patient was known to have such episodes. No other arrhythmias were recorded. Another HFrEF patient (randomized to placebo followed by 3-OHB) reported a brief episode of nausea during the placebo infusion and another volunteer had an episode of nausea at the very end of the study after the PET examination and termination of the 3-OHB–infusion.
Discussion
The current study is the first to evaluate the hemodynamic effects of 3-OHB infusion in HFrEF patients, and to assess and compare its effect on MEE in HFrEF patients and age-matched volunteers. First, we demonstrated that 3-OHB infusion in patients with HFrEF improved CO by 2.0 L/min and LVEF by 8%, and reduced SVR by 30% compared with placebo infusion. Second, we observed that the effect was dose-dependent, reaching significant hemodynamic effects at a circulating 3-OHB concentration in the physiological range. Third, despite the increase in cardiac work, 3-OHB infusion did not deteriorate mechanoenergetic coupling in terms of MEE. Fourth, even though MEE was lower in HFrEF patients than in age-matched volunteers, the response to 3-OHB infusion did not vary among groups. Hence, the cardiovascular response to elevated P-3-OHB levels seems ubiquitous, and modulation of circulating P-3-OHB levels may constitute a novel treatment principle in HFrEF patients.
Improvement in Hemodynamics and Cardiac Function by 3-OHB Infusion
In early experimental studies, CO decreased in the isolated perfused rat heart at high concentrations of 3-OHB when ketone bodies were administered as the sole energy substrate.16 Adding glucose to the perfusate reversed this detrimental effect. Coadministration of 3-OHB (4 mM) and glucose actually increased cardiac work17 and output compared with glucose alone in an isolated rat heart model.18 Knock-out of the gene responsible for control of flux through the enzymes of cardiac ketone metabolism resulted in pathological remodeling and cardiac dysfunction in the failing heart,19 suggesting a pivotal role for sustained ketone oxidation in HF.20 Moreover, increased myocardial ketone utilization has been demonstrated in explanted hearts from patients with severe HF.5
The observed improvement of cardiac function was not caused by increments in preload, as PCWP decreased and BNP remained unchanged. Lactate levels increased with the rise in ketone levels, presumably because of energy substrate competition with increased lactate production from pyruvate,21–23 in combination with reduced lactate oxidation in peripheral tissues.24 The changes in circulating lactate levels were minor (1.1 versus 1.5 mM) and well below the levels between 2.4 to 4.8 mM that affects hemodynamics.25
The observed increase in CO and LVEF may partly be caused by reduced SVR. In fact, other studies are supportive of vasodilatory effects of ketones.26 However, in the present study, blood pressure did not change definitively, despite a decline in SVR of 30%. In addition, we observed no major change in preload during 3-OHB infusion. Consequently, the Frank–Starling relationship shifted upward because of vasodilatation, increased contractility, or a combination thereof. Invasive pressure–volume measurements were not performed since the main scope of the present study was to investigate the clinical, cardiovascular and hemodynamic effects of 3-OHB. Therefore, we cannot definitively determine whether 3-OHB increases CO by a direct effect on the myocardium, by vasodilation or by a combination thereof. Since 3-OHB interacts with G-protein coupled receptors there is a plausible mechanism for peripheral, vascular effects. Finally, 3-OHB may also work as an agonist for cellular receptors known to increase HR,27 which could be involved in the observed increase in HR.
Irrespective of the mechanism, our data demonstrate that 3-OHB has beneficial cardiovascular effects in HF as it improves cardiac function and hemodynamics, causes vasodilatation, and thus, improves organ perfusion.
3-OHB Has Cardiovascular Effects in the Physiological Concentration Range
In healthy subjects, circulating P-3-OHB levels range from 0.1 to 0.3 mM following an overnight fast. Levels increase to 2 to 3 mM after 48 to 72 hours of fasting and may reach 5 to 10 mM after prolonged fasting.28,29 Thus, in the dose-response study, circulating P-3-OHB concentrations were in the physiological range from 0.7 to 1.6 mM, and we observed a dose-dependent cardiovascular response to 3-OHB infusion (Table 3 and Figure 2C). At the lowest infusion rate of 0.045 g · kg-1 · min-1, P-3-OHB increased from 0.3 mM at baseline to 0.7 mM, and this increase was associated with significant increases in CO and SVO2. The hemodynamic response increased at higher infusion rates and concentrations. In HFrEF patients, circulating 3-OHB concentrations are increased in proportion to the severity of cardiac dysfunction.30,31 This may be caused partly by increased FFA mobilization in response to stimulation by stress hormones and insulin resistance.31 Our observations suggest that increased levels of ketones bodies may be adaptive and act as endogenous mediators of cardiovascular function in HFrEF patients. This finding may be relevant in the context of sodium-glucose cotransporter 2 inhibitors. These glucose-lowering drugs increase ketogenesis and circulating P-3-OHB concentrations to approximately 0.6 mM.32 Hence, it is conceivable that the associated reduction in HF hospitalizations during sodium-glucose cotransporter 2 treatment7 is partly attributed to the properties of 3-OHB, as demonstrated in the current study. Thus, the current study demonstrates that the hemodynamic response to 3-OHB infusion is dose-dependent, observed in the physiological concentration range, and can be adjusted by changing the infusion rate (Figure 2C).
Increased 3-OHB Levels Do Not Change Mechanoenergetic Coupling in HFrEF Patients
Reduced MEE is believed to be causally involved in the development of HF33 and is a prognostic factor in HF patients.34 Ketone bodies improve myocardial efficiency of the working rat heart by 25%.18 However, the results of animal experiments are inconsistent,5,16,18 and the relationship between myocardial ketone body oxidation, cardiac work, and myocardial efficiency in patients with HF remains undetermined.4,6,35 We observed that 3-OHB infusion increased MVO2 and cardiac work proportionally, leaving MEE unaltered. Thus, short-term treatment with 3-OHB did not impair the coupling between myocardial energy generation and cardiac work although hemodynamics were improved. This is clinically important as reduced MEE could worsen the prognosis.34 Our experiments were conducted during a low-dose insulinemic euglycemic clamp, partly to mimic ordinary, fed insulin-stimulated conditions of every-day life and partly to suppress endogenous ketone production and impose clear-cut differences in 3-OHB levels between the two infusion periods. Thus, the findings cannot be extrapolated to a setting of prolonged fasting where FFA levels are higher and myocardial FFA uptake is increased;36 under such conditions, a metabolic shift in substrate oxidation from FFA to 3-OHB oxidation could theoretically improve oxygen efficiency and MEE.
The effect of inotropic agents on MEE have been equivocal in previous studies.37–39 The present study demonstrates that despite the hemodynamic effects of 3-OHB infusion, there was no detrimental effects on MEE. Consequently, 3-OHB improves CO and organ perfusion without MEE deterioration.
Hemodynamic Effect of 3-OHB Does Not Differ Between HFrEF Patients and Age-Matched Control Subjects
Like others,40 we observed that MEE was approximately 50% lower in HFrEF patients than in age-matched volunteers (Figure 3). We have previously shown that in healthy subjects a short-term increase in circulating 3-OHB levels reduces myocardial glucose uptake by 50% without affecting FFA oxidation,41 suggesting that the healthy human heart rapidly increases 3-OHB utilization for mitochondrial ATP generation. We now extend our findings and demonstrate that infusion of 3-OHB increases CO and SV in subjects without heart disease. MBF was increased, most likely by autoregulatory mechanisms in the coronary vasculature, in response to increased external work and MVO2.42 Both cardiac work and MVO2 increased during 3-OHB infusion. Thus, MEE remained unchanged in both HFrEF patients and age-matched volunteers. In highly trained endurance athletes, ingestion of a ketone ester modestly increases exercise capacity.43 Although this effect may have been caused by an increased muscular capacity to oxidize fatty-acid–derived carbon moieties, it remains possible that increased cardiac performance may have contributed.
Overall, the effects of modulation of circulating 3-OHB in age-matched controls did not differ from the effects observed in HFrEF patients. These findings suggest that the cardiovascular response to 3-OHB infusion is universal, and that the metabolic shift toward increased ketone metabolism5 during metabolic stress in evolutionary terms may be viewed as a beneficial species-preserving mechanism.
Safety Issues
The changes in pH and potassium by 3-OHB infusion have previously been shown.41,44 The dissolved Na-3-OHB dissociates and allows 3-OHB to act as a conjugated base, which increases pH.28 In the present study, pH increased only marginally and unlikely influenced the results. Major changes in P-potassium were avoided by administering a potassium supplement. In future clinical trials, changes in pH and potassium levels should be taken into consideration.
HR increased by 7 bpm during 3-OHB infusion at a rate of 0.18 g · kg-1 · min-1. Since increased HR is associated with a worse prognosis in chronic HFrEF,45 the increase might raise concerns for future use of 3-OHB in HFrEF patients. Importantly, the increase in HR was only 2 to 4 bpm at an infusion rate of 3-OHB 0.045 g · kg-1 · h-1 and 3-OHB 0.09g · kg-1 · h-1, corresponding to 3-OHB levels in the range of 0.7 to 1.6 mM. These findings may be useful for establishing the optimal dosage in clinical trials.
One episode of asymptomatic idioventricular arrhythmia was observed in a patient with advanced HFrEF. The patient was known to present with such episodes. Whether the arrhythmia was related to cellular hyperpolarization due to increased ketone metabolism,46 placement of the Swan–Ganz catheter or merely a coincidence is unknown. Episodes of arrhythmias were not recorded in the remaining HFrEF patients.
Fluid load is of importance in HFrEF. In the present study, no patients had acute HFrEF, and a Na-3-OHB load for 3 hours seems to be acceptable as we observed no increase in PCWP, BNP, or high sensitive cardiac troponinT levels. However, the Na+ conjugated to the 3-OHB may have detrimental effects in patients with severe, acute HF. The used Na-3-OHB consisted of a racemic mixture of both L- and D-isomer in which only the D-isomer was metabolized. Hence, a pure D-isomer of 3-OHB conjugated with another low-molecular–weight combustible carbon moiety could decrease the infused volume and avoid Na+ load.21,43
Study Limitations
Studies 1, 3, and 4 were crossover studies which is a design with potential carry-over effect. Patients who received 3-OHB infusion initially had slightly elevated P-3-OHB levels at the end of the placebo period (Figure 2), as well as persisting elevated pH (Figure VIIF in the online-only Data Supplement). However, there was no interaction between infusion order and changes in SV and SVO2 (Study 1), nor was there interaction between infusion order and changes in MEE and CO in the PET studies.
In Studies 1 and 2, the investigator performing the cardiac output assessment by thermodilution was not blinded to the randomization. Echocardiographic measurements were blinded during analyses and confirmed the invasive findings (Table 3). CO measurements by PET in Studies 3 and 4 also confirmed the increase in cardiac output (Table 4) during 3-OHB infusion. The investigator performing the PET analyses was not involved in the experiments and was blinded to randomization.
The decrease in filling pressures (CVP, PCWP) were small despite improvement in cardiac performance. This is in consistence with previous studies concerning dobutamine in stable HFrEF patients.47 It is also conceivable that the volume loading given during the study outbalanced the decrease in filling pressures induced by vasodilation.
Insulin was coadministered during the interventions. However, it seems unlikely that insulin caused the observed effects as insulin levels did not differ when end point measurements were assessed in any of the studies (Table 2, Table I in the online-only Data Supplement). In terms of relative differences, FFA levels were approximately 60% lower during 3-OHB infusion. However, absolute changes in FFA levels were minimal because of suppression by the low-dose insulinemic euglycemic clamp (medians between 0.04 and 0.27 mM; Figures VIIB, VIIIC, and IXC in the online-only Data Supplement). Therefore, it seems unlikely that the small absolute differences in FFA levels had any impact on the hemodynamic findings.
Conclusion
3-OHB demonstrated dose-dependent beneficial cardiac and hemodynamic effects in HFrEF patients without deteriorating mechanoenergetic coupling and without causing any safety issues. We observed similar response to 3-OHB infusion in age-matched test subjects and HFrEF patients. Thus, modulation of circulating 3-OHB may represent a novel treatment principle in patients with HF.
Sources of Funding
The study was investigator-initiated and investigator-designed. The investigators received an unrestricted grant from the Lundbeck Foundation.
Disclosures
None.
Supplementary Material
Footnotes
Sources of Funding, see page 2140
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.118.036459.
References
- 1.Cahill GF., Jr. Starvation in man. N Engl J Med. 1970;282:668–675. doi: 10.1056/NEJM197003192821209. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- 2.Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RV, Jr, de Cabo R, Ulrich S, Akassoglou K, Verdin E. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–214. doi: 10.1126/science.1227166. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolwicz SC, Jr, Airhart S, Tian R. Ketones step to the plate: A game changer for metabolic remodeling in heart failure? Circulation. 2016;133:689–691. doi: 10.1161/CIRCULATIONAHA.116.021230. doi: 10.1161/CIRCULATIONAHA.116.021230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME Trial: A “thrifty substrate” hypothesis. Diabetes Care. 2016;39:1108–1114. doi: 10.2337/dc16-0330. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 5.Bedi KC, Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016;133:706–716. doi: 10.1161/CIRCULATIONAHA.115.017545. doi: 10.1161/CIRCULATIONAHA.115.017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopaschuk GD, Ussher JR. Evolving concepts of myocardial energy metabolism: More than just fats and carbohydrates. Circ Res. 2016;119:1173–1176. doi: 10.1161/CIRCRESAHA.116.310078. doi: 10.1161/CIRCRESAHA.116.310078. [DOI] [PubMed] [Google Scholar]
- 7.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 8.Javed B, Carine H, Gerasimos F, Stuart P, Richard B, Martina B, Alfred C, Jyothis G, Jennifer G, James J, Sanjay K, Carolyn L, Gregory L, Nikolaus M, Peter M, Cyrus M, Piotr P, Julio R, Naveed S, Afshin S, Benjamin S, Sanjiv S, Hiroyuki T, Subodh V, Christoph W, Hans-Juergan W, Faiez Z, D AS. The potential role and rationale for treatment of heart failure with sodium–glucose co-transporter 2 inhibitors. Eur J Heart Fail. 2017;19:1390–1400. doi: 10.1002/ejhf.933. [DOI] [PubMed] [Google Scholar]
- 9.Sattar N, McLaren J, Kristensen SL, Preiss D, McMurray JJ. SGLT2 Inhibition and cardiovascular events: Why did EMPA-REG Outcomes surprise and what were the likely mechanisms? Diabetologia. 2016;59:1333–1339. doi: 10.1007/s00125-016-3956-x. doi: 10.1007/s00125-016-3956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen R, Nørrelund H, Kampmann U, Kim WY, Ringgaard S, Schär M, Møller N, Bøtker HE, Wiggers H. Failing heart of patients with type 2 diabetes mellitus can adapt to extreme short-term increases in circulating lipids and does not display features of acute myocardial lipotoxicity. Circ Heart Fail. 2013;6:845–852. doi: 10.1161/CIRCHEARTFAILURE.113.000187. doi: 10.1161/CIRCHEARTFAILURE.113.000187. [DOI] [PubMed] [Google Scholar]
- 11.Hansson NH, Tolbod L, Harms HJ, Wiggers H, Kim WY, Hansen E, Zaremba T, Frøkiær J, Jakobsen S, Sørensen J. Evaluation of ECG-gated [(11)C]acetate PET for measuring left ventricular volumes, mass, and myocardial external efficiency. J Nucl Cardiol. 2016;23:670–679. doi: 10.1007/s12350-015-0331-0. doi: 10.1007/s12350-015-0331-0. [DOI] [PubMed] [Google Scholar]
- 12.Harms HJ, Knaapen P, de Haan S, Halbmeijer R, Lammertsma AA, Lubberink M. Automatic generation of absolute myocardial blood flow images using [15O]H2O and a clinical PET/CT scanner. Eur J Nucl Med Mol Imaging. 2011;38:930–939. doi: 10.1007/s00259-011-1730-3. doi: 10.1007/s00259-011-1730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansson NH, Harms HJ, Kim WY, Nielsen R, Tolbod LP, Frøkiær J, Bouchelouche K, Poulsen SH, Wiggers H, Parner ET, Sörensen J. Test-retest repeatability of myocardial oxidative metabolism and efficiency using standalone dynamic 11C-acetate PET and multimodality approaches in healthy controls. J Nucl Cardiol. 2018;25:1929–1936. doi: 10.1007/s12350-018-1302-z. doi: 10.1007/s12350-018-1302-z. [DOI] [PubMed] [Google Scholar]
- 14.Marinho NV, Keogh BE, Costa DC, Lammerstma AA, Ell PJ, Camici PG. Pathophysiology of chronic left ventricular dysfunction. New insights from the measurement of absolute myocardial blood flow and glucose utilization. Circulation. 1996;93:737–744. doi: 10.1161/01.cir.93.4.737. [DOI] [PubMed] [Google Scholar]
- 15.Kroon M, Groeneveld AB, Smulders YM. Cardiac output measurement by pulse dye densitometry: Comparison with pulmonary artery thermodilution in post-cardiac surgery patients. J Clin Monit Comput. 2005;19:395–399. doi: 10.1007/s10877-005-6865-y. doi: 10.1007/s10877-005-6865-y. [DOI] [PubMed] [Google Scholar]
- 16.Taegtmeyer H, Hems R, Krebs HA. Utilization of energy-providing substrates in the isolated working rat heart. Biochem J. 1980;186:701–711. doi: 10.1042/bj1860701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashiwaya Y, Sato K, Tsuchiya N, Thomas S, Fell DA, Veech RL, Passonneau JV. Control of glucose utilization in working perfused rat heart. J Biol Chem. 1994;269:25502–25514. [PubMed] [Google Scholar]
- 18.Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9:651–658. doi: 10.1096/fasebj.9.8.7768357. [DOI] [PubMed] [Google Scholar]
- 19.Schugar RC, Moll AR, André d’Avignon D, Weinheimer CJ, Kovacs A, Crawford PA. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol Metab. 2014;3:754–769. doi: 10.1016/j.molmet.2014.07.010. doi: 10.1016/j.molmet.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Krüger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133:698–705. doi: 10.1161/CIRCULATIONAHA.115.017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cotter DG, Schugar RC, Crawford PA. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2013;304:H1060–H1076. doi: 10.1152/ajpheart.00646.2012. doi: 10.1152/ajpheart.00646.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi K, Neely JR. Control of maximum rates of glycolysis in rat cardiac muscle. Circ Res. 1979;44:166–175. doi: 10.1161/01.res.44.2.166. [DOI] [PubMed] [Google Scholar]
- 23.Jeffrey FM, Diczku V, Sherry AD, Malloy CR. Substrate selection in the isolated working rat heart: Effects of reperfusion, afterload, and concentration. Basic Res Cardiol. 1995;90:388–396. doi: 10.1007/BF00788500. [DOI] [PubMed] [Google Scholar]
- 24.Berger M, Hagg SA, Goodman MN, Ruderman NB. Glucose metabolism in perfused skeletal muscle. Effects of starvation, diabetes, fatty acids, acetoacetate, insulin and exercise on glucose uptake and disposition. Biochem J. 1976;158:191–202. doi: 10.1042/bj1580191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nalos M, Leverve X, Huang S, Weisbrodt L, Parkin R, Seppelt I, Ting I, Mclean A. Half-molar sodium lactate infusion improves cardiac performance in acute heart failure: A pilot randomised controlled clinical trial. Crit Care. 2014;18:R48. doi: 10.1186/cc13793. doi: 10.1186/cc13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fioretto P, Trevisan R, Velussi M, Cernigoi A, De Riva C, Bressan M, Doria A, Pauletto N, Angeli P, De Dona C, Nosadini R. Glomerular filtration rate is increased in man by the infusion of both D,L-3-hydroxybutyric acid and sodium D,l-3-hydroxybutyrate. J Clin Endocrinol Metab. 1987;65:331–338. doi: 10.1210/jcem-65-2-331. [DOI] [PubMed] [Google Scholar]
- 27.Gadegbeku CA, Dhandayuthapani A, Shrayyef MZ, Egan BM. Hemodynamic effects of nicotinic acid infusion in normotensive and hypertensive subjects. Am J Hypertens. 2003;16:67–71. doi: 10.1016/s0895-7061(02)03196-5. [DOI] [PubMed] [Google Scholar]
- 28.Balasse EO. Kinetics of ketone body metabolism in fasting humans. Metabolism. 1979;28:41–50. doi: 10.1016/0026-0495(79)90166-5. [DOI] [PubMed] [Google Scholar]
- 29.Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980;60:143–187. doi: 10.1152/physrev.1980.60.1.143. doi: 10.1152/physrev.1980.60.1.143. [DOI] [PubMed] [Google Scholar]
- 30.Lommi J, Kupari M, Koskinen P, Näveri H, Leinonen H, Pulkki K, Härkönen M. Blood ketone bodies in congestive heart failure. J Am Coll Cardiol. 1996;28:665–672. doi: 10.1016/0735-1097(96)00214-8. [DOI] [PubMed] [Google Scholar]
- 31.Nørrelund H, Wiggers H, Halbirk M, Frystyk J, Flyvbjerg A, Bøtker HE, Schmitz O, Jørgensen JO, Christiansen JS, Møller N. Abnormalities of whole body protein turnover, muscle metabolism and levels of metabolic hormones in patients with chronic heart failure. J Intern Med. 2006;260:11–21. doi: 10.1111/j.1365-2796.2006.01663.x. doi: 10.1111/j.1365-2796.2006.01663.x. [DOI] [PubMed] [Google Scholar]
- 32.Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–1195. doi: 10.2337/db15-1356. doi: 10.2337/db15-1356. [DOI] [PubMed] [Google Scholar]
- 33.Neubauer S. The failing heart–an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 34.Kim IS, Izawa H, Sobue T, Ishihara H, Somura F, Nishizawa T, Nagata K, Iwase M, Yokota M. Prognostic value of mechanical efficiency in ambulatory patients with idiopathic dilated cardiomyopathy in sinus rhythm. J Am Coll Cardiol. 2002;39:1264–1268. doi: 10.1016/s0735-1097(02)01775-8. [DOI] [PubMed] [Google Scholar]
- 35.Taegtmeyer H. Failing heart and starving brain: Ketone bodies to the rescue. Circulation. 2016;134:265–266. doi: 10.1161/CIRCULATIONAHA.116.022141. doi: 10.1161/CIRCULATIONAHA.116.022141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mäki MT, Haaparanta M, Nuutila P, Oikonen V, Luotolahti M, Eskola O, Knuuti JM. Free fatty acid uptake in the myocardium and skeletal muscle using fluorine-18-fluoro-6-thia-heptadecanoic acid. J Nucl Med. 1998;39:1320–1327. [PubMed] [Google Scholar]
- 37.Vanoverschelde JL, Wijns W, Essamri B, Bol A, Robert A, Labar D, Cogneau M, Michel C, Melin JA. Hemodynamic and mechanical determinants of myocardial O2 consumption in normal human heart: Effects of dobutamine. Am J Physiol. 1993;265(6 Pt 2):H1884–H1892. doi: 10.1152/ajpheart.1993.265.6.H1884. doi: 10.1152/ajpheart.1993.265.6.H1884. [DOI] [PubMed] [Google Scholar]
- 38.Ukkonen H, Saraste M, Akkila J, Knuuti J, Karanko M, Iida H, Lehikoinen P, Någren K, Lehtonen L, Voipio-Pulkki LM. Myocardial efficiency during levosimendan infusion in congestive heart failure. Clin Pharmacol Ther. 2000;68:522–531. doi: 10.1067/mcp.2000.110972. doi: 10.1067/mcp.2000.110972. [DOI] [PubMed] [Google Scholar]
- 39.Beanlands RS, Bach DS, Raylman R, Armstrong WF, Wilson V, Montieth M, Moore CK, Bates E, Schwaiger M. Acute effects of dobutamine on myocardial oxygen consumption and cardiac efficiency measured using carbon-11 acetate kinetics in patients with dilated cardiomyopathy. J Am Coll Cardiol. 1993;22:1389–1398. doi: 10.1016/0735-1097(93)90548-f. [DOI] [PubMed] [Google Scholar]
- 40.Tuunanen H, Engblom E, Naum A, Scheinin M, Någren K, Airaksinen J, Nuutila P, Iozzo P, Ukkonen H, Knuuti J. Decreased myocardial free fatty acid uptake in patients with idiopathic dilated cardiomyopathy: evidence of relationship with insulin resistance and left ventricular dysfunction. J Card Fail. 2006;12:644–652. doi: 10.1016/j.cardfail.2006.06.005. doi: 10.1016/j.cardfail.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Gormsen LC, Svart M, Thomsen HH, Sondergaard E, Vendelbo MH, Christensen N, Tolbod LP, Harms HJ, Nielsen R, Wiggers H, Jessen N, Hansen J, Botker HE, Moller N. Ketone body infusion with 3-hydroxybutyrate reduces myocardial glucose uptake and increases blood flow in humans: A positron emission tomography study. J Am Heart Assoc. 2017;6:1–11. doi: 10.1161/JAHA.116.005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–1086. doi: 10.1152/physrev.00045.2006. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- 43.Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, Murray AJ, Stubbs B, West J, McLure SW, King MT, Dodd MS, Holloway C, Neubauer S, Drawer S, Veech RL, Griffin JL, Clarke K. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24:256–268. doi: 10.1016/j.cmet.2016.07.010. doi: 10.1016/j.cmet.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 44.Hasselbalch SG, Madsen PL, Hageman LP, Olsen KS, Justesen N, Holm S, Paulson OB. Changes in cerebral blood flow and carbohydrate metabolism during acute hyperketonemia. Am J Physiol. 1996;270(5 Pt 1):E746–E751. doi: 10.1152/ajpendo.1996.270.5.E746. doi: 10.1152/ajpendo.1996.270.5.E746. [DOI] [PubMed] [Google Scholar]
- 45.Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R BEAUTIFUL investigators. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): A subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817–821. doi: 10.1016/S0140-6736(08)61171-X. doi: 10.1016/S0140-6736(08)61171-X. [DOI] [PubMed] [Google Scholar]
- 46.Ma W, Berg J, Yellen G. Ketogenic diet metabolites reduce firing in central neurons by opening K(ATP) channels. J Neurosci. 2007;27:3618–3625. doi: 10.1523/JNEUROSCI.0132-07.2007. doi: 10.1523/JNEUROSCI.0132-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egstrup M, Gustafsson I, Andersen MJ, Kistorp CN, Schou M, Tuxen CD, Møller JE. Haemodynamic response during low-dose dobutamine infusion in patients with chronic systolic heart failure: Comparison of echocardiographic and invasive measurements. Eur Heart J Cardiovasc Imaging. 2013;14:659–667. doi: 10.1093/ehjci/jes234. doi: 10.1093/ehjci/jes234. [DOI] [PubMed] [Google Scholar]


