SUMMARY
Aims: It is unknown whether hypothermia can disrupt the progress of epileptogenesis. The present study aimed to determine the effect of hypothermia on brain edema and epileptogenesis and to establish whether brain edema is associated with epileptogenesis after severe status epilepticus (SE). Methodology: Rats were injected with a single dose of Kainic acid (KA) to produce either chronic epileptic rats (rats with spontaneous recurrent seizure, SRS) or rats without spontaneous recurrent seizure (no‐SRS rats). A second KA injection was used to induce SE in SRS rats and in no‐SRS rats. The number of SRS was counted and the brain edema induced by SE was assessed by brain water content measurement. The cognitive function was assessed by the radial‐arm maze (RAM) test. Results: A second KA injection resulted in brain edema that was more severe in SRS rats than in no‐SRS rats. After second injection of KA, hypothermia treatment attenuated the KA induced brain edema and reduced the SRS attack in SRS rats. Additionally cognitive function was better in hypothermia‐treated SRS rats than in nomothermia treated SRS rats 1 month after the second KA injection. Conclusions: Hypothermia treatment immediately after SE not only exhibited protective effects against the chronic spontaneous recurrent convulsant seizures but also improved cognitive function. These antiepileptogenic properties of hypothermia may be related to its attenuating effect on brain edema induced by SE. They therefore suggest that brain edema may be involved in the progress of epileptogenesis.
Keywords: Brain edema, Cognitive function, Hypothermia, Kainic acid, Spontaneous recurrent seizure
Introduction
Status epilepticus (SE) is a neurological emergency associated with well‐known acute complications and serious long‐term consequences of epileptogenesis and cognitive dysfunction. Thus, SE should be terminated immediately to reduce long‐term deleterious consequences. However, 31–43% of SE is refractory to current anticonvulsant drug treatment [1, 2]. The currently available antiepileptic drugs (AEDs) exert their seizure suppressing effects either through directly affecting synaptic interactions or through reducing the generation of action potentials [3]. Brain edema has also been suggested to play a role in epileptogenesis. Diffusion‐weighted magnetic resonance imaging (MRI) imaging showed that not only cytotoxic edema but also vasogenic edema exists in partial or general convulsive SE in humans [4] and in animals [5]. Further, the edema reducing agents, furosemide and mannitol, significantly suppressed spontaneous epileptic spikes and electrical stimulation‐evoked epileptiform discharges and even completely blocked all epileptic activity in some patients without suppressing normal electroencephalographic activity [6], suggesting that brain edema plays a critical role in modulating the epileptogenicity of the human brain.
Hypothermia has been clinically used to reduce brain damage in cerebral hemorrhage and infarction [7] or in traumatic brain injury [8]. One of the mechanisms underlying the neuroprotective effects of hypothermia in acute stroke is reduction of brain edema [9, 10]. Currently, hypothermia, a supplementary treatment regimen for epilepsy, has been shown to significantly diminish epileptic activity in patients [11] and in animals [12]. However, the mechanisms through which hypothermia treatment is effective in treating epilepsy are unclear. It is not known whether hypothermia suppresses epileptogenesis partially through reducing brain edema. In this study, we focus on the effects of hypothermia on brain edema induced by generalized convulsive SE. In order to mimic a clinically relevant scenario, we induced hypothermia after SE was elicited in epileptic rats (rats with spontaneous recurrent seizure, SRS) and monitored morphological and functional outcomes after hypothermia treatment.
Materials and Methods
Experimental Animals
Adult Sprague–Dawley rats aged 10–12 weeks weighing 240–300 g were used to induce SE. Rats were housed under standard conditions including a 12‐h light‐dark cycle with free access to laboratory food and water. Room temperature was maintained at 20 ± 1°C and the air humidity of the room kept at around 50%. The research complied with national legislation on the Care and Use of animals.
Experiment 1
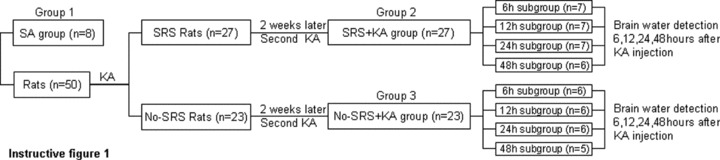
Group 1 (SA group, n = 8): Saline was injected subcutaneously (s.c.).
The experimental rats were injected with Kainic acid (KA, 12 mg/kg, 5 mg/mL, s.c. pH 7.3) to elicit SE and then video monitored 6 h per day for 2 weeks for SRS recording. Those rats showing SRS were regarded as SRS rats (n = 27), those without SRS as no‐SRS rats (n = 23).
Group 2 (SRS + KA group, n = 27): 2 weeks after the initial SE was elicited, the SRS rats were re‐injected with KA (12 mg/kg, 5 mg/mL, s.c. pH 7.3) to elicit a second SE. SRS rats were randomly divided into four subgroups: 6 (n = 7), 12 (n = 7), 24 (n = 7), and 48 h (n = 6) after the second SE.
Group 3 (no‐SRS + KA group, n = 23): 2 weeks after the initial SE was elicited, the no‐SRS rats were re‐injected with KA (12 mg/kg, 5 mg/mL, s.c. pH 7.3) to elicit the second SE. The no‐SRS rats were also randomly divided into four subgroups: 6 (n = 6), 12 (n = 6), 24 (n = 6), and 48 h (n = 5) after the second SE.
At 6, 12, 24, and 48 h after the second SE, rats were sacrificed and the brain water content of group 2 and 3 rats was determined. The brain water content of group 1 rats was also determined. Group division and experimental steps were illustrated as in instructive Figure 1.
Figure 1.
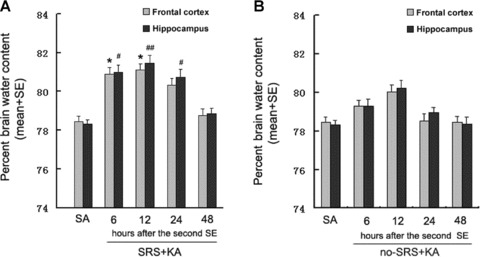
(A) Brain water content after KA‐induced SE in rats with SRS. The water content (%) is significantly increased in SRS + KA group compared with SA group both in the frontal cortex (6 and 12 h after KA treatment) and in the hippocampus (6, 12, and 24 h after KA treatment). different than SA groups in the frontal cortex, *P < 0.05; different than SA group in the hippocampus, #P < 0.05, ##P < 0.01. (B) Brain water content after KA‐induced SE in rats without SRS. The water content (%) is increased but not significantly in no‐SRS + KA group compared with the SA group both in the frontal cortex and in the hippocampus. different than SA groups in the frontal cortex, P > 0.05; different than SA groups in the hippocampus, P > 0.05.
Experiment 2
The experimental groups are as outlined in instructive Figure 2.
Figure 2.
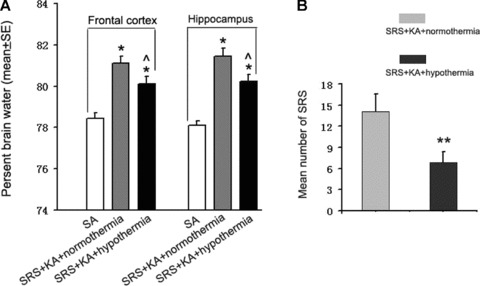
(A) Brain water content 12 h after KA‐induced SE with treatment of normothermia and hypothermia in rats with SRS. The water content (%) is significantly decreased in SRS + KA + hypothermia group compared with SRS + KA + normothermia group but still significantly increased compared with the SA group. different than SA group, *P < 0.05; different than SRS + KA + normothermia group, ∧ P < 0.05. (B) SRS number in 1 month after second KA injection in chronic epileptic rats. The SRS number is significantly decreased in SRS + KA + hypothermia group compared with SRS + KA + normothermia group. **P < 0.01.
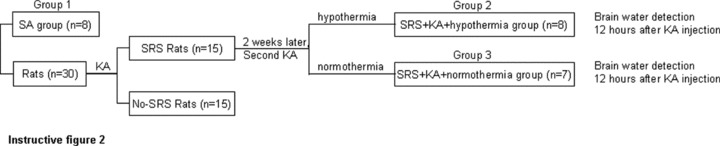
Group 1 (SA group, n = 8): Saline was injected (s.c.) and kept at room temperature.
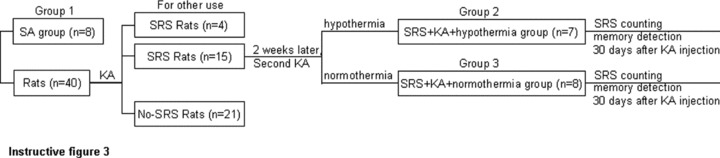
The experimental rats (n = 30) were injected with KA (12 mg/kg, 5 mg/mL, s.c. pH 7.3) to elicit SE and then video monitored 6 h per day for 2 weeks. Those showing SRS were (n = 15) randomly divided into Groups and Group 3.
Group 2 (SRS + KA + hypothermia group, n = 8): 2 weeks after the initial SE was elicited, the SRS rats were re‐injected with KA (12 mg/kg, 5 mg/mL, s.c. pH 7.3) to elicit a second SE. After the second SE was elicited the rats were immediately treated with hypothermia.
Group 3 (SRS + KA + normothermia group, n = 7): 2 weeks after the initial SE was elicited, the SRS rats were re‐injected with KA (12 mg/kg, 5 mg/mL, s.c. pH 7.3) to elicit the second SE. After the second SE was elicited the rats were not treated with hypothermia but kept at room temperature. Twelve hours after the second SE was elicited, brain water content was determined in all groups.
Experiment 3
The experimental groups are as outlined in instructive Figure 3. The experimental groups were the same as in experiment 2 with varying numbers of rats in each group (group 1, n = 8; group 2, n = 7; group 3, n = 8).
Figure 3.
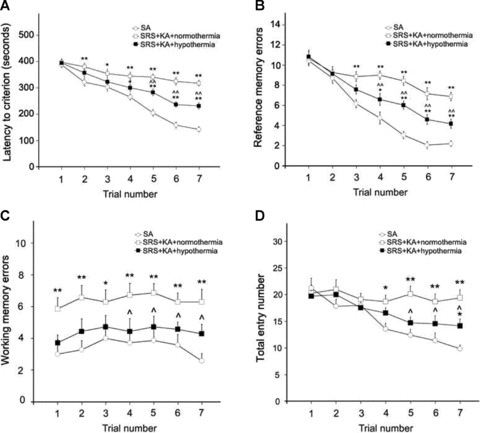
(A) The latency to criterion is significantly longer in SRS + KA + normothermia group compared with the SA group by the second trial. The latency to criterion is significantly shorter in SRS + KA + hypothermia group compared with SRS + KA + normothermia group by fifth trial, but still longer compared with SA group by fourth trial. (B) More reference errors were found in the SRS + KA + normothermia group compared with the SA group by the third trial. More reference errors were found in the SRS + KA + hypothermia group compared with the SA group by the fourth trial. Fewer reference errors were found in the SRS + KA + hypothermia group compared with the SRS + KA + normothermia group by the fourth trial. (C) More working memory errors were found in the SRS + KA + normothermia group compared with the SA group at all trials. No significant difference in working memory errors was found between the SRS + KA + hypothermia group and the SA group. But fewer working memory errors were found in the SRS + KA + hypothermia group compared with the SRS + KA + normothermia group by the fourth trial. (D) More total entries were found in the SRS + KA + normothermia group compared with the SA group by the fourth trial. More total entries were found in the SRS + KA + hypothermia group compared with the SA group by the fifth trial. But fewer total entries were found in the SRS + KA + hypothermia group compared with the SRS + KA + normothermia group by the fourth trial, different than SA group, **P < 0.01, *P < 0.05; different than SRS + KA + normothermia groups, ∧ ∧ P < 0.01, ∧ P < 0.05.
The number of SRS occuring in rats within 1 month after the second SE were counted and compared between the “SRS + KA + hypothermia” group and the “SRS + KA + normothermia” group. The number of SRS was counted through video monitoring 6 h per day. At the end of SRS counting, 1 month after the second SE, the rats were tested in the radial‐arm maze (RAM). Their performance was compared between the three groups.
Induction of SE—In the first three experiments, rats were injected with KA (Sigma Company, USA, 12 mg/kg, 5 mg/mL, s.c.). Seizure was recorded in 5 stages as defined by Racine [13]: stage 1, chewing; stage 2, head nodding; stage 3, unilateral forelimb clonus; stage 4, rearing with bilateral forelimb clonus; stage 5, rearing with bilateral forelimb clonus and falling back. After seizure reached stage 5, it was regarded as “one seizure cycle.” Only those rats that had more continuous seizures scaled in any stage of the 5 Racine stages after one seizure cycle were regarded as SE rats. To get a prolonged seizure attack model, SE model, dosage of KA injection was elevated a little to 12 mg/kg. All rats that were injected with KA developed SE ‐including KA injected normal rats, KA injected no‐SRS rats and KA injected SRS rats. The ending of SE was defined as the stopping of continuous seizures. After SE, rats were treated with hypothermia or kept at room temperature of 20 ± 1°C.
Introduction of hypothermia—Immediately after the second SE, the rats were treated with hypothermia for 1 h per time, three times daily with an interval of 7 h, for 2 days. Hypothermia treatment was conducted according to Schmitt et al. [12] with little modification. A plastic cage with gauze of 5 mm thickness nailed to the floor of the inner cage was put in a larger styrofoam box. The space between the cage and the styrofoam box was filled completely with −20°C ice packs. The gauze of 5 mm thickness nailed to the floor of the inner cage was used to protect against frostbite damage to the bodies of the rats as rats fall down after the seizures reach stage 5. The ice packs were replaced every 10 min to keep the rats’ body temperatures maintained at 30 ± 0.6°C. The normothermic rats were maintained at 37 ± 0.6°C by intermittent use of a heating lamp. Body temperature was measured rectally to reflect changes in body core temperature. This is a valid approach to measure epidural temperature as epidural temperature changes parallel changes in body core temperature measured rectally, and the epidurally measured temperature was only 0.4 ± 0.3 °C higher compared to that measured rectally [14, 15].
Brain water content—The wet–dry weight method [16] was used to measure the brain water content. Briefly, at different time points after the second KA treatment, the normothermic and hypothermic rats were anesthetized with ketamine/xylazine solution (50 mg/kg ketamine/7.5 mg/kg xylazine in 0.9% NaCl solution) then euthanized via decapitation. Brains were removed and were dissected on cold 0°C ice and water mixture. About 2 mm3 samples in volume from brain regions including hippocampus, prefrontal cortex were dissected bilaterally. After dissection, each sample of the brains was immediately weighed using an Electronic Analytical Balance (China, model FA1004, measured accuracy ±0.001%) and then placed into a 80°C oven for drying for 12 h. Brain regions were then re‐weighed and the percent water content was calculated using the formula: ([wet weight − dry weight]/wet weight) × 100.
Radial‐arm maze (RAM) test—The RAM was located in a testing room, and illuminated by a 75‐Wt incandescent lamp hung directly above the center of the maze. Adapted from Roberge et al. [17], the RAM apparatus was set up with a central octagonal area, eight arms radiated from the open central octagonal area with equal inter‐space, and the sliding doors at the conjunction with the central area. Each arm was 60 cm in length and 12 cm in width, and has a 5 cm lip around each arm. The central octagonal area was 32 cm in diameter with a 30 cm high clear plastic wall. The sliding doors were made with transparent plastic which allowed the animals see the entry into the arms. The sliding doors could be operated by the experimenter by means of overhead lines to open or close entry into each arm. Large color geometric shapes were attached at the entrance of each arm in which a pellet was located as visual clues to assist the rats in learning the task. The floor of the arms and the central area was covered with black rubber lining. The maze was elevated 50 cm above the floor and placed in a standard experimental room. A food well (3 cm in diameter, 1 cm in depth) with a small pellet inside was placed at the end of four arms. Before use, and between each trial, the maze was wiped down with 70% ethanol and dried for 1 min to prevent the use of odor cues.
To ensure adequate motivation in this maze test, some of the rats weighted more than 280 g were calorically restricted until they lost 15% of their body weight before the start of the experiment. Before the formal maze test, habituation was conducted. The rats were put into the central area of the RAM for 5 seconds with all the sliding doors closed and were then allowed 5 min to learn to navigate the apparatus and find and eat food pellets (bait) hidden in recesses at the ends of four arms of the maze. Arm entry was counted once the rat completely stepped into the arm. After 5 min habituation, the formal tests were conducted. The formal test consisted of seven trials, 30 min apart, for a total testing time of ∼210 min. The same arms remained baited for habituating trials and all the formal test trials. The rat was placed at the center of the RAM for 5 seconds with all the sliding doors closed. Then all the sliding doors were opened to allow the rat to explore the maze until the four baits were consumed. The time to consume the four pellets was recorded as the “latency” and the number of entries into unbaited arms as “reference memory errors.” Entries into an arm in which the baits had already been previously consumed were recorded as “working memory errors.” The total number of entries was the number of entries into baited, unbaited, and previously baited arms.
Stastistical Analysis
All data are presented as mean ± SE. The data among the groups were compared using one‐way or two‐way analysis of variance (ANOVA). Between‐groups variance was determined by a Fisher's least significant difference post hoc test after ANOVA. Student's t‐test was used to compare the means of SRS between the SRS + KA + hypothermia and the SRS + KA + nomothermia groups. All statistical analyses were carried out using SPSS statistical software. Probability values of <0.05 were considered significant.
Results
SE Induced More Severe Brain Edema in SRS Rats than in No‐SRS Rats (Experiment 1)
In SRS rats, there were significant increases in brain water content after KA injection as compared to saline injected rats (Figure 1A). These increases were detected in the hippocampus 6, 12, and 24 h after KA injection and in the frontal cortex after 6 and 12 h. Forty‐eight hours after KA treatment, the brain water content had returned to control levels. No difference in brain water content was found between the right and the left cortex or hippocampus after KA treatment (data not shown). We therefore measured the brain water content of both left and right hippocampi and cortexes for each animal. In no‐SRS rats (group 3), brain water content (Figure 1B) showed an increased trend that was not statistically significant. The increase in brain water content in the hippocampus in SRS rats was significantly greater than in no‐SRS rats by 6 h after KA treatment (P < 0.05). These results indicate that epileptic rats (those undergoing SRS) are more prone to develop brain edema after SE than the rats without SRS after SE (no‐SRS rats).
Hypothermia Treatment Reduced Brain Edema Induced by SE (Experiment 2)
As the brain water content increased most significantly 12 h after KA treatment, this time point was chosen to study the effect of hypothermia on brain edema induced by SE (Figure 2A). Brain water content was significantly decreased in KA‐injected SRS rats that were subsequently treated with hypothermia as compared with the KA‐injected SRS rats treated with normothermia. However, the water content of the frontal cortex and the hippocampus of KA‐injected, hypothermia treated SRS rats was still elevated relative to those of saline‐injected rats. These results indicate that hypothermia attenuated the formation of KA‐induced brain edema in the frontal cortex as well as in the hippocampus of epileptic rats (SRS rats).
Hypothermia Treatment Reduced the Number of Spontaneous Reoccurring Seizures in Epileptic Rats (Experiment 3)
The number of spontaneous reoccurring seizures was counted after the re‐induction of SE with KA in epileptic rats (SRS rats) treated with hypothermia or normothermia. There was a significant decrease in the number of SRS in KA‐injected, hypothermia‐treated epileptic rats as compared with the KA‐injected, normothermia‐treated epileptic rats (Figure 2B). These results indicate that hypothermia treatment immediately after SE can reduce SRS in epileptic rats.
Hypothermia Treatment Improved Performance in the RAM After SE (Experiment 3)
To determine whether hypothermia treatment affected cognitive function after SE we tested all rats for their ability to remember where food baits were located in a RAM test. All groups of rats learned to find and consume the baits in each trial. Both groups of KA‐injected SRS rats took more time to find the four baits than the control SA injected animals (SA group) and had more reference memory errors. However, hypothermia treatment reduced the latency time to find the four baits in the KA‐injected SRS rats (Figure 3A). By the fourth trial, hypothermia treated KA‐injected SRS rats also showed significantly fewer reference memory errors than their normothermia treated counterparts (Figure 3B).
Hypothermia treatment had a more dramatic effect on the testing of working memory errors. No significant difference was seen in the number of working memory errors committed between hypothermia treated KA‐injected SRS rats and SA‐injected control rats. However, those SRS rats that were injected with KA without hypothermia treatment showed significant increases in working memory errors compared with SA‐injected control rats in the first trial, and with their hypothermia treated counterparts by the fourth trial (Figure 3C).
More trials were required to show differences in the total number of entries into the maze arms between the experimental groups. Hypothermia treatment led to a reduction in the total number of entries into the maze arms in comparison to the normothermia treated KA‐injected SRS rats by the fifth trial. However, while the SA‐injected control rats continued to reduce their number of entries in trials 6 and 7, both groups of KA‐injected SRS rats appeared to stabilize, causing a more significant difference between control and normothermia treated KA‐injected SRS rats as the trials progressed and in trial 7, a significant difference between the control and the hypothermia treated KA‐injected SRS rats (Figure 3D). Thus these results show that hypothermia treatment reduced the cognitive deficits caused by KA treatment in SRS rats.
Discussion
Our data provide evidence that hypothermia treatment immediately following SE can reduce the number of spontaneous seizure attacks as well as improve cognitive function that was impaired by SE in SRS rats. This therapeutic improvement for experimental epilepsy may be related to the reduced brain edema after hypothermia treatment, as SRS rats are prone to more severe brain edema than no‐SRS rats and hypothermia can reduce the brain edema induced by SE.
SRS Rats Were Prone to More Severe Brain Edema than No‐SRS Rats After Acute Seizures
Neuronal activity itself is associated with increases in cell volume as water accompanies movement of ions during action potential generation [18, 19]. KA injection can induce a 10% increase in tissue volume [20] and a similar increase in regional brain water content [18]. One published report showed that this brain edema after kainate injection was due to the massive swelling of perineuronal and perivascular astroglia [21]. This astroglial swelling and the concomitant reduction in extra‐cellular volume are important in epileptiform spike generation [22]. In the early phase of brain edema development, a brain edema reducing agent, mannitol, prevents the development of KA‐induced seizures in addition to preventing irreversible brain lesions and neurochemical changes [23]. This evidence supports an old hypothesis that brain edema play an important role in the processes of epileptogenesis. Thus, we speculated that if brain edema contributes to epileptogenesis there will be more severe brain edema in rats that are capable of developing epileptogenesis in response to KA than in rats that are unable to develop epileptogenesis after KA induced SE. In our study, an initial KA injection led to the development of SRS in 27 out of a total of 50 rats. The proportion of rats that developed SRS after a single KA injection is similar to other reports [24]. In order to examine the difference in brain edema induced by SE in rats with epileptogenesis (with SRS) with that of rats without epileptogenesis (without SRS), a second KA injection was employed to induce SE in rats with and without SRS and brain water content measurement was used to evaluate the brain edema. As we expected, we found that acute seizure (KA injection) produced more regional brain water content in rats with SRS (chronic epileptic rats) than in rats without SRS. This brain water content change could at least partially reflect the level of brain edema as in other report [16], though we could mot exclude the possibility that the increased the blood content within the vascular network of the brain in KA‐treated rats in SE due to the increases in blood pressure, blood volume, and vessel dilation in their brains may affect the brain edema evaluation. This result of water content measurement did not show any clue of cell swelling or extracellular space changes, thus, an combination of brain water content and extracellular space measurement is needed to show the cell swelling [25], but the present result provides supportive evidence that SRS rats were prone to more severe brain edema than no‐SRS rats after acute seizures. The mechanism of this difference in brain edema formation between the SRS rats and the no‐SRS rats is unknown. Genetic variability may be invoked to explain why the same dose of seizure inducing agent produced a different level of brain edema. Indeed, a recent report showed that a genetic polymorphism of AQP4, one of the aquaporin water channels (AQPs), which are highly expressed around epileptic focus in animal with chronic epilepsy [26] and in humans with refractory epilepsy [27], was correlated with the severity of brain edema that developed after middle cerebral artery occlusion in human [28]. Thus, our findings indicate that brain edema may play a role in the generation of chronic epilepsy from acute seizures. The suppression of brain edema induced by seizures may decrease the number of SRS.
Hypothermia Reduced Brain Edema and the Number of SRS in Epileptic Rats
In 1956, Rosomoff reported the marked protective effect of hypothermia on cerebral infarcts of dogs with middle cerebral artery occlusion, when body temperature was reduced to 22–24°C [29]. In 1987, Busto et al. demonstrated that mild hypothermia at 34°C markedly protected neurons in the hippocampus CA1 and striatum from ischemic damage [30]. Even lowering the body temperature only 1 degree provides a protective effect against ischemic damage [31]. The same protective effect was also seen in epileptic seizures [12, 32]. However, previous research was mainly focused on evaluation of the effects of hypothermia on acute convulsant seizures. This lack of long‐term assessment is a serious concern as treatments may not provide lasting benefit. Here, it is encouraging to note that hypothermia treatment immediately after SE can attenuate the number of spontaneous seizure attacks in chronic epileptic rats. This result indicates that early hypothermia treatment may disturb the process of epileptogenesis leading to lower cerebral excitability.
The neurophysiological processes involved in reducing the number of spontaneous seizures with hypothermia treatment are largely unclear. Hypothermia treatment both attenuated brain edema and reduced the number of spontaneous reoccurring seizures. Though the hypothermia was introduced immediately after SE, we were still able to detect the attenuation of brain edema. This may be because maximum edema formation was detected 12 h after SE, providing a time window during which interventions may be effective. The correlation between reduction in brain edema and the reduction in number of SRS, together with the finding that mannitol is able to significantly suppress spontaneous epileptic spikes [6] in humans as well as motor seizures in animals [23], suggests that hypothermia mediated attenuation in brain edema may contribute to the suppression of SRS. However, we cannot exclude the possibility that hypothermia may also act to reduce the number of SRS through other mechanisms including alteration of membrane properties and ion pumps [33] and reduction of excitatory transmitter release [34]. Although increased brain edema is a transient event lasting less than 48 h in this study, there are longer lasting effects of hypothermia treatment on spontaneous motor seizures. These may be explained by longer lasting secondary changes of neural plasticity within the brain regions that have edema [35].
Hypothermia Protected Against SE Induced Impairment of Cognitive Function
It is well known that hippocampus is highly vulnerable to KA induced excitotoxicity [36], which results in long lasting learning and memory deficits in adult rats [37, 38]. As expected, our seizure animals showed a deficiency in visual spatial learning in the RAM test. One month after KA induced SE, chronic epileptic rats took longer to perform the test, showed more working memory errors and more reference memory errors compared with controls. Hypothermia treatment reduced the latency, working memory errors and reference memory errors in chronic epileptic rats in comparison to similar epileptic rats without hypothermia treatment. These data shows that hypothermia treatment even in chronic epileptic rats still can protect against impairment of cognitive performance.
Control SA‐injected rats and SRS + KA + hypothermia rats showed the ability to learn the task, as they entered the arms of the radial maze less on the seventh trial compared with the first trial. However, SRS + KA + hypothermia group rats did not learn the task as well as the SA control group. Therefore hypothermia treated KA‐injected chronic epileptic rats had impaired ability to acquire spatial information. In contrast, SRS + KA + nomothermia group rats did not learn the task, having a similar number of arm entries on the seventh trial as on the first. Therefore, KA‐induced seizures in chronic epileptic rats not only impaired but also precluded the acquisition of spatial information. Our data conflict with a different report that showed that seizures impair but do not preclude the acquisition of spatial information [39]. This discrepancy may result from the age of rats tested: our study used adult rats, whereas young animals were employed in this other study. The brains in younger animals are more resistant to excitatory damage [40].
Conclusions
In the current model of SE in SRS animal, brain edema plays an important role in the generation of chronic epilepsy from SE. Hypothermia‐treatment immediately after SE not only exhibited protective effects against the chronic spontaneous recurrent convulsant seizures but also improved cognitive function. These antiepileptogenic properties of hypothermia may be related to its attenuating effect on brain edema induced by SE. Our findings require confirmation in other animal models of experimental epilepsy, before possible clinical trials to explore the value of hypothermia treatment in clinical epilepsy protocols.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgment
This work was supported by an Academic Natural Science Research Grant to Y Wang from the Educational Department of Anhui Province, China (Grant no: 2000j1132zd) and a Medical Science Research Grant to Y Wang from the Health Department of Anhui Province, China (Grant no: 09B140). The authors thank Dr. Aviva J. Symes for editorial assistance.
References
- 1. Holtkamp M, Othman J, Buchheim K, Meierkord H. Predictors and prognosis of refractory status epilepticus treated in a neurological intensive care unit. J Neurol Neurosurg Psychiatry 2005;76:534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: Frequency, risk factors, and impact on outcome. Arch Neurol 2002;59:205–210. [DOI] [PubMed] [Google Scholar]
- 3. LaRoche SM, Helmers SL. The new antiepileptic drugs: Scientific review. JAMA 2004;291:605–614. [DOI] [PubMed] [Google Scholar]
- 4. Hong KS, Cho YJ, Lee SK, Jeong SW, Kim WK, Oh EJ. Diffusion changes suggesting predominant vasogenic oedema during partial status epilepticus. Seizure 2004;13:317–321. [DOI] [PubMed] [Google Scholar]
- 5. Sztriha L, Joo F, Szerdahelyi P, Eck E, Koltai M. Effects of dexamethasone on brain edema induced by Kainic acid seizures. Neuroscience 1986;17:107–114. [DOI] [PubMed] [Google Scholar]
- 6. Haglund MM, Hochman DW. Furosemide and mannitol suppression of epileptic activity in the human brain. J Neurophysiol 2005;94:907–918. [DOI] [PubMed] [Google Scholar]
- 7. Hemmen TM, Lyden PD. Induced hypothermia for acute stroke. Stroke 2007;38:794–799. [DOI] [PubMed] [Google Scholar]
- 8. Adelson PD, Ragheb J, Kanev P, et al Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery 2005;56:740–754; discussion 740–754. [DOI] [PubMed] [Google Scholar]
- 9. Kawai N, Kawanishi M, Okauchi M, Nagao S. Effects of hypothermia on thrombin‐induced brain edema formation. Brain Res 2001;895:50–58. [DOI] [PubMed] [Google Scholar]
- 10. Kawai N, Nakamura T, Nagao S. Effects of brain hypothermia on brain edema formation after intracerebral hemorrhage in rats. Acta Neurochir Suppl 2002;81:233–235. [DOI] [PubMed] [Google Scholar]
- 11. Karkar KM, Garcia PA, Bateman LM, Smyth MD, Barbaro NM, Berger M. Focal cooling suppresses spontaneous epileptiform activity without changing the cortical motor threshold. Epilepsia 2002;43:932–935. [DOI] [PubMed] [Google Scholar]
- 12. Schmitt FC, Buchheim K, Meierkord H, Holtkamp M. Anticonvulsant properties of hypothermia in experimental status epilepticus. Neurobiol Dis 2006;23:689–696. [DOI] [PubMed] [Google Scholar]
- 13. Racine RJ. Modification of seizure activity by electrical stimulation. Part II. Motor seizure. Electroencephalogr Clin Neurophysiol 1972;32:281–294. [DOI] [PubMed] [Google Scholar]
- 14. Schmitt FC, Matzen J, Buchheim K, Meierkord H, Holtkamp M. Limbic self‐sustaining status epilepticus in rats is not associated with hyperthermia. Epilepsia 2005;46:188–192. [DOI] [PubMed] [Google Scholar]
- 15. Mellergard P. Changes in human intracerebral temperature in response to different methods of brain cooling. Neurosurgery 1992;31:671–677; discussion 677. [DOI] [PubMed] [Google Scholar]
- 16. Clough RW, Neese SL, Sherill LK, et al Cortical edema in moderate fluid percussion brain injury is attenuated by vagus nerve stimulation. Neuroscience 2007;147:286–293. [DOI] [PubMed] [Google Scholar]
- 17. Roberge MC, Hotte‐Bernard J, Messier C, Plamondon H. Food restriction attenuates ischemia‐induced spatial learning and memory deficits despite extensive CA1 ischemic injury. Behav Brain Res 2008;187:123–132. [DOI] [PubMed] [Google Scholar]
- 18. Korf J, Postema F. Rapid shrinkage of rat striatal extracellular space after local kainate application and ischemia as recorded by impedance. J Neurosci Res 1988;19:504–510. [DOI] [PubMed] [Google Scholar]
- 19. Olsson T, Broberg M, Pope KJ, et al Cell swelling, seizures and spreading depression: An impedance study. Neuroscience 2006;140:505–515. [DOI] [PubMed] [Google Scholar]
- 20. Emerson MR, Nelson SR, Samson FE, Pazdernik TL. Hypoxia preconditioning attenuates brain edema associated with Kainic acid‐induced status epilepticus in rats. Brain Res 1999;825:189–193. [DOI] [PubMed] [Google Scholar]
- 21. Lassmann H, Petsche U, Kitz K, Baran H, Sperk G, Seitelberger F, Hornykiewicz O. The role of brain edema in epileptic brain damage induced by systemic Kainic acid injection. Neuroscience 1984;13:691–704. [DOI] [PubMed] [Google Scholar]
- 22. Traynelis SF, Dingledine R. Role of extracellular space in hyperosmotic suppression of potassium‐induced electrographic seizures. J Neurophysiol 1989;61:927–938. [DOI] [PubMed] [Google Scholar]
- 23. Baran H, Lassmann H, Sperk G, Seitelberger F, Hornykiewicz O. Effect of mannitol treatment on brain neurotransmitter markers in Kainic acid‐induced epilepsy. Neuroscience 1987;21:679–684. [DOI] [PubMed] [Google Scholar]
- 24. Yin S, Guan Z, Tang Y, Zhao J, Hong J, Zhang W. Abnormal expression of epilepsy‐related gene ERG1/NSF in the spontaneous recurrent seizure rats with spatial learning memory deficits induced by Kainic acid. Brain Res 2005;1053:195–202. [DOI] [PubMed] [Google Scholar]
- 25. Hrabetova S, Chen KC, Masri D, Nicholson C. Water compartmentalization and spread of ischemic injury in thick‐slice ischemia model. J Cereb Blood Flow Metab 2002;22:80–88. [DOI] [PubMed] [Google Scholar]
- 26. Kim JE, Ryu HJ, Yeo SI, et al Differential expressions of aquaporin subtypes in astroglia in the hippocampus of chronic epileptic rats. Neuroscience 2009;163:781–789. [DOI] [PubMed] [Google Scholar]
- 27. Zhou S, Sun X, Liu L, Wang X, Liu K. Increased expression of aquaporin‐1 in the anterior temporal neocortex of patients with intractable epilepsy. Neurol Res 2008;30:400–405. [DOI] [PubMed] [Google Scholar]
- 28. Kleffner I, Bungeroth M, Schiffbauer H, Schabitz WR, Ringelstein EB, Kuhlenbaumer G. The role of aquaporin‐4 polymorphisms in the development of brain edema after middle cerebral artery occlusion. Stroke 2008;39:1333–1335. [DOI] [PubMed] [Google Scholar]
- 29. Rosomoff HL. Hypothermia and cerebral vascular lesions. Part I. Experimental interruption of the middle cerebral artery during hypothermia. J Neurosurg 1956;13:244–255. [DOI] [PubMed] [Google Scholar]
- 30. Busto R, Dietrich WD, Globus MY, Valdes I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab 1987;7:729–738. [DOI] [PubMed] [Google Scholar]
- 31. Wass CT, Lanier WL, Hofer RE, Scheithauer BW, Andrews AG. Temperature changes of > or = 1 degree C alter functional neurologic outcome and histopathology in a canine model of complete cerebral ischemia. Anesthesiology 1995;83:325–335. [DOI] [PubMed] [Google Scholar]
- 32. Legriel S, Bruneel F, Sediri H, et al Early EEG monitoring for detecting postanoxic status epilepticus during therapeutic hypothermia: A pilot study. Neurocrit Care 2009, doi: 10.1007/s12028-009-9246-4. [DOI] [PubMed] [Google Scholar]
- 33. Aihara H, Okada Y, Tamaki N. The effects of cooling and rewarming on the neuronal activity of pyramidal neurons in guinea pig hippocampal slices. Brain Res 2001;893:36–45. [DOI] [PubMed] [Google Scholar]
- 34. Yang XF, Ouyang Y, Kennedy BR, Rothman SM. Cooling blocks rat hippocampal neurotransmission by a presynaptic mechanism: Observations using 2‐photon microscopy. J Physiol 2005;567:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arismendi‐Morillo GJ, Castejon OJ, Castellano‐Ramirez A. Ultrastructural features of the synaptic plasticity in peritumoral cerebral oedema in humans. Rev Neurol 2007;45:587–593. [PubMed] [Google Scholar]
- 36. Lothman EW, Collins RC. Kainic acid induced limbic seizures: Metabolic, behavioral, electroencephalographic and neuropathological correlates. Brain Res 1981;218:299–318. [DOI] [PubMed] [Google Scholar]
- 37. Mikulecka A, Krsek P, Mares P. Nonconvulsive Kainic acid‐induced seizures elicit age‐dependent impairment of memory for the elevated plus‐maze. Epilepsy Behav 2000;1:418–426. [DOI] [PubMed] [Google Scholar]
- 38. Yin H, Bardgett ME, Csernansky JG. Kainic acid lesions disrupt fear‐mediated memory processing. Neurobiol Learn Mem 2002;77:389–401. [DOI] [PubMed] [Google Scholar]
- 39. Mikati MA, Tarif S, Lteif L, Jawad MA. Time sequence and types of memory deficits after experimental status epilepticus. Epilepsy Res 2001;43:97–101. [DOI] [PubMed] [Google Scholar]
- 40. Stafstrom CE, Chronopoulos A, Thurber S, Thompson JL, Holmes GL. Age‐dependent cognitive and behavioral deficits after Kainic acid seizures. Epilepsia 1993;34:420–432. [DOI] [PubMed] [Google Scholar]


