Abstract
Oral cholinesterase inhibitors (ChEIs) are associated with side effects such as nausea and vomiting. The use of transdermal patches for ChEI delivery may help to minimize these problems. The objective of this review was to consider available data from patients switching from oral ChEIs to transdermal rivastigmine treatment, and to suggest practical guidelines for patients wishing to do this. Literature database and reference list searches were performed to identify suitable publications. Data from two clinical trials and a series of open observational studies, in which patients were switched to the rivastigmine patch from oral rivastigmine, donepezil tablets, or galantamine, were evaluated. Adverse events were tabulated. In the studies reported here, nausea was reported in up to 3.2% and vomiting in up to 1.9% of patients switching to the rivastigmine patch from oral rivastigmine. Similar rates (up to 3.8% of patients for nausea and 0.8% of patients for vomiting) were reported when switching to the rivastigmine patch from donepezil tablets, and no nausea or vomiting was reported in a case study of patients switching to the rivastigmine patch from galantamine tablets. Switching regimes used in clinical trials appeared well tolerated. Data support recommendations for patients on high rivastigmine capsule doses to switch directly to the 9.5 mg/24 h rivastigmine patch, while those on lower oral rivastigmine doses should start on the 4.6 mg/24 h patch for 4 weeks before increasing to the 9.5 mg/24 h patch. This latter regimen is recommended for patients on other oral cholinesterase inhibitors if switching is medically indicated or requested by the patient or the caregiver.
Keywords: Alzheimer's disease, Cholinesterase inhibitor, Switching, Transdermal
Introduction
Alzheimer's disease (AD) is the most prevalent neurodegenerative dementia. Up to 75% of all late‐onset cases of dementia are attributable to AD, with or without vascular contribution [1]. AD results in a loss of presynaptic cholinergic nerve terminals and a subsequent decrease in the levels of acetylcholine in the brain [2], which is associated with progressive deterioration of cognitive performance, the ability to perform activities of daily living, and the development of neuropsychiatric symptoms [3].
Cholinesterase inhibitors (ChEIs) have been shown to be effective in improving cognitive and global functioning in AD patients, and are the main pharmacological intervention in the clinical management of the disease [4]. A number of early studies on ChEIs reported beneficial effects of the drugs in animal models [5, 6, 7]. Although studies in animals were extremely important for the initial assessments of efficacy, safety, and tolerability of ChEIs, the results obtained cannot be directly translated to the results of clinical trials in human healthy volunteers and patients with AD [8]. Differences between animal and human enzymes, and drug–host interactions, mean that a meaningful comparison of the data cannot always be made. However, further successful clinical trials in humans corroborated the results of the animal studies, and the first oral ChEIs became available in the mid‐1990s. Oral ChEIs have since been associated with side effects such as nausea and vomiting [9], and practical issues such as lack of treatment compliance [10] in patients with AD. The various benefits of transdermal therapies [10, 11, 12] have led to the evaluation of transdermal patches for ChEI delivery. Designed to minimize the problems often encountered with classic oral treatments, transdermal patch delivery may represent the next generation of anticholinesterase treatment [13]. The first such therapy, the rivastigmine transdermal patch, became available in many countries in 2007. Pharmacokinetic studies have shown that approximately half of the total dose loaded onto the rivastigmine patch is absorbed into the bloodstream over a 24‐h period. For the target dose patch, 18 mg of rivastigmine is loaded, which releases 9.5 mg in 24 h [14]. Rivastigmine exposure with the 9.5 mg/24 h patch is comparable to that with 12 mg/day rivastigmine capsule dose [15]. By delivering the drug through the skin directly into the bloodstream, transdermal patches avoid the first‐pass effect, where a significant proportion of the amount of drug absorbed through the gastrointestinal tract undergoes biotransformation or excretion by the liver.
Transdermal rivastigmine delivery also results in reduced rates of nausea and vomiting compared with rivastigmine capsules, due to differences in pharmacokinetics between the two modes of drug administration [16]. The incidence of the cholinergic gastrointestinal side effects commonly experienced with oral ChEIs is related to dose and the resulting degree of cholinesterase inhibition [17]. Increased levels of nausea and vomiting have been associated with high maximum blood plasma drug levels, which occur rapidly following capsule administration [18]. When administered orally, rivastigmine levels in blood plasma peak approximately 1 h after administration. In contrast, following patch administration, rivastigmine plasma levels rise more slowly, with the maximum level reached after around 8 h. The maximum level of rivastigmine in the plasma after administration of the 9.5 mg/24 h patch is around 70% lower than with 12 mg/day capsules, yet the elimination half‐life for rivastigmine patch is about twice as long as that seen with capsules [14]. The smoother, more continuous drug delivery with the rivastigmine patch means that gastrointestinal side effects are greatly reduced [16].
With continued use, therapeutic doses of ChEI therapy can stabilize a patient's clinical status and delay deterioration [19]. Maintaining patients on optimal target doses over the long term is therefore an important goal [20]. In practice, however, if AD patients discontinue or reduce their initial ChEI treatment due to problems with safety and tolerability, or due to loss of therapeutic benefit, they may be denied optimal treatment [21]. Yet switching medications is a therapeutic option often employed for other nervous system disorders, such as epilepsy, schizophrenia, or headache, when patients encounter problems with efficacy or safety/tolerability. The strategy of switching to another ChEI in such cases has already been suggested by experts in the field of AD [21, 22, 23]. Although different agents within a therapeutic class of drugs often share the same mechanism of action, their pharmacological properties – and thus their efficacy, safety and tolerability profiles – may differ. In addition, the relatively new availability of the rivastigmine patch offers the opportunity of switching from oral to patch therapy. A shift in the AD treatment paradigm from pills to patches offers potential practical advantages, and allows access to optimal dose with excellent tolerability without sacrificing efficacy [6, 9, 24, 25].
A number of recent publications have described available clinical trial data in AD patients receiving rivastigmine patch therapy [25, 26, 27]. The challenges now facing many clinicians are those relating to the practical use of a patch in this disease setting. Specifically, while prescribing information provides guidance on initiating patch treatment in de novo patients, it is currently less clear how to initiate patch treatment if patients are already receiving oral treatment and wish to switch to the patch. The present objective is to review available data from patients switching from oral ChEIs to transdermal rivastigmine treatment, and to consider practical guidelines for patients wishing to do this, or in cases where switching is medically indicated for any reason.
Methods
A systematic search of literature indexed by MEDLINE or PubMed during the past 10 years was carried out, using the term “transdermal” in combination with one or more of the following terms: switch, switching, Alzheimer's, rivastigmine, dementia. The bibliographies of included publications were used to supplement the search. Inclusion criteria were: English language, human studies (65+ years), and relevance to AD, PDD, and ChEIs. The literature search returned six publications regarding switching from oral to transdermal ChEI delivery, which are reviewed here.
Results
Of the six communications highlighted by the literature search, four were poster presentations giving relatively brief details of case studies by one research group [28, 29, 30, 31]. Results from these studies will be mentioned here, but the major focus of this review will be the remaining two publications. These were both peer‐reviewed journal articles. One described the open‐label extension to a double‐blind, randomized trial in which one group of patients was switched from rivastigmine capsules to the rivastigmine patch (the IDEAL study) [27]. The other was an open‐label, randomized, parallel‐group study looking at the switching of patients from oral donepezil to the rivastigmine patch (the SWAP study) [32].
IDEAL Open‐Label Extension and SWAP Studies: Switching Patients from Oral Rivastigmine and Donepezil Tablets to the Rivastigmine Patch
The IDEAL and SWAP studies shared similar entry criteria, both including males or females aged ≥50 years (50–85 years for the IDEAL study) with a diagnosis of probable AD, and a Mini‐Mental State Examination (MMSE) score indicating mild‐to‐moderate dementia. Patients with any neurodegenerative disorder other than AD or any serious or unstable illness that could interfere with the study or put the patient at special risk were excluded [25, 27].
Switching Patients from Oral Rivastigmine to Rivastigmine Patch
The IDEAL study was a 24‐week, double‐blind trial in which 1195 patients with probable AD were randomized to either 12 mg/day rivastigmine capsules, 9.5 mg/24 h rivastigmine patch, 17.4 mg/24 h rivastigmine patch, or placebo [25]. The study compared the efficacy, safety, and tolerability of the rivastigmine transdermal patch with rivastigmine capsules and placebo. All patients completing the double‐blind study were eligible to enter the 28‐week open‐label extension phase, irrespective of their treatment group [27]. In the open‐label extension, all patients were immediately switched to 9.5 mg/24 h rivastigmine patch (including those patients previously on 12 mg/day rivastigmine capsules). The patch dose was then increased every 4 weeks to a maximum of 17.4 mg/24 h, dependent upon tolerability, and maintained at this or the highest tolerated dose for the remainder of the extension. Safety evaluations, made at weeks 24, 28, 32, 36, 40, 46, and 52, consisted of recording and rating all adverse events (AEs) and serious adverse events (SAEs), recording vital signs, and assessing skin irritation.
Overall baseline patient demographics and background characteristics were similar between the patients entering the IDEAL open‐label extension and those involved in the initial double‐blind study (Table 1). Of the 870 patients that entered the IDEAL open‐label extension (including 209 who switched to rivastigmine patch from capsules), 704 completed the study (Figure 1). The full results of this study are published elsewhere [27].
Table 1.
Baseline characteristics and demographics of patients switching from rivastigmine capsules to rivastigmine patch (the IDEAL study; safety population [25]) and from donepezil tablets to rivastigmine patch (the SWAP study; safety population [32])
| IDEAL studya[25] Rivastigmine capsule (n = 294) | SWAP study [32] | ||
|---|---|---|---|
| Donepezil tablets to rivastigmine patch – immediate switch (n = 131) | Donepezil tablets to rivastigmine patch – delayed switch (n = 130) | ||
| Mean age (years) ± SD | 72.8 ± 8.2 | 77.8 ± 7.7 | 76.7 ± 8.4 |
| % Female | 65.6 | 60.3 | 55.4 |
| % Caucasian | 74.5 | 90.1 | 85.4 |
| Mean weight (kg) ± SD | – | 72.0 ± 13.2 | 72.8 ± 16.4 |
| Duration dementia (years) ± SD | 1.1 ± 1.4 | 4.0 ± 2.5 | 3.8 ± 2.7 |
| Duration donepezil treatment (months) ± SD | – | 30.2 ± 25.3 | 27.9 ± 20.2 |
| Mean baseline MMSE ± SD | 16.4 ± 3.1 | 18.6 ± 4.0 | 18.1 ± 4.0 |
aBaseline characteristics of patients given rivastigmine capsules, prior to commencement of the 24‐week double‐blind phase that preceded the open‐label phase in which they were switched to patch.
Figure 1.
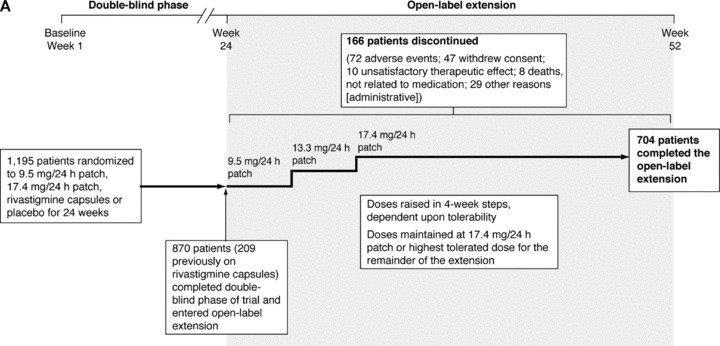
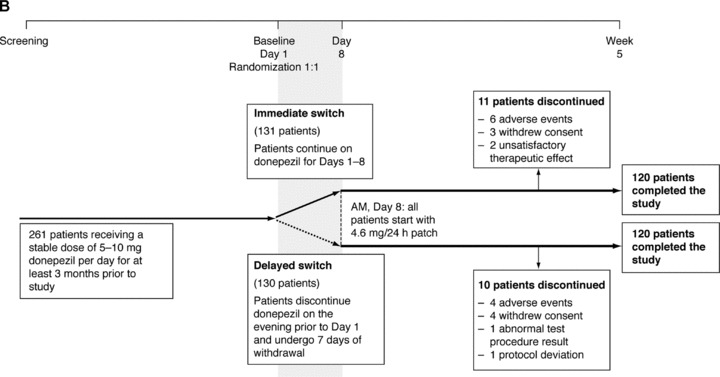
Study profiles of (A) the IDEAL study, switching from rivastigmine capsules to rivastigmine patch plus open‐label extension [25, 27]; and (B) the SWAP study, switching from donepezil tablets to rivastigmine patch [32].
The rivastigmine patch was well tolerated by the patients switching from rivastigmine capsules in the IDEAL study open‐label extension (Figure 2). The AEs reported most frequently during weeks 1–4 of the open‐label extension (the period of time that all patients were on the 9.5 mg/24 h patch) are presented in Table 2. During the first 4 weeks of the open‐label extension, patients formerly randomized to rivastigmine capsules reported fewer AEs than those formerly randomized to placebo (14.4% vs. 28.2%). This effect was particularly marked for nausea (2.4% vs. 8.5%) and vomiting (1.9% vs. 6.0%). Skin tolerability at the patch application site was good, with over 90% of all patients experiencing “none, slight, or mild” irritation as their most severe skin reaction. Serious AEs occurred in nine (1.0%) of patients during weeks 1–4 of the open‐label extension phase, and 82 (9.4%) of patients during the full open‐label extension phase [27]. Six patients (2.9%) from the rivastigmine capsule group discontinued due to AEs during the first 4 weeks of the open‐label extension, five of these (2.4%) due to skin irritation.
Figure 2.
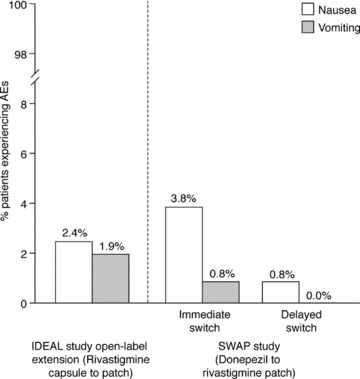
Percentages of patients who experienced the adverse events of nausea and vomiting after switching from rivastigmine capsules to rivastigmine patch (nausea and vomiting reported during the “switch” phase to the 9.5 mg/24 h patch [weeks 1–4 of the open‐label extension] only), and from donepezil tablets to rivastigmine patch (safety populations).
Table 2.
Most frequently reported adverse eventsa and all reported serious adverse events (safety populations)
| A. IDEAL study open‐label extension, switching from rivastigmine capsule to rivastigmine patch (n = 209)b | ||
|---|---|---|
| n (%) | ||
| Any adverse event | 30 (14.4%) | |
| Nausea | 5 (2.4%) | |
| Vomiting | 4 (1.9%) | |
| Any serious adverse event | 0 | |
| B. SWAP study, switching from donepezil tablets to rivastigmine patch | ||
| n (%) | ||
| Donepezil tablets to rivastigmine patch – immediate switch (n = 131) | Donepezil tablets to rivastigmine patch – delayed switch (n = 130) | |
| Any adverse event | 36 (27.5%) | 45 (34.6%) |
| Nausea | 5 (3.8%) | 1 (0.8%) |
| Vomiting | 1 (0.8%) | 0 |
| Decreased appetite | 4 (3.1%) | 0 |
| Bradycardia | 3 (2.3%) | 0 |
| Hallucination | 3 (2.3%) | 0 |
| Constipation | 0 | 6 (4.6%) |
| Application site reaction | 1 (0.8%) | 5 (3.8%) |
| Somnolence | 2 (1.5%) | 4 (3.1%) |
| Agitation | 3 (2.3%) | 3 (2.3%) |
| Any serious adverse event | 4 (3.1%) | 2 (1.5%) |
| Anemia | 0 | 1 (0.8%) |
| Bradycardia | 1 (0.8%) | 0 |
| Abscess limb | 1 (0.8%) | 0 |
| Fall | 0 | 1 (0.8%) |
| Hip fracture | 0 | 1 (0.8%) |
| Dehydration | 1 (0.8%) | 0 |
| Benign vaginal neoplasm | 0 | 1 (0.8%) |
| Lethargy | 1 (0.8%) | 0 |
| Mental status changes | 1 (0.8%) | 0 |
| Dyspnea | 0 | 1 (0.8%) |
aIn addition to nausea and vomiting, adverse events occurring in at least 2% of patients in any treatment group are reported.
bAdverse and serious adverse events reported during the “switch” phase (weeks 1–4 of the open‐label extension).
Switching Patients from Donepezil Tablets to Rivastigmine Patch
The SWAP study was a 5‐week, open‐label, randomized, parallel‐group study, evaluating the tolerability and safety of switching from oral donepezil to the rivastigmine transdermal patch [32]. Patients with probable mild‐to‐moderate AD who had been receiving donepezil tablets for ≥6 months and taking a stable dose of 5–10 mg/day donepezil tablets for ≥3 months prior to participating in the study were included. Patients were randomized in a 1:1 ratio to either an immediate switch or a delayed switch from donepezil tablets to the 4.6 mg/24 h rivastigmine transdermal patch. The two switching protocols are presented in Figure 1. The primary outcome of the study was discontinuation due to any reason. Secondary safety measures included discontinuation due to AEs and the incidence of AEs.
The immediate switch and the delayed switch SWAP study groups shared similar baseline patient demographics and background characteristics (Table 1). Of the 261 patients randomized into the SWAP study (n = 131, immediate switch group; n = 130, delayed switch group), a total of 240 patients completed the 5‐week core phase (Figure 1).
In this study, the rivastigmine transdermal patch was generally well tolerated in both treatment groups (Figure 2). The most frequently reported side effects are presented in Table 2. A total of 10 patients discontinued due to AEs (n = 6, immediate switch group; n = 4, delayed switch group), and 36 (27.5%) patients in the immediate switch group and 45 (34.6%) patients in the delayed switch group experienced at least one AE during the 5‐week study. Three (2.3%) patients in the immediate switch group reported bradycardia (of these, two were either taking β blockers or calcium‐channel blockers, or had a prior history of bradycardia; the third was receiving concomitant treatment with an α‐adrenoreceptor antagonist for hypertension). Serious AEs were reported in four (3.1%) and two (1.5%) patients in the immediate and delayed switch groups, respectively. Only two of these (one case of lethargy and one case of bradycardia; both in the immediate switch group) were considered by the investigators to be related to the study medication. The incidences of nausea or vomiting in both treatment groups were low. Application–site reactions were experienced by six patients during the 5‐week core phase (n = 1, immediate switch group; n = 5, delayed switch group) and were generally mild in severity.
Case Studies: Switching Patients from Oral Donepezil, Galantamine and Rivastigmine to the Rivastigmine Patch
At the 2008 International Conference on AD, Shua‐Haim et al. presented a series of open, observational studies evaluating a total of 400 patients over the course of 2 months as they were switched from donepezil tablets, galantamine ER, or oral rivastigmine to the rivastigmine patch [28, 29, 30, 31]. All consecutive patients switching from any of these treatments to the rivastigmine patch (at the request of the family or caregiver) between September and November (December in the case of patients switching from donepezil tablets) 2007 were included. Patients underwent monthly follow‐ups, at which times they and their caregivers were questioned as to the incidence of any side effects, and any perceived noticeable cognitive improvement or deterioration. The studies were mainly oriented towards tolerability, with no detail provided on the use of any formal scale for measuring change in cognitive performance. Table 3 shows the switching protocols followed by the patients, and the side effects that were reported. No patients reported experiencing any AEs while undergoing treatment with the lower dose (4.6 mg/24 h) patch. At the higher 9.5 mg/24 h dose, patients reported experiencing tiredness/sleepiness, skin rash, and nausea. No discontinuations were recorded for any of the switching protocols.
Table 3.
Switching protocols used in case studies by Shua‐Haim et al., switching a total of 400 patients from oral donepezil, galantamine, and rivastigmine to the rivastigmine patch, and all adverse events reported [28, 29, 30, 31]
| Previous medication | Switching protocola | Patients reporting AEs after switching to 9.5 mg/24 h rivastigmine patch, n (%)b | ||
|---|---|---|---|---|
| Tiredness/sleepiness | Skin rash | Nausea | ||
| Donepezil 5–10 mg/day (n = 116) | 4.6 mg/24 h rivastigmine patch, titrated to 9.5 mg/24 h patch after one month | 18 (15.5) | 3 (2.5) | – |
| Donepezil 10 mg/day (n = 56) | 4.6 mg/24 h rivastigmine patch plus 5 mg/day donepezil, titrated to 9.5 mg/24 h patch and donepezil tablets discontinued after one month | 7 (12.5) | 3 (5.3) | – |
| Galantamine 8–24 mg/day (n = 136) | 4.6 mg/24 h rivastigmine patch titrated to 9.5 mg/24 h patch after one month | 11 (8.1) | 1 (0.7) | – |
| Rivastigmine capsules 3–6 mg/day (n = 16) | 4.6 mg/24 h rivastigmine patch titrated to 9.5 mg/24 h patch after one month | 7 (7.6) | 4 (4.3) | 3 (3.2) |
| Rivastigmine capsules 9–12 mg/day (n = 76) | 9.5 mg/24 h rivastigmine patch | |||
aEach switching protocol required patients to discontinue original medication on the day prior to the switch, starting on the new regime on the following day.
bOnly pooled data were available for patients switching from rivastigmine capsules 3–6 and 9–12 mg/day.
Conclusions
Patients switching from oral ChEI treatment to the rivastigmine patch generally tolerated the change in medication well, for all the variations in switching protocol that were described in the literature reviewed here.
The most commonly reported side effects of oral ChEIs tend to be those that are cholinergic in nature, such as nausea and vomiting [33]. This is true for oral rivastigmine, with rates of 23% and 17% (respectively) reported in a recent study [25]. In the studies reviewed here, nausea and/or vomiting were reported in no more than 3.2% of patients switching to the rivastigmine patch from oral rivastigmine. Patients switching from placebo directly to the 9.5 mg/24 h rivastigmine patch reported a higher incidence of gastrointestinal side effects during the first 4 weeks following the switch. This suggests that patients on high rivastigmine capsule doses may be switched directly to the 9.5 mg/24 h patch, whereas de novo patients and those on low doses should undergo 4 weeks of 4.6 mg/24 h patch treatment before raising their dose to 9.5 mg/24 h.
Similarly low rates of nausea and vomiting were reported when switching from donepezil tablets to rivastigmine patch, and no mention of any nausea or vomiting was made in the case study report of patients switching from galantamine to rivastigmine patch. We would therefore recommend that the majority of patients receiving donepezil tablets could be switched to rivastigmine transdermal patch immediately, without a withdrawal period, if switching is clearly indicated for any reason. Some patients, however, may benefit from a delayed switch (i.e., those with a very low body weight or a history of bradycardia). Likewise, most patients switching to the rivastigmine patch from galantamine could be switched immediately, unless there are factors that suggest that a 1‐week withdrawal period would be beneficial. Figure 3 shows a suggested treatment algorithm for switching patients to the rivastigmine patch, based on their prior medication regimen.
Figure 3.
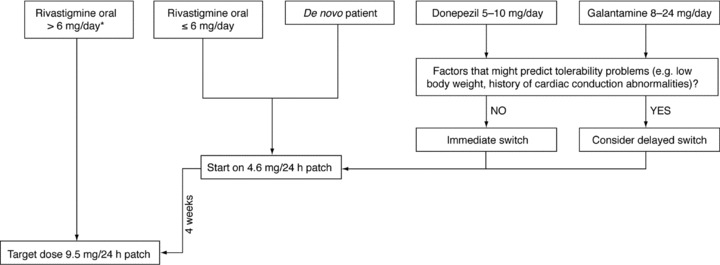
Suggested treatment algorithm for starting patients on the rivastigmine patch. *Regional differences in guidelines apply. The above represents the current European Union switching guidelines (according to the EU rivastigmine patch summary of product characteristics [34]). In the United States, if a patient is receiving <6 mg/day oral rivastigmine they can be switched to 4.6 mg/24 h patch; if a patient is receiving ≥6 mg/day they may be directly switched to 9.5 mg/24 h patch, dependent on tolerability.
A certain incidence of skin irritation, mostly irritant dermatitis, is an acknowledged consequence of treatment with any transdermal medication [35, 36, 37]. The signs and symptoms experienced by those patients who suffer skin irritation while using transdermal medications are usually mild to moderate in severity and transient in nature [38]. Most are restricted to the area of application, and resolve spontaneously following removal of the patch. The rivastigmine patch demonstrated good skin tolerability in both the IDEAL and SWAP studies, and reported reactions were usually in the form of erythema and mild in severity. In the studies reviewed here, at 4–8 weeks after switching to the 4.6 mg/24 h and 9.5 mg/24 h rivastigmine patch, skin rash or application site reaction was reported in 0.74% of patients switching to the patch from oral galantamine, in 0.8–5.3% of patients previously treated with oral donepezil, and in 0.5–4.3% of patients changing their medication from oral to transdermal rivastigmine (Tables 2 and 3).
Relatively high numbers of patients reported experiencing tiredness/sleepiness in the series of case studies presented by Shua‐Haim et al., compared with the numbers of patients reporting somnolence in the IDEAL and SWAP studies (7.6–15.5% compared with 0–3.1% of patients, respectively). The reasons for this seeming discrepancy are unclear, as the levels of methodological detail reported in the case study posters were, of necessity, low. However, there were no obvious differences in switching regimes or other practices that might be expected to explain these differences. The incidences of other side effects were similar between the Shua‐Haim case studies, the IDEAL OLE, and the SWAP study, where comparisons were applicable, although these are different studies.
The IDEAL OLE was a 28‐week extension to the original 24‐week study. During the initial 4 weeks of the extension, all patients were given the 9.5 mg/24 h patch, before being titrated to higher doses according to tolerability. As doses higher than the 9.5 mg/24 h patch are not widely approved, only results from the first 4 weeks of the study are reported here.
The limitations of this review include the small number of studies of patients switching from oral to transdermal ChEIs currently available, in comparison to the relative wealth of data published on switching between oral ChEIs. The single‐center, nonrandomized nature of the case studies means that the potential for interpretation of these data is limited. Another limitation of the case studies is that while AE data were presented separately for patients switching from two dose ranges of donepezil tablets (5–10 mg/day and 10 mg/day), data from patients switching from galantamine were pooled for all patients who had received a relatively wide dose range of 8–24 mg/day. Likewise, AEs are presented from all patients switching from rivastigmine capsule (both 3–6 and 9–12 mg/day), with no way to determine whether patients who had been receiving a lower dose of rivastigmine capsule had a lesser or greater incidence of AEs than those receiving a higher dose, upon switching to the patch. Also to be considered is the fact that to date, only one transdermal ChEI patch is available, although others are expected to come onto the market in the future. Finally, it should be noted that the data reviewed in this study came from patients included in clinical trials, and therefore might not be indicative of the AD population in general.
The purpose of this review is to provide some guidance for those physicians who believe that their patients would benefit from switching to patch therapy for any reason, rather than to focus on the potential reasons for switching. Physicians considering switching patients from oral cholinesterase inhibitor therapy to rivastigmine patch may find it useful to consider the switching algorithm suggested here when making treatment decisions.
Author Contributions
All authors contributed to, reviewed, and approved this article. The decision to submit this article to peer review, for publication, was reached by consensus among all of the authors.
Disclosure
C.S. has served as a consultant to Novartis, has served as a speaker for Novartis and Forest Pharmaceuticals, and has received honoraria from both companies. J.A.D.P. has no conflicts of interest to disclose. R.W.B. has received financial funding for clinical trials, honoraria for consultation services and lectures, and financial support for attending international meetings on dementia from pharmaceutical companies including Novartis. I.G. is on speaker bureaus for Novartis, Pfizer, Forrest, and Accera Pharmaceuticals. S.T. is an employee of Novartis.
Acknowledgments
Alpha‐Plus Medical Communications Ltd. (UK) provided editorial assistance with the production of the article. This assistance was sponsored by Novartis Pharma AG.
References
- 1. Rosenstein LD. Differential diagnosis of the major progressive dementias and depression in middle and late adulthood: A summary of the literature of the early 1990s. Neuropsychol Rev 1998;8:109–167. [DOI] [PubMed] [Google Scholar]
- 2. Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet 1976;2:1403. [DOI] [PubMed] [Google Scholar]
- 3. Cummings JL, Kaufer D. Neuropsychiatric aspects of Alzheimer's disease: The cholinergic hypothesis revisited. Neurology 1996;47:876–883. [DOI] [PubMed] [Google Scholar]
- 4. Doody RS, Stevens JC, Beck C, et al Practice parameter: Management of dementia (an evidence‐based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1154–1166. [DOI] [PubMed] [Google Scholar]
- 5. Enz A, Boddeke H, Gray J, Spiegel R. Pharmacologic and clinicopharmacologic properties of SDZ ENA 713, a centrally selective acetylcholinesterase inhibitor. Ann N Y Acad Sci 1991;640:272–275. [DOI] [PubMed] [Google Scholar]
- 6. Bickel U, Thomsen T, Fischer JP, Weber W, Kewitz H. Galanthamine: Pharmacokinetics, tissue distribution and cholinesterase inhibition in brain of mice. Neuropharmacology 1991;30:447–454. [DOI] [PubMed] [Google Scholar]
- 7. Kasa P, Papp H, Kasa P, Jr ., Torok I. Donepezil dose‐dependently inhibits acetylcholinesterase activity in various areas and in the presynaptic cholinergic and the postsynaptic cholinoceptive enzyme‐positive structures in the human and rat brain. Neuroscience 2000;101:89–100. [DOI] [PubMed] [Google Scholar]
- 8. Poirier J. Evidence that the clinical effects of cholinesterase inhibitors are related to potency and targeting of action. Int J Clin Pract Suppl 2002;127:6–19. [PubMed] [Google Scholar]
- 9. Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int J Clin Pract Suppl 2002;127:45–63. [PubMed] [Google Scholar]
- 10. Small G, Dubois B. A review of compliance to treatment in Alzheimer's disease: Potential benefits of a transdermal patch. Curr Med Res Opin 2007;23:2705–2713. [DOI] [PubMed] [Google Scholar]
- 11. Nitti VW, Sanders S, Staskin DR, Dmochowski RR, Sand PK, MacDiarmid S, Maibach H. Transdermal delivery of drugs for urologic applications: Basic principles and applications. Urology 2006;67:657–664. [DOI] [PubMed] [Google Scholar]
- 12. Priano L, Gasco MR, Mauro A. Transdermal treatment options for neurological disorders: Impact on the elderly. Drugs Aging 2006;23:357–375. [DOI] [PubMed] [Google Scholar]
- 13. Winblad B, Machado JC. Use of rivastigmine transdermal patch in the treatment of Alzheimer's disease. Expert Opin Drug Deliv 2008;5:1377–1386. [DOI] [PubMed] [Google Scholar]
- 14. Kurz A, Farlow M, Lefèvre G. Pharmacokinetics of a novel transdermal rivastigmine patch for the treatment of Alzheimer's disease: A review. Int J Clin Pract 2009;63:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mercier F, Lefèvre G, Huang HL, Schmidli H, Amzal B, Appel‐Dingemanse S. Rivastigmine exposure provided by a transdermal patch versus capsules. Curr Med Res Opin 2007;23:3199–3204. [DOI] [PubMed] [Google Scholar]
- 16. Cummings J, Lefèvre G, Small G, Appel‐Dingemanse S. Pharmacokinetic rationale for the rivastigmine patch. Neurology 2007;69:S10–S13. [DOI] [PubMed] [Google Scholar]
- 17. Imbimbo BP. Pharmacodynamic‐tolerability relationships of cholinesterase inhibitors for Alzheimer's disease. CNS Drugs 2001;15:375–390. [DOI] [PubMed] [Google Scholar]
- 18. Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet 2002;41:719–739. [DOI] [PubMed] [Google Scholar]
- 19. Winblad B, Brodaty H, Gauthier S, et al Pharmacotherapy of Alzheimer's disease: Is there a need to redefine treatment success? Int J Geriatr Psychiatry 2001;16:653–666. [DOI] [PubMed] [Google Scholar]
- 20. Gauthier S. Long‐term efficacy of cholinesterase inhibitors. Brain Aging 2002;2:9–22. [Google Scholar]
- 21. Gauthier S, Emre M, Farlow MR, Bullock R, Grossberg GT, Potkin SG. Strategies for continued successful treatment of Alzheimer's disease: Switching cholinesterase inhibitors. Curr Med Res Opin 2003;19:707–714. [DOI] [PubMed] [Google Scholar]
- 22. Farlow MR. Pharmacokinetic profiles of current therapies for Alzheimer's disease: Implications for switching to galantamine. Clin Ther 2001;23:A13–A24. [DOI] [PubMed] [Google Scholar]
- 23. Bullock R, Connolly C. Switching cholinesterase inhibitor therapy in Alzheimer's disease – donepezil to rivastigmine, is it worth it? Int J Geriatr Psychiatry 2002;17:288–289. [DOI] [PubMed] [Google Scholar]
- 24. Cummings J, Winblad B. A rivastigmine patch for the treatment of Alzheimer's disease and Parkinson's disease dementia. Expert Rev Neurother 2007;7:1457–1463. [DOI] [PubMed] [Google Scholar]
- 25. Winblad B, Cummings J, Andreasen N, et al A six‐month double‐blind, randomized, placebo‐controlled study of a transdermal patch in Alzheimer's disease – rivastigmine patch versus capsule. Int J Geriatr Psychiatry 2007;22:456–467. [DOI] [PubMed] [Google Scholar]
- 26. Lefèvre G, Sedek G, Jhee SS, et al Pharmacokinetics and pharmacodynamics of the novel daily rivastigmine transdermal patch compared with twice‐daily capsules in Alzheimer's disease patients. Clin Pharmacol Ther 2008;83:106–114. [DOI] [PubMed] [Google Scholar]
- 27. Grossberg G, Sadowsky C, Forstl H, et al Safety and tolerability of the rivastigmine patch: Results of a 28‐week open‐label extension. Alzheimer Dis Assoc Disord 2009;23:158–164. [DOI] [PubMed] [Google Scholar]
- 28. Shua‐Haim J, Yap C, Kretov A, Lee P. Results of next day crossover study of donepezil (Aricept) to rivastigmine patch (Exelon Patch) in Alzheimer's disease patients: A two‐month clinical experience. Poster presented at the International Conference on Alzheimer's Disease 2008; Chicago , IL , USA .
- 29. Shua‐Haim J, Yap C, Kretov A, Lee P. Results of next‐day crossover study of rivastigmine oral capsules (Exelon) to rivastigmine patch (Exelon Patch) in Alzheimer's disease patients: A two‐month clinical experience. Poster presented at the International Conference on Alzheimer's Disease 2008; Chicago , IL , USA .
- 30. Shua‐Haim J, Yap C, Kretov A, Lee P. Results of a two‐step crossover study of donepezil (Aricept) to rivastigmine patch (Exelon Patch) in Alzheimer's disease patients: A two‐month clinical experience. Poster presented at the International Conference on Alzheimer's Disease 2008; Chicago , IL , USA .
- 31. Shua‐Haim J, Yap C, Kretov A, Lee P, Patel S. Results of next‐day crossover study of galantamine ER (Razadyne ER) to rivastigmine patch (Exelon Patch) in Alzheimer's disease patients: A two‐month clinical experience. Poster presented at the International Conference on Alzheimer's Disease 2008; Chicago , IL , USA .
- 32. Sadowsky CH DA, Olin JT, Koumaras B. Meng X, Brannan S, on behalf of the US38 Study Group. Switching from donepezil tablets to rivastigmine transdermal patch in Alzheimer's disease. Am J Alz Dis Other Dement 2009;24:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nordberg A, Svensson AL. Cholinesterase inhibitors in the treatment of Alzheimer's disease: A comparison of tolerability and pharmacology. Drug Saf 1998;19:465–480. [DOI] [PubMed] [Google Scholar]
- 34. Summary of product characteristics : Exelon 4.6 mg/24 h and 9.5 mg/24 h transdermal patch. May 2008.
- 35. LeWitt PA, Lyons KE, Pahwa R. Advanced Parkinson disease treated with rotigotine transdermal system: PREFER Study. Neurology 2007;68:1262–1267. [DOI] [PubMed] [Google Scholar]
- 36. Bodkin JA, Amsterdam JD. Transdermal selegiline in major depression: A double‐blind, placebo‐controlled, parallel‐group study in outpatients. Am J Psychiatry 2002;159:1869–1875. [DOI] [PubMed] [Google Scholar]
- 37. Wolf R, Tuzun B, Tuzun Y. Adverse skin reactions to the nicotine transdermal system. Clin Dermatol 1998;16:617–623. [DOI] [PubMed] [Google Scholar]
- 38. Ale I, Lachapelle J‐M, Maibach HI. Skin tolerability associated with transdermal drug delivery systems: An overview. Adv Ther 2009;26:920–935. [DOI] [PubMed] [Google Scholar]


