Abstract
Multiple sclerosis (MS) is a chronic, inflammatory, and degenerative neurological illness with no cure. It has been suggested that Hyperbaric Oxygen Therapy (HBO2T) may slow or reverse the progress of the disease. This article summarizes the clinical evidence for the use of HBO2T in the treatment of MS. We conducted a literature review focused on the interaction of hyperbaric oxygenation and MS. In particular, we appraised the clinical data regarding treatment and performed a meta‐analysis of the randomized evidence using the methodology of the Cochrane Collaboration. We found 12 randomized studies in the area, all of which were performed between 1983 and 1987. A meta‐analysis of this evidence suggests there is no clinically significant benefit from the administration of HBO2T. The great majority of randomized trials investigated a course of 20 treatments at pressures between 1.75ATA and 2.5ATA daily for 60–120 min over 4 weeks against a placebo regimen. None have tested the efficacy of HBO2T against alternative current best practice. No plausible benefit of HBO2T on the clinical course of MS was identified in this review. It remains possible that HBO2T is effective in a subgroup of individuals not clearly identified in the trials to date, but any benefit is unlikely to be of great clinical significance. There is some case for further human trials in selected subgroups and for prolonged courses of HBO2T at modest pressures, but the case is not strong. At this time, the routine treatment of MS with HBO2T is not recommended.
Keywords: Hyperbaric oxygen, Meta‐analysis, Multiple sclerosis, Systematic review
Introduction
Multiple sclerosis (MS) is a chronic neurological disease in which there is patchy inflammation, demyelination, and gliosis in the central nervous system (CNS). MS occurs most widely in races of Northern European Ancestry (lifetime risk about 1 in 400) [1, 2] and is the commonest cause of chronic neurological disability in such people. The disease frequently affects young adults, with a mean age at onset in the late 20s [3, 4].
There is considerable variability in both presenting clinical features and the progression of disability across the spectrum of MS. Since the 1980s, clinical and laboratory criteria have been used to classify the disease and establish a diagnosis (the McDonald criteria). These have been updated by consensus several times, and most recently summarized by Polman in 2005 [5]. These new criteria allow earlier diagnosis by the addition of specific magnetic resonance imaging (MRI) findings, and therefore, early institution of therapies designed to slow the progress of the disease.
Despite many recent advances in immunology, genetics, molecular biology, and related fields, the etiology of MS remains uncertain [6]. The view that MS is an inflammatory, autoimmune demyelinating disease in genetically susceptible individuals has been challenged for some years, but remains the generally accepted model [6, 7]. The prevailing hypothesis is that exposure to unknown environmental antigens in genetically susceptible individuals results in activation of certain T‐cell populations toward myelin antigens, oligodendrocytes, and/or neurons. This triggers a massive inflammatory process that results in tissue destruction within the CNS.
The histological changes described in MS are remarkably constant [6]. Discrete areas of inflammation appear and evolve within the CNS, showing a marked perivenular distribution. The lesions are mainly in the white matter, but extend into the gray matter and may occur in the cerebral hemispheres, cerebellum, spinal cord, and optic nerves. Perivascular cuffing with lymphocytes, breakdown of the blood–brain barrier (BBB) and egress of inflammatory cells from the intravascular compartment are followed by cascading inflammatory activation. The area in which these series of events occur is known as a plaque. Damage to myelin sheaths, oligodendrocytes, and degeneration of axons causes the neurological deficits by which the disease becomes apparent. The presence of thinly myelinated sheaths in some chronic lesions suggests that partial remyelination may occur.
The most obvious feature of the acute lesion is a vigorous inflammatory response with abundant lymphocytes and macrophages, along with some plasma cells and eosinophils. The proinflammatory cytokines TNF‐α, γ‐interferon, and IL‐2 can be shown on cells within the lesion. Frohman has recently published an account of the pathogenesis of the inflammatory and neurodegenerative aspects of MS that summarizes the case for MS as an immunological disease [8].
Some authors have noted that inflammation is a feature of neurodegenerative diseases of the CNS and they go on to suggest that the inflammatory changes summarized above are reactive rather than causative [9, 10]. As Chaudhuri points out, immune cells are a feature of a number of neurological disorders including stroke, where a 7‐fold rise in circulating and CSF myelin‐antigen reactive T cells is accepted as a response to acute brain injury rather than its cause. Further, several features of MS are highly suggestive of a disorder of metabolic regulation including the protective effect of sunlight and sex steroids during pregnancy. Following histopathologic analysis of a series of very early lesions, Barnett and Prineas have proposed that all MS lesions may start with apoptosis of oligodendrocytes secondary to an ischaemic or metabolic insult yet to be identified, rather than inflammation being the primary event [11].
Multiple Sclerosis as a Vascular/ischaemic Event
The similarity noted between the diffuse neurological abnormalities associated with gas embolism and decompression illness on the one hand, and MS on the other, has led some to suggest that MS is of vascular origin. Several features of the disease support this position, including the observation of perivenular lesions [12], abnormal permeability of vessels in MS [13], and abnormal vessel reactivity [14]. The close anatomical relationship between MS plaques and venules in the CNS was first remarked upon in 1863 [15]. Acute lesions often extend along the vessels in a sleeve‐like manner and both thrombosis and perivascular haemorrhages have been described [12].
In a 1982 review, James suggested a novel mechanism to explain the typical lesions [16]. Noting the sudden onset of neurological symptoms in the absence of generalized illness could be explained as an embolic phenomenon, James postulated that a subacute form of fat embolization may be responsible. Such emboli could be triggered by a number of stimuli, and in theory at least might lead to downstream hypoxia, endothelial damage and leakage of both reactive oxygen species and hydrolysed fats into the interstitium. Damage to myelin might then produce the typical plaque over time [16, 17]. This mechanism is summarized in Figure 1.
Figure 1.
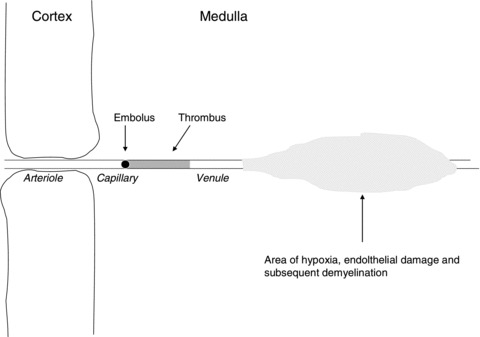
Theoretical pathology of plaque formation from James [16] and based on data from Dow and Berglund [17] Fat embolus causes downstream hypoxia, thrombus formation, and endothelial damage. Leakage of reactive species into the interstitium damages myelin and promotes plaque formation.
More recently, a modified vascular hypothesis has been proposed, with attempts to include both immunological and vascular processes in the general pathogenesis of MS [18]. In this hypothesis, Minagar suggests that breaching of the BBB is a consequence of endothelial dysfunction, in turn mediated by leukocyte–endothelial interactions. Either leucocytes or cerebral vascular endothelial cells may act as the primary antigen‐presenting cells in this process, but the result is chemotaxis between them, opening of the endothelial tight junctions (TJs) that characterize the BBB and entry of activated T cells and macrophages to the cerebral interstitium. The resulting cascade of inflammatory response damages both cellular elements and myelin. There is some experimental evidence to suggest a high incidence of TJ abnormality in MS compared to both other neuropathologies and control white matter [19]. Pharmacological agents designed to specifically target adhesion molecules along the BBB have already been introduced into clinical practice, although the first, Natalizumab, has recently been withdrawn due to reports of progressive multifocal leucoencephalopathy while on the drug [20, 21].
Therapy for MS
MS is currently an incurable disease. HBO2T has been postulated to modify disease progression and to reduce relapse rate, therefore this discussion is limited to those measures designed to produce similar treatment effects. For the most part, such measures are immunosuppressive and/or immunomodulatory. Drugs used in MS include interferon beta (IFNB), Glatiramer acetate (GA), intravenous immunoglobulin, mitoxantrone, methotrexate, and corticosteroids. The most commonly employed options have been evaluated by the American Academy of Neurology and the MS Council for Clinical Practice Guidelines [22].
IFNB is the agent for which there is the best evidence of efficacy, and several large, placebo‐controlled RCTs have been published over the last few years [23, 24, 25, 26, 27, 28, 29, 30]. These trials suggest a limited benefit in relapsing–remitting and secondary progressive MS, although all the trials have methodological limitations. Benefits are achieved at high cost with the annual cost per patient in the United Kingdom estimated to be between £10,000 and £20,000 [31]. Side effects are common, particularly flu‐like symptoms and injection site reactions. GA, also known as copolymer 1, has been used as an alternative to IFNB and is probably the second most commonly prescribed disease‐modifying therapy. The annual drug cost per patient is estimated to be about £10,000 [31].
Current best practice consists of the administration of one of these partially effective disease‐modifying treatments to appropriate patients. The identification of nonresponders is problematic, and there are no absolute criteria by which to plan the timing of new or addition therapy [32]. In any case, the evidence for efficacy is difficult to interpret and clinical trials in this area are fraught with difficulty, not the least of which is the design and application of instruments to evaluate clinical outcomes [33, 34].
Outcome Assessment
While MRI findings are now widely accepted as surrogate outcomes for disease extent and progression, clinical outcomes were the standard measure by which the success or failure of therapeutic interventions were judged at least until the early 1990s. While there are several such clinical assessment schemes, by far the most popular are those developed by Kurtzke et al. The Kurtzke Extended Disability Status Score (EDSS) and the Kurtzke Functional Status Score (FSS) were intended to be used together to reproducibly describe the degree of functional impairment across seven systems (FSS) and a score for overall disability (EDSS) [35, 36].
In the overall EDSS, each 0.5 of a point is given a different descriptor from zero indicating normal examination to ten indicating death. These scales are categorical and should not be treated as indicating “equal” decrements in functional ability or symptoms for each numerically equal decrement on the scale. Most of the clinical literature examining the effectiveness of HBO2T for MS used one or both of these scales to compare functional and global impairment.
The Clinical Evidence for the use of Hyperbaric Oxygen
In his 1982 article suggesting MS was a vascular‐ischaemic event, James proposed the use of HBO2T based on the demonstrated ability of hyperbaric oxygen to produce vasoconstriction with increased oxygen delivery and some anecdotal evidence of efficacy [16, 37, 38, 39]. The clinical evidence is summarised in Table 1.
Table 1.
Selected clinical evidence for the treatment of MS with HBO2T
| Level of evidence [40] | Author | Study design | Subjects | Conclusion |
|---|---|---|---|---|
| Level Ia | Bennett and Heard [41] | Meta‐analysis | 14 controlled trials | No net benefit shown |
| Level 1a* | Kleijnen et al. [42] | Semiquantitative review | 14 controlled trials | Majority of trials showed no benefit |
| Level 1b | Fischer et al. [43] | RCT, double‐blind | 40 chronic severe | Positive benefit, some transient |
| Level 1b | Neiman et al. [44] | RCT, double‐blind | 24 chronic progressive | No benefit |
| Level 1b | Wood et al. [45] | RCT, double‐blind | 44 chronic progressive | No benefit |
| Level 1b | Slater et al. [46] | RCT, double‐blind | 57 chronic stable or progressive | No benefit |
| Level 1b | Erwin et al. Massey et al. [47, 48] | RCT, double‐blind, crossover | 18 | No benefit |
| Level 1b | Confavreux et al. [49] | RCT, double‐blind | 17 chronic progressive | No benefit |
| Level 1b | Wiles et al. [50] | RCT, double‐blind | 88 chronic progressive | No benefit |
| Level 1b | Harpur et al. [51] | RCT double‐blind | 82 definite MS | No benefit |
| Level 1b | Barnes et al. [52] | RCT double‐blind | 120 chronic stable | Transient symptomatic sphincter improvement |
| Level 1b | Oriani et al. [53] | RCT, double‐blind | 44 chronic stable | Improved symptoms and disability scores |
| Level 1b | L’Hermitte et al. [54] | RCT, double‐blind | 49 chronic | No benefit |
| Level 1b | Murthy et al. [55] | RCT, double‐blind | 40 no details | Some benefit in mild disease |
| Level 2b | Worthington et al. [56] | Comparative study, HBO v HBAir in crossover design, nonrandom | 51 (all types) | Minor benefit from HBO2 |
| Level 2b | Pallotta et al. [57] | Cases compared with untreated controls | 22 | Reduced relapse |
| Level 4 | Boschetty and Cernoch [37] | Case series | 26 | Transient symptomatic improvement (15/26) |
| Level 4# | No authors [58] | Case series | 703 (417 chronic progressive, 43 chronic static, 167 relapsing) | Improved disability scores and symptomatology |
| Level 5 | Gottlieb and Neubauer [7] | Qualitative review | 14 trials | Poor trials, data misinterpreted |
*No meta‐analysis but some attempt to synthesise data.
#Internet publication only. No authors formally recognized, but advice of James and Perrin acknowledged.
Early reports had for the most part supported a role for HBO2T in preventing progression and indeed reducing disability across a wide range of patients. Neurologists and hyperbaricists were divided into enthusiasts or staunch opponents of this approach, and the place of HBO2T was controversial. In the late 1980s, Kindwall initiated a national data register for MS patients having HBO2T [59]. One hundred and seventy neurologists across 22 institutions in the United States of America contributed to this 2 years longitudinal study and a total of 312 patients were enrolled. Kindwall described a high drop‐out rate (only 76% finished the initial course of 20 treatments) and at completion of the 2 years study period, only 28 of the original 312 patients remained in treatment (9%). The mean deterioration on the Kurtzke EDSS score was 0.93 or almost a full step from the beginning of treatment until the last evaluation. These disappointing results led the Undersea and Hyperbaric Medical Society to suggest that MS should not be an approved indication.
On the other hand, an informal longitudinal case series published only on the web suggested significant benefit from the application of hyperbaric oxygen to patients with a variety of MS presentations (The experience of MS National in treating MS with prolonged courses of high dosage oxygenation. Accessed at http://www.ms‐selfhelp.org/html/oxygen_3.html). In particular, those data suggested HBO2T was associated with the prevention of long‐term deterioration by regular maintenance therapy. This analysis was not attributed to any individual author and has since been removed. The results were likely to be significantly biased in favor of apparent effectiveness as the only patients for whom we had late assessments are those who continued treatment over several years. As one might speculate was the case with the Kindwall study, those dropping out are likely to be those who found little or no benefit from HBO2T.
The evidence from comparative trials has been far less positive. For example, Worthington, in a nonrandomized crossover trial involving 51 patients with chronic—progressive and relapsing—remitting disease, found some minor benefits after 20 hyperbaric oxygen treatment sessions (peak flow and finger tapping improved), although walking and mobility were improved after the placebo sessions. Self‐care activities decreased during the course of the trial for each group [56].
Between 1983 and 1990, there was a flurry of activity resulting in the publication of several small RCTs with mixed results. The clinical impact of these trials has subsequently been examined in three reviews. In a qualitative review, Gottlieb and Neubauer suggested many of the RCTs were methodologically flawed and suggested the authors may have misinterpreted their trial data [7]. Of particular concern was the possibility that the dose of oxygen was too high and that few trials included ongoing “top‐up” treatments after the original course of HBO2T. Neubauer recommended a starting pressure of 1.5 ATA with graduated introduction of higher pressures titrated to the patient response [60]. Of note, however, is that the only positive RCT published at that time (Fischer 1983) used 2 ATA of oxygen and showed positive results at 1 year follow‐up despite not including “top‐up” treatments [43]. Neubauer and Gottlieb concluded that despite generally poor results, these trials justified the use of HBO2T when interpreted in the light of their own vascular‐ischaemic pathophysiological model.
In 1995, Kleijnen and Knipschild published a semi‐quantitative analysis and concluded: “the majority of controlled trials could not show positive effects”[57]. They considered 8 of the 14 trials to be of reasonable to high quality and of these, only one trial (Fischer) showed a result in favour of HBO2T. In 2004, we published a Cochrane systematic review with meta‐analysis [41]. This review is discussed below in more detail.
Summary of the Cochrane Review
This review included any RCT, regardless of allocation concealment and blinding, where HBO2T was part of the randomized methodology. Trials enrolling any MS patients (based on clinical criteria) irrespective of the disease state or course were considered for inclusion. The methodology conformed to that of the Collaboration, and has been described in detail elsewhere [41]. Our search strategies were repeated in July 2009 but did not locate further relevant studies.
The primary outcomes of interest were objective assessments by neurologist or hyperbaric physician, specifically: Kurtzke EDSS at completion of the intervention, 6 months and/or 1 year [35], the numbers of participants suffering at least one exacerbation and the numbers of participants suffering sideeffects or adverse events associated with treatment.
Analysis of data were performed most recently using RevMan 5.0 software (Version 5.0. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2008). In general, continuous data were analyzed using comparison of group means (difference between means across trials) and standard deviation (SD), whilst dichotomous data were presented as an odds ratio (OR). Where meaningful, the number needed to treat to achieve one extra favorable outcome was calculated and presented with 95% confidence intervals. On the basis of comments of Gottlieb and Neubauer [7], subgroup analysis was considered by individual treatment session nitrogen dose (nature of sham treatment—PiN2), individual treatment oxygen dose (treatment pressure), and length of therapy—(1 month [20 treatment sessions] vs. 6 months or 1 year).
Description of Studies
The search identified 36 relevant publications and initial examination suggested 19 possible comparative trials. After appraisal of the full reports, nine publications were excluded because they were not reports of RCTs or did not contain new data [7, 42, 46, 47, 48, 55, 56, 57, 59].
Ten reports of nine trials contributed to this review. In total, these trials include data on 504 participants, 260 receiving HBO2T and 244 control or sham therapy. The lowest dose of oxygen administered was 1.75 ATA for 90 min (Harpur) [51], while the highest dose was 2.5 ATA for 90 min (Confavreux; Oriani) [49, 53]. All others used 2.0 ATA for 90 min. All trials used an initial course of 20 treatment sessions over 4 weeks, while two (Harpur 1986; Oriani 1990) continued to administer “top‐up” treatments [51, 53]. Overall, there were 31 participants lost to final follow‐up (7.7% of the total number enrolled).
Results
There were no significant improvements in mean EDSS at the completion of 20 treatments (HBO2T 0.07 points better than sham, 95%CI –0.09 to 0.23, P= 0.4; Fig. 2), nor at 6 months (0.22, 95%CI –0.09 to 0.54, P= 0.17), however there was a statistically significant benefit at 1 year after completion of initial course (0.85, 95%CI 0.42 to 1.28, P= 0.0001). Subgroup analysis by PIN2 during treatment and the oxygen dose did not explain these findings. The only two out of nine trials that reported mean EDSS at 1 year were also the only two generally positive trials.
Figure 2.
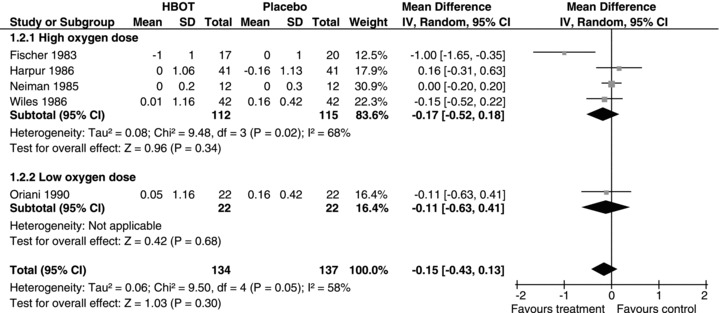
Forest plot for improvement in EDSS after 20 treatments—subgroup analysis by oxygen dose.
Similarly, the proportion of participants improved by at least one point on EDSS did not differ at completion of 20 treatments. Few participants improved in either group: 11 (5%) in the HBO2T group and 3 (1.5%) in the sham group. The OR for benefiting from sham was 0.33, 95% CI 0.09 to 1.18, P= 0.09, and at 6 months 0.42, 95%CI 0.16 to 1.08, P= 0.07. Again, there was a statistically significant benefit from HBO2T at 1 year (OR 0.2, 95%CI 0.06 to 0.72, P= 0.01). Thirteen participants (14.3%) improved in the HBO2T group and four participants (4.5%) in the sham group. This analysis largely reflects the Oriani study, to which it contributes 84.7% of the weight. This result was sensitive to the allocation of drop‐outs with a loss of any significant advantage from the administration of HBO2T with worst case assumptions (OR 1.34, 95% CI 0.08 to 21.75, P= 0.21). The analysis suggests we would need to treat 10 participants with HBO2T to achieve one extra patient with an improvement in EDSS of one point at 1 year, but we may have to treat as many as 71 (NNT = 10, 95%CI 5 to 71).
There was no significant reduction in the odds of experiencing an exacerbation at completion of initial course of HBO2T (OR 0.31, 95%CI 0.01 to 7.80, P= 0.5), 6 months (OR 0.74, 95%CI 0.25 to 2.22, P= 0.6), nor at 1 year (OR 0.38, 95%CI 0.04 to 3.22, P= 0.4). At the final follow‐up, 25.9% of patients in the HBO2T group had suffered an exacerbation versus 36.9% in the sham group.
On analysis of individual functional systems, there was no significant improvement or slowing of deterioration in bladder/bowel control or pyramidal signs after 20 treatments (sphincter control: OR 0.72, 95%CI 0.33 to 1.60, P= 0.4, pyramidal signs OR 0.30, 95%CI 0.06 to 1.47, P= 0.14), but at 6 months and 1 year there was a reduced chance of deterioration in pyramidal signs (at 6 months OR 0.17, 95%CI 0.07 to 0.78, P= 0.02, 1 year OR 0.13, 95%CI 0.03 to 0.58, P= 0.007). At one year, 13.2% of patients improved in the HBO2T group compared to 4.5% in the sham group. These results largely reflect the outcome in a single trial (Oriani 1990) and suggest we would need to treat at least six patients with HBO2T in order to improve one extra individual, but perhaps as many as 197 patients (NNT = 11, 95% CI 6 to 197).
Adverse Effects
There were significantly increased odds of deteriorating vision following the administration of HBO2T (OR 24.87, 95% CI 1.44 to 428.50, P= 0.03). The analysis suggests the number need to treat with HBO2T to get one further complaint of visual disturbance is very low (NNT = 1; 95%CI 1 to 2).
Discussion
We concluded there is little evidence of a significant effect for the administration of HBO2T in this review. Whilst there was a modest benefit demonstrated in mean EDSS at 12 months, this result is uncertain given that only two trials reported on this outcome at this time (16% of the total participants in this review) and they were the only trials of the nine in this review to suggest benefit. There was similarly little consistent evidence for benefit with respect to the secondary outcomes relating to improvements in function systems. No analysis indicated benefit in global FSS or the bladder/bowel sphincter function element of this scale. The benefit in the pyramidal function element at 6 months and 12 months reflected one single positive trial (Oriani 1990) [56].
Of the 20 separate outcome factors where meta‐analysis was possible, significant benefit was only suggested in three. Where appropriate we made up to three previously planned subgroup analyses with respect to treatment length, nature of the sham, and oxygen dose per treatment session. None could explain the heterogeneity between the results of Fischer and Oriani and the other seven trials, because those two trials were in alternative subgroups in all three analyses.
We recognise this review is hampered by the modest total number of participants enrolled, variation in entry criteria and treatment length between studies and the large number of analyses where there were no individuals with the outcome of interest in either arm of the study. Nevertheless, the entry EDSS scores indicated the majority of participants had mild or moderate disabilities and the analyses are most likely to reflect outcomes in these patient groups.
The first RCT published (Fischer) [43] defined a reduction of one point on the EDSS as a “major improvement”, and a similar reduction of one point on the FSS as a “minor improvement”. Most subsequent authors followed this example. The reduction in mean EDSS in the HBO2T group at 12 months was 0.84 points. This magnitude of improvement is of dubious clinical benefit. Over all these trials, only a small proportion of participants improved by a full point in either group (at 20 treatments 6.8% HBO2T group, 3% sham).
These results should be interpreted with great caution for a number of reasons. It is possible, for example, there is bias toward later reporting in those trials showing successful outcomes compared to those in which the initial findings were unpromising. It is also biologically implausible that a benefit be absent at 6 months after treatment and present at 12 months. Proponents of HBO2T suggest that a long course of treatment may be required to demonstrate benefit [61] and that those trials giving only 20 treatments are flawed in this regard. Others maintain that treatments over 2 ATA are toxic and unhelpful [7, 60]. Both these assertions are difficult to sustain however, in that of the two trials contributing to this significant result, one gave a short course at only 2 ATA (Fischer) [43], while the other continued with “top‐up” treatments to 12 months and used 2.5 ATA (Oriani) [53], and both showed benefits after 20 treatments and 6 months. Furthermore, the only other trial to administer a longer course of treatments (Harpur) [51] failed to suggest any benefit in EDSS at 20 treatment or 6 months (no data at 12 months).
Anecdotally, most improvements reported have been in sphincter function and pyramidal system function, and these trials focus particularly on these areas. There was no evidence from this review to support the improvement of bladder/bowel function following HBO2T when compared to a sham. Pyramidal function was improved at 6 months only, and are due to the findings of a single study.
Cost‐effectiveness of HBO2T
The patient charge for providing HBO2T is highly variable and in part dependent on the type of facility, the presence or absence of physician supervision and the healthcare system within which it is delivered. Whilst the true cost of HBO2T is difficult to establish, a range of likely cost to benefit can be estimated from recently published data from an Australian facility [62].
These authors calculated the dollar cost of HBO sessions and the total HBO2T costs per diagnosis treated in their unit in 2003 to 2004—defining the financial cost as the expenditure on goods and services purchased. On the basis that MS patients would be outpatients for the most part and involve a relatively low level of complexity, a reasonable estimate of the true cost of a single treatment for MS in a hospital setting was likely to be $AUD304. The first annual cost for a course of 20 sessions with “top‐up” treatments of twice each month (42 treatments in total) is estimated at $AUD12,768.
On the basis of the results of the meta‐analysis above, the likely cost for one extra individual to achieve an increase of a single point on the Kurtzke EDSS at one year is $AUD127,680, with a 95% CI of $AUD63,840 to $AUD906,528 (NNT 10, 95%CI 5 to 71).
Such estimates assume the cost of provision of HBO2T will be similar to those of a tertiary HBO2T facility. The actual cost of provision in centers operated and designed solely for the provision of HBO2T to ambulatory MS patients is likely to be considerably lower. If the cost was $100/treatment, for example, the equivalent figures for an improvement in EDSS would be $AUD42,000 (95%CI $AUD21,000 to $AUD298,200).
On the other hand, on the basis of an initial course of 20 treatments and “top‐up” treatments weekly, each patient would require 68 treatments in the first year rather than the 42 estimated earlier, with a corresponding rise in the total costs.
Conclusions
There is no consistent evidence to confirm a beneficial effect of HBO2T for the treatment of MS and routine use does not seem justified on the available evidence. The small number of analyses suggestive of benefit in the Cochrane meta‐analysis are isolated, difficult to ascribe with biological plausibility and would need to be confirmed in future well‐designed trials. The cost to achieve any benefit is likely to be high.
The published clinical evidence, and in particular the randomized clinical trials concerning HBO2T for MS are somewhat dated and difficult to interpret compared to contemporary investigations. Whilst there is some case for further research, there is little indication that strong and clinically useful treatment effects are likely. It is possible, however, that modest treatment benefits may be present in a subset of disease severity or classification.
Any future trials would need to be carefully planned. They would need to enrol appropriate sample sizes with power to detect expected differences in carefully selected target patients. Inclusion criteria and outcome measures will both need to include MRI data and validated quality of life instruments. Finally, any future trials should assess both the safety and cost of therapy. It is our opinion that such trials are difficult to justify given the existing evidence, and it is likely that only staunch advocates would be willing to pursue such investigations.
Contribution of Authors
The conception and design of systematic review, development of search strategy, appraisal and selection of studies for inclusion, data analysis, and drafting article (M.B.). The appraisal and selection of studies for inclusion, data extraction and entry, critical review of article (R.H.).
Conflict of Interest
The authors have no conflict of interest to declare in relation to this review.
Acknowledgments
The authors wish to acknowledge the assistance of the Cochrane Multiple Sclerosis Review Group in the preparation of this review. In particular, we wish to thank Graziella Filippini and Liliana Coco for their guidance. This review received no external funding.
This review includes material from a Cochrane review published originally in 2004, Issue 1 (see http://www.thecochranelibrary.com). Cochrane reviews are regularly updated as new evidence emerges, and the Cochrane library should be consulted for the most recent version of the review.
References
- 1. Compston D. The genetic epidemiology of multiple sclerosis In: Compston D, Ebers GC, Lassmann H, McDonald WI, Matthews WB, Wekerle H, editors. McAlpine's Multiple Sclerosis, 3rd ed London : Churchill Livingstone, 1998;45–142. [Google Scholar]
- 2. Flachenecker P, Stuke K. National MS registries. J Neurol 2008;255(Suppl 6):102–108. [DOI] [PubMed] [Google Scholar]
- 3. Weinschenker BG, Bass B, Rice GP. The natural history of multiple sclerosis: A geographically based study. Part 1. Clinical course and disability. Brain 1989;112:133–146. [DOI] [PubMed] [Google Scholar]
- 4. Pittock SJ, Mayr WT, McClelland RL. Disability profile of MS didn't change over 10 years in a population‐based prevalence cohort. Neurology 2004;62:601–606. [DOI] [PubMed] [Google Scholar]
- 5. Polman CH, Reingold SC, Edan G, et al Diagnostic criteria for multiple sclerosis 2005 revisions to the “McDonald Criteria”. Ann Neurol 2005;58:840–846. [DOI] [PubMed] [Google Scholar]
- 6. Ludwin SK. The pathogenesis of multiple sclerosis: Relating human pathology to experimental studies. J Neuropathol Exp Neurol 2006;65:305–318. [DOI] [PubMed] [Google Scholar]
- 7. Gottlieb SF, Neubauer Ra. Multiple sclerosis: Its etiology, pathogenesis, and therapeutics with emphasis on the controversial use of HBO. J Hyperbaric Med 1988;3:143–164. [Google Scholar]
- 8. Frohman EM, Racke MK, Raine CS. Multiple sclerosis—the plaque and its pathogenesis. New England J Med 2006;354:942–955. [DOI] [PubMed] [Google Scholar]
- 9. Hemmer B, Archelos JJ, Hartung HP. New concepts in the immunopathogenesis of multiple sclerosis. Nature Rev Neurosci 2002;3:291–301. [DOI] [PubMed] [Google Scholar]
- 10. Chaudhuri A, Behan PO. Multiple sclerosis is not an autoimmune disease. Arch Neurol 2004;61:1610–1612. [DOI] [PubMed] [Google Scholar]
- 11. Barnett MH, Prineas J. Relapsing and remitting multiple sclerosis: Pathology of the newly forming lesion. Ann Neurol 2004;55:458–468. [DOI] [PubMed] [Google Scholar]
- 12. Scheinker M. Histogenesis of the early lesions of multiple sclerosis. Arch Neurol 1943;49:178–185. [Google Scholar]
- 13. Aita JF, Bennett DR, Anderson RE, Ziter F. Cranial CT appearance of acute multiple sclerosis. Neurology 1978;28:251–255. [DOI] [PubMed] [Google Scholar]
- 14. Brickner RM. The significance of localized vasoconstrictions in multiple sclerosis. Transient sudden miniature attacks of multiple sclerosis. Assoc Respiratory, Nervous, and Mental Dis Proc 1950;29:236–244. [PubMed] [Google Scholar]
- 15. Rindfleisch E. Histologische detail zu der degeneration von gehirn und ruckenmark. Virchows Arch (Pathol Anat) 1863;26:478–483. [Google Scholar]
- 16. James PB. Evidence for subacute fat embolism as the cause of multiple sclerosis. Lancet 1982;1:380–386. [DOI] [PubMed] [Google Scholar]
- 17. Dow RS, Berglund G. Vascular pattern of lesions of multiple sclerosis. Arch Neurol 1942;47:1–18. [Google Scholar]
- 18. Minagar A, Wenche J, Jiminez JJ, Alexander JS. Multiple sclerosis as a vascular disease. Neurol Res 2006;28:230–235. [DOI] [PubMed] [Google Scholar]
- 19. Kirk J, Plumb J, Mirakhur M. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood–brain barrier leakage and active demyelination. J Pathol 2003;210:319–327. [DOI] [PubMed] [Google Scholar]
- 20. Miller D, Khan OA, Sheremata WA. A controlled trial of natalizumab for relapsing multiple sclerosis. New England J Med 2003;348:15–23. [DOI] [PubMed] [Google Scholar]
- 21. Chaudhuri A. Lessons for clinical trials from natalizumab in multiple sclerosis. BMJ 2006;332:416–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goodin DS, Frohman EM, Garmany GP, et al Disease modifying therapies in multiple sclerosis. Neurology 2002;58:169–178. [DOI] [PubMed] [Google Scholar]
- 23. Group PS. Randomized double‐blind placebo‐controlled study of interferon beta‐1a in relapsing/remitting multiple sclerosis. Lancet 1998;352:1498–1504. [PubMed] [Google Scholar]
- 24. The Once Weekly Interferon for MSSG . Evidence of interferon beta‐1a dose response in relapsing–remitting MS: The OWIMS Study. Neurology 1999;53:679–686. [DOI] [PubMed] [Google Scholar]
- 25. Patti F, L’Episcopo MR, Cataldi ML, Reggio A. Natural interferon‐beta treatment of relapsing‐remitting and secondary‐progressive multiple sclerosis patients. A two‐year study. Acta Neurol Scand 1999;100:283–289. [DOI] [PubMed] [Google Scholar]
- 26. The European Study Group on interferon beta‐1b in secondary progressive MS . Placebo‐controlled multicenter randomized trial of interferon beta‐1b in treatment of secondary progressive multiple sclerosis. Lancet 1998;352:1491–1497. [PubMed] [Google Scholar]
- 27. Simon JH, Lull J, Jacobs LD, Rudick RA. A longitudinal study of T1 hypointense lesions in relapsing MS. MSCRG trial of interferon beta‐1a. Multiple Sclerosis Collaborative Research Group. Neurology 2000;55:185–192. [DOI] [PubMed] [Google Scholar]
- 28. Group SS. Randomized controlled trial of interferon‐beta‐1a in secondary progressive MS: Clinical results. Neurology 2001;56:1496–1504. [DOI] [PubMed] [Google Scholar]
- 29. Li DKB, Zhao GJ, Paty DW. Randomized controlled trial of interferon‐beta‐1a in secondary progresssive MS: MRI results. Neurology 2001;56:1505–1513. [DOI] [PubMed] [Google Scholar]
- 30. Barbero P, Bergui M, Versino E, et al Every‐other‐day interferon beta‐1b versus once‐weekly interferon beta‐1a for multiple sclerosis (INCOMIN Trial) II: Analysis of MRI responses to treatment and correlation with NAb. Multiple Sclerosis 2006;12:72–76. [DOI] [PubMed] [Google Scholar]
- 31. Clegg A, Bryant J, Milne R. Disease‐modifying drugs for multiple sclerosis: A rapid and systematic review. Health Technol Assess 2000;4:1–101. [PubMed] [Google Scholar]
- 32. Stangel M, Gold R, Gass A, Haas J, Jung S, Elias W, Zettl UK. Current issues in immunomodulatory treatment of multiple sclerosis. A practical appraoch. J Neurol 2006;253:32–36. [DOI] [PubMed] [Google Scholar]
- 33. Waubant E, Goodkin K. Methodological problems in evaluating efficacy of a treatment for multiple sclerosis. Pathol Biol (Paris) 2000;48:104–113. [PubMed] [Google Scholar]
- 34. Liu C, Blumhardt LD. Disability outcome measures in therapeutic trials of relapsing/remitting multiple sclerosis: Effects of heterogeeity of disease in placebo cohorts. J Neurol Neurosurg Psych 2000;68:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kurtzke JF. Rating neurological impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 36. Kurtzke JF. Further notes on disability evaluation in multiple sclerosis with scale modifications. Neurology (Minneapolis) 1965;15:654–661. [DOI] [PubMed] [Google Scholar]
- 37. Boschetty V, Dostal J, Holek J. Use of hyperbaric oxygenation in acute cerebrovascular accidents. (Preliminary report). Bratislavske lekarske listy 1970;53:160–164. [PubMed] [Google Scholar]
- 38. Neubauer RA. Treatment of multiple sclerosis with monoplace hyperbaric oxygenation. J Fla Med Assoc 1978;65:101. [PubMed] [Google Scholar]
- 39. Neubauer RA. Exposure of multiple sclerosis patients to hyperbaric oxygen at 1.5–2 ATA. A preliminary report. J Fla Med Assoc 1980;67:498–504. [PubMed] [Google Scholar]
- 40. Howick J (Updating: Phillips B, Ball C, Sackett D, Straus S, Haynes B, Dawes M, 1998). Oxford center for evidence‐based medicine levels of evidence, 2009. Available from: http://www.cebm.net/?o=1025 [Accessed 13 January 2010.
- 41. Bennett MH, Heard R. Hyperbaric oxygen therapy for multiple sclerosis. Cochrane Database Syst Rev 2004: doi: 10.1002/14651858.CD003057.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kleijnen J, Knipschild P. Hyperbaric oxygen for multiple sclerosis. Review of controlled trials. Acta Neurol Scand 1995;91:330–334. [DOI] [PubMed] [Google Scholar]
- 43. Fischer BH, Marks M, Reich T. Hyperbaric‐oxygen treatment of multiple sclerosis. A randomized, placebo‐controlled, double‐blind study. New England J Med 1983;308:181–186. [DOI] [PubMed] [Google Scholar]
- 44. Neiman J, Nilsson BY, Barr PO, Perrins DJD. Hyperbaric oxygen in chronic progressive multiple sclerosis: Visual evoked potentials and clinical effects. J Neurol Neurosurg Psychiatry 1985;48:497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wood J, Stell R, Unsworth I, Lance JW, Skuse N. A double‐blind trial of hyperbaric oxygen in the treatment of multiple sclerosis. Med J Aust 1985;143:238–241. [DOI] [PubMed] [Google Scholar]
- 46. Slater GE, Anderson DA, Sherman R, Ettiger MG, Haglin J, Hitchcock C. Hyperbaric oxygen and multiple sclerosis: A double‐blind, controlled study. Neurology 1985;35:315. [Google Scholar]
- 47. Erwin CW, Massey EW, Brendle AC, Shelton DL, Bennett PB. Hyperbaric oxygen influences on the visual evoked potentials in multiple sclerosis patients. Neurology 1985;35:104. [Google Scholar]
- 48. Massey EW, Shelton DL, Pact V, Greenburg J, Erwin W, Satzman H. Hyperbaric oxygen in multiple sclerosis: A double‐blind crossover study of 18 patients. Neurology 1985;35:104. [Google Scholar]
- 49. Confavreux C, Mathieu C, Cacornac R, Aimard G, Devic M. Hyperbaric oxygen in multiple sclerosis. La Presse M, dicale 1986;15:1319–1322. [PubMed] [Google Scholar]
- 50. Wiles CM, Clarke CRA, Irwin HP, Edgar EF, Swan AV. Hyperbaric oxygen in multiple sclerosis: A double blind trial. BMJ (Clinical Research Edition) 1986;292:367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Harpur GD, Suke R, Bass BH, et al Hyperbaric oxygen therapy in chronic stable multiple sclerosis: Double‐blind study. Neurology 1986;36:988–991. [DOI] [PubMed] [Google Scholar]
- 52. Barnes MP, Bates D, Cartlidge NEF, French JM, Shaw DA. Hyperbaric oxygen and multiple sclerosis: Final results of a placebo‐controlled, double‐blind trial. J Neurol Neurosurg Psychiatry 1987;50:1402–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oriani G, Barbieri S, Cislaghi G, Albonico G, Scarlato G, Mariani C. Long‐term hyperbaric oxygen in multiple sclerosis: A placebo‐controlled, double‐blind trial with evoked potentials studies. J Hyperbaric Med 1990;5:237–245. [Google Scholar]
- 54. L’Hermitte F, Roullet E, Lyon‐Caen O, et al Hyperbaric oxygen treatment of chronic multiple sclerosis. Results of a placebo‐controlled double‐blind study in 49 patients. Revue Neurologique (Paris) 1986;142:201–206. [PubMed] [Google Scholar]
- 55. Murthy KN, Maurice PB, Wilmeth JB. Double‐blind randomised study of hyperbaric oxygen (HBO) versus placebo in multiple sclerosis (MS). Neurology 1985;35:104. [Google Scholar]
- 56. Worthington J, DeSouza L, Forti A, Jones R, Modarres‐Sadeghi H, Blaney A. A double‐blind controlled cross‐over trial investigating the efficacy of hyperbaric oxygen in patients with multiple sclerosis In: Rose FC, Jones R, editors. Multiple sclerosis, immunological, diagnostic and therapeutic aspects. Current problems in neurology 3. London : John Libbey, 1987;229–240. [Google Scholar]
- 57. Pallotta R. Hyperbaric therapy of multiple sclerosis. Minerva-Medica 1982;73:2947–2954. [PubMed] [Google Scholar]
- 58. Centers MSNT . The experience of MS National in treating MS with prolonged courses of high dosage oxygenation 2009.
- 59. Kindwall EP, McQuillen MP, Khatri BO, Grucho HW, Kindwall ML. Treatment of multiple sclerosis with hyperbaric oxygen. Results of a national registry. Arch Neurol 1991;48:195–199. [DOI] [PubMed] [Google Scholar]
- 60. Neubauer RA. Hyperbaric oxygen therapy of multiple sclerosis. A multi‐center survey. Lauderdale‐By‐The‐Sea, Florida : RA Neubauer, 1983. [Google Scholar]
- 61. James PB. Hyperbaric oxygen and multiple sclerosis. Lancet 1985;1:572. [PubMed] [Google Scholar]
- 62. Gomez‐Castillo JD, Bennett MH. The cost of hyperbaric therapy at the Prince of Wales Hospital, Sydney. South Pacific Underwater Med J 2005;35:194–198. [Google Scholar]


