Abstract
Apathy is defined as a disorder of motivation. There is wide acknowledgement that apathy is an important behavioral syndrome in Alzheimer's disease and in various neuropsychiatric disorders. In light of recent research and the renewed interest in the correlates and impacts of apathy and in its treatments, it is important to develop criteria for apathy that will be widely accepted, have clear operational steps, and be easy to apply in clinical practice and in research settings. Meeting these needs was the focus for a task force that included members of the European Psychiatric Association, the European Alzheimer's Disease Consortium and experts from Europe, Australia and North America.
Keywords: Apathy, Alzheimer's disease, Diagnostic criteria, Neuropsychiatry, Neurotransmitters, Pharmacological treatment
Neuropsychiatric symptoms form part of the clinical picture of Alzheimer's disease (AD) and other dementias. Irrespective of the severity of the disease, the most frequently encountered symptom is apathy [1]. As indicated by Starkstein and Leentjens [2], apathy is increasingly diagnosed in patients with neurological and psychiatric conditions in spite of the lack of a proper definition. Marin was the first [3, 4] to present apathy as a disorder of motivation, defined as “the direction, intensity and persistence of goal‐directed behavior.” Most of the current descriptions acknowledge this point and consider apathy in terms of a lack of goal‐directed behaviour, cognition, or emotion [5, 6, 7, 8] or as “an absence of responsiveness to stimuli as demonstrated by lack of self initiated action”[9].
An extensive review of prevalence [10] found that apathy seemed to be very common in disorders that directly involve the cortex (averaged point prevalence of approximately 60%) and disorders of subcortical structures (averaged point prevalence of approximately 40%). Once again these results must be interpreted with caution taking into account the absence of a common definition and common diagnostic criteria in the different studies.
Apathy during the Course of AD
Several studies have shown that in addition to the impairment of cognitive performance neuropsychiatric symptoms are present very early in the disease process. Apathy and depressive symptoms are the most frequent neuropsychiatric symptoms observed in mild cognitive impairment (MCI) [11, 12, 13]. In a prospective study on predictive factors for AD [14] we reported that the presence of mild signs of apathy in MCI patients was cross‐sectionally associated with a higher degree of memory impairment [15].
The same study also examined the influence of the apathy dimensions on the risk of developing of AD in 214 patients with MCI during a 3‐year follow‐up. Anxiety, depression, and apathy assessment were included in the battery of neuropsychiatric tests. After 3 years the risk of conversion to AD was significantly higher for patients with lack of interest, which is one of the core apathetic symptoms [16]. It was recently demonstrated that apathetic but not depressive symptoms are a major risk factor for conversion to dementia in MCI subjects [17]. In a 2‐year follow‐up study the rates of conversion to dementia were 24% for MCI without depression and apathy, 7.9% for MCI depressed, 19% for MCI depressed‐apathetic and 60% for MCI apathetic. The authors’ hypothesis was that depression could have a different pathophysiological basis from dementia.
From a patient and caregiver perspective, these results serve as a reminder of the very important place of the clinical interview associated with the cognitive assessment.
Apathy is also present during the course of the disease. In the REAL‐FR cohort study the prevalence of apathy and hyperactivity symptoms increased significantly during the 4‐year follow‐up period, while the prevalence of affective and psychotic symptoms did not [18]. Several studies have also indicated that apathy explains at least partially the loss of autonomy in activities of daily living [19, 20, 21].
In summary, apathy is important from the beginning of the disorder and is increasingly persistent during the disease process, which explains the need to consider the therapeutic options.
Current Pharmacological Treatment
Drijgers et al. [22] recently published a very interesting systematic review of studies assessing the effects of pharmacological treatment on apathy in neurodegenerative diseases. From the review process 35 articles were selected, including two meta‐analyses, 13 randomized controlled trials (RCTs), 14 open label studies, and six case studies. Only nine of the studies had treatment of apathy as a primary outcome. Cholinesterase inhibitors were investigated in 24 studies, methylphenidate in five studies and other drugs (paroxetine, amantadine, memantine, levodopa, tianeptine, and gingko biloba extract) in one study each. For the cholinesterase inhibitors positive effects were reported in one of the two meta‐analyses, in six of the nine RCTs and in five of the six open label studies. Concerning the other drugs a single RCT indicated a positive effect of Gingko biloba extract and one small RCT and three case reports suggested positive effects for methyphenidate. The authors concluded that they “found limited and inconsistent evidence for the efficacy of any specific drug in treating apathy.”
Prior to this review article another study [23] indicated that donepezil delayed progression to AD only among MCI depressed subjects. Interestingly, in this double‐blind placebo‐controlled multicenter study an MCI depressive subgroup was characterized by a cut‐off score on the Beck Depression Inventory [24]. It should be noted that previous studies using the same scale [25, 26] had demonstrated the higher predictive value of motivation‐related symptoms included in the Beck depression Inventory.
As indicated by Drijgers et al. [22], large‐scale placebo‐controlled RCTs with apathy as primary outcome measure are needed in the near future. To facilitate this research reliable and validated diagnostic criteria for apathy and validated scales are a prerequisite.
Diagnostic Criteria for Apathy
In light of recent research and the renewed interest in the correlates and impacts of apathy, and in its treatments, it was considered important to develop criteria for apathy that would be widely accepted, have clear operational steps, and be easy to apply in clinical practice and research settings. To this end, a task force including members of the Association Française de Psychiatrie Biologique, the European Psychiatric Association and the European Alzheimer's Disease Consortium and experts from Europe, Australia and North America has developed diagnostic criteria for apathy [27].
Diagnostic criteria for apathy require (see Table 1): first (A), a general statement on the core feature of apathy being diminished motivation; second (B 1–3), a description of the three dimensions of apathy; third (C), functional impairments attributable to apathy; and fourth (D), specific exclusion criteria.
Table 1.
Diagnostic criteria for apathy
| For a diagnosis of apathy the patient should fulfil criteria A, B, C and D |
|---|
| A – Loss of, or diminished, motivation in comparison to the patient's previous level of functioning and which is not consistent with his/her age or culture. |
| These changes in motivation may be reported by the patient or by the observations of others. |
| B – Presence of at least one symptom in at least two of the three following domains for a period of at least four weeks and present most of the time |
| Domain B1‐ Behavior: |
| Loss of, or diminished, goal‐directed behavior as evidenced by at least one of the following: |
| Initiation symptom: Loss of self‐initiated behavior (for example, starting conversation, doing basic tasks of day‐to‐day living, seeking social activities, |
| communicating choices); |
| ‐ Responsiveness symptom: Loss of environment‐stimulated behavior (for example: Responding to conversation, participating in social activities). |
| Domain B2 – Cognition: |
| Loss of, or diminished, goal‐directed cognitive activity as evidenced by at least one of the following: |
| ‐ Initiation symptom: Loss of spontaneous ideas and curiosity for routine and new events (e.g., challenging tasks, recent news, social opportunities, |
| personal/family and social affairs); |
| ‐ Responsiveness symptom: Loss of environment‐stimulated ideas and curiosity for routine and new events (e.g., in the person's residence, neighbor‐ |
| hood or community). |
| Domain B3 – Emotion: |
| Loss of, or diminished, emotion as evidenced by at least one of the following: |
| ‐ Initiation symptom: Loss of spontaneous emotion, observed or self‐reported (e.g., subjective feeling of weak or absent emotions, or observation by |
| others of a blunted affect); |
| ‐ Responsiveness symptom: Loss of emotional responsiveness to positive or negative stimuli or events (e.g., observer reports of unchanging affect or |
| of little emotional reaction to exciting events, personal loss, serious illness, emotional‐laden news). |
| C – The symptoms in criteria A and B cause clinically significant impairment in personal, social, occupational, or other important areas of functioning. |
| D – The symptoms in criteria A and B are not exclusively explained by or due to any of the following: Physical disabilities (e.g., blindness and loss of |
| hearing), motor disabilities, diminished level of consciousness or the direct physiological effects of a substance (e.g., drug abuse, medication) |
Criterion B describes the three core domains of apathy, that is, behavior, cognition, and emotion. Criterion B is based on the premise that change in motivation can be measured by examining a patient's responsiveness to internal or external stimuli. Therefore, each of the three domains within criterion B includes two symptoms. The first symptom pertains to self‐initiated or “internal” behaviors, cognitions, and emotions (Initiation symptom) and the second symptom to the patient's responsiveness to “external” stimuli (Responsiveness symptom). To be fulfilled, criterion B requires the presence of at least one symptom in at least two of the three domains for a period of at least 4 weeks, and to be present for most of the time.
The first results of an as yet unpublished multicenter study aimed at validating the diagnostic criteria indicate that apathy was present in 55% of the AD patients (n = 132) and that responsiveness symptoms were less frequent than initiation symptoms in MCI, AD and mixed dementia patients.
Apathy Assessment
Ideally, the assessment should be structured, with input from the patient and the carer, and should also incorporate the physician's perspective. The instrument also needs to allow robust measures of severity and change, and to reliably distinguish apathy from other syndromes, particularly depression, which may co‐exist in these disorders. Using a variety of measures, apathy, and depression can in fact be reliably distinguished as separate syndromes in AD [28]. In a study using Marin's [3] criteria to define apathy, 37% of 319 subjects with AD had apathy; of these, 24% had apathy and depression, whilst 13% had apathy alone [6]. Using the apathy and depression items of the Neuropsychiatric Inventory (NPI) in 216 patients with AD, Benoit et al. [29] found that 8% of this sample had depression alone, 30.5% had apathy alone and 12.5% had both depression and apathy, whilst 49% had neither.
The most widely used instrument in clinical research on apathy is the NPI apathy item [30]. Today the following specific instruments can be used for ascertainment of apathy in patients with dementia and other neurodegenerative diseases both in clinical practice and research; the Structured Clinical Interview for Apathy (SCIA) [28], the Apathy Evaluation Scale (AES) [31], the Apathy Scale (AS) [32], the Apathy Inventory (AI) [33], the Lille Apathy Rating Scale (LARS) [34]. The AES, AS, AI, and LARS have the relevant psychometric properties for measuring the level of apathy. A forthcoming version of the NPI designed specifically for clinicians (NPI‐C), currently in development, will incorporate for the apathy item, symptoms belonging to the diagnostic criteria.
The classical neuropsychiatric symptom assessments described earlier are subjective structured interview‐based, using input from the caregiver and/or the patient. In the future, new technologies are likely to provide us with a more objective measure. An example is ambulatory actigraphy, consisting of a piezoelectric accelerometer designed to record arm movement in three dimensions. Experiments using this instrument have been conducted in several disorders, including sleep/wake disorders [35], and attempts are being made to establish its usefulness as a means of measuring agitation [36].
This technology was recently used for the assessment of apathy [37] in 30 AD subjects and 15 healthy controls. AD patients were divided into two subgroups (with and without apathy) according to the AI. Locomotor activity was assessed using a wrist‐worn actigraph for 75 minutes, during which neuropsychological and behavioral examinations were performed systematically in the same order (60 minutes), followed by 15 minutes of free activity. As shown in Figure 1, AD patients had significantly lower motor activity than healthy subjects. In addition, actigraphic data clearly differentiated between AD patients with and without apathy, thus confirming the interest of such tools. However, it must be underlined that actigraphy may not be useful in patients with mobility problems such as parkinsonism or arthritis.
Figure 1.
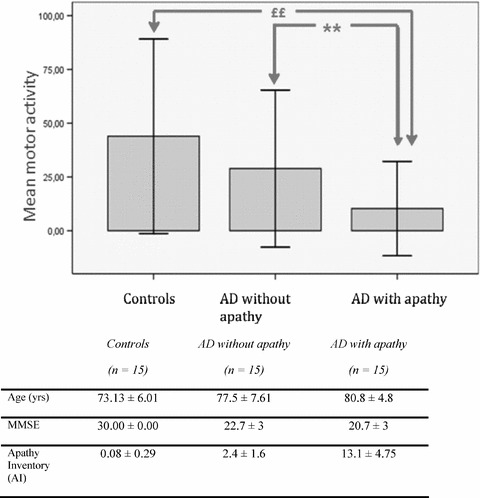
Actigraphic parameters for the three groups and comparison between AD patients without apathy and AD patients with apathy. *p<0.05; **p<0.01 AD with apathy vs. AD without apathy; £p<0.05; ££p<0.01 AD without apathy vs; controls. AD with apathy: AI total score >3 (range 0–12); AD with apathy: AI total score <3 (range 0–12).
Better Understanding for Better New Treatment Strategies
As indicated by Lavretsky [38], the field of old age psychiatry is in urgent need of new paradigms of therapeutic development to treat neuropsychiatric symptoms. This obviously includes new classes of drugs but also the innovative use of available drugs. The first step is to develop a better understanding of these behavioral disturbances.
Neural Substrate of Motivation and Apathy
Figure 2 summarizes some of the neuroanatomical results described later. Brain imaging studies in AD have shown relationships between apathy and abnormal perfusion in the frontal cortex and in the cingulate area [39, 40, 41, 42]. Compared to healthy subjects, apathetic AD patients had significantly decreased perfusion in the anterior cingulate, the inferior and medial gyrus frontalis and the orbitofrontal gyrus [43]. In addition, AD apathetic and AD depressive patients had a distinctive pattern of regional perfusion metabolism [44]. Using structural magnetic resonance imaging (MRI) it has also been shown [45] that a high apathy score correlated significantly with low gray matter density values in the anterior cingulate cortex, orbitofrontal cortex, and regions of the dorsolateral prefrontal cortex in both hemispheres, and bilateral correlations were found in the putamen and in the head of the left caudate nucleus.
Figure 2.
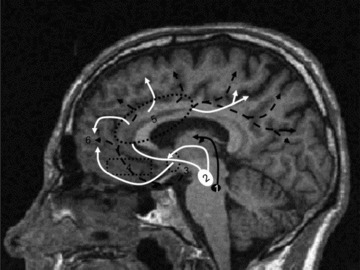
Anatomical structures involved in motivation The meso‐cortico‐limbic pathway is a dopaminergic network from the ventral tegmentum [2] to the different anterior cortical regions (frontal and parietal) connecting via the anterior cingulate [5]. According to Levy and Dubois [8], lesions or dysfunctions of this system could be involved in disruption of “emotional‐affective” processing. The nigro‐striatal pathway corresponds to a dopaminergic connection between the substantia nigra [1] and the striatum. [50]. A nigrostriatal dopaminergic loss should impair the capacity to select and initiate goal‐directed actions. The cortical pathway connects the basal nucleus of Meynert [3] to frontal, temporal, parietal, and occipital regions and is essentially involved in cholinergic neurotransmission. A lesion affecting the prefrontal cortex [6] and especially the dorsolateral pre‐frontal cortex and basal ganglia connections would lead to the cognitive mechanism of apathy [8]. White line: Meso‐cortico‐limbic dopaminergic pathway. Black line: Nigrostiatal dopaminergic pathway. Dot black line: Cortical cholinergic pathway.
Atrophy of elements of the limbic circuit, including the cingulate gyrus together with the more prominent atrophy in mediotemporal structures, is observed early in AD, as well as atrophy in the periventricular nuclei and the striatum [46, 47]. This regional atrophy could explain the temporal coincidence between the early appearance of apathy and the first stage of AD. The involvement of frontostriatal structures is in line with the two general frameworks for the neural substrate of motivation.
In the first of these frameworks, motivation is related to goal‐directed behavior, defined as a set of related and integrated motivational, emotional, cognitive and motor processes allowing for the translation of an internal state through action into the attainment of a goal [48]. Brown and Pluck [7] stated that; “the goal object can be immediate and physical such as relieving thirst, or long term and abstract, such as being successful in one's job. By “directed” it is meant that the action is mediated by knowledge of the contingency between the action and the outcome”.
Reward and the dopamine hypothesis is the second general framework. Mesolimbic and neostriatal dopamine projection have been suggested to mediate reward. Rolls [49] uses equivalently reward and “positive reinforcer” in order to describe stimuli which an animal wants to obtain. Berridge and Robinson [50] argue that reward is a constellation of multiple processes many of which can be separately identified in behavior. In animal studies, Berridge [51] had suggested that dopamine‐related neural systems mediate more specifically one component of reward. Their incentive salience hypothesis is built on earlier incentive theory formulation of motivation [52, 53]. Incentive salience transforms the brain's neural representations of conditioned stimuli, converting an event or stimulus from a neutral representation into an attractive and wanted incentive that can “grab attention” and is able to elicit voluntary action [50]. Incentive salience can be dissociated into the complementary but separate components “liking” and “wanting,” dopamine systems only mediating the latter. In this theory, “wanting” refers specifically to the underlying core process that instigates goal‐directed behavior, attraction to an incentive stimulus, and consumption of the goal object with as behavioral manifestation the interest of the animal in the goal object and the initiative to obtain it.
From a behavioral point of view, it is interesting to discuss this point in relation to the diagnostic criteria for apathy. Lack of initiative and interest, as described in criterion B, could be related to the “wanting” defined in animal studies as the underlying implicit motivational component of reward mediated by the mesolimbic dopamine circuitry [50]. However, this relationship must be considered carefully because in animal studies the term “wanting” is used for concrete and particular stimuli such as food, drugs and their cues, while in the present study we are dealing with more abstract and cognitively loaded incentives. This major difference also has anatomical implications. The importance of subcortical brain structures is frequently indicated by investigators whose research has primarily been on brain mechanisms of emotion and motivation in animals [54]. In contrast, the overwhelming majority of human studies underline the importance of the prefrontal and cingulate structures [54]. In fact, cognitive incentives and “wanting” appear to operate simultaneously at different levels [55]. Using the AI it has been demonstrated that AD patients with lack of initiative and interest have a significantly lower perfusion in the right anterior cingulate than AD patients without lack of initiative and interest [56]. In addition, David et al. [57] found a significant correlation between lack of initiative and dopamine transporter striatal uptake SPECT levels independently of motor activity in a small group patients presenting either AD or Lewy body dementia.
Neuropharmacological Approach to Motivation
The neural substrate of motivation and attention is a network involving the cingulate, dorsolateral prefrontal and inferior parietal cortices. Dopamine is the principal neurotransmitter in the mesocorticolimbic systems and an association between age‐related decline in dopamine activity and impairment in cingulate metabolism has already been demonstrated [58]. In parallel, the cholinergic projections to limbic and other cortical areas appear to participate in a number of cognitive disturbances and dysfunctions. The correlated response of the cholinergic and dopamine neurotransmitter systems in the striatum [59] suggests that the two systems could be related. Martorana [60] tested whether cholinergic dysfunction could be modified by dopamine, using short latency afferent inhibition (SLAI) before and after a single l‐dopa challenge. SLAI was reduced in AD patients and preserved in normal subjects. l‐Dopa administration was able to restore SLAI modification only in AD, thus confirming the relationship between the acetylcholine and dopamine systems.
Another player is serotonin. There is growing evidence for serotoninergic influences on dopamine transmission. The majority of studies have demonstrated that 5‐HT transmission plays an inhibitory role on dopaminergic activity [61, 62, 63], but some studies have suggested the opposite view [64, 65, 66]. These divergences could be partially explained by the variety of subtypes and actions of 5‐HT receptors. For example, 5‐HT2C agonists inhibit dopaminergic effects [67], whereas 5‐HT1B and 5‐HT3 agonists enhance dopamine release [68].
These interactions are important for frontal lobe function. One of the major dopaminergic pathways is mesocortical, with numerous terminations in the prefrontal cortex. Their synapses are regulated by frontal 5‐HT2 heteroreceptors activated by serotoninergic neurons projecting from the medial raphe [69]. Serotoninergic projections also inhibit dopaminergic activity in the striatum. The distribution and interaction of dopaminergic and serotoninergic neurons supports the hypothesis of a serotonin–dopamine balance, which could thus play a major role in the regulation of transmission between the prefrontal cortex and subcortical structures [70]. In fact, it seems possible to enhance dopaminergic activity in the prefrontal cortex with 5‐HT2A and 5‐HT2C inhibitors. The serotoninergic system is also indirectly involved in reward processes, as demonstrated using the reversal‐learning paradigm, which requires the adaptation of behavior according to changes in stimulus‐reward contingencies, a capacity relevant to social and emotional behavior [49]. Consistent with animal studies indicating that reversal learning is modulated by 5HT manipulations [71], two studies in healthy human volunteers showed that 5HT suppression by acute tryptophan depletion impairs reversal learning [72, 73]. In another study, Tanaka et al. [74] demonstrated that when human subjects learned actions on the basis of immediate rewards, significant activity was seen in the lateral orbitofrontal cortex and the striatum, whereas when subjects learned to act in order to obtain large future rewards while incurring a small immediate loss, the dorsolateral prefrontal cortex, inferior parietal cortex, dorsal raphe nucleus, and cerebellum were also activated. The authors suggest that different sub‐loops of the cortico‐basal ganglia network are specialized for reward prediction at different time scales and that they are differently activated by the ascending serotoninergic system.
More specifically in the field of AD, a cholinergic modulation of the cerebral metabolic response, measured with positron emission tomography (PET), to acute administration of citalopram, a selective serotonin reuptake inhibitor, has recently been demonstrated [75]. It has also been suggested that antidepressants may have neuroprotective abilities by increasing the proliferation of neural progenitors in the subgranulate zone of the hippocampus as well as the survival of these new‐born neurons [76]. A short‐term preliminary study indicated that a combination of cholinergic treatment and serotonin reuptake inhibitor may improve cognitive function and daily living in patients with AD [77]. A recent epidemiological study using the register of all prescribed antidepressants and diagnoses of dementia in Denmark during the period 1995–2005 indicated that only long‐term antidepressant treatment was associated with a reduction in the rate of dementia, however not to the rate among the general population [78].
Possible Implications for Future Therapeutic Studies
Various options exist for future research, including high quality RCTs with apathy as primary outcome measure.
-
1
In connection with atrophy data, research based on cholinergic treatment, specifically targeting early AD patients with apathy.
-
2
In connection with the goal‐directed behavior and reward hypothesis, research aimed at correcting the dopaminergic deficit in the brain reward system, using a single dopaminergic agent or, more likely, a more subtle approach targeting the activity of a combination of neurotransmitters (e.g., cholinergic–dopaminergic neurotransmitters).
-
3
In connection with the serotoninergic interaction, a careful study of the use of antidepressants in combination with anti‐dementia drugs.
Lastly, it must be remembered that pharmacological treatments are not the only option. As indicated in the diagnostic criteria for apathy, there are two types of symptoms (initiation and responsiveness). In practice, observation of preserved responsiveness abilities may suggest to the clinician the importance of using environmental and social stimulation, if possible specifically oriented to the individual's own interests. In our view, this is the most important factor in the treatment of apathy and the main objective of pharmacological treatment should simply be to ensure that the patient is in the best possible state to derive the greatest benefit from these stimulations.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
This study was supported by a grant from the Conseil general des Alpes Maritimes (Alzheimer Disease 06 plan). P. Robert contributed to the conception/design and drafting of the article. R. David, E. Mullin, and P. Malléa contributed to data collection and analysis.
References
- 1. Robert P, Verhey F, Byrne EJ, et al Grouping for behavioral and psychological symptoms in dementia: Clinical and biological aspects. Consensus paper of the European Alzheimer Disease Consortium. Eur Psychiatry 2005;20:490–496. [DOI] [PubMed] [Google Scholar]
- 2. Starkstein SE, Leentjens AFG. The nosological position of apathy. J Neurol Neurosurg Psychiatry 2008;79:1088–1092. [DOI] [PubMed] [Google Scholar]
- 3. Marin RS. Apathy: A neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci 1991;3:243–254. [DOI] [PubMed] [Google Scholar]
- 4. Marin RS. Differential diagnosis and classification of apathy. Am J Psychiatry 1990;147:22–30. [DOI] [PubMed] [Google Scholar]
- 5. Marin RS, Fogel BS, Hawkins J, Duffy J, Krupp B. Apathy: A treatable syndrome. J Neuropsychiatry Clin Neurosci 1995;7:23–30. [DOI] [PubMed] [Google Scholar]
- 6. Starkstein SE, Petracca G, Chemerinski E, Kremer J. Syndromic validity of apathy in Alzheimer's disease. Am J Psychiatry 2001;158:872–877. [DOI] [PubMed] [Google Scholar]
- 7. Brown RG, Pluck G. Negative symptoms: The pathology of motivation and goal‐directed behavior. Trends Neurosci 2000;23:412–417. [DOI] [PubMed] [Google Scholar]
- 8. Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex‐basal ganglia circuits. Cereb Cortex 2006;16:916–928. [DOI] [PubMed] [Google Scholar]
- 9. Stuss DT, Van Reekum R, Murphy KJ. Differentiation of states and causes of apathy. In: Borod J, editor. The neuropsychology of emotion . New York : Oxford University Press, 2000. [Google Scholar]
- 10. Van Reekum R, Stuss DT, Ostrander R. Apathy: Why care? J Neuropsychiatry Clin Neurosci 2005;17:7–19. [DOI] [PubMed] [Google Scholar]
- 11. Lopez OL, Becker JT, Sweet RA. Non‐cognitive symptoms in mild cognitive impairment subjects. Neurocase 2005;11:65–71. [DOI] [PubMed] [Google Scholar]
- 12. Feldman H, Scheltens P, Scarpini E, et al Behavioral symptoms in mild cognitive impairment. Neurology 2004;62:1199–1201. [DOI] [PubMed] [Google Scholar]
- 13. Gabryelewicz T, Styczynska M, Pfeffer A, et al Prevalence of major and minor depression in elderly persons with mild cognitive impairment–MADRS factor analysis. Int J Geriatr Psychiatry 2004;19:1168–1172. [DOI] [PubMed] [Google Scholar]
- 14. Sarazin M, Berr C, De Rotrou J, et al Amnestic syndrome of the medial temporal type identifies prodromal AD: A longitudinal study. Neurology 2007;69:1859–1867. [DOI] [PubMed] [Google Scholar]
- 15. Robert P, Berr C, Volteau M, et al Neuropsychological performance in mild cognitive impairment with and without apathy. Dement Geriatr Cogn Dis 2006;21:192–197. [DOI] [PubMed] [Google Scholar]
- 16. Robert PH, Berr C, Volteau M, et al Importance of lack of interest in patients with mild cognitive impairment. Am J Geriatr Psychiatry 2008;16:770–776. [DOI] [PubMed] [Google Scholar]
- 17. Vicini Chilovi B, Conti M, Zanetti M, Mazzu I, Rozzini L, Padovani A. Differential impact of apathy and depression in the development of dementia in mild cognitive impairment patients. Dement Geriatr Cogn Disord 2009;27:390–398. [DOI] [PubMed] [Google Scholar]
- 18. Gonfrier S, Andrieu S, David R, et al Course of neuropsychiatric symptoms during a four year follow‐up in the REAL‐FR cohort. The Journal of the Alzheimer's Association 2008;4(4 Suppl 2):135. [Google Scholar]
- 19. Boyle PA, Malloy PF. Treating apathy in Alzheimer's disease. Dement Geriatr Cogn Disord 2004;17:91–99. [DOI] [PubMed] [Google Scholar]
- 20. Starkstein SE, Jorge R, Mizrahi R, Robinson RG. A prospective longitudinal study of apathy in Alzheimer's disease. J Neurol Neurosurg Psychiatry 2006;77:8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lechowski L, Benoit M, Chassagne P, et al Persistent apathy in Alzheimer's disease as an independent factor of rapid functional decline: The REAL longitudinal cohort study. Int J Geriatri Psychiatry 2009;24:341–346. [DOI] [PubMed] [Google Scholar]
- 22. Drijgers RL, Aalten P, Wionogrodzka A, Verhey FRJ, Leentjens AFG. Pharmacological treatment of apathy in neurodegenerative diseases: A systematic review. Dement Geriatr Cogn Disord 2009;28:13–22. [DOI] [PubMed] [Google Scholar]
- 23. Lu PH, Edland E, Tingus K, Petersen RC, Cummings JL; Alzheimer's Disease Cooperative Study Group . Donepezil delays progression to AD in MCI with depressive symptoms. Neurology 2009;72:2115–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Am J Geriatr Psychiatry 1961;4:561–571. [DOI] [PubMed] [Google Scholar]
- 25. Bartolini M, Coccia M, Luzzi S, Provinciali L, Ceravolo MG. Motivational symptoms of depression mask preclinical Alzheimer's disease in elderly subjects. Dement Geriatr Cogn Disord 2005;19:31–36. [DOI] [PubMed] [Google Scholar]
- 26. Berger AK, Fratiglioni L, Forsell Y, Winblad B, Bäckman L. The occurrence of depressive symptoms in the preclinical phase of AD. A population‐based study. Neurology 1999;53:1998–2002. [DOI] [PubMed] [Google Scholar]
- 27. Robert PH, Onyike CU, Leentjens AFG, et al Proposed diagnostic criteria for apathy in Alzheimer's disease and other neuropsychiatric disorders. European Psychiaty 2009;24:98–104. [DOI] [PubMed] [Google Scholar]
- 28. Starkstein SE, Ingram L, Garau ML, Mizrahi R. On the overlap between apathy and depression in dementia. J Neurol Neurosurg Psychiatry 2005;76:1070–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benoit M, Andrieu S, Lechowski L, Gillette‐Guyonnet S, Robert PH, Vellas B. Apathy and depression in Alzheimer's disease are associated with functional deficit and psychotropic prescription. Int J Geriatric Psychiatry 2008;23:409–414. [DOI] [PubMed] [Google Scholar]
- 30. Cummings JL, Mega MS, Gray K, Rosemberg‐Thompson S, Gornbein T. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–2314. [DOI] [PubMed] [Google Scholar]
- 31. Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 1991;38:143–162. [DOI] [PubMed] [Google Scholar]
- 32. Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity and clinical correlates of apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci 1992;4:134–139. [DOI] [PubMed] [Google Scholar]
- 33. Robert PH, Clairet S, Benoit M, et al The Apathy Inventory: Assessment of apathy and awareness in Alzheimer's disease, Parkinson's disease and mild cognitive impairment. Int J Geriatric Psychiatry 2002;17:1099–1105. [DOI] [PubMed] [Google Scholar]
- 34. Sockeel P, Dujardin K, Devos D, Denève C, Destée A, Defebvre L. The Lille apathy rating scale (LARS), a new instrument for detecting and quantifying apathy: Validation in Parkinson's disease. J Neurol Neurosurg Psychiatry 2006;77:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yesavage JA, Friedman L. A follow‐up study of actigraphic measures in home‐residing Alzheimer's disease patients. J Geriatr Psychiatry Neurol 1998;11:7–10. [DOI] [PubMed] [Google Scholar]
- 36. Mahlberg R, Walther S. Actigraphy in agitated patients with dementia: Monitoring treatment outcomes. Z Gerontol Geriatr 2007;40:178–184. [DOI] [PubMed] [Google Scholar]
- 37. Mulin E, David R, Rivet A, et al Apathy assessment using ambulatory actigraphy short‐time recording in mild Alzheimer's disease. Int Psychogeriatrics Assoc 2009;21:S116. [Google Scholar]
- 38. Lavretsky H. Neuropsychiatric symptoms in Alzheimer disease and related disorders: Why do treatments work in clinical practice but not in the randomized trials? Am J Geriatr Psychiatry 2008;16:523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Craig AH, Cummings JL, Fairbanks L, et al Cerebral blood flow correlates of apathy in Alzheimer disease. Arch Neuro 1996;53:1116–1120. [DOI] [PubMed] [Google Scholar]
- 40. Benoit M, Dygai I, Migneco O, et al Behavioral and psychological symptoms in Alzheimer's disease. Dement Geriatr Cogn Dis 1999;10:511–517. [DOI] [PubMed] [Google Scholar]
- 41. Migneco O, Benoît M, Koulibaly PM, et al Perfusion brain SPECT and statistical parametric mapping analysis indicate that apathy is a cingulate syndrome: A study in Alzheimer's disease and nondemented patients. NeuroImage 2001;13:896–902. [DOI] [PubMed] [Google Scholar]
- 42. Benoit M, Clairet S, Koulibaly M, Darcourt J, Robert PH. Brain perfusion correlates of the Apathy Inventory dimensions in Alzheimer's disease. Int J Geriatric Psychiary 2004;19:864–869. [DOI] [PubMed] [Google Scholar]
- 43. Benoit M, Koulibaly PM, Migneco O, Darcourt J, Pringuey DJ, Robert PH. Brain perfusion in Alzheimer's disease with and without apathy: A SPECT study with statistical parametric mapping analysis. Psychiatry Res: Neuroimaging 2002;114:103–111. [DOI] [PubMed] [Google Scholar]
- 44. Holthoff V, Baumann B, Kalbe E, et al Regional cerebral metabolism in early Alzheimer's disease with clinically significant apathy or depression. Biol Psychiatry 2005;57:412–421. [DOI] [PubMed] [Google Scholar]
- 45. Bruen PD, McGeown WJ, Shanks MF, Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer's disease. Brain 2008;131:2455–2463. [DOI] [PubMed] [Google Scholar]
- 46. Jack CRJ, Shiung MM, Weigand SD, et al Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology 2005;65:1227–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shino A, Watababe T, Maeda K, Kotani E, Akiguchi I, Matsuda M. Four subgroups of Alzheimer's disease based on patterns of atrophy using VBM and a unique pattern for early onset disease. Neuroimage 2006;33:17–26. [DOI] [PubMed] [Google Scholar]
- 48. Schultz W. The primate basal ganglia and the voluntary control of behaviour. J Consc Stud 1999;6:31–45. [Google Scholar]
- 49. Rolls E. The brain and emotion. Oxford : Oxford University Press, 1999. [Google Scholar]
- 50. Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Rev 1998;28:309–369. [DOI] [PubMed] [Google Scholar]
- 51. Berridge KC. Food reward: Brain substrates of wanting and liking. Neurosci Biobehav Rev 1996;20:1–25. [DOI] [PubMed] [Google Scholar]
- 52. Blackburn JR, Phillips AG, Jakubovic A, Fibiger HC. Dopamine and preparatory behavior. Behav Neurosci 1989; 103 :15–23. [DOI] [PubMed] [Google Scholar]
- 53. Toates F. Motivational systems. Cambridge : Cambridge University Press, 1986. [Google Scholar]
- 54. Berridge KC. Comparing the emotional brains of humans and other animals In: Davidson R, Goldsmith H, Scherer K, editors. Handbook of affective sciences. Oxford : Oxford University Press, 2003;25–51. [Google Scholar]
- 55. Berridge KC, Robinson T. Parsing reward. Trends Neurosci 2003;26:507–513. [DOI] [PubMed] [Google Scholar]
- 56. Robert P, Darcourt G, Koulibaly M, et al Lack of initiative and interest in Alzheimer's disease. A single photon computed tomography study. European Neurol 2006;13:729–735. [DOI] [PubMed] [Google Scholar]
- 57. David R, Koulibaly MP, Benoit M, et al Striatal dopamine transporter levels correlate with apathy in neurodegenerative diseases. A SPECT study with partial volume effect correction. Clin Neurol Neurosurg 2007;110:19–24. [DOI] [PubMed] [Google Scholar]
- 58. Volkow ND, Logan J, Fowler JS, et al Association between age‐related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am J Psychiatry 2000;157:75–80. [DOI] [PubMed] [Google Scholar]
- 59. Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. Neurobiology 2002;53:590–605. [DOI] [PubMed] [Google Scholar]
- 60. Martorana AF, Mori Z, Esposito H, et al Dopamine modulates cholinergic cortical excitability in Alzheimer's patients. Neuropsychopharmacology 2009;34:2323–2328. [DOI] [PubMed] [Google Scholar]
- 61. Kapur S, Remington G. Serotonin–dopamine interaction and its relevance to schizophrenia. Am J Psychiatry 1996;153:466–476. [DOI] [PubMed] [Google Scholar]
- 62. Korsgaard S, Gerlach J, Christensson E. Behavioral aspects of serotonin–dopamine interaction in the monkey. Eur J Pharmacol 1985;118:245–252. [DOI] [PubMed] [Google Scholar]
- 63. Sasaki‐Adams DM, Kelley AE. Serotonin–dopamine interactions in the control of reinforcement and motor behavior. Neuropsychopharmacology 2001;25:440–452. [DOI] [PubMed] [Google Scholar]
- 64. Yoshimoto K, Yayama K, Sorimachi Y, et al Possibility of 5‐HT3 receptor involvement in alcohol dependence: A microdialysis study of nucleus accumbens dopamine and serotonin release in rats with chronic alcohol consumption. Alcohol Clin Exp Res 1996;20:311A–319A. [PubMed] [Google Scholar]
- 65. De Deurwaerdere P, Bonhomme N, Lucas G, Le Moal M, Spampinato U. Serotonin enhances striatal dopamine outflow in vivo through dopamine uptake sites. J Neurochem 1996;66:210–215. [DOI] [PubMed] [Google Scholar]
- 66. Hallbus M, Magnusson T, Magnusson O. Influence of 5‐HT1B/1D receptors on dopamine release in the guinea pig nucleus accumbens: A microdialysis study. Neurosci Lett 1997;225:57–60. [DOI] [PubMed] [Google Scholar]
- 67. Walsh SL, Cunningham KA. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacology 1997;130:41–58. [DOI] [PubMed] [Google Scholar]
- 68. De Deurwaerdere P, Stinus L, Spampinato U. Opposite change of in vivo dopamine release in the rat nucleus accumbens and striatum that follows electrical stimulation of dorsal raphe nucleus: Role of 5‐HT3 receptors. J Neurosci 1998;18:6528–6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ugedo L, Grenhoff J, Svensson TH. Ritanserin, a 5‐HT2 receptor antagonist, activate midbrain dopamine neurons by blocking serotonergic inhibition. Psychopharmacology 1989;98:45–50. [DOI] [PubMed] [Google Scholar]
- 70. Kapur S, Zipursky RB, Remington G. Comparison of the 5‐HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia: Clinical and theoretical implications. Am J Psychiatry 1999;156:286–293. [DOI] [PubMed] [Google Scholar]
- 71. Millan MJ, Dekeyne A, Gobert A. Serotonin (5‐HT)2C receptors tonically inhibit dopamine (DA) and noradrenaline (NA), but not 5‐HT, release in the frontal cortex in vivo. Neuropharmacology 1998;37:953–955. [DOI] [PubMed] [Google Scholar]
- 72. Park SB, Coull JT, McShane RH, et al Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacology 1994;33:575–588. [DOI] [PubMed] [Google Scholar]
- 73. Rogers RD, Blackshaw AJ, Middleton HC, et al Tryptophan depletion impairs stimulus‐reward learning while methylphenidate disrupts attentional control in healthy young adults: Implications for the monoaminergic basis of impulsive behaviour. Psychopharmacology (Berl) 1999;146:482–491. [DOI] [PubMed] [Google Scholar]
- 74. Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico‐basal ganglia loops. Nature Neurosci 2004;7:887–893. [DOI] [PubMed] [Google Scholar]
- 75. Smith GS, Kramer E, Yilong MA, et al Cholinergic modulation of the cerebral metabolic response to citalopram in Alzheimer's disease. Brain 2009;132:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dranovsky A, Hen R. Hippocampal neurogenesis: Regulation by stress and antidepressants. Biol Psychiatry 2006;59:1136–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mowla A, Mosavinasab M, Haghshenas H, Haghighi AB. Does serotonin augmentation have any effect on cognition and activities of daily living in Alzheimer's dementia? A double‐blind, placebo‐controlled clinical trial. J Clin Psychopharmacol 2007;27:484–487. [DOI] [PubMed] [Google Scholar]
- 78. Kessing LV, Sondergard L, Forman JL, Andersen PK. Antidepressants and dementia. J Affect Disord 2009;117:24–29. [DOI] [PubMed] [Google Scholar]


