Abstract
Breast cancer (BC) and thyroid cancer (TC) are common malignancies among females. However, the connection between TC and BC is not well understood. To explore the relationship between these two cancers and to determine the effect of second metachronous TC on BC survival, we compared BC patients with or without second primary TC using data from the Surveillance, Epidemiology, and End Results (SEER) database. We extracted data from patients with only BC or TC and from BC patients with a second metachronous cancer from 2000–2014. Differences in the clinicopathological and treatment characteristics between BC patients with or without second metachronous TC were analyzed by chi-square tests. Multivariate analyses of BC survival were performed by using Cox regression models. Comparison of disease-specific survival (DSS) curves between these cohorts was performed with the log-rank (Mantel-Cox) test. Survival analyses were also performed using data from 1980–1994. Within this dataset, we found 1,262 BC cases in which a second metachronous TC (BC2TC) developed, accounting for 3.1% of all metachronous cancers following BC from 2000–2014. No significant differences were found in molecular markers. In addition, the mean age at BC diagnosis was younger in the BC2TC group than in the BC group (55.418 y vs 60.273 y). Half of the BC2TC patients developed TC in the first three years following BC diagnosis. Patients with BC2TC showed better DSS than those with BC alone from 2000–2014 (P<0.001). However, this superiority was not significant from 1980–1994 (P = 0.579) or for TNM stage I BC (P = 0.927) and grade I BC (P = 0.431) from 2000–2014. In conclusion, the incidence of BC2TC has increased dramatically during the past 15 years. In addition, patients with BC2TC showed better DSS than patients with BC alone, especially in cases from 2000–2014.
Introduction
Breast cancer (BC) and thyroid cancer (TC) are common malignancies. Although the mortality rate of BC fell between 1989 and 2014, BC remains the second most common cause of cancer-related death among females [1]. In addition, the incidence of TC in the United States has tripled over the past 30 years, with a particularly rapid rise since the 1990s [2].
Many studies have attempted to demonstrate an association between thyroid disease and BC as both diseases principally appear in women. Smyth reviewed the association between thyroid disease and BC and confirmed that hypothyroidism or autoimmune thyroid disease potentially contributes to an increased risk or alters the outcome of BC [3]. In addition, the overall risk of second primary TC or BC is increased in patients with prior BC or TC, respectively [4–6]. However, the interrelationship between TC and BC has not been definitively elucidated [7].
The criteria for diagnosing a second primary tumor or multiple primary tumors have been discussed for many years [8]. Metachronous cancer is considered a secondary cancer diagnosed more than 6 months after the primary cancer diagnosis [9, 10]. In this study, we used data from the Surveillance, Epidemiology, and End Results (SEER) database, which followed the SEER definition of multiple primary tumors [11].
In 1973, Moossa considered that patients with BC and a history of thyroid disease had a lower average survival rate at both 5 and 10 years than those without any evidence of thyroid disease [12]. In addition, it seems logical to consider that adding TC to primary BC would result in a worse prognosis than that of BC alone, but is this true? Here, we used data from the SEER database to analyze the incidence rate of second malignancies following BC and the differences in the clinical, pathological and treatment characteristics and disease-specific survival (DSS) of BC with or without second primary TC. Our results show that the incidence of TC as a second malignancy following BC has increased dramatically during the past 15 years and that BC patients with a second TC have better DSS than patients with BC only, especially in the past 15 years.
Materials and methods
We used data from the SEER Cancer Registry database, which collects data on every case of cancer reported from 19 U.S. geographical areas. These areas cover approximately 34% of the U.S. population and are representative of the demographics of the entire U.S. population (https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf). We used the following database: Incidence—SEER 18 Regs Custom Data (with additional treatment fields), submitted November 2016 (1973–2014 varying)—Linked to County Attributes—Total U.S., 1969–2015 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017. The majority of patients in this study were diagnosed between 1 January 2000 and 31 December 2014. We extracted three cohorts of patients with definite survival months from this database. For cohort one, the total number of in situ/malignant tumors for each patient was 2, with BC as the first malignancy and a second metachronous cancer. In the other cohorts, each patient had only BC or TC. There were 40,520 patients in cohort one, 629,976 patients with only BC and 120,339 patients with only TC. We also extracted cases from 1 January 1980 to 31 December 1994 following the former formula with the second primary TC diagnosed before 1995. All data from SEER database were fully anonymized. The patient records used in our study are provided as supporting databases. The S1 database provides records of the BC cohort from 2000–2014. The S2 database provides records of other cohorts from 2000–2014. The S3 database includes data from all cohorts from 1980–1994.
The following parameters were evaluated for BC: age, race, TNM stage, tumor grade, estrogen receptor (ER) expression, progesterone receptor (PR) expression, Her-2 expression, histopathological subtype, radiation therapy and chemotherapy history. There were 36,825 cases without definite TNM data for the BC only (BC) group and 60 cases without definite TNM data for the BC followed by TC (BC2TC) group. There were 56,532 cases without definite grade data for the BC group and 90 cases without definite grade data for the BC2TC group. There were some cases lacking ER expression data (58,409 for BC; 131 for BC2TC) or PR expression data (66,077 for BC; 150 for BC2TC). There were 214,258 cases with Her-2 expression data for the BC group and 237 cases for the BC2TC group. Radiation therapy included beam radiation, combinations of beam radiation with implants or isotopes, unspecified radiation methods or sources, radioactive implants, and radioisotopes.
We used the Surveillance Research Program, National Cancer Institute SEER*Stat software (www.seer.cancer.gov/seerstat) version 8.3.5 to obtain the incidence rates of patients with TC only or generate lists of cases with the desired data. Data analysis was performed using GraphPad Prism 7 (GraphPad Software, Inc., CA, USA). The differences in clinical characteristics were analyzed by chi-square test. Multivariate analyses of BC survival were performed by using Cox regression models. Comparison of DSS curves was performed using the log-rank (Mantel-Cox) test. P<0.05 was considered significant.
Results
TC is one of the top ten metachronous cancers following BC
The results of the incidence ratio analysis of a second metachronous cancer following BC are shown in Table 1. The most common metachronous cancer of BC was second primary BC. TC developed in 1,262 cases, a rate of 3.1%. During the same period, the incidence of TC alone was 9.5 per 100,000, age-adjusted to the 2000 US Standard Population. However, the rate of a second TC after BC was 145.7 per 100,000 (1,262/865,886). The total number of BC cases was 865,886 from the database.
Table 1. The top ten types of second metachronous cancer (SMC) in breast cancer patients.
| SMC type | No. | Percentage (%) |
|---|---|---|
| Breast | 14,321 | 35.3 |
| Lung and Bronchus | 5,207 | 12.9 |
| Colon/Rectum | 3,683 | 9.1 |
| Corpus Uteri | 2,448 | 6.0 |
| Skin | 1,476 | 3.6 |
| Pancreas | 1,300 | 3.2 |
| NHL | 1,263 | 3.1 |
| Thyroid | 1,262 | 3.1 |
| Leukemia | 1,246 | 3.1 |
| Ovary | 1,153 | 2.9 |
Clinicopathological characteristics of the BC and BC2TC groups
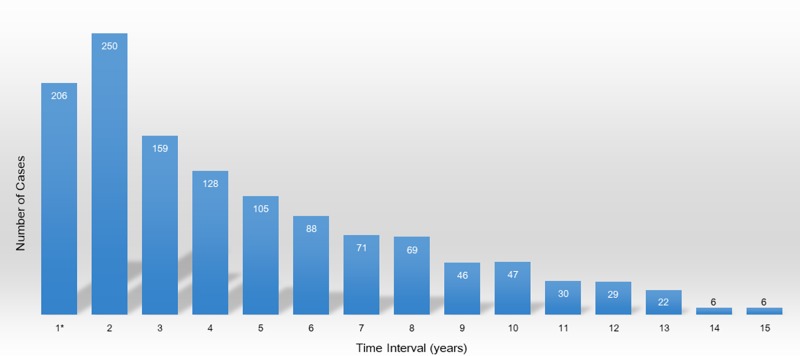
The clinicopathological characteristics of BC were compared between the BC and BC2TC groups. There were 629,976 cases in the BC group and 1,262 cases in the BC2TC group. Between these groups, significant differences were found in race, TNM stage, tumor grade, histological classification, radiation therapy and chemotherapy history but not in molecular markers including ER, PR and Her-2 expression (Table 2). In addition, the mean age at BC diagnosis was younger in the BC2TC group than in the BC group (55.418 y vs 60.273 y, respectively). The numbers of patients who developed TC during the second half of the first year, the second and the third year after BC diagnosis were 206, 250 and 159, respectively (Fig 1).
Table 2. Clinical characteristics of patients with only BC (BC group) and those with BC followed by TC (BC2TC group).
| Variable | BC (n = 629,976) | BC2TC (n = 1,262) | chi-square P-value |
|---|---|---|---|
| Race | P< 0.001 | ||
| White | 506,105 (80.3%) | 1,010 (80.0%) | |
| Black | 67,537 (10.7%) | 102 (8.1%) | |
| Other | 51,708 (8.2%) | 145 (11.5%) | |
| Unknown | 4,626 (0.7%) | 5 (0.4%) | |
| TNM Stage | P< 0.001 | ||
| IV | 32,393 (5.1%) | 18 (1.4%) | |
| III | 77,388 (12.3%) | 172 (13.6%) | |
| II | 209,060 (33.2%) | 451 (35.7%) | |
| I | 274,310 (43.5%) | 561 (44.5%) | |
| Grade | p = 0.044 | ||
| IV | 6,765 (1.1%) | 23 (1.8%) | |
| III | 203,557 (32.3%) | 428 (33.9%) | |
| II | 241,996 (38.4%) | 494 (39.1%) | |
| I | 121,126 (19.2%) | 227 (18.0%) | |
| ER-positive | p = 0.234 | ||
| No | 115,782 (18.4%) | 213 (16.9%) | |
| Yes | 455,785 (72.3%) | 918 (72.7%) | |
| PR-positive | p = 0.539 | ||
| No | 177,243 (28.1%) | 340 (26.9%) | |
| Yes | 386,656 (61.4%) | 772 (61.2%) | |
| Her-2-positive | p = 0.062 | ||
| No | 180,368 (28.6%) | 189 (15.0%) | |
| Yes | 33,890 (5.4%) | 48 (3.8%) | |
| Breast histology | p = 0.036 | ||
| Infiltrating ductal carcinoma | 452,423 (71.8%) | 898 (71.2%) | |
| Infiltrating lobular carcinoma | 50,069 (7.9%) | 79 (6.3%) | |
| Mixed invasive | 60,781 (9.6%) | 146 (11.6%) | |
| Inflammatory | 3,953 (0.6%) | 6 (0.5%) | |
| Other | 62,750 (10.0%) | 133 (10.5%) | |
| Radiation therapy | p = 0.033 | ||
| Yes | 307,043 (48.7%) | 653 (51.7%) | |
| No/Unknown | 322,933 (51.3%) | 609 (48.3%) | |
| Chemotherapy | P< 0.001 | ||
| Yes | 260,797 (41.4%) | 637 (50.5%) | |
| No/Unknown | 369,180 (58.6%) | 625 (49.5%) |
Fig 1. Time interval between breast cancer and thyroid cancer 1*: Only the second half of the first year.
Survival analysis
Multivariate analyses of BC survival were performed by using Cox regression models for SMC patients first. Second primary TC significantly influenced both OS and DSS of BC (with a second metachronous TC vs. without a second metachronous TC: hazard ratio [HR] 0.248, 95% confidence interval [CI] 0.209 to 0.294, P<0.001 for OS; [HR] 0.227, 95% [CI] 0.181 to 0.284, P<0.001 for DSS) (S1 Table).
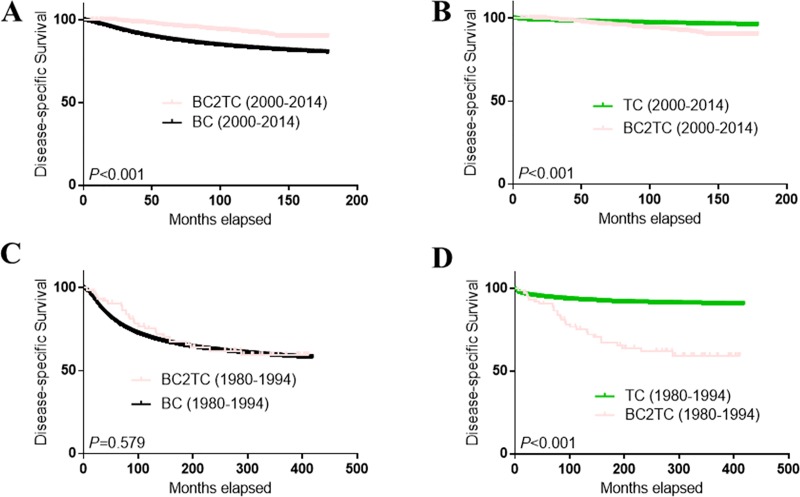
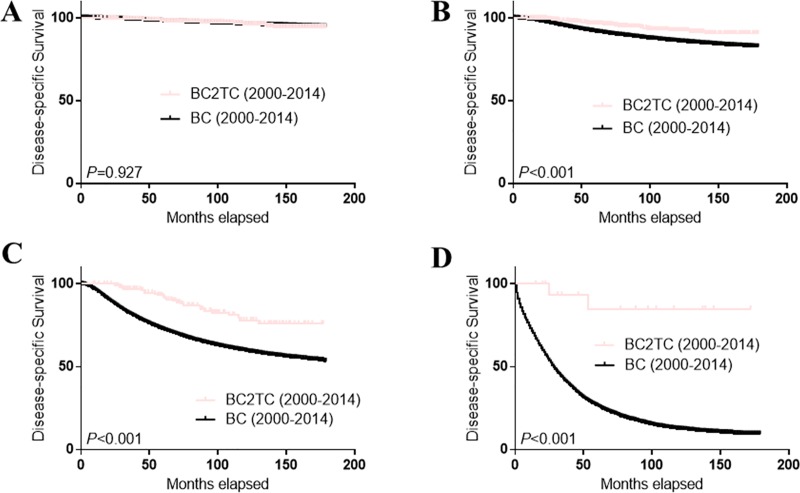
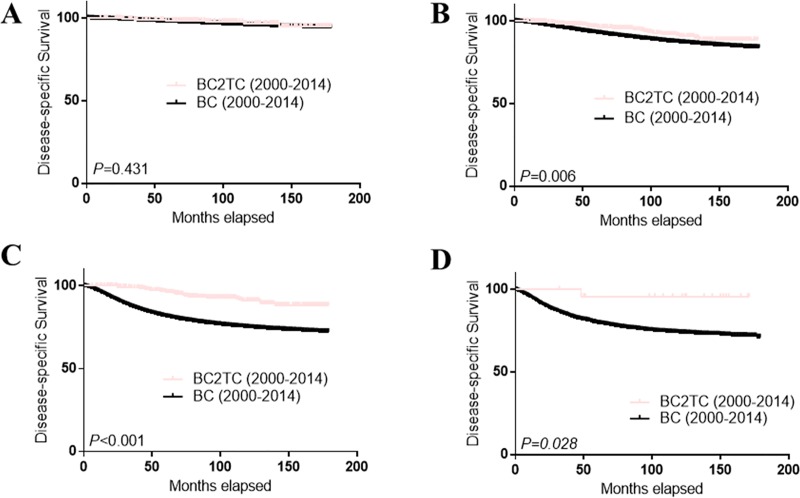
The survival curves of the BC2TC, BC and TC groups (TC only patients) are shown in Fig 2. DSS in the BC2TC group was much better than that in the BC group (A) but worse than that in the TC group (B) from 2000–2014. However, the survival advantage in the BC2TC group over the BC group was not statistically significant from 1980–1994 (C) (P = 0.579) (Fig 2). When we stratified the cases from 2000–2014 by TNM stage (Fig 3) and tumor grade (Fig 4), we observed a significant survival advantage for all groups except stage I (P = 0.927, Fig 3A) and grade I (P = 0.431, Fig 4A). In addition, the improved survival was obvious in all of the cases regardless of radiation therapy (S1 Fig) or chemotherapy (S2 Fig) history.
Fig 2. Comparison of DSS among the BC2TC, BC and TC groups.
BC vs BC2TC from 2000 to 2014 (A); TC vs BC2TC from 2000 to 2014 (B); BC vs BC2TC from 1980 to 1994 (C); TC vs BC2TC from 1980 to 1994 (D).
Fig 3. Comparison of DSS among different TNM stages in the BC2TC and BC groups.
BC vs BC2TC for stage I (A); BC vs BC2TC for stage II (B); BC vs BC2TC for stage III (C); BC vs BC2TC for stage IV (D).
Fig 4. Comparison of DSS among different grades in the BC2TC and BC groups.
BC vs BC2TC for grade I (A); BC vs BC2TC for grade II (B); BC vs BC2TC for grade III (C); BC vs BC2TC for grade IV (D).
Discussion
Is it true that adding TC to primary BC will lead to a worse prognosis than that of BC alone? Our results indicated a better DSS in the BC2TC group than in the BC group, especially for cases between 1 January 2000, and 31 December 2014. In addition, the incidence of TC as a second malignancy following BC has dramatically increased in the last 15 years.
To the best of our knowledge, this is the first paper to address the effect of second TC on BC survival.
Many studies have focused on the possible relationship between BC and thyroid disease, but the results have been conflicting. Hyperthyroidism was found to be associated with an increased risk of BC compared with the general population, and hypothyroidism was associated with a slightly decreased risk of BC compared with the general population. Thus, it appears that thyroid function levels may be associated with BC risk [13, 14]. However, another study found no statistically significant association between hypothyroidism or hyperthyroidism and BC [15]. In addition, using samples collected from participants at one time point after an overnight fast, Chan et al. found no associations of thyroid-stimulating hormone (TSH), free thyroxine (FT4), or anti-thyroid peroxidase (TPO) with BC [16]. Thyroid hormone substitution treatment did not reduce the incidence of BC [17]. Regarding the effect of thyroid disease on BC survival, in the largest and longest observational study to date, no evidence was found for a prognostic role of anti-TPO antibodies and/or thyroid function in moderate-to-high-risk early BC [18]. However, another study showed a positive association between FT4 levels and improved BC survival [19], whereas data over 10 years from one institute showed that patients with co-existing BC and TC generally exhibited worse survival than those with only BC or TC [20]. What is the actual effect of TC as a second cancer following BC? Our data from the SEER database revealed better DSS for patients with BC2TC than for those with BC alone between 2000 and 2014. In addition, while DSS may be more appropriate in terms of prediction, it does not include all causes [21]. We consider our results to be robust and more representative of the population. However, this survival advantage was not significant in data from 1980 to 1994. To explore reasons for this difference, we retrieved data regarding changes in TC therapy and found that TSH suppression using supraphysiologic doses of levothyroxine (LT4) to suppress the level of TSH without hyper- or hypothyroidism has become increasingly prevalent as a treatment for TC in the past 15 years.
In 1994, Mazzaferri found that thyroid hormone therapy confers a distinct outcome advantage in TC; this was a very important long-term study with a major influence on TC treatment [22]. Then, from 1996 to 2009, TSH suppression strategies became increasingly recommended in the American Thyroid Association (ATA) guidelines for TC therapy [23, 24].
However, whether TSH suppression using LT4 is the reason for the better survival for BC in the BC2TC group remains to be determined. As early as 1896, Beatson used thyroid extract to treat BC and explore the relationship between BC and thyroid dysfunction, and determined that they were correlated [25]. Loeser reported the use of thyroid hormone to treat inoperable BC or BC after radical surgery and found that massive thyroid hormone dosages decreased the rate of BC growth [26]. Now that evidence did not suggest that thyroid hormone causes BC [27]. We shift our sights to TSH. TSH is a hormone of the hypothalamus-pituitary-thyroid axis, and the hypothalamus-pituitary-thyroid and hypothalamus-pituitary-gonadal axes are linked [28]. Endogenous estrogens and progestins, which are regulated by the hypothalamic-pituitary-gonadal axis, have been proved to be carcinogenic and increase the risk of breast cancer [29]. Based on these observations, we inferred that TSH suppression therapy might lead to improved survival for BC due to the interaction between the hypothalamus-pituitary-thyroid axis and hypothalamus-pituitary-gonadal axis.
BC is the most common second primary cancer following BC [30], in agreement with our results. In addition, there is a significantly increased risk of TC after diagnosis with primary BC [31, 32]. Compared with Raymond’s results for data before 2000 [33], our data showed that the incidence of TC has increased more than 4-fold. This is consistent with a true increase in the occurrence of TC in the United States [34], and might also reflect an influence of access to care also [35].
Because it was based on data from the SEER database, our study was retrospective. We found that patients in the BC2TC group had better DSS, but we can only speculate that TSH suppression therapy is the reason for the better survival for BC in these patients. In addition, stage I and grade I BC did not show any survival advantages for subsequent TC; thus, studies to identify the BC patients who might in fact achieve improved survival for second metachronous TC should be performed. The results of this study should be verified in a prospective population-based study, and the underlying mechanisms should be investigated.
In conclusion, despite these limitations, our results showed that the incidence of TC as a second malignancy following BC has dramatically increased during the past 15 years. Notably, our study found better DSS in the BC2TC group than the BC group, especially in cases diagnosed between 1 January 2000, and 31 December 2014. We speculate that TSH suppression therapy is the underlying reason for better DSS in the BC2TC group. TSH suppression therapy may be another potential regimen for BC.
Supporting information
(XLSX)
BC vs BC2TC with radiation therapy (A); BC vs BC2TC without radiation therapy (B).
(TIF)
BC vs BC2TC with chemotherapy (A); BC vs BC2TC without chemotherapy (B).
(TIF)
(RAR)
(RAR)
(RAR)
Acknowledgments
We thank Matthew Waitkus from the Department of Pathology and the Preston Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, U.S.A. and QiuRan Xu from Zhejiang Provincial People's Hospital, Hangzhou Medical College, Hangzhou, China for critical revision of the manuscript. This manuscript was edited for proper English language, grammar, punctuation, spelling, and overall style by one or more highly qualified native English speaking editors at American Journal Experts.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was funded by the National Natural Science Foundation of China (grant no. 81502463), the Natural Science Foundation of Zhejiang Province of China (grant nos. LY13H160039 and LY18H160039). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. 10.3322/caac.21387 . [DOI] [PubMed] [Google Scholar]
- 2.Morris LG, Tuttle RM, Davies L. Changing Trends in the Incidence of Thyroid Cancer in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(7):709–11. 10.1001/jamaoto.2016.0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyth PP. The thyroid and breast cancer. Curr Opin Endocrinol Diabetes Obes. 2016;23(5):389–93. 10.1097/MED.0000000000000273 . [DOI] [PubMed] [Google Scholar]
- 4.Chalstrey LJ, Benjamin B. High incidence of breast cancer in thyroid cancer patients. Br J Cancer. 1966;20(4):670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hung MH, Liu CJ, Teng CJ, Hu YW, Yeh CM, Chen SC, et al. Risk of Second Non-Breast Primary Cancer in Male and Female Breast Cancer Patients: A Population-Based Cohort Study. PLoS One. 2016;11(2):e0148597 10.1371/journal.pone.0148597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An JH, Hwangbo Y, Ahn HY, Keam B, Lee KE, Han W, et al. A Possible Association Between Thyroid Cancer and Breast Cancer. Thyroid. 2015;25(12):1330–8. 10.1089/thy.2014.0561 . [DOI] [PubMed] [Google Scholar]
- 7.Kuo JH, Chabot JA, Lee JA. Breast cancer in thyroid cancer survivors: An analysis of the Surveillance, Epidemiology, and End Results-9 database. Surgery. 2016;159(1):23–9. 10.1016/j.surg.2015.10.009 . [DOI] [PubMed] [Google Scholar]
- 8.Eisenstaedt JS. Multiple Primary Malignant Tumors. JAMA. 1938;110(25):2056–9. [Google Scholar]
- 9.Warren S, Gates O. Multiple primary malignant tumors: a survey of the literature and a statistical study. Am J Cancer. 1932;16:1358–414. [Google Scholar]
- 10.Swaroop VS, Winawer SJ, Kurtz RC, Lipkin M. Multiple primary malignant tumors. Gastroenterology. 1987;93(4):779–83. . [DOI] [PubMed] [Google Scholar]
- 11.Adamo M, Dickie L, Ruhl J. SEER Program Coding and Staging Manual 2016. National Cancer Institute, Bethesda: January 2016. [Google Scholar]
- 12.Moossa AR, Evans DA, Brewer AC. Thyroid status and breast cancer. Reappraisal of an old relationship. Ann R Coll Surg Engl. 1973;53(3):178–88. [PMC free article] [PubMed] [Google Scholar]
- 13.Chaker L, Visser TJ. Thyroid function: Thyroid dysfunction and breast cancer risk—an unfinished story. Nat Rev Endocrinol. 2016;12(6):313–4. 10.1038/nrendo.2016.48 . [DOI] [PubMed] [Google Scholar]
- 14.Sogaard M, Farkas DK, Ehrenstein V, Jorgensen JO, Dekkers OM, Sorensen HT. Hypothyroidism and hyperthyroidism and breast cancer risk: a nationwide cohort study. Eur J Endocrinol. 2016;174(4):409–14. 10.1530/EJE-15-0989 . [DOI] [PubMed] [Google Scholar]
- 15.Chiappa C, Rovera F, Rausei S, Del Ferraro S, Fachinetti A, Lavazza M, et al. Breast cancer and thyroid diseases: analysis of 867 consecutive cases. J Endocrinol Invest. 2017;40(2):179–84. 10.1007/s40618-016-0543-4 . [DOI] [PubMed] [Google Scholar]
- 16.Chan YX, Knuiman MW, Divitini ML, Brown SJ, Walsh J, Yeap BB. Lower TSH and higher free thyroxine predict incidence of prostate but not breast, colorectal or lung cancer. Eur J Endocrinol. 2017;177(4):297–308. 10.1530/EJE-17-0197 . [DOI] [PubMed] [Google Scholar]
- 17.Fang Y, Yao L, Sun J, Yang R, Chen Y, Tian J, et al. Does thyroid dysfunction increase the risk of breast cancer? A systematic review and meta-analysis. J Endocrinol Invest. 2017. 10.1007/s40618-017-0679-x . [DOI] [PubMed] [Google Scholar]
- 18.Muller I, Kilburn LS, Taylor PN, Barrett-Lee PJ, Bliss JM, Ellis P, et al. TPOAb and Thyroid Function Are Not Associated with Breast Cancer Outcome: Evidence from a Large-Scale Study Using Data from the Taxotere as Adjuvant Chemotherapy Trial (TACT, CRUK01/001). Eur Thyroid J. 2017;6(4):197–207. 10.1159/000460246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandt J, Borgquist S, Almquist M, Manjer J. Thyroid function and survival following breast cancer. Br J Surg. 2016;103(12):1649–57. 10.1002/bjs.10284 . [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Wu Y, Liu F, Fu L, Tong Z. Characteristics and survival of patients with metachronous or synchronous double primary malignancies: breast and thyroid cancer. Oncotarget. 2016;7(32):52450–9. 10.18632/oncotarget.9547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke HB. Predicting Clinical Outcomes Using Molecular Biomarkers. Biomark Cancer. 2016;8:89–99. 10.4137/BIC.S33380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97(5):418–28. . [DOI] [PubMed] [Google Scholar]
- 23.Singer PA, Cooper DS, Daniels GH, Ladenson PW, Greenspan FS, Levy EG, et al. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. American Thyroid Association. Arch Intern Med. 1996;156(19):2165–72. . [PubMed] [Google Scholar]
- 24.American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid C, Cooper DS, Doherty GM, Haugen BR, Kloos RT, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–214. 10.1089/thy.2009.0110 . [DOI] [PubMed] [Google Scholar]
- 25.Beatson GT. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment, with illustrative cases. Glasgow: Printed by Alex. Macdougall; 1896. 35; 23 cm. p. [PMC free article] [PubMed] [Google Scholar]
- 26.Loeser AA. A new therapy for prevention of post-operative recurrences in genital and breast cancer; a six-years study of prophylactic thyroid treatment. Br Med J. 1954;2(4901):1380–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hercbergs A, Mousa SA, Leinung M, Lin HY, Davis PJ. Thyroid Hormone in the Clinic and Breast Cancer. Horm Cancer. 2018;9(3):139–43. 10.1007/s12672-018-0326-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrera MP, Ramirez-Exposito MJ, Valenzuela MT, Garcia MJ, Mayas MD, Arias de Saavedra JM, et al. Pyrrolidon carboxypeptidase activities in the hypothalamus-pituitary-thyroid and hypothalamus-pituitary-ovary axes of rats with mammary gland cancer induced by N-methyl nitrosourea. Horm Metab Res. 2005;37(2):74–8. 10.1055/s-2005-861158 . [DOI] [PubMed] [Google Scholar]
- 29.Vaz-Luis I, Partridge AH. Exogenous reproductive hormone use in breast cancer survivors and previvors. Nat Rev Clin Oncol. 2018;15(4):249–61. 10.1038/nrclinonc.2017.207 . [DOI] [PubMed] [Google Scholar]
- 30.Vural O, Dizdar O, Petekkaya I, Alnak A, Babacan T, Altundag K. Frequency of thyroid disease among breast cancer patients: a descriptive study of breast cancer patients. J BUON. 2013;18(1):294–5. . [PubMed] [Google Scholar]
- 31.Silverman BG, Lipshitz I, Keinan-Boker L. Second Primary Cancers After Primary Breast Cancer Diagnosis in Israeli Women, 1992 to 2006. J Glob Oncol. 2017;3(2):135–42. 10.1200/JGO.2016.003699 www.asco.org/rwc or ascopubs.org/jco/site/ifc. Barbara G. SilvermanNo relationship to discloseIrena LipshitzNo relationship to discloseLital Keinan-BokerNo relationship to disclose. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Fossen VL, Wilhelm SM, Eaton JL, McHenry CR. Association of thyroid, breast and renal cell cancer: a population-based study of the prevalence of second malignancies. Ann Surg Oncol. 2013;20(4):1341–7. 10.1245/s10434-012-2718-3 . [DOI] [PubMed] [Google Scholar]
- 33.Raymond JS, Hogue CJ. Multiple primary tumours in women following breast cancer, 1973–2000. Br J Cancer. 2006;94(11):1745–50. 10.1038/sj.bjc.6603172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA. 2017;317(13):1338–48. 10.1001/jama.2017.2719 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris LG, Sikora AG, Tosteson TD, Davies L. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. 2013;23(7):885–91. 10.1089/thy.2013.0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
BC vs BC2TC with radiation therapy (A); BC vs BC2TC without radiation therapy (B).
(TIF)
BC vs BC2TC with chemotherapy (A); BC vs BC2TC without chemotherapy (B).
(TIF)
(RAR)
(RAR)
(RAR)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.