SUMMARY
During the last decade, the identification of a number of novel drug targets led to the development of promising new compounds which are currently under evaluation for their therapeutic prospective in CNS related disorders. Besides the established pleiotropic regulatory functions in the periphery, the interest in the potential homeostatic role of histamine in the brain was revived following the identification of H3 and H4 receptors some years ago. Complementing classical CNS pharmacology, the development of selective histamine receptor agonists, antagonists, and inverse agonists provides the lead for the potential exploitation of the histaminergic system in the treatment of brain pathologies. Although no CNS disease entity has been associated directly to brain histamine dysfunction until now, the H3 receptor is recognized as a drug target for neuropathic pain, sleep‐wake disorders, including narcolepsy, and cognitive impairment associated with attention deficit hyperactivity disorder, schizophrenia, Alzheimer’s, or Parkinson's disease, while the first H3 receptor ligands have already entered phase I–III clinical trials. Interestingly, the localization of the immunomodulatory H4 receptor in the nervous system exposes attractive perspectives for the therapeutic exploitation of this new drug target in neuroimmunopharmacology. This review focuses on a concise presentation of the current “translational research” approach that exploits the latest advances in histamine pharmacology for the development of beneficial drug targets for the treatment of neuronal disorders, such as neuropathic pain, cognitive, and sleep‐wake pathologies. Furthermore, the role of the brain histaminergic system(s) in neuroprotection and neuroimmunology/inflammation remains a challenging research area that is currently under consideration.
Keywords: Cognitive impairment, Histamine, H3 and H4 receptors, Multiple sclerosis, Neuropathic pain, Neuroprotection, New drug targets, Sleep‐wake cycle
Introduction
Histamine [2‐(4‐imidazolyl)‐ethylamine] is an endogenous short‐acting biogenic amine synthesized from l‐histidine through the catalytic activity of the rate‐limiting enzyme histidine decarboxylase (HDC, EC 4.1.1.22) [1]. Following its discovery 100 years ago [2], histamine has been one of the most studied substances in medicine for a century, possessing a wide spectrum of activities, including its function in neurotransmission (Fig. 1) [3].
Figure 1.
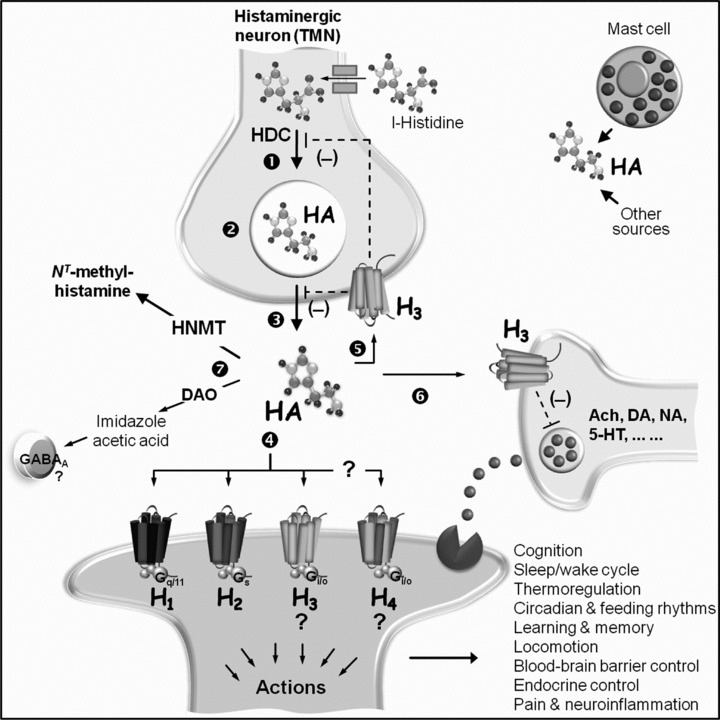
Schematic representation of central histaminergic components. In the adult vertebrate brain, histamine (HA) is formed in histaminergic neurons of the tuberomammillary nucleus (TMN) of the posterior hypothalamus from l‐histidine through one‐step catalytic action of histidine decarboxylase (HDC) (1). HA is stored in presynaptic secretory vesicles (2), and released into the extracellular space (3) to activate four subtypes of postsynaptic G protein (G)‐coupled receptors (4) designated H1 to H4, thus eliciting a variety of physiological responses as indicated in the figure. Activation of postsynaptic H1 receptors has been associated with enhanced vigilance and attention and decreased feeding, while stimulation of the postsynaptic H2 receptors has been linked to enhanced working memory [18]. Binding to postsynaptic H3 and, possibly, H4 receptors leads to as yet poorly defined actions (?), which are currently undergoing rigorous investigation. Stimulation of presynaptic H3 autoreceptors (5) inhibits (‐) HA synthesis (1) and/or release (3), while HA binding to presynaptic H3 receptors on nonhistaminergic neurons (6) inhibits the release of a number of neurotransmitters, including acetylcholine (ACh), dopamine (DA), noradrenaline (NA), serotonin (5‐HT), and others in a pathway‐dependent manner. HA is inactivated (7) predominantly by methylation through neuronal histamine N‐methyltransferase (HNMT), without excluding the contribution of diamine oxidase (DAO) as a salvage pathway to produce imidazole acetic acid, a γ‐aminobutyric acid (GABA)A receptor agonist. Additional sources of brain HA may include (hypothalamic) mast cells and other nonneuronal components [cf. 3].
Histamine is synthesized in several cell types of peripheral and central tissues. The classical source of histamine is the pluripotent heterogeneous mast cell where it is stored in cytosolic granules and released by exocytosis to exert several actions in response to various immunological and nonimmunological stimuli [4]. Nonmast cell histamine is derived from numerous sources, indicative examples being gastric enterochromaffin‐like cells [5], various types of blood cells [6] and neurons [7, 8]. The pleiotropic regulatory character of histamine in cellular events is attributed to its binding to four distinct subtypes of G‐protein‐coupled receptors (GPCRs), designated H1, H2, H3, and H4 that are differentially expressed in various cell types [9]. In humans, the endogenous ligand shows low affinity for H1 and H2 receptors, whereas its potency on H3 and H4 receptors is considerably higher. H3 and H4 receptors are most closely related to each other and they have a closer phylogenetic relationship with peptide ligand GPCRs, while they are remotely related to other biogenic amine receptors, including H1 and H2 receptors [10].
It is currently accepted that all four subtypes of histamine receptors play as yet ill‐defined roles in the central nervous system (CNS) [3]. While data on the contribution of histamine receptors in brain physiology and disease states continue to emerge in the literature [11], the H3 receptor is a recognized drug target for neuronal diseases, such as cognitive impairment, schizophrenia, epilepsy, sleep‐wake disorders and neuropathic pain, and the first H3 receptor ligands have been taken into clinical studies (Table 1) [cf. 12]. Interestingly, the very recent demonstration of H4 receptor functional expression in the CNS (Fig. 2) [13] may have implications for the therapeutic potential of these receptors in central disorders, in addition to their topicality as a potential drug target for inflammatory conditions [14].
Table 1.
Histamine H3 receptor ligands in clinical evaluation
| Disorder | Compound | Condition | Phase | Study identifiera |
|---|---|---|---|---|
| Narcolepsy | BF2.649 | EDS | III | NCT01067222 |
| BF2.649 | Cataplexy (add on Modafinil) | III | NCT01067235 | |
| GSK‐189254 | IIc | NCT00366080 | ||
| JNJ‐17216498 | IIb | NCT00424931 | ||
| PF‐03654746 | EDS | II | NCT01006122 | |
| ADHD | MK‐0249 | IIb | NCT00475735 | |
| PF‐03654746 | IIb | NCT00531752 | ||
| Alzheimer's disease | GSK‐239512 | (mild to moderate) | II | NCT01009255 |
| MK‐0249 | Cognitive function | Ic | NCT00874939 | |
| MK‐0249 | IIb | NCT00420420 | ||
| PF‐03654746 | (mild to moderate) | I | NCT01028911 | |
| Schizophrenia | BF2.649 | Cognitive function | II | NCT00690274 |
| GSK‐239512 | Cognitive function | II | NCT01009060 | |
| MK‐0249 | Cognitive function | IIb | NCT00506077 | |
| Parkinson's disease | BF2.649 | EDS | IIb | NCT00642928 |
| BF2.649 | EDS | III | NCT01036139 | |
| Neuropathic Pain | GSK‐189254 | Hyperalgesia | Ib | NCT00387413 |
ADHD, attention deficit hyperactivity disorder; EDS, excessive daytime sleepiness; BF2.649, tiprolisant, [1‐{3‐[3‐(4‐chlorophenyl)propoxy]propyl}piperidine hydrochloride][46,47]; GSK‐189254, 6‐[(3‐cyclobutyl‐2,3,4,5‐tetrahydro‐1H‐3‐benzazepin‐7‐yl)oxy]‐N‐methyl‐3‐pyridinecarboxamide hydrochloride [54].
awww.clinicaltrials.gov.
bCompleted.
cTerminated.
Figure 2.
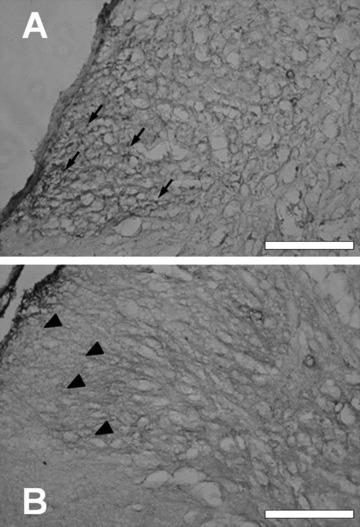
Histamine (A) H3 and (B) H4 receptors expressed in the mouse spinal dorsal horn. Paraformaldehyde (4%)‐fixed adult murine spinal cords were dehydrated using sucrose infiltration, with 10% sucrose, then 20% sucrose made up with phosphate buffered saline, and snap‐frozen in iso‐pentane −70°C for 1 minute and stored at −80°C. Cryoslices (20 μm thickness) were probed with either (A) anti‐H3 and (B) anti‐mH4 receptor antibodies essentially as described previously [35, 38]. Arrows and arrowheads indicate H3 and H4 receptor expression, respectively. Bar = 200 μm.
The complex brain histamine neurophysiology [3, 15, 16] and its receptor ligand pharmacology [12, 17, 18] have been extensively reviewed in the recent literature. This contribution highlights the challenging novel new CNS drug targets introduced by the latest advances in histamine pharmacology, predominantly pertaining to the H3 and H4 receptors, and focuses on a concise presentation of the very recent data supporting the “bench‐to‐bedside” concept and future directions for the potential exploitation of the histaminergic system in the treatment of brain pathologies.
Histamine in the Brain
The presence of histamine in the brain was first shown more than 60 years ago [19, 20]. Early indirect pharmacological evidence for the role of histamine in the brain was provided by the sedative side effects of classic antihistamines used for the treatment of allergies and by the interactions, particularly with the H1‐receptor, of a number of drugs commonly used in neuropsychiatry, including typical or atypical antipsychotics and antidepressants [21]. However, research into the significance of histamine in the CNS has been delayed for many decades, as a possible correlation of these interactions to the therapeutic actions of neuropsychiatric medication was overlooked and any histamine‐related effects of these agents were regarded solely as side effects resulting from their complex pharmacology [22]. More importantly, the well‐established strong association of histamine with peripheral mast cells and with the pathophysiology of atopic diseases [4] seems to have deterred neuroscientists from the investigation of this biogenic amine.
Despite the structural and functional identification of the mostly excitatory H1 and H2 receptors in the CNS, the important actions of histamine in the brain were recognized in the 1980s [8, 23] in parallel with the identification of H3 autoreceptors that control the activity of histaminergic neurons [7]. Since then, ongoing related research continues to provide evidence for the (patho)physiological significance of the histaminergic system in the CNS and for a better understanding of the actions of therapeutic agents, such as the H4 receptor binding of several neuroactive drugs, including amitriptyline and clozapine and the recently identified H1 receptor‐mediated orexigenic actions of the antipsychotic clozapine in the hypothalamus [24].
Histamine does not penetrate the blood–brain barrier; hence its biosynthesis in the brain is elicited in one step by HDC (Fig. 1) and is controlled by the availability of l‐histidine [cf. 3]. Unlike other biogenic amines, histamine is not a direct inhibitor of its biosynthetic enzyme, S‐α‐fluoromethylhistidine being a selective and potent HDC “suicide” inhibitor [25]. In the adult vertebrate brain, histamine is formed in histaminergic neurons of the tuberomammillary (TM) nucleus of the posterior hypothalamus that project to all major areas of the brain, whereas data on the afferent connections are relatively limited [3, 15]. Neuronal histamine is stored in cell somata and in axon varicosities, while its synthesis and release are controlled mainly through H3 autoreceptors on the somata, dendrites and axons (Fig. 1). Histamine acts postsynaptically via H1, H2, H3, and H4 receptors, and it is inactivated by methylation through neuronal histamine N‐methyltransferase (HNMT, EC 2.1.1.8), without excluding the contribution of diamine oxidase (DAO, EC 1.4.3.6), which converts histamine into imidazole acetic acid, a γ‐aminobutyric acid (GABA)A receptor agonist [cf. Ref. 3].
So far, no direct association between a neuronal disease and brain histamine dysfunction has been identified. In a mutual interaction network with other transmitter systems, brain histamine is implicated in basic homeostatic and higher brain functions, including sleep‐wake regulation, circadian and feeding rhythms, body temperature, locomotor activity, learning, and memory [3]. Relatively recently, attention has been drawn to the role of histamine in autoimmune diseases and neuroinflammation [26, 27], while H4 receptors, which are primarily, but not exclusively, distributed in immune cells [10] may be involved in a number of elaborate homeostatic and integrative interactions between the immune and nervous systems.
Besides neuronal histamine, mast cells, which are fundamental in peripheral immune and atopic responses [4], seem to be an additional source of brain histamine (Fig. 1). Mast cells appear to act as gatekeepers at interfaces between the CNS and the endocrine and immune systems [28, 29, 30, 31], as they can migrate into the brain, particularly during development [32] and under some pathological conditions [33].
Histamine H3 Receptors
Studies on histamine function in the CNS have been focused largely on the effects mediated via H3 receptor signaling [7]. H3 receptors are located on TM neurons and are mainly expressed in cerebral cortex, hippocampus, amygdala, nucleus accumbens, globus pallidus, striatum, thalamus, and hypothalamus [34, 35]. The human H3 receptor gene is located on chromosome 20q13.33 and encodes a 70 kDa 445 amino acid peptide belonging to the GPCR family [36], showing very low sequence similarity with other GPCRs and only 22% and 20% similarity with H1 and H2 receptors, respectively [17]. Downstream signalling is mediated through Gi/o proteins and consequently the negative coupling of the receptor to adenylyl cyclase downregulates cAMP‐dependent activation of protein kinase A and cAMP‐response‐element‐binding protein‐induced gene transcription. Additional interactions with other effector signalling cascades include the activation of phospholipase A2, as well as the mitogen‐activated protein kinase and phosphatidylinositol 3‐kinase pathways (PI3K), which activate extracellular signal‐regulated kinases and Akt and subsequently inhibit the action of glycogen synthase kinase 3β (GSK3β), thus playing important roles in neuronal apoptosis, axonal and synaptic plasticity and being associated with Alzheimer's disease, ischemia or Parkinson's disease, diabetes, and/or insulin resistance [cf. 3, 17].
The H3 autoreceptor function in the negative feedback control of histamine synthesis and release from histaminergic neurons [7], as well as the cross‐talk of the H3 heteroreceptor with other neurotransmitters, including acetylcholine, dopamine, serotonin, and noradrenaline (Fig. 1) [18], play a major role in brain physiology [3], illustrated by the high H3 receptor constitutive or spontaneous activity in vivo[37]. However, H3 receptors show species differences and pronounced molecular and functional heterogeneity achieved through differential splicing. The large number of isoforms exhibit differential distribution and pharmacological patterns that have complicated the extrapolation of preclinical data into the clinic [17]. The challenge is even greater than initially expected because of the complexity of the central histaminergic system, the diversity of actions, the overlapping structure‐affinity/efficacy relationships and pharmacology of H3 and H4 receptor‐targeting compounds and the differential opposing effects of some H3 ligands in a number of experimental models [16]. Yet, the usefulness of H3 receptor ligands in the treatment of pathophysiological states evoked by an imbalance of histaminergic interactions are currently under intense investigation, while a number of H3 receptor ligands are on their way to the clinic (Table 1).
Despite the evidence on the contribution of postsynaptic H1 and/or H2 receptors in histamine‐mediated transmission and in cellular mechanisms involved in arousal and cognitive functions [16], translational research has been directed towards the therapeutic potential of antagonists and inverse agonists targeting selectively the H3 receptor, based on a relatively complex rationale, without any proof of concept for an H3 receptor (patho)physiological function reported so far [12].
Histamine H4 Receptors
Following identification of the H4 receptor some 10 years ago by several groups simultaneously, accumulating evidence points to its regulatory role in immune and inflammatory responses [14]. Contrary to the H3 receptor predominant neuronal localization, H4 receptors are primarily, but not exclusively, distributed in immune cells, including mast cells, eosinophils, dendritic, and T cells in peripheral tissues such as spleen, thymus, colon, blood and bone marrow, thereby eliciting chemotactic actions [cf. 14].
Importantly, however, the recently reported functional expression of H4 receptors on human and rodent neurons (Fig. 2) highlights their implication in neuronal functions [13, 38, 39, 40, 41]. H4 receptor expression was detected in distinct deep laminae in the human cortex [13], hippocampus, thalamus, amygdala, and spinal cord [39]. Expression of H4 receptors has been reported in mouse thalamus, hippocampal CA4 stratum lucidum, and layer IV of the cerebral cortex, where they appear to induce hyperpolarization and promotion of outwardly rectifying currents [13], as well as in motor neuron subpopulations of the murine spinal cord [38]. In the rat, H4 mRNA has been detected in cortex, cerebellum, brainstem, amygdala, thalamus, striatum, dorsal root ganglia, and spinal cord, while very low levels were detected in the hypothalamus and no H4 mRNA signal in the hippocampus [39]. Recently, the H4 receptor antagonist, JNJ‐7777120, subjected intraperitoneally (i.p.) in rats reduced exploratory behavior in a dose‐dependent manner [41], which may indicate a role of the H4 receptor in motor function and/or anxiety responses, consistent with its anatomical profile. This requires further investigation in appropriately validated behavioral tests which discriminate these activities.
The human H4 receptor gene is located in a single copy on chromosome 18q11.2 and encodes a 390 amino acid peptide belonging to the GPCR family, sharing ∼35% amino acid identity with the H3 receptor, with ∼58% homology in its transmembrane regions, and only ∼19% sequence similarity to the H1 and H2 receptors. Similarly to the H3 receptor, the H4 receptor is coupled to pertussis toxin‐sensitive Gi/o proteins consequently inhibiting forskolin‐induced cAMP and eventually modulating the transcription of genes regulated by cAMP‐responsive elements [cf. 10]. At present only two nonsignalling H4 receptor isoforms have been identified, which may act as regulatory elements [42].
The similarities between H3 and H4 receptors and the overlap in their pharmacological profiles along with the high structural and pharmacological species differences displayed by the receptors and their ligands, particularly in terms of their potency, efficacy and functional selectivity [10], have resulted in frequent misinterpretations of preclinical data. Efforts are currently focused on the development of selective compounds that would provide effective pharmacological tools to assess the central and peripheral physiological role and the therapeutic potential of the H4 receptor [12]. The high patent filing activity in this field resulted in the advancement of the H4 receptor antagonist UR‐63325 to Phase I clinical trials for respiratory diseases, while further H4 receptor‐targeting agents developed by many pharmaceutical companies and academic departments show promise for potential entry into clinical studies.
Histamine Receptors as New CNS Drug Targets
Sleep‐Wake Disorders
The TM nucleus and the adjacent posterior hypothalamus are crucial for wakefulness. TM neurons, tuned via GABAA receptors activated by the GABAergic input from the ventrolateral preoptic area, project to various brain regions that control the sleep‐wake cycle, such as the cortex, thalamus, preoptic and anterior hypothalamus, brainstem and forebrain, while histamine release in the prefrontal cortex is strictly correlated to waking [3, 16]. The concerted action of this biogenic amine on H1, H2 and H3 receptors exerts a tonic wake‐selective activity pattern in the brain, which seems to be responsible for the qualitative cognitive aspects and cortical electroencephalogram activation in wakefulness, whereas orexin elicits a distinct, yet synergistic control with histamine by being involved mainly in the behavioral aspects of wakefulness [16, 43]. H1 receptor agonists and/or H3 antagonists are promising targets for the treatment of hypersomnia, while H1 receptor antagonists and/or H3 agonists could be useful in controlling insomnia [44]. Despite the sedative effect of most clinically used antihistamines, some of which are marketed as sleep aids due to their daytime drowsiness side effects, they are of limited use in controlling insomnia as they have long half‐lives, peripheral side effects and show inconsistent sedative patterns [45].
Selective ligands targeting the H3 autoreceptor have been shown to be modulators of sleep phenomena and associated pathologies, such as hypersomnia, daytime somnolence and the rare but unsatisfactorily treated sleep disorder, narcolepsy [12, 45]. Among the H3 receptor antagonists that have been used in related preclinical studies, JNJ‐17216498 and GSK‐189254 advanced to the phase II clinical trials for the treatment of narcolepsy [18]. The cyclobutyl amide PF‐03654746 is under clinical evaluation for its efficacy in improving alertness and awakeness in patients with narcolepsy‐associated excessive daytime sleepiness (EDS) (Table 1). Furthermore, the H3 receptor nonimidazole inverse agonist BF2.649 (tiprolisant) significantly reduced EDS in narcoleptic patients and currently running clinical trials are showing that it is a very promising alerting drug in Parkinson's Disease (Table 1) [46, 47]. Tiprolisant passed clinical phase II studies and approved orphan drug status by the European Medicines Agency (EMEA) for the therapeutic treatment of narcolepsy.
Cognitive Disorders
Impaired cognitive functions accompany neuropsychiatric and neurodegenerative diseases such as Alzheimer's disease, attention deficit hyperactivity disorder (ADHD), or schizophrenia, where most treatment strategies have focused on a single neurotransmitter system, even though disruption of multiple neurotransmitter systems is apparently involved. Histamine acting on postsynaptic H1 and H2 receptors functions as an excitatory neurotransmitter and plays a key role in attention and vigilance. Blockade of presynaptic H3 autoreceptors would therefore indirectly enhance histamine‐mediated attention in cognitive disorders such as ADHD and Alzheimer's disease [48]. Besides controlling histamine release through the presynaptic H3 autoreceptors, H3 ligands may control the release of other transmitter systems (Fig. 1) involved in cognitive processes [16, 18].
An ongoing phase II clinical trial aims to evaluate the cognitive enhancing effects of the H3 receptor antagonist GSK‐239512 in patients with mild to moderate Alzheimer's disease. In this regard, [3H]GSK‐189254 binding in hippocampal and cortical sections from patients with advanced Alzheimer's disease is an important observation, suggesting that H3 expression is still prevalent even in severe late stages of the disease [48]. Other compounds, such as MK‐0249 and PF‐03654746 await evaluation for their effectiveness in the treatment of ADHD (Table 1).
Furthermore, tiprolisant having completed phase II trials and showing efficacy in patients suffering from antipsychotic‐induced weight gain, is currently under evaluation for its cognitive enhancing effects in patients with schizophrenia and schizoaffective disorder (Table 1). In addition to the histaminergic and dopaminergic hyperactivity observed in schizophrenia, postsynaptic H3 receptors show additive activation with striatal dopamine D2 receptors in generating some of the disease symptoms, thus supporting the interest of H3 receptor inverse agonists as antipsychotics, either alone or as adjunctive treatment with classical neuroleptics [49]. A very interesting feature of H3 receptor antagonists/inverse agonists is that several of them produce cognitive enhancing effects at much lower doses than those required to elicit a robust wake enhancement [50, 51]. Ideally, different compounds could be used for the treatment of sleep‐wake cycle disorders or cognitive impairments.
Neuropathic Pain
Neuropathic pain is relatively common and largely resistant to treatment mainly because of the poorly understood underlying mechanisms, which include ectopic excitability of sensory neurones, sensitization of neurones in the dorsal horn of the spinal cord and, more recently, inflammatory and immune pathways [52]. Histamine as well as mast cells have been strongly implicated in the pathophysiology of neuropathic pain, while both H3[53] and H4[41] receptors seem to contribute to the underlying mechanisms. The existing data have been somewhat conflicting, mostly because of the use of experimental models with questionable relevance to the clinical situation and of the species‐differences and complex pharmacology of the compounds used, such as thioperamide, which it is now known to possess both H3 and H4 receptor activity [12, 52].
Novel selective H3 receptor antagonists/inverse agonists, such as GSK‐189254 and GSK‐334429, are effective in surgically induced and virally induced rat models of neuropathic pain [54]. Histamine produces itch which might be converted into pain in neuropathic hyperalgesia [55]. Regarding H4 receptors, the antihyperalgesic and antinociceptive effects of the H4 receptor antagonist JNJ‐7777120 have been suggested to be secondary to its antiinflammatory action [41]. JNJ‐7777120 exhibited robust antinociceptive activity in persistent inflammatory (complete Freund's adjuvant‐induced) pain, effectively reversed monoiodoacetate‐induced osteoarthritic joint pain and produced dose‐dependent antiallodynic effects in the spinal nerve ligation and sciatic nerve constriction injury models of chronic neuropathic pain, as well as in a skin‐incision model of acute postoperative pain, with ED50 values ranging from 29–88 mg/kg i.p. [41]. Although investigations utilizing H3 and/or H4 receptor ligands have to be evaluated carefully, H3 receptors in the human dorsal root ganglia and dorsal horn of the spinal cord seem to play a role in linking peripheral and central sensitization pathways [54]. Moreover, the recent demonstration of the functional expression of H4 receptors in the brain and the strong expression in human and rodent spinal cord (Fig. 2) [13, 38, 39], together with the involvement of inflammatory and immune components in neuropathic pain is directing current research to the investigation of a wider contribution of the H4 receptors in itch and pain [56].
The overall outcome of preclinical investigations led to phase I clinical trials of the highly potent histamine H3 receptor antagonist GSK‐189254 (Table 1), which demonstrated efficacy in the reduction of mechanical hyperalgesia and allodynia in the chronic constriction injury preclinical model of neuropathic pain, possibly through enhanced release of monoamines in the CNS [54].
Future Directions
Histamine and Neuroprotection
Antagonists/inverse agonists of the H3 receptor have raised much interest in the scientific community for their potential clinical use [12], yet there are potential therapeutic applications for H3 receptor agonists as well, that deserve consideration.
In addition to the modulation of the wake promoting effect of histamine, novel effects of H3 receptor activation have appeared in the recent literature, including an antinociceptive role for spinal H3 receptor activation [57]. Recently, several studies have hinted at a role of the histaminergic system and H3 receptor in neuroprotection. The hypothesis came from Panula and collaborators who provided clear indications of the plasticity of brain histaminergic system following injury or neurotoxic insults. H3 receptor mRNA was shown to be upregulated in the rat caudate and putamen following induction of transient global cerebral ischemia [58], or in the rat cortex following kainic acid‐induced seizures [59], although with different time courses and recovery. More recently the same group elegantly demonstrated that histamine protects hippocampal neurons from damage induced by kainic acid in organotypic cocultures of hypothalamic and hippocampal tissue [60], where histamine released from hypothalamic neurons affords the neuroprotective effect of hippocampal neurons presumably by activating postsynaptic H1 receptors. On the other hand, blockade of presumably presynaptic autoinhibitory H3 receptors ameliorates the protective effect of histaminergic neurons [60].
In cultured murine cortical neurons, though, where no histaminergic neurons are present, and H3 receptors are abundantly expressed, H3 receptor stimulation activates antiapoptotic signaling cascades, such as the PI3K/Akt/GSK‐3β pathway [61]. The Akt pathway has been implicated in regulating several important cellular processes, including cell plasticity and survival, proliferation and metabolism. Indeed, in cultured cortical cells, H3 receptor agonists increase the expression of the inhibitor of apoptosis Bcl‐2 and decrease the expression of proapoptotic elements such as caspase‐3, following neurotoxic insults [61]. Hence, stimulation of H3 receptors protects cortical neurons from N‐methyl‐d‐aspartate‐induced neurotoxic insults and this observation may have relevance in the prevention of, for instance, ischemic neuronal damage or neurodegenerative diseases. As a matter of fact, evidence points to a key role for GSK‐3β in promoting neurodegeneration [62] and it is involved in a cascade of events, such as hyperphosphorylation of tau protein, increased production of β‐amyloid, local cerebral inflammatory responses that may culminate in Alzheimer's disease [63]. In this regard, binding studies showed that the expression of H3 receptors is spared in the brain of Alzheimer's and Lewy Body Dementia patients [48, Lethbridge, Medhurst, & Chazot, unpublished]. To fully understand the impact of H3 receptor‐induced activation of antiapoptotic pathways in the CNS, in vivo experiments are necessary, the more so as H3 receptor antagonists are now viewed as potential therapeutics for schizophrenia and Alzheimer's disease (Table 1).
Multiple Sclerosis
Multiple sclerosis (MS) is the most common nontraumatic cause of neurological disability among young adults in Western Europe and North America and existing therapies are partly effective in halting ongoing inflammatory tissue damage and clinical progression. MS pathogenesis is complex and probably heterogeneous among patients, suggesting that combination therapy strategies that target a range of disease mechanisms might be more effective than medications used as monotherapy [64].
Increasing evidence indicates that histamine is also involved in the pathophysiology of MS and its most widely used animal model, experimental autoimmune encephalomyelitis (EAE) [27, 65]. The interest in histaminergic compounds for the treatment of MS stems mostly from preclinical studies although in a small pilot study as well, a cohort of MS patients treated with the H1 receptor antagonist hydroxyzine showed signs of neurological amelioration [66]. Recent evidence indicates a role for H1 receptors in autoimmune demyelination and extensive involvement of elements of the immune response associated with allergy in EAE [67]. H2 receptors also seem to regulate in part encephalitogenic T cell responses and EAE susceptibility, as mice lacking H2 receptors present less severe acute, early‐phase of the disease [68]. Moreover, H3 receptor knock‐out mice developed a more severe form of EAE than their wild type littermates, an effect that the authors attributed to altered blood–brain barrier permeability [68]. Considering the immunoregulatory role of the H4 receptor in inflammatory responses and the compelling evidence associating H4 receptors with atopic and autoimmune disorders [14, 56], it is intriguing to speculate an H4 receptor role in EAE and the possible attenuation of the immune response and amelioration of the neurological signs following blockade of the H4 receptor, a hypothesis that is currently under investigation.
Concluding Remarks
The identification of the histamine H3 and H4 receptors is pivotal in refining our understanding of the pharmacology and the therapeutic potential of this biogenic amine. Classically established as a “peripherally” important mediator of inflammation, histamine is now accepted to play a role in neurotransmission (Fig. 1) and to provide new CNS drug targets, similarly to the other biogenic amine systems exploited by neuropharmacology, including noradrenalin, dopamine and serotonin, which are major targets for medication commonly used in neuropsychiatry. Histamine exerts its central effects through H1, H2, and H3 receptors. The latter, whether presynaptic autoreceptors that inhibit the synthesis and release of histamine in the histaminergic neurones or postsynaptic heteroreceptors, are predominately distributed in the CNS and seem to be a suitable target for CNS drug development. Furthermore, the recent intriguing observation of the functional expression in enteric and central neurons of the predominantly peripheral H4 receptor (Fig. 2), which is undergoing rigorous characterization at present, adds further complexity to the role of histamine in the CNS. Thus, a potential interaction of the histamine receptors in the overall brain (patho)physiolology cannot be excluded at present.
Although important questions regarding the (patho)physiology of the central histaminergic system remain to be answered, H3 receptors have gained widespread attention by various academic and industrial laboratories for the development of ligands currently undergoing extensive preclinical pharmacological profiling for clinical candidate selection in a plethora of experimental models of human diseases. A number of antagonists and inverse agonists have advanced into clinical assessment (Table 1) for their safety and effectiveness in disorders of the sleep‐wake cycle such as narcolepsy, in neuropathic pain and as cognition enhancers in ADHD, schizophrenia, Alzheimer’s, and Parkinson's disease, the results of these clinical trials being eagerly awaited. Moreover, the increasing evidence for a role of the histaminergic system in neuroprotection and in the pathophysiology of MS has started to motivate vigorous related investigations in order to progress our understanding of the properties of histamine and its receptors, and to meet the challenge of identifying potential new drug targets in the neuropsychiatric and neuroimmunological arenas.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgements
This review was supported by ESF COST Action BM0806 “Recent advances in histamine receptor H4R research” financed by EU‐FP7.
References
- 1. Moya‐Garcia AA, Medina MA, Sánchez‐Jiménez F. Mammalian histidine decarboxylase: From structure to function. Bioessays 2005;27:57–63. [DOI] [PubMed] [Google Scholar]
- 2. Dale HH, Laidlaw PP. The physiological action of β‐imidazolylethylamine. J Physiol 1910;41:318–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev 2008;88:1183–1241. [DOI] [PubMed] [Google Scholar]
- 4. Kakavas S, Zampeli E, Papamichael K, Delitheos B, Tiligada E. The mast cell pathway to inflammation and homeostasis: Pharmacological insights. Anti-Inflamm Anti-Allergy Agents Med Chem 2006;5:323–334. [Google Scholar]
- 5. Prinz C, Zanner R, Gratzl M. Physiology of gastric enterochromaffin‐like cells. Annu Rev Physiol 2003;65:371–382. [DOI] [PubMed] [Google Scholar]
- 6. Falcone FH, Zillikens D, Gibbs BF. The 21st century renaissance of the basophil? Current insights into its role in allergic responses and innate immunity. Exp Dermatol 2006;15:855–864. [DOI] [PubMed] [Google Scholar]
- 7. Arrang JM, Garbarg M, Schwartz JC. Auto‐inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature 1983;302:832–837. [DOI] [PubMed] [Google Scholar]
- 8. Panula P, Yang HY, Costa E. Histamine‐containing neurons in the rat hypothalamus. Proc Natl Acad Sci USA 1984;81:2572–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parsons ME, Ganellin CR. Histamine and its receptors. Br J Pharmacol 2006;147(S1):S127–S135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leurs R, Chazot PL, Shenton FC, Lim HD, de Esch IJ. Molecular and biochemical pharmacology of the histamine H4 receptor. Br J Pharmacol 2009;157:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ogawa S, Yanai K, Watanabe T, Wang ZM, Akaike H, Ito Y, Akaike N. Histamine responses of large neostriatal interneurons in histamine H1 and H2 receptor knock‐out mice. Brain Res Bull 2009;78:189–194. [DOI] [PubMed] [Google Scholar]
- 12. Tiligada E, Zampeli E, Sander K, Stark H. Histamine H3 and H4 receptors as novel drug targets. Expert Opin Investig Drugs 2009;18:1519–1531. [DOI] [PubMed] [Google Scholar]
- 13. Connelly WM, Shenton FC, Lethbridge N, et al The histamine H4 receptor is functionally expressed on neurons in the mammalian CNS. Br J Pharmacol 2009;157:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zampeli E, Tiligada E. The role of histamine H4 receptor in immune and inflammatory disorders. Br J Pharmacol 2009;157:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci 2003;4:121–130. [DOI] [PubMed] [Google Scholar]
- 16. Passani MB, Lin JS, Hancock A, Crochet S, Blandina P. The histamine H3 receptor as a novel therapeutic target for cognitive and sleep disorders. Trends Pharmacol Sci 2004;25:618–625. [DOI] [PubMed] [Google Scholar]
- 17. Leurs R, Bakker RA, Timmerman H, de Esch IJ. The histamine H3 receptor: From gene cloning to H3 receptor drugs. Nat Rev Drug Discov 2005;4:107–120. [DOI] [PubMed] [Google Scholar]
- 18. Esbenshade TA, Browman KE, Bitner RS, Strakhova M, Cowart MD, Brioni JD. The histamine H3 receptor: An attractive target for the treatment of cognitive disorders. Br J Pharmacol 2008;154:1166–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kwiatkowski H. Histamine in nervous tissue. J Physiol 1943;102:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. White T. Formation and catabolism of histamine in brain tissue in vitro. J Physiol 1959;149:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richelson E. Tricyclic antidepressants block histamine H1 receptors of mouse neuroblastoma cells. Nature 1978;274:176–177. [DOI] [PubMed] [Google Scholar]
- 22. Maurer I, Volz HP. Cell‐mediated side effects of psychopharmacological treatment. Arzneimittelforschung 2001;51:785–792. [DOI] [PubMed] [Google Scholar]
- 23. Watanabe T, Taguchi Y, Hayashi H, et al Evidence for the presence of a histaminergic neuron system in the rat brain: An immunohistochemical analysis. Neurosci Lett 1983;39:249–254. [DOI] [PubMed] [Google Scholar]
- 24. Kim SF, Huang AS, Snowman AM, Teuscher C, Snyder SH. Antipsychotic drug‐induced weight gain mediated by histamine H1 receptor‐linked activation of hypothalamic AMP‐kinase. Proc Natl Acad Sci USA 2007;104:3456–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Watanabe T, Yamatodani A, Maeyama K, Wada H. Pharmacology of α‐fluoromethylhistidine, a specific inhibitor of histidine decarboxylase. Trends Pharmacol Sci 1990;11:363–367. [DOI] [PubMed] [Google Scholar]
- 26. Teuscher C, Subramanian M, Noubade R, Gao JF, Offner H, Zachary JF, Blankenhorn EP. Central histamine H3 receptor signaling negatively regulates susceptibility to autoimmune inflammatory disease of the CNS. Proc Natl Acad Sci USA 2007;104:10146–10151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Musio S, Gallo B, Scabeni S, et al A key regulatory role for histamine in experimental autoimmune encephalomyelitis: Disease exacerbation in histidine decarboxylase‐deficient mice. J Immunol 2006;176:17–26. [DOI] [PubMed] [Google Scholar]
- 28. Matsumoto I, Inoue Y, Shimada T, Aikawa T. Brain mast cells act as an immune gate to the hypothalamicpituitary‐adrenal axis in dogs. J Exp Med 2001;194:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol 2004;5:575–581. [DOI] [PubMed] [Google Scholar]
- 30. Kakavas S, Tiligada E. Hypothalamic histamine levels in hyperthyroid, arthritic and C48/80‐treated rats. Inflamm Res 2005;54(S1):S30–S31. [DOI] [PubMed] [Google Scholar]
- 31. Kakavas S, Tiligada E. Time course of thyroxine on hypothalamic histamine in the rat. Inflamm Res 2006;55(S1):S32–S33. [DOI] [PubMed] [Google Scholar]
- 32. Lambracht‐Hall M, Dimitriadou V, Theoharides TC. Migration of mast cells in the developing rat brain. Brain Res 1990;56:151–159. [DOI] [PubMed] [Google Scholar]
- 33. Theoharides TC, Konstantinidou AD. Corticotropin‐releasing hormone and the blood‐brain‐barrier. Front Biosci 2007;12:1615–1628. [DOI] [PubMed] [Google Scholar]
- 34. Pollard H, Moreau J, Arrang JM, Schwartz JC. A detailed autoradiographic mapping of histamine H3 receptors in rat brain areas. Neuroscience 1993;52:169–189. [DOI] [PubMed] [Google Scholar]
- 35. Chazot PL, Hann V, Wilson C, Lees G, Thompson CL. Immunological identification of the mammalian H3 histamine receptor in the mouse brain. Neuroreport 2001;12:259–262. [DOI] [PubMed] [Google Scholar]
- 36. Lovenberg TW, Roland BL, Wilson SJ, et al Cloning and functional expression of the human histamine H3 receptor. Mol Pharmacol 1999;55:1101–1107. [PubMed] [Google Scholar]
- 37. Arrang JM, Morisset S, Gbahou F. Constitutive activity of the histamine H3 receptor. Trends Pharmacol Sci 2007;28:350–357. [DOI] [PubMed] [Google Scholar]
- 38. Lethbridge NL, Chazot PL. Immunological identification of the mouse H4 histamine receptor on spinal cord motor neurons using a novel anti‐mouse H4 antibody. Inflamm Res 2009;59(S2):S197–S198. [DOI] [PubMed] [Google Scholar]
- 39. Strakhova MI, Nikkel A, Manelli AM, Hsieh G, Esbenshade TA, Brioni JD, et al Localization of histamine H4 receptors in the central nervous system of human and rat. Brain Res 2009;1250:41–48. [DOI] [PubMed] [Google Scholar]
- 40. Breunig E, Michel K, Zeller F, Seidl S, Weyhern CW, Schemann M. J Histamine excites neurones in the human submucous plexus through activation of H1, H2, H3 and H4 receptors. J Physiol 2007;583:731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hsieh GC, Chandran P, Salyers AK, et al H4 receptor antagonism exhibits anti‐nociceptive effects in inflammatory and neuropathic pain models in rats. Pharmacol Biochem Behav 2010;95:41–50. [DOI] [PubMed] [Google Scholar]
- 42. van Rijn RM, van Marle A, Chazot PL, et al Cloning and characterization of dominant negative splice variants of the human histamine H4 receptor. Biochem J 2008;414:121–131. [DOI] [PubMed] [Google Scholar]
- 43. Anaclet C, Parmentier R, Ouk K, et al Orexin/hypocretin and histamine: Distinct roles in the control of wakefulness demonstrated using knock‐out mouse models. J Neurosci 2009;29:14423–14438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stahl SM. Selective histamine H1 antagonism: Novel hypnotic and pharmacologic actions challenge classical notions of antihistamines. CNS Spectr 2008;13:1027–1038. [DOI] [PubMed] [Google Scholar]
- 45. Barbier AJ, Bradbury MJ. Histaminergic control of sleep‐wake cycles: Recent therapeutic advances for sleep and wake disorders. CNS Neurol Disord Drug Targets 2007;6:31–43. [DOI] [PubMed] [Google Scholar]
- 46. Lin JS, Dauvilliers Y, Arnulf I, et al. An inverse agonist of the histamine H3 receptor improves wakefulness in narcolepsy: Studies in orexin−/− mice and patients. Neurobiol Dis 2008;30:74–83. [DOI] [PubMed] [Google Scholar]
- 47. Arnulf I. Results of clinical trials of tiprolisant in narcolepsy and Parkinson's disease. Eur Neuropsychopharmacol 2009;19:S204. [Google Scholar]
- 48. Medhurst AD, Atkins AR, Beresford IJ, et al GSK189254, a novel H3 receptor antagonist that binds to histamine H3 receptors in Alzheimer's disease brain and improves cognitive performance in preclinical models. J Pharmacol Exp Ther 2007;321:1032–1045. [DOI] [PubMed] [Google Scholar]
- 49. Humbert‐Claude M, Morisset S, Gbahou F, Arrang JM. Histamine H3 and dopamine D2 receptor‐mediated [35S]GTPgamma[S] binding in rat striatum: Evidence for additive effects but lack of interactions. Biochem Pharmacol 2007;73:1172–1181. [DOI] [PubMed] [Google Scholar]
- 50. Fox GB, Pan JB, Radek RJ, et al Two novel and selective nonimidazole H3 receptor antagonists A‐304121 and A‐317920: II. In vivo behavioral and neurophysiological characterization. J Pharmacol Exp Ther 2003;305:897–908. [DOI] [PubMed] [Google Scholar]
- 51. Fox GB, Esbenshade TA, Pan JB, et al Pharmacological properties of ABT‐239 [4‐(2‐{2‐[(2R)‐2‐methylpyrrolidinyl]ethyl}‐benzofuran‐5‐yl)benzonitrile]: II. Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 receptor antagonist. J Pharmacol Exp Ther 2005;313:176–190. [DOI] [PubMed] [Google Scholar]
- 52. Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev 2006;51:240–264. [DOI] [PubMed] [Google Scholar]
- 53. Cannon KE, Leurs R, Hough LB. Activation of peripheral and spinal histamine H3 receptors inhibits formalin‐induced inflammation and nociception, respectively. Pharmacol Biochem Behav 2007;88:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Medhurst SJ, Collins SD, Billinton A, et al Novel histamine H3 receptor antagonists GSK 189254 and GSK 334429 are efficacious in surgically‐induced and virally‐induced rat models of neuropathic pain. Pain 2008;138:61–69. [DOI] [PubMed] [Google Scholar]
- 55. Baron R, Schwarz K, Kleinert A, Schattschneider J, Wasner G. Histamine‐induced itch converts into pain in neuropathic hyperalgesia. Neuroreport 2001;12:3475–3478. [DOI] [PubMed] [Google Scholar]
- 56. Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: The search for new antihistamines. Nat Rev Drug Discov 2008;7:41–53. [DOI] [PubMed] [Google Scholar]
- 57. Cannon KE, Hough LB. Inhibition of chemical and low‐intensity mechanical nociception by activation of histamine H3 receptors. J Pain 2005;6:193–200. [DOI] [PubMed] [Google Scholar]
- 58. Lozada A, Munyao N, Sallmen T, Lintunen M, Leurs R, Lindsberg PJ, Panula P. Postischemic regulation of central histamine receptors. Neuroscience 2005;136:371–379. [DOI] [PubMed] [Google Scholar]
- 59. Kukko‐Lukjanov TK, Soini S, Taira T, Michelsen KA, Panula P, Holopainen IE. Histaminergic neurons protect the developing hippocampus from kainic acid‐induced neuronal damage in an organotypic coculture system. J Neurosci 2006;26:1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lintunen M, Sallmen T, Karlstedt K, Panula P. Transient changes in the limbic histaminergic system after systemic kainic acid‐induced seizures. Neurobiol Dis 2005;20:155–169. [DOI] [PubMed] [Google Scholar]
- 61. Mariottini C, Scartabelli T, Bongers G, et al Activation of the histaminergic H3 receptor induces phosphorylation of the Akt/GSK‐3 beta pathway in cultured cortical neurons and protects against neurotoxic insults. J Neurochem 2009;110:1469–1478. [DOI] [PubMed] [Google Scholar]
- 62. Kaytor MD, Orr HT. The GSK3 beta signaling cascade and neurodegenerative disease. Curr Opin Neurobiol 2002;12:275–278. [DOI] [PubMed] [Google Scholar]
- 63. Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer's disease. J Neurochem 2008;104:1433–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Conway D, Cohen JA. Combination therapy in multiple sclerosis. Lancet Neurol 2010;9:299–308. [DOI] [PubMed] [Google Scholar]
- 65. Linker RA, Lee DH. Models of autoimmune demyelination in the central nervous system: On the way to translational medicine. Exp Transl Stroke Med 2009;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Logothetis L, Mylonas IA, Baloyannis S, et al A pilot, open label, clinical trial using hydroxyzine in multiple sclerosis. Int J Immunopathol Pharmacol 2005;18:771–778. [DOI] [PubMed] [Google Scholar]
- 67. Pedotti R, DeVoss JJ, Houssef S, Mitchell D, Wedemeyer J, Madanat R. Multiple elements of the allergic arm of the immune response modulate autoimmune demyelination. Proc Nat Acad Sci USA 2003;100:1867–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Teuscher C, Poynter ME, Offner H, et al Attenuation of Th1 effector cell responses and susceptibility to experimental allergic encephalomyelitis in histamine H2 receptor knockout mice is due to dysregulation of cytokine production by antigen‐presenting cells. Am J Pathol 2004;164:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]


