SUMMARY
Aim: Clinically electroacupuncture (EA) is proved an effective therapy for vascular dementia (VD), but their mechanisms remain uncertain. The aim of this study was to determine whether EA protects pyramidal cells from apoptosis in hippocampus of a VD rat model by inhibiting the expression of p53 and Noxa. Methods: One month after a VD animal model was established by bilateral occlusion of common carotid arteries, EA treatment was given at “Baihui” (DU20), “Dazhui” (DU14), and “Shenshu” (BL23). The learning and memory ability was assessed by Morris water maze. Neuronal apoptosis in hippocampus was evaluated with hematoxylin–eosin (HE) staining, and the expression of p53 and Noxa was analyzed by confocal laser scanning microscope with immunofluorescence staining. Results: Expressions of p53 and Noxa in the EA group and sham‐operated group were less than in the VD model group (P < 0.01), and the expression of p53 was positively correlated to expression of Noxa in hippocampus of VD rats (r = 0.918, P < 0.01). EA treatment could reduce the amount of apoptotic neurons in hippocampal CA1 area of rats with VD. The average latency in the Morris water maze test was significantly shorter, and escape strategies improved from edge and random searches to more linear swim pathway in the EA group compared with the VD model group (P < 0.01). Conclusions: The increasing expressions of p53 and Noxa play important roles in the pathogenesis of VD. EA improves learning and memory ability and protects pyramidal cells from apoptosis by blocking expression of p53 and Noxa in the hippocampal CA1 region of VD rats. These results suggest a novel mechanism of EA treatment to VD.
Keywords: Apoptosis, Electroacupuncture, p53, Noxa, Vascular dementia
Introduction
Vascular dementia (VD) is a clinical syndrome of cognitive decline caused by ischemic, hemorrhagic, or oligemic injury to the brain as a consequence of cardiovascular disease or cerebrovascular disease. It is the second most common form of dementia ranking after Alzheimer disease (AD). In China, it accounts for approximately 45.8% of dementia cases and has an increasing impact on an aging population. Although officially approved medications for vascular dementia (VD) remain limited [1], clinical studies have revealed that electroacupuncture (EA) is an effective therapy for VD in China [2].
Evidence has recently accumulated indicating the significant effect of EA on hippocampus of VD rats by antagonizing apoptosis. For instance, EA has been shown to suppress the increase of Glu content, downregulate NMDAR 1 mRNA expression in VD rats, increase the expression of Bcl‐2, and attenuate the expression of Bax in nerve cells of VD rats. All these data suggest the therapeutic potential of EA against apoptosis.
P53 is a tumor suppressor. Data show that p53 and its downstream effector Noxa play important roles in regulating apoptosis and mediate hypoxic cell death in cerebral ischemia rats [3, 4, 5]. In the present study, to assess the role of p53 and Noxa in VD, we have further analyzed the expression of p53 and Noxa in hippocampus of VD rats by confocal laser scanning microscope with immunofluorescence staining. We have also investigated effects of EA on the expression of p53 and Noxa of VD rats.
Material and Methods
Animal Selection
A total of 40 healthy, male, Sprague‐Dawley (SD) rats, 9 months old, and of clean grade (460 ± 30) g, were provided by the Research Center of Laboratory Animals, Fujian Medical University (Permission No. SCXK [min] 2004‐0002, China). Thirty‐eight rats with good learning and memory abilities were selected by Morris water maze criteria, two animals were eliminated.
Preparation and Grouping of Animal Models
Twelve rats were selected to form the sham‐operated group, and 26 rats were used for VD model establishment, which was induced by bilateral occlusion of common carotid arteries. Under anesthesia (3 mg/kg choralhydrate), rats were fixed in a supine position, and a midline incision of the cervical skin was made to expose the bilateral carotid arteries, which were ligated with double silk suture, then the rats were fed and regularly observed. After induction of the VD model, the surviving rats were randomly assigned into an EA group (n = 11) and a VD model group (n = 12). The sham‐operated group received the same surgical procedures; however, the bilateral common carotid arteries were not occluded.
Therapeutic Methods
One month after surgery, rats in the EA group were electroacupunctured with an electronic acupuncture treatment instrument (SDZ‐II electronic acupuncture treatment instruments, Suzhou, China). No. 30, 1.5 cm filiform needles were inserted obliquely 1.5 cm at Baihui (DU20) of the model rats, perpendicularly 1.5 cm at Dazhui (DU14), and obliquely 0.6 cm at both Shenshu (BL23).
The electronic acupuncture treatment instrument was connected to supply continuous waveform with a frequency of 4 Hz, the stimulation intensity was within rat‐resting tolerance (about 2.0 mA). This treatment was administered once a day for 30 days, and the needle was retained for 20 min during each treatment. The other groups received no treatment after surgery.
Behavioral Test
The behavioral test was conducted using Morris water maze. A circular tank (150 cm diameter, 50 cm deep) was filled with 17‐cm deep water of 22∼26°C. Four entering points were marked on the wall of the pool, and the pool was divided into four quadrants. The platform (12.5 cm diameter, 15 cm high) was placed in the third quadrant, and was submerged 2 cm below the water surface. The behavioral test began immediately right after treatment.
Navigation Test
The test lasted for 5 days with four trials per day. Each trial began by releasing the rat into the water, with its face toward the pool wall at the one of the four placement points. The time to find the platform within a 2‐min limit was recorded. If the rat failed to find the platform within 2 min, the experimenter moved the animal to the platform, where it remained for 10 seconds. The escape latency was recorded as 2 min.
Spatial Probe Test
The platform was removed from the pool, and rats were allowed to search for the platform for 120 seconds. Swim paths were recorded to determine search strategies, which including search along the edge (edge type), random swim path (random type), a trend toward a straight swim path (trend type), and a linear swim path (line type).
Preparation of Paraffin Section and Hematoxylin–Eosin (HE) Staining
One rat was randomly selected from each group. After anesthesia, the rats were injected with 100 mL normal saline and 200 mL 10% neutral formalin via ventricle. Tissue blocks, including hippocampus, were collected, postfixed in 10% neutral formalin for 12 h, embedded in paraffin, and serially sectioned (5‐μm thick coronal sections). HE staining was used to observe neuropathological changes in hippocampus with opticalmicroscope.
Immunofluorescence Staining
Ten rats were randomly selected from each group. Following anesthesia, the rats were injected with 300 mL normal saline via ventricle. Tissue blocks, including hippocampus, were collected and sectioned coronally on cryostat microtome at 10 μm thickness. Frozen brain sections, at the level of the hippocampus were placed on slides and postfixed in acetone for 8 min.
To detect expression of p53 and Noxa in hippocampal CA1 region, we performed indirect immunofluorescence. Sections were washed three times with phosphate buffered saline (PBS) for 5 min, treated with 0.1% Triton for 10 min, blocked with serum albumin, and incubated with primary antibodies rabbit anti‐p53 polyclonal antibody (1:300; SantaCruz, USA) and goat anti‐Noxa polyclonal antibody (1:200; Santa Cruz, USA) overnight at 4°C. After being washed three times in PBS, sections were incubated with secondary antibodies, fluorescein‐isothiocyanate (FICT)‐conjugated goat antirabbit IgG (1:100; Jackson, USA) and FICT‐conjugated rabbit anti‐goat IgG (1:100; Jackson, USA), for 40 min at 37°C and washed three times with PBS.
Confocal Laser Scanning Microscopy
Subsequent to immunofluorescence staining, sections were immediately examined using confocal laser scanning microscope (sp5, Leica, Germany). The excitation source was an argon ion laser at 488 nm. Sections with similar hippocampal CA1 regions were randomly selected to observe p53 and Noxa expression and to count the number of p53‐or Noxa‐immunopositive cell.
Statistical Analysis
The data were statistically evaluated by the first author using SPSS13.0 software. Due to nonhomogeneous variance, data for escape latency were analyzed with rank sum test. The results of spatial probe test were analyzed with chi‐square test. The number of the cells that express p53 or Noxa was analyzed using One‐Way ANOVA and LSD post hoc test, and the relation between p53 and Noxa was analyzed using linear correlation analysis.
Results
Morris Water Maze Test
The average latency in the VD model group, sham‐operated group, and EA group was 28.96, 10.42, and 16.13, VD model group was weaker than sham‐operated group and EA improved learning ability in VD rats (Table 1).
Table 1.
Comparison of mean escape latency in Morris water maze test at 2 months after surgery
| Group | n | Mean rank |
|---|---|---|
| Sham‐operated group | 12 | 10.42 |
| VD model group | 12 | 28.96a |
| EA group | 11 | 16.13b |
a P < 0.01, versus sham‐operated group; b P < 0.01, versus VD model group.
Escape strategies improved from edge and random searches to more linear swim pathways in the EA group, compared to the VD model group (Figure l).
Figure 1.
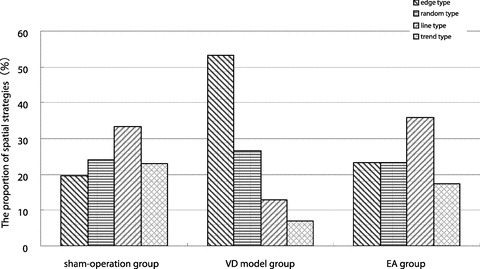
The result of search strategies.
HE Staining
Sham‐operated group: Pyramidal cells exhibited regular and compact arrangement in the hippocampal CA1 region. The cell boundary was clear. The cytoplasm was stained and well distributed. The cell nucleus was large and round with a visible nucleolus (Figure 2A).
Figure 2.
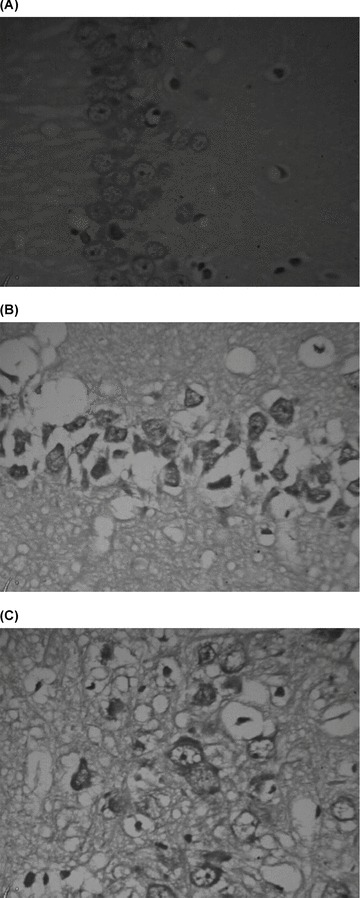
Pathological changes in the hippocampal CA1 region (hematoxylin‐eosin staining × 400). (A) Sham‐operated group. (B) VD model group. (C): EA group.
VD model group: The pyramidal cell layer was loosely arranged, and the cell outline disappeared in many pyramidal cells. The boundary between cytoplasm and nucleus was obscured. Some cells had shrunken and exhibited pyknosis. The nucleolus disappeared. Vacuoles were left in the hippocampal CA1 region (Figure 2B).
EA group: The pyramidal cell layer was loosely arranged. The cell boundary was clear in most cells and the cytoplasm stained light. The boundary between cytoplasm and nucleus was clear and the nucleolus was visible. Vacuoles were in the hippocampal CA1 region (Figure 2C).
Immunofluorescence Staining
p53‐Positive cells, as well as Noxa‐positive cells, were detectable using confocal laser scanning microscopy following immunofluorescence staining (Table 2 and Figures 3 and 4).
Table 2.
Immunopositive cells for p53 and Noxa in hippocampal CA1 region at 2 months after surgery in every group 
| Group | p53 | Noxa |
|---|---|---|
| Sham‐operated group | 34.80 + 3.23 | 33.20 + 7.48 |
| VD model group | 53.80 + 3.12a | 68.70 + 6.75a |
| EA group | 42.70 + 3.09b | 45.50 + 5.17b |
a P < 0.01, versus sham‐operated group; b P < 0.01, versus VD model group.
Figure 3.
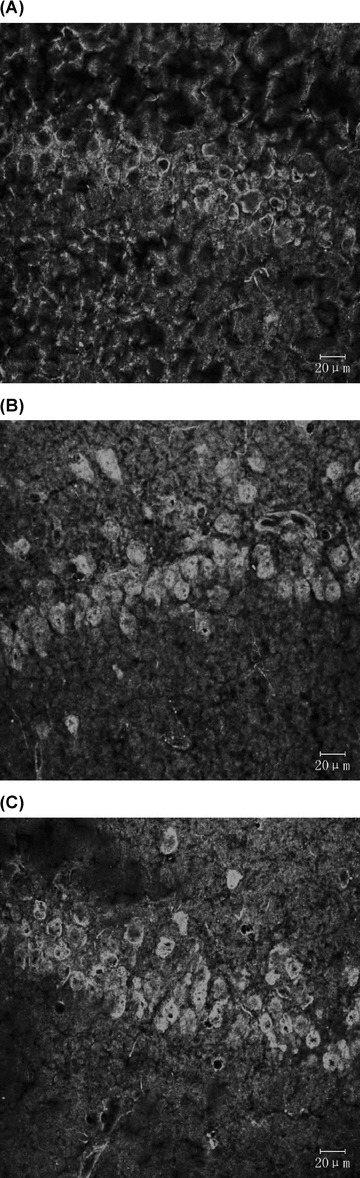
Expression of p53‐positive cells (immunofluorescence staining). (A) Sham‐operated group. (B) VD model group. (C) EA group.
Figure 4.
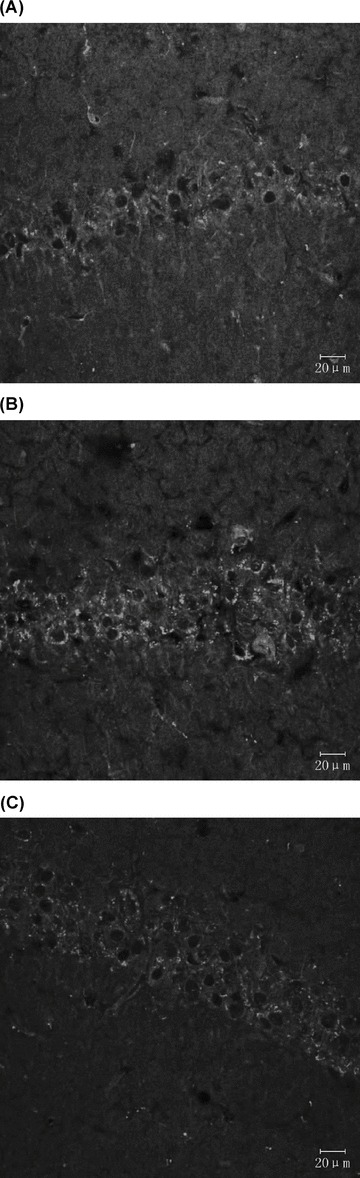
Expression of Noxa‐positive cells (immunofluorescence staining). (A) Sham‐operated group. (B) VD model group. (C) EA group.
In Figure 3, a slight p53 immunoreactivity exhibited in cytoplasm of sham‐operated group and an increasing cytosolic and nuclear p53 immunoreactivity, as cake‐like, exhibited in both VD model group and EA group. In Figure 4, Noxa immunoreactivity was in cytoplasm of groups. Table 2 showed that expression of p53 and Noxa in the EA group and sham‐operated group was less than in the VD model group (P < 0.01).
Linear correlation analysis between p53‐positive cell number and Noxa‐positive cell number in hippocampus indicated that the expression of p53 was positively correlated to expression of Noxa in hippocampus of VD rats (r = 0.918, P < 0.01), and suggested close interaction between p53 and Noxa in VD rats.
Discussion
Although necrotic cell death occurs at the core of an infarction where oxygen supply is most reduced, apoptotic cell death largely predominates in peri‐infarct areas [6, 7], and apoptosis is also involved in delayed ischemic injury after transient focal cerebral ischemia in rats [8]. Autopsy evidences conformed that apoptosis in hippocampus was the most common pathology of patients with VD [9]. In the present, HE staining test demonstrated apoptosis in the hippocampal CA1 region of VD rats and revealed that apoptosis was the main pathogenic mechanism of VD.
P53 is a central player in apoptosis by transcription‐independent mechanisms and by transcriptional activation of proapoptotic genes. In the first process, p53 induces permeabilization of the outer mitochondrial membrane by forming a complex with a Bcl‐2 family protein, resulting in cytochrome c release [10, 11]. In the latter process, p53 encodes a sequence‐specific transcription factor that controls the expression of genes whose products mediate apoptosis, and these products include Bax, Noxa, PUMA, Fas, and so on [3, 12, 13]. Data proved that the increasing p53 immunoreactivity as small particles in the cytosol was observed and the mitochondrial p53 pathway was one of the mechanisms mediating apoptosis of hippocampal neurons after reperfusion [4]. Our study showed increasing p53 immunoreactivity as cake‐like was observed in cytosol and nuclei of VD rat and the p53‐positive cells were elevated in a rat model of VD. These indicated p53 played an important role in the pathogenesis of VD, and transcription‐independent and transcriptional p53 activation mechanisms might be both involved in pathogenesis of VD.
Noxa is a proapoptotic member of BH3‐only Bcl‐2 family proteins [3]. It can be activated in p53‐dependent way or in p53‐independent way [5, 14, 15]. A study on cerebral ischemic proved upregulated expression of Noxa. The present results showed that expression of Noxa in the hippocampal CA1 region was elevated in a rat model of VD, and the expression of p53 was positively correlated to expression of Noxa in hippocampus of VD rats. These indicated that Noxa‐induced apoptosis pathway was involved in the pathogenesis of VD and there was a close interaction between p53 and Noxa in VD rat.
Traditional Chinese Medicine believes that deficiency of marrow‐reservoir is the main pathogenesis of VD and Shenjing point masters spinal marrow. So we electroacupunctured at Shenshu, the back‐shu‐acupoint of kidney, to invigorate the kidney and replenish the essence. In accordance with this finding, the present data demonstrated that EA could shorten the average latency and improve search strategies in VD rats. These results indicated that EA improved the learning and spatial memory in these rats. In addition, EA tagonized the occurrence of neuronal apoptosis in the hippocampal CA1 region, thereby providing effective neuroprotection. To further explore its mechanism, we confirmed that EA was able to reduce the expression of p53 and Noxa, which might protect neurons from apoptosis in the hippocampal CA1 region. These results provide a new mechanism for EA in prevention and treatment of VD and provide conclusive evidences for the clinical application of EA.
In conclusion, data from this study demonstrated that increasing expressions of p53 and Noxa played important roles in the pathogenesis of VD. We also found that EA protected pyramidal cells in hippocampal CA1 region of VD rats by inhibiting expression of p53 and Noxa.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
This study was supported by fund for Youth Teacher from Fujian Provincial Health Department, No.2005‐2‐40.
References
- 1. Erkinjuntti T, Román G, Gauthier S, Feldman H, Rockwood K. Emerging therapies for vascular dementia and vascular cognitive impairment. Stroke 2004;35:1010–1017. [DOI] [PubMed] [Google Scholar]
- 2. Chen ZH, Lai XS, Jiang GH. Effects of electro‐acupuncture on electroencephalography in patients with vascular dementia. Zhongguo Zhong Xi Yi Jie He Za Zhi 2006;26:738–740. [PubMed] [Google Scholar]
- 3. Oda E, Ohki R, Murasawa H, et al Noxa, a BH3‐only member of the Bcl‐2 family and candidate mediator of p53‐induced apoptosis. Science 2000;288:1053–1058. [DOI] [PubMed] [Google Scholar]
- 4. Endo H, Kamada H, Nito C, Nishi T, Chan PH. Mitochondrial translocation of p53 mediates release of cytochrome c and hippocampal CA1 neuronal death after transient global cerebral Ischemia in Rats. J Neurosci 2006;26:7974–7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim JY, Ahn HJ, Ryu JH, Suk K, Park JH. BH3‐only protein Noxa is a mediator of hypoxic cell death induced by hypoxia‐inducible factor 1a. J Exp Med 2004;199:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med 2003;348:1365–1375. [DOI] [PubMed] [Google Scholar]
- 7. Sairanen T, Karjalainen‐Lindsberg ML, Paetau A, Ijäs P, Lindsberg PJ. Apoptosis dominant in the periinfarct area of human ischaemic stroke: A possible target of antiapoptotic treatments. Brain 2006;129:189–199. [DOI] [PubMed] [Google Scholar]
- 8. Lee SH, Kim M, Kim YJ, Kim YA, Chi JG, Roh JK, Yoon BW. Ischemic intensity influences the distribution of delayed infarction and apoptotic cell death following transient focal cerebral ischemia in rats. Brain Res 2002;956:14–23. [DOI] [PubMed] [Google Scholar]
- 9. Kril JJ, Patel S, Harding AJ, Halliday GM. Patients with vascular dementia due to microvascular pathology have significant hippocampal neuronal loss. J Neurol Neurosurg Psychiatry 2002;72:747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marchenko ND, Zaika A, Moll UM. Death signal‐induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J Biol Chem 2000;275:16202–16212. [DOI] [PubMed] [Google Scholar]
- 11. Chipuk JE, Maurer U, Green DR, Schuler M. Pharmacologic activation of p53 elicits Bax‐dependent apoptosis in the absence of transcription. Cancer Cell 2003;4:371–381. [DOI] [PubMed] [Google Scholar]
- 12. Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995;80:293–299. [DOI] [PubMed] [Google Scholar]
- 13. Nakano K, Vousden KH. Puma,a novel proapoptotic gene, is induced by p53. Mol Cell 2001;7:683–694. [DOI] [PubMed] [Google Scholar]
- 14. Sun Y, Leaman DW. Involvement of Noxa in cellular apoptotic responses to interferon, double‐stranded RNA, and virus infection. J Biol Chem 2005;280:15561–15568. [DOI] [PubMed] [Google Scholar]
- 15. Hershko T, Ginsberg D. Up‐regulation of Bcl‐2 homology 3(BH3)‐only proteins by E2F1 mediates apoptosis. J Biol Chem 2004;279:8627–8634. [DOI] [PubMed] [Google Scholar]


