SUMMARY
Naltrexone is an opioid receptor antagonist that blocks the reinforcing effects of opioids and reduces alcohol consumption and craving. It has no abuse potential, mild and transient side effects, and thus appears an ideal pharmacotherapy for opioid dependence. Its effectiveness in alcohol dependence is less evident, but compliance with naltrexone combined with psychosocial support has been repeatedly shown to improve drinking outcomes. Limited compliance with oral naltrexone treatment is a known drawback. Several naltrexone implant and injectable depot formulations are being investigated and provide naltrexone release for at least 1 month. Studies among opioid‐dependent patients indicate significant reductions in heroin use, but sample sizes are usually small. In alcohol dependence, two large multicenter trials report alcohol and craving reductions for naltrexone and placebo groups, indicating a significant but moderate effect. The pharmacokinetic profile of the injectable formulation indicates reliable naltrexone release over 1 month at therapeutic levels. Implant formulations releasing naltrexone up to 7 months are reported. Findings on safety and tolerability confirm the generally mild adverse effects described for naltrexone tablets. However, further research on therapeutic levels (i.e., opioid blocking) is warranted. The majority of naltrexone implants lacks approval for regular clinical use and larger longitudinal studies are needed. The available naltrexone depot formulations have the potential to significantly improve medication compliance in opioid and alcohol dependence. In certain circumstances, they may constitute a promising new treatment option.
Keywords: Alcoholism, Naltrexone, Opioid dependence, Systematic review
Introduction
The endogenous opioid system has several important regulatory functions. The three main opioid receptor (OR) subtypes (μ, γ, and δ) modulate the transmission of dopaminergic, noradrenergic, and serotonergic neurons. Consequently, endogenous opioids influence the motivational, the stress‐regulating, and the mood‐regulating systems [1]. The motivational system and the neurotransmitter dopamine have primary importance in substance dependence. Behaviors that increase dopamine levels such as the use of psychoactive substances will be linked to wanting and liking. Repeated stimulation with psychoactive substances is followed by neuroadaptation that may lead to a decrease in liking and an increase in wanting[2]. These changes are believed to be maintained by substance induced genetic rearrangements of cell functioning, and appear to take a major part in maintaining addictive behavior, that is, the individual's impaired ability to regulate or limit the use of the psychoactive substance [3].
An ideal pharmacotherapy to prevent and treat substance dependence would lie in blocking the reinforcing, agonistic effects of the substance of abuse. Relapse to substance use would not have a reinforcing effect. Continuously blocking the receptors might even allow reversal of the cellular adaptations and reinstatement of normal functioning. For decades, several antagonist pharmacotherapies for drug dependence have been investigated. The primary focus has been the impaired OR system as hypothesized for opioid dependence and this rationale has resulted in extensive research on OR antagonists [4]. Naltrexone, developed in the 1970s by National Institute on Drug Abuse (NIDA), has been the most widely investigated opioid antagonist and its metabolism is well characterized [5, 6, 7]. It is a competitive, nonselective, specific OR antagonist with highest affinity at the μ‐receptor [8].
Naltrexone has been shown to effectively block heroin effects in humans [9, 10]. Plasma levels of 1–2 ng/mL are suggested to block clinically relevant doses (e.g., 25 mg) of intravenously administered heroin (diacetylmorphine), but this issue needs further investigation [7, 11]. Naltrexone has been found to reduce craving for alcohol in rhesus monkeys [12] and among alcohol‐dependent patients [13]. In amphetamine dependence, reduced craving during naltrexone treatment has recently been reported [14, 15]. The neurobiological mechanisms by which naltrexone reduces craving are not fully understood, but two main hypotheses have emerged. Since the effects of alcohol and amphetamine in the motivational system are partly mediated through ORs [16, 17], the receptor blocking with naltrexone could explain the reduction in craving. In alcohol‐dependent subjects, naltrexone has been found to augment plasma cortisol and adrenocorticotropic hormone (ACTH) levels through activation of the hypothalamic–pituitary–adrenal (HPA)‐axis, which is found to be related to reduced urge and amount of alcohol drinking [18].
Limited compliance with oral naltrexone in opioid and alcohol‐dependent patients is described as the main drawback [19, 20]. Two strategies seem promising in order to secure long‐term naltrexone treatment [21]. One is contingency management [22] and the other is naltrexone depot formulations [23]. Achieving good tolerability in conjunction with sufficient naltrexone release (above 1–2 ng/mL) over at least 4 weeks has been a major challenge [23, 24]. Recently, an injectable, once‐a‐month depot (Vivitrol®, Alkermes, Waltham Massachusetts, USA) has been Food and Drug Administration (FDA)‐approved for the treatment of alcoholism and it is under FDA review to receive approval for opioid dependence. Naltrexone implants with 3–6 months duration are also being investigated for the treatment of opioid dependence.
This article systematically reviews the literature on naltrexone depot or implant treatment for alcohol and opioid dependence. Apart from treatment effect outcomes such as reduction in heroin use and alcohol consumption, data on safety and tolerability were considered. Also, the pharmacokinetic (PK) characteristics of the various formulations are described.
Literature Search
A systematic search of seven electronic databases from their inception to December 2009 was performed: CINAHL, The Cochrane Library, EMBASE, ISI Web of Knowledge, LILACS, MEDLINE, and PsycINFO. The search strategy was developed in cooperation with the Cochrane Drugs and Alcohol Group, and the first search was performed in November 2007. The following terms were used for the MEDLINE and EMBASE search: (1) naltrexone.mp; (2) exp sustained release preparation/; (3) exp sustained drug release/; (4) implant$.ab,ti; (5) depot$.ab,ti; (6) (delay$ adj2 action).ti,ab; (7) 2 or 3 or 4 or 5 or 6; (8) 1 and 7; (9) limit 8 to human. The review including the search strategy in detail for each database is available online [25]. The search was updated in December 2009, and the inclusion criteria were extended to any report on the use of naltrexone depot formulations for opioid or alcohol dependence. Besides randomized controlled trials, longitudinal studies with or without a control condition and case reports were included. Excluded were review articles, comments, letters, editorials, press releases, and abstracts from conference proceedings unless previously unpublished data was reported or a full text report was available. Two authors independently assessed all potentially eligible reports. Disagreement was resolved by discussion, if necessary with a third author. Figure 1 gives an overview on study identification and inclusion process. Table 1 briefly summarizes the included studies. Since several different study designs, types of depot formulations, and patient populations were included, the methodological heterogeneity among the studies was high. Therefore, meta‐analyses of pooled outcome measures across study samples were considered inappropriate and not performed.
Figure 1.
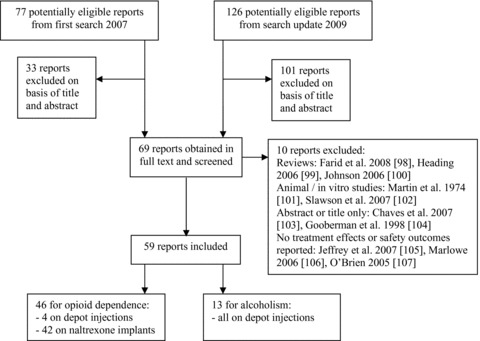
Study identification process from the systematic literature search in November 2007 and the update performed in December 2009.
Table 1.
Included reports on treatment effects and tolerability by first author and year of publication
| Randomized‐controlled trials | Target population (n) and comparison group | Type of naltrexone depot formulation | Main findings for the investigated formulation |
|---|---|---|---|
| Kranzler 2004 | Alcohol‐dependent (315), placebo‐controlled | Monthly injectable during 3 months follow‐up | Reduced drinking and greater abstinence rate |
| Garbutt 2005 | Alcohol‐dependent (624), placebo‐controlled | Monthly injectable during 6 months follow‐up | Reduced heavy drinking |
| Comer 2006 | Opioid‐dependent (60), placebo‐controlled | Monthly injectable during 2 months follow‐up | Reduced heroin use and more time spent in treatment |
| Hulse 2009 | Opioid‐dependent (70), oral naltrexone | Three months implants | Reduced heroin relapse rates |
| Kunøe 2009 | Opioid‐dependent (56), usual‐treatment aftercare | Six months implants | Reduced heroin use |
| Other effect studies | Condition/intervention | Comparison group | Main outcomes |
| Ciraulo 2008, Lapham 2009, O’Malley 2007, Pettinati 2009 | Alcohol dependence/injectable naltrexone | Placebo‐controlled trial; same sample as in Garbutt 2005 | Quality of life and treatment response |
| Carreno 2003, Colquhoun 2005 | Opioid dependence/naltrexone implants | Oral naltrexone | Opioid use and time spent in treatment |
| Foster 2003, Gölz 2000, Grusser 2006, Hulse 2003a and b, Reece 2007 and 2009a, Waal 2003 and 2006 | Opioid dependence/naltrexone implants | Single group studies; some with a control group of convenience | Opioid use, feasibility in research and primary care settings, naltrexone blood levels |
| Hulse 2005, Ngo 2007 and 2008, Tait 2008a and b | Opioid dependence/naltrexone implants | Cohorts receiving naltrexone or methadone, database linkage design | Morbidity and mortality |
| Safety and tolerability | Condition/Intervention | Comparison group | Main outcomes |
| Lucey 2008 | Alcohol dependence/injectable naltrexone | Placebo‐controlled trial; same sample as in Garbutt 2005 | Hepatic safety |
| Brewer 2004, Gibson 2007, Hamilton 2002, He 2008 and 2009, Hulse 2008, Lintzeris 2008, Oliver 2005, Reece 2009b | Opioid dependence/naltrexone implants | None | Overdose death and other severe adverse events, hepatic safety, cognitive impairment, biodegradability |
| Brewer 2002, Fishman 2008, Kruptisky 2007, O’Brien 2006, Sullivan 2006 | Opioid dependence/naltrexone implants or injectables | None | Blockade overriding with opioid agonists |
| Hulse 2002a and b, 2003c, 2004c | Opioid dependence during pregnancy/naltrexone implants | None | Obstetric and neonatal |
Treatment Effects in Alcohol Dependence
Two multicenter, double‐blind, placebo‐controlled trials were conducted in the US investigating three different dosages of once‐a‐month injectable naltrexone [26, 27]. In both trials participants who received naltrexone or placebo injections reported reductions in alcohol consumption. These reductions were inconsistent in regard of treatment condition indicating a moderate effect size of depot naltrexone.
In the first trial with 315 participants over 3 months, the naltrexone (300 mg) group reported fewer days to the first drink and more abstinent days compared to placebo [26]. However, time to first heavy drinking day showed no difference between the groups. In both groups the γ‐ glutamyl transferase (GT) values improved throughout the study indicating clinically significant reductions in alcohol consumption. In the subsequent trial 624 alcohol‐dependent subjects received up to six monthly injections of naltrexone depot in two dosages (190 or 380 mg) or placebo [27]. The rate of heavy drinking days was found to be 25% lower in the 380‐mg group compared to placebo. However, naltrexone treatment did not show a significant advantage in terms of reduction in risky drinking (more than one or two drinks per day) or any drinking. Also, γ‐GT values showed no additional improvement in the naltrexone groups compared to placebo. Attrition rates did not differ across the three groups. Quick treatment response after the first 380‐mg naltrexone injection predicted a sustained reduction in alcohol consumption over the 6 months follow‐up [28]. In the subgroup that achieved abstinence before treatment start, the 380‐mg naltrexone group maintained abstinence significantly longer and reported a greater reduction in alcohol consumption and craving than the placebo group [29, 30]. In the full sample, quality of life showed improvement on mental health but not on physical health scores for the 380‐mg naltrexone group compared to placebo [31].
The reduction in alcohol drinking and craving that was found in both studies did not unequivocally support the advantages of the naltrexone injections compared to placebo. This finding is in line with inconsistent results from oral naltrexone studies [20, 32]. A possible explanation of the mixed results may be performance bias, that is, the participants in double‐blinded trials receiving a new, experimental pharmacotherapy may be more likely to report superior outcomes. Another explanation could lie in the psychosocial counseling that was given to placebo and naltrexone participants, which may have made it more difficult to detect a specific effect of the pharmacotherapy. Genetic polymorphism in the μ‐OR gene has been related to differential patient response to oral naltrexone treatment and a single transcription factor has been suggested to predict improved drinking outcomes [33, 34]. These findings may explain the mixed results and need to be replicated for depot formulations. Further, longitudinal studies of naltrexone depot formulations with or without psychosocial counseling for alcohol‐dependent outpatients are warranted and objective markers for alcohol consumption such as ethyl glucoronide should be used. Reasons for nonreturn to treatment should also be investigated considering gender, motivational, and tolerability aspects. Future research on naltrexone depot formulations need to assess the possible impact of reduced blood level variations on drinking outcomes and the possible benefits of receiving monthly (depot) versus daily (oral) medication to compliance, treatment retention, and dropout. Optimal naltrexone blood levels to reduce craving for alcohol need to be established.
Treatment Effects in Opioid Dependence
Findings from three randomized‐controlled trials suggest that patients receiving treatment with sustained‐release naltrexone use less opioids than patients who receive placebo injections [35], usual‐treatment aftercare [36], or oral naltrexone treatment [37]. This is consistent with findings from controlled trials [38, 39] and cohort studies or case reports [40, 41, 42, 43, 44, 45, 46, 47]. In a pilot study of abstinence 6 months postexpiry of naltrexone release, 8 of 12 naltrexone implant patients had remained abstinent [48].
A reduction in the number of hospital presentations of opioid‐dependent patients for physical as well as psychiatric reasons has been shown in several registry‐based studies [49, 50, 51, 52]. These reductions appear to be greater than those produced by treatment with opioid agonists like methadone or buprenorphine [53, 54, 55], although the absence of randomization means these differences could also be due to selection factors.
Retention in treatment is a main concern in the treatment of opioid dependence, as relapse to opioid use following a period of abstinence is associated with greatly elevated risk of overdose and overdose death [56]. Only one study has reported data on retention in naltrexone depot treatment: 32% of the opioid‐dependent patients discontinued treatment between the first and the second monthly naltrexone depot injection [35]. The sample receiving therapeutic dosages of naltrexone was small (n = 20), and no data on posttreatment overdoses were provided. One case study has documented that deaths and overdoses do occur following treatment discontinuation [57]. Preclinical literature indicates that naltrexone may cause OR upregulation [58, 59]. However, the significance of this finding for human samples is still unclear; longitudinal controlled studies that investigate overdose death rates in former naltrexone patients compared to other abstinent patients at risk of relapse are lacking. Before naltrexone induction, one case of relapse and subsequent fatal overdose has been reported in a patient who discharged himself from detoxification [36]. Autopsy studies have also identified cases of naltrexone implant patients who have died during and after ultra rapid opioid detoxification in outpatient settings [60], or experienced nonfatal adverse events during treatment or induction onto naltrexone [61, 62].
Self‐testing of the competitive antagonist blockade with heroin or other agonists may occur; two case studies reported patients who challenged the antagonist blockade with very high doses of opioids and experienced an agonist effect [63, 64]. As these case reports lack data on plasma levels of naltrexone, opioids, or other substances, it is unclear whether or not these findings are consistent with human laboratory studies reporting the effective blocking provided by naltrexone depot formulations [7, 11, 65].
For opioid dependence, treatment effect findings give reason for cautious optimism: several small studies, a few of which are randomized‐controlled trials (RCTs), suggest that treatment with naltrexone depot formulations leads to significant reductions in opioid use and improved secondary outcomes such as reduced hospital presentations for overdose or psychiatric reasons. Larger controlled trials comparing naltrexone depot formulations with naltrexone tablets or agonist maintenance treatment are needed to confirm these findings and to provide prospective data on safety and tolerability.
Mode of Administration
Naltrexone depot formulations are available as injectables or implants. The implants are surgically inserted into the subcutaneous tissue and release naltrexone for several months. They can be removed surgically upon patient request or if opioid analgesia becomes necessary. Apart from the Russian Federation, no country has approved regular use of naltrexone implants outside of research settings. The injectable formulation Vivitrol is FDA approved for alcohol dependence. It needs to be administered monthly to maintain sufficient naltrexone blood levels. Injectable naltrexone cannot be removed if opioid analgesia becomes necessary. However, the analgesic blockade provided by naltrexone is competitive and during general anesthesia it can be overridden with potent opioid agonists such as fentanyl. The clinical challenge of pain management during treatment with naltrexone depot formulations has been acknowledged [93]. The systematic search did not retrieve a report that directly compared injectable and implant formulations.
Injectable Depots: Development and PK Properties
Five studies investigated PK and safety outcomes among alcohol‐dependent subjects, one among healthy volunteers, and one among an opioid‐dependent sample. The injectable naltrexone depots were administered intramuscularly in the gluteal region in dosages ranging from 75 to 400 mg [66, 67, 68, 69, 70, 71, 72]. In these studies, three different pharmaceutical companies supplied injectables, which contained naltrexone loaded microspheres of poly‐lactide (PLA) or poly‐lactide‐co‐glycolide (PLA‐PGA) polymers.
The (PLA‐PGA) polymer formulation Vivitrol containing 380 mg of naltrexone has been agreed upon and received FDA approval for treatment of alcohol dependence in April 2006. This formulation releases naltrexone at levels above 1 ng/mL for about 4–5 weeks [71] and findings from alcohol‐ and opioid‐dependent patients suggest that it is not necessary to adjust the dosage to weight, age, gender, or health status [70].
Implants: Development and PK Properties
Although several types of naltrexone implants are commercially available and used in private clinic settings, usually for opioid dependence, our systematic literature search retrieved PK data on only three formulations, with the most extensive data on an Australian‐produced implant (O'Neil Implant®, Go Medical Industries, Perth, Australia) [48, 73, 74]. This formulation consists of 10 pellets containing a poly‐lactic‐based polymer and naltrexone in dosages ranging from 1.1 to 1.8 g. The single, 10‐pellets implant is found to release naltrexone above 1–2 ng/mL for about 3 months. It is surgically inserted, usually in the subcutaneous tissue of the lower abdominal wall. Use of the double implant with 20 pellets extends the period of naltrexone release to approximately 5–6 months. A comparable triple implant containing 3.3 g of naltrexone has been found to provide blood levels above 1–2 ng/mL for up to 7 months.
An implant formulation containing 1 g of naltrexone compounded with magnesium stearate (Wedgewood Implant®; Wedgewood Pharmacy, Sewell, NJ, USA) was found to release naltrexone at levels above 1 ng/mL during 30–80 days, indicating significant interindividual variation [75]. A case report from this type of implant suggests blocking levels at 5 ng/mL about 3 weeks after surgical insertion [65]. Another type of implant containing 1 g of naltrexone (Prodetoxon®, Fidelity Capital, Moscow, Russia) has been approved for the treatment of opioid dependence in the Russian Federation. The PK data suggest naltrexone levels above 20 ng/mL despite considerable interindividual variation [76].
Safety and Tolerability
General adverse effects such as nausea, vomiting, and muscle twitches show a high degree of consistency between depot formulations and oral naltrexone [77, 78]. Most adverse affects have been found to be transient and mild to moderate [35, 37]. They were more likely in patients receiving depot injections compared to placebo [26, 27]. Two Chinese studies reported that naltrexone implants do not negatively affect measures of cognitive functioning [79, 80]. The lower plasma concentrations and fewer peaks in the PK profile of naltrexone depot formulations may reduce the intensity of adverse effects, but direct comparisons of safety outcomes between implant or depot formulations and tablets are still lacking. Depressive mood related to oral naltrexone treatment has been hypothesized [81]. However, several subsequent reports did not support depression induced by naltrexone tablets [82, 83]. On the contrary, depressive symptoms were found to improve among patients who stayed in treatment [84]. This is in line with the findings from one study among heroin‐dependent outpatients that did not report deterioration in depression scores during naltrexone implant treatment [38].
Site‐related adverse effects such as pain, induration, infection, and allergic tissue reaction may occur when administering naltrexone depots or implants. In studies of injectable naltrexone, these reactions appear more common among patients receiving naltrexone than placebo injections [26, 27, 35, 67]. In line with these findings, the FDA has issued an alert for Vivitrol concerning increased risk of administration site reactions. Data on site‐related adverse effects of naltrexone implants are scarce, but single group studies indicate a low rate of site‐related adverse effects [48, 85]. The Australian implant formulation was found to biodegrade completely during a period of up to 3.3 years [86] and this implant was well tolerated in a prospective study [37]. In naltrexone implant treatment, attempts at self‐removal of naltrexone pellets can cause wounds, infections, and scarring. Although no incident has been reported, attempted self‐removal cannot be ruled out as a possible cause of two ruptured implant sites [36].
Hepatic impairment is common among alcohol‐ and opioid‐dependent populations. Excessive alcohol consumption is considered the leading cause of liver cirrhosis in the western world [87]. Up to 35% of heavy drinkers suffer from alcoholic hepatitis and of those about 70% will subsequently develop cirrhosis [88]. Among injecting drug users, high prevalence (up to 94%) of hepatitis C virus (HCV) infection [89] is likely to cause liver cirrhosis in up to 30% of the infected individuals [90]. Since impaired liver functioning is common among the target populations for naltrexone treatment, possibly reduced hepatic capacity to metabolize naltrexone needs to be taken into account. Naltrexone depot has been investigated in patients with alcohol‐induced liver impairment [68]. Findings on plasma levels did not show significant deterioration. Liver enzyme tests had improved during follow‐up, supposedly due to reduction in alcohol drinking. Adverse effects such as headache were generally rated mild and nonspecific; these occurred more frequently among patients with alcoholic liver disease compared to healthy controls. The same depot injection was found to have good hepatic tolerability in a large alcohol‐dependent sample during 6 months of treatment [91]. Another depot injection showed comparable improvements in terms of reduced γ‐GT levels in a placebo‐controlled trial [26]. A case report from an opioid‐dependent patient presenting with acute hepatitis during treatment with a 1‐g naltrexone implant suggests that naltrexone did not affect the patient's recovery [92].
Pain management during treatment with sustained‐release naltrexone may become a challenge in an outpatient setting, because analgesia with oral opioids such as codeine is not feasible. Patient cases are reported where nonopioid analgesics or a regional nerve blockade were used and provided effective analgesia [93].
Several cases of naltrexone implant treatment among opioid‐dependent pregnant women are reported [94, 95, 96, 97]. The obstetric and neonatal data were found to be relatively unproblematic despite the rapid detoxification procedures that occasionally were performed during pregnancy. A post hoc comparison between methadone‐maintained and naltrexone‐implanted women suggests no clinically significant differences in obstetric and neonatal outcomes.
Conclusions
Naltrexone depot formulations have been developed to increase the well‐known low compliance with oral naltrexone. Currently available naltrexone injectables and implants have been shown to significantly reduce heroin use and alcohol consumption in patient populations. Several formulations with overall good tolerability release naltrexone above the suggested therapeutic plasma levels for between 1 and 7 months. Further research is required on the level of naltrexone needed to effectively block clinically relevant heroin doses. The duration of naltrexone release at blocking levels from injectable and implant formulations is crucial, because naltrexone promotes abstinence from opioids and the risk of death from opioid overdose is increased upon relapse after prolonged abstinence. Longitudinal multicenter studies that compare naltrexone depot formulations with agonist maintenance treatment or oral naltrexone in opioid dependence are still lacking. In alcohol dependence, the neurobiological mechanisms that reduce craving and alcohol consumption are not fully understood. However, alcohol‐dependent patients benefit from treatment with naltrexone injectables whereas longer acting naltrexone implants have not been investigated. An injectable naltrexone formulation is FDA approved for alcohol dependence. The majority of naltrexone implant formulations still lack approval for regular clinical use; more data on safety and tolerability are needed. Naltrexone depot formulations constitute an interesting development in delivering pharmacotherapy to selected opioid‐ or alcohol‐dependent patients.
Conflict of Interest
The authors have no conflict of interest.
References
- 1. Koob GF, LeMoal M. Addiction and the brain antireward system. Annu Rev Psychol 2008;59:29–53. [DOI] [PubMed] [Google Scholar]
- 2. Robinson TE, Berridge KC. The incentive sensitization theory of addiction: Some current issues. Philos Trans R Soc Lond B Biol Sci 2008;363:3137–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nestler EJ. Transcriptional mechanisms of addiction: Role of DeltaFosB. Philos Trans R Soc Lond B Biol Sci 2008;363:3245–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin WR, Gorodetzky CW, McClane TK. An experimental study in the treatment of narcotic addicts with cyclazocine. Clin Pharmacol Ther 1966;7:455–465. [DOI] [PubMed] [Google Scholar]
- 5. Cone EJ, Gorodetzky CW, Yeh SY. The urinary excretion profile of naltrexone and metabolites in man. Drug Metab Dispos 1974;2:506–512. [PubMed] [Google Scholar]
- 6. Lee MC, Wagner HN Jr, Tanada S, Frost JJ, Bice AN, Dannals RF. Duration of occupancy of opiate receptors by naltrexone. J Nucl Med 1988;29:1207–1211. [PubMed] [Google Scholar]
- 7. Verebey K, Volavka J, Mule SJ, Resnick RB. Naltrexone: Disposition, metabolism, and effects after acute and chronic dosing. Clin Pharmacol Ther 1976;20:315–328. [DOI] [PubMed] [Google Scholar]
- 8. Gonzalez JP, Brogden RN. Naltrexone. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of opioid dependence. Drugs 1988;35:192–213. [DOI] [PubMed] [Google Scholar]
- 9. Resnick RB, Volavka J, Freedman AM, Thomas M. Studies of EN‐1639A (naltrexone): A new narcotic antagonist. Am J Psychiatry 1974;131:646–650. [DOI] [PubMed] [Google Scholar]
- 10. Volavka J, Resnick RB, Kestenbaum RS, Freedman AM. Short‐term effects of naltrexone in 155 heroin ex‐addicts. Biol Psychiatry 1976;11:679–685. [PubMed] [Google Scholar]
- 11. Sullivan MA, Vosburg SK, Comer SD. Depot naltrexone: Antagonism of the reinforcing, subjective, and physiological effects of heroin. Psychopharmacology 2006;189:37–46. [DOI] [PubMed] [Google Scholar]
- 12. Altshuler HL, Phillips PE, Feinhandler DA. Alteration of ethanol self‐administration by naltrexone. Life Sci 1980;26:679–688. [DOI] [PubMed] [Google Scholar]
- 13. Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry 1992;49:876–880. [DOI] [PubMed] [Google Scholar]
- 14. Jayaram‐Lindstrom N, Konstenius M, Eksborg S, Beck O, Hammarberg A, Franck J. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology 2008;33:1856–1863. [DOI] [PubMed] [Google Scholar]
- 15. Jayaram‐Lindstrom N, Wennberg P, Hurd YL, Franck J. Effects of naltrexone on the subjective response to amphetamine in healthy volunteers. J Clin Psychopharmacol 2004;24:665–669. [DOI] [PubMed] [Google Scholar]
- 16. Widdowson PS, Holman RB. Ethanol‐induced increase in endogenous dopamine release may involve endogenous opiates. J Neurochem 1992;59:157–163. [DOI] [PubMed] [Google Scholar]
- 17. Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychological Review 1987;94:469–492. [PubMed] [Google Scholar]
- 18. O’Malley SS, Krishnan‐Sarin S, Farren C, Sinha R, Kreek J. Naltrexone decreases craving and alcohol self‐administration in alcohol‐dependent subjects and activates the hypothalamo‐pituitary‐adrenocortical axis. Psychopharmacology 2002;160:19–29. [DOI] [PubMed] [Google Scholar]
- 19. Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev 2006;1: doi: 10.1002/14651858.CD001333.pub2. [DOI] [PubMed] [Google Scholar]
- 20. Chick J, Anton R, Checinski K, et al A multicentre, randomized, double‐blind, placebo‐controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcohol 2000;35:587–593. [DOI] [PubMed] [Google Scholar]
- 21. Waal H, Kornor H. Abstinence‐oriented therapies for opiate addicts. Current Opinion in Psychiatry 2004;17:169–174. [Google Scholar]
- 22. Carroll KM, Ball SA, Nich C, et al Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: Efficacy of contingency management and significant other involvement. Arch Gen Psychiatry 2001;58:755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Willette RE. The development of sustained action preparations of narcotic antagonists. NIDA Res Monogr 1978;19:333–339. [PubMed] [Google Scholar]
- 24. Chiang CN, Hollister LE, Gillespie HK, Foltz RL. Clinical evaluation of a naltrexone sustained‐release preparation. Drug Alcohol Depend 1985;16:1–8. [DOI] [PubMed] [Google Scholar]
- 25. Lobmaier P, Kornør H, Kunøe N, Bjørndal A. Sustained‐release naltrexone for opioid dependence. Cochrane Database Syst Rev 2008;2: doi: 10.1002/14651858.CD006140.pub2. [DOI] [PubMed] [Google Scholar]
- 26. Kranzler HR, Wesson DR, Billot L; DrugAbuse Sciences Naltrexone Depot Study Group . Naltrexone depot for treatment of alcohol dependence: A multicenter, randomized, placebo‐controlled clinical trial. Alcohol Clin Exp Res 2004;28:1051–1059. [DOI] [PubMed] [Google Scholar]
- 27. Garbutt JC, Kranzler HR, O’Malley SS, et al Efficacy and tolerability of long‐acting injectable naltrexone for alcohol dependence: A randomized controlled trial. JAMA 2005;293:1617–1625. [DOI] [PubMed] [Google Scholar]
- 28. Ciraulo DA, Dong Q, Silverman BL, Gastfriend DR, Pettinati HM. Early treatment response in alcohol dependence with extended‐release naltrexone. J Clin Psychiatry 2008;69:190–195. [DOI] [PubMed] [Google Scholar]
- 29. O’Malley SS, Garbutt JC, Gastfriend DR, Dong Q, Kranzler HR. Efficacy of extended‐release naltrexone in alcohol‐dependent patients who are abstinent before treatment. J Clin Psychopharmacol 2007;27:507–512. [DOI] [PubMed] [Google Scholar]
- 30. Lapham S, Forman R, Alexander M, Illeperuma A, Bohn MJ. The effects of extended‐release naltrexone on holiday drinking in alcohol‐dependent patients. J Subst Abuse Treat 2009;36:1–6. [DOI] [PubMed] [Google Scholar]
- 31. Pettinati HM, Gastfriend DR, Dong Q, Kranzler HR, O’Malley SS. Effect of extended‐release naltrexone (XR‐NTX) on quality of life in alcohol‐dependent patients. Alcohol Clin Exp Res 2009;33:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gastpar M, Bonnet U, Böning J, et al Lack of efficacy of naltrexone in the prevention of alcohol relapse: Results from a German multicenter study. J Clin Psychopharmacol 2002;22:592–598. [DOI] [PubMed] [Google Scholar]
- 33. Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu‐opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: Results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry 2008;65:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O’Brien CP. A functional polymorphism of the mu‐opioid receptor gene is associated with naltrexone response in alcohol‐dependent patients. Neuropsychopharmacology 2003:28:1546–1552. [DOI] [PubMed] [Google Scholar]
- 35. Comer SD, Sullivan MA, Yu E, et al Injectable, sustained‐release naltrexone for the treatment of opioid dependence: A randomized, placebo‐controlled trial. Arch Gen Psychiatry 2006;63:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kunoe N, Lobmaier P, Vederhus JK, et al Naltrexone implants after in‐patient treatment for opioid dependence: Randomised controlled trial. Br J Psychiatry 2009;194:541–546. [DOI] [PubMed] [Google Scholar]
- 37. Hulse GK, Morris N, Arnold‐Reed D, Tait RJ. Improving clinical outcomes in treating heroin dependence: Randomized, controlled trial of oral or implant naltrexone. Arch Gen Psychiatry 2009;66:1108–1115. [DOI] [PubMed] [Google Scholar]
- 38. Carreno JE, Alvarez CE, San Narciso GL, Bascarán MT, Díaz M, Bobes J. Maintenance treatment with depot opioid antagonists in subcutaneous implants: An alternative in the treatment of opioid dependence. Addict Biol 2003;8:429–438. [DOI] [PubMed] [Google Scholar]
- 39. Colquhoun R, Tan DY, Hull S. A comparison of oral and implant naltrexone outcomes at 12 months. J Opioid Manag 2005;1:249–256. [DOI] [PubMed] [Google Scholar]
- 40. Foster J, Brewer C, Steele T. Naltrexone implants can completely prevent early (1‐month) relapse after opiate detoxification: A pilot study of two cohorts totalling 101 patients with a note on naltrexone blood levels. Addict Biol 2003;8:211–217. [DOI] [PubMed] [Google Scholar]
- 41. Grusser SM, Thalemann CN, Platz W, Golz J, Partecke G. A new approach to preventing relapse in opiate addicts: A psychometric evaluation. Biol Psychol 2006;71:231–235. [DOI] [PubMed] [Google Scholar]
- 42. Gölz J, Partecke G. Katamnestische Entwicklung Opiatabhängiger nach Naltrexoninduziertem Entzug unter Narkose, naltrexongestützter Rückfallprophylaxe und ambulanter psychosozialer Nachsorge. Suchttherapie 2000;1:166–172. [Google Scholar]
- 43. Hulse GK, O’Neil G, Hatton M, Paech MJ. Use of oral and implantable naltrexone in the management of the opioid impaired physician. Anaesth Intensive Care 2003;31:196–201. [DOI] [PubMed] [Google Scholar]
- 44. Hulse GK, Tait RJ. A pilot study to assess the impact of naltrexone implant on accidental opiate overdose in ‘high‐risk’ adolescent heroin users. Addict Biol 2003;8:337–342. [DOI] [PubMed] [Google Scholar]
- 45. Waal H, Christophersen AS, Frogopsahl G, Olsen LH, Morland J. Naltrexone implants: A pilot project. Tidsskr Nor Laegeforen 2003;123:1660–1661. [PubMed] [Google Scholar]
- 46. Reece AS. Psychosocial and treatment correlates of opiate free success in a clinical review of a naltrexone implant program. Subst Abuse Treat Prev Policy 2007; 2: doi: 10.1186/1747-597X-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reece AS. Chronic ulcers caused by injection of substances: Healing aided by naltrexone. Arch Dermatol 2009;145:375–377. [DOI] [PubMed] [Google Scholar]
- 48. Waal H, Frogopsahl G, Olsen L, Christophersen AS, Morland J. Naltrexone implants: Duration, tolerability and clinical usefulness. A pilot study. Eur Addict Res 2006;12:138–144. [DOI] [PubMed] [Google Scholar]
- 49. Hulse GK, Tait RJ, Comer SD, Sullivan MA, Jacobs IG, Arnold‐Reed D. Reducing hospital presentations for opioid overdose in patients treated with sustained release naltrexone implants. Drug Alcohol Depend 2005;79:351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ngo HT, Tait RJ, rnold‐Reed DE, Hulse GK. Mental health outcomes following naltrexone implant treatment for heroin‐dependence. Prog Neuropsychopharmacol Biol Psychiatry 2007;31:605–612. [DOI] [PubMed] [Google Scholar]
- 51. Ngo HT, Tait RJ, Hulse GK. Comparing drug‐related hospital morbidity following heroin dependence treatment with methadone maintenance or naltrexone implantation. Arch Gen Psychiatry 2008;65:457–465. [DOI] [PubMed] [Google Scholar]
- 52. Tait RJ, Hulse GK. Hospital morbidity associated with the natural history of heroin use. J Opioid Manag 2008;4:321–327. [DOI] [PubMed] [Google Scholar]
- 53. Hulse GK, Arnold‐Reed DE, O’Neil G, Chan CT, Hansson RC. Achieving long‐term continuous blood naltrexone and 6‐beta‐naltrexol coverage following sequential naltrexone implants. Addict Biol 2004;9:67–72. [DOI] [PubMed] [Google Scholar]
- 54. Tait RJ, Ngo HT, Hulse GK. Mortality in heroin users 3 years after naltrexone implant or methadone maintenance treatment. J Subst Abuse Treat 2008;35:116–124. [DOI] [PubMed] [Google Scholar]
- 55. Reece AS. Comparative treatment and mortality correlates and adverse event profile of implant naltrexone and sublingual buprenorphine. J Subst Abuse Treat 2009;37:256–265. [DOI] [PubMed] [Google Scholar]
- 56. Ravndal E, Amundsen EJ. Mortality among drug users after discharge from inpatient treatment: An 8‐year prospective study. Drug Alcohol Depend; 2010;108:65–69. [DOI] [PubMed] [Google Scholar]
- 57. Oliver P, Horspool M, Keen J. Fatal opiate overdose following regimen changes in naltrexone treatment. Addiction 2005;100:560–561. [DOI] [PubMed] [Google Scholar]
- 58. Yoburn BC, Sierra V, Lutfy K. Simultaneous development of opioid tolerance and opioid antagonist‐induced receptor upregulation. Brain Res 1990;529:143–148. [DOI] [PubMed] [Google Scholar]
- 59. Yoburn BC, Sierra V, Lutfy K. Chronic opioid antagonist treatment: Assessment of receptor upregulation. Eur J Pharmacol 1989;170:193–200. [DOI] [PubMed] [Google Scholar]
- 60. Gibson AE, Degenhardt LJ, Hall WD. Opioid overdose deaths can occur in patients with naltrexone implants. Med J Australia 2007;186:152–153. [DOI] [PubMed] [Google Scholar]
- 61. Hamilton RJ, Olmedo RE, Shah S, et al Complications of ultrarapid opioid detoxification with subcutaneous naltrexone pellets. Acad Emerg Med 2002;9:63–68. [DOI] [PubMed] [Google Scholar]
- 62. Lintzeris N, Lee S, Scopelliti L, Mabbutt J, Haber PS. Unplanned admissions to two Sydney public hospitals after naltrexone implants. Med J Australia 2008;188:441–444. [DOI] [PubMed] [Google Scholar]
- 63. Kruptisky EM, Burakov AM, Tsoy MV, et al Overcoming opioid blockade from depot naltrexone (Prodetoxon). Addiction 2007;102:1164–1165. [DOI] [PubMed] [Google Scholar]
- 64. Fishman M. Precipitated withdrawal during maintenance opioid blockade with extended release naltrexone. Addiction 2008;103:1399–1401. [DOI] [PubMed] [Google Scholar]
- 65. Brewer C. Serum naltrexone and 6‐beta‐naltrexol levels from naltrexone implants can block very large amounts of heroin: A report of two cases. Addict Biol 2002;7:321–323. [DOI] [PubMed] [Google Scholar]
- 66. Galloway GP, Koch M, Cello R, Smith DE. Pharmacokinetics, safety, and tolerability of a depot formulation of naltrexone in alcoholics: An open‐label trial. BMC Psychiatry 2005;5: doi: 10.1186/1471-244X-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Johnson BA, Ait‐Daoud N, Aubin HJ, et al A pilot evaluation of the safety and tolerability of repeat dose administration of long‐acting injectable naltrexone (Vivitrex) in patients with alcohol dependence. Alcohol Clin Exp Res 2004;28:1356–1361. [DOI] [PubMed] [Google Scholar]
- 68. Turncliff RZ, Dunbar JL, Dong Q, Silverman BL, Ehrich EW, Dilzer SC, Lasseter KC. Pharmacokinetics of long‐acting naltrexone in subjects with mild to moderate hepatic impairment. J Clin Pharmacol 2005;45:1259–1267. [DOI] [PubMed] [Google Scholar]
- 69. Kranzler HR, Modesto‐Lowe V, Nuwayser ES. Sustained‐release naltrexone for alcoholism treatment: A preliminary study. Alcohol Clin Exp Res 1998;22:1074–1079. [PubMed] [Google Scholar]
- 70. Dunbar JL, Turncliff RZ, Hayes SC, Farrell CB. Population pharmacokinetics of extended‐release injectable naltrexone (XR‐NTX) in patients with alcohol dependence. J Stud Alcohol Drugs 2007;68:862–870. [DOI] [PubMed] [Google Scholar]
- 71. Dunbar JL, Turncliff RZ, Dong Q, Silverman BL, Ehrich EW, Lasseter KC. Single‐ and multiple‐dose pharmacokinetics of long‐acting injectable naltrexone. Alcohol Clin Exp Res 2006;30:480–490. [DOI] [PubMed] [Google Scholar]
- 72. Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fischman MW. Depot naltrexone: Long‐lasting antagonism of the effects of heroin in humans. Psychopharmacology 2002;159:351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hulse GK, Arnold‐Reed DE, O’Neil G, Chan CT, Hansson R, O’Neil P. Blood naltrexone and 6‐beta‐naltrexol levels following naltrexone implant: Comparing two naltrexone implants. Addict Biol 2004;9:59–65. [DOI] [PubMed] [Google Scholar]
- 74. Ngo HT, rnold‐Reed DE, Hansson RC, Tait RJ, Hulse GK. Blood naltrexone levels over time following naltrexone implant. Prog Neuropsychopharmacol Biol Psychiatry 2008;32:23–28. [DOI] [PubMed] [Google Scholar]
- 75. Olsen L, Christophersen AS, Frogopsahl G, Waal H, Morland J. Plasma concentrations during naltrexone implant treatment of opiate‐dependent patients. Brit J Clin Pharmacol 2004;58:219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ramenskaya GV, Shikh EV, Arzamastsev AP, Kukes VG. Molecular‐biological problems of drug design and mechanism of drug action: Pharmacokinetic study of the new domestic hypodermic form of naltrexone: Prodetoxon depot tablets. Pharmaceutical Chemistry Journal 2005;1:1–3. [Google Scholar]
- 77. Martin WR, Jasinski DR, Mansky PA. Naltrexone, an antagonist for the treatment of heroin dependence. Effects in man. Arch Gen Psychiatry 1973;28:784–791. [DOI] [PubMed] [Google Scholar]
- 78. Oncken C, Van Kirk J, Kranzler HR. Adverse effects of oral naltrexone: Analysis of data from two clinical trials. Psychopharmacology 2001;154:397–402. [DOI] [PubMed] [Google Scholar]
- 79. He S‐X, Yu L‐C, Wang D‐M, Hu S, Jia S‐W. Cue‐elicited event‐related potential evaluation of long‐term sustained release naltrexone treatment for opioid dependence. [Chinese]. J Clin Rehab Tissue Eng Res 2008;12:1027–1030. [Google Scholar]
- 80. He S‐X, Yu L‐C, Chen Q, Wang D‐M, Hu S, Jia S‐W. Effect of long‐term sustained release naltrexone on semantic recognition of opioid addicts. J Clin Rehab Tissue Eng Res 2009;13:1573–1576. [Google Scholar]
- 81. Miotto K, McCann MJ, Rawson RA, Frosch D, Ling W. Overdose, suicide attempts and death among a cohort of naltrexone‐treated opioid addicts. Drug Alcohol Depend 1997;45:131–134. [DOI] [PubMed] [Google Scholar]
- 82. Ritter AJ. Naltrexone in the treatment of heroin dependence: Relationship with depression and risk of overdose. Aust N Z J Psychiatry 2002;36:224–228. [DOI] [PubMed] [Google Scholar]
- 83. Miotto K, McCann M, Basch J, Rawson R, Ling W. Naltrexone and dysphoria: Fact or myth? Am J Addiction 2002;11:151–160. [DOI] [PubMed] [Google Scholar]
- 84. Dean AJ, Saunders JB, Jones RT, Young RM, Connor JP, Lawford BR. Does naltrexone treatment lead to depression? Findings from a randomized controlled trial in subjects with opioid dependence. J Psychiatry Neurosci 2006;31:38–45. [PMC free article] [PubMed] [Google Scholar]
- 85. Hulse GK, Stalenberg V, McCallum D, Smit W, O'Neil G, Morris N, Tait RJ. Histological changes over time around the site of sustained release naltrexone‐poly(DL‐lactide) implants in humans. J Control Release 2005;108:43–55. [DOI] [PubMed] [Google Scholar]
- 86. Hulse GK, Low VH, Stalenberg V, et al Biodegradability of naltrexone‐poly(DL) lactide implants in vivo assessed under ultrasound in humans. Addict Biol 2008;13:364–372. [DOI] [PubMed] [Google Scholar]
- 87. Tilg H, Day CP. Management strategies in alcoholic liver disease. Nat Clin Pract Gastroenterol Hepatol 2007;4:24–34. [DOI] [PubMed] [Google Scholar]
- 88. NIAAA . Alcoholic liver disease. Alcohol Alert 2005;64:1–6. [Google Scholar]
- 89. Grebely J, Genoway KA, Raffa JD, et al Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug Alcohol Depend 2008;93:141–147. [DOI] [PubMed] [Google Scholar]
- 90. NIH . Managment of Hepatitis C NIH Consensus Conference Statement 2002. Available from: http://consensus.nih.gov/2002/2002HepatitisC2002116html.htm [Accessed 2 September 2010]. [Google Scholar]
- 91. Lucey MR, Silverman BL, Illeperuma A, O’Brien CP. Hepatic safety of once‐monthly injectable extended‐release naltrexone administered to actively drinking alcoholics. Alcohol Clin Exp Res 2008;32:498–504. [DOI] [PubMed] [Google Scholar]
- 92. Brewer C, Wong VS. Naltrexone: Report of lack of hepatotoxicity in acute viral hepatitis, with a review of the literature. Addict Biol 2004;9:81–87. [DOI] [PubMed] [Google Scholar]
- 93. O’Brien B, Cody C. Analgesia and sedation in the presence of a naltrexone implant: A novel pharmacological challenge. Eur J Emerg Med 2006;13:315–316. [DOI] [PubMed] [Google Scholar]
- 94. Hulse GK, O’Neill G. A possible role for implantable naltrexone in the management of the high‐risk pregnant heroin user. Aust N Z J Obstet Gynaecol 2002;42:93–94. [DOI] [PubMed] [Google Scholar]
- 95. Hulse GK, Arnold‐Reed DE, O’Neil G, Hansson RC. Naltrexone implant and blood naltrexone levels over pregnancy. Aust N Z J Obstet Gynaecol 2003;43:386–388. [DOI] [PubMed] [Google Scholar]
- 96. Hulse G, O’Neil G. Using naltrexone implants in the management of the pregnant heroin user. Aust N Z J Obstet Gynaecol 2002;42:569–573. [DOI] [PubMed] [Google Scholar]
- 97. Hulse GK, O’Neil G, Arnold‐Reed DE. Methadone maintenance vs. implantable naltrexone treatment in the pregnant heroin user. Int J Gynecol Obstet 2004;85:170–171. [DOI] [PubMed] [Google Scholar]
- 98. Farid WO, Dunlop SA, Tait RJ, Hulse GK. The effects of maternally administered methadone, buprenorphine and naltrexone on offspring: Review of human and animal data. Curr Neuropharmacol 2008;6:125–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Heading CE. Vivitrex (alkermes/cephalon). Curr Opin Investig Drugs 2006;7:81–88. [PubMed] [Google Scholar]
- 100. Johnson BA. A synopsis of the pharmacological rationale, properties and therapeutic effects of depot preparations of naltrexone for treating alcohol dependence. Expert Opin Pharmacother 2006;7:1065–1073. [DOI] [PubMed] [Google Scholar]
- 101. Martin WR, Sandquist VL. A sustained release depot for narcotic antagonists. Arch Gen Psychiatry 1974;30:31–33. [DOI] [PubMed] [Google Scholar]
- 102. Slawson MH, Chen M, Moody D, Comer SD, Nuwayser ES, Fang WB, Foltz RL. Quantitative analysis of naltrexone and 6beta‐naltrexol in human, rat, and rabbit plasma by liquid chromatography‐electrospray ionization tandem mass spectrometry with application to the pharmacokinetics of Depotrex in rabbits. J Anal Toxicol 2007;31:453–461. [DOI] [PubMed] [Google Scholar]
- 103. Chaves ME, Chávez MA, Gerez M, Vázquez MT, Maatouk M. Experiencia interdisciplinaria en la problemática del alcoholismo. [Interdisciplinary experience in the problematic one of the alcoholism.] Vis Enferm Actual 2007;3:18–21. [Google Scholar]
- 104. Gooberman LL, Bradway DW. Depot naltrexone vs oral naltrexone postdetoxification. J Addict Dis 1998;17:150. [Google Scholar]
- 105. Jeffrey GP, MacQuillan G, Chua F, et al Hepatitis C virus eradication in intravenous drug users maintained with subcutaneous naltrexone implants. Hepatology 2007;45:111–117. [DOI] [PubMed] [Google Scholar]
- 106. Marlowe DB. Depot naltrexone in lieu of incarceration: A behavioral analysis of coerced treatment for addicted offenders. J Subst Abuse Treat 2006;31:131–139. [DOI] [PubMed] [Google Scholar]
- 107. O’Brien CP. Efficacy and tolerability of long‐acting injectable naltrexone for alcohol dependence. Curr Psychiatry Rep 2005;7:327–328. [DOI] [PubMed] [Google Scholar]


