Abstract
Medial prefrontal cortical areas have been hypothesized to underlie altered contextual processing in posttraumatic stress disorder (PTSD). We investigated brain signaling of contextual information in this disorder. Eighteen PTSD subjects and 16 healthy trauma‐exposed subjects underwent a two‐day fear conditioning and extinction paradigm. On day 1, within visual context A, a conditioned stimulus (CS) was followed 60% of the time by an electric shock (conditioning). The conditioned response was then extinguished (extinction learning) in context B. On day 2, recall of the extinction memory was tested in context B. Skin conductance response (SCR) and functional magnetic resonance imaging (fMRI) data were collected during context presentations. There were no SCR group differences in any context presentation. Concerning fMRI data, during late conditioning, when context A signaled danger, PTSD subjects showed dorsal anterior cingulate cortical (dACC) hyperactivation. During early extinction, when context B had not yet fully acquired signal value for safety, PTSD subjects still showed dACC hyperactivation. During late extinction, when context B had come to signal safety, they showed ventromedial prefrontal cortex (vmPFC) hypoactivation. During early extinction recall, when context B signaled safety, they showed both vmPFC hypoactivation and dACC hyperactivation. These findings suggest that PTSD subjects show alterations in the processing of contextual information related to danger and safety. This impairment is manifest even prior to a physiologically‐measured, cue‐elicited fear response, and characterized by hypoactivation in vmPFC and hyperactivation in dACC.
Keywords: Amygdala, Contextual signaling, dACC, Fear extinction, fMRI, Hippocampus, vmPFC
Introduction
In classical fear conditioning, a conditioned stimulus (CS) acquires emotional significance when paired with an aversive unconditioned stimulus (US). This pairing induces various conditioned responses (CR), such as freezing in rodents and enhanced skin conductance response (SCR) in humans. These CRs can then be extinguished if the CS is repeatedly presented in the absence of the US. These two types of training lead to the formation of two distinct memories: the conditioning memory and the extinction memory. Numerous studies have shown that neuronal signaling of contextual information is critically involved in the expression or inhibition of conditioned fear responses [1, 2, 3, 4]. Specifically, contextual information gates the expression of the potentially conflicting conditioning and extinction memories toward the same CS. For example, when humans are conditioned in context A and shortly thereafter extinguished in context B, the CR on the next day depends upon the context in which in the CS is presented. Specifically, when the CS is presented in the “safe” context B, the CR is smaller than when it is presented in the “dangerous” context A [5].
Deficient contextual signaling facilitates the development and maintenance of exaggerated fear responses in anxiety disorders [6]. Liberzon and colleagues recently hypothesized that impaired contextualization represents a pathophysiological mechanism in posttraumatic stress disorder (PTSD). Specifically, impaired contextual signaling might confer reduced ability to use external and internal contextual cues to select the most appropriate behavioral response out of a variety of competing options [7].
In both rodents and humans, the brain structures involved in contextual signaling during conditioning and extinction include hippocampus, amygdala, and ventromedial prefrontal cortex (vmPFC). In particular, hippocampus plays a crucial role in mediating the activation of a specific memory by its associated context [8, 9, 10, 11, 12]. One target structure of hippocampal mediation of contextual selectivity is the amygdala, which plays a central role not only in the acquisition and expression of fear, but also in extinction learning [13, 14, 15, 16]. Another region that modulates amygdala responses is the vmPFC. This structure receives strong hippocampal projections and is itself densely connected to the amygdala [17, 18, 19]. Inhibitory control by vmPFC over amygdala has been revealed in numerous studies [20, 21]. In the rat, the PFC is subdivided into infralimbic (IL) and prelimbic (PL) regions. IL facilitates fear inhibition [22], whereas PL facilitates fear expression [23]. The human homologues of IL and PL are thought to be vmPFC and dorsal anterior cingulate cortex (dACC), respectively [24]. Both structures receive modulating input from the hippocampus [18, 25, 26].
The above‐mentioned structures are dysfunctional in PTSD subjects across a variety of paradigms [7]. Using a two‐day fear conditioning paradigm in which on day 1 a cue (or CS) was conditioned in context A and then extinguished in context B (extinction learning), and on day 2 presented (without the US) again in context B (extinction recall), we recently published data related to the CS presentation: When the CS was presented during extinction learning, PTSD subjects exhibited more amygdala and less vmPFC activation than trauma exposed normal controls (TENC), whereas during extinction recall, they displayed less activation in hippocampus and vmPFC, as well as more activation in dACC [27]. Data analysis of SCR following presentation of the CS showed a significant group × stimulus interaction during extinction recall. The TENC group displayed smaller SCRs to the CS‐stimulus which was extinguished previously during extinction learning in comparison to responses of the PTSD group suggesting that the recall of the extinction memory was impaired in the PTSD group. Moreover, we found that CS‐induced vmPFC and hippocampal activations were significantly correlated with the expression of the extinction memory during recall, as manifest in decreased SCRs.
The present analysis addresses the question of whether PTSD subjects show an altered pattern of brain activation even prior to the appearance of the CS in the above paradigm, that is, in response to the presentation of the context that precedes the cue. This question is of substantial interest in light of the impaired contextual signaling hypothesis of PTSD described above [7]. In order to address it, we conducted a novel analysis of the data set from the above study of Milad et al. [27]. We assessed brain responses to the visual contextual stimuli across the different phases of the experiment in PTSD versus TENC subjects. The fact that the CS is followed by the US during conditioning but not during extinction learning or recall creates a situation in which subjects have to cope with conflicting information, which means that they have to assess, whether or not the CS will be followed by a shock. In order to resolve this conflict, the subject should draw upon the context, that is, evaluate whether this is a context in which the CS is likely, or unlikely, to be followed by the US. We hypothesized that PTSD subjects would be deficient in their ability to retain and use this contextual information, and that this deficit would be reflected in altered activations in the brain regions reviewed above. We expected stronger hippocampal and vmPFC activations in TENC versus than PTSD subjects especially during the extinction recall phase, during which the nervous system should be preparing the activation of the extinction memory based upon the learning that had taken place the previous day. The presence of such activation would suggest ongoing contextual signaling to inhibit amygdala‐driven fear responses to the CS.
Methods
Subjects
Eighteen patients diagnosed with PTSD (9 males, 9 females) and 16 TENC subjects (7 males, 9 females) contributed data to the present study. This sample largely overlaps with the one studied in Milad et al. [27] but with three former subjects excluded on the basis of an updated motion correction algorithm and six subjects added as a result of ongoing recruitment.
All subjects participating completed the Clinician‐Administered PTSD Scale (CAPS [28]) and the Structured Clinical Interview for DSM‐IV Axis I Disorders (SCID [29]). TENC subjects with any Axis I psychiatric disorder were excluded. PTSD subjects using any psychotropic medication within 4 weeks of study participation (or within 1 year for neuroleptics) were excluded. Table 1 displays demographic and psychometric data. All study participants received a thorough explanation of the experimental procedure, and written consent was obtained in accordance with the requirements of the Partners Healthcare System Human Research Committee.
Table 1.
Group mean demographic and psychometric measures, types of trauma exposure, and psychiatric comorbidities
| Demographic and psychometric measures | |||
|---|---|---|---|
| PTSD | TENC | ||
| Age | 34.1 (±2.86) | 29.0 (±2.35) | P= 0.20 |
| Years of education | 14.9 (±0.50) | 15.9 (±0.63) | P= 0.28 |
| Mean age at trauma exposure | 14.9 (±3.04) | 18.0 (±2.16) | P= 0.48 |
| CAPS scores | 63.1 (±4.99) | 9.6 (±2.24) | P < 0.0001 |
| Type of trauma (numbers of subjects) | |||
| PTSD | TENC | ||
| Motor vehicle accidents | 1 | 2 | |
| Sexual assaults | 3 | 1 | |
| Physical assaults | 5 | 3 | |
| Child abuse | 6 | 2 | |
| Combat | 2 | 1 | |
| Witness to traumatic events | 4 | 6 | |
| Otherwise life threatening situation | 0 | 2 | |
| Current psychiatric comorbidities (numbers of subjects) | |||
| PTSD | TENC | ||
| Major depression | 3 | 0 | |
| Panic disorder | 1 | 0 | |
| Simple phobia | 1 | 0 | |
| Alcohol abuse | 1 | 0 | |
| Eating disorder | 2 | 0 | |
The number of types of trauma and comorbidities shown may exceed the number of subjects, because a subject may have had more than one comorbid disorder or type of traumatic event. Data in brackets indicate standard error of the mean (SEM).
Fear Conditioning, Extinction, and Testing Procedures
The experimental protocol was identical to that of previous studies described by Milad et al. [30]. Briefly, the US consisted of an electric shock to the fingers previously selected by the subject to be “highly annoying but not painful.” The US was applied only during the conditioning phase, where it followed the offset of the CS at a partial reinforcement rate of 60%. The CS consisted of the switch‐on of a colored light in a virtual lamp, which was positioned in the virtual context image. The shock electrodes remained attached to the subject's fingers during all phases, and subjects were instructed throughout the experiment that they “may or may not receive an electric shock.”
Day 1 consisted of a conditioning phase shortly followed by an extinction phase; day 2 consisted of an extinction recall phase. Either of two counterbalanced, distinct context images were displayed during each phase. One was a virtual library, the other a virtual office; one of which (A) was displayed during conditioning and the other (B) during extinction learning and recall. Because context A was presented during a phase in which shock was given, it constituted a “dangerous” context, whereas context B, which was displayed during phases in which there was no electric shock, constituted a “safe” context.
Each phase contained 32 trials, with an inter‐trial interval of 12 to 18 seconds. The context (A or B) was presented for 9 seconds, alone for 3 seconds, and then in combination with the CS for 6 seconds. Duration of context and CS presentation was not jittered in order to minimize behavioral variation of the effects of interest due to a varying predictability of the presented stimuli. Given that the SCR and fMRI responses to the CS have already been published [27] and that fMRI responses to the CS were treated as effects of no interest in the present analysis, this work focuses on data related to the context presentations only. Data from the 32 trials of each phase were blocked into the first 16 (early) trials and the last 16 (late) trials.
Psychophysiological Measures
SCR to each context trial was calculated by subtracting the mean SCR level during the 2 seconds prior to context onset from the highest SCR level during the subsequent 3‐second context display, that is, the period before the cue was presented. Student's t‐tests were performed to test for statistically significant group differences in each block.
Image Acquisition
The image acquisition parameters were identical to those in our previous studies [30]. Scans were performed using a Siemens Trio 3.0 Tesla whole body, high‐speed MRI system (Siemens Medical Systems, Iselin, NJ, USA) with an 12‐channel head coil. After acquisition of a scout image and automated shimming procedures, high resolution 3D MPRAGE sequences (TR/TE/flip angle = 7.25 ms/3 ms/7°; 1 × 1 mm in plane × 1.3 mm) were collected in the sagittal plane for spatial normalization and positioning of the subsequent scans. Functional MRI images of blood oxygenation level dependent (BOLD) contrast signal changes were collected using gradient echo T2*‐weighted echo planar imaging (EPI; TR/TE/flip angle = 3 second/30 ms/90°). The EPI images were acquired in 45 axial oblique slices tilted 30° to the anterior–posterior commissural line, with a slice thickness of 3 mm.
Functional MRI Data Analysis
For data preprocessing, first‐ and second‐level modeling and analyses, SPM8 software was used (http://www.fil.ion.ucl.ac.uk/spm/software/spm8). Imaging time series were examined for excessive motion artifacts using the Artifact Detection Tool (ART) software package. Outliers where identified by assessing between‐scan differences (Z‐ threshold: 3.0, scan to scan movement threshold 0.6 mm; rotation threshold: 0.004 radians; http://web.mit.edu/swg/art/art.pdf). Data from subjects with more than 10% outliers were excluded from further analyses (two TENC, one PTSD subject). Images from each functional run were slice‐timing corrected and realigned to the first image of the run. This procedure generated realignment parameters for each run that were later used as covariates of no interest in the first‐level model, as well as a mean image for each run. Then, the mean images from each run were aligned with the first image of each imaging run to facilitate transformation into MNI space. The subject's MPRAGE was then coregistered with the realigned mean image from the first run. Next, the MPRAGE was segmented and spatially normalized to the T1 MNI305 template included in SPM8 (Montreal Neurological Institute, MNI). The resulting spatial transformation parameters were then applied to the EPI time series in order to transform them to the common anatomical coordinate space (MNI305). Finally, to mitigate the effects of residual spatial transformation noise, the normalized functional images were smoothed using an 8 mm full‐width at half maximum Gaussian kernel.
For the first level, an epoch model was chosen with durations of 3 seconds for the context and of 6 seconds for the CS. The US during conditioning was modeled with a 0.5‐second duration. Modeled responses to the CS and the US were treated as effects of no interest. Each modeled regressor was convolved with the SPM canonical hemodynamic response function (HRF). A high pass filter (128 seconds) was included in the first‐level model to correct for low frequency signal drift.
Statistical parametric maps were calculated using the general linear model. In this analysis, the contrast of principal interest was the 3‐second duration of either the first or the last 16 context trials of each run compared to the implicit baseline. Contrast maps from the first level analysis were modeled in a second level mixed effects analysis using a 3‐way factorial design (factor 1: Subjects, modeled independently with equal variance; factor 2: Groups, PTSD or TENC, modeled independently with unequal variance; factor 3: Condition, that is, first 3 seconds of context presentation vs. baseline, modeled dependently with equal variance). Total degrees of freedom were 96. Group × Condition interactions were examined using voxel‐wise t‐statistic maps.
A Priori Brain Areas, Functional Regions of Interest (ROIs)
A priori brain areas examined consisted of the vmPFC, dACC, amygdala, and hippocampus. VmPFC was defined as the medial wall of the prefrontal cortex inferior to the genu of the corpus callosum, with z‐coordinates below 0 [31]. dACC was defined as ACC superior to the corpus callosum, with y coordinates between 0 and +30 mm [32] and z coordinates up to +50 mm [33]. Localization of activations in the amygdala and hippocampus were verified using the WFU pickatlas (Wake Forest University, School of Medicine Winston‐Salem, NC, USA). For these four areas, a significance threshold of P < 0.001 (one‐tailed, uncorrected) was applied, whereas for activations outside the a priori areas a more stringent threshold of P < 0.0001 was employed. Once significant main effects of Condition had been identified within the a priori brain areas, functional regions of interest (ROIs) were defined as clusters of activation with at least four contiguous voxels exceeding the aforementioned thresholds. Beta values were then extracted from these ROIs using Marsbar (http://marsbar.sourceforge.net). These values were then used to generate plots representing the beta values of the PTSD and TENC groups in the contrast of interest.
Results
Psychophysiology
No SCR group differences during context presentations were found in any experimental block. In general SCRs during context presentations were much lower than the previously reported SCRs in response to CS presentations within the same paradigm [27]. Thus, no additional analyses of the SCR data were performed.
BOLD Responses during Fear Conditioning
Table 2 presents all brain areas that showed activations or deactivations meeting the threshold criteria for the two parts of each of the three experimental runs.
Table 2.
Significant activations (+ sign) and deactivations (− sign) during the different phases of the experiment in a priori areas of interest (threshold for peak voxel P < 0.001, one‐tailed, uncorrected, Z = 3.09) and in other brain areas (threshold for peak voxel P < 0.0001, one‐tailed, uncorrected, Z = 3.72); number of voxels in clusters computed for threshold of P= 0.001. Contrast: PTSD +/− TENC, context versus baseline.
| Area of activation | MNI coordinates | Number of voxels | Z value | P value | ||
|---|---|---|---|---|---|---|
| x | Y | z | ||||
| Conditioning early | ||||||
| A priori areas | ||||||
| None | ||||||
| Areas outside the a priori areas | ||||||
| Occipital cortex (+) | −36 | −62 | −8 | 217 | 4.19 | <0.0001 |
| Conditioning late | ||||||
| A priori areas | ||||||
| dACC/SFG (+) | −12 | 26 | 50 | 54 | 4.05 | <0.0001 |
| Areas outside the a priori areas | ||||||
| Frontal cortex, middle frontal gyrus (+) | 42 | 4 | 52 | 158 | 4.31 | <0.0001 |
| Posterior cingulate gyrus (+) | −14 | −32 | 32 | 76 | 4.23 | <0.0001 |
| Extinction learning early | ||||||
| A priori areas | ||||||
| dACC (+) | 10 | 22 | 48 | 56 | 3.84 | 0.0001 |
| Areas outside the a priori areas | ||||||
| None | ||||||
| Extinction learning late | ||||||
| A priori areas | ||||||
| vmPFC (–) | −6 | 20 | −6 | 176 | 4.07 | <0.0001 |
| Areas outside the a priori areas | ||||||
| Frontal cortex, ant. medial part (–) | 14 | 62 | 14 | 156 | 4.37 | <0.0001 |
| Occipital cortex and parahippocampal gyrus (–) | 18 | −74 | 14 | 349 | 4.17 | <0.0001 |
| Occipital cortex (–) | 46 | −68 | −4 | 182 | 4.16 | <0.0001 |
| Extinction recall early | ||||||
| A priori areas | ||||||
| dACC (+) | 4 | 22 | 44 | 42 | 3.49 | 0.0002 |
| vmPFC (–) | 2 | 36 | −14 | 66 | 3.62 | 0.0001 |
| Areas outside the a priori areas | ||||||
| Frontal cortex, middle frontal gyrus (+) | −42 | 6 | 32 | 131 | 4.11 | <0.0001 |
| Extinction recall late | ||||||
| A priori areas and areas outside the a priori areas | ||||||
| None | ||||||
During early conditioning, there were no significant group activation differences in any a priori area. During late conditioning, PTSD subjects showed significantly greater dACC activation than TENCs (Figure 1). This cluster was adjacent to the superior frontal gyrus (SFG). Analysis of the corresponding beta values revealed that this group difference is in fact attributable to the PTSD subjects’ failure to deactivate dACC, whereas a deactivation was seen in TENCs.
Figure 1.
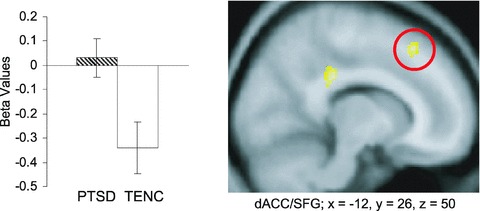
Conditioning: dACC/SFG activation during the last 16 (late) context presentations. Graph shows comparison of beta values in relation to baseline activity of the two groups (error bars indicate SEM). Numbers following x, y, and z refer to MNI coordinates. Figure shows map of brain activation at a threshold of P= 0.0001 displayed on a group averaged T1‐template. Yellow = increased activation in PTSD versus TENC subjects. Red circle indicates dACC.
Bold Responses during Extinction Learning
During early extinction learning, greater dACC activation was observed in PTSD versus TENC subjects (Figure 2A), with the difference attributable to dACC activation in the PTSD group in contrast to slight deactivation in the TENC group. During late extinction, there was lesser vmPFC activation in PTSD than in TENC subjects (Figure 2B), attributable to PTSD subjects’ failure to activate the vmPFC in contrast to activation in the TENC group.
Figure 2.
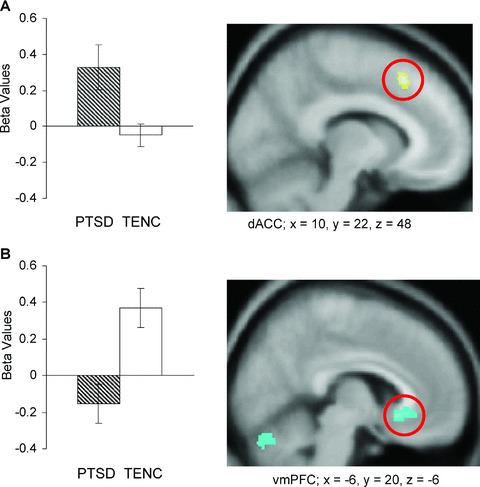
Extinction learning: A. dACC activation during the first 16 (early) context presentations. B. vmPFC deactivation during the last 16 (late) context presentations. Same description of plots and figures as in Figure 1. Yellow = increased activation, blue = decreased activation in PTSD versus TENC subjects. Red circles indicate dACC and vmPFC.
Bold Responses during Extinction Recall
During early extinction recall, both greater dACC activation and lesser vmPFC activation were observed in PTSD versus TENC subjects (Figure 3). The plots show that the dACC group difference was due to activation in the PTSD group but not in the TENC group (Figure 3A), whereas the vmPFC group difference was due to deactivation in the PTSD group but activation in the TENC group (Figure 3B). There were no significant group activation differences during late extinction recall.
Figure 3.
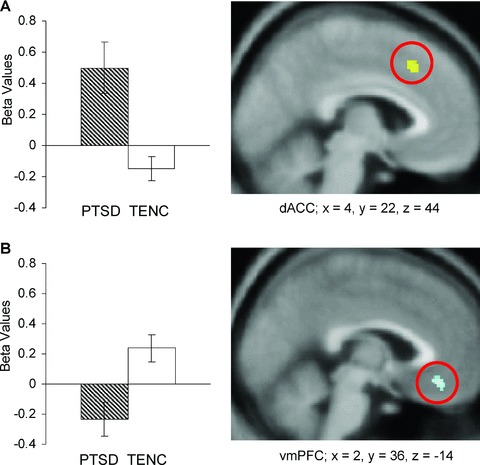
Extinction recall: A. dACC activation and B. vmPFC deactivation during context presentation for the first 16 (early) context presentations. Same description of plots and figures as in Figure 1. Red circles indicate dACC and vmPFC.
Discussion
The present analysis revealed a consistent pattern of activation differences in response to contextual information between study groups involving regions in the medial wall of the prefrontal cortex. Relative to trauma‐exposed but psychiatrically healthy subjects, PTSD subjects showed deactivation of the vmPFC and increased activation in the dACC. These between‐group differences were most pronounced in the early extinction recall phase (which occurred after both conditioning and extinction learning had taken place), during which contextual information is needed to guide the choice of a response to the now‐ambiguous CS. Thus, in addition to the previous finding of dysfunctional responses to extinguished cues in PTSD subjects [27], our current analysis reveals that the function of these structures is also impaired even during the processing of contextual information prior to cue presentation.
Our results concerning the vmPFC seem to be in line with literature describing the reactivity of this structure to conditioned cues. The vmPFC is known to inhibit fear responses by exerting top‐down control over the amygdala [20, 21, 34], and persons with PTSD have been found to be deficient in this regard [35, 36]. What is noteworthy from the present analysis is that deficiencies in vmPFC activation (and exaggerated dACC activation discussed below) are evident even in the absence of an autonomous fear response. In contrast to the significant group and stimulus interactions of the SCRs found in response to the CS in these same subjects [27], SCRs in response to the preceding context presentations were weak and no group differences were observed. This is consistent with low fear, thereby suggesting that the role of the vmPFC extends beyond merely inhibiting the fear response. In the paradigm employed here, we propose that vmPFC activation also corresponds to recognition of a safe context. In conjunction with the data presented by Milad et al. [27] our data suggest the following: whereas identification of the context sets the stage for the selection of a specific memory association, the actual expression or suppression of the fear reaction as represented by SCR depends on the occurrence of the CS. Therefore, the lack of vmPFC activation during both late extinction learning and early extinction recall in PTSD subjects may be reflecting failure to learn, or retain learning, that a context in which shock does not occur is safe.
A surprising finding in the present study is the extent of dACC involvement in signaling contextual information throughout the different phases. The dACC has been implicated in processes such as reward, pain, uncertainty, and cognitive interference [37, 38, 39, 40, 41]. It has also been shown that activity of the dACC, which projects to the amygdala [42], correlates positively with the expression of fear in healthy humans [43]. There is emerging evidence that dACC mediates the exaggerated signaling of threat commonly found in people with anxiety disorders such as PTSD [44, 45]. Our finding of reduced dACC deactivation during late conditioning in PTSD subjects may indicate sustained dACC reactivity in this group. This interpretation is supported by the observation that in rodents the prelimibic cortex (PL, which is a purported homologue of the dACC in humans) mediates the expression of a learned fear response during conditioning [46]. Furthermore, the persistence of PL responses after extinction training is associated with failure to express extinction memory [23]. Thus, the increased dACC activity observed in PTSD subjects during extinction learning and recall may be related to failure to learn, or retain learning, that a new context in which shock is no longer presented is not dangerous. Interestingly, dACC activation in response to contextual stimuli was situated more dorsally and cranially than the dACC activation observed in response to cue stimuli in the same paradigm, using virtually the same sample [27]. This difference in localization may be due to the fact that different types of stimuli were processed leading to different psychophysiological outcomes (weak SCR to contextual stimuli, strong SCR toward cue stimuli).
Limitations of this study mainly pertain to the small sample sizes. It is possible that significant between‐group differences in hippocampal and amygdala activation would have emerged had larger sample sizes been studied. The same limitation applies to the SCR finding: a higher number of subjects and greater statistical power might have resulted in a significant group difference between PTSD and TENC subjects in response to the context presentation. Also, a longer duration of the context presentation might have allowed unveiling group differences as the SCR typically has a latency of 2–3 seconds after the sympathetic activation occurs.
In conclusion, the main finding consists of a pattern of vmPFC hyporeactivity and dACC hyperreactivity to contexts signaling either safety or danger in persons with PTSD which is manifest even prior to the fear response. The former may be interpreted as conveying a lesser ability to make use of safety information, and the latter as conveying a greater ability to make use of danger information in this disorder, thereby representing opposite sides of a coin. This interpretation appears in line with the concept proposed by Liberzon and colleagues of a “contextualization network” situated in the medial wall of the prefrontal cortex that is aberrant in PTSD [7]. Those authors suggest that the structures within this network (such as the dACC and vmPFC) are not merely involved in threat‐related signaling but further play a crucial role in the appropriate discrimination of a large variety of stimuli. This discrimination would enable appropriate assessment of each stimulus with regard to its cognitive, social, and internal context—allowing the individual to express an optimally adaptive behavioral response. With regard to PTSD, impaired functioning of this network could result in such symptoms as the often‐encountered expression of trauma‐related memories and affects outside the traumatic context, and to the symptom of emotional numbing, which might be understood as an inability to experience various affective states in accordance with their relevant contexts.
Conflict of Interest
Ansgar Rougemont‐Bücking: No conflict to report.
Clas Linnman: No conflict to report.
Thomas A. Zeffiro: No conflict to report.
Mohamed A. Zeidan: No conflict to report.
Kelimer Lebron‐Milad: No conflict to report.
Jose Rodriguez‐Romaguera: No conflict to report.
Scott L. Rauch: Dr. Rauch has received honoraria and/or consultation fees from Neurogen, Sepracor, Novartis and Medtronic. He has also participated in research funded by Medtronic, Cyberonics, Cephalon, and Northstar.
Roger K. Pitman: No conflict to report.
Mohammed R. Milad: No conflict to report.
Author Contributions
Ansgar Rougemont‐Bücking: data analysis, writing; Clas Linnman: data collection, data analysis, review of the manuscript; Thomas A. Zeffiro: monitoring of data analysis, review of the manuscript; Mohamed A. Zeidan: review of the manuscript; Kelimer Lebron‐Milad: data analysis; Jose Rodriguez‐Romaguera: data analysis, review of the manuscript; Scott L. Rauch: obtaining grant, design of study paradigm, review of the manuscript; Roger K. Pitman: scientific editing of the manuscript; Mohammed R. Milad: design of study paradigm, data collection, data analysis, review of the manuscript.
Acknowledgments
This study was funded by a grant from the National Institute of Mental Health (1R21MH072156‐1) to Scott L. Rauch and a Young Investigator Award from NARSAD to Mohammed R. Milad. Ansgar Rougemont‐Bücking received funding from the Centre Hospitalier Universitaire Vaudois and University of Lausanne, Switzerland, as well as a grant from the Société Académique Vaudoise, Lausanne, Switzerland. Clas Linnman received funding from the Sweden‐America foundation. Jose Rodriguez‐Romaguera received funding from the Latin American Network for the Training and Exchange of Researchers in Neuroscience (LANTERN) and the Summer Research Training Program (SRTP) from the Multicultural Affairs Office from Massachusetts General Hospital. The authors wish to thank Jacques Besson for institutional support to the first author, as well as Scott P. Orr and Gregory J. Quirk for helpful discussion on data interpretation.
References
- 1. Bouton ME, King DA. Contextual control of the extinction of conditioned fear: Tests for the associative value of the context. J Exp Psychol Anim Behav Process 1983;9:248–265. [PubMed] [Google Scholar]
- 2. Harris JA, Jones ML, Bailey GK, Westbrook RF. Contextual control over conditioned responding in an extinction paradigm. J Exp Psychol Anim Behav Process 2000;26:174–185. [DOI] [PubMed] [Google Scholar]
- 3. Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biol Psychiatry 2002;52:976–986. [DOI] [PubMed] [Google Scholar]
- 4. Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biol Psychiatry 2006;60:352–360. [DOI] [PubMed] [Google Scholar]
- 5. Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology 2005;42:456–464. [DOI] [PubMed] [Google Scholar]
- 6. Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychol (Amst) 2008;127:567–580. [DOI] [PubMed] [Google Scholar]
- 7. Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: A critical review. Prog Brain Res 2008;167:151–169. [DOI] [PubMed] [Google Scholar]
- 8. Holt W, Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. J Neurosci 1999;19:9054–9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus 2007;17:749–758. [DOI] [PubMed] [Google Scholar]
- 10. LaBar KS, Phelps EA. Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behav Neurosci 2005;119:677–686. [DOI] [PubMed] [Google Scholar]
- 11. Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci 2005;25:8978–8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maren S, Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav Brain Res 2000;110:97–108. [DOI] [PubMed] [Google Scholar]
- 13. Hobin JA, Goosens KA, Maren S. Context‐dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci 2003;23:8410–8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron 2009;62:757–771. [DOI] [PubMed] [Google Scholar]
- 15. Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 2008;33:56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barad M, Gean PW, Lutz B. The role of the amygdala in the extinction of conditioned fear. Biol Psychiatry 2006;60:322–328. [DOI] [PubMed] [Google Scholar]
- 17. Conde F, Maire‐Lepoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol 1995;352:567–593. [DOI] [PubMed] [Google Scholar]
- 18. Ishikawa A, Nakamura S. Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex in the rat. J Neurosci 2003;23:9987–9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris‐leucoagglutinin. J Comp Neurol 1991;313:574–586. [DOI] [PubMed] [Google Scholar]
- 20. Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci 2002;22:324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 2003;23:8800–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 2002;420:70–74. [DOI] [PubMed] [Google Scholar]
- 23. Burgos‐Robles A, Vidal‐Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci 2009;29:8474–8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biol Psychol 2006;73:61–71. [DOI] [PubMed] [Google Scholar]
- 25. Sotres‐Bayon F, Bush DE, LeDoux JE. Emotional perseveration: An update on prefrontal‐amygdala interactions in fear extinction. Learn Mem 2004;11:525–535. [DOI] [PubMed] [Google Scholar]
- 26. Corcoran KA, Quirk GJ. Recalling safety: Cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectr 2007;12:200–206. [DOI] [PubMed] [Google Scholar]
- 27. Milad MR, Pitman RK, Ellis CB, et al Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 2009;66:1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weathers FW, Keane TM, Davidson JR. Clinician‐administered PTSD scale: A review of the first ten years of research. Depress Anxiety 2001;13:132–156. [DOI] [PubMed] [Google Scholar]
- 29. First MB, Spitzer RL, Gibbon M, Williams GB. Structured Clinical Interview for DSM‐IV. New York: Biometrics Research, 1995. [Google Scholar]
- 30. Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 2007;62:446–454. [DOI] [PubMed] [Google Scholar]
- 31. Shin LM, Whalen PJ, Pitman RK, et al An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry 2001;50:932–942. [DOI] [PubMed] [Google Scholar]
- 32. Bush G, Vogt BA, Holmes J, et al Dorsal anterior cingulate cortex: A role in reward‐based decision making. Proc Natl Acad Sci USA 2002;99:523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shin LM, Bush G, Whalen PJ, et al Dorsal anterior cingulate function in posttraumatic stress disorder. J Trauma Stress 2007;20:701–712. [DOI] [PubMed] [Google Scholar]
- 34. Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Ann N Y Acad Sci 2007;1121:546–561. [DOI] [PubMed] [Google Scholar]
- 35. Heim C, Nemeroff CB. Neurobiology of posttraumatic stress disorder. CNS Spectr 2009;14:13–24. [PubMed] [Google Scholar]
- 36. Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat‐related posttraumatic stress disorder. Biol Psychiatry 2008;63:550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting Stroop: An interference task specialized for functional neuroimaging–validation study with functional MRI. Hum Brain Mapp 1998;6:270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci 2008;11:389–397. [DOI] [PubMed] [Google Scholar]
- 39. Doya K. Modulators of decision making. Nat Neurosci 2008;11:410–416. [DOI] [PubMed] [Google Scholar]
- 40. Holroyd CB, Coles MG. Dorsal anterior cingulate cortex integrates reinforcement history to guide voluntary behavior. Cortex 2008;44:548–559. [DOI] [PubMed] [Google Scholar]
- 41. Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 2005;6:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brinley‐Reed M, Mascagni F, McDonald AJ. Synaptology of prefrontal cortical projections to the basolateral amygdala: An electron microscopic study in the rat. Neurosci Lett 1995;202:45–48. [DOI] [PubMed] [Google Scholar]
- 43. Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry 2007;62:1191–1194. [DOI] [PubMed] [Google Scholar]
- 44. Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010;35:169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bryant RA, Felmingham KL, Kemp AH, et al Neural networks of information processing in posttraumatic stress disorder: A functional magnetic resonance imaging study. Biol Psychiatry 2005;58:111–118. [DOI] [PubMed] [Google Scholar]
- 46. Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci 2007;27:840–844. [DOI] [PMC free article] [PubMed] [Google Scholar]


