Abstract
We investigated a carbohydrate‐rich nutrient‐drink mix for treatment of seasonal affective disorder (SAD). This mixture may contribute to brain serotonin synthesis, potentially exerting an antidepressant effect and controlling carbohydrate cravings. Two successive double‐blind placebo‐controlled studies were performed. In Study 1, 18 subjects (50% women; mean age 43 ± 15 years) with SCID‐diagnosed SAD were randomized to 12 days of twice daily carbohydrate beverage (CHO) containing mixed starches, or a placebo beverage (PRO) containing the CHO mix plus casein protein to dampen serotonin synthesis. Following a 2‐day washout, subjects were crossed over to the other treatment for 12 days. In Study 2, 32 subjects (63% women; mean age 46 ± 14 years) with SCID‐diagnosed SAD were randomized to 21 days of CHO or PRO. Efficacy in both studies was determined by the first 17 items of the Hamilton Depression Rating Scale (HAM‐D‐28), an appetite questionnaire, and regular weighing. In Study 1, response rates were 50% for both groups. Remission rates favored CHO (50% vs. 38%), as did the decrease in the HAM‐D‐17 score, but differences were nonsignificant. In Study 2, response rates were 71% for CHO and 76% for PRO, and remission rates were 71% for each group. Both treatment groups experienced significant improvement in HAM‐D‐17 scores within 1 week of treatment, which continued through the entire study period. Weight change did not differ significantly between treatment groups in either study. The drink mix was well tolerated and treatment adherence was high. Both the active and placebo intervention were effective in alleviating symptoms of SAD. Replication studies in larger samples appear warranted.
Keywords: Carbohydrate, Depression, SAD, Seasonal affective disorder, Serotonin
Introduction
Seasonal affective disorder (SAD) is a subcategory of major depressive disorder (MDD), characterized by a temporal relationship between the onset of the depressive episode and a particular time of the year, most often the fall or winter, with full remissions usually occurring in the spring [1, 2]. Prevalence of SAD increases with higher latitudes and tends to affect younger individuals, with women comprising 60–90% of persons with SAD [1].
Research so far has identified various effective treatments for SAD. Several studies have yielded encouraging results with conventional antidepressant agents [1, 3, 4, 5, 6, 7, 8, 9, 10], and bupropion is approved by the FDA for this indication [9, 11]. Other promising treatments include the stimulant modafinil [12] and the melatonin analog agomelatine [13]. Research on light therapy, the most popular treatment for SAD, has yielded generally positive results, with up to 53% of patients responding to light therapy [1, 14, 15, 16, 17, 18, 19, 20]. Effect sizes have been suggested to be comparable between antidepressants and light therapy [21]. Light therapy has also been combined with antidepressants [4, 22] and cognitive behavioral therapy [20, 23], with encouraging results. However, the body of research studies as a whole is limited by small sample sizes, relatively few randomized controlled designs, and other methodological issues. There is therefore a need for continued rigorous characterization of the established therapies for SAD as well as the development of new ones.
Carbohydrate metabolism has been proposed to have a strong relationship with mood regulation and disorders, particularly SAD [24]. Associated features of SAD include lack of energy, hypersomnia, overeating—particularly carbohydrate craving—and weight gain [25]. A few small studies have shown evidence of disturbances in carbohydrate metabolism [26, 27], which may result in increased carbohydrate consumption in seasonally depressed individuals. Whether carbohydrates should be avoided in SAD, say, in favor of more protein‐rich diets, remains uncertain [25], but given the concern about weight gain, a treatment approach that targets appetite and weight control in addition to mood regulation would be especially valuable.
Serotonin metabolism abnormalities have likewise been implicated in the etiology of SAD with carbohydrate craving [28, 29, 30, 31, 32]. We have previously found that the serotonin agonist dexfenfluramine was highly effective in improving mood, energy, motivation, and decreasing food intake in a sample of patients with SAD [29]. It is therefore possible that increasing serotonin activity by another mechanism, that is, by enhancing serotonin synthesis, might provide a less robust but still effective way of treating SAD. We have developed a serotonin‐producing carbohydrate mixed beverage (CHO) that has demonstrated a beneficial effect on mood, appetite, and cognitive function in women with premenstrual syndrome (PMS) [33]. The beverage contains a mixture of potato starch, maltodextrin, dextrose, and dextrin, which are metabolized and utilized at different rates. The mixture is expected to contribute to serotonin synthesis in the brain, potentially exerting mood enhancing effects as well as controlling carbohydrate cravings [33] by providing a regular, slowly delivered influx of carbohydrate.
Given the symptomatic overlap between seasonal depression and PMS, this carbohydrate beverage could potentially have a beneficial effect on individuals with SAD, by alleviating depressive symptoms and minimizing carbohydrate cravings. We therefore tested the feasibility, acceptability, efficacy, and safety of the carbohydrate‐rich beverage (CHO) in individuals with SAD in two pilot double blind placebo‐controlled clinical trials, one of which used a crossover design.
We hypothesized that, given the nutraceutical nature and easy administration of the carbohydrate mix, there would be strong acceptability among potential subjects, resulting in high recruitment rates over a short seasonal window, and high rates of treatment adherence and study completion, making this a viable alternative to conventional antidepressants or light therapy for SAD. We predicted that subjects receiving the carbohydrate drink (CHO) would experience greater alleviation of depression and carbohydrate cravings compared with those on a protein‐enriched placebo drink (PRO). Likewise, we predicted that the CHO group would experience less (or no) weight gain compared to PRO group.
Finally, given that atypical depressive symptoms, such as fatigue, carbohydrate cravings, and hypersomnia, are common in SAD [12, 34, 35], we carried out an exploratory analysis of the prevalence of atypical depression in our study samples, and the impact of the treatment on subjects with this subtype. We hypothesized that subjects meeting criteria for atypical depression would experience a greater benefit from the carbohydrate drink mix compared with those with the melancholic subtype.
Methods
Inclusion and Exclusion Criteria for both Studies
The study was conducted at the Massachusetts Institute of Technology's Clinical Research Center (CRC). Inclusion criteria for both studies included meeting SAD criteria by the DSM‐IV Structured Clinical Interview for Depression SCID [36] and having a score between 10 and 25 on the first 17 items of the Hamilton‐D‐28 scale. The HAMD‐28 scale [37, 38, 39] was used in order to characterize efficacy of the carbohydrate beverage in symptoms of atypical depression and also to allow characterization of specific benefits of the treatment on a broader range of depressive symptoms. Subjects were required to understand and sign an IRB‐approved informed consent prior to participation.
Exclusion criteria included: diabetes or other medical disorders (e.g., hypoglycemia) that would prevent subject from consuming a high carbohydrate drink; having a history of milk allergies; a body mass index (BMI) greater than 35; concomitant treatment for SAD (i.e., with medication or light therapy); having a history of eating disorders, exercise bulimia, alcoholism, recreational drug use, or smoking within 6 months of study entry; taking medications that may have an effect on mood and appetite, including antidepressants, mood stabilizers, antipsychotic drugs, or steroids. Also excluded were potential volunteers whose lifestyle would make it difficult to come to the study venue regularly, who would find it difficult to adhere to eating three meals a day at relatively traditional times (for example, students), or whose dietary habits severely limited their ability or desire to make typical food choices. Additionally, anyone who planned to travel to the southern latitudes during the study was excluded.
Interventions for both Studies
The active beverage (CHO) contained 40 g of a mixture of potato starch, maltodextrin, dextrose, and dextrin and is expected to contribute to serotonin synthesis in the brain, potentially exerting mood enhancing effects as well as controlling carbohydrate cravings [30]. The CHO beverage mix in the second study contained an additional 400 IU of vitamin D for general health benefits. The control intervention (PRO) in both studies was a carbohydrate‐protein mix (PRO) consisting of 15 g of the milk protein casein and 25 g of the carbohydrate mixture used in the active beverage. The addition of 15 g of casein prevents tryptophan uptake in the brain, hence dampening the synthesis of serotonin.
Subjects were allowed to continue with their regular diet, and no specific monitoring of eating habits was performed. However, special snack muffins were provided to match the composition of the drink mixes, essentially representing an “extension” of each drink mix. These muffins were intended to alleviate carbohydrate cravings and prevent the urge to snack on unhealthy foods, thus reducing any confounding effects from extraneous snacks. The CHO snack (122 Kcal) consisted of 3 g of protein, 18 g of carbohydrate, and 4 g of fat. The control (PRO) snack (125 Kcal) consisted of 7 g of protein, 15 g of carbohydrate, and 4 g of fat.
Study One: A Double‐Blind Randomized Placebo‐Controlled Crossover Pilot Study of the Carbohydrate‐Rich Nutrient Mixture for Treatment of SAD
Thirty‐one subjects between the ages of 18 and 70 years, in good general health with a BMI of no more than 35 (weighed in a hospital gown) were recruited and screened from October to December of 2004 by placing IRB‐approved advertisements in local newspapers. Individuals who responded to the advertisements underwent a brief telephone interview with a research assistant to schedule a screening interview and signed an IRB‐approved consent form prior to the intake assessment. Twenty‐one of these subjects met criteria for study entry, and 18 (50% women; mean age 43 ± 15 years) chose to enter (86% acceptability). Subjects who completed the entire study were remunerated $300 for their participation.
Following the screen visit, and prior to the onset of treatment, subjects underwent a 1‐week single‐blind placebo run‐in with the protein‐carbohydrate drink (PRO). The run‐in period (Visits 1 and 2) was used primarily to familiarize the subjects with the intervention schedule. Subjects were instructed in the guidelines for consuming the beverage twice daily, before lunch and dinner, prior to beginning the 1‐week run‐in phase of the study.
After completing the run‐in period, subjects were randomized in a double‐blind 1 : 1 manner to either the carbohydrate (CHO) or the control (PRO) drink for 12 days (Phase 1), during which time they were seen every 6 days (Visits 3 and 4). At the completion of this phase and after a 2‐day washout period, subjects were crossed over to the other treatment for an additional 12 days (Phase 2), during which they were seen every 6 days (Visits 5 and 6) (Figure 1). Subjects were assessed for depressive symptoms, appetite and food cravings, and weight changes at each visit. The short treatment and washout periods were used because the compound had produced relatively rapid results in our PMS study [33], but the effect of carbohydrates on serotonin is very short‐lived; the carbohydrate mix would therefore not be expected to have lasting effects following discontinuation, and subjects who received CHO in Phase 1 would quickly lose the benefit in Phase 2 once switched to placebo. By examining whether the CHO drink mix had a similar rapid‐onset mood elevation, we sought to determine whether longer‐term studies were warranted.
Figure 1.
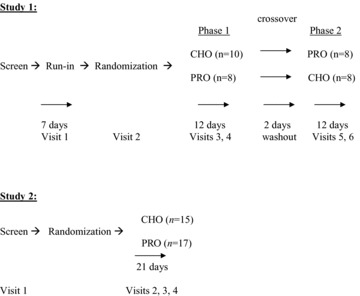
Treatment schemas for both studies. CHO, Active carbohydrate beverage with 40 g of a mixture of potato starch, maltodextrin, dextrose and dextrin; PRO, Control carbohydrate‐protein mix with 15 g of the milk protein casein and 25 g of the carbohydrate mixture used in the active (CHO) beverage.
Outcome Measures
The primary endpoints in this study were the feasibility of the intervention and the change in depressive severity. Feasibility was assessed based on the ease of recruitment, treatment adherence and tolerability, and completion rates for the study. Alleviation of depression was measured by changes in the first 17 items of the HAM‐D‐28 scale. Response rates were defined as an improvement of 50% or greater in the HAM‐D‐17 score from the screening visit to the end of each treatment period. Remission was defined as a final HAM‐D‐17 score of 7 or less. We performed a power calculation to determine the optimal sample size to allow detection of a significant difference in response or remission rates between the two treatment conditions. Assuming a conservative response rate differential of 60% for the CHO intervention and 30% for the PRO mix, a sample of 96 subjects would be necessary to obtain the desired power of 80% at a two‐tailed alpha level of 0.05. A more robust difference in response rates of 70% for CHO and 20% for PRO would yield an 83% power to detect a difference with 36 subjects (or 18 in a crossover design). While this pilot study would be underpowered to detect a significant difference in response between the two interventions if a modest effect size were assumed [40], we hoped that by determining an effect size, this would allow us to design larger, adequately powered studies in the future.
The secondary endpoints were changes in weight, appetite, and food cravings. These were assessed at each visit by weight measurements and by a hunger questionnaire. The questionnaire inquired about adherence with the drink mix (based on number of doses missed), effect of the drink mix on hunger before lunch and dinner (“very hungry,”“normally hungry,”“much less hungry,” and “having trouble finishing meal”), and changes in cravings (“increased,”“same,” or “decreased”) for fat, protein, carbohydrate, and fruit.
Improvement in Hamilton‐D scores and comparisons between the two treatment groups and between depressive subtypes were assessed for significance by repeated measures ANOVA. Partial eta squared (ηp 2) was used as the measure of effect size. Comparisons in response rates between treatment groups and between depressive subtypes were made using the Fisher's exact test and odds ratio of response. Significance of differences in appetite and cravings between the two treatment groups was determined by the nonparametric Mann Whitney U‐test, in view of the ordinal nature of responses. Two‐tailed statistical significance was set at P < 0.05. Statistical analyses were performed with SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA). Power analyses were performed using G*Power software version 3.0.10 (Edgar Erdfelder, Lehrstuhl für Psychologie III, Universität Mannheim, Schloss Ehrenhof Ost 255, 68131, Mannheim, Germany) [41].
Results for Study 1
All 18 subjects completed Phase 1, and 16 completed Phase 2, with one subject discontinuing at the completion of Phase 1, and another after the first visit of Phase 2. The carbohydrate drink (CHO) and the placebo drink (PRO) were equally well tolerated. The only side effects reported were mild bloating, and some subjects did not like the orange flavor of the carbohydrate and control drinks. All subjects reported liking the snack muffins. All completers in both treatment groups reported adherence rates of greater than 80% (range 83–100%), based on proportion of doses missed. Seventy‐nine percent of CHO subjects and 59% of PRO subjects reported no missed doses during the entire study period, and the difference was not significant (Fisher's P= 0.14).
Subjects in both treatment groups experienced statistically significant improvement in HAM‐D‐17 scores over both phases of the study (Table 1). Subjects on CHO in Phase 1 experienced a somewhat greater decrease in HAM‐D‐17 scores compared with PRO subjects, and this improvement continued into the second phase of the study after the crossover, though the differences between treatment groups did not reach significance by repeated measures ANOVA (Table 1).
Table 1.
HAMD‐17 Improvement, Response and Remission Rates, and Changes in Weight in Study Completers
| Study 1 | Study 2 | ||||
|---|---|---|---|---|---|
| CHO‐1/PRO‐2 (n = 10) | PRO‐1/CHO‐2 (n = 8) | CHO (n = 14) | PRO (n = 17) | ||
| HAMD17 (Screen) | 17.0 ± 4.2 | 17.4 ± 4.3 | HAMD‐17 (Screen) | 17.7 ± 2.9 | 17.4 ± 2.9 |
| HAMD‐17 (End Phase 1) | 6.5 ± 3.8a | 9.3 ± 4.4a | HAMD‐17 (End week 1) | 12.3 ± 4.1g | 11.1 ± 3.0g |
| HAMD‐17 (End Phase 2) | 4.6 ± 3.9a | 7.9 ± 5.3a | HAMD‐17 (End week 2) | 9.0 ± 5.1g | 7.8 ± 3.4g |
| HAMD‐17 (End week 3) | 6.4 ± 5.8g | 6.1 ± 4.4g | |||
| Response Rate (End Phase 1) | 50% (n = 5/10)b | 50% (n = 4/8)b | Response Rate | 71% (n = 10)h | 76% (n = 13)h |
| Remission Rate (End Phase 1) | 50% (n = 5/10)c | 38% (n = 3/8)c | Remission Rate | 71% (n = 10)i | 71% (n = 12)i |
| Response Rate (End Phase 2) | 88% (n = 7/8)d | 63% (n = 5/8)d | |||
| Remission Rate (End Phase 2) | 88% (n = 7/8)e | 50% (n = 4/8)e | |||
| Weight (Screen) (kg) | 75.4 ± 20.0 | 72.8 ± 11.3 | Weight (Screen)(kg) | 67.2 ± 7.6 | 73.1 ± 14.6 |
| Weight (End Phase 1) (kg) | 76.2 ± 20.3f | 73.2 ± 10.7f | Weight (End week 1) (kg) | 67.8 ± 7.8j | 73.0 ± 14.5j |
| Weight (End Phase 2) (kg) | 73.4 ± 21.4f | 73.2 ± 9.7f | Weight (End week 2) (kg) | 67.9 ± 7.6j | 74.3 ± 14.9j |
| Weight (End week 3) (kg) | 68.2 ± 7.4j | 73.4 ± 14.4j | |||
| Notes | |
| Study 1 | Study 2 |
| CHO‐1/PRO‐2 = CHO in Phase 1, followed by PRO in Phase 2. | CHO = CHO drink |
| PRO‐1/CHO‐2 = PRO in Phase 1, followed by CHO in Phase 2. | PRO = PRO drink |
| a Repeated measures ANOVA: | g Repeated measures ANOVA: |
| Time effect: F = 76.99, df(2,28), P<0.001, η2 p= 0.85 | Time effect: F = 91.48, df(3,87), P<0.001, η2 p= 0.76 |
| Time X Treatment effect: F = 2.67, df(2,28), P = 0.09, η2 p= 0.16 | Time X Treatment effect: F = 0.22, df(3,87), P = 0.88, η2 p= 0.01 |
| b P = 1.00, OR = 1.00, 95% CI(0.16–6.42) | h P = 1.00, OR = 0.77, 95% CI(0.15–3.86) |
| c P = 0.66, OR = 1.67, 95% CI(0.25–11.11) | i P = 1.00, OR = 1.04, 95% CI(0.22–4.96) |
| d P = 0.57, OR = 4.17, 95% CI(0.33–50.0) | j Repeated measures ANOVA: |
| e P = 0.28, OR = 7.14, 95% CI(0.57–100.00) | Time effect: F = 2.36, df(3,84), P = 0.08, η2 p= 0.08 |
| f Repeated measures ANOVA: | Time X Treatment effect: F = 0.73, df(3,84), P = 0.54, η2 p= 0.03 |
| Time effect: F = 1.01, df(2,28), P = 0.38, η2 p= 0.07 | η2 p= Partial eta squared |
| Time X Treatment effect: F = 0.08, df(2,28), P = 0.93, η2 p= 0.01 | |
| η2 p= Partial eta squared | |
Response rates in Phase 1 were the same (50%) for both treatment groups (P= 1.00, OR = 1.00, 95% CI [0.16–6.42]). Remission rates favored the CHO group (50%) over the PRO group (38%), but this difference was nonsignificant (P= 0.66, OR = 1.67, 95% CI [0.25–11.11]). After the crossover, responders from both groups maintained their benefit from Phase 1, with no relapses occurring. Final response rates, based on the difference in HAM‐D‐17 score between the screening visit and the final study visit, increased in both groups, with a nonsignificant advantage (P= 0.57, OR = 4.17, 95% CI [0.33–50.0]) for the group that received CHO in Phase 1 and PRO in Phase 2 (88%) compared with the group that received PRO in Phase 1 and CHO in Phase 2 (63%). Remission rates also favored the group that received CHO in Phase 1 and PRO in Phase 2, by a nonsignificant margin (88% vs. 50%; P= 0.28, OR = 7.14, 95% CI [0.57–100.00]). Results are summarized in Table 1.
Baseline BMIs for the entire sample ranged from 22 to 34 with a mean of 25.9 ± 3.4. Seven subjects had BMIs of 24 or less (normal), nine from 25 to 29 (overweight), and two from 30 to 34 (obese). Baseline weights ranged from 48.5 to 115.3 kg, with a mean of 74.3 ± 16.3 kg. Subjects who received PRO in Phase 1 experienced nonsignificant weight change in both phases of the study (Table 1). Subjects who received CHO in Phase 1 gained a small, nonsignificant amount of weight, and then lost it in Phase 2 when switched to PRO (Table 1). Differences in weight change between treatment groups were nonsignificant by repeated measures ANOVA (Table 1).
Subjects taking CHO reported less evening hunger and greater morning hunger compared with those taking PRO. These differences were not significant by the Mann Whitney U‐test at any time point (P > 0.05). Subjects taking CHO reported a greater impact on food cravings in general, compared with those on PRO, though this difference was not significant at any time point (P > 0.05). Both drinks appeared to have beneficial effects on specific cravings for fat, protein, carbohydrate, and fruit, but no significant differences in cravings for any of these food groups were observed between CHO and PRO subjects at any time point (P > 0.05).
Fifty‐six percent of subjects (n = 10) met criteria for atypical depression; 39% (n = 7) met criteria for melancholic depression; and 6% (n = 1) met criteria for neither subtype. Subjects with atypical depression had a nonsignificantly higher baseline weight than those with melancholic depression and a significantly lower baseline HAM‐D‐17 score compared with melancholic subjects (Table 2). Atypical and melancholic subjects experienced a significant decrease in HAM‐D‐17 scores in both treatment arms (F = 75.06, df (2,24), P < 0.001, η2P= 0.86). No interaction effect was observed between depressive subtypes and treatment (F = 0.83, df (2,24), P= 0.45, η2P= 0.07). Response and remission rates favored PRO among atypical subjects and CHO among melancholic subjects, but no differences between the subgroups reached significance (Table 2). Atypical subjects in both treatment arms gained a small, nonsignificant amount of weight in Phase 1 and Phase 2 (Table 1). Melancholic subjects experienced no significant weight changes in either treatment arm (Table 2). We found no significant treatment‐related differences between atypical and melancholic subjects by repeated measures ANOVA (Table 2).
Table 2.
Comparison of Treatment Effects in Study Completers with Atypical vs Melancholic Depressive Subtypes
| HAM‐D‐17 (Screen) | Study 1 | HAM‐D‐17 (Screen) | Study 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Atypical (n = 10) | Melancholic (n = 7) | Atypical (n = 15) | Melancholic (n = 7) | ||||||
| 15.2 ± 3.2a | 20.4 ± 3.5a | 17.7 ± 3.1n | 17.9 ± 2.7 n | ||||||
| Wt (Kg) (Screen) | 76.2 ± 16.7b | 68.7 ± 15.0b | Wt (Kg) (Screen) | 68.5 ± 10.7p | 74.7 ± 11.4p | ||||
| CHO‐1/ PRO‐2 (n = 4) | PRO‐1/ CHO‐2 (n = 6) | CHO‐1/ PRO‐2 (n = 5) | PRO1/ CHO2 (n = 2) | CHO (n = 7) | PRO (n = 8) | CHO (n = 3) | PRO (n = 4) | ||
| HAM‐D‐17 (Screen) | 14.5 ± 3.0 | 15.7 ± 3.5 | 19.6 ± 3.9 | 22.5 ± 0.7 | HAM‐D‐17 (Screen) | 18.0±3.3 | 17.4 ± 3.1 | 16.7 ±1.2 | 18.8 ± 3.4 |
| HAM‐D‐17 (End Phase 1) | 8.0 ± 3.4c | 8.3 ± 4.8c | 5.0 ± 4.4c | 12.0 ± 1.4c | HAM‐D‐17 (End wk 1) | 12.3 ±5.3q | 10.5 ±2.9 q | 12.3 ±2.1 q | 11.8 ± 3.5 q |
| HAM‐D‐17 (End Phase 2) | 6.7 ± 6.0c | 6.7 ± 5.4c | 3.4 ± 1.8c | 11.5 ± 3.5c | HAM‐D‐17 (End wk 2) | 9.0 ± 5.9q | 7.3 ± 3.6 q | 8.3 ± 6.5 q | 10.0 ± 2.6 q |
| HAM‐D‐17 (End wk 3) | 6.9 ± 6.6q | 5.0 ± 4.3 q | 7.3 ± 4.7 q | 8.0 ± 3.4 q | |||||
| Response Rate (Phase 1) (%) | 25% (n = 1)d,f | 50% (n = 3)d,g | 80% (n = 4)e,f | 50% (n = 1)e,g | Response Rate (%) | 86% (n = 6)r,t | 88% (n = 7)r,u | 33% (n = 1)s,t | 75% (n = 3)s,u |
| Remission Rate (Phase 1) (%) | 25% (n = 1)h,j | 50% (n = 3)h,k | 80% (n = 4)i,j | 0% (n = 0)i,k | Remission Rate (%) | 86% (n = 6)v,x | 75% (n = 6)v,y | 33% (n = 1)w,x | 75% (n = 3)w,y |
| Weight (Screen) | 79.7 ± 24.9 | 73.8 ± 10.7 | 68.2 ± 16.2 | 69.9 ± 17.3 | Weight (Screen) | 65.9 ± 2.9 | 70.7 ±14.4 | 69.3 ±7.6 | 78.7 ±13.1 |
| Weight (End Phase 1) | 80.9±25.1m | 74.2 ± 9.6m | 68.7 ± 16.7m | 70.3 ± 17.7m | Weight (End wk 1) | 66.1 ± 3.0z | 70.5 ±13.9z | 71.0 ±9.6z | 78.7 ±13.1z |
| Weight (End Phase 2) | 82.3 ± 29.7m | 74.2 ± 8.6m | 68.1±16.3m | 70.2 ± 16.3m | Weight (End wk 2) | 66.4 ± 3.2z | 72.2 ±15.0z | 71.5 ±8.0z | 79.9 ±13.2z |
| Weight (End wk 3) | 66.7 ± 3.1z | 71.4 ±14.2z | 71.3 ±8.4z | 79.4 ±13.7z | |||||
| Notes | |
| Study 1 | Study 2 |
| CHO‐1/PRO‐2 = CHO in Phase 1, followed by PRO in Phase 2. | CHO = CHO drink |
| PRO‐1/CHO‐2 = PRO in Phase 1, followed by CHO in Phase 2. | PRO = PRO drink |
| a U = 9.00, Z =−2.56, P = 0.01 (difference in initial HAMD‐17 score between | n U = 57.50, Z =−0.16, P = 0.87 (difference in initial HAMD‐17 score between. |
| Atypical and Melancholic subjects). | Atypical and Melancholic subjects). |
| b U = 27.00, Z =−0.78, P = 0.44 (difference in initial weight | p U = 36.00, Z =−1.55, P = 0.12 (difference in initial weight between |
| between Atypical and Melancholic subjects). | Atypical and Melancholic subjects). |
| c Repeated measures ANOVA: | q Repeated Measures ANOVA: |
| Time effect: F = 75.06, df (2,24), P<0.001, ηp 2= 0.86 | Time effect: F = 72.37, df (3,75), P<0.001, ηp 2= 0.74 |
| Time X Treatment effect: F = 1.07, df (2,24), P = 0.36, ηp 2= 0.08 | Time X Treatment effect: F = 0.22, df (3,75), P = 0.89, ηp 2= 0.01 |
| Time X Treatment X Subtype effect: F = 0.83, df(2,24), P = 0.45, ηp 2= 0.07 | Time X Treatment X Subtype effect: F = 0.41, df(6,75), P = 0.87, ηp 2= 0.03 |
| d Atypical: CHO1‐PRO2 vs PRO1‐CHO2: P = 0.57, OR = 0.33, 95% CI(0.02–5.26) | r Atyp: CHO vs PRO: P = 1.00, OR = 0.86. 95% CI(0.04–16.85) |
| e Melancholic: CHO1‐PRO2 vs PRO1‐CHO2: P = 1.00, OR = 4.00, 95% CI(0.12–100.00) | s Mel: CHO vs PRO: P = 0.49, OR = 0.17, 95% CI(0.01–4.52) |
| f CHO1‐PRO2: Atyp vs Mel: P = 0.21, OR = 0.08, 95% CI(0.0004–1.96) | t CHO: Atyp vs Mel: P = 0.18, OR = 12.00, 95% CI (0.49–294.57) |
| g PRO1‐CHO2: Atyp vs Mel: P = 1.00, OR = 1.00, 95% CI(0.04–24.55) | u PRO: Atyp vs Mel: P = 1.00, OR = 2.33, 95% CI(0.11–50.98) |
| h Atyp: CHO1‐PRO2 vs vs PRO1‐CHO2: P = 0.57, OR = 0.33, 95% CI(0.02–5.26) | v Atyp: CHO vs PRO: P = 1.00, OR = 2.00 95% CI(0.14–28.42) |
| i Mel: CHO1‐PRO2 vs vs PRO1‐CHO2: P = 0.14, OR = NA, 95% CI(NA) | w Mel: CHO vs PRO: P = 0.49, OR = 0.17, 95% CI(0.01–4.53) |
| j CHO: Atyp vs Mel: P = 0.21, OR = 0.08, 95% CI(0.0004–1.96) | x CHO: Atyp vs Mel: P = 0.18, OR = 12.00, 95% CI(0.49–294.57) |
| k PRO: Atyp vs Mel: P = 0.46, OR = NA, 95% CI(NA) | y PRO: Atyp vs Mel: P = 1.00,OR = 1.00, 95% CI(0.06–15.99) |
| m Repeated measures ANOVA: | z Repeated measures ANOVA: |
| Time effect: F = 1.09, df (2,24), P = 0.35, ηp 2= 0.08 | Time effect: F = 2.65, df (3,72), P = 0.06, ηp 2= 0.10 |
| Time X Treatment effect: F = 0.17, df (2,24), P = 0.84, ηp 2= 0.01 | Time X Treatment effect: F = 0.91, df (3,72), P = 0.44, ηp 2= 0.04 |
| Time X Treatment X Subtype effect: F = 0.62, df (2,24), P = 0.55, ηp 2= 0.05 | Time X Treatment X Subtype effect: F = 0.43, df (6,72), P = 0.86, ηp 2= 0.03 |
| ηp 2= Partial eta squared | ηp 2= Partial eta squared |
Study 2: A Double‐Blind Randomized Placebo‐Controlled Pilot Study of the Carbohydrate‐Rich Nutrient Mixture for Treatment of SAD
Thirty‐four subjects between the ages of 18 and 70 years, in good general health with a BMI of no more than 35 (weighed in a hospital gown) were recruited from November 2005 to January 2006 by placing IRB‐approved advertisements in local newspapers. Potential subjects who responded to the ad underwent a brief telephone interview with a research assistant to schedule a screening interview and signed an IRB‐approved consent form prior to the intake assessment. Thirty‐two of these subjects (63% women; mean age 46 ± 14 years) met criteria for study entry, and all 32 were randomized (100% acceptability). Subjects who completed the entire study were remunerated $300 for their participation.
Subjects were randomized in a double‐blind, 1 : 1 manner to either the carbohydrate (CHO) or the control (PRO) drink for 21 days. Subjects were assessed weekly for depressive symptoms, appetite and food cravings, and weight changes. A longer treatment period was used here compared with the first study to increase the chance of separation between the active intervention and placebo if there was a difference in efficacy.
Outcome measures were the same ones used in Study 1. In view of the high placebo‐response rate observed in the first study, we added a questionnaire at the end of the second study, asking subjects and study physicians to guess which intervention they had received. Power analysis considerations were essentially the same as for Study 1.
Results for Study 2:
Thirty‐one of the 32 subjects completed the study, with one subject dropping out after week 2. The carbohydrate drink (CHO) and the placebo drink (PRO) were equally well tolerated, as in the first study. All completers in the PRO group reported adherence rates greater than 80% (range 83–100%), based on proportion of doses missed. In the CHO group, all but one subject reported an adherence rate greater than 80% (range 67–100%). Sixty‐seven percent of CHO subjects, and 64% of PRO subjects reported no missed doses during the entire study period, and these differences were not significant (Fisher's P= 0.83).
Subjects in both treatment groups experienced statistically significant improvement in HAM‐D‐17 scores within 1 week of starting treatment, and the improvement continued steadily through the end of the third week (F = 91.48, df (3,87), P < 0.001, η2 p= 0.76) (Table 1). The differences in improvement between treatment groups by the end of 3 weeks were not significant by repeated measures ANOVA (F = 0.22, df (3,87), P= 0.88, η2 p= 0.01).
Response rates were similar for both treatment groups (71% for CHO and 76% for PRO), remission rates were equivalent (71% for each), and these differences were nonsignificant by Fisher's exact test (Table 1).
Baseline BMIs ranged from 18 to 35 with a mean of 25.5 ± 4.3. Fifteen subjects had BMIs of 24 or less, eleven from 25 to 29, and six from 30 to 35. Baseline weights ranged from 42.4 to 102.8 kg, with a mean of 71.4 ± 13.3 kg. Neither treatment group experienced a significant weight change throughout the course of treatment (F = 2.36, df(3,84), P= 0.08, η2 p= 0.08), and the difference in weight change between the treatment groups was not significant by repeated measures ANOVA (F = 0.73, df (3,84), P= 0.54, η2 p= 0.03). Results are summarized on Table 1.
Subjects taking CHO tended to report less evening hunger and greater morning hunger compared with those taking PRO. These differences were not significant by the Mann Whitney U‐test at any time point (P > 0.05). Subjects taking CHO reported a greater impact on food cravings, in general, compared with those on PRO. PRO appeared to have a greater beneficial effect on cravings for fat, protein, and carbohydrate, with CHO having an advantage with regard to fruit cravings. None of these comparisons reached significance (P > 0.05).
Forty‐seven percent of subjects (n = 15) met criteria for atypical depression; 25% (n = 8) met criteria for melancholic depression; and 28% (n = 9) met criteria for neither subtype. Completers with atypical depression (n = 15) had a nonsignificantly lower baseline weight compared with those with melancholic depression (n = 7), and both groups had equivalent baseline HAM‐D‐17 scores (Table 2).
Atypical and melancholic subjects experienced a significant decrease in HAM‐D‐17 scores from week 1 onward, independent of treatment (F = 72.37, df (3,75), P < 0.001, η2 p= 0.74) (Table 2). The overall improvement in HAM‐D‐17 score was higher for atypical subjects than for melancholic subjects, particularly among CHO recipients, but none of these differences were significant by repeated measures ANOVA (F = 0.41, df (6,75), P= 0.87, η2 p= 0.03) (Table 2).
Among atypical subjects, response and remission rates were very similar regardless of treatment (Table 2). Among melancholic subjects, response rates favored PRO over CHO, but the difference between the treatment groups was not significant (Table 2). Atypical subjects showed a stronger response rate to CHO than melancholic subjects, and both had comparable response rates to PRO, but no comparisons yielded significant differences (Table 2).
Neither atypical nor melancholic patients experienced a significant weight change, and the difference between groups was not significant by repeated measures ANOVA (F = 0.43, df (6,72), P= 0.86, η2 p= 0.03) (Table 2).
Among the 14 study completers who received CHO, 71% (n = 10) correctly guessed their assigned treatment, and the study clinicians guessed correctly in 79% of cases (n = 11). Among the 17 completers who received PRO, 18% (n = 3) correctly guessed their assigned treatment, and the study clinicians guessed correctly in 29% of cases (n = 5). Correct guess rate differences between treatment groups were nonsignificant for patients (Fisher's P= 0.67) and for clinicians (Fisher's P= 0.70).
Discussion
Several lines of research have examined the impact of carbohydrate administration in individuals with SAD. In a small study by Rosenthal et al. [26], SAD patients fed a carbohydrate‐rich meal, as opposed to an isocaloric protein‐rich meal, experienced an increase in energy, whereas nondepressed control subjects experienced sedation. SAD patients, when depressed as opposed to euthymic, have a faster glycemic response and secrete more insulin in response to an oral glucose load [27]. This response may, in theory, result in a vicious circle whereby increased carbohydrate consumption lowers blood glucose to subnormal levels, thus triggering more carbohydrate cravings and consumption, and hence lowering blood glucose levels even further. Given the concerns over excessive carbohydrate intake in patients with SAD and their potential impact on weight and cardiovascular health, many clinicians tend to recommend low‐carbohydrate diets for individuals with SAD.
On the other hand, Danilenko et al. [25] found no significant differences in antidepressant effects between short‐term carbohydrate or protein‐rich diets in women with SAD; clinical improvement in each group was significant but modest, and subject to other factors such as exposure to sunshine, menstrual cycle, and initial percentage fat. The above studies are limited by small sample sizes, thus the question of whether carbohydrates should be minimized or simply better regulated requires further investigation.
As mentioned in the Introduction, the finding that enhanced serotonin activity by dexfenfluramine alleviated SAD symptoms [29], and that the carbohydrate mixture of our study had beneficial effects on mood in women with PMS [33] encouraged us to investigate it as a possible therapy for SAD. If such a therapy were to allow SAD patients to obtain a carefully regulated carbohydrate intake while alleviating depressive symptoms without causing weight gain, it would be a potentially valuable treatment.
Given the narrow clinical window for SAD, we were pleased by the robust recruitment rates of about nine patients per month over a 2‐month period in the first study, and about 10 patients per month over a 3‐month period in the second study, both of which were considerably higher than recruitment rates observed in many outpatient clinical trials [42]. We unfortunately do not have enough available information about patient acceptability per se (relative to patients deemed eligible) in prior studies of SAD to compare the acceptability of our therapy against other treatments. Likewise, we did not inquire about whether our subjects chose these studies because of the specific intervention or because they wished to obtain any treatment for their condition. Nonetheless, the high acceptability rates (86% in Study 1 and 100% in Study 2) are encouraging.
Both drink mixes and the snack muffins were well tolerated, and the adherence and completion rates were excellent, though it must be emphasized that both treatment periods were shorter than in standard depression studies, which may have contributed to the higher completion rates. Our findings overall suggest strong acceptability, tolerability, and feasibility of this therapy in clinical and research applications among depressed populations.
Both studies were characterized by significant, early improvement in the HAM‐D‐17 scores and comparable response and remission rates for both the active treatment (CHO) and the placebo intervention (PRO). Effect sizes in Study 1, based on change in HAM‐D‐17 score were robust for the sample as a whole (η2 p= 0.85) but weak when comparing CHO against PRO (η2 p= 0.16), and the pattern in Study 2 was similar. This suggests little difference in efficacy between CHO and PRO. The placebo response rates of 50% and 76% in the two respective studies were notably higher than the usual 20–30% observed in most depression studies. The odds ratio for response for CHO was 1.00 in Study 1, and 0.77 in Study 2, again suggesting little difference with PRO. These pilot investigations were designed to examine robust effect sizes, that is, large by Cohen's criteria [40]. Given the effect sizes observed, the study is clearly underpowered. The small effect size would require a larger sample size to be detectable.
Study 1, the first one performed, used a crossover design to enrich each treatment arm by having each subject act as his or her own control, hence increasing statistical power. However, because both treatment groups maintained their benefit from Phase 1 and continued to improve during Phase 2, the pooled analysis could not be carried out as originally proposed, and instead, we analyzed each treatment phase for each patient cohort. The continued improvement in Phase 2 was initially thought to be a carryover effect from Phase 1 (perhaps due to a short washout period), and/or a strong placebo response for those who received the PRO drink in Phase 1, perhaps further aided by the active CHO drink in Phase 2. However, the comparable response rates for both interventions in Study 2, which did not use a crossover design, would appear to argue against a carryover effect. The low correct‐treatment guess rate among placebo subjects and clinicians in Study 2 suggests that the placebo drink was well masked, and that unblinding did not occur.
Given the high response rates for both interventions, we must ask whether this can be explained simply as a high placebo response rate, as an unexpected but real antidepressant effect of the PRO drink, or whether the protein component of PRO is not enough to dampen the proposed serotonergic effect of the carbohydrate component, thus resulting in a placebo that is essentially the same as the active intervention. Factors contributing to the high placebo response rate in these studies may include treatment periods shorter than those typically set for antidepressant studies. There is evidence that response to antidepressants tends to occur early in the course of treatment [43], which suggested that we should be able to observe a reasonable signal of efficacy within the allotted time. This turned out to be the case for both the active treatment and the placebo. Longer term investigations will be needed to determine whether the benefit of either intervention would be maintained over a longer period of time.
Because SAD is not present year‐round like other depressive conditions, it may be more tractable and therefore subject to a greater placebo effect, though this is speculative, given the limited number of placebo comparison studies in SAD. Other explanations for the lack of separation between CHO and PRO may be in the design limitations of the two studies: the small samples limited the statistical power; the study venue, the MIT CRC, has a small infrastructure with limited clinician hours and appointment times and may have resulted in a different self‐selection of study subjects; for many study participants, the act of entering treatment may have a beneficial effect on mood, particularly over the short term; finally, the offer of remuneration may have impacted on subjects' choice to participate, as well as having its own mood‐elevating effect. In view of growing numbers of investigative groups that offer remuneration to entice potential subjects [44], these findings might raise a red flag with regard to that practice.
In both studies, neither CHO nor PRO had a significant impact on weight. We did not institute rigorous control over the subjects' diet in these pilot studies, though a more regulated diet would certainly be appropriate in larger, more definitive investigations. It was hoped that by encouraging use of the snack muffins as opposed to unhealthy snacks, a secondary benefit of improved weight control could be obtained. By requiring subjects to return any uneaten muffins at each study visit, it minimized the chances of weight gain as a result of stockpiling and eating too many muffins.
Appetite and food cravings were subject to a generally positive impact from both interventions. In both studies, CHO tended to reduce evening hunger but had less impact on morning hunger compared with PRO. It is possible that if these subjects ate less at night (when most people tend to overeat), it may explain their being hungrier in the morning. The caloric supplementation and presence of carbohydrates and/or protein may have been enough to curb appetite in most participants. Again, replication in larger samples is necessary for further clarification.
Prevalence of atypical (56% in Study 1; 47% in Study 2; 50% in the pooled sample) and melancholic depression (39% in Study 1; 25% in Study 2; 30% in the pooled sample) were higher than those reported in the depression literature—about 40% for atypical depression [45] and 17% for melancholic depression [46]. Many SAD symptoms overlap with those of atypical depression, and one study has shown a high rate of melancholic symptoms such as depression worse in the morning in SAD patients [47]. Given these overlaps, investigation into treatment effects in these subtypes appeared worthwhile, though cautious interpretation is necessary. For example, sleeping better and eating more may represent improvement for melancholic subjects but the opposite for atypical subjects.
Our exploratory analysis of treatment response to CHO and PRO in atypical and melancholic subjects was limited by the smallness of each subgroup, with no significant differences found. Atypical depressed subjects in Study 1 had a greater proclivity to weight gain compared with melancholic subjects, which may be a reflection of the depressive subtypes, though the difference between subtypes was not significant. An additional item‐by‐item analysis of the individual HAM‐D‐28 items was carried out to investigate any differential effects between the active and placebo treatment in both studies, particularly with regard to symptoms common in SAD and atypical depression, such as weight gain, food cravings, and hypersomnia, but after correction for multiple comparisons, no significant differences were found (data not shown). While these pilot studies sought primarily to establish efficacy in SAD in general, improved characterization of the prevalence of atypical and melancholic subtypes in SAD and their response to treatment may eventually yield strategies for tailoring therapy to the depressive subtype observed.
Additional limitations of this investigation include examining two independent studies, which raises the question of whether the samples and treatment conditions are similar enough for generalizable conclusions. In Study 1, subjects were recruited from October to December 2004, and for Study 2 from November 2004 to January 2005, with the same recruitment methods, diagnostic instruments, study clinicians, and remuneration. In theory, the second group could have been more severely ill in view of the recruitment occurring somewhat later in the fall/winter season, but the baseline HAM‐D‐17 scores were comparable for both study samples, which suggests that there was no major difference between the two subject cohorts. Study 2 recruited more women than Study 1 (63% vs. 50%). An examination of the study samples by gender (not shown) demonstrated no significant gender‐related differences in depressive severity or treatment response rates, which was expected, given that men and women respond equally well to antidepressants.
To conclude, we have found good acceptability, tolerability, and therapeutic effects for two different drink mixes in SAD. Given the high response rates for both and the absence of a differentiation between the treatments, further investigation would seem warranted to clarify whether the benefit of these drink mixes is real or a placebo effect. The fact that most subjects had symptomatic improvement and remained at a stable weight by the end of the study suggests that both interventions were beneficial, at least in the short term. Perhaps having a healthy drink mix and snack on a daily basis is a good intervention for this mood disorder, and may prevent patients from snacking on bad foods, which in itself may have therapeutic benefits. These preliminary results should set the stage for larger‐scale, longer‐term comparisons against a potentially less active placebo, and if findings of these studies are positive, eventual comparisons against established therapies, such as an FDA‐approved antidepressants or light therapy.
Conflict of Interest
Drs. Mischoulon, Wurtman (J), Wurtman (R), and Vangel have received license royalties from the Back Bay Scientific Company for the product used in these studies (patent application pending).
Dr. Mischoulon has received research support for other clinical trials, in the form of donated medications from Amarin (Laxdale), NordicNaturals, Lichtwer Pharma GmbH, Bristol‐Myers Squibb Company, Cederroth, Ganeden, and SwissMedica. He has received consulting and writing honoraria from Pamlab. He has received speaking honoraria from Bristol‐Meyers Squibb Company, Pfizer, Pamlab, Virbac, and NordicNaturals. He has received speaker's honoraria from the MGH‐Psychiatry Academy. Commercial entities currently supporting the MGH Psychiatry Academy are listed on the Academy's website http://www.mghcme.org and include Astra Zeneca, Eli Lilly, and Janssen Pharmaceuticals.
Dr. Pedrelli reports no conflicts of interest.
Acknowledgments
This research was supported in part by a General Clinical Research Center grant from the National Institutes of Health (MO1‐RR01066) awarded to the Massachusetts Institute of Technology General Clinical Research Center, and by SwissMedica. SwissMedica was not involved in the study design, data collection or analysis, manuscript preparation, or publication decisions.
The authors thank Janine McDermott, M.S., and Rita Tsay, R.D., for their technical assistance, and the nursing and nutritional staff of the MIT Clinical Research Center for their help with patient care. We also thank Alisabet Clain, M.S., and Lee Baer, Ph.D., for additional statistical advice.
References
- 1. Roecklein KA, Rohan KJ. Seasonal affective disorder: An overview and update. Psychiatry 2005;Jan:20–26. [PMC free article] [PubMed] [Google Scholar]
- 2. American Psychiatric Association . Diagnostic and statistical manual of mental disorders, 4th ed., Washington , DC : APPI, 1994;389–390. [Google Scholar]
- 3. Moscovitch A, Blashko CA, Eagles JM, et al International Collaborative Group on Sertraline in the treatment of outpatients with seasonal affective disorders: A placebo‐controlled study of sertraline in the treatment of outpatients with seasonal affective disorder. Psychopharmacology (Berl) 2004;171:390–397. [DOI] [PubMed] [Google Scholar]
- 4. Murray G, Michalak EE, Levitt AJ, Levitan RD, Enns MW, Morehouse R, Lam RW. Therapeutic mechanism in seasonal affective disorder: Do fluoxetine and light operate through advancing circadian phase? Chronobiol Int 2005;22:937–943. [DOI] [PubMed] [Google Scholar]
- 5. Michalak EE, Murray G, Levitt AJ, et al Quality of life as an outcome indicator in patients with seasonal affective disorder: results from the Can‐SAD study. Psychol Med 2007;37:727–736. [DOI] [PubMed] [Google Scholar]
- 6. Pjrek E, Winkler D, Stastny J, Praschak‐Rieder N, Willeit M, Kasper S. Escitalopram in seasonal affective disorder: Results of an open trial. Pharmacopsychiatry 2007;40:20–24. [DOI] [PubMed] [Google Scholar]
- 7. Gaynes BN. Light therapy and fluoxetine similarly effective for improving seasonal affective disorder. Evid Based Ment Health 2007;10:26. [DOI] [PubMed] [Google Scholar]
- 8. Pjrek E, Willeit M, Praschak‐Rieder N, Konstantinidis A, Semlitsch HV, Kasper S, Winkler D. Treatment of seasonal affective disorder with duloxetine: An open‐label study. Pharmacopsychiatry 2008;41:100–105. [DOI] [PubMed] [Google Scholar]
- 9. Modell JG, Rosenthal NE, Harriett AE, et al Seasonal affective disorder and its prevention by anticipatory treatment with bupropion XL. Biol Psychiatry 2005;58:658–667. [DOI] [PubMed] [Google Scholar]
- 10. Shen J, Kennedy SH, Levitan RD, Kayumov L, Shapiro CM. The effects of nefazodone on women with seasonal affective disorder: clinical and polysomnographic analyses. J Psychiatry Neurosci 2005;30:11–16. [PMC free article] [PubMed] [Google Scholar]
- 11. Food and Drug Administration . FDA approves the first drug for seasonal depression. FDA News June 12, 2006. Available at: http://www.fda.gov/bbs/topics/NEWS/2006/NEW01388.html. Accessed Aug 1, 2008.
- 12. Lundt L. Modafinil treatment in patients with seasonal affective disorder/winter depression: An open‐label pilot study. J Affect Disord 2004;81:173–178. [DOI] [PubMed] [Google Scholar]
- 13. Pjrek E, Winkler D, Konstantinidis A, Willeit M, Praschak‐Rieder N, Kasper S. Agomelatine in the treatment of seasonal affective disorder. Psychopharmacology (Berl) 2007;190:575–579. [DOI] [PubMed] [Google Scholar]
- 14. Aan Het Rot M, Benkelfat C, Boivin DB, Young SN. Bright light exposure during acute tryptophan depletion prevents a lowering of mood in mildly seasonal women. Eur Neuropsychopharmacol 2008;18:14–23. [DOI] [PubMed] [Google Scholar]
- 15. Desan PH, Weinstein AJ, Michalak EE, et al A controlled trial of the Litebook light‐emitting diode (LED) light therapy device for treatment of Seasonal Affective Disorder (SAD). BMC Psychiatry 2007;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rastad C, Ulfberg J, Lindberg P. Light room therapy effective in mild forms of seasonal affective disorder—a randomised controlled study. J Affect Disord 2008;108:291–296. [DOI] [PubMed] [Google Scholar]
- 17. Even C, Schröder CM, Friedman S, Rouillon F. Efficacy of light therapy in nonseasonal depression: A systematic review. J Affect Disord 2008;108:11–23. [DOI] [PubMed] [Google Scholar]
- 18. Golden RN, Gaynes BN, Ekstrom RD, et al The efficacy of light therapy in the treatment of mood disorders: A review and meta‐analysis of the evidence. Am J Psychiatry 2005;162:656–662. [DOI] [PubMed] [Google Scholar]
- 19. Pjrek E, Winkler D, Stastny J, Konstantinidis A, Heiden A, Kasper S. Bright light therapy in seasonal affective disorder—Does it suffice? Eur Neuropsychopharmacol 2004;14:347–351. [DOI] [PubMed] [Google Scholar]
- 20. Rohan KJ, Lindsey KT, Roecklein KA, Lacy TJ. Cognitive‐behavioral therapy, light therapy, and their combination in treating seasonal affective disorder. J Affect Disord 2004;80:273–283. [DOI] [PubMed] [Google Scholar]
- 21. Poirrier R, Cambron L. Bright light therapy. Rev Med Liege 2007;62:25–32. [PubMed] [Google Scholar]
- 22. Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: Efficacy, protocol, safety, and side effects. CNS Spectr 2005;10:647–663. [DOI] [PubMed] [Google Scholar]
- 23. Rohan KJ, Roecklein KA, Tierney Lindsey K, Johnson LG, Lippy RD, Lacy TJ, Barton FB. A randomized controlled trial of cognitive‐behavioral therapy, light therapy, and their combination for seasonal affective disorder. J Consult Clin Psychol 2007;75:489–500. [DOI] [PubMed] [Google Scholar]
- 24. Wurtman RJ, Wurtman JJ. Carbohydrates and depression. Sci Am 1989;Jan:68–75. [DOI] [PubMed] [Google Scholar]
- 25. Danilenko KV, Plisov IL, Hébert M, Kräuchi K, Wirz‐Justice A. Influence of timed nutrient diet on depression and light sensitivity in seasonal affective disorder. Chronobiol Int 2008;25:51–64. [DOI] [PubMed] [Google Scholar]
- 26. Rosenthal NE, Genhart MJ, Caballero B, et al Psychobiological effects of carbohydrate‐ and protein‐rich meals in patients with seasonal affective disorder and normal controls. Biol Psychiatry 1989;25:1029–1040. [DOI] [PubMed] [Google Scholar]
- 27. Kräuchi K, Keller U, Leonhardt G, Brunner DP, Van Der Velde P, Haug HJ, Wirz‐Justice A. Accelerated post‐glucose glycaemia and altered alliesthesia‐test in Seasonal Affective Disorder. J Affect Disord 1999;53:23–26. [DOI] [PubMed] [Google Scholar]
- 28. O'Rourke D, Wurtman JJ, Wurtman RJ. Serotonin implicated in the etiology of seasonal affective disorder with carbohydrate craving In: Pirke KM, Vandereycken W, Ploog D, editors. The psychobiology of bulimia nervosa, Heidelberg : Springer Verlag, 1988; 13–17. [Google Scholar]
- 29. O'Rourke D, Wurtman JJ, Wurtman RJ, Chebli R, Gleason R. Treatment of seasonal depression with d‐fenfluramine. J Clin Psychiatry 1989;50:343–347. [PubMed] [Google Scholar]
- 30. Levitan RD. The chronobiology and neurobiology of winter seasonal affective disorder. Dialogues Clin Neurosci 2007;9:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Willeit M, Sitte HH, Thierry N, et al Enhanced serotonin transporter function during depression in seasonal affective disorder. Neuropsychopharmacology 2008;33:1503–1513. [DOI] [PubMed] [Google Scholar]
- 32. Swiecicki L, Bidziński A, Tonderska A. Platelet serotonin transport in the group of outpatients with seasonal affective disorder before and after light treatment, and in remission (in the summer). Psychiatr Pol 2005;39:459–468. [PubMed] [Google Scholar]
- 33. Sayegh, R , Schiff, I , Wurtman J, Spiers, P , McDermott J, Wurtman R. The effect of a carbohydrate‐rich beverage on mood, appetite, and cognitive function in women with premenstrual syndrome. Obstet and Gynecol 1995;86:520–528. [DOI] [PubMed] [Google Scholar]
- 34. Westrin A, Lam RW. Seasonal affective disorder: A clinical update. Ann Clin Psychiatry 2007;19:239–246. [DOI] [PubMed] [Google Scholar]
- 35. Juruena MF, Cleare AJ. Overlap between atypical depression, seasonal affective disorder and chronic fatigue syndrome. Rev Bras Psiquiatr 2007;29(Suppl. 1):S19–S26. [DOI] [PubMed] [Google Scholar]
- 36. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM‐IV Axis I disorders—Patient edition (SCID‐I/P, Version 2.0). New York : Biometrics Research Department, New York State Psychiatric Institute, 1995. [Google Scholar]
- 37. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967;6:278–296. [DOI] [PubMed] [Google Scholar]
- 39. Williams JB. A structured interview guide for the Hamilton depression rating scale. Arch Gen Psychiatry 1988;45:742–747. [DOI] [PubMed] [Google Scholar]
- 40. Cohen J. Statistical power analysis for the behavioral sciences, 2nd Ed., Hillsdale , NJ : Lawrence Erlbaum, 1988. [Google Scholar]
- 41. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–191. [DOI] [PubMed] [Google Scholar]
- 42. Fava M, Mischoulon D. Evaluating the data: Limitations of research and quality assurance issues regarding natural remedies In: Mischoulon D, Rosenbaum J, editors. Natural medications for psychiatric disorders: considering the alternatives, 2nd Ed Philadelphia : Lippincott Williams & Wilkins, 2008; 11–23. [Google Scholar]
- 43. Nierenberg AA, McLean NE, Alpert JE, Worthington JJ, Rosenbaum JF, Fava M. Early nonresponse to fluoxetine as a predictor of poor 8‐week outcome. Am J Psychiatry 1995;152:1500–1503. [DOI] [PubMed] [Google Scholar]
- 44. Elliott C. Guinea‐pigging: healthy human subjects for drug‐safety trials are in demand. But is it a living? New Yorker 2008, January 7:36–41. [PubMed] [Google Scholar]
- 45. Farabaugh AH, Mischoulon D, Fava M, Green C, Guyker W, Alpert J. The potential relationship between levels of perceived stress and subtypes of major depressive disorder (MDD). Acta Psychiatr Scand 2004;110:465–470. [DOI] [PubMed] [Google Scholar]
- 46. Lafer B, Nierenberg AA, Rosenbaum JF, Fava M. Outpatients with DSM‐III‐R versus DSM‐IV melancholic depression. Compr Psychiatry 1996;37:37–39. [DOI] [PubMed] [Google Scholar]
- 47. Graw P, Kräuchi K, Wirz‐Justice A, Pöldinger W. Diurnal variation of symptoms in seasonal affective disorder. Psychiatry Res 1991;37:105–111. [DOI] [PubMed] [Google Scholar]


