Abstract
Insomnia is a common condition that affects one's ability to sleep comfortably and consequently to work effectively. Its etiology is multifactorial and involves plethora of risk factors. Consequences can vary from mild sleepiness to more sever psychiatric disturbances and ischemic stroke. Despite several diagnostic criteria it is poorly diagnosed and less often treated. Benzodiazepines formed the mainline therapy for many years till the advent of newer nonbenzodiazepine group of drugs including zolpidem. Zolpidem is an imidazo‐pyridine compound that enhances the GABAA receptor function by interaction with Omega‐1 receptor subtype. Its pharmacokinetic profile allows the patients to use it later in the night when having trouble falling asleep without any residual cognitive impairment the next morning. It has rapid onset of action, improves total sleep duration, and reduces night‐time awakenings. Its adverse effect profile is satisfactory as it appears to have low addiction potential. This review will focus on the current role of zolpidem in the management of insomnia.
Keywords: Insomnia, Management, Zolpidem
Insomnia
Introduction and Implications
Insomnia is a distressing condition that affects not only the ability to sleep adequately at night, but also to function effectively during one's desired waking time [1]. Population‐based studies estimate that 10% to 40% of American adults have intermittent insomnia and 10% to 15% have long‐term sleep difficulties [2]. More than one‐third of adults report some degree of insomnia within any given year, and 2 to 6% use medications to aid sleep [3]. It is also estimated that 10 to 15% of the adult population suffers from chronic insomnia; an additional 25 to 35% has transient or occasional insomnia [4]. Approximately one in four adults experiences insomnia at some time and at least 10% of the general population considers the problem to be chronic [5]. Cost estimates for lost productivity and insomnia‐related accidents exceed $100 billion per year [6].
Despite these high prevalence rates, evidence suggests that insomnia is underrecognized, underdiagnosed and undertreated. Several factors hinder the appropriate recognition of insomnia and its adequate and appropriate management. This includes lack of physician's education, time‐constrained patient visits, beliefs among patients and physicians that sleep complaints are not important, the belief that treatment is not effective or causes more problems, and the lack of research evidence that treating insomnia improves outcomes of comorbid conditions [7].
Symptoms of insomnia include difficulty in initiating sleep, difficulty in maintaining sleep, or waking too early without being able to return to sleep causing clinically significant daytime distress and functional impairment [8, 9]. Risk factors for insomnia include increasing age, female sex, psychiatric illness, medical comorbidities, impaired social relationships, lower socioeconomic status, substance abuse, environmental factors, circadian rhythm disturbances, and poor sleep hygiene [10].
Insomnia can be very limiting in both short and long term. Consequences may vary from daytime acute consequences like sleepiness to most severe consequences like stroke. (Table 1)
Table 1.
Insomia short‐term and long‐term consequences
| Daytime consequences [11] | Chronic severe consequences [12, 13, 14, 15, 16] |
|---|---|
| Sleepiness | Cardiac morbidity |
| Fatigue | Ischemic stroke |
| Increased absence from work | Diabetes |
| Diminished ability to accomplish tasks | Glucose intolerance |
| Relationship problems | Arthritis |
| Nodding off during daily activities | Weight gain |
| Irritability | Psychiatric disturbances |
| Poor concentration | Impaired psychomotor functioning |
| Limited enjoyment of family and social life | Impaired performance, memory, and alertness |
Insomnia has also been associated with decrease in work performance, increase in motor vehicle accidents and hospitalization rates, and reduction in overall quality of life and cognitive and occupational functioning of the patient [17, 18].
Diagnosis
Research studies often define insomnia as a sleep latency (time taken to fall asleep) that is greater than 30 min, sleep efficiency (time asleep/time in bed) less than 85%, or sleep disturbance more than three times a week [19]. More recently, the International Classification of Sleep Disorders documented that the person is said to be suffering from insomnia if one criterion from class I and one criterion from class II is present in a person [20].
Class I (any one):
-
•
difficulty initiating and/or maintaining sleep;
-
•
sleep that is poor in quality;
-
•
trouble sleeping despite adequate opportunity and circumstances for sleep;
-
•
waking up too early.
and Class II (any one):
-
•
daytime impairment related to sleep difficulty problems including attention, concentration, or memory impairment;
-
•
concerns or worries about sleep;
-
•
daytime sleepiness;
-
•
errors or accidents at work or while driving;
-
•
fatigue or malaise;
-
•
gastrointestinal symptoms;
-
•
lack of motivation;
-
•
mood disturbance or irritability;
-
•
social or vocational dysfunction; or
-
•
poor school performance or tension headaches.
Management
In the past, bromides, barbiturates, paraldehyde, and methaqualone were among the medications that were employed as hypnotics in the treatment of insomnia. Though they had marked sedating properties, due to significant toxicity profile, they ran out of use [21]. Many drug treatment modalities are now available. Treatment strategies for insomnia should ideally alleviate night‐time symptoms, the feeling of nonrestorative sleep, and impaired daytime function. Benzodiazepines (BZD) formed the mainline therapy for insomnia for many years.
First developed in the 1980s, the nonbenzodiazepine hypnotics have pharmacological profiles distinct from those of the classical BDZ as they are highly selective for the GABA‐chloride channel within the type I‐BDZ (BZ1) receptors in the CNS, thereby producing a strong sedative and hypnotic profile that predominates over the anticonvulsivant and anxiolytic activity and, moreover, appears practically devoid of myorelaxant properties. Nonbenzodiazepine hypnotics approved by the Food and Drugs Administration for the treatment of insomnia include zolpidem, zaleplon, zopiclone, and eszopiclone.
Zopiclone is not commercially available in United States, although its active stereoisomer, eszopiclone, is sold. It is also a controlled substance in Canada, Japan, and some European countries, and thus may be illegal to possess without a prescription. Zaleplon has been discontinued in Canada and is only prescribed privately (though rarely) in the United Kingdom.
Zolpidem
Zolpidem is a nonbenzodiazepine hypnotic agent belonging to a new class of psychotropic drugs, the imidazopyridines, which enhance the GABAA receptor function by binding selectively to the omega‐1 receptor subtype. On April 23, 2007 the US Food and Drugs Administration approved 13 generic versions of zolpidem tartrate.
Chemistry
Chemically, zolpidem is N,N,6‐trimethyl‐2‐p‐tolylimidazo [1, 2‐a] pyridine‐3‐acetamide L‐(+)‐tartrate (2:1).
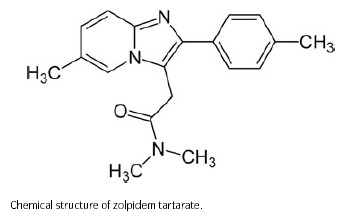
Zolpidem tartrate is a white to off‐white crystalline powder that is sparingly soluble in water, alcohol, and propylene glycol. It has a molecular weight of 764.88.
Pharmacodynamics
ω1 and ω2 are the two main subtypes of GABAA receptors. ω1 type GABAA receptors are the α1 containing GABAA receptors and ω2 GABAA receptors are the α2, α3, α4, α5 and α6 containing GABAA receptors. ω1 GABAA receptors are primarily found in the brain whereas ω2 receptors are primarily found in the spine. Zolpidem binds with high affinity to the α1 containing GABAA receptors, about 10‐fold lower affinity for those containing the α2—and α3—GABAA receptor subunits, and with no appreciable affinity for α5 subunit containing receptors [22]. Due to its selective binding, it has very weak anxiolytic, myorelaxant, and anticonvulsant properties but very strong hypnotic properties [23]. Thus, it has a preferential binding for the GABAA‐benzodiazepine receptor complex in the brain but a low affinity for the GABAA‐benzodiazepine receptor complex in the spine [24]. Zolpidem positively modulates GABAA receptors, likely by increasing the GABAA receptor complexes apparent affinity for GABA, without affecting desensitization or peak current [25].
Pharmacokinetics and Metabolism
It is rapidly absorbed following oral administration, with peak plasma concentrations attained in about 1.6 h. Absolute bioavailability is about 70%. Food decreases AUC by 15%, decreases peak plasma concentration by 25%, and prolongs time to peak plasma concentration by 60%. These characteristics allow patients to take zolpidem later in the night when having trouble falling asleep without worrying about residual cognitive impairment the next morning. It is metabolized in the liver via oxidation and hydroxylation, principally by CYP3A4 and to a lesser extent by CYP1A2 and CYP2D6. No active metabolites are formed and it is excreted principally in urine as inactive metabolites.
Clinical Efficacy
Zolpidem is characterized by a rapid onset of action as well as minimal residual and rebound effects. Its effects on sleep‐maintenance parameters, however, have been rather inconsistent. Zolpidem decreases sleep‐onset latency, improves sleep quality, increases stage 2 and slow‐wave sleep and does not exhibit tolerance or rebound following 5 weeks of continuous use of recommended dosages [26]. This agent significantly improves total sleep duration and reduces night‐time awakenings; therefore it is an appropriate treatment for patients having trouble achieving sustained sleep.
Zolpidem as a Sleep Onset Agent
Zolpidem is primarily effective in inducing sleep with minimal effect on sleep duration or sleep maintenance [27]. It was the most commonly prescribed agent for insomnia in 2001 [28] despite the absence of studies of nightly use for longer than 5 weeks [29]. One of the two studies conducted with 15 patients with nonorganic insomnia related to neurotic or stress‐related disorders, indicated that although statistical analysis of polysomnographic variables demonstrated a significant lengthening of the total sleep period and total sleep time (TST), an improvement in sleep efficiency and a shortening of sleep latencies after 10 mg zolpidem as compared with placebo, there were no statistically significant differences in wake time during the sleep period or number of awakenings (NAW) [30].
In one other multicenter study, hypnotic effects of zolpidem 10 mg was compared with both, temazepam 15 mg and placebo in healthy adults [31]. Subjects with normal sleep histories and without prior sleep laboratory experience were randomly assigned to treatment groups. Medications were administered 15 min before lights out, with polysomnographic monitoring for 7.5 h. Subjective questionnaires and performance tests, digit symbol substitution test and symbol copying test were administered at study entry and after arising. Six hundred and thirty subjects completed the study. Zolpidem reduced awakenings and wake after sleep onset (WASO); temazepam did not. Both agents improved sleep efficiency and most subjective sleep measures relative to placebo, with zolpidem superior for five of six subjective outcome measures compared to temazepam. Symbol copying test, morning sleepiness, and morning concentration were not altered by any treatment. Zolpidem 10 mg provided greater subjective hypnotic efficacy than temazepam 15 mg, with reduced polysomnographic awakenings and WASO. Though, impairment of digit symbol substitution test was seen with zolpidem and not with temazepam.
The hypnotic activity of zolpidem has also been explored in different patient populations and assessment included both objective and subjective measures of hypnotic efficacy for different treatment durations, with results confirming that 10 mg is superior to placebo [32]. Consequently, 10 mg is the recommended dose for the short‐term treatment of insomnia in the nonelderly; in elderly patients 5 mg has been shown to be effective at inducing sleep while giving an optimum safety profile [33].
Zolpidem in Middle of the Night (MOTN) Insomnia
Insomnia characterized by difficulty returning to sleep following a nocturnal awakening is called middle of the night (MOTN) insomnia. It is the most common form of insomnia in adults, and has been shown to become more prevalent with increasing age [34]. In one of the studies conducted by Roth et al., subjects were given dosing period of two consecutive nights separated by a washout of 5 to 12 days. They were awakened 4 h after lights out, dosed with sublingual zolpidem tartrate (3.5 mg or 1.75 mg) or placebo, kept awake for 30 min, and then returned to bed for an additional 4 h. Sleep parameters were assessed by polysomnography and postsleep questionnaires. It was concluded that low‐dose sublingual zolpidem tartrate may be suitable for treatment of patients who have difficulty resuming sleep after MOTN awakenings [35]. Following this, novel low‐dose (1.75 mg and 3.5 mg) sublingual formulation of zolpidem tartrate is currently being evaluated.
Zolpidem When “As Needed”
“As needed” use of hypnotic medication is often recommended for the treatment of chronic insomnia [36]. Zolpidem 10 mg taken “as needed,” with the provision that it should be taken from three to five times per week, is very efficacious and safe in the treatment of chronic insomnia over a period of 8 weeks. There is minimal evidence of rebound insomnia [37].
Zolpidem in Comorbid Insomnia
Insomnia is a multifaceted clinical entity and can either be the specific condition of primary insomnia or be associated with one or more of a variety of medical or psychiatric disorders [38]. Comorbidities that exist with insomnia are often bidirectional; not only does insomnia increase the odds of developing psychiatric and medical problems, but the latter two also increase the likelihood of insomnia [39]. Although 10 medications currently are approved by the US Food and Drug Administration for the treatment of insomnia [40], only two among them that is, eszopiclone and zolpidem, have been evaluated for efficacy in patients with chronic comorbid insomnia.
Studies suggest clear benefits of zolpidem in comorbid insomnia. Asnis et al. evaluated zolpidem in 190 patients treated with either fluoxetine, sertraline, or paroxetine for a depressive disorder [41]. The patients had recovered from their depressed mood, but experienced persistent insomnia. After a 1‐week, single‐blind placebo period, the patients received either placebo or zolpidem (10 mg) nightly for 4 weeks. The zolpidem‐treated cohort showed longer sleep times (P < 0.05), better sleep quality (P < 0.01), and reduced WASO (P < 0.05). All effects except the WASO improvement were for weeks 1 through 4; WASO showed improvement at weeks 1, 2, and 4. Also, study of zolpidem together with fluoxetine or sertraline found no clinically significant pharmacokinetic and pharmacodynamic interactions in healthy individuals [42].
Fava et al. presented data on a study in which patients received daily escitalopram (10 mg) and zolpidem extended release (ER) (12.5) or placebo. The first phase (8 weeks) assessed sleep variables; patients whose depression responded were treated for another 16 weeks. Patients receiving zolpidem ER showed significant improvements in TST, WASO, NAW, sleep quality and sleep latency compared with placebo (P < 0.0003) at each 2‐week assessment during the first phase; and also showed significant improvements in TST, WASO, NAW, and sleep quality from weeks 12 to 24 (P < 0.05). Zolpidem ER did not significantly affect improvements in depression and there was no evidence of rebound insomnia upon discontinuation [43].
Zolpidem in Chronic Insomnia
In one of the 30‐year meta‐analysis study done by Nowell et al. in which they evaluated the efficacy of benzodiazepines and zolpidem tartrate in all the randomized, double‐blind, placebo‐controlled, parallel or crossover designs with benzodiazepines or zolpidem in adults younger than 65 years with chronic insomnia, they found out that benzodiazepines and zolpidem produced reliable improvements in commonly measured parameters of sleep in patients with chronic insomnia [44]. Even, zolpidem in the dose of 10 mg is found to be effective in treating chronic insomnia when used intermittently, without evidence of discontinuation effects or increased frequency of pill taking [45].
Zolpidem and the Next‐Day Benefits
Although next‐day benefits with zolpidem use have not been clearly evaluated or demonstrated, a study by Saletu‐Zyhlarz indicated that there was significant improvement in somatic complaints versus placebo [46]. In this single‐blind, placebo‐controlled crossover study, 15 patients diagnosed as having nonorganic insomnia related to neurotic and stress‐related disorders were included. Objective and subjective sleep and awakening quality measures were investigated in three subsequent nights in the sleep laboratory (adaptation, baseline/placebo, and zolpidem 10 mg night), utilizing clinical, polysomnographic, psychometric, and psychophysiological methods. Statistical analysis of polysomnographic variables demonstrated a significant lengthening of the total sleep period and TST, an improvement in sleep efficiency and a shortening of sleep latencies after zolpidem as compared with placebo. These changes were opposite to the differences between patients and controls. All other tests of psychomotor function, attention, and memory, as well as subjective reports of well‐being, showed no difference compared with placebo. A meta‐analysis of more than 30 international clinical trials of single or repeated dosing, in healthy subjects or insomniac patients, showed that zolpidem appears to induce minimal next‐day residual effects [47].
One of the other studies evaluated the effect of zolpidem‐related effects on performance and mood during simulated night‐shift work [48]. Seven participants completed this 23‐day, within‐participant design study. They received a single oral zolpidem dose (0, 5, or 10 mg) 1 hr before bedtime for three consecutive days under two‐shift conditions: day shift and night shift. When participants received placebo, next‐day performance and subjective effects were disrupted, and food intake was decreased during the night shift. Zolpidem on the other hand improved subjective reports of sleep quality and, to a lesser extent, next‐day performance. Next‐day mood, however, was worsened and food intake was unaffected by zolpidem.
Zolpidem in Posttraumatic Stress Disorder (PTSD)
Zolpidem may confer advantages over conventional medications for sleep induction and maintenance in PTSD. In one of the studies done by Abramowitz et al., they evaluated the benefits of add‐on hypnotherapy with zolpidem in patients with chronic PTSD [49]. Thirty‐two PTSD patients treated by SSRI antidepressants and supportive psychotherapy were randomized to two groups: 15 patients in the first group received zolpidem 10 mg nightly for 14 nights, and 17 patients in the hypnotherapy group were treated by symptom‐oriented hypnotherapy, twice‐a‐week 1.5‐h sessions for 2 weeks. It was found that there was a significant main effect of the hypnotherapy treatment with PTSD symptoms as measured by the Posttraumatic Disorder Scale. This effect was preserved at follow‐up 1 month later. Also, in other studies as well, zolpidem appears to be beneficial in treating PTSD‐associated insomnia in most veterans and in alleviating nightmares in some veterans with PTSD [50, 51].
Zolpidem in Jet Lag
Jet lag commonly affects air travelers who cross several time zones. It results from the body's internal rhythms being out of step with the day–night cycle at the destination. Using the first‐night effect in a sleep laboratory as a model of transient insomnia, this placebo‐controlled, double‐blind, parallel‐group study evaluated the efficacy and safety of zolpidem in 462 normal volunteers [52]. Zolpidem was tested at doses of 5, 7.5, 10, 15, and 20 mg, and statistical analysis of 7.5 mg and 10 mg was compared with placebo. Compared with placebo, the 7.5 mg and 10 mg doses of zolpidem decreased sleep latency and increased sleep duration and maintenance (i.e., reduced NAW). Zolpidem (7.5 mg or 10 mg) had no significant effect on next‐day psychomotor performance. This study demonstrated that zolpidem at 7.5 mg and 10 mg is effective in the treatment of transient insomnia like the one experienced in jet lag. In one more study zolpidem 10 mg produced significant improvement in sleep following rapid transmeridian travel [53].
Effectiveness and tolerability of melatonin and zolpidem for the alleviation of jet lag was evaluated in one of the studies done by Suhner et al. [54]. The participants either received melatonin 5 mg (n = 35), zolpidem 10 mg (n = 34), a combination thereof (n = 29) or placebo (n = 39) and once daily at bedtime on four consecutive days after the flight. Subjects taking zolpidem reported significantly less jet lag and zolpidem was rated as the most effective jet lag medication.
Modified‐Release (MR) Formulation of Zolpidem
Sleep is not an acute event, but a complete 6–8‐h process. This concept makes it obvious to look for modified controlled release formulation of hypnotics. Zolpidem has been developed as zolpidem‐MR, marketed in the United States in 2005 and indicated in the treatment of insomnia [55]. Zolpidem‐MR was designed so that initial zolpidem plasma levels were comparable to those obtained with the standard formulation, followed by an extended release of zolpidem in order to maintain plasma concentrations through the middle of the night [56]. A two‐layer coated tablet was developed with biphasic zolpidem release: immediate release followed by prolonged release. Based on pharmacokinetic and pharmacodynamic studies, the optimal dose of 12.5 mg zolpidem‐MR was identified for adults and 6.25 mg for the elderly.
All the trial findings establish the efficacy of three to seven nights per week dosing of zolpidem extended‐release 12.5 mg for up to 6 months. Treatment provided sustained and significant improvements in sleep onset and maintenance and also improved next‐day concentration and morning sleepiness [57]. On the other hand concurrent use of zolpidem ER preparation with escitalopram when compared with placebo and escitalopram, though significantly improved insomnia and sleep‐related next‐day symptoms, but not the anxiety symptoms, in patients with comorbid insomnia and generalized anxiety disorder [58].
Zolpidem extended‐release has a safety profile comparable to immediate release. ER zolpidem did not significantly affect psychomotor and cognitive performance 8 h post dose compared with placebo, on the basis of neurocognitive tests of vigilance and motor and memory [59]. There are a minimal number of drug‐drug interactions with zolpidem ER, and, although they are well characterized, the pharmacology of any central nervous system‐active drug should be considered prior to administration.
Safety Profile
Zolpidem has a rapid onset, a short duration of action, and a low incidence of adverse effects. Over 30 placebo‐controlled studies demonstrate a satisfactory safety profile of zolpidem (5–10 mg) on daytime cognitive functions as compared to other hypnotics, that is, flunitrazapam, nitrazepam, and triazolam [60]. It appears to have low addictive potential and few drug interactions [61]. Number of studies suggest that zolpidem in the dose of 5 to 10 mg is safe even for longer treatment periods of up to 35 days to 3 months of treatment [62, 63]. It has also been shown not to interfere with the nocturnal sleep‐induced improvement of memory [64]. Also, in one other study insomniac patients tended to prefer zolpidem to zaleplon on both nocturnal and diurnal assessments in a randomized, double‐blind, crossover study done on 53 patients [65].
Common side effects of the drug include drowsiness (5%), dizziness (5%), headache (3%), gastrointestinal symptoms (4%), memory problems (1–2%), nightmares (1–2%), and confusion (1–2%) [66]. Few other side effects include ataxia or poor motor coordination, difficulty maintaining balance, euphoria and/or dysphoria, increased appetite, increased libido, impaired judgment and reasoning, uninhibited extroversion in social or interpersonal settings and increased impulsivity.
One retrospective case‐control study demonstrated that use of zolpidem by older people was associated with nearly twice the risk of hip fracture [67], although evidence generally points to the fact that longer acting hypnotic agents are more likely to be associated with falls and hip fractures [68]. This became evident with one of the reviews that aimed to establish the relationship between treatment with hypnotics and the risk of postural instability and as a consequence, falls and hip fractures, in the elderly. The review concluded that benzodiazepines are the major class of hypnotics involved in the causation of falls and fractures, whereas Z‐compounds are less frequently reported in this context, with zolpidem considered at risk only in one study [69]. The relationship between hypnotic use and falls is complicated by the fact that sleep problems among elderly people are independently associated with an increased risk of falls [70].
Hallucinatory phenomena and other sensory distortions have been reported even with therapeutic doses of zolpidem [71]. Possibly because of its limited efficacy for sleep maintenance problems, patients may take higher than recommended doses or take a second dose during the night, which may increase the risk of both acute side effects and next‐day residual effects. Psychotic reactions with predominant visual hallucinations after the ingestion of zolpidem have been reported in the literature [72, 73]. Retrograde amnestic effects shortly after administration have also been observed, a likely consequence of zolpidem's pharmacological action on α1 GABAA receptors [74].
One of the studies conducted by Zammit et al. evaluated the effect of ramelteon and zolpidem (as positive control) on middle‐of‐the‐night balance, mobility, and memory in older insomniacs. Thirty‐three older adults (age ≥65 years) with insomnia were enrolled in a single‐dose, 3‐way crossover study of balance after bedtime administration of ramelteon, 8 mg; zolpidem, 10 mg (positive control); or placebo. Subjects were administered study medication 30 min before bedtime and were awakened 2 h after dosing to evaluate balance (Sensory Organization Test), turning speed and stability, memory (immediate and delayed word recall), and adverse events. Ramelteon or zolpidem (positive control) was compared with placebo. It was seen that there were no differences between placebo and ramelteon on the Sensory Organization Test (P= 0.837), turn time (P= 0.776), or turn sway (P= 0.982). Though, the positive control (zolpidem) did reveal significant impairments on the Sensory Organization Test, turn time, and turn sway (P < 0.001, all). Immediate recall declined significantly with zolpidem (P= 0.002).
The effects of therapeutic oral doses of prolonged release melatonin (PR‐M) (2 mg), zolpidem (10 mg) and their combination administered at bedtime on cognitive functions in healthy subjects aged 55 years and older were assessed in a randomized, double‐blind, placebo‐controlled, and four‐way crossover study [75]. Psychomotor functions, memory recall, and driving skills were assessed at 1 and 4 h following administration and the next morning. It was seen that compared to placebo, PR‐M alone did not impair performances on any cognitive tasks, but zolpidem significantly impaired psychomotor and driving performance 1 h and 4 h post dosing, and early memory recall. This impairment was also exacerbated with PR‐M coadministration.
Chiang et al. report two cases in which amnestic sleep‐related eating disorder (SRED) occurred with ER zolpidem but not with the immediate‐release formulation. These cases illustrate how even relatively small differences such as formulation can affect the likelihood of experiencing such events [76]. Further to this, a case was observed in which zolpidem was associated with amnestic nocturnal eating [77].
The evidence linking zolpidem with bizarre sleep‐related behaviors consists of postmarketing surveillance and isolated case reports. Although sleep‐related behaviors have been reported with other hypnotics, the therapeutic goods administration considers that the pattern of reports with zolpidem signals an increased risk of these events with this drug. The incidence of these events is not known, but the wide international use of zolpidem over the last 15 years suggests that they are rare. Published reports of sleepwalking, phone conversations, and hallucinations emerged with zolpidem in the mid 1990s. Since then, other unusual sleep‐related events have been described internationally, including preparing and eating food, compulsive house cleaning, sleep‐driving, and house painting, in addition to reports of sleepwalking [78, 79]. People exhibiting these sleep‐related behaviors have consistently reported no memory of the event. Most events occurred after the first dose of zolpidem or within a few days of starting therapy. In most cases the behavior resolved when zolpidem was stopped. Alcohol use, other CNS depressants, and taking more than the recommended dose of zolpidem are likely to increase the risk of these events. Adult onset sleep walking in psychiatric patients has also been related to the life time use of zolpidem [80].
On looking out for acute effects of zolpidem on daytime alertness, psychomotor and physical performance in a double‐blind crossover study where seven athletes received zolpidem (10 mg) or placebo in two sessions over 2 nights, it was found that zolpidem has a hypnotic activity without disturbing psychomotor and physical performance on the following day when given to healthy adults. This suggests that zolpidem may be used in healthy athletes to adjust their extrinsic sleep disturbances and their consecutive psychomotor and physical impairments [81]. On the other hand, on looking at the residual effects of middle‐of‐the‐night administration of zaleplon 10 or 20 mg, zolpidem 10 or 20 mg, or placebo on driving ability, memory functions, and psychomotor performance in a double‐blind, five‐period crossover design, it was seen that memory and psychomotor test performance was unaffected after both doses of zaleplon and zolpidem 10 mg. But, in contrast, zolpidem 20 mg significantly increased standard deviation of lateral position and speed variability. Also, zolpidem 20 mg significantly impaired performance on all psychomotor and memory tests [82]. On comparison of the effects of zaleplon, zolpidem, and triazolam on memory, learning, and psychomotor performance, it was seen that zaleplon 10 mg did not produce any significant changes in memory or learning compared with placebo, whereas all other active treatments, including zolpidem 10 mg, caused psychomotor impairment at the 1.25‐h test battery. Zolpidem 20 mg (twice the therapeutic dose) produced more psychomotor impairment at the 1.25‐h assessment than did any of the other active treatments, including zaleplon 20 mg [83].
One more study comparing psychomotor, cognitive, and subjective effects of triazolam (0.125, 0.25, and 0.5 mg/70 kg) and zolpidem (5, 10, and 20 mg/70 kg) in a double‐blind, placebo‐controlled, crossover design in healthy volunteers showed that triazolam produced significantly more impairment than zolpidem in time estimation. Triazolam, but not zolpidem, produced significant impairment on a short‐term memory task and zolpidem produced significantly more impairment than triazolam on several novel measures of performance on a computerized trail‐making test. Whereas, one further study suggests that the effects of daytime administration of zolpidem (5, 10, or 15 mg) and triazolam (0.125, 0.25, or 0.5 mg) on performance‐impairing effects are dose‐dependent and functionally coupled to their sleep‐inducing properties [84].
Most side effects are dose‐related, occurring at doses above 20 mg per day. Hence patients should fully comply with the prescription instructions of zolpidem, that is, to take the medication just prior to a full 8 h of uninterrupted sleep. If this strategy is adopted, zolpidem is a safe alternative to benzodiazepine hypnotics that do show significant driving impairment the morning following bedtime administration.
Zolpidem Withdrawal, Abuse, and Seizures
Although substance abusers may abuse benzodiazepines, they rarely abuse nonbenzodiazepines [85]. Zolpidem is assumed to have a lower potential to develop tolerance and dependence than benzodiazepines. However, cases of zolpidem abuse and dependence have been reported since 1993 [86]. Seizure is a rare and serious withdrawal symptom of zolpidem and was first reported in 1996. This seems to develop in those patients who had taken supratherapeutic doses for a long time and discontinued it abruptly. Even successful zolpidem detoxification with a benzodiazepine has been reported [87].
In addition, there have been case reports of dependence following prolonged use in patients with histories of substance abuse [88], and the World Health Organization has indicated that rates of abuse and dependency for zolpidem appear to be similar to those for some of the benzodiazepines (World Health Organization Expert Committee 2001, 2003) [89]. This report highlights the fact that zolpidem has drug abuse potential and dependence liability.
Cost‐Effectiveness
The cost of nonbenzodiazepines is considerably higher than benzodiazepines. An economic evaluation comparing the cost‐effectiveness of nonpharmacologic treatment, benzodiazepines, eszopiclone, and no treatment in older adults found that, compared with benzodiazepines, nonpharmacologic therapy produced a net gain of 0.37 quality‐adjusted life‐years at a savings of $2,781 over 10 years [90]. The short‐acting drugs seem equally effective and safe.
On the other hand, when a comparative review of temazepam and zolpidem use in managing insomnia in the hospice patient was undertaken by Bain et al., to determine whether treatment with temazepam is a more cost‐effective approach for their patient population, they found out that after review of the primary literature and the prescribing patterns in their hospital setting, they had no evidence to support that zolpidem is superior to benzodiazepines for the treatment of insomnia [91].
Conclusions
Insomnia may appear with different presentations: sleep onset, sleep maintenances, sleep offset, nonrestorative sleep, or a combination of these symptoms. Untreated symptoms result in clinically significant distress or impairment in social, occupational, or other important areas of following‐day functionality. Benzodiazepine hypnotic agents were the mainstream pharmacotherapy for insomnia from the 1960s to the 1980s, but their safety profile proved to be not quite as perfect as originally expected.
The nonbenzodiazepine compounds generally represent an improvement over benzodiazepines as a result of improved binding selectivity and pharmacokinetic profiles. However, the enduring potential for amnestic effects, next‐day residual sedation, and abuse and physical dependence, particularly at higher doses, underscores the need for new treatment strategies.
Physicians, pharmacists, and other clinicians should be aware of the conditions that contribute to, are antecedent to, and associated with insomnia. These pathophysiological conditions include advanced age; female gender; respiratory, gastrointestinal, vascular, and rheumatologic pain syndromes; and other conditions such as depression and/or anxiety. Additional health factors contributing to insomnia include chronic pain, stressors, grief reaction, pharmacotherapeutic side effects, lifestyle contributors such as social/recreational drugs, phytopharmaceuticals, and ethanol use.
Zolipidem is a nonbenzidiazepine imidazo‐pyridine compound that acts by enhancing GABAA receptor function. It has high affinity toward α1 containing omega 1 receptor subtype. This selective binding confers upon it a strong hypnotic property. It has rapid onset of action with minimal rebound effects. It has been established as an effective sleep onset agent and studies have shown its efficacy in inducing sleep with minimal effect on sleep duration or sleep maintainance. Low‐dose sublingual zolpidem is shown to be effective in middle‐of‐the‐night insomnia. A study by Hajak et al. shows promising results if zolpidem is given three to five times a week for chronic insomnia. It is incidentally one of the two drugs, which has been evaluated with benefit in chronic comorbid insomnia. Zolpidem has also shown some benefits in posttraumatic stress disorder in few studies. On the whole the drug demonstrates a good safety profile in comparison with other hypnotics and has low addictive potential and few drug interactions. Common adverse effects reported include drowsiness, dizziness, headache, and gastrointestinal symptoms. Zolpidem seems to be a good new hypnotic with a lot of hope and has a higher safety profile unlike the traditional benzodiazepines but is a little more expensive.
Limitations of the Review
Authors of the manuscript admit to various limitations of the present review being done by them. This includes the publication bias that tends to lead to the publication of mostly positive studies as the negative findings about a given drug are less well disseminated. This becomes clear with one of the reviews done by Hopewell et al., who assessed the extent to which publication of a cohort of clinical trials is influenced by the statistical significance, perceived importance, or direction of their results [92]. They did a thorough “Cochrane Methodology Register” review and found that trials with positive findings are published more often, and more quickly than trials with negative findings or null findings. Also, it has been seen that clinical trial design can influence treatment outcome in patients. This becomes evident with one of the studies conducted by Rutherford et al., where they showed that response and remission rates to antidepressants are significantly affected by study type as well as duration [93]. This too limits our work of review in the present form.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
Authors would like to acknowledge SciEdit reviewers for critically analyzing and editing the manuscript before the final submission.
References
- 1. Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychintr Res 2003;27:9–15. [DOI] [PubMed] [Google Scholar]
- 2. Kiley J. Insomnia research and future opportunities. Sleep 1999;22(Suppl 1):S344–S345. [PubMed] [Google Scholar]
- 3. Ohayon MM. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med Rev 2002;6:97–111. [DOI] [PubMed] [Google Scholar]
- 4. Roth T. New developments for treating sleep disorders. J Clin Psychiatr 2001;10:3–4. [PubMed] [Google Scholar]
- 5. Sateia MJ, Nowell PD. Insomnia. Lancet 2004;364:1959–1973. [DOI] [PubMed] [Google Scholar]
- 6. Eddy M, Walbroehl G. Insomnia. Am Fam Physician 1999;59:1911–1916. [PubMed] [Google Scholar]
- 7. Ruth M, Benca . Diagnosis and treatment of chronic insomnia: A review. Psychiatr Serv 2005;56:332–343. [DOI] [PubMed] [Google Scholar]
- 8. National Institute of Health . National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults. Sleep 2005;28:1049–1057. [DOI] [PubMed] [Google Scholar]
- 9. American Psychiatric Association . Diagnostic and statistical manual of mental disorders, 4th ed Text Revision. Washington , DC : American Psychiatric Association, 2002. [Google Scholar]
- 10. Buscemi N, Vandermeer B, Friesen C, et al Manifestations and management of chronic insomnia in adults. Evid Rep Technol Assess 2005;125:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ohayon MM, Paiva T. Global sleep dissatisfaction for the assessment of insomnia severity in the general population of Portugal. Sleep Med 2005;6:435–441. [DOI] [PubMed] [Google Scholar]
- 12. Elwood P, Hack M, Pickering J, et al Sleep disturbance, stroke, and heart disease events: Evidence from the Caerphilly cohort. J Epidemiol Commun Health 2006;60:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drake CL, Roehrs TA, Burduvali E. Effects of rapid versus slow accumulation of eight hours of sleep loss. Psychophysiology 2001;38:979–987. [DOI] [PubMed] [Google Scholar]
- 14. Rosenthal L, Roehrs TA, Rosen A. Level of sleepiness and total sleep time following various time in bed conditions. Sleep 1993;16:226–232. [DOI] [PubMed] [Google Scholar]
- 15. Gottlieb DJ, Punjabi NM, Newman AB, et al Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 2005;165:863–867. [DOI] [PubMed] [Google Scholar]
- 16. Varkevisser M, Kerkhof GA. Chronic insomnia and performance in a 24‐h constant routine study. J Sleep Res 2005;14:49–59. [DOI] [PubMed] [Google Scholar]
- 17. Meyer TJ. Evaluation and management of insomnia. Hosp Pract (Off Ed) 1998;33:75–78, 83–86. [DOI] [PubMed] [Google Scholar]
- 18. Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc 2001;49:1185–1189. [DOI] [PubMed] [Google Scholar]
- 19. Lacks P, Morin C. Recent advances in the assessment and treatment of insomnia. J Consult Clin Psychol 1992;60:586–594. [DOI] [PubMed] [Google Scholar]
- 20. American Academy of Sleep Medicine . International classification of sleep disorders: Diagnostic and coding manual, 2nd ed Westchester , IL : American Academy of Sleep Medicine, 2005. [Google Scholar]
- 21. NHLBI Working Group on Insomnia . Insomnia: Assessment and Management in Primary Care 1998; NIH Publication No. 98‐4088.
- 22. Pritchett DB, Seeburg PH. Gamma‐aminobutyric acidA receptor alpha 5‐subunit creates novel type II benzodiazepine receptor pharmacology. J Neurochem 1990;54:1802–1804. [DOI] [PubMed] [Google Scholar]
- 23. Salvà P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications. Clin Pharmacokinet 1995;29:142–153. [DOI] [PubMed] [Google Scholar]
- 24. Rowlett JK, Woolverton WL. Assessment of benzodiazepine receptor heterogeneity in vivo: Apparent pA2 and pKB analyses from behavioral studies. Psychopharmacology 1996;128:1–16. [DOI] [PubMed] [Google Scholar]
- 25. Perrais D, Ropert N. Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABAA receptors in central synapses. J Neurosci 1999;19:578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buscemi N, Vandermeer B, Friesen C, et al Manifestations and management of chronic insomnia in adults. Evid Rep Technol Assess 2005;125:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holm KJ, Goa KL. Zolpidem: An update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs 2000;59:865–889. [DOI] [PubMed] [Google Scholar]
- 28. IMS Health . National prescription audit plus. Fairfield , CT: IMS Health, 2003. [Google Scholar]
- 29. Scharf MB, Roth T, Vogel GW, et al A multicenter, placebo‐controlled study evaluating zolpidem in the treatment of chronic insomnia. J Clin Psychiat 1994;55:192–199. [PubMed] [Google Scholar]
- 30. Saletu‐Zyhlarz G, Anderer P, Brandstatter N, et al Placebo‐controlled sleep laboratory studies on the acute effects of zolpidem on objective and subjective sleep and awakening quality in nonorganic insomnia related to neurotic and stress‐related disorder. Neuropsychobiology 2000;41:139–148. [DOI] [PubMed] [Google Scholar]
- 31. Erman MK, Erwin CW, Gengo FM, et al Comparative efficacy of zolpidem and temazepam in transient insomnia. Hum Psychopharmacol 2001;16:169–176. [DOI] [PubMed] [Google Scholar]
- 32. Priest RG, Terzano MG, Parrino L, Boyer P. Efficacy of zolpidem in insomnia. Eur Psychiatry 1997;12(Suppl 1):5s–14s. [DOI] [PubMed] [Google Scholar]
- 33. Holm KJ, Goa KL. Zolpidem: An update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs 2000;59:865–889. [DOI] [PubMed] [Google Scholar]
- 34. Ohayon MM, Roth T. What are the contributing factors for insomnia in the general population? J Psychosom Res 2001;51:745–755. [DOI] [PubMed] [Google Scholar]
- 35. Roth T, Hull SG, Lankford DA, Rosenberg R, Scharf MB. Low‐dose sublingual zolpidem tartrate is associated with dose‐related improvement in sleep onset and duration in insomnia characterized by middle‐of‐the‐night (MOTN) awakenings. Sleep 2008;31:1277–1284. [PMC free article] [PubMed] [Google Scholar]
- 36. Kupfer DF, Reynolds CF. Management of insomnia. N Engl Med 1997;336:341–346. [DOI] [PubMed] [Google Scholar]
- 37. Hajak G, Geisler P. Experience with zolpidem ‘as needed’ in primary care settings. CNS Drugs 2004;18(Suppl 1):35–40; discussion 41, 43–45. [DOI] [PubMed] [Google Scholar]
- 38. Moul DE, Nofzinger EA, Pilkonis PA, et al Symptom reports in severe chronic insomnia. Sleep 2002;25:553–563. [PubMed] [Google Scholar]
- 39. Riemann D, Berger M, Voderholzer U. Sleep and depression – results from psychobiological studies: An overview. Biol Psychol 2001;57:67–103. [DOI] [PubMed] [Google Scholar]
- 40. Neubauer DN. The evolution and development of insomnia pharmacotherapies. J Clin Sleep Med 2007;3(5 suppl):S11–S15. [PMC free article] [PubMed] [Google Scholar]
- 41. Asnis GM, Chakraburtty A, DuBoff EA, et al Zolpidem for persistent insomnia in SSRI‐treated depressed patients. J Clin Psychiatry 1999;60:668–676. [DOI] [PubMed] [Google Scholar]
- 42. Allard S, Sainati SM, Roth‐Schechter BE. Coadministration of short‐term zolpidem with sertraline in healthy women. J Clin Pharmucol 1999;39:184–191. [DOI] [PubMed] [Google Scholar]
- 43. Fava M, Asnuis G, Shrivastava R. Zolpidem extended release, co‐administered with escitalopram, improves insomnia in patients with comorbid insomnia and major depressive disorder. Paper presented at: American Psychiatric Association Annual Meeting; 2008 ; San Francisco , CA .
- 44. Nowell PD, Mazumdar S, Buysse DJ, et al Benzodiazepines and zolpidem for chronic insomnia: A meta‐analysis of treatment efficacy. JAMA 1997;278:2170–2177. [PubMed] [Google Scholar]
- 45. Cluydts R, Peeters K, de Bouyalsky I, et al Comparison of continuous versus intermittent administration of zolpidem in chronic insomniacs: A double‐blind, randomized pilot study. J Int Med Res 1998;26:13–24. [DOI] [PubMed] [Google Scholar]
- 46. Saletu‐Zyhlarz G, Anderer P, Brandstatter N, et al Placebo‐controlled sleep laboratory studies on the acute effects of zolpidem on objective and subjective sleep and awakening quality in nonorganic insomnia related to neurotic and stress‐related disorder. Neuropsychobiology 2000;41:139–148. [DOI] [PubMed] [Google Scholar]
- 47. Uden M, Schechter BR. Next day effects after night‐time treatment with zolpidem: A review. Eur Psychiatry 1996;11(Suppl 1):21s–30s. [Google Scholar]
- 48. Hart CL, Ward AS, Haney M, et al Zolpidem‐related effects on performance and mood during simulated night‐shift work. Exp Clin Psychopharmacol 2003;11:259–268. [DOI] [PubMed] [Google Scholar]
- 49. Abramowitz EG, Barak Y, Ben‐Avi I, et al Hypnotherapy in the treatment of chronic combat‐related PTSD patients suffering from insomnia: A randomized, zolpidem‐controlled clinical trial. Int J Clin Exp Hypn 2008;56:270–280. [DOI] [PubMed] [Google Scholar]
- 50. Dieperink ME, Drogemuller L. Zolpidem for insomnia related to PTSD. Psychiatr Serv 1999;50:421. [DOI] [PubMed] [Google Scholar]
- 51. Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post‐traumatic stress disorder: Epidemiology, impact and approaches to management. CNS Drugs 2006;20:567–590. [DOI] [PubMed] [Google Scholar]
- 52. Roth T, Roehrs T, Vogel G. Zolpidem in the treatment of transient insomnia: A double‐blind, randomized comparison with placebo. Sleep 1995;18:246–251. [DOI] [PubMed] [Google Scholar]
- 53. Jamieson AO, Zammit GK, Rosenberg RS, et al Zolpidem reduces the sleep disturbance of jet lag. Sleep Med 2001;2:423–430. [DOI] [PubMed] [Google Scholar]
- 54. Suhner A, Schlagenhauf P, Höfer I, et al Effectiveness and tolerability of melatonin and zolpidem for the alleviation of jet lag. Aviat Space Environ Med 2001;72:638–646. [PubMed] [Google Scholar]
- 55. Hindmarch I, Legangneux E, Stanley N. A randomized double‐blind placebo‐controlled 10‐way cross‐over study shows that a new modified‐release formulation improves sleep maintenance compared to standard zolpidem. Sleep 2004;27:A55. [Google Scholar]
- 56. Weinling E, Andre F, Dubruc C, et al Pharmacokinetic profile of a new modified‐release formulation of zolpidem designed to improve sleep maintenance. Sleep 2004;27:A271. [DOI] [PubMed] [Google Scholar]
- 57. Krystal AD, Erman M, Zammit GK, et al Long term efficacy and safety of zolpidem extended‐release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: A 6‐month, randomized, doubleblind, placebo‐controlled, parallel‐group, multicenter study. Sleep 2008;31:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fava M, Asnis GM, Shrivastava R, et al Zolpidem extended‐release improves sleep and next‐day symptoms in comorbid insomnia and generalized anxiety disorder. J Clin Psychopharmacol 2009;29:222–230. [DOI] [PubMed] [Google Scholar]
- 59. Blin O, Micallef‐Roll J, Audebert C, et al A double‐blind, placebo‐ and flurazepam‐controlled investigation of the residual psychomotor and cognitive effects of modified release zolpidem in young healthy volunteers. J Clin Psychopharmacol 2006;26:284–289. [DOI] [PubMed] [Google Scholar]
- 60. DeClerk AC, Bisserbe JC. Short‐term safety profile of zolpidem: Objective measures of cognitive effects. Eur Psychiatry 1997;12(Suppl I):15s–20s. [DOI] [PubMed] [Google Scholar]
- 61. Evans SM, Funderburk FR, Griffiths RR. Zolpidem and triazolam in humans: Behavioral and subjective effects and abuse liability. J Pharmacol Exp Theraputics 1990;255:1246–1255. [PubMed] [Google Scholar]
- 62. Scharf MB, Roth T, Vogel GW, et al A multicenter, placebo‐controlled study evaluating zolpidem in the treatment of chronic insomnia. J Clin Psychiatry 1994;55:192–199. [PubMed] [Google Scholar]
- 63. Perlis ML, McCall WV, Krystal AD, et al Long‐term, non‐nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry 2004;65:1128–1137. [DOI] [PubMed] [Google Scholar]
- 64. Meléndez J, Galli I, Boric K, et al Zolpidem and triazolam do not affect the nocturnal sleep‐induced memory improvement. Psychopharmacology (Berl) 2005;181:21–26. [DOI] [PubMed] [Google Scholar]
- 65. Allain H, Bentué‐Ferrer D, Breton SL, et al Preference of insomniac patients between a single dose of zolpidem 10 mg versus zaleplon 10 mg. Hum Psychopharmacol 2003;18:369–374. [DOI] [PubMed] [Google Scholar]
- 66. Diagnostic Classification Steering Committee of the America Sleep Disorders Association . International classification of sleep disorders—diagnostic and coding manual. Rochester, MN : American Sleep Disorders Association, 1990. [Google Scholar]
- 67. Wang PS, Bohn RL, Glynn RJ, et al Zolpidem use and hip fractures in older people. J Am Geriatrics Soc 2001;49:1685–1690. [DOI] [PubMed] [Google Scholar]
- 68. Mendelson WB. Clinical distinctions between long‐acting and short‐acting benzodiazepines. J Clin Psychiatry 1992;53(suppl):4–7. [PubMed] [Google Scholar]
- 69. Allain H, Bentué‐Ferrer D, Polard E, et al Postural instability and consequent falls and hip fractures associated with use of hypnotics in the elderly: A comparative review. Drugs Aging 2005;22:749–765. [DOI] [PubMed] [Google Scholar]
- 70. Brassington GS, King AC, Bliwise DL. Sleep problems as a risk factor for falls in a sample of community‐dwelling adults aged 64–99 years. J Am Geriatrics Soc 2000;48:1234–1240. [DOI] [PubMed] [Google Scholar]
- 71. Pies RW. Dose‐related sensory distortions with zolpidem. J Clin Psychiatry 1995;56:35–36. [PubMed] [Google Scholar]
- 72. Ansseau M, Pichot W, Hansenne M, Moreno AG. Psychotic reactions to zolpidem. Lancet 1992;339:809. [DOI] [PubMed] [Google Scholar]
- 73. Iruela LM, Ibanez‐Rojo V, Bace E. Zolpidem‐induced macropsia in anorexic woman. Lancet 1993;342:443–444. [DOI] [PubMed] [Google Scholar]
- 74. Patat A, Paty I, Hindmarch I. Pharmacodynamic profile of Zaleplon, a new non‐benzodiazepine hypnotic agent. Hum Psychopharmacol 2001;16:369–392. [DOI] [PubMed] [Google Scholar]
- 75. Otmani S, Demazières A, Staner C, et al Effects of prolonged‐release melatonin, zolpidem, and their combination on psychomotor functions, memory recall, and driving skills in healthy middle aged and elderly volunteers. Hum Psychopharmacol 2008;23:693–705. [DOI] [PubMed] [Google Scholar]
- 76. Chiang A, Krystal A. Report of two cases where sleep related eating behavior occurred with the extended‐release formulation but not the immediate‐release formulation of a sedative‐hypnotic agent. J Clin Sleep Med 2008;4:155–156. [PMC free article] [PubMed] [Google Scholar]
- 77. Dang A, Garg G, Rataboli PV. Zolpidem induced nocturnal sleep‐related eating disorder (NSRED) in a male patient. Int J Eat Disord 2009;42:385–386. [DOI] [PubMed] [Google Scholar]
- 78. Doan JA, Dalpiaz AZ. Zolpidem‐induced sleep‐driving. Am J Med 2008;121:e5. [DOI] [PubMed] [Google Scholar]
- 79. Adverse Drug Reactions Advisory Committee . Zolpidem and bizarre sleep related effects. Austr Adverse Drug React Bull 2007. Available from: http://www.tga.gov.au/adr/aadrb/aadr0702.htm [Accessed 7 August 2009. [Google Scholar]
- 80. Lam SP, Fong SY, Yu MW, et al Sleepwalking in psychiatric patients: Comparison of childhood and adult onset. Aust N Z J Psychiatry 2009;43:426–430. [DOI] [PubMed] [Google Scholar]
- 81. Ito SU, Kanbayashi T, Takemura T, et al Acute effects of zolpidem on daytime alertness, psychomotor and physical performance. Neurosci Res 2007;59:309–313. [DOI] [PubMed] [Google Scholar]
- 82. Verster JC, Volkerts ER, Schreuder AH, et al Residual effects of middle‐of‐the‐night administration of zaleplon and zolpidem on driving ability, memory functions, and psychomotor performance. J Clin Psychopharmacol 2002;22:576–583. [DOI] [PubMed] [Google Scholar]
- 83. Troy SM, Lucki I, Unruh MA, et al Comparison of the effects of zaleplon, zolpidem, and triazolam on memory, learning, and psychomotor performance. J Clin Psychopharmacol 2000;20:328–337. [DOI] [PubMed] [Google Scholar]
- 84. Wesensten NJ, Balkin TJ, Belenky GL. Effects of daytime administration of zolpidem and triazolam on performance. Aviat Space Environ Med 1996;67:115–120. [PubMed] [Google Scholar]
- 85. Darcourt G, Pringuey D, Salliere D, et al The safety and tolerability of zolpidem—an update. J Psychopharmacol 1999;13:81–93. [DOI] [PubMed] [Google Scholar]
- 86. Cavallaro R, Regazzetti MG, Covelli G, et al Tolerance and withdrawal with zolpidem. Lancet 1993;342:374–375. [DOI] [PubMed] [Google Scholar]
- 87. Ravishankar A, Carnwath T. Zolpidem tolerance and dependence: Two case reports. J Psychopharmacol 1998; 12:103–104. [DOI] [PubMed] [Google Scholar]
- 88. Hajak G, Muller WE, Wittchen HU, et al Abuse and dependence potential for the non‐benzodiazepine hypnotics zolpidem and zopiclone: A review of case reports and epidemiological data. Addiction 2003;98:1371–1378. [DOI] [PubMed] [Google Scholar]
- 89. World Health Organization Expert Committee on Drug Dependence . Thirty‐third report, 915 World Health Organ Tech Rep Ser 915, pp. 1–26. World Health Organization, 2003.
- 90. Bell L, Tousignant P. The treatment of insomnia in the elderly: A cost utility analysis [meeting abstract]. Med Decis Making 1999;18:487. [Google Scholar]
- 91. Bain KT, Weschules DJ, Knowlton CH, et al Toward evidence‐based prescribing at end of life: A comparative review of temazepam and zolpidem for the treatment of insomnia. Am J Hosp Palliat Care 2003;20:382–388. [DOI] [PubMed] [Google Scholar]
- 92. Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst Rev 2009; DOI: 10.1002/14651858.MR000006.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rutherford BR, Sneed JR, Roose SP. Does study design influence outcome? The effects of placebo control and treatment duration in antidepressant trials. Psychother Psychosom 2009;78:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]


