SUMMARY
Behavioral sensitization to psychostimulants in rodents is associated with the alteration of dopaminergic neurotransmission, and has been proposed as a useful model of schizophrenia due to its progressively intensifying, easily relapsing, and long‐lasting features. Pharmacological treatments that reverse the established sensitization may have potential therapeutic values for schizophrenia. The present aim is to review pharmacological treatments that induce the reversal of established sensitization to psychostimulants. In addition, we discuss possible mechanisms for the reversal of sensitization. Reversal of sensitization is induced by chronic dopamine D1 receptor agonism, D2 or D1/D2 receptor agonism combined with mild N‐methyl‐D‐aspartate (NMDA) receptor antagonism or serotonin (5‐HT2A or 5‐HT3) receptor antagonism, 5‐HT1A receptor agonism, and 5‐HT2A or 5‐HT3 receptor antagonism. Chronic treatments with these drugs likely adjust altered dopaminergic neurotransmission in sensitized animals. Especially, chronic dopamine D1 receptor agonism, which may adjust mesolimbic hyperdopaminergic and mesocortical hypodopaminergic functions in sensitized animals, is an attractive therapeutic approach for schizophrenia.
Keywords: Behavioral sensitization, Dopamine, Schizophrenia
Introduction
Repeated administration of psychostimulants such as methamphetamine (MAP), amphetamine (AMPH), and cocaine induces a progressive and enduring enhancement of locomotor activity in rodents, which is known as behavioral sensitization (development of sensitization). Behavioral sensitization easily reappears with the injection of small doses of psychostimulants (expression of sensitization) after a long period of abstinence following initial drug exposure [1, 2]. Since the sensitized state is maintained for long time after psychostimulant withdrawal, it is important to find therapeutic agents that reverse the established behavioral sensitization.
Chronic exposure to psychostimulants in human can induce a psychotic state indistinguishable from paranoid schizophrenia. Moreover, acute AMPH administration can produce or enhance a psychotic reaction in patients with schizophrenia at doses that are ineffective in healthy controls [3, 4]. The progressively intensifying, easily relapsing, and long‐lasting features of behavioral sensitization in rodents are similar to clinical features in psychostimulant psychosis and schizophrenia [2]. In addition, alterations in dopaminergic neurotransmission induced by psychostimulants in rodents are similar to those hypothesized in schizophrenia [5, 6, 7]. Therefore, the psychostimulant‐sensitized animal model is very useful to understand mechanisms of increased susceptibility to relapse and long duration of the vulnerability to relapse in schizophrenia [8]. Furthermore, psychostimulant sensitization, which is thought as a model of positive symptoms of schizophrenia associated with the mesolimbic hyperdopaminergic state, is now demonstrated to serve as a model of cognitive dysfunction of schizophrenia associated with the mesocortical hypodopaminergic state, based on the long‐lasting cognitive deficits in domains of attention/vigilance and reasoning and problem solving observed in sensitized animals [9].
Most research in this field has focused on mechanisms underlying development and expression of sensitization. Development of sensitization is inhibited by dopamine D1 or D2 receptor antagonism, and expression of sensitization by psychostimulant challenge is inhibited by dopamine D2 receptor antagonism and in some cases by D1 receptor antagonism [7, 10, 11, 12, 13, 14]. Dopamine D2 receptor antagonism suppresses the expression of sensitized behavior to psychostimulant challenge, but the sensitized state is maintained. For this meaning, it is necessary to develop therapeutic agents that reverse the established sensitization long time after withdrawal of therapeutic agents, resulting in cure rather than control of the sensitized state (Figure 1). Several drugs, previously shown to inhibit the development of sensitization, have been tested whether to reverse sensitization, but so far these attempts have failed. The drugs reported not effective were a dopamine D1 receptor antagonist (SCH23390 [10, 11, 15]), dopamine D2 receptor antagonists (chlorpromazine [16], haloperidol [10], sulpiride [17], and nemonapride (YM‐09151‐2 [11, 15, 18]), and an N‐methyl‐D‐aspartate (NMDA) receptor antagonist (MK‐801 [19, 20, 21]).
Figure 1.
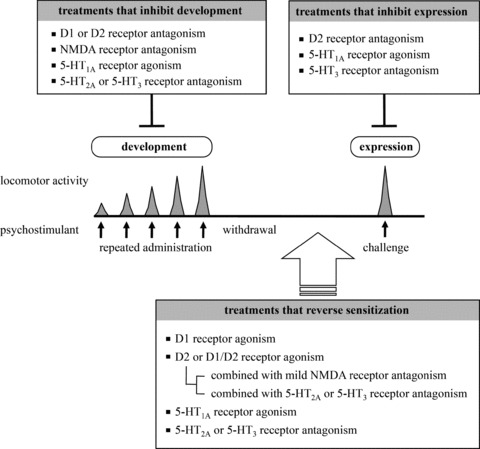
Pharmacological treatments that inhibit development and expression of sensitization and reverse established sensitization.
Interestingly, administration of cocaine itself is demonstrated to reverse behavioral sensitization to cocaine, when cocaine administration is combined with the NMDA receptor antagonist [22]. Furthermore, there are several reports showing the reversal of psychostimulant‐induced behavioral alterations in animal models, for example, drug‐self‐administration and seeking behaviors for cocaine, by dopamine D1 receptor agonists [23, 24, 25]. Based on these observations, many studies aiming the reversal of sensitization have focused on agonism of dopamine receptors. Activation of dopamine D1 receptors alone or activation of dopamine D2 or D1/D2 receptors in combination with NMDA receptor antagonists or serotonin receptor antagonists is shown to have therapeutic potentials, as summarized in Table 1. Drugs that modulate serotonergic neurotransmission are also shown to reverse behavioral sensitization. Since an excellent review on serotonergic drugs [26] is available and spaces are limited, we mainly focus on the reversal of behavioral sensitization by dopamine receptor agonism.
Table 1.
Summary of pharmacological treatments that reverse behavioral sensitization to psychostimulants
| Reversal treatment | Inducer of sensitization | Animal | Study |
|---|---|---|---|
| D1 receptor agonism | |||
| SKF81297 | Cocaine | Rat | [21] |
| R‐(+)‐SKF38393 | Methamphetamine | Rat | [27, 28] |
| SKF38393 | Amphetamine | Rat | [15] |
| D2 or D1/D2 receptor agonism combined with mild NMDA receptor antagonism | |||
| Cocaine + MK‐801 | Cocaine | Rat | [21, 22] |
| Quinpirole + MK‐801 | Cocaine | Rat | [21] |
| Quinpirole + CGS19755 | Cocaine | Rat | [21] |
| Pergolide + memantine | Cocaine | Rat | [21] |
| D1/D2 receptor agonism combined with 5‐HT2A receptor antagonism | |||
| Cocaine + clozapine | Cocaine | Rat | [67] |
| Cocaine + mianserin | Cocaine | Rat | [67] |
| Cocaine + ketanserin | Cocaine | Rat | [67] |
| Aripiprazole | Methamphetamine | Mouse | [17] |
| D1/D2 receptor agonism combined with 5‐HT3 receptor antagonism | |||
| Cocaine + ondansetron | Cocaine | Rat | [62] |
| Pergolide + ondansetron | Cocaine | Rat | [64, 65] |
| Pergolide + ondansetron | Methamphetamine | Rat | [63] |
| 5‐HT1A receptor agonism | |||
| Osemozotan | Methamphetamine | Mouse | [87] |
| 5‐HT2A receptor antagonism | |||
| Ritanserin | Methamphetamine | Mouse | [88] |
| Clozapine | Cocaine | Mouse | [91] |
| 5‐HT3 receptor antagonism | |||
| ondansetron | cocaine | Rat | [89, 90] |
| Selective serotonin receptor reuptake inhibitor | |||
| Fluoxetine | Methamphetamine | Mouse | [97] |
| Paroxetine | Methamphetamine | Mouse | [97] |
| Opioid receptor‐like 1 (ORL1) receptor agonism | |||
| Orphanin FQ/nociceptin | Cocaine | Mouse | [98] |
| Environmental conditions | |||
| Environmental enrichment | Cocaine | Mouse | [99] |
Effect of Dopamine D1 Receptor Agonism on Established Sensitization
Repeated administration of dopamine D1 receptor agonists has been demonstrated to reverse the established behavioral sensitization to MAP [27, 28], AMPH [15], and cocaine [21] in rats (Table 1). We previously reported that intermittent administration of MAP (once every 3 days for a total of five times) induced the behavioral sensitization in rats [27]. After development of sensitization, the rats received a dopamine D1 agonist, R‐(+)‐SKF38393, once a day for seven consecutive days. The chronic administration of R‐(+)‐SKF38393 reversed MAP‐induced behavioral sensitization, and the reversal was maintained at least for 14 days after the last injection of R‐(+)‐SKF38393 (Figure 2). Thus, chronic dopamine D1 receptor agonism reverses the established behavioral sensitization to MAP, lasting for at least 2 weeks.
Figure 2.

Effect of chronic dopamine D1 receptor agonism on methamphetamine (MAP)‐induced behavioral sensitization. Rats were injected with MAP 1.0 mg/kg or saline (SAL) once every 3 days for total of 5 times (days 1–13), and then challenged with MAP 0.5 mg/kg after a 7‐day withdrawal period (on day 20) to verify the development of sensitization. The rats subsequently received R‐(+)‐SKF38393 at 1.0 mg/kg (1 SKF) or 3.0 mg/kg (3 SKF) or SAL once a day for 7 days (on days 21–27), and then challenged with MAP 0.5 mg/kg. After a 14‐day withdrawal period (on day 41), locomotor activity was recorded. Rats were subdivided into five groups (treatment on days 1–13/treatment on days 21–27), and number of rats was indicated in parentheses: SAL/SAL (5), SAL/3 SKF (6), MAP/SAL (9), MAP/1 SKF (5), MAP/3 SKF (7). Bars represent means ± S.E.M. ***P < 0.05 versus SAL/SAL group; †P < 0.05 versus MAP/SAL group. (Reproduced from Ref. [27] with permission.)
The mechanisms for the expression and maintenance of MAP sensitization have been intensively investigated, and alterations of presynaptic and postsynaptic dopaminergic neurotransmission have been reported [2, 29]. Analysis of dopamine release in the striatum by in vivo microdialysis revealed that the behavioral sensitization to MAP is in part mediated through the enhanced release of dopamine in response to MAP (Figure 3A) [27]. The findings are in agreement with previous reports [30, 31]. Chronic administration of R‐(+)‐SKF38393 reversed the enhanced dopamine release in the striatum, and the reversal lasted for 2 weeks (Figure 3B). The results suggest that chronic dopamine D1 receptor agonism adjusts the enhanced dopamine release in the striatum of MAP‐sensitized rats.
Figure 3.

Effect of chronic dopamine D1 receptor agonism on the enhanced dopamine release in the striatum of MAP‐sensitized rats. Dopamine release in the striatum after MAP 0.5 mg/kg challenge was analyzed using in vivo microdialysis on day 41, and compared between drug naïve and MAP/SAL groups (A) and between MAP/SAL and MAP/3 SKF groups (B). Dopamine release after MAP challenge was calculated relative to the basal values in each group (100%). Basal values in MAP/SAL or MAP/3 SKF group were not different from that in drug naïve group. Each point is the mean ± S.E.M., and number of rats was: drug naïve (6), MAP/SAL (8), MAP/3 SKF (10). *P < 0.05 versus drug naïve group; † P < 0.01 versus MAP/SAL‐treated group. (Reproduced from Ref. [27] with permission.)
As a postsynaptic mechanism, we reported the alteration of dopamine receptor affinity states in MAP‐sensitized rats. The dopamine receptor exists in high‐ and low‐affinity states. High‐affinity states of dopamine D1 (D1High) and D2 (D2High) receptors have ∼1000‐fold greater affinity for dopamine than low‐affinity states of dopamine D1 and D2 receptors [32, 33], and dopamine D1High and D2High receptors have been proposed as functional states of dopamine receptors [34]. In our study, repeated administration of MAP increased the proportions of D1High and D2High receptors in the rat striatum by 2.4‐fold and 2.6‐fold, respectively [28], as previously reported [35]. Behavioral sensitization can be explained by the increased proportion of D1High and D2High receptors in the striatum, which may result in supersensitivity to dopaminergic drugs and psychostimulants [36, 37]. Chronic administration of R‐(+)‐SKF38393 after development of sensitization reversed the increased proportions of both D1High and D2High receptors. In agreement with our findings, an electrophysiological study revealed that chronic administration of a dopamine D1 receptor agonist SKF81297 reversed the dopamine D1 receptor supersensitivity in NAc neurons of cocaine‐sensitized rats [21]. Thus, adjustment of the high affinity states of D1 and D2 receptors in striatal neurons and the dopamine D1 receptor supersensitivity in NAc neurons as well as the enhanced dopamine release by chronic D1 receptor agonism likely result in the attenuation of sensitized behavior to MAP or cocaine.
The mechanisms by which dopamine D1 receptor agonism reverses the increased release of dopamine and the increased proportions of D1High and D2High receptors in MAP‐sensitized rats are currently unknown. Dopamine release from axon terminals of nigrostriatal dopaminergic neurons is negatively regulated by stiratonigral GABAergic neurons [38]. Activation of striatonigral GABAergic neurons by dopamine D1 receptor agonism likely inhibits nigrostriatal dopaminergic neurons and dopamine release in the striatum. Alternatively, the inhibition of glutamate release from corticostriatal glutamatergic neurons via activation of the striatonigral GABAergic neurons by dopamine D1 receptor agonism and subsequent changes in activity of nigro‐thalamo‐cortical loop [39] may result in the attenuation of dopamine release in the striatum. Thus, dopamine release from axon terminals of nigrostriatal dopaminergic neurons seems to be indirectly downregulated via activation of striatonigral GABAergic neurons by dopamine D1 receptor agonism, but mechanisms for its reversal lasting for a long time need to be clarified.
There are several reports suggesting attenuated intracellular signaling of dopamine D1 receptors in sensitized rats. Whereas a dopamine D2 receptor agonist induces augmented behavioral responses in sensitized rats, a dopamine D1 receptor agonist fails to induce augmented responses [36, 37]. It is possible that activity of the dopamine D1 receptor/cAMP/PKA signaling cascade in the striatum is downregulated after the establishment of sensitization, in spite of the increased proportion of dopamine D1High receptors [28] and the supersensitivity of dopamine D1 receptors [21]. The fact that PKA activity is reduced in the NAc of AMPH‐ and cocaine‐sensitized rats [40] supports the hypothesis. In addition, the phosphorylation of dopamine and cAMP‐regulated phosphoprotein of Mr 32 kDa (DARPP‐32) at Thr34 by PKA is reduced in the striatum of MAP‐sensitized rats and cocaine‐sensitized mice [41]. These findings raise a plausible mechanism that chronic dopamine D1 receptor agonism counteracts the decreased dopamine D1 receptor/cAMP/PKA signaling in the sensitized state, leading to the reversal of sensitization.
Dopamine D1 receptors are required for the development of behavioral sensitization, but dopamine D1 receptor agonism shows ability to reverse the sensitized state. It has been proposed that mesocortical hypodopaminergic function (causing negative symptoms), induced by lesioning of dopaminergic neurons, results in mesolimbic hyperdopaminergic function (causing positive symptoms), which is important in the etiology of schizophrenia [42, 43, 44]. Systemic administration or local administration to prefrontal cortex (PFC) of the dopamine D1 receptor agonist is shown to improve the behavioral impairments related to the function of PFC such as the AMPH‐induced impairment of attentional set shifting [45] and the phencyclidine (PCP)‐induced impairments in the novel object recognition test and the reversal learning test [46], suggesting the dysfunction of dopamine D1 receptors in the PFC of sensitized animals. Thus, chronic dopamine D1 receptor agonism, targeting hypofunctional D1 receptor signaling in the PFC as well as the striatum, is an attractive therapeutic approach, and may adjust the altered dopaminergic neurotransmission in sensitized animal models of schizophrenia.
Effect of Dopamine D2 or D1/D2 Receptor Agonism on Established Sensitization
Dopamine D2 receptor antagonism is an essential future of antipsychotics alleviating or controlling positive symptoms of schizophrenia, but less effective for negative symptoms or cognitive dysfunction. In sensitized animal models of schizophrenia, dopamine D2 receptor antagonism blocks the development and expression of behavioral sensitization, but fails to reverse the sensitized state, suggesting the limitation of dopamine D2 receptor antagonism for the treatment of sensitization.
Dopamine D2 receptor agonism or dopamine D1/D2 receptor agonism are also tried, but the agonism alone does not show the ability to reverse the established sensitization. However, when dopamine D2 or D1/D2 receptor agonism is combined with additional manipulations, reversal of sensitization is achieved, as described below.
Combination with Mild NMDA Receptor Antagonism
Glutamate as well as dopamine plays critical roles in the development of sensitization [47]. This notion is based on the finding that the NMDA receptor antagonist prevents the development of sensitization to cocaine or AMPH [48]. Glutamate signaling may be altered in the established sensitization, and NMDA receptors can be therapeutic targets for the reversal of sensitization.
Chronic dopamine D2 receptor agonism by quinpirole and chronic dopamine D1/D2 receptor agonism by cocaine or pergolide are shown to reverse the established sensitization to cocaine, when the function of NMDA receptors are partially suppressed by low doses of MK‐801 or CGS19755 and by a relatively weak NMDA receptor antagonist, memantine [21]. The combined treatments also reverse the dopamine D1 receptor supersensitivity in the NAc of cocaine‐sensitized rats [21].
Stimulation of dopamine D1/D2 receptors by systemic administration of cocaine is known to increase glutamate levels in the NAc [49]. Although highly speculative, combination of glutamatergic signal activated by dopamine D1/D2 receptor agonism and mild NMDA receptor antagonism may be required for the reversal of sensitization. Electrophysiological studies at corticostriatal synapses demonstrate that mild NMDA receptor activation and a subsequent, relatively small increase in intracellular Ca2+ induce long‐term depression (LTD) [50, 51]. The inhibition of glutamatergic neurotransmission via LTD may counteract long‐term potentiation (LTP)‐like plasticity, which is induced during development of sensitization [52, 53, 54, 55, 56]. The finding that D2 receptors are required for induction of LTD at corticostriatal synapses [57, 58], supports the hypothesis that dopamine D2 or D1/D2 receptor agonism combined with mild NMDA receptor antagonism may adjust LTP‐like plasticity of glutamatergic neurotransmission in sensitized animals via LTD‐mediated mechanisms.
Combination with Serotonin Receptor Antagonism
Serotonin receptors (5‐HT1A, 5‐HT2A and 5‐HT3 receptors) are implicated in the development and/or expression of sensitization [59, 60, 61], indicating that serotonin receptors are also therapeutic targets for the reversal of sensitization. Chronic 5‐HT3 receptor antagonism (ondansetron) [62, 63, 64, 65, 66] and chronic 5‐HT2A receptor antagonism (clozapine, mianserin, and ketanserin) [67], combined with dopamine D1/D2 receptor agonism (cocaine or pergolide), reverse sensitization. Interestingly, an antipsychotic drug, aripiprazole, with properties of the partial dopamine D2 receptor agonist and 5‐HT2A receptor antagonist [68], but not risperidone with properties of the dopamine D2 receptor antagonist and 5‐HT2A receptor antagonist, is reported to reverse MAP sensitization [17].
The mechanism by which 5‐HT2A or 5‐HT3 receptor antagonism reverses sensitization is still poorly understood. Chronic administration of 5‐HT3 antagonists reduces activity of dopaminergic neurons in the ventral tegmental area (VTA) [69, 70, 71, 72, 73, 74] and basal extracellular dopamine levels in the NAc, but not in the PFC [71, 75]. Atypical antipsychotics with a property of 5‐HT2A receptor antagonist are known to induce the profound increase in dopamine release in the PFC presumably by interacting with 5‐HT1A receptor signaling [76, 77, 78, 79, 80], although the effects of the selective 5‐HT2A receptor agonist or antagonist on dopamine release in the PFC are controversial [81, 82]. Thus, chronic 5‐HT3 or 5‐HT2A receptor antagonism may induce neuroadaptive changes in mesolimbic hyperdopaminergic and/or mesocortical hypodopaminergic function in sensitized animals [83].
The regulatory roles of 5‐HT2 and 5‐HT3 receptors are also implicated in glutamatergic neurotransmission. Activation of 5‐HT2 receptors enhances NMDA receptor function via phosphoinositol hydrolysis and subsequent stimulation of protein kinase C [84]. Activation of 5‐HT3 receptors induces Ca2+ influx at presynaptic nerve terminals and then enhances the release of neurotransmitters including glutamate [83, 85, 86]. These findings obtained with 5‐HT2 and 5‐HT3 receptor agonists suggest that chronic 5‐HT2A or 5‐HT3 receptor antagonism combined with dopamine D1/D2 receptor agonism may also induce LTD and adjust LTP‐like plasticity of glutamatergic neurotransmission in sensitized animals.
Effect of Serotonin Receptor Agonism or Antagonism alone on Established Sensitization
A 5‐HT1A receptor agonist (osemozotan) [87], a 5‐HT2A receptor antagonist (ritanserin) [88], a 5‐HT3 receptor antagonist (ondansetron) [89, 90], and an atypical antipsychotic with a property of 5‐HT2 receptor antagonist (clozapine) [91] were demonstrated to reverse the established sensitization by themselves. Osemozotan and ritanserin were also shown to reverse the enhanced serotonin release in the PFC of MAP‐sensitized mice [26, 87, 88].
Conclusions
Reversal of sensitization is likely achieved by treatments that adjust mesolimbic hyperdopaminergic and mesocortical hypodopaminergic functions in sensitized animals. As means of treatment for schizophrenia in human, dopamine D1 receptor agonists and drugs that regulate serotonergic receptors may have potential therapeutic values, since D2 receptor agonists [92] or NMDA receptor antagonists [45, 93, 94, 95, 96], which would exacerbate symptoms of schizophrenia, can not be used. We have demonstrated that chronic D1 receptor agonism reverses the established sensitization via presynaptic and postsynaptic mechanisms [27, 28]. Future studies are required to elucidate mechanisms for long‐lasting reversal of sensitization by chronic D1 receptor agonism, possibly involving the remodeling of neuronal circuits mediated through neurogenesis and epigenetic modification.
Conflict of Interest
The authors have no conflict of interest.
References
- 1. Nestler EJ. Molecular basis of long‐term plasticity underlying addiction. Nat Rev Neurosci 2001;2:119–128. [DOI] [PubMed] [Google Scholar]
- 2. Ujike H. Stimulant‐induced psychosis and schizophrenia: The role of sensitization. Curr Psychiatry Rep 2002;4:177–184. [DOI] [PubMed] [Google Scholar]
- 3. Curran C, Byrappa N, McBride A. Stimulant psychosis: Systematic review. Br J Psychiatry 2004;185:196–204. [DOI] [PubMed] [Google Scholar]
- 4. Lieberman JA, Kane JM, Alvir J. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology (Berl) 1987;91:415–433. [DOI] [PubMed] [Google Scholar]
- 5. King GR, Joyner C, Ellinwood EH, Jr . Continuous or intermittent cocaine administration: Effects of amantadine treatment during withdrawal. Pharmacol Biochem Behav 1994;47:451–457. [DOI] [PubMed] [Google Scholar]
- 6. King GR, Joyner C, Ellinwood EH, Jr . Continuous or intermittent cocaine administration: Effects of flupenthixol treatment during withdrawal. Pharmacol Biochem Behav 1994;49:883–889. [DOI] [PubMed] [Google Scholar]
- 7. Vezina P, Stewart J. The effect of dopamine receptor blockade on the development of sensitization to the locomotor activating effects of amphetamine and morphine. Brain Res 1989;499:108–120. [DOI] [PubMed] [Google Scholar]
- 8. Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann N Y Acad Sci 2004;1025:279–287. [DOI] [PubMed] [Google Scholar]
- 9. Featherstone RE, Kapur S, Fletcher PJ. The amphetamine‐induced sensitized state as a model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2007;31:1556–1571. [DOI] [PubMed] [Google Scholar]
- 10. Kuribara H, Uchihashi Y. Dopamine antagonists can inhibit methamphetamine sensitization, but not cocaine sensitization, when assessed by ambulatory activity in mice. J Pharm Pharmacol 1993;45:1042–1045. [DOI] [PubMed] [Google Scholar]
- 11. Kuribara H, Uchihashi Y. Effects of dopamine antagonism on methamphetamine sensitization: Evaluation by ambulatory activity in mice. Pharmacol Biochem Behav 1994;47:101–106. [DOI] [PubMed] [Google Scholar]
- 12. Vezina P. D1 dopamine receptor activation is necessary for the induction of sensitization by amphetamine in the ventral tegmental area. J Neurosci 1996;16:2411–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karper PE, De la Rosa H, Newman ER, et al Role of D1‐like receptors in amphetamine‐induced behavioral sensitization: A study using D1A receptor knockout mice. Psychopharmacology (Berl) 2002;159:407–414. [DOI] [PubMed] [Google Scholar]
- 14. Kuczenski R, Segal DS. Sensitization of amphetamine‐induced stereotyped behaviors during the acute response. J Pharmacol Exp Ther 1999;288:699–709. [PubMed] [Google Scholar]
- 15. Moro H, Sato H, Ida I, et al Effects of SKF‐38393, a dopamine D1 receptor agonist on expression of amphetamine‐induced behavioral sensitization and expression of immediate early gene arc in prefrontal cortex of rats. Pharmacol Biochem Behav 2007;87:56–64. [DOI] [PubMed] [Google Scholar]
- 16. Hirabayashi M, Tadokoro S. Effect of chlorpromazine on mouse ambulatory activity sensitization caused by (+)‐amphetamine. J Pharm Pharmacol 1993;45:481–483. [DOI] [PubMed] [Google Scholar]
- 17. Futamura T, Akiyama S, Sugino H, Forbes A, McQuade RD, Kikuchi T. Aripiprazole attenuates established behavioral sensitization induced by methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry 2010;34:1115–1119. [DOI] [PubMed] [Google Scholar]
- 18. Kuribara H, Tadokoro S. Effects of YM‐09151–2, a potent and selective dopamine D2 antagonist, on the ambulation‐increasing effect of methamphetamine in mice. Jpn J Pharmacol 1990;52:489–492. [DOI] [PubMed] [Google Scholar]
- 19. Kuribara H, Asami T, Ida I, Iijima Y, Tadokoro S. Effects of repeated MK‐801 on ambulation in mice and in sensitization following methamphetamine. Psychopharmacology (Berl) 1992;108:271–275. [DOI] [PubMed] [Google Scholar]
- 20. Ida I, Asami T, Kuribara H. Inhibition of cocaine sensitization by MK‐801, a noncompetitive N‐methyl‐D‐aspartate (NMDA) receptor antagonist: Evaluation by ambulatory activity in mice. Jpn J Pharmacol 1995;69:83–90. [DOI] [PubMed] [Google Scholar]
- 21. Li Y, White FJ, Wolf ME. Pharmacological reversal of behavioral and cellular indices of cocaine sensitization in the rat. Psychopharmacology (Berl) 2000;151:175–183. [DOI] [PubMed] [Google Scholar]
- 22. De Montis MG, Gambarana C, Ghiglieri O, Tagliamonte A. Reversal of stable behavioural modifications through NMDA receptor inhibition in rats. Behav Pharmacol 1995;6:562–567. [PubMed] [Google Scholar]
- 23. Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1‐like and D2‐like agonists and antagonists on cocaine self‐administration and food maintained responding in rats. Neuropharmacology 2004;47(Suppl 1):256–273. [DOI] [PubMed] [Google Scholar]
- 24. Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D(1‐like) and D(2‐like) agonists in rats that self‐administer cocaine. J Pharmacol Exp Ther 1999;291:353–360. [PubMed] [Google Scholar]
- 25. Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine‐seeking behavior by D1‐ and D2‐like dopamine receptor agonists. Science 1996;271:1586–1589. [DOI] [PubMed] [Google Scholar]
- 26. Ago Y, Nakamura S, Baba A, Matsuda T. Neuropsychotoxicity of abused drugs: Effects of serotonin receptor ligands on methamphetamine‐ and cocaine‐induced behavioral sensitization in mice. J Pharmacol Sci 2008;106:15–21. [DOI] [PubMed] [Google Scholar]
- 27. Shuto T, Kuroiwa M, Hamamura M, et al Reversal of methamphetamine‐induced behavioral sensitization by repeated administration of a dopamine D1 receptor agonist. Neuropharmacology 2006;50:991–997. [DOI] [PubMed] [Google Scholar]
- 28. Shuto T, Seeman P, Kuroiwa M, Nishi A. Repeated administration of a dopamine D1 receptor agonist reverses the increased proportions of striatal dopamine D1High and D2High receptors in methamphetamine‐sensitized rats. Eur J Neurosci 2008;27:2551–2557. [DOI] [PubMed] [Google Scholar]
- 29. Bowers MS, McFarland K, Lake RW, et al Activator of G protein signaling 3: A gatekeeper of cocaine sensitization and drug seeking. Neuron 2004;42:269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arai I, Shimazoe T, Yoshimatsu A, Inoue H, Shibata S, Watanabe S. Vulnerability of synaptic plasticity in the striatum of methamphetamine‐sensitized rats. Jpn J Pharmacol 1998;78:105–108. [DOI] [PubMed] [Google Scholar]
- 31. Yoshimatsu A, Shimazoe T, Kawashimo A, et al Effects of adenosine A1‐ and A2A‐receptor agonists on enhancement of dopamine release from the striatum in methamphetamine‐sensitized rats. Jpn J Pharmacol 2001;86:254–257. [DOI] [PubMed] [Google Scholar]
- 32. Niznik HB. Dopamine receptors: Molecular structure and function. Mol Cell Endocrinol 1987;54:1–22. [DOI] [PubMed] [Google Scholar]
- 33. Seeman P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse 1987;1:133–152. [DOI] [PubMed] [Google Scholar]
- 34. George SR, Watanabe M, Di Paolo T, Falardeau P, Labrie F, Seeman P. The functional state of the dopamine receptor in the anterior pituitary is in the high affinity form. Endocrinology 1985;117:690–697. [DOI] [PubMed] [Google Scholar]
- 35. Seeman P, Tallerico T, Ko F, Tenn C, Kapur S. Amphetamine‐sensitized animals show a marked increase in dopamine D2 high receptors occupied by endogenous dopamine, even in the absence of acute challenges. Synapse 2002;46:235–239. [DOI] [PubMed] [Google Scholar]
- 36. Levy AD, Kim JJ, Ellison GD. Chronic amphetamine alters D‐2 but not D‐1 agonist‐induced behavioral responses in rats. Life Sci 1988;43:1207–1213. [DOI] [PubMed] [Google Scholar]
- 37. Ujike H, Akiyama K, Otsuki S. D‐2 but not D‐1 dopamine agonists produce augmented behavioral response in rats after subchronic treatment with methamphetamine or cocaine. Psychopharmacology (Berl) 1990;102:459–464. [DOI] [PubMed] [Google Scholar]
- 38. Saklayen SS, Mabrouk OS, Pehek EA. Negative feedback regulation of nigrostriatal dopamine release: Mediation by striatal D1 receptors. J Pharmacol Exp Ther 2004;311:342–348. [DOI] [PubMed] [Google Scholar]
- 39. Mark KA, Soghomonian JJ, Yamamoto BK. High‐dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long‐term dopamine toxicity. J Neurosci 2004;24:11449–11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crawford CA, Choi FY, Kohutek JL, Yoshida ST, McDougall SA. Changes in PKA activity and Gs alpha and Golf alpha levels after amphetamine‐ and cocaine‐induced behavioral sensitization. Synapse 2004;51:241–248. [DOI] [PubMed] [Google Scholar]
- 41. Borgkvist A, Fisone G. Psychoactive drugs and regulation of the cAMP/PKA/DARPP‐32 cascade in striatal medium spiny neurons. Neurosci Biobehav Rev 2007;31:79–88. [DOI] [PubMed] [Google Scholar]
- 42. Carter CJ, Pycock CJ. Behavioural and biochemical effects of dopamine and noradrenaline depletion within the medial prefrontal cortex of the rat. Brain Res 1980;192:163–176. [DOI] [PubMed] [Google Scholar]
- 43. Pycock CJ, Carter CJ, Kerwin RW. Effect of 6‐hydroxydopamine lesions of the medial prefrontal cortex on neurotransmitter systems in subcortical sites in the rat. J Neurochem 1980;34:91–99. [DOI] [PubMed] [Google Scholar]
- 44. Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: A review and reconceptualization. Am J Psychiatry 1991;148:1474–1486. [DOI] [PubMed] [Google Scholar]
- 45. Fletcher PJ, Tenn CC, Rizos Z, Lovic V, Kapur S. Sensitization to amphetamine, but not PCP, impairs attentional set shifting: Reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Psychopharmacology (Berl) 2005;183:190–200. [DOI] [PubMed] [Google Scholar]
- 46. McLean SL, Idris NF, Woolley ML, Neill JC. D(1)‐like receptor activation improves PCP‐induced cognitive deficits in animal models: Implications for mechanisms of improved cognitive function in schizophrenia. Eur Neuropsychopharmacol 2009;19:440–450. [DOI] [PubMed] [Google Scholar]
- 47. Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol 1998;54:679–720. [DOI] [PubMed] [Google Scholar]
- 48. Karler R, Calder LD, Chaudhry IA, Turkanis SA. Blockade of “reverse tolerance” to cocaine and amphetamine by MK‐801. Life Sci 1989;45:599–606. [DOI] [PubMed] [Google Scholar]
- 49. Reid MS, Hsu K, Jr ., Berger SP. Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system: Studies on the involvement of dopamine. Synapse 1997;27:95–105. [DOI] [PubMed] [Google Scholar]
- 50. Artola A, Singer W. Long‐term depression of excitatory synaptic transmission and its relationship to long‐term potentiation. Trends Neurosci 1993;16:480–487. [DOI] [PubMed] [Google Scholar]
- 51. Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine‐mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci 2007;30:211–219. [DOI] [PubMed] [Google Scholar]
- 52. Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol 2008;154:327–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Centonze D, Costa C, Rossi S, et al Chronic cocaine prevents depotentiation at corticostriatal synapses. Biol Psychiatry 2006;60:436–443. [DOI] [PubMed] [Google Scholar]
- 54. Tashev R, Moura PJ, Venkitaramani DV, et al A substrate trapping mutant form of striatal‐enriched protein tyrosine phosphatase prevents amphetamine‐induced stereotypies and long‐term potentiation in the striatum. Biol Psychiatry 2009;65:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pu L, Liu QS, Poo MM. BDNF‐dependent synaptic sensitization in midbrain dopamine neurons after cocaine withdrawal. Nat Neurosci 2006;9:605–607. [DOI] [PubMed] [Google Scholar]
- 56. Li Y, Kauer JA. Repeated exposure to amphetamine disrupts dopaminergic modulation of excitatory synaptic plasticity and neurotransmission in nucleus accumbens. Synapse 2004;51:1–10. [DOI] [PubMed] [Google Scholar]
- 57. Wang Z, Kai L, Day M, et al Dopaminergic control of corticostriatal long‐term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron 2006;50:443–452. [DOI] [PubMed] [Google Scholar]
- 58. Centonze D, Grande C, Saulle E, et al Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J Neurosci 2003;23:8506–8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Przegalinski E, Siwanowicz J, Baran L, Filip M. Activation of serotonin (5‐HT)1A receptors inhibits amphetamine sensitization in mice. Life Sci 2000;66:1011–1019. [DOI] [PubMed] [Google Scholar]
- 60. Auclair A, Drouin C, Cotecchia S, Glowinski J, Tassin JP. 5‐HT2A and alpha1b‐adrenergic receptors entirely mediate dopamine release, locomotor response and behavioural sensitization to opiates and psychostimulants. Eur J Neurosci 2004;20:3073–3084. [DOI] [PubMed] [Google Scholar]
- 61. Yoo JH, Cho JH, Yu HS, et al Involvement of 5‐HT receptors in the development and expression of methamphetamine‐induced behavioral sensitization: 5‐HT receptor channel and binding study. J Neurochem 2006;99:976–988. [DOI] [PubMed] [Google Scholar]
- 62. Davidson C, Lee TH, Xiong Z, Ellinwood EH. Ondansetron given in the acute withdrawal from a repeated cocaine sensitization dosing regimen reverses the expression of sensitization and inhibits self‐administration. Neuropsychopharmacology 2002;27:542–553. [DOI] [PubMed] [Google Scholar]
- 63. Davidson C, Gopalan R, Ahn C, et al Reduction in methamphetamine induced sensitization and reinstatement after combined pergolide plus ondansetron treatment during withdrawal. Eur J Pharmacol 2007;565:113–118. [DOI] [PubMed] [Google Scholar]
- 64. Zhang X, Mi J, Wetsel WC, et al PI3 kinase is involved in cocaine behavioral sensitization and its reversal with brain area specificity. Biochem Biophys Res Commun 2006;340:1144–1150. [DOI] [PubMed] [Google Scholar]
- 65. Zhang X, Lee TH, Davidson C, Lazarus C, Wetsel WC, Ellinwood EH. Reversal of cocaine‐induced behavioral sensitization and associated phosphorylation of the NR2B and GluR1 subunits of the NMDA and AMPA receptors. Neuropsychopharmacology 2007;32:377–387. [DOI] [PubMed] [Google Scholar]
- 66. Chen Q, Lee TH, Wetsel WC, et al Reversal of cocaine sensitization‐induced behavioral sensitization normalizes GAD67 and GABAA receptor alpha2 subunit expression, and PKC zeta activity. Biochem Biophys Res Commun 2007;356:733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Davidson C, Lazarus C, Xiong X, Lee TH, Ellinwood EH. 5‐HT2 receptor antagonists given in the acute withdrawal from daily cocaine injections can reverse established sensitization. Eur J Pharmacol 2002;453:255–263. [DOI] [PubMed] [Google Scholar]
- 68. Jordan S, Koprivica V, Chen R, Tottori K, Kikuchi T, Altar CA. The antipsychotic aripiprazole is a potent, partial agonist at the human 5‐HT1A receptor. Eur J Pharmacol 2002;441:137–140. [DOI] [PubMed] [Google Scholar]
- 69. Rasmussen K, Stockton ME, Czachura JF. The 5‐HT3 receptor antagonist zatosetron decreases the number of spontaneously active A10 dopamine neurons. Eur J Pharmacol 1991;205:113–116. [DOI] [PubMed] [Google Scholar]
- 70. Prisco S, Pessia M, Ceci A, Borsini F, Esposito E. Chronic treatment with DAU 6215, a new 5‐HT3 receptor antagonist, causes a selective decrease in the number of spontaneously active dopaminergic neurons in the rat ventral tegmental area. Eur J Pharmacol 1992;214:13–19. [DOI] [PubMed] [Google Scholar]
- 71. Invernizzi R, Pozzi L, Samanin R. Selective reduction of extracellular dopamine in the rat nucleus accumbens following chronic treatment with DAU 6215, a 5‐HT3 receptor antagonist. Neuropharmacology 1995;34:211–215. [DOI] [PubMed] [Google Scholar]
- 72. Sorensen SM, Humphreys TM, Palfreyman MG. Effect of acute and chronic MDL 73,147EF, a 5‐HT3 receptor antagonist, on A9 and A10 dopamine neurons. Eur J Pharmacol 1989;163:115–118. [DOI] [PubMed] [Google Scholar]
- 73. Minabe Y, Ashby CR, Jr ., Wang RY. Effects produced by acute and chronic treatment with granisetron alone or in combination with haloperidol on midbrain dopamine neurons. Eur Neuropsychopharmacol 1992;2:127–133. [DOI] [PubMed] [Google Scholar]
- 74. Palfreyman MG, Schmidt CJ, Sorensen SM, et al Electrophysiological, biochemical and behavioral evidence for 5‐HT2 and 5‐HT3 mediated control of dopaminergic function. Psychopharmacology (Berl) 1993;112:S60–S67. [DOI] [PubMed] [Google Scholar]
- 75. Liu W, Thielen RJ, McBride WJ. Effects of repeated daily treatments with a 5‐HT3 receptor antagonist on dopamine neurotransmission and functional activity of 5‐HT3 receptors within the nucleus accumbens of Wistar rats. Pharmacol Biochem Behav 2006;84:370–377. [DOI] [PubMed] [Google Scholar]
- 76. Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O’Laughlin IA, Meltzer HY. 5‐HT(2A) and D(2) receptor blockade increases cortical DA release via 5‐HT(1A) receptor activation: A possible mechanism of atypical antipsychotic‐induced cortical dopamine release. J Neurochem 2001;76:1521–1531. [DOI] [PubMed] [Google Scholar]
- 77. Moghaddam B, Bunney BS. Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: An in vivo microdialysis study. J Neurochem 1990;54:1755–1560. [DOI] [PubMed] [Google Scholar]
- 78. Volonte M, Monferini E, Cerutti M, Fodritto F, Borsini F. BIMG 80, a novel potential antipsychotic drug: Evidence for multireceptor actions and preferential release of dopamine in prefrontal cortex. J Neurochem 1997;69:182–190. [DOI] [PubMed] [Google Scholar]
- 79. Li XM, Perry KW, Wong DT, Bymaster FP. Olanzapine increases in vivo dopamine and norepinephrine release in rat prefrontal cortex, nucleus accumbens and striatum. Psychopharmacology (Berl) 1998;136:153–161. [DOI] [PubMed] [Google Scholar]
- 80. Kuroki T, Meltzer HY, Ichikawa J. Effects of antipsychotic drugs on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens. J Pharmacol Exp Ther 1999;288:774–781. [PubMed] [Google Scholar]
- 81. Bortolozzi A, Diaz‐Mataix L, Scorza MC, Celada P, Artigas F. The activation of 5‐HT receptors in prefrontal cortex enhances dopaminergic activity. J Neurochem 2005;95:1597–1607. [DOI] [PubMed] [Google Scholar]
- 82. Schmidt CJ, Fadayel GM. The selective 5‐HT2A receptor antagonist, MDL 100,907, increases dopamine efflux in the prefrontal cortex of the rat. Eur J Pharmacol 1995;273:273–279. [DOI] [PubMed] [Google Scholar]
- 83. Engleman EA, Rodd ZA, Bell RL, Murphy JM. The role of 5‐HT3 receptors in drug abuse and as a target for pharmacotherapy. CNS Neurol Disord Drug Targets 2008;7:454–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Blank T, Zwart R, Nijholt I, Spiess J. Serotonin 5‐HT2 receptor activation potentiates N‐methyl‐D‐aspartate receptor‐mediated ion currents by a protein kinase C‐dependent mechanism. J Neurosci Res 1996;45:153–160. [DOI] [PubMed] [Google Scholar]
- 85. Ronde P, Nichols RA. High calcium permeability of serotonin 5‐HT3 receptors on presynaptic nerve terminals from rat striatum. J Neurochem 1998;70:1094–1103. [DOI] [PubMed] [Google Scholar]
- 86. Funahashi M, Mitoh Y, Matsuo R. Activation of presynaptic 5‐HT3 receptors facilitates glutamatergic synaptic inputs to area postrema neurons in rat brain slices. Methods Find Exp Clin Pharmacol 2004;26:615–622. [DOI] [PubMed] [Google Scholar]
- 87. Ago Y, Nakamura S, Uda M, et al Attenuation by the 5‐HT1A receptor agonist osemozotan of the behavioral effects of single and repeated methamphetamine in mice. Neuropharmacology 2006;51:914–922. [DOI] [PubMed] [Google Scholar]
- 88. Ago Y, Nakamura S, Kajita N, et al Ritanserin reverses repeated methamphetamine‐induced behavioral and neurochemical sensitization in mice. Synapse 2007;61:757–763. [DOI] [PubMed] [Google Scholar]
- 89. King GR, Xiong Z, Ellinwood EH, Jr . Blockade of the expression of sensitization and tolerance by ondansetron, a 5‐HT3 receptor antagonist, administered during withdrawal from intermittent and continuous cocaine. Psychopharmacology (Berl) 1998;135:263–269. [DOI] [PubMed] [Google Scholar]
- 90. King GR, Xiong Z, Douglass S, Ellinwood EH. Long‐term blockade of the expression of cocaine sensitization by ondansetron, a 5‐HT(3) receptor antagonist. Eur J Pharmacol 2000;394:97–101. [DOI] [PubMed] [Google Scholar]
- 91. Park HJ, Cui FJ, Hwang JY, Kang UG. Effects of clozapine on behavioral sensitization induced by cocaine. Psychiatry Res 2010;175:165–170. [DOI] [PubMed] [Google Scholar]
- 92. Seeman P. All Roads to Schizophrenia Lead to Dopamine Supersensitivity and Elevated Dopamine D2 Receptors. CNS Neurosci Ther 2010. doi: 10.1111/j.1755-5949.2010.00162.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 1991;148:1301–1308. [DOI] [PubMed] [Google Scholar]
- 94. Takahata R, Moghaddam B. Activation of glutamate neurotransmission in the prefrontal cortex sustains the motoric and dopaminergic effects of phencyclidine. Neuropsychopharmacology 2003;28:1117–1124. [DOI] [PubMed] [Google Scholar]
- 95. Sams‐Dodd F. A test of the predictive validity of animal models of schizophrenia based on phencyclidine and D‐amphetamine. Neuropsychopharmacology 1998;18:293–304. [DOI] [PubMed] [Google Scholar]
- 96. Martinez ZA, Ellison GD, Geyer MA, Swerdlow NR. Effects of sustained phencyclidine exposure on sensorimotor gating of startle in rats. Neuropsychopharmacology 1999;21:28–39. [DOI] [PubMed] [Google Scholar]
- 97. Kaneko Y, Kashiwa A, Ito T, Ishii S, Umino A, Nishikawa T. Selective serotonin reuptake inhibitors, fluoxetine and paroxetine, attenuate the expression of the established behavioral sensitization induced by methamphetamine. Neuropsychopharmacology 2007;32:658–664. [DOI] [PubMed] [Google Scholar]
- 98. Bebawy D, Marquez P, Samboul S, Parikh D, Hamid A, Lutfy K. Orphanin FQ/nociceptin not only blocks but also reverses behavioral adaptive changes induced by repeated cocaine in mice. Biol Psychiatry 2010;68:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M. Reversal of cocaine addiction by environmental enrichment. Proc Natl Acad Sci U S A 2008;105:17145–17150. [DOI] [PMC free article] [PubMed] [Google Scholar]


