SUMMARY
Background: The dopamine D2 receptor is the common target for antipsychotics, and the antipsychotic clinical doses correlate with their affinities for this receptor. Antipsychotics quickly enter the brain to occupy 60–80% of brain D2 receptors in patients (the agonist aripiprazole occupies up to 90%), with most clinical improvement occurring within a few days. The D2 receptor can exist in a state of high‐affinity (D2High) or in a state of low‐affinity for dopamine (D2Low). Aim: The present aim is to review why individuals with schizophrenia are generally supersensitive to dopamine‐like drugs such as amphetamine or methyphenidate, and whether the D2High state is a common basis for dopamine supersensitivity in the animal models of schizophrenia. Results: All animal models of schizophrenia reveal elevations in D2High receptors. These models include brain lesions, sensitization by drugs (amphetamine, phencyclidine, cocaine, corticosterone), birth injury, social isolation, and gene deletions in pathways for NMDA, dopamine, GABA, acetylcholine, and norepinephrine. Conclusions: These multiple abnormal pathways converge to a final common pathway of dopamine supersensitivity and elevated D2High receptors, presumably responsible for psychotic symptoms. Although antipsychotics alleviate psychosis and reverse the elevation of D2High receptors, long‐term antipsychotics can further enhance dopamine supersensitivity in patients. Therefore, switching from a traditional antipsychotic to an agonist antipsychotic (aripiprazole) can result in psychotic signs and symptoms. Clozapine and quetiapine do not elicit parkinsonism or tardive dyskinesia because they are released from D2 within 12 to 24 h. Traditional antipsychotics remain attached to D2 receptors for days, preventing relapse, but allowing accumulation that can lead to tardive dyskinesia. Future goals include imaging D2High receptors and desensitizing them in early‐stage psychosis.
Keywords: Antipsychotics, Dopamine D2 receptor, Dopamine supersensitivity, D2High receptors, Psychosis, Schizophrenia
Multiple Causes of Schizophrenia
There are many risk factors, genetic and nongenetic, for the development of schizophrenia. Genetic risk factors are numerous, with 50 to 100 gene mutations and variations reported each year as associated with schizophrenia. Nongenetic risk factors include infection during fetal life, brain injury, and anoxia at birth, trauma in childhood, abuse of street drugs and steroids, brain lesions, psychosocial stress, isolation, smoking, and excess coffee.
It is difficult to decide whether a factor is actually causative because, in the end, the signs and symptoms are the same, regardless of the cause. They are the same whether one has had a brain injury and developed schizophrenia, or whether one has smoked too much cannabis and developed schizophrenia.
In other words, despite the many different bio‐psychosocial origins of schizophrenia, the clinical signs, symptoms, and natural progress of the illness are more or less similar. Such similarities suggest that there may be a common pathway through which the various causal factors operate.
Common Biomarker for Schizophrenia? Dopamine Supersensitivity
Although many biomarkers have been suggested as indicative of schizophrenia, none have stood the test of time.
Possibly, the most consistent biological aspect of schizophrenia is that the majority of patients, regardless of the apparent cause, are behaviorally supersensitive to dopamine‐like drugs such as amphetamine, methamphetamine, cocaine, apomorphine, or methylphenidate, whether or not they are taking antipsychotics [1, 2].
As reviewed by Lieberman et al. [1], 74–78% of patients with schizophrenia become worse with new or intensified psychotic symptoms after being given amphetamine or methylphenidate. Psychotic symptoms can also be elicited in this way in control subjects, but only in about 25% of individuals.
In addition, the worsening of symptoms caused by the psychostimulants also occurs when patients are taking antipsychotics. Altogether, psychotogens elicit or enhance psychotic symptoms in 40% of schizophrenia patients compared to ∼2% of control individuals.
Because amphetamine increases the release of dopamine from dopamine neuron terminals, the psychotic action of amphetamine could arise from enhanced release of dopamine in schizophrenia [3] or from supersensitivity of postsynaptic dopamine receptors. Furthermore, by stimulating postsynaptic dopamine receptors, apomorphine can increase thought disorder [4], Thus, the common psychotogenic actions of these dopamine‐like drugs is their net stimulation of postsynaptic dopamine receptors, especially D2 receptors.
The Common Target for Antipsychotics Is the Dopamine D2 Receptor. Comparison with D3 and D4
Five dopamine receptors have been found: D1, D2, D3, D4, and D5. The D2 receptor has three main variants, D2Short, D2Long, and D2Longer [5].
Of the five different dopamine receptors, D2 is the most relevant clinically, because it is the main target for antipsychotics and for dopamine‐like stimulants used to alleviate Parkinson's disease [6, 7].
In fact, the action of amphetamine and its blockade by antipsychotics led Van Rossum to propose that antipsychotics selectively targeted dopamine receptors [8, 9, 10, 11]. Before 1967, antipsychotics were known to affect metabolism of adrenaline, noradrenaline, and serotonin, but no selective action on these or any other neurotransmission systems had been shown, nor were neurotransmitter receptors then directly detectable. Van Rossum's suggestion of an isolated tissue that would selectively respond to dopamine later materialized with the advent of a radioreceptor assay, using [3H]haloperidol on homogenized tissue [12, 13, 14, 15, 16] and later on cloned D2 receptors [17]. For the first time, this work provided direct evidence that all antipsychotics selectively blocked dopamine receptors with clinical potencies that correlated with their affinity for a dopamine receptor in vitro[13, 14]. Based on these early findings, the target for the antipsychotic drugs was named “the antipsychotic/dopamine receptor”[14], but was later renamed the dopamine D2 receptor [18, 19].
Apart from D2, the other four dopamine receptors are not central to the action of antipsychotics or anti‐Parkinson medication. For example, the concentrations of antipsychotics that block D1 and D5 receptors are in the micromolar range [20, 21, 22, 23], concentrations that would be lethal if found in the plasma of patients. Moreover, there is no correlation between the clinical doses of antipsychotics and their affinities for D1 or D5 [22, 23].
Similarly, the dopamine D3 and D4 receptors are not clinically relevant in the action of antipsychotics, because neither of these receptors serves as a clinically consistent target for antipsychotic drugs [24, 25]. Although the affinities of antipsychotics are similar for D2 and D3 receptors [26], D3 is less sensitive to haloperidol, molindone, olanzapine, quetiapine, risperidone, and especially remoxipride.
It is only the D2 receptor that is universally occupied to a therapeutic level of 60–80% by the antipsychotics [22]. The D3 receptors are not occupied by therapeutic doses of antipsychotics [24]. This observation suggests that D3 may not be a treatment target in schizophrenia. In fact, BP8947, a partial agonist with a Ki of 0.9 nM for D3, was not effective in schizophrenia (10 mg/day for 10 days)[25].
Many antipsychotic drugs have similar potencies on D2 and D4, except clozapine, which is about 10 times more potent on D4. Compared to their potency on D2, raclopride, remoxipride, sulpiride, flupentixol, and fluphenazine are all much weaker on D4 [22, 27, 28].
The dissociation constant of a particular antipsychotic often differs among laboratories. One reason for this is that the final concentration of tissue is different in different laboratories [29]. Another reason is that an antipsychotic shows a higher dissociation constant when competing versus a highly fat‐soluble ligand, as compared to its competition versus a more water‐soluble ligand [30, 31]. For example, haloperidol has a dissociation constant of 0.74 nM with [3H]raclopride, 2.7 nM with [3H]spiperone, and 8.4 nM with [3H]nemonapride (from Table 1).
Table 1.
Antipsychotic dissociation constants, Ki, at dopamine receptors
| Human clone | D1 | D2 | D2 | D2 | D3 | D4 | 5HT2A |
|---|---|---|---|---|---|---|---|
| nM | nM | nM | nM | nM | nM | nM | |
| [3H]ligand used Kd of ligand, nM | Sch. | Raclo. 1.9 | Spip. 0.065 | Nem. 0.068 | Raclo. 1.6 | Spip. 0.086 | Ket. |
| Amisulpride‐(‐)‐S | — | 1.8 | 4.6 | 8 | 3 | — | — |
| Amoxapine | — | 21 | 56 | 140 | — | 5.5 | 0.6 |
| Aripiprazole | — | 1.8 | — | — | — | — | — |
| Bifeprunox | — | 3.8 | — | — | — | — | — |
| Butaclamol–(+) | — | 0.14 | 0.9 | 2.3 | — | 70 | — |
| Chlorpromazine | 16.5 | 1.2 | 4.6 | 14 | 1.4 | 9.6 | 2 |
| Clozapine | 90 | 76 | 180 | 385 | 190 | 22 | 4 |
| Clozapine‐iso | 120 | 15 | 60 | 130 | 21 | 21 | 2.2 |
| [3H]Domperidone | — | 0.35 | — | — | 1.2 | — | — |
| Droperidol | — | 0.54 | 2.3 | 4 | — | 2 | — |
| Flupentixol‐cis | 1.8 | 0.38 | 0.7 | 2.8 | 0.7 | 15 | — |
| Fluphenazine | 2.6 | 0.55 | 1.2 | 1.9 | 0.17 | 30 | 3.8 |
| Haloperidol | 55 | 0.74 | 2.7 | 8.4 | 8.8 | 2 | 74 |
| Iloperidone (HP873) | 5.6 | 5.4 | 10 | 20 | 20 | 9.6 | 0.2 |
| Loxapine | 18 | 9.8 | 23 | 41 | 7.2 | 8 | 1.9 |
| Melperone | 148 | 152 | 375 | 564 | 315 | 720 | 180 |
| Metoclopramide | — | 16 | — | — | — | — | — |
| Molindone | 1558 | 4.9 | 15 | 33 | 44 | 3900 | 5200 |
| Moperone | — | 1.6 | 3.6 | 5.6 | — | 7 | — |
| Nemonapride | — | 0.014 | 0.038 | 0.076 | — | — | — |
| Norclozapine | 73 | 180 | 300 | 650 | — | 120 | — |
| Olanzapine | 9.2 | 7.4 | 21 | 46 | 14 | 15 | 3.4 |
| Perlapine | — | 138 | 450 | 1300 | 168 | 186 | 22 |
| Perphenazine | 4.5 | 0.27 | 0.47 | 0.9 | 0.23 | 32 | — |
| Pimozide | — | 1.4 | 0.95 | — | — | — | — |
| Prochlorperazine | 7.7 | 1.7 | 4 | 5.3 | — | 89 | — |
| Quetiapine | 290 | 140 | 680 | 1400 | 240 | 2000 | 135 |
| Raclopride | — | 1.6 | 7.1 | 22 | 2.9 | 2400 | 4400 |
| Remoxipride | 4900 | 67 | 800 | 900 | 960 | 2400 | 6600 |
| Risperidone | 42 | 1.09 | 4 | 19 | 3.5 | 4.4 | 0.2 |
| Risperidone‐9‐OH | — | 1.6 | — | — | — | — | — |
| Sertindole | 22 | 1.9 | 6.5 | 8.2 | 3 | 11 | 0.28 |
| Spiperone | — | 0.018 | 0.06 | 0.13 | — | — | 0.57 |
| Sulpiride‐S | — | 9.9 | 8 | 20 | 10 | 1000 | — |
| Thioridazine | 5.8 | 1.1 | 5.2 | 18.5 | 1.9 | 11 | 1.3 |
| Trifluperazine | 2.9 | 1.4 | 3.8 | 6.8 | 0.7 | 39 | 8.8 |
| Trifluperidol | — | 0.44 | — | — | — | 0.9 | — |
| Ziprasidone | 9 | 2.7 | 6 | 11 | 1.5 | 8 | 3 |
Using the data in Table 1, Figure 1 shows that the daily oral doses of antipsychotic drugs are related to their affinities for D2, using [3H]raclopride on the human cloned D2 [31, 32]. Although the doses for chlorpromazine and thioridazine are off the correlation, these two drugs are highly bound to plasma proteins. When allowance is made for this high binding to plasma proteins, the dissociation constants and their unbound (or free) concentrations of the antipsychotics (in the plasma water of patients) have almost identical values [21, 22].
Figure 1.
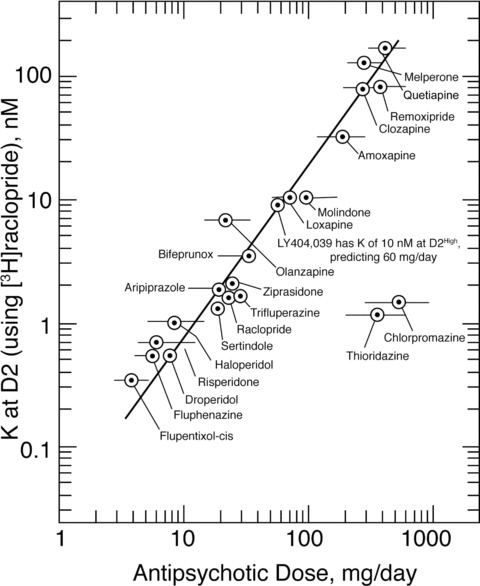
The clinical doses of antipsychotic medications are related to their affinities for the dopamine D2 receptor. The antipsychotic dissociation constants at D2, obtained using [3H]raclopride, are shown on the ordinate. The glutamate agonist LY404,039 [33] has an affinity for the dopamine D2High receptor with a dissociation constant of 10 nM at D2High (using [3H]domperidone, because [3H]raclopride does not readily reveal D2High receptors)[34]. This value of 10 nM predicts a clinical dose of approximately 60–100 mg/day, in general agreement with the dose of 80 mg/day used by Patil et al. [33]. Because of the very high binding (exceeding 98%) of chlorpromazine and thioridazine to plasma proteins [21], these antipsychotics require high daily doses. However, the final concentrations of all the antipsychotics (including chlorpromazine and thioridazine) in the plasma water in treated patients are almost identical to their dissociation constants [21, 23](Adapted from [23]; with permission from Scholarpedia; Reproduced from [31], with permission of Walsh Medical Media LLC.)
Also, the glutamate agonist LY404,039 is included in the correlation in Figure 1. Although it is a glutamate agonist, this compound has an antipsychotic effect [33]. However, using the D2‐selective ligand [3H]domperidone, LY404,039 has an affinity for the dopamine D2High receptor with a dissociation constant of about 10 nM, as shown in Figure 2[23, 34], a concentration that would predict a clinical daily dose of approximately 60–100 mg per day for psychosis [32]; Patil et al. [33] used a daily dose of 80 mg. Therefore, because D2 is a central target for antipsychotic action and anti‐Parkinson action, it is reasonable to consider whether any particular property of D2 may be related to dopamine supersensitivity and schizophrenia.
Figure 2.
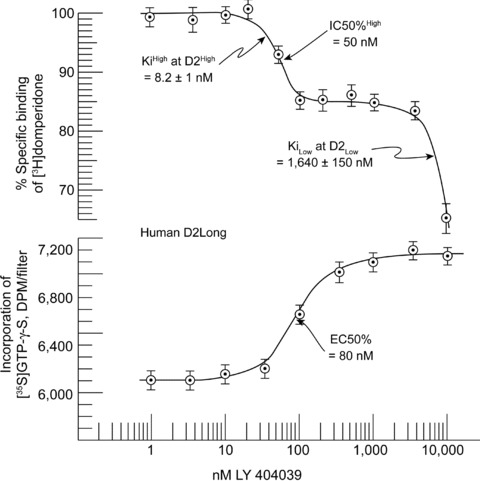
Top: The glutamate agonist LY404,039 inhibited the binding of 2 nM [3H]domperidone on dopamine D2Long receptors (in CHO cells). The inhibition occurred in two concentration phases of LY404,039, with 15.5% inhibition for the high‐affinity phase. 120 mM NaCl present. Data points are mean values (with SE; n = 3). The inhibition of 15.5% occurred at D2High receptors, all of which were converted to low‐affinity D2Low receptors in the presence of GN (200 μM guanylylimidodiphosphate). The dissociation constant, Ki High of LY404,039 was 8.2 ± 1 nM. Nonspecific binding was defined in the presence of 10 μM S‐sulpiride. Bottom: LY404,039 stimulated the incorporation of [35S]GTP‐γ‐S into dopamine D2Long receptors with 50% incorporation occurring at 80 ± 15 nM. The maximum amount of stimulation was 43% of that caused by 10 μM dopamine. The stimulation was blocked by 10 μM S‐sulpiride. Data points are means ± SE (n = 3). (Reproduced from [34], with permission of Wiley‐Liss, Inc.).
Is Dopamine Supersensitivity Related to D2 Density?
Since 1975 when the D2 receptor was discovered, it has been difficult to relate the total density of D2 receptors with behavioral dopamine supersensitivity. This is because sensitivity to a dopamine agonist increases 3‐fold after denervation or after long‐term antipsychotics, but the total density of D2 receptors increases by only 1.3‐fold. Moreover, even though most patients with schizophrenia are supersensitive to dopamine, the density of the total population of D2 receptors is elevated by only 1.4‐fold in postmortem human schizophrenia striatal tissues [35].
Apart from the modest elevations in D2 receptors in schizophrenia, a more relevant question is whether the functional state of D2, or D2High, is elevated in dopamine supersensitive animal models and in schizophrenia.
Is Dopamine Supersensitivity Related to D2High Receptors?
Dopamine has different affinities for the two states of D2. Dopamine attaches to D2High below 100 nM, and attaches to D2Low above 100 nM [36]. The D2High receptor is the functionally active state of the dopamine D2 receptor [37]. Antipsychotic drugs, however, have identical affinity for the high‐affinity and low‐affinity states of D2.
In the amphetamine‐sensitized animal model of psychosis, the animal is supersensitive to dopamine‐like drugs, but the density of D2 in the brain striatum is normal [36, 38]. Surprisingly, however, D2High receptors in the striatum were found to be markedly elevated, by 250%.
This type of elevation in D2High receptors also occurred in other animal models of psychosis [36, 38]. For example, mice with specific gene deletions have now been examined. A typical example is shown in Figure 3 for striata from mice with the metabotropic glutamate receptor‐2 (mGluR2) gene knocked out [39]. Such mice are dopamine‐supersensitive, and, while the D2 density in the striata is normal in these mice, the proportion of D2High receptors increased 3‐ or 4‐fold, as shown in Figure 3.
Figure 3.
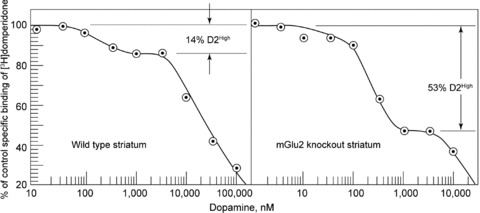
Left: Representative experiment on a control mouse striatal homogenate (average of duplicate measurements) showing competition between dopamine and [3H]domperidone for dopamine D2 receptors. The proportion of high‐affinity D2 receptors, D2High, was 14% in this tissue, with the average being 16.2 ± 2.5% in all the control tissues. The final concentration of [3H]domperidone was 2 nM. Nonspecific binding was defined by the presence of 1 μM S‐sulpiride. Right: Representative experiment showing competition between dopamine and [3H]domperidone for dopamine D2 receptors in a striatal homogenate from an mGlu2 receptor knockout mouse. The proportion of high‐affinity D2 receptors, D2High, was 53% in this tissue. The final concentration of [3H]domperidone was 2 nM. (Reproduced from [39] with permission of Wiley‐Liss, Inc.)
Additional data for many other animal models of schizophrenia are summarized in Figure 4. In general, the gene‐deleted mice (gene knockout mice) showed behavioral dopamine supersensitivity and revealed elevated levels of D2High receptors in the striata. Gene knockout mice with no behavioral supersensitivity did not reveal any elevation in the proportion of D2High receptors (Figure 4, right side).
Figure 4.
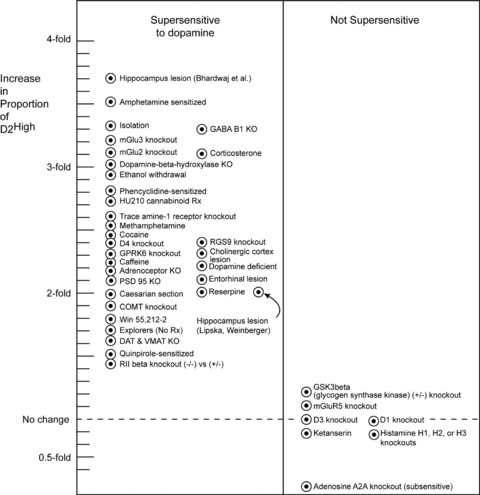
Animals that are supersensitive to dopamine‐like drugs (e.g., apomorphine, cocaine, methylphenidate, amphetamine) reveal elevated proportions of dopamine D2 receptors that are in the high‐affinity state for dopamine, D2High (left). The data summarized here were obtained on striata from animals found to be dopamine supersensitive under the following conditions (listed from the upper left top down; unless otherwise specified, details are found in [36, 38]: Mature rats with neonatal lesion of the hippocampus [46]. Rats sensitized by long‐term treatment with amphetamine. Rats socially isolated after weaning [40]. Knockouts of GABA B1(–/–) receptors in mice (B. Bettler and P. Seeman, unpublished). Knockouts of metabotropic glutamate mGlu3 receptors in mice [39]. Knockouts of metabotropic glutamate mGlu2 receptors in mice [39]. Five days of 10 mg/kg corticosterone treatment to rats. Knockouts of the DBH (dopamine‐beta hydroxylase) gene in mice. Long‐term ethanol treatment of rats [47]. Rats sensitized to phencyclidine. Rats treated for 14 days with cannabinoid HU210 at 20 μg/kg (Moreno et al., 2005; F.J. Bermudez Silva, F. Rodriguez de Fonseca, J. Suarez, and P. Seeman, unpublished). Knockouts of trace amine‐1 receptors in mice [48]. Rats sensitized by long‐term treatment with methamphetamine [49]. Rats sensitized and addicted by long‐term self‐treatment with cocaine [50]. Knockouts of the RGS9–2 (regulator of G protein signaling‐9) gene in mice. Knockouts of the dopamine D4 receptor gene in mice. Knockouts of the GPRK6 (G protein‐coupled receptor kinase) gene in mice. Cholinergic lesion in the cerebral cortex of rats. Long‐term high‐dose treatment of rats with caffeine [51]. Mice made dopamine‐deficient by tyrosine hydroxylase knockouts. Knockouts of alpha‐1b‐adrenoceptors in mice [52]. Rats with entorhinal lesions of the hippocampus [53]. Knockouts of PSD95 (postsynaptic density 95) gene in mice (M. Beaulieu, M. Caron, and P. Seeman, unpublished). Rats born by Caesarian section with anoxia. Rats treated with reserpine (5 mg/kg for 3 days; 2 days no drug). Mice with COMT (catechol‐O‐methyl transferase) gene knockouts. Rats with neonatal lesion of the hippocampus [54]. Rats treated with cannabinoid WIN 55,212–2 (4 mg/day for 14 days; F.J. Bermudez Silva, F. Rodriguez de Fonseca, J. Suarez, and P. Seeman, unpublished). Rats spontaneously active and explorative (no treatment)[55]. Mice with knockouts of dopamine transporter DAT or vesicle monoamine transporter‐2 VMAT‐2 [56]. Rats sensitized to quinpirole. Mice with knockouts of RIIbeta protein kinase A. The right side [36, 38] shows either the lack of elevation, a minor elevation, or an actual fall in the proportion of D2High receptors in mice with knockouts in the genes for glycogen synthase kinase (GSK3beta), metabotropic glutamate receptor mGluR5, dopamine D1 or D3 receptors, histamine H1, H2 or H3 receptors, and adenosine A2A receptors. Nine days of ketanserin treatment also had no effect on D2High receptors. (Adapted and extended from [38]; with permission from Wiley & Sons, Inc.; Reproduced from [31] with permission from Walsh Medical Media LLC).
Deletions of GABA, Glutamate, and Nondopamine Genes Increase D2High
Deletions of genes that are not related to the dopamine system also yield animal models of behavioral dopamine supersensitivity and at the same time reveal marked elevations in D2High receptors [36, 38]. These genes include those for the GABAB1 receptor, RIIβ protein kinase A, PSD95 (Post‐Synaptic Density protein 95), GPRK6 (G‐Protein Receptor Kinase 6), the trace amine‐1 receptor, and RGS9–2 (Regulator of G protein Signaling 9–2)(Figure 4).
Many gene knockouts, of course, do not result in dopamine supersensitivity, because knockouts of some genes, such as those for adenosine A2A receptors, lead to dopamine subsensitivity [36, 38]. In keeping with this reduction in dopamine sensitivity, the D2High receptors are reduced by 75% in the striata of adenosine A2A knockout mice. Similarly, mice with knockouts of the metabotropic glutamate receptor‐5 (mGluR5) are not supersensitive, and the proportion of D2High receptors does not increase (Figure 4).
Other Psychosis Models: Caesarian Birth with Anoxia, Psychostimulants, Social Isolation, Steroids
In another model for schizophrenia, adult rats that had been born by Caesarian section (with or without added anoxia) exhibit dopamine supersensitivity. Striata from these rats reveal a 2‐fold to 5‐fold elevation in the proportion of D2High receptors (Figure 4), but no increase in the total population of D1 or D2 receptors [36, 38].
Rats that have been sensitized by amphetamine, phencyclidine, quinpirole, or caffeine also become supersensitive to dopamine agonists. The striata from such supersensitive rats do not reveal any increase in dopamine D2 receptors, but show a 2‐fold to 4‐fold elevation in the proportion of D2High receptors (Figure 4).
Social isolation is a risk factor for psychosis, and rats isolated from birth reveal dopamine supersensitivity and elevated D2High receptors [40] (Figure 4).
Consistent with the hypothesis of D2High being the convergent target for various psychoses is the fact that most psychoses respond to treatment with D2 antagonists. This includes phencyclidine psychosis [32, 41]. The treatment of phencyclidine psychosis by haloperidol is significant, because haloperidol does not block NMDA receptors, indicating that the D2 target contributes to phencyclidine psychosis.
Measurement of D2High Receptors in Humans
Because D2High receptors are consistently elevated in the animal models of the various human psychoses, it appears reasonable to consider D2High a common target for the convergence of the various psychosis pathways. Moreover, it is reasonable to hypothesize that risk factors or altered genes that lead to dopamine supersensitivity can also increase the risk for psychosis or schizophrenia.
The elevated D2High receptors may be related to the clinical signs and symptoms of psychosis. Such a relation will need to be tested when the selective imaging of D2High in patients becomes possible by radioactive D2High‐selective agonists [42].
Do D2High States Exist In Vivo?
Despite data showing that high‐affinity and low‐affinity states of the D2 receptor can be detected in homogenized tissues, as well as the removal of D2High by guanine nucleotides, the existence of D2High states in vivo is less well established. For example, Sibley et al. [43] and Skinbjerg et al. [44] could not detect D2High sites in intact cells, although such sites could be detected in intact cells by others [45]. In particular, Skinbjerg et al. [44] found that dopamine inhibited the binding of [3H]sulpiride at a single binding site in intact tissue culture cells, but only detected D2High in homogenized cells with [3H]methylspiperone.
A search to detect D2High states in vivo by means of positron emission tomography is encountering inconsistencies. Intravenous amphetamine, for example, inhibited the binding of agonists (methoxy‐NPA or radioactive (+)PHNO) more than it inhibited the binding of an antagonist (raclopride) [57, 58].
However, Finnema et al. [59] found that apomorphine injected intravenously was about equally effective in displacing the agonist [11C]methoxy‐NPA and the antagonist [11C]raclopride, data that suggest that there is no distinction between the binding of agonist and antagonist to D2 and that there may be no detectable D2High state in vivo. McCormick et al. [60, 61] made a similar finding.
However, both Finnema et al. [59] and McCormick et al. [60, 61] injected the cold agonist (apomorphine or NPA) intravenously 3 or 30 min before the intravenous injection of the radioligands. This procedure may result in an identical pattern of inhibition of the radioactive agonist and the radioactive antagonist.
In contrast, Ross and Jackson [62] simultaneously co‐injected a dopamine agonist and the radioligand intravenously to measure D2 in vivo. By this method, Ross and Jackson successfully identified high‐affinity and low‐affinity D2 receptors occupied by the ligand but displaced by the agonist (such as pergolide), similarly to the pattern obtained in vitro.
Using the co‐injection method, therefore, it was found that an intravenous injection of the agonist NPA inhibited the binding of [3H](+)PHNO more than the D2 antagonist [3H]raclopride [63], as measured ex vivo. This finding supports the idea that the sites for the binding of agonist and antagonist differ and that D2High sites exist in vivo. Overall, the greater inhibition (by 17%) of [3H](+)PHNO than [3H]raclopride by NPA suggests that the additional inhibition of 17% may reflect competition at D2High receptors [63]. This would agree with in vitro work that shows that between 10% and 20% of the D2 population exists in the D2High state [36, 38]. In fact, the data of Finnema et al. [59] show that their lowest dose of 10 μg/kg apomorphine inhibited up to 15% more radioactive agonist than radioactive antagonist. This amount of 15% matches the proportion of D2 receptors that are normally in the D2High state.
The major biological difference between the pre‐injection method [60, 61] and the co‐injection method [62, 63] is that the co‐injection method ensures that the arrival of the ligand and the NPA agonist occur at precisely the same moment. Considering that a receptor agonist can rapidly desensitize a receptor or internalize receptors within seconds or minutes [64, 65], the arrival of NPA prior to that of [3H](+)PHNO would reduce the amount of D2High receptors available to [3H](+)PHNO.
Moreover, Seneca et al. [58] injected amphetamine 20 min before the radioligand; amphetamine‐released dopamine may here contribute to desensitization or receptor internalization with consequent fewer D2High states. In addition, the protocol of Finnema et al. [59] may also lead to a rapid desensitization or receptor internalization by apomorphine with a reduction in D2High states in the 3 min before the agonist radioligand arrives.
Finally, the injection of a G‐protein inhibitor directly into the striatum to block the G proteins that mediate dopamine‐inhibited cyclase attenuates apomorphine‐induced stereotyped behavior [66], demonstrating that the high‐affinity state of D2 may be the functional state in the nervous system.
It is also possible to demonstrate directly that an agonist ligand such as [3H](+)PHNO actually binds to D2High sites in vivo. This is shown in Figure 6, where guanine nucleotide accelerated the in vitro release of [3H]PHNO that had been injected immediately before the demise of the animal, indicating that a significant amount of this ligand attached to D2High receptors in vivo moments before the striatum was removed. Note that the high‐affinity state was reduced in matter of seconds by the nucleotide.
Figure 6.
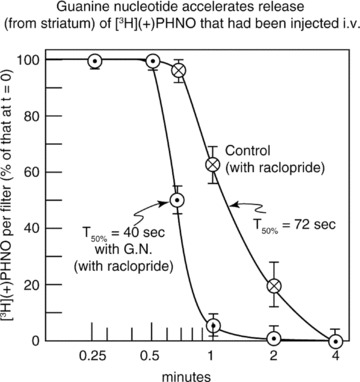
Showing that the agonist ligand, [3H](+)PHNO, binds to D2High receptors in vivo. Ten μCi of [3H](+)PHNO was injected into the rat tail vein. Five minutes later, the brain striatum was removed, rapidly homogenized, and the [3H](+)PHNO allowed to dissociate (in the presence of 200 μM raclopride to prevent re‐binding). The time for 50% dissociation of [3H](+)PHNO was 72 seconds. However, in the presence of 200 μM guanine nucleotide (GN) to convert the receptors into their D2Low state, the dissociation of [3H](+)PHNO was much more rapid with a 50% dissociation time of 40 seconds. The ability of GN to accelerate the release of [3H]PHNO indicated that a significant amount of this ligand had been attached to D2High receptors in vivo moments before the striatum was removed [Seeman, unpublished].
Multiple Pathways, Multiple Genes, Multiple Causes
If there are multiple neural pathways that mediate psychosis by converging onto a similar set of brain D2High targets, it suggests that there can be multiple causes and multiple genes associated with psychosis in general and schizophrenia in particular. It is even likely that different pedigrees have different sets of risk genes for schizophrenia.
Although the elevation of D2High receptors may be a necessary minimum for psychosis, it is not likely to be sufficient for full expression of psychotic features. For example, Hirvonen et al. [67] found D2 receptors elevated in healthy co‐twins of schizophrenia individuals, suggesting that the elevation of D2 was necessary but not sufficient for psychosis to develop. The elevation of D2 is becoming recognized as a valuable biomarker for prognosis and outcome in first‐episode psychosis [68].
While dopamine supersensitivity may be a basis for the positive signs and symptoms of psychosis, the biology underlying negative aspects of psychosis, such as cognition, is not known. Recent work, however, has found that overexpression of D2 in the striatum [69] leads to cognitive deficits in animals.
Elevation of D2High and the Dopamine Hypothesis of Schizophrenia
In summary, the overactivity of dopamine in schizophrenia [10, 71] is supported by the following evidence, including the elevation of D2High in animal models:
-
1
Antipsychotics, including partial agonist antipsychotics, reduce dopamine transmission by blocking D2 receptors in relation to their affinities for D2.
-
2
The reduction of dopamine transmission is relatively quick, a matter of a few days, and consistent with D2 blockade. Although patients with schizophrenia require 2 to 3 weeks before they are discharged from hospital, Delay et al. [72] observed that it only required about 3 days for chlorpromazine to attenuate the acute psychosis. Agid et al. [73, 74] and Kapur et al. [75] report that antipsychotic clinical effects have their onset within the first 24 h of administration, and reach a peak of improvement within the first few days or first week, supporting earlier reports [76, 77, 78, 79, 80, 81, 82].
-
3
Although antipsychotics have different profiles of receptor blockade, they share a common action in blocking D2 receptors at the predicted concentrations in plasma water [22]. Moreover, antipsychotics such as remoxipride and amisulpiride are highly selective for D2 receptors, largely precluding the contribution of other receptors to the antipsychotic clinical action.
-
4
Antipsychotic occupancies of D2 are between 60% and 80%[83, 84], with the exception of the aripiprazole which internalizes D2 receptors into the cytoplasm, and occupies D2 in excess of 90%. The antipsychotic occupancies of D3 and D4 receptors are highly variable, suggesting that these two receptors are not consistently targeted by antipsychotics [22].
Antipsychotics such as clozapine and quetiapine elicit negligible parkinsonism, even at relatively high doses. The blockade of serotonin‐2 receptors or stimulation of serotonin‐1 receptors may alleviate the parkinsonism of D2 blockade [85]. But when one calculates the ratio of the Ki values for D2 receptors divided by the Ki values for serotonin‐2A or ‐1 receptors, there does not appear to be any obvious relation between parkinsonism and the ratio of the Ki values [31](ratios can be obtained from Table 1).
A factor may be the speed of dissociation of antipsychotics from D2. For example, all eight antipsychotics that result in low or negligible parkinsonism (remoxipride, clozapine, quetiapine, norclozapine, perlapine, S‐(‐)‐amisulpiride, aripiprazole, and amoxapine) dissociate rapidly from the D2 receptor [22, 86, 87]. The attachment of clozapine, quetiapine, or amisulpiride to D2 is transient in the sense that these drugs quickly dissociate from D2 after 12 to 24 h. The patient's signs and symptoms, however, remain abated.
-
5
Although D2 receptors are elevated in postmortem brains of individuals who died with schizophrenia [20, 35, 88], findings with positron emission tomography in living patients did not find an elevation of D2 receptors [89, 90]. These negative results do not invalidate the dopamine hypothesis of schizophrenia, because Hirvonen et al. [67] and Corripio et al. [68] show that D2 receptors are elevated in individuals with schizophrenia who have never been treated with antipsychotic drugs.
Although the elevation of D2High in the animal models supports the dopamine hypothesis of schizophrenia, such D2High receptors have yet to be detected in humans.
-
6
Of the various polymorphisms in D2, the variation at amino acid 311 in D2 was found associated with schizophrenia in a meta‐analysis of 3,707 individuals [91, 92]. Allen et al. [93] found the silent polymorphism at amino acid position Proline319Proline (nucleotide 957) in D2 to be associated with schizophrenia (P < 0.00004).
-
7
Selective overactivity of D2 in the striatum can also be the basis of cognitive difficulties [69].
-
8
As noted, further support for the dopamine hypothesis is that the majority of individuals with schizophrenia are supersensitive to the dopamine‐like actions of amphetamine or methylphenidate [1][see also supersensitivity psychosis in 94, 95, 96, 97, 98, 99, 100].
-
9
Antipsychotic clinical effects are clearly related to the occupancy of dopamine D2 receptors in the striatal region and not in the extra‐striatal regions [101].
-
10
Although the binding of radioactive PHNO, a dopamine‐like agonist, is normal in schizophrenia patients [102], this study only used a single brain scan and did not measure the “displaceable PHNO.” The displaceable radio‐PHNO can be determined using two brain scans per patient: the first brain scan is taken when injecting radio‐PHNO alone, while the second brain scan entails co‐injecting radio‐PHNO and a low dose of apomorphine to block the attachment of radio‐PHNO. The difference between the two scans would represent the binding of radio‐PHNO to the D2High receptors. This co‐injection method has yet to be tried in control subjects and in individuals with schizophrenia.
-
11
It has been found that a glutamate agonist alleviates the signs and symptoms of schizophrenia [33], suggesting that there are nondopamine pathways to schizophrenia and to treatment [103]. However, these glutamate agonists, including LY404039, have a significant agonist affinity for D2High, and would, therefore, be expected to have an aripiprazole‐like agonist action [32]. Although Fell et al. [103] did not find a dopamine in vitro component of action for mGluR2 agonists, as compared to other studies [32, 34], the tissue membranes used by Fell et al. were extensively washed, a procedure known to cause a major loss of dopamine receptors [Refs. in 32 and 34].
-
12
Although it is known that long‐term use of antipsychotics can lead to tardive dyskinesia, dopamine supersensitivity, and antipsychotic‐induced supersensitivity psychosis [94, 95, 96, 97, 98, 99, 100], antipsychotics can reverse the elevation of D2High, as shown in Figure 5. The development of tardive dyskinesia depends on the long‐term accumulation of the antipsychotic in the neuromelanin of the substantia nigra, with secondary injury to the nigral cells and axon terminal sprouting [105].
Figure 5.
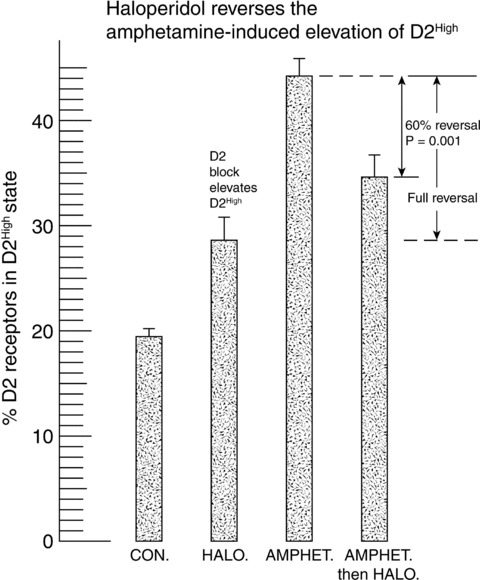
Elevation of D2High receptors by amphetamine, and reversal by haloperidol. The control level of D2High receptors was 19.5 ± 0.6% (N = 20 rats). Haloperidol alone (0.25 mg/kg/day i.p. for 9 days) raised D2High to 28.6 ± 2% (N = 6 rats), while amphetamine alone (1.6 mg/kg/day i.p. for 9 days followed by a drug holiday for 10 days) resulted in a D2High level of 44.2 ± 1.3% (N = 6 rats). Haloperidol (0.25 mg/kg/day for 9 days), given after amphetamine, brought D2High down to 34.8 ± 1.6%, a reversal of 60%, where a full reversal would have corresponded to the level brought about by haloperidol alone. (Reproduced from [70] with permission of Elsevier Inc. and Copyright Clearance Center.)
Finally, a summary of the factors elevating D2High and possibly leading to schizophrenia is shown in Figure 7.
Figure 7.
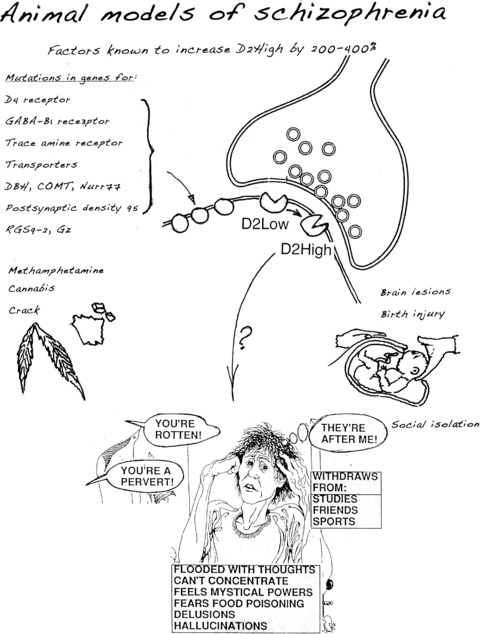
Summary of biological roads to schizophrenia [104]. Many risk factors lead to elevated D2High receptors and dopamine supersensitivity that underly signs and symptoms of schizophrenia (from [104], reproduced with permission from SZ Publications).
Conflict of Interest
The author is also affiliated with Clera, Inc., a pharmaceutical company, but has no other competing interests.
References
- 1. Lieberman JA, Kane JM, Alvir J. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology (Berl) 1987;91:415–433. [DOI] [PubMed] [Google Scholar]
- 2. Curran C, Byrappa N, McBride A. Stimulant psychosis: Systematic review. Brit J Psychiat 2004;185:196–204. [DOI] [PubMed] [Google Scholar]
- 3. Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: A critical review. J Cereb Blood Flow Metab 2000;20:423–451. [DOI] [PubMed] [Google Scholar]
- 4. Zemlan FP, Hirschowitz J, Garver DL. Relation of clinical symptoms to apomorphine‐stimulated growth hormone release in mood‐incongruent psychotic patients. Arch Gen Psychiat 1986;43:1162–1167. [DOI] [PubMed] [Google Scholar]
- 5. Seeman P, Nam D, Ulpian C, Liu IS, Tallerico T. New dopamine receptor, D2Longer, with unique TG splice site, in human brain. Brain Res Mol Brain Res 2000;76:132–141. [DOI] [PubMed] [Google Scholar]
- 6. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets 2006;10:515–531. [DOI] [PubMed] [Google Scholar]
- 7. Seeman P. Anti‐Parkinson therapeutic potencies correlate with their affinities for dopamine D2High receptors. Synapse 2007;61:1013–1018. [DOI] [PubMed] [Google Scholar]
- 8. Van Rossum JM. Different types of sympathomimetic alpha‐receptors. J Pharm Pharmacol 1965;17:202–216. [DOI] [PubMed] [Google Scholar]
- 9. Van Rossum JM. The significance of dopamine‐receptor blockade for the mechanism of action of neuroleptic drugs. Arch Int Pharmacodyn Ther 1966;160:492–494. [PubMed] [Google Scholar]
- 10. Van Rossum JM. The significance of dopamine‐receptor blockade for the action of neuroleptic drugs In: Brill H, Cole JO, Deniker P, Hippius H, Bradley PB, editors. Neuro‐Psycho‐Pharmacology, Proceedings of the Fifth International Congress of the Collegium Internationale Neuro‐Psycho‐pharmacologicum Amsterdam : Excerpta Medica Foundation, 1967;321–329. [Google Scholar]
- 11. Van Rossum JM, Hurkmans JAThM. Mechanism of action of psychomotor stimulant drugs. Significance of dopamine in locomotor stimulant action. Int J Neuropharmacol 1964;3:227–239. [DOI] [PubMed] [Google Scholar]
- 12. Seeman P, Wong M, Lee T. Dopamine receptor‐block and nigral fiber‐impulse blockade by major tranquilizers. Fed Proc 1974;33:246 Abstr 243. [Google Scholar]
- 13. Seeman P, Chau‐Wong M, Tedesco J, Wong K. Brain receptors for antipsychotic drugs and dopamine: Direct binding assays. Proc Natl Acad Sci USA 1975;72:4376–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seeman P, Lee T, Chau‐Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature 1976;261:717–719. [DOI] [PubMed] [Google Scholar]
- 15. Snyder SH, Creese I, Burt DR. The brain's dopamine receptor: Labeling with [3H]dopamine and [3H]haloperidol. Psychopharmacol Commun 1975;1:663–673. [PubMed] [Google Scholar]
- 16. Burt DR, Creese I, Snyder SH. Properties of [3H]haloperidol and [3H]dopamine binding associated with dopamine receptors in calf brain membranes. Mol Pharmacol 1976;12:800–812. [PubMed] [Google Scholar]
- 17. Bunzow JR, Van Tol HH, Grandy DK, et al Cloning and expression of a rat D2 dopamine receptor cDNA. Nature 1988;336:783–787. [DOI] [PubMed] [Google Scholar]
- 18. Spano PF, Govoni S, Trabucchi M. Studies on the pharmacological properties of dopamine receptors in various areas of the central nervous system. Adv Biochem Psychopharmacol 1978;19:155–165. [PubMed] [Google Scholar]
- 19. Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature 1979;277:93–96. [DOI] [PubMed] [Google Scholar]
- 20. Seeman P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse 1987;1:133–152. [DOI] [PubMed] [Google Scholar]
- 21. Seeman P. Dopamine receptor sequences. Therapeutic levels of neuroleptics occupy D2, clozapine occupies D4. Neuropsychopharmacology 1992;7:261–284. [PubMed] [Google Scholar]
- 22. Seeman P. Atypical antipsychotics: Mechanism of action. Can J Psychiatry 2002;47:27–38. [PubMed] [Google Scholar]
- 23. Seeman P. Dopamine and schizophrenia. Scholarpedia 2007;2:3634 Available from: http://www.scholarpedia.org/article/Dopamine_and_schizophrenia [Accessed 12 April 2010. [Google Scholar]
- 24. Graff‐Guerrero A, Mamo D, Shammi CM, et al The effect of antipsychotics on the high‐affinity state of D2 and D3 receptors: A positron emission tomography study with [11C]‐(+)‐PHNO. Arch Gen Psychiatry 2009;66:606–615. [DOI] [PubMed] [Google Scholar]
- 25. Lecrubier Y. A partial D3 receptor agonist in schizophrenia. Neuropsychopharmacology 2003;13:S167–S168. [Google Scholar]
- 26. Seeman P. Antipsychotic drugs, dopamine receptors, and schizophrenia. Clin Neurosci Res 2001;1:53–60. [Google Scholar]
- 27. Van Tol HHM, Bunzow JR, Guan H‐C, Sunahara RK, Seeman P, Niznik HB, Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature 1991;350:610–614. [DOI] [PubMed] [Google Scholar]
- 28. Seeman P, Corbett R, Van Tol HH. Atypical neuroleptics have low affinity for dopamine D2 receptors or are selective for D4 receptors. Neuropsychopharmacology 1997;16:93–110. [DOI] [PubMed] [Google Scholar]
- 29. Seeman P, Ulpian C, Wreggett KA, Wells J. Dopamine receptor parameters detected by 3H‐spiperone depend on tissue concentration: Analysis and examples. J Neurochem 1984;43:221–235. [DOI] [PubMed] [Google Scholar]
- 30. Seeman P, Van Tol HHM. Deriving the therapeutic concentrations for clozapine and haloperidol: The apparent dissociation constant of a neuroleptic at the dopamine D2 or D4 receptor varies with the affinity of the competing radioligand. Eur J Pharmacol–Mol Pharmacol Section 1995;291:59–66. [DOI] [PubMed] [Google Scholar]
- 31. Seeman P. Dopamine D2 receptors as treatment targets in schizophrenia. Clin Schizophrenia & Related Psychoses 2010;4:56–73. [PubMed] [Google Scholar]
- 32. Seeman P. Glutamate and dopamine components in schizophrenia. J Psychiatry Neurosci 2009;34:143–149. [PMC free article] [PubMed] [Google Scholar]
- 33. Patil ST, Zhang L, Martenyi F, et al Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: A randomized Phase 2 clinical trial. Nat Med 2007;13:1102–1107. [DOI] [PubMed] [Google Scholar]
- 34. Seeman P, Guan H‐C. Glutamate agonist LY404039 for treating schizophrenia has affinity for the dopamine D2High receptor. Synapse 2009;63:935–939. [DOI] [PubMed] [Google Scholar]
- 35. Seeman P, Ulpian C, Bergeron C, et al Bimodal distribution of dopamine receptor densities in brains of schizophrenics. Science 1984;225:728–731. [DOI] [PubMed] [Google Scholar]
- 36. Seeman P, Schwarz J, Chen JF, et al Psychosis pathways converge via D2high dopamine receptors. Synapse 2006;60:319–346. [DOI] [PubMed] [Google Scholar]
- 37. George SR, Watanabe M, Di Paolo T, Falardeau P, Labrie F, Seeman P. The functional state of the dopamine receptor in the anterior pituitary is in the high‐affinity form. Endocrinology 1985;117:690–697. [DOI] [PubMed] [Google Scholar]
- 38. Seeman P, Weinshenker D, Quirion R, et al Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci USA 2005;102:3513–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seeman P, Battaglia G, Corti C, Corsi M, Bruno V. Glutamate receptor mGlu2 and mGlu3 knockout striata are dopamine supersensitive, with elevated D2High receptors and marked supersensitivity to the dopamine agonist (+)PHNO. Synapse 2009;63:247–251. [DOI] [PubMed] [Google Scholar]
- 40. King MV, Seeman P, Marsden CA, Fone KC. Increased dopamine D2High receptors in rats reared in social isolation. Synapse 2009;63:476–483. [DOI] [PubMed] [Google Scholar]
- 41. Giannini AJ, Nageotte C, Loiselle RH, Malone DA, Price WA. Comparison of chlorpromazine, haloperidol and pimozide in the treatment of phencyclidine psychosis: DA‐2 receptor specificity. J Toxicol Clin Toxicol 1984;22:573–579. [DOI] [PubMed] [Google Scholar]
- 42. Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P, Wilson AA. High‐affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]‐(+)‐PHNO. Biol Psychiat 2006;59:389–394. [DOI] [PubMed] [Google Scholar]
- 43. Sibley DR, Mahan LC, Creese I. Dopamine receptor binding on intact cells. Absence of a high‐affinity agonist‐receptor binding state. Mol Pharmacol 1983;23:295–302. [PubMed] [Google Scholar]
- 44. Skinbjerg M, Namkung Y, Halldin C, Innis RB, Sibley DR. Pharmacological characterization of 2‐methoxy‐N‐propylnorapomorphine's interactions with D2 and D3 dopamine receptors. Synapse 2009;63:462–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seeman P. Dopamine D2High receptors on intact cells. Synapse 2008;62:314–318. [DOI] [PubMed] [Google Scholar]
- 46. Bhardwaj SK, Beaudry G, Quirion R, Levesque D, Srivastava LK. Neonatal ventral hippocampus lesion leads to reductions in nerve growth factor inducible‐B mRNA in the prefrontal cortex and increased amphetamine response in the nucleus accumbens and dorsal striatum. Neuroscience 2003;122:669–676. [DOI] [PubMed] [Google Scholar]
- 47. Seeman P, Tallerico T, Ko F. Alcohol‐withdrawn animals have a prolonged increase in dopamine D2high receptors, reversed by general anesthesia: Relation to relapse? Synapse 2004;52:77–83. [DOI] [PubMed] [Google Scholar]
- 48. Wolinsky TD, Swanson CJ, Smith KE, et al The Trace Amine 1 receptor knockout mouse: An animal model with relevance to schizophrenia. Genes Brain Behav 2007;6:628–639. [DOI] [PubMed] [Google Scholar]
- 49. Shuto T, Seeman P, Kuroiwa M, Nishi A. Repeated administration of a dopamine D1 receptor agonist reverses the increased proportions of striatal dopamine D1High and D2High receptors in methamphetamine‐sensitized rats. Eur J Neurosci 2008;27:2551–2557. [DOI] [PubMed] [Google Scholar]
- 50. Briand LA, Flagel SB, Seeman P, Robinson TE. Cocaine self‐administration produces a persistent increase in dopamine D2High receptors. Eur Neuropsychopharmacol 2008;18:551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Simola N, Morelli M, Seeman P. Increase of dopamine D2High receptors in the striatum of rats sensitized to caffeine motor effects. Synapse 2008;62:394–397. [DOI] [PubMed] [Google Scholar]
- 52. Tassin JP, Torrens Y, Salomon L, Lanteri C, Seeman P. Elevated dopamine D2High receptors in alpha‐1b‐adrenoceptor knockout supersensitive mice. Synapse 2007;61:569–572. [DOI] [PubMed] [Google Scholar]
- 53. Sumiyoshi T, Seeman P, Uehara T, Itoh H, Tsunoda M, Kurachi M. Increased proportion of high‐affinity dopamine D2 receptors in rats with excitotoxic damage of the entorhinal cortex, an animal model of schizophrenia. Brain Res Mol Brain Res 2005;140:116–119. [DOI] [PubMed] [Google Scholar]
- 54. Lipska BK, Lerman DN, Khaing ZZ, Weinberger DR. The neonatal ventral hippocampal lesion model of schizophrenia: Effects on dopamine and GABA mRNA markers in the rat midbrain. Eur J Neurosci 2003;18:3097–3104. [DOI] [PubMed] [Google Scholar]
- 55. Alttoa A, Seeman P, Kõiv K, Eller M, Harro J. Rats with persistently high exploratory activity have both higher extracellular dopamine levels and higher proportion of D2High receptors in the striatum. Synapse 2009;63:443–446. [DOI] [PubMed] [Google Scholar]
- 56. Seeman P, Hall FS, Uhl G. Increased dopamine D2High receptors in knockouts of the dopamine transporter and the vesicular monoamine transporter may contribute to spontaneous hyperactivity and dopamine supersensitivity. Synapse 2007;61:573–576. [DOI] [PubMed] [Google Scholar]
- 57. Ginovart N, Galineau L, Willeit M, et al Binding characteristics and sensitivity to endogenous dopamine of [11C]‐(+)‐PHNO, a new agonist radiotracer for imaging the high‐affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem 2006;97:1089–1103. [DOI] [PubMed] [Google Scholar]
- 58. Seneca N, Finnema SJ, Farde L, Gulyás B, Wikström HV, Halldin C, Innis RB. Effect of amphetamine on dopamine D2 receptor binding in nonhuman primate brain: A comparison of the agonist radioligand [11C]MNPA and antagonist [11C]raclopride. Synapse 2006;59:260–269. [DOI] [PubMed] [Google Scholar]
- 59. Finnema SJ, Halldin C, Bang‐Andersen B, Gulyás B, Bundgaard C, Wikström HV, Farde L. Dopamine D2 /3 receptor occupancy of apomorphine in the nonhuman primate brain–a comparative PET study with [11C]raclopride and [11C]MNPA. Synapse 2009;63:378–389. [DOI] [PubMed] [Google Scholar]
- 60. McCormick PN, Kapur S, Seeman P, Wilson AA. Dopamine D2 receptor radiotracers [11C](+)‐PHNO and [3H]raclopride are indistinguishably inhibited by D2 agonists and antagonists ex vivo. Nucl Med Biol 2008;35:11–17. [DOI] [PubMed] [Google Scholar]
- 61. McCormick PN, Kapur S, Reckless G, Wilson AA. Ex vivo[11C]‐(+)‐PHNO binding is unchanged in animal models displaying increased high‐affinity states of the D2 receptor in vitro. Synapse 2009;63:998–1009. [DOI] [PubMed] [Google Scholar]
- 62. Ross SB, Jackson DM. Kinetic properties of the in vivo accumulation of 3H‐(‐)‐N‐n‐propylnorapomorphine in mouse brain. Naunyn Schmiedebergs Arch Pharmacol 1989;340:13–20. [DOI] [PubMed] [Google Scholar]
- 63. Seeman P. Dopamine D2High receptors measured ex vivo are elevated in amphetamine‐sensitized animals. Synapse 2009;63:186–192. [DOI] [PubMed] [Google Scholar]
- 64. von Zastrow M, Kobilka BK. Ligand‐regulated internalization and recycling of human beta 2‐adrenergic receptors between the plasma membrane and endosomes containing transferrin receptors. J Biol Chem 1992;267:3530–3538. [PubMed] [Google Scholar]
- 65. Insel PA, Mahan LC, Motulsky HJ, Stoolman LM, Koachman AM. Time‐dependent decreases in binding affinity of agonists for beta‐adrenergic receptors of intact S49 lymphoma cells. A mechanism of desensitization. J Biol Chem 1983;258:13597–13605. [PubMed] [Google Scholar]
- 66. Fujita N, Nakahiro M, Fukuchi I, Saito K, Yoshida H. Effects of pertussis toxin on D2‐dopamine receptor in rat striatum: Evidence for coupling of Ni regulatory protein with D2‐receptor. Brain Res 1985;333:231–236. [DOI] [PubMed] [Google Scholar]
- 67. Hirvonen J, van Erp TG, Huttunen J, et al Increased caudate dopamine D2 receptor availability as a genetic marker for schizophrenia. Arch Gen Psychiatry 2005;62:371–378. [DOI] [PubMed] [Google Scholar]
- 68. Corripio I, Perez V, Catafau AM, Mena E, Carrio I, Alvarez E. Striatal D2 receptor binding as a marker of prognosis and outcome in untreated first‐episode psychosis. Neuroimage 2006;29:662–666. [DOI] [PubMed] [Google Scholar]
- 69. Kellendonk C, Simpson EH, Polan HJ, et al Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron 2006;49:603–615. [DOI] [PubMed] [Google Scholar]
- 70. Seeman P. Schizophrenia model of elevated D2High receptors: Haloperidol reverses the amphetamine‐induced elevation in dopamine D2High . Schizophrenia Res 2009;91:191–192. [DOI] [PubMed] [Google Scholar]
- 71. Baumeister AA, Francis JL. Historical development of the dopamine hypothesis of schizophrenia. J Hist Neurosci 2002;11:265–277. [DOI] [PubMed] [Google Scholar]
- 72. Delay J, Deniker P, Harl J‐M. Traitement des états d’excitation et d’agitation par une méthode médicamenteuse dérivée de l’hibernithérapie. [Therapeutic method derived from hiberno‐therapy in excitation and agitation states]. Ann Méd-Psychol (Paris) 1952;110:267–273. [PubMed] [Google Scholar]
- 73. Agid O, Kapur S, Arenovich T, Zipursky RB. Delayed‐onset hypothesis of antipsychotic action: A hypothesis tested and rejected. Arch Gen Psychiatry 2003;60:1228–1235. [DOI] [PubMed] [Google Scholar]
- 74. Agid O, Seeman P, Kapur S. The ‘delayed onset’ of antipsychotic action – an idea whose time has come and gone. J Psychiatry Neurosci 2006;31:93–100. [PMC free article] [PubMed] [Google Scholar]
- 75. Kapur S, Arenovich T, Agid O, Zipursky R, Lindborg S, Jones B. Evidence for onset of antipsychotic effects within the first 24 hours of treatment. Am J Psychiatry 2005;162:939–946. [DOI] [PubMed] [Google Scholar]
- 76. Leucht S, Busch R, Hamann J, Kissling W, Kane JM. Early‐onset hypothesis of antipsychotic drug action: A hypothesis tested, confirmed and extended. Biol Psychiatry 2005;57:1543–1549. [DOI] [PubMed] [Google Scholar]
- 77. Anderson WH, Kuehnle JC, Catanzano DM. Rapid treatment of acute psychosis. Am J Psychiatry 1976;133:1076–1078. [DOI] [PubMed] [Google Scholar]
- 78. Carter RG. Psychotolysis with haloperidol. Rapid control of the acutely disturbed psychotic patient. Dis Nerv Syst. 1977;38:237–239. [PubMed] [Google Scholar]
- 79. Glovinsky D, Kirch DG, Wyatt RJ. Early antipsychotic response to resumption of neuroleptics in drug‐free chronic schizophrenic patients. Biol Psychiatry 1992;31:968–970. [DOI] [PubMed] [Google Scholar]
- 80. Keck PE Jr., Cohen BM, Baldessarini RJ, McElroy SL. Time course of antipsychotic effects of neuroleptic drugs. Am J Psychiatry 1989;146:1289–1292. [DOI] [PubMed] [Google Scholar]
- 81. Emsley R, Rabinowitz J, Medori R. Time course for antipsychotic treatment response in first‐episode schizophrenia. Am J Psychiatry 2006;163:743–745. [DOI] [PubMed] [Google Scholar]
- 82. Lieberman J, Jody D, Geisler S, et al Time course and biologic correlates of treatment response in first‐episode schizophrenia. Arch Gen Psychiatry 1993;50:369–376. [DOI] [PubMed] [Google Scholar]
- 83. Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry 1992;49:538–544. [DOI] [PubMed] [Google Scholar]
- 84. Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5‐HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry 1999;156:286–293. [DOI] [PubMed] [Google Scholar]
- 85. Meltzer HY, Huang M. In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog Brain Res 2008;172:177–197. [DOI] [PubMed] [Google Scholar]
- 86. Seeman P, Tallerico T. Rapid release of antipsychotic drugs from dopamine D2 receptors: An explanation for low receptor occupancy and early clinical relapse upon drug withdrawal of clozapine or quetiapine. Am J Psychiat 1999;156:876–884. [DOI] [PubMed] [Google Scholar]
- 87. Kapur S, Seeman P. Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics?– A new hypothesis. Am J Psychiatry 2001;158:360–369. [DOI] [PubMed] [Google Scholar]
- 88. Lee T, Seeman P. Elevation of brain neuroleptic/dopamine receptors in schizophrenia. Am J Psychiatry 1980;137:191–197. [DOI] [PubMed] [Google Scholar]
- 89. Farde L, Wiesel FA, Stone‐Elander S, Halldin C, Nordström AL, Hall H, Sedvall G. D2 dopamine receptors in neuroleptic‐naive schizophrenic patients. A positron emission tomography study with [11C]raclopride. Arch Gen Psychiatry 1990;47:213–219. [DOI] [PubMed] [Google Scholar]
- 90. Nordström AL, Farde L, Eriksson L, Halldin C. No elevated D2 dopamine receptors in neuroleptic‐naive schizophrenic patients revealed by positron emission tomography and [11C]N‐methylspiperone. Psychiatry Res 1995;61:67–83. [DOI] [PubMed] [Google Scholar]
- 91. Glatt SJ, Faraone SV, Tsuang MT. Meta‐analysis identifies an association between the dopamine D2 receptor gene and schizophrenia. Mol Psychiat 2003;8:911–915. [DOI] [PubMed] [Google Scholar]
- 92. Glatt SJ, Jönsson EG. The Cys allele of the DRD2 Ser311Cys polymorphism has a dominant effect on risk for schizophrenia: Evidence from fixed‐ and random‐effects meta‐analyses. Am J Med Genet B Neuropsychiatr Genet 2006;141:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Allen NC, Bagade S, McQueen MB, et al Systematic meta‐analyses and field synopsis of genetic association studies in schizophrenia: The SzGene database. Nat Genet 2008;40:827–834. [DOI] [PubMed] [Google Scholar]
- 94. Chouinard G, Jones BD, Annable L. Neuroleptic‐induced supersensitivity psychosis. Am J Psychiatry 1978;135:1409–1410. [DOI] [PubMed] [Google Scholar]
- 95. Chouinard G. Severe cases of neuroleptic‐induced supersensitivity psychosis. Diagnostic criteria for the disorder and its treatment. Schizophr Res 1991;5:21–33. [DOI] [PubMed] [Google Scholar]
- 96. Chouinard G, Jones BD. Schizophrenia as dopamine‐deficiency disease. Lancet 1978; II :99–100. [DOI] [PubMed] [Google Scholar]
- 97. Chouinard G, Jones BD. Evidence of brain dopamine deficiency in schizophrenia. Can J Psychiatr 1979;24:661–667. [DOI] [PubMed] [Google Scholar]
- 98. Miller R, Chouinard G. Loss of striatal cholinergic neurons as a basis of tardive and L‐dopa‐induced dyskinesias, and neuroleptic‐induced supersensitivity psychosis. Biol Psychiatry 1993;34:713–738. [DOI] [PubMed] [Google Scholar]
- 99. Chouinard G, Miller R. A new Rating Scale for Psychotic Symptoms (RSPS). Part I. Theoretical Principles and Subscale 1: Perception Symptoms (Hallucinations and Illusions). Schizophrenia Res 1999;38:101–122. [DOI] [PubMed] [Google Scholar]
- 100. Chouinard G, Chouinard VA. Atypical antipsychotics: CATIE study, drug‐induced movement disorder and resulting iatrogenic psychiatric‐like symptoms, supersensitivity rebound psychosis and withdrawal discontinuation syndromes. Psychother Psychosom 2008;77:69–77. [DOI] [PubMed] [Google Scholar]
- 101. Mizrahi R, Rusjan P, Agid O, Graff A, Mamo DC, Zipursky RB, Kapur S. Adverse subjective experience with antipsychotics and its relationship to striatal and extrastriatal D2 receptors: A PET study in schizophrenia. Am J Psychiatry 2007;164:630–637. [DOI] [PubMed] [Google Scholar]
- 102. Graff‐Guerrero A, Mizrahi R, Agid O, et al The dopamine D2 receptors in high‐affinity state and D3 receptors in schizophrenia: A clinical [11C]‐(+)‐PHNO PET study. Neuropsychopharmacology 2009;34:1078–1086. [DOI] [PubMed] [Google Scholar]
- 103. Fell MJ, Perry KW, Falcone JF, et al In vitro and in vivo evidence for a lack of interaction with dopamine D2 receptors by the metabotropic glutamate 2/3 receptor agonists 1S,2S,5R,6S‐2‐aminobicyclo[3.1.0]hexane‐2,6‐bicaroxylate monohydrate (LY354740) and (‐)‐2‐oxa‐4‐aminobicyclo[3.1.0] Hexane‐4,6‐dicarboxylic acid (LY379268). J Pharmacol Exp Ther 2009;331:1126–1136. [DOI] [PubMed] [Google Scholar]
- 104. Seeman N, Seeman P. Psychosis: Discovery of the antipsychotic receptor. Toronto : SZ Publications, 2009;148. [Google Scholar]
- 105. Seeman P. Tardive dyskinesia, dopamine receptors, and neuroleptic damage to cell membranes. J Clin Psychopharmacol 1988;8(4 Suppl):3S–9S. [DOI] [PubMed] [Google Scholar]


