Abstract
The interaction between serotonin (5‐HT) and dopamine (DA)‐containing neurons in the brain is a research topic that has raised the interest of many scientists working in the field of neuroscience since the first demonstration of the presence of monoamine‐containing neurons in the mid 1960. The bulk of neuroanatomical data available clearly indicate that DA‐containing neurons in the brain receive a prominent innervation from serotonin (5‐hydroxytryptamine, 5‐HT) originating in the raphe nuclei of the brainstem. Compelling electrophysiological and neurochemical data show that 5‐HT can exert complex effects on the activity of midbrain DA neurons mediated by its various receptor subtypes. The main control seems to be inhibitory, this effect being more marked in the mesocorticolimbic DA system as compared to the DA nigrostriatal system. In spite of a direct effect of 5‐HT by its receptors located on DA cells, 5‐HT can modulate their activity indirectly, modifying γ‐aminobutyric (GABA)‐ergic and glutamatergic input to the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc). Although 5‐HT/DA interaction in the brain has been extensively studied, much work remains to be done to clarify this issue. The recent development of subtype‐selective ligands for 5‐HT receptors will not only allow a detailed understanding of this interaction but also will lead to the development of new treatment strategies, appropriate for those neuropsychiatric disorders in which an alteration of the 5‐HT/DA balance is supposed.
Keywords: Antidepressants, Antipsychotics, Dopaminergic function, Drug addiction, 5‐HT receptors, Mesocorticolimbic DA system, Microdialysis, Nigrostriatal DA system, Parkinson disease, Single cell recording
Introduction
Dopamine (DA)‐containing neurons of the ventral mesencephalon have been designated as A8, A9, and A10 cell groups: these neurons can be collectively designated as the mesotelencephalic DA system [1]. Historically, the mesolimbic DA system was defined as originating in the A10 cells of the ventral tegmental area (VTA) and projecting to structures closely associated with the limbic system. This system was considered to be separated from the nigrostriatal DA system, which originates from the more lateral substantia nigra pars compacta (SNc)(A9 cell group) [1, 2, 3, 4, 5] (Figure 1). The mesolimbic and mesocortical DA system appear critically involved in modulation of the functions subserved by cortical and limbic regions, such as motivation, emotional control, and cognition [6]. Substantial evidence indicates that the mesolimbic pathway, particularly the DA cells innervating accumbal areas, is implicated in the reward value of both natural and drug reinforcers, such as sexual behavior or psychostimulants, respectively [7, 8, 9]. The medial prefrontal cortex (mPFC) is generally associated with cognitive functions including working memory, planning and esecution of behavior, inhibitory response control, and maintenance of focused attention [6]. In addition, the mesolimbic DA pathway is sensitive to a variety of physical and psychological stressors [10]. Indeed, recent studies have indicated that stress‐induced activation of the mesocortical DA neurons may be obligatory for the behavioral expression of such stimuli [11].
Figure 1.
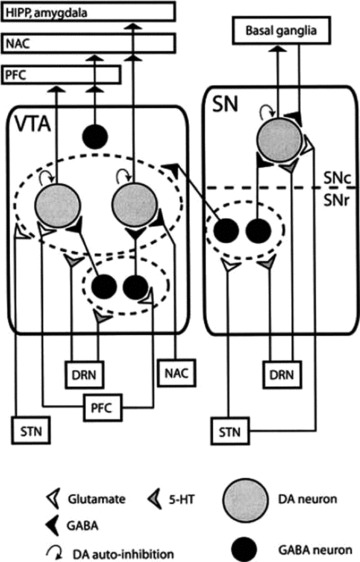
A schematic view of SN and VTA connections. DA neurons receive input from the local GABA‐ergic interneurons and input from other brain areas. Both areas receive serotonergic input from the DEN. The dotted line indicates the subdivision of the SN: the SN pars reticulata (SNr) and the SN pars compacta (SNC). Neurons receiving a collective input are grouped (dotted circles). GABA‐ergic, glutamatergic and serotonergic inputs are indicated by black, white and gray symbols, respectively. HIPP, hippocampus; NAC, nucleus accumbens; PFC, prefrontal cortex; STN, subthalamic nucleus.
The nigrostriatal DA system, which originates from the substantia nigra (A9 cell group), is one of the best studied because of its involvement in the pathogenesis of Parkinson's disease [12]. In mammals, the substantia nigra is a heterogeneous structure that includes two distinct compartments: the SNc and the substantia nigra pars reticulata (SNr). The SNc represent the major source of striatal DA and, as already mentioned, its degeneration causes Parkinson's disease. On the contrary, the SNr mainly contains γ‐aminobutyric (GABA)‐ergic neurons which constitute one of the major efferences of the basal ganglia [12]. These DA‐ergic systems receive innervation from serotonergic (5‐HT) fibers arising from cell bodies of the two main subdivisions of the midbrain 5‐HT‐ergic nuclei, the dorsal raphe nuclei (DRN) and the medial raphe (MRN) [13, 14, 15, 16, 17, 18, 19]. Serotonin‐containing cell bodies of the raphe nuclei send projections to dopaminergic cells both in the VTA and the SN, and to their terminal fields in the nucleus accumbens, prefrontal cortex, and striatum [14, 15, 16, 17, 18, 19] (Figure 1). Moreover, electron microscopy demonstrates the presence of synaptic contacts of [3H]5‐HT labeled terminals with both DA‐ergic and non‐DA‐ergic dendrites in all subnuclei of the VTA, and the SN pars compacta and reticulata [4, 16, 19] (Figure 1). The diverse physiological effects of serotonin in the brain are mediated by a variety of distinct receptors. These receptors are presently divided into seven classes (5‐HT1–5‐HT7), which are then subdivided into subclasses with a total of at least 14 different receptors, based upon their pharmacological profiles, cDNA‐deduced primary sequences and signal transduction mechanisms [20, 21, 22] (Figure 2). Several investigators began to study the behavioral, biochemical, and electrophysiological relevance of these neuroanatomical findings. This research was further spurred by the increasing evidences that most psychotropic drugs, including antidepressants, antipsychotics, opioids, anxiolytics, and psychostimulants exerted their pharmacological actions by interfering with 5‐HT‐ergic and DA‐ergic transmission.
Figure 2.
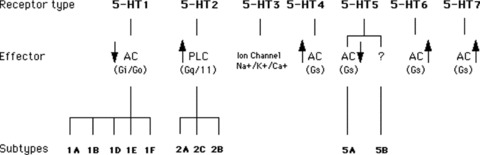
Serotonin receptors.
Early studies showed that experimental manipulations aimed at decreasing central 5‐HT function, such as selective nerve lesions by neurotoxin, inhibition of 5‐HT synthesis, or 5‐HT receptor blockade tended to potentiate the behavioral and neurochemical effects of drugs enhancing the dopaminergic transmission such as amphetamine and related compounds, thus leading to the conclusion that central serotonergic systems inhibits DA functions [23]. The interest of our laboratory to investigate 5‐HT/DA interaction sprang from these early studies. Our approach was based on in vivo electrophysiological and neurochemical techniques. The experimental data gathered over a period of almost 20 years lead to the conclusion that several 5‐HT receptor subtypes, including the 5‐HT1A, 5‐HT1B, 5‐HT2A, 5‐HT3, and 5‐HT4 receptors, act to facilitate neuronal DA function and release, while the 5‐HT2C receptor mediates an inhibitory effect of 5‐HT on the basal electrical activity of dopaminergic neurons and on DA release.
Central serotonergic and dopaminergic systems play an important role in regulating normal and abnormal behaviors [24, 25]. Moreover, dysfunctions of 5‐HT and DA neurotransmission are involved in the pathophysiology of various neuropsychiatric disorders including schizophrenia, depression, and drug abuse [24, 25, 26]. Thus, the development of a number of relatively selective pharmacological agents with agonist or antagonist activity at a specific 5‐HT receptor subtype, has allowed investigators to better understand the functional role of these receptors in the control of central DA‐ergic function, as serotonin widely contributes to the regulation of a number of behavioral and physiological processes involving both limbic, cortical, and striatal DA pathways [27, 28, 29, 30].
Serotonin–Dopamine Interactions in Neuropsychiatric Disorders
Depression
Although dopamine has received little attention in biological research on depression, as compared to other monoamines such as serotonin and noradrenaline, current research on the dopaminergic system is about to change this situation. It is now well‐established that disturbances of mesolimbic and nigrostriatal DA function are involved in the pathophysiology of depression [25, 26]. Moreover, stress promotes profound and complex alterations involving DA release, metabolism, and receptor densities in the mesolimbic system [31, 32]. It seems that exposure to unavoidable/uncontrollable aversive experiences leads to inhibition of DA release in the mesoaccumbens DA system as well as impaired responding to rewarding and aversive stimuli. These alterations could elicit stress‐induced expression and exacerbation of some depressive symptoms in humans [32]. Thus, in view of the hypothesis that disinhibition of the mesocorticolimbic DA system underlies the mechanism of action of several antidepressant drugs, the disinhibitory effect of SB 206553 and SB 242084 on the mesolimbic DA system might open new possibilities for the employment of 5‐HT2C receptor antagonists as antidepressants [33, 34]. This hypothesis is consistent with the suggestion that 5‐HT2C receptor blockers might exert antidepressant activity [27, 29, 34, 35, 36, 37]. In this respect, it is interesting to note that several antidepressant drugs have been shown to bind with submicromolar affinity to 5‐HT2C receptors in the pig brain and to antagonize mCPP‐induced penile erections in rats, an effect mediated through the stimulation of central 5‐HT2C receptors [37, 38, 39]. Based on those findings, Matteo et al. [34] have carried out experiments showing that acute administration of amitriptyline and mianserin, two antidepressants with high affinity for 5‐HT2C receptors, enhances DA release in the rat nucleus accumbens by blocking these receptor subtypes, in addition to their other pharmacological properties. Interestingly, amitriptyline and mianserin have been tested in the chronic mild stress‐induced anhedonia model of depression and were found to be effective in reversing the stress effects [40, 41]. The antianhedonic effects of tricyclic antidepressants, mianserin, and fluoxetine were blocked by pretreatment with D2/D3 receptor antagonists, thus indicating an involvement of DA in the antidepressant effect of various drugs in this model [40, 42]. The ability of antidepressants, such as tricyclics, SSRIs, and mianserin, to affect DA systems, via indirect mechanisms, was also reported by studies of Tanda et al. [43, 44] suggesting that potentiation of DA release in the rat cortex may play a role in the therapeutic action of antidepressants. The chronic mild stress procedure, which induces a depression‐like state in animals, was shown to enhance 5‐HT2C receptor‐mediated function, as measured in vivo by mCPP induced penile erections. In contrast, two different antidepressant treatments (72‐h REM sleep deprivation and 10‐day administration of moclobemide, a reversible inhibitor of monoamine oxidase type A) resulted in a reduction of this 5‐HT2C receptor‐mediated function [45]. This was interpreted as an indication that the 5‐HT2C receptor may be altered, and preasumably may exist in a dysregulated (hypersensitive) state in depressive illness. Thus, adaptive processes resulting from chronic antidepressant treatment (i.e., desensitization and/or downregulation of 5‐HT2C receptors) may play an important role in reversing the 5‐HT2C receptor system supersensitivity resulting from a depressive state [37, 46]. In contrast to most other receptors, 5‐HT2C is not classically regulated. Indeed, 5‐HT2C receptors appear not only to decrease their responsiveness upon chronic agonist stimulation, but also and paradoxically after chronic treatment with antagonists [47, 48]. This mechanism appears to be related to an internalisation process that removes activated cell surface receptors from the plasma membrane involving a phosphorylation step and possible degradation in lysosomes [47]. As a large number of psychotropic drugs, including atypical antipsychotics, antidepressants, and anxiolitics, can all induce down‐regulation of 5‐HT2C receptors, it has been suggested that this receptor adaptation plays a role in the therapeutic action of these drugs [47, 48]. In this respect, it is interesting to note that chronic treatment with 5‐HT2 agonists or antagonists resulted in a paradoxical down‐regulation at the 5‐HT2A and 5‐HT2C receptors [47, 48, 49, 50, 51] and it seems that the down‐regulation state occurring after chronic exposure to mianserin in isolated systems as well as in cell cultures, is a direct receptor‐mediated mechanism of this drug at these receptors [51]. Therefore, the down‐regulating capacity of 5‐HT2C agonists and antagonists may play a particularly important role in treating the supersensitivity of 5‐HT2C receptors resulting from a depressive state [37, 46, 48]. The possible involvement of 5‐HT2C receptors in the pathogenesis of depressive disorders and in the mode of action of antidepressants is further substantiated by several other observations. For example, acute administration of fluoxetine caused a dose‐dependent inhibition of the firing rate of VTA DA neurons [52], and decreased DA release in both the nucleus accumbens and the striatum [53], but it did not affect the activity of DA cells in the SNc [52]. A similar effect, though less pronounced, has been observed with citalopram [52]. Furthermore, mesulergine, an unselective 5‐HT2C receptor antagonist, as well as the lesion of 5‐HT neurons by the neurotoxin 5,7‐dihydroxytryptamine (5,7‐DHT), prevented fluoxetine‐induced inhibition of VTA DA cells [52]. These results indicate that fluoxetine inhibits the mesolimbic DA pathway by enhancing the extracellular level of 5‐HT, which would act through 5‐HT2C receptors [52]. This study also demonstrated that fluoxetine‐induced inhibition of DA neurons in the VTA was no longer observed after chronic treatment (21 days) with this drug. Interestingly, mCPP inhibited the firing activity of VTA DA neurons in control animals but not in those chronically treated with fluoxetine [52]. The authors suggested that 5‐HT2C receptors might be down‐regulated after repeated fluoxetine administration. Consistent with this hypothesis is the evidence that chronic treatment with sertraline and citalopram, two selective serotonin reuptake inhibitors (SSRIs), induced tolerance to the hypolocomotor effect of mCPP [54]. This hyposensitivity of 5‐HT2C receptors might be a key step for the achievement of an antidepressant effect. Indeed, it is possible to argue that the acute inhibitory effect of fluoxetine on the mesolimbic DA system would mask its clinical efficacy in the early stage of treatment. This masking effect would disappear when the hyposensitivity of 5‐HT2C receptors occurs. A series of studies carried out in our laboratory have shown that acute administration of SSRIs such as paroxetine, sertraline, and fluvoxamine causes a slight but significant decrease in the basal firing rate of VTA DA neurons [55]. Therefore, it is conceivable that, similar to fluoxetine, these SSRIs could reduce mesocorticolimbic DA transmission by activating 5‐HT2C receptors. Furthermore, employing complementary electrophysiological and neurochemical approaches, and both acute and chronic administration routes, it was found that mirtazapine, nefazodone, and agomelatine, three effective and innovative antidepressants, elicit a robust and pronounced enhancement in the activity of mesocorticolimbic DA pathways. These actions were ascribed to their antagonistic properties at inhibitory, tonically active 5‐HT2C receptors, that desensitize after repeated drug administration [56, 57, 58].
Interestingly, agomelatine, has shown antidepressant efficacy in clinical trials [59, 60, 61], and, indeed, it was found to be effective in treating severe depression associated with anxiety symptoms, with a better tolerability and lower adverse effects than other antidepressants such as paroxetine [59]. Recent experimental evidence suggests that 5‐HT2A and 5‐HT2C receptors can be constitutively active (agonist‐independent activity) in vivo, and alterations in the constitutive activity of these receptor systems could be involved in the mechanisms underlying anxiety and depression or exploited for therapeutic benefit. Therefore, drugs with inverse agonist properties at these receptors may have more activity in vivo to regulate DA neurotransmission than that afforded by simple competitive antagonism [62].
Other serotonin receptors such as 5HT3 receptors may also contribute to the changes in 5‐HT‐induced dopamine release [63]. Thus, antidepressant drugs that will block 5‐HT2C and activate 5‐HT3 receptors will probably restore serotonin induced DA release in the nucleus accumbens, and normalize depressive‐like behavior faster than classical antidepressant drugs [58, 63, 64]. This suggestion agrees with clinical studies that demonstrated that mirtazapine, which acts on 5‐HT2C and 5‐HT3 receptors, and nefazodone, which acts on 5‐HT2C, and whose metabolite acts on 5‐HT3 receptors, are characterized by a more rapid onset of behavioral effects of treatment [65]. Further, valproic acid, carbamazepine, and zonisamide, three anticonvulsant mood stabilizers, have been reported to preferentially increase DA release in the mPFC of rats, sharing a common mechanism of action mediated by 5‐HT1A receptor activation [66, 67]. Thus, both anticonvulsant mood stabilizers and atypical antipsychotic drugs, would be expected to ameliorate or prevent depression, at least in part, via reversal of decreased prefrontal cortical activity by facilitating 5‐HT1A activation and its resultant increase in mPFC DA release. A number of antidepressant agents, such as ipsapirone [68], mirtazapine [69], fluoxetine, and buspirone [70, 71], all drugs showing agonistic properties at 5‐HT1A receptors, raised extracellular DA in mPFC, and were markedely attenuated by pretreatment with WAY 100635. Also, the combination of atypical antipsychotic drugs in addition to serotonin reuptake inhibitors has recently proven to be beneficial in a number of neuropsychiatric disorders, such as resistant depression, schizophrenia, and obsessive‐compulsive disorder, as this method markedly potentiates mPFC DA release elicited by the administration of a single drug alone. Interestingly, acute combination of fluoxetine and olanzapine caused a synergistic and selective effect on the extracellular concentration of dopamine in the medial prefrontal cortex [72, 73], and this regional selectivity may account for the mood‐stabilizing properties of these drugs. Nevertheless, the combination of long‐term fluoxetine with acute olanzapine did not show the synergistic effect on the extracellular of DA observed following acute administration [74] suggesting that the therapeutic benefit of this pharmacological combination may not be associated with changes in the cortical concentration of monoamines, but to postsynaptic blockade of monoaminergic receptors. Thus, activation of 5‐HT1A receptors secondary to the combined blockade of 5‐HT2A/2C and D2/3 receptors seems to be relevant for this action [73, 74, 75, 76, 77, 78, 79, 80, 81, 82].
Schizophrenia
Both hypo‐ and hyperfunction of dopaminergic systems may occur in schizophrenic patients, perhaps even simultaneusly, albeit in a region specific manner [83, 84, 85]. Thus, whereas a dopaminergic hyperfunction of the mesolimbic system may underlie the development of positive symptoms, a dopaminergic hypofunction of the cortical projections may well be related to the negative symptomatology in schizophrenia. Given the critical role of cortical DA in cognitive functioning [86, 87], the hypothesized cortical DA hypofunction may therefore also be implicated in the cognitive disturbances frequently experienced by schizophrenic patients. Hence, it appears likely that both the negative symptoms and cognitive disturbances of schizophrenia may be associated with a hypofunction of the mesocortical DA system.
Currently used antipsychotic drugs are usually divided into two main classes, on the basis of their liability to induce neurological side effects after long‐term treatment. Drugs defined as typical antipsychotics (APDs) (e.g., chlorpromazine, haloperidol, trifluopromazine) are known to induce, following repeated administration, various extrapyramidal side effects (EPS) including Parkinson‐like syndrome and tardive dyskinesia [88]. On the other hand, chronic treatment with atypical antipsychotic drugs (e.g., clozapine, risperidone, sertindole, zotepine) is associated with a low incidence of neurological side effects [88]. Moreover, atypical antipsychotic drugs do not increase plasma prolactin levels in humans [88]. The hypothesis that typical antipsychotics produce their clinical effects, as well as EPS, by blocking DA D2 receptors in the mesolimbic and nigrostriatal systems, respectively [88], is now generally accepted. In contrast, the mechanisms responsible for the clinical effects of atypical antipsychotic drugs are still not clear. Numerous studies have shown that the so‐called “atypical antipsychotics” such as clozapine, amperozide, olanzapine, risperidone and others, compared to the typical antipsychotics haloperidol or (‐) sulpiride, stimulate the release of DA more potently in the mPFC and mesocorticolimbic innervated areas, than in the striatum [89, 90, 91, 92, 93, 94, 95, 96]. This selective action is associated with a lower incidence of EPS, and with a greater ability to improve negative symptoms and cognitive functions in schizophrenia [24, 88, 97, 98]. As a common property of these drugs, that distinguishes them from the typical antipsychotics, is their high affinity for the 5‐HT2A receptor, it has been suggested that potent 5‐HT2A antagonism, in relation to a weaker DA D2 receptor antagonism, contributes to their beneficial effects [99]. Thus, pretreatment with the selective 5‐HT2A antagonist M100907 before administration of the D2 antagonists haloperidol, sulpiride or raclopride produced an increase in mPFC DA release, wich was not observed by these compounds administered alone [89, 95, 100, 101]. Interestingly, M100907 potentiated low but not high dose haloperidol‐induced DA release in the mPFC and inhibited that in the nucleus accumbens [100, 101], thus, weak D2 and potent 5‐HT2A receptor blockade may have an important influence on the preferential increase of mPFC DA release by the atypical antipsychotics and on their clinical effectiveness. Further, evidence has been provided that this effect may be mediated by actions of released 5‐HT interacting with 5‐HT1A receptors. In fact, reversal by WAY100635 of the potentiation of DA release in mPFC induced by selective antagonism at 5‐HT2A and D2 receptors suggested that facilitation of 5‐HT1A receptor stimulation is essential to the simultaneus blockade of 5‐HT2A and D2 receptors to increase cortical DA release [89, 100].
Interestingly, 8‐OH‐DPAT, a 5‐HT1A agonist, or amperozide and M100907, two 5HT2A receptor antagonists, inhibited the ability of amphetamine to increase DA release in rat nucleus accumbens and striatum [101, 102, 103]. Thus attenuation of stimulated DA release in the nucleus accumbens and striatum by 5‐HT1A receptor agonism and/or 5‐HT2A antagonism, may contribute to reverse neuroleptic‐induced catalepsy in rats. In this respect, combining antagonist/partial agonist activity at dopamine D2 and agonist activity at serotonin 5‐HT1A receptors is one of the approaches that has recently been chosen to develop the new generation of antipsychotics, including bifeprunox, SSR181507 and SLV313, in that 5‐HT1A receptor activation greatly reduces or prevents the cataleptogenic potential of these novel antipsychotics [104, 105, 106]. Interestingly, 5‐HT1A receptor activation and dopamine D2 receptor antagonism underlie the electrophysiological and neurochemical profile of F15063 as measured by determination of DRN and VTA electrical activity that increased catecholamine levels in the medial prefrontal cortex and decreased 5‐HT levels in the hippocampus [107].
As already mentioned, preferential increase of DA release in the mPFC seems to be a common mechanism of action of atypical antipsychotic drugs, an effect which might be relevant for their therapeutic action on negative symptoms of schizophrenia [94]. In this respect, it is important to note that the selective 5‐HT2C receptor antagonist SB 242084 markedly increases DA release in the frontal cortex of awake rats [108, 109]. Thus, it is possible to argue that blockade of 5‐HT2C receptors might contribute to the preferential effect of atypical antipsychotics on DA release in the prefrontal cortex. It is noteworthy to mention recent data showing that atypical antipsychotic drugs (clozapine, sertindole, olanzapine, ziprasidone, risperidone, zotepine, tiospirone, fluperlapine, tenilapine), which produce little or no EPS while improving negative symptoms of schizophrenia, exert substantial inverse agonist activity at 5HT2C receptors [110, 111, 112]. Thus, 5‐HT2C receptor inverse agonism might underlie the unique clinical properties of atypical antipsychotic drugs [111]. Interestingly, there is preclinical evidence indicating that 5‐HT2C receptor blockade is responsible for reducing EPS: 5‐HT2C but not 5‐HT2A receptor antagonists were capable of inhibiting haloperidol‐induced catalepsy in rats [113]. On the other hand, the blockade of DA‐ergic neurotransmission in the nucleus accumbens via D2 receptor antagonism or partial agonism is considered the primary mechanism underlying antipsychotic efficacy for the positive symptoms (i.e., hallucinations, delusions, and thought disorder) of schizophrenia. Thus, an alternative approach to blocking dopamine D2 receptors may be to reduce the activity of the mesolimbic pathway without affecting that of the nigrostriatal system, thus avoiding potential extrapyramidal side effect liabilities. The selective effects shown by the 5‐HT2C receptor agonists on the mesolimbic DA pathway suggest that 5‐HT2C receptor agonists should have antipsychotic efficacy without the EPS associated with typical antipsychotics. To this end, recently, the antipsychotic efficacy of the selective 5‐HT2C receptor agonist WAY‐163909 was preclinically evaluated by in vivo microdialysis, electrophysiology, and various animal models of schizophrenia [114], showing selectivity for the mesolimbic system and an interesting profile similar to that of an atypical antipsychotic, when given acutely or chronically in mice and rats, facilitating cortical DA‐ergic neurotransmission and reducing that of the nucleus accumbens, without affecting the nigrostriatal DA activity.
Agents acting at multi‐receptor sites appear to be more promising as antipsychotic drugs, and recent data show that blockade of DA receptors and combined antagonism at 5‐HT2A as well as 5‐HT2C receptors may be involved in the therapeutic effects of novel antipsychotics [97, 98, 100, 115]. Interestingly, ritanserin, a mixed 5‐HT2A/2C receptor antagonist, has been reported to potentiate the D2/3 receptor antagonist raclopride‐induced DA release in the medial prefrontal cortex and nucleus accumbens, but not in the striatum [116]. Another putative atypical antipsychotic drug SR46349B, that shares both 5‐HT2A and 5‐HT2C receptor antagonism, increased cortical DA release and potentiated haloperidol induced DA release in both mPFC and nucleus accumbens, suggesting that 5‐HT2C receptor antagonism may also contribute to the potentiation of DA release produced by haloperidol [100]. A novel putative atypical antipsychotic ACP‐103, inverse agonist at both 5‐HT2A and 5‐HT2C receptors, increased DA relese in the mPFC but not in the nucleus accumbens, and potentiated low dose of haloperidol‐induced DA release in the mPFC, while inhibiting that in the nucleus accumbens [117]. Taken together, these data suggest that combined 5‐HT2A/2C receptor antagonism may be more advantageous than selective 5‐HT2A antagonism alone as an adjunct to D2 antagonism and 5‐HT1A stimulation to improve cogniton end negative symptoms in schizophrenia.
Parkinson's Disease
The major pathology in Parkinson's disease is the degeneration of pigmented dopamine‐producing neurons, particularly within the SNc, which causes a consequent reduction of dopamine levels in the striatum, and changes in the basal ganglia–thalamo–cortical network activity [118, 119, 120]. The neural mechanisms underlying the generation of parkinsonian symptoms are thought to involve reduced activation of primary motor and premotor cortex and supplementary motor areas, secondary to overactivation of the output regions of the basal ganglia, that is, SNr and globus pallidus internus (GPi) [121], largely because of excessive excitatory drive from the subthalamic nucleus (STN), consequent to dopamine loss in the striatum [119, 120]. Therapy for Parkinson's disease consists mainly of amelioration of the symptoms with classical dopaminomimetics [122]. This treatment, however, is characterized by declining efficacy and the occurrence of disabling side‐effects [123]. Functional inhibition of GPi or STN, has provided an alternative to lesioning, by deep brain stimulation associated with modest side‐effects [124]. As serotonergic projections from the DRN innervate all components of the basal ganglia circuitry [119, 120], it is likely that 5‐HT plays a role in regulating the basal ganglia's activities, and of particular interest with respect to the development of new treatments for Parkinson's disease are 5‐HT1A, 5‐HT1B, and 5‐HT2C receptors [119, 125]. Stimulation of 5‐HT1A receptors might be expected to reduce 5‐HT release in several brain regions, including the basal ganglia, and reduce the activity of glutamatergic inputs to the striatum, that may have both antiparkinsonian and antidyskinetic actions [119]. Moreover, stimulation of striatal 5‐HT1B receptors may modulate levodopa metabolism to dopamine in 5‐HT terminals [126]. 5‐HT1B receptors in the pallidum and substantia nigra reduce GABA release thus having either antiparkinsonian or antidyskinetic actions [119]. Another interesting application of the data regarding the functional role of 5‐HT2C receptors in the basal ganglia is the possible use of 5‐HT2C receptor antagonists in the treatment of Parkinson's disease, and 5‐HT2C agonists to reduce the problems of levodopa‐induced dyskinesia [119, 127]. As already mentioned, 5‐HT2C receptors are located in the SNr and medial segment of the pallidal complex in the rat and human brain [13, 128], and enhanced 5‐HT2C receptor‐mediated transmission within the output regions of the basal ganglia in Parkinsonism appears to contribute to their overactivity [127]. In addition, 5‐HT2C‐like receptor binding is increased in a rat model of Parkinsonism [129] and in human Parkinsonian patients [130]. Interestingly, systemic administration of the 5‐HT2C receptor antagonist SB 206553 enhanced the anti‐Parkinsonian action of the DA D1 and D2 agonists in the 6‐hydroxydopamine‐lesioned rats [131, 132], suggesting that the use of a 5‐HT2C receptor antagonist in combination with a DA receptor agonist may reduce the reliance upon dopamine replacement therapies and may thus reduce the problems associated with long term use of currently available antiparkinsonian agents [127].
Drugs of Abuse
Substantial evidence indicates that the mesolimbic pathway, particularly the dopaminergic system innervating accumbal areas, is implicated in the reward value of both natural and drug reinforcers, such as sexual behavior or psychostimulants, respectively [7, 8, 133]. Therefore, blocking or stimulating several 5‐HT receptor subtypes, including the 5‐HT1B, 5‐HT2A, 5‐HT2C, and 5‐HT3 subtypes, modulates both the neurochemical and the behavioral effects of addictive drugs. 5‐HT3 antagonism has been shown to counteract the increase of accumbal DA release induced by various drugs of abuse, such as ethanol, nicotine, morphine, or cocaine [134, 135, 136, 137, 138, 139]. Systemically, ICS205‐930, ondansetron, and MDL72222 attenuated morphine‐induced DA release in the nucleus accumbens [134, 135, 137, 140], nevertheless, intra VTA, but not intra‐accumbal, infusion of ICS205‐930, was able to counteract the action of morphine [140], suggesting that selective antagonism on VTA 5‐HT3 receptors is able to modulate the morphine's action in the mesolimbic system. Furthermore, intra‐accumbal infusion of ondansetron strongly reduced the enhancement of DA release elicited by a high but not a low dose of morphine in the same area [137] so, it was proposed that in addition to increased DA tone, increased 5‐HT release is required to trigger the excitatory action of accumbal 5‐HT3 receptors on DA release. Systemic pretreatment with ICS205‐930 also attenuated ethanol‐induced increase of DA efflux in the nucleus accumbens [134, 141]. Furthermore, local infusion of ICS205‐930 into the VTA [142] or in the nucleus accumbens [143] prevented ethanol's action in both areas. In addition, the 5‐HT3 agonist mCPBG had additive effect on DA release when infused in the nucleus accumbens concomitantly to the systemic injection of ethanol in rats [143]. Therefore, Yoshimoto et al. [144] showed that chronic alcohol intake increases the sensitivity of accumbal 5‐HT3 receptors in rats, thus suggesting their involvement in alcohol dependence.
There are, however, contrasting results in the case of amphetamine or cocaine. Systemic administration of the 5‐HT3 antagonists MDL72222 and zacopride has been shown to attenuate both cocaine‐ or amphetamine‐induced DA release in the nucleus accumbens [138, 139, 145], while, other studies have shown that systemic 5‐HT3 receptor antagonism had no effect on DA efflux enhanced by these drugs in the same area [137, 146, 147]. Therefore, the fact that drugs of abuse stimulate DA release through different cellular mechanisms leads to the possibility that their effect on DA function could be modulated by the 5‐HT3 receptor only under specific conditions, in that it requires a concomitant increase in both endogenous DA and 5‐HT tones and operates selectively on the depolarization‐dependent exocytosis of DA.
Pharmacological studies have shown that the acute administration of 5‐HT1B receptor agonists augments cocaine‐evoked DA overflow within the nucleus accumbens [148]. The ability of extracellular 5‐HT to facilitate mesolimbic DA release through 5‐HT1B receptors has implications for psychostimulant abuse. Likewise, studies have shown that systemic or intra‐VTA 5‐HT1B receptor agonism (CP 93129 and RU 24969, respectively) potentiated cocaine‐induced increase in DA efflux in the nucleus accumbens and decreased GABA release in the VTA [148, 149]. Dopaminergic activity from the mesolimbic pathway may then be disinhibited by the stimulation of 5‐HT1B receptors on GABA‐ergic projection neurons from the nucleus accumbens to the VTA, resulting in a potentiated response to cocaine. There is also evidence that VTA 5‐HT1B receptors may be involved, in part, in mediating the activating effects of ethanol on mesolimbic DA neurons, in that activation and blockade of VTA 5‐HT1B receptors potentiated and attenuated, respectively, the ethanol‐induced increases in extracellular DA concentrations in both the VTA and the ipsilateral nucleus accumbens [150]. Taken together, these studies suggest that 5‐HT1B and/or 5‐HT3 receptor antagonism could be beneficial in treating psychostimulant abuse.
5‐HT2A and 5‐HT2C receptor subtypes have also been implicated in modulating responses to psychostimulants and may play a role in their rewarding effects. The fact that addictive drugs act through different cellular mechanisms leads to the possibility that their effects on DA release could be modulated differentially by each of the 5‐HT2A or 5‐HT2C receptor subtypes. For example, it has been reported that the increased locomotor activity, as well as the accumbal DA release, elicited by phencyclidine is further enhanced by the blockade of 5‐HT2C receptors [109], while antagonism at 5‐HT2A receptors had opposite effects [151]. A similar picture emerges when considering the influence of these receptors on 3,4‐methylenedioxymethamphetamine (MDMA, ecstasy)‐induced effects on DA neuron activity. Thus, the selective 5‐HT2A antagonist MDL 100,907 significantly reduced hyperlocomotion and stimulated DA release produced by MDMA while the selective 5‐HT2C antagonists SB 242084 and SB 206553 potentiated it [152, 153, 154, 155].
It was recently found that SB 206553 administration potentiates both the enhancement of DA release in the nucleus accumbens and striatum, and the increased DA neuron firing rate induced by morphine both in theVTA and the SNc [156]. Consistent with these findings, stimulation of central 5‐HT2C receptors has been shown to inhibit morphine‐induced increase in DA release in the nucleus accumbens of freely moving rats [157]. A series of studies showed that blockade of 5‐HT2A or 5‐HT2C receptors had opposite effects on cocaine‐induced locomotor activity. Thus, 5‐HT2A receptor blockade with M100,907 attenuated cocaine‐induced locomotion, whereas 5‐HT2C blockade with SB 242084 or SB 206553 enhanced cocaine‐induced activity [158, 159, 160, 161]. Consistent with these data obtained in rats, 5‐HT2C receptor null mutant mice showed enhanced cocaine‐induced elevations of DA levels in the nucleus accumbens, and marked increase in locomotor response to cocaine as compared to wild‐type mice, suggesting that selective 5‐HT2C receptor agonist treatments may represent a promising novel approach for treating cocaine abuse and dependence [162]. In line with this hypothesis, it was previously found that RO 60‐0175 reduced cocaine‐reinforced behavior by stimulating 5‐HT2C receptors [163]. Moreover, these authors also showed that RO 60‐0175 reduced ethanol‐ and nicotine‐induced self‐administration and hyperactivity [164, 165]. Consistent with this evidence, we showed that the selective activation of 5‐HT2C receptors by RO 60‐0175 blocks the stimulatory action of nicotine on SNc DA neuronal activity and DA release in the corpus striatum [166, 167]. The mesolimbic DA system appeared to be less sensitive to the inhibitory effect of 5‐HT2C receptors activation on nicotine‐induced stimulation, indeed a higher dose of RO 60‐0175 was necessary to prevent the enhancement of VTA DA neuronal firing elicited by acute nicotine. Furthermore, pretreatment with the 5‐HT2C agonist did not affect nicotine‐induced DA release in the nucleus accumbens [166]. Interestingly, in animals treated repeatedly with nicotine, pretreatment with RO 60‐0175 reproduced the same pattern of effects on the enhancement in DA neuronal firing caused by challenge with nicotine, and as a result was effective only at a higher dose in preventing nicotine excitation in the VTA compared to the SNc. Furthermore, the 5‐HT2C receptors agonist counteracted nicotine‐induced DA release both in the striatum and in the nucleus accumbens in rats chronically treated with this alkaloid, even if this effect was observed only with the highest dose of RO 60‐0175 [166, 167]. Therefore, we hypothesized that after repeated nicotine exposure an up‐regulation of 5‐HT2C receptors occurs only in the DA mesolimbic system and the blocking of its hyperfunction by 5‐HT2C receptor activation might be a useful approach in reducing nicotine reward, and eventually helping in smoking cessation.
Conclusion
Serotonergic and dopaminergic systems are closely related in the central nervous system, and the involvement of 5‐HT receptors in the control of central DA activity is now well established. Recent evidence suggests that dysfunction of dopaminergic and serotoninergic neurotransmitter systems contributes to various disorders including depression, schizophrenia, Parkinson's disease and drug abuse. Thus, the use of a complementary dialysis and electrophysiological approach, together with several highly selective ligands, has permitted important insights into the complex pattern of reciprocal interactions via which multiples classes of 5‐HT receptors control the activity of central dopaminergic pathways. These data facilitate interpretation of the influence upon central DA‐ergic systems exerted by 5‐HT and diverse classes of antidepressant, antipsychotic and psychostimulant agents, and suggest numerous possible receptorial strategies for modulation (potentiation) of their therapeutic actions.
Moreover, the scenario is complicated by some experimental caveats. Most of the agonists and antagonists used in the studies reviewed here are selective but are not specific enough to identify receptors involved, especially when used in relatively high concentrations. Most of the in vivo data, obtained by extracellular recordings are from putative DA neurons identified by their firing properties. Nevertheless, these classical identification criteria are deceptive. In fact, the presence of a functionally distinct non‐dopaminergic (but not GABA‐ergic) population of neurons in the VTA with overlapping characteristics has been demonstrated [168], which is likely to have been included in the analysis of DA neurons in the electrophysiological studies that we reviewed. In addition, these studies are carried out in anaesthetized rats. Although DA neurons are autoactive, their impulse activity and response to natural neurochemical inputs are strongly affected by general anaesthesia. Some alterations appear to be specific to the general anaesthetic used, while others probably reflect changes in the activity of afferent inputs, brain metabolism, and neurotransmitter uptake that are typical to any type of general anaesthesia. Most of the data are obtained using chloral hydrate that is known to exert subtle effects on the basal activity and pharmacological responsiveness of midbrain DA neurons [169]. Therefore, taking consideration of the aforementioned, when extracellular unit activity from single DA neurons in anaesthetized rats are performed, it is of paramount importance that they are subsequently labelled with the use of the juxtacellular technique, and neurochemically characterized with immunofluorescence for Tyrosine Hydroxylase. Moreover, the utilization of microiontophoresis or reverse dialysis might be encouraged. In fact, despite their methodological constraints, they remain almost the only way of applying relatively few molecules rapidly into the vicinity of central synapses. The techniques get closer to mimicking synaptically released neurotransmitters than any other, and can show direct or indirect effects. Despite the large body of data available on this subject there still a number of points that need to be elucidated. Thus, it would be important to establish the exact brain site(s) involved in the control of DA neuronal activity by the various 5‐HT receptor subtypes. For example, it would important to determine whether or not the effects of the selective activation of 5‐HT subtypes are mediated at the level of the nuclei of origin of the DA‐ergic systems (i.e., in the SNc or the VTA); whether or not their effects are direct or indirect (e.g., mediated by GABA‐ergic transmission) and whether or not feed‐back pathways originating from projection areas of the nigro‐striatal and mesocorticolimbic systems are involved in their overall effect upon DA neuronal function. Lastly, the majority of the effects described herein were acquired upon acute drug administration. This approach is eminently suitable to the characterization of the functional roles of various auto‐ and heteroreceptor subtypes. However, inasmuch as the majority of the therapeutic agents are often administered chronically, and may trigger adaptive changes, it would be interesting to expand the present observations with studies of long‐term drug administration.
The intensive research in medicinal chemistry will help this field of investigation. In fact, more selective ligands for 5‐HT receptors are currently produced. In the future, the use of such selective ligands, especially agonists of 5‐HT receptors, would certainly be helpful in determining their functional importance and their involvement in the pathogenesis of diseases, not exclusively of the CNS. However, it should be kept in mind that although selective receptor ligands are an important and indispensable research tool, they rarely happen, in practice, to be drugs. This is likely due to a need to act on different neurotransmitter systems to obtain a therapeutic effect since neuropsychiatric disorders are not pure DAergic dysfunction. Many questions need to be answered before we can truly understand how these serotonin receptors regulate DA neuronal activity in the brain. The challenge ahead is to build on this foundation and keep up this engaging adventure: the interaction between serotonin and dopamine systems is far from being completely revealed.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors wish to thank Dr. Clare Austen for the English revision and Ms. Barbara Mariani for her help in preparing the manuscript.
References
- 1. Dahlström A, Fuxe K. Evidence for the existence of monoamine‐containing neurons in the central nervous system. Part I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand 1964;62:1–55. [PubMed] [Google Scholar]
- 2. Bannon MJ, Roth RH. Pharmacology of mesocortical dopamine neurons. Pharmacol Rev 1983;35:53–68. [PubMed] [Google Scholar]
- 3. Roth RH, Wolf ME, Deutch AY. Neurochemistry of midbrain dopamine systems In: Meltzer HY, editor. Psychopharmacology: The third generation of progress. New York : Raven Press, 1987;81–94. [Google Scholar]
- 4. Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Rev 1993;18:75–113. [DOI] [PubMed] [Google Scholar]
- 5. White FJ. Synaptic regulation of mesocorticolimbic dopamine neurons. Annu Rev Neurosci 1996;19:405–436. [DOI] [PubMed] [Google Scholar]
- 6. Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: Functional and regulatory roles. Physiol Rev 1991;71:155–234. [DOI] [PubMed] [Google Scholar]
- 7. Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 1988;85:5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koob GF. Drugs of abuse: Anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci 1992;13:177–184. [DOI] [PubMed] [Google Scholar]
- 9. Salamone JD, Correa M, Farrar A, Mingote SM. Effort‐related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology 2007;191:461–482. [DOI] [PubMed] [Google Scholar]
- 10. Roth RH, Elsworth JD. Biochemical pharmacology of midbrain dopamine neurons In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The fourth generation of progress. New York : Raven Press, 1995;227–243. [Google Scholar]
- 11. Morrow BA, Elsworth JD, Zito C, Roth RH. Biochemical and behavioral anxiolytic‐like effects of R(+) HA‐966 at the level of the ventral tegmental area in rats. Psychopharmacology 1999;143:227–234. [DOI] [PubMed] [Google Scholar]
- 12. Grace A, Bunney B. Dopamine In: Rogawski MA, Barker JL, editors. Neurotransmitter action in the vertebrate nervous system. New York : Plenum Press, 1985;285–319. [Google Scholar]
- 13. Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol 1978;179:641–659. [DOI] [PubMed] [Google Scholar]
- 14. Van Der Kooy D, Attori T. Dorsal raphé cells with collateral projections to the caudate‐putamen and substantia nigra: A fluorescent retrograde double labeling study in the rat. Brain Res 1980;186:1–7. [DOI] [PubMed] [Google Scholar]
- 15. Steinbush HWM. Serotonin‐immunoreactive neurons and their projections in the CNS In: Björklund A, Hökfelt T, Kuhar MJ, editors. Handbook of chemical neuroanatomy: Classical transmitter receptors in the CNS, Part II. Amsterdam : Elsevier Science Publishers BV, 1984;68–125. [Google Scholar]
- 16. Hervé D, Pickel VM, Joh TH, Beaudet A. Serotonin axon terminals in the ventral tegmental area of the rat: Fine structure and synaptic input to dopaminergic neurons. Brain Res 1987;435:71–83. [DOI] [PubMed] [Google Scholar]
- 17. Van Bockstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphé nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res 1993;624:188–198. [DOI] [PubMed] [Google Scholar]
- 18. Van Bockstaele EJ, Cestari DM, Pickel VM. Synaptic structure and connectivity of serotonin terminals in the ventral tegmental area: Potential sites for modulation of mesolimbic dopamine neurons. Brain Res 1994;647:307–322. [DOI] [PubMed] [Google Scholar]
- 19. Moukhles H, Bosler O, Bolam JP, Vallée A, Umbriaco D, Geffard M, Doucet G. Quantitative and morphometric data indicate precise cellular interactions between serotonin terminals and postsynaptic targets in rat substantia nigra. Neuroscience 1997;76:1159–1171. [DOI] [PubMed] [Google Scholar]
- 20. Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5‐HT receptors. Pharmacol Biochem Behav 2002;71:533–554. [DOI] [PubMed] [Google Scholar]
- 21. Hoyer D, Clarke DE, Fozard JR, Harting PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA. VII. International union of pharmacology classification of receptors for 5‐hydroxytryptamine (serotonin). Pharmacol Rev 1994;46:157–203. [PubMed] [Google Scholar]
- 22. Barnes NM, Sharp T. A review of central 5‐HT receptors and their function. Neuropharmacology 1999;38:1083–1152. [DOI] [PubMed] [Google Scholar]
- 23. Samanin R, Garattini S. The serotonergic system in the brain and its possible functional connections with other aminergic systems. Life Sci 1975;8:1201–1209. [DOI] [PubMed] [Google Scholar]
- 24. Roth BL, Roland D, Ciaranello D, Meltzer HY. Binding of typical and atypical antipsychotic agents to transiently expressed 5‐HT1C receptors. J Pharmacol Exp Ther 1992;260:1361–1365. [PubMed] [Google Scholar]
- 25. Fibiger HC. Neurobiology of depression: Focus on dopamine In: Gessa G, Fratta W, Pani L, Serra G, editors. Depression and mania: From neurobiology to treatment. New York : Raven Press, 1995;1–17. [Google Scholar]
- 26. Brown AS, Gershon S. Dopamine and depression. J Neural Transm 1993;91:75–109. [DOI] [PubMed] [Google Scholar]
- 27. Di Matteo V, De Blasi A, Di Giulio C, Esposito E. Role of 5‐HT2C receptors in the control of central dopamine function. Trends Pharmacol Sci 2001;22:229–232. [DOI] [PubMed] [Google Scholar]
- 28. Higgins GA, Fletcher PJ. Serotonin and drug reward: Focus on 5‐HT2C receptors. Eur J Pharmacol 2003;480:151–162. [DOI] [PubMed] [Google Scholar]
- 29. Giorgetti M, Tecott L. Contribution of 5‐HT2C receptors to multiple action of central serotonin systems. Eur J Pharmacol 2004;488:1–9. [DOI] [PubMed] [Google Scholar]
- 30. Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther 2007;113:296–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Puglisi‐Allegra S, Imperato A, Angelucci L, Cabib S. Acute stress induces time‐dependent responses in dopamine mesolimbic system. Brain Res 1991;554:217–222. [DOI] [PubMed] [Google Scholar]
- 32. Cabib S, Puglisi‐Allegra S. Stress, depression and the mesolimbic dopamine system. Psychopharmacology 1996;128:331–342. [DOI] [PubMed] [Google Scholar]
- 33. Di Matteo V, Di Giovanni G, Esposito E. SB 242084: A selective 5‐HT2C receptor antagonist. CNS Drug Reviews 2000;6:195–205. [Google Scholar]
- 34. Di Matteo V, Di Mascio M, Di Giovanni G, Esposito E. Acute administration of amitriptyline and mianserin increases dopamine release in the rat nucleus accumbens: Possible involvement of serotonin2C receptors. Psychopharmacology 2000;150:45–51. [DOI] [PubMed] [Google Scholar]
- 35. Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. Selective blockade of serotonin 2C /2B receptors enhances dopamine release in the rat nucleus accumbens. Neuropharmacology 1998;37:265–272. [DOI] [PubMed] [Google Scholar]
- 36. Baxter GS, Kennett GA, Blaney F, Blackburn T. 5‐HT2 receptor subtypes: A family reunited? Trends Pharmacol Sci 1995;16:105–110. [DOI] [PubMed] [Google Scholar]
- 37. Jenck F, Bös J, Wichmann J, Stadler H, Martin JR, Moreau JL. The role of 5‐HT2C receptors in affective disorders. Exp Opin Invest Drugs 1998;7:1587–1599. [DOI] [PubMed] [Google Scholar]
- 38. Jenck F, Moreau JL, Mutel V, Martin JR, Haefely WE. Evidence for a role of 5‐HT1C receptors in the antiserotonergic properties of some antidepressant drugs. Eur J Pharmacol 1993;231:223–229. [DOI] [PubMed] [Google Scholar]
- 39. Jenck F, Moreau JL, Mutel V, Martin JR. Brain 5‐HT1C receptors and antidepressants. Prog Neuropsychopharmacol Biol Psychiatry 1994;18:563–574. [DOI] [PubMed] [Google Scholar]
- 40. Sampson D, Muscat R, Willner P. Reversal of antidepressant action by dopamine antagonists in an animal model of depression. Psychopharmacology 1991;104:491–495. [DOI] [PubMed] [Google Scholar]
- 41. Moreau JL, Bourson A, Jenck F, Martin JR, Mortas P. Curative effects of the atypical antidepressant mianserin in the chronic mild stress‐induced anhedonia model of depression. J Psychiatr Neurosci 1994;19:51–56. [PMC free article] [PubMed] [Google Scholar]
- 42. Willner P. Animal models of depression: Validity and applications In: Gessa G, Fratta W, Pani L, Serra G, editors. Depression and mania: From neurobiology to treatment. New York : Raven Press, 1995;19–41. [Google Scholar]
- 43. Tanda G, Carboni E, Frau R, Di Chiara G. Increase of extracellular dopamine in the prefrontal cortex: A trait of drugs with antidepressant potential? Psychopharmacology 1994;155:285–288. [DOI] [PubMed] [Google Scholar]
- 44. Tanda G, Bassareo V, Di Chiara G. Mianserin markedly and selectively increases extracellular dopamine in the prefrontal cortex as compared to the nucleus accumbens of the rat. Psychopharmacology 1996;123:127–130. [DOI] [PubMed] [Google Scholar]
- 45. Moreau JL, Jenck F, Martin JR, Perrin S, Haefely WE. Effect of repeated mild stress and two antidepressant treatments on the behavioral response to 5‐HT1C receptor activation in rats. Psychopharmacology 1993;110:140–144. [DOI] [PubMed] [Google Scholar]
- 46. Moreau JL, Bös M, Jenck F, Martin JR, Mortas P, Wichmann J. 5‐HT2C receptor agonists exhibit antidepressant‐like properties in the anhedonia model of depression in rats. Eur Neuropsychopharmacol 1996;6:169–175. [DOI] [PubMed] [Google Scholar]
- 47. Van Oekelen D, Luyten WH, Leysen JE. 5‐HT2A and 5‐HT2C receptors and their atypical regulation properties. Life Sci 2003;72:2429–2449. [DOI] [PubMed] [Google Scholar]
- 48. Serretti A, Artioli P, De Ronchi D. The 5‐HT2C receptor as a target for mood disorders. Expert Opin Ther Targets 2004;8:1–9. [DOI] [PubMed] [Google Scholar]
- 49. Barker EL, Sanders‐Bush E. 5‐Hydroxytryptamine1C receptor density and mRNA levels in choroid plexus epithelial cells after treatment with mianserin and (‐)‐1‐(4‐bromo‐2,5‐dimethoxyphenyl)‐2‐aminopropane. Mol Pharmacol 1993;44:725–730. [PubMed] [Google Scholar]
- 50. Pranzatelli MR, Murthy JN, Tailor PT. Novel regulation of 5‐HT1C receptors: Down‐regulation induced both by 5‐HT1C /2 receptor agonists and antagonists. Eur J Pharmacol 1993;244:1–5. [DOI] [PubMed] [Google Scholar]
- 51. Newton RA, Elliott JM. Mianserin‐induced down‐regulation of human 5‐hydroxytryptamine2A and 5‐Hydroxytryptamine2C receptors stably expressed in the human neuroblastoma cell line SH‐SY5Y. J Neurochem 1997;69:1031–1038. [DOI] [PubMed] [Google Scholar]
- 52. Prisco S, Esposito E. Differential effects of acute and chronic fluoxetine administration on the spontaneous activity of dopaminergic neurones in the ventral tegmental area. Br J Pharmacol 1995;116:1923–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ichikawa J, Meltzer HY. Effect of antidepressants on striatal and accumbens extracellular dopamine levels. Eur J Pharmacol 1995;281:255–261. [DOI] [PubMed] [Google Scholar]
- 54. Maj J, Moryl E. Effects of sertraline and citalopram given repeatedly on the responsiveness of 5‐HT receptor subpopulations. J Neural Transm: Gen Sec 1992;88:143–156. [DOI] [PubMed] [Google Scholar]
- 55. Di Mascio M, Di Giovanni G, Di Matteo V, Prisco S, Esposito E. Selective serotonin reuptake inhibitors reduce the spontaneous activity of dopaminergic neurons in the ventral tegmental area. Brain Res Bull 1998;46:547–554. [DOI] [PubMed] [Google Scholar]
- 56. Millan MJ, Gobert A, Rivet JM, et al Mirtazapine enhances frontocortical dopaminergic and corticolimbic adrenergic, but not serotonergic, transmission by blockade of α2‐adrenergic and serotonin2C receptors: A comparison with citalopram. Eur J Neurosci 2000;12:1079–1095. [DOI] [PubMed] [Google Scholar]
- 57. Millan MJ, Gobert A, Lejeune F, et al The novel melatonin agonist agomelatine (S20098) is an antagonist at 5‐hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther 2003;306:954–964. [DOI] [PubMed] [Google Scholar]
- 58. Dremencov E, Newman ME, Kinor N, Blatman‐Jan G, Schindler CJ, Overstreet DH, Yadid G. Hyperfunctionality of serotonin‐2C receptor‐mediated inhibition of accumbal dopamine release in an animal model of depression is reversed by antidepressant treatment. Neuropharmacology 2005;48:34–42. [DOI] [PubMed] [Google Scholar]
- 59. Lôo H, Hale A, D’Haenen H. Determination of the dose of agomelatine, a melatonergic agonist and selective 5‐HT2C antagonist, in the treatment of major depressive disorder: A placebo‐controlled dose range study. Int Clin Psychoparmacol 2002;17:239–247. [DOI] [PubMed] [Google Scholar]
- 60. Pandi‐Perumal SR, Srinivasan V, Cardinali DP, Monti MJ. Could agomelatine be the ideal antidepressant? Expert Rev Neurother 2006;6:1595–1608. [DOI] [PubMed] [Google Scholar]
- 61. Zupancic M, Guilleminault C. Agomelatine: A preliminary review of a new antidepressant. CNS Drugs 2006;20:981–992. [DOI] [PubMed] [Google Scholar]
- 62. Aloyo VJ, Berg KA, Spampinato U, Clarke WP, Harvey JA. Current status of inverse agonism at serotonin2A (5‐HT2A) and 5‐HT2C receptors. Pharmacol Ther 2009;121:160–173. [DOI] [PubMed] [Google Scholar]
- 63. Dremencov E, Weizmann Y, Kinor N, Gispan‐Herman I, Yadid G. Modulation of dopamine transmission by 5HT2C and 5HT3 receptors: A role in the antidepressant response. Curr Drug Targets 2006;7:165–175. [DOI] [PubMed] [Google Scholar]
- 64. Dremencov E, Gispan‐Herman I, Rosenstein M, Mendelman A, Overstreet DH, Zohar J, Yadid G. The serotonin‐dopamine interaction is critical for fast‐onset action of antidepressant treatment: In vivo studies in an animal model of depression. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:141–147. [DOI] [PubMed] [Google Scholar]
- 65. Artigas F, Nutt DJ, Shelton R. Mechanism of action of antidepressants. Psychopharmacol Bull 2002;36(Suppl 2):123–132. [PubMed] [Google Scholar]
- 66. Ichikawa J, Meltzer HY. Valproate and carbamazepine increase prefrontal dopamine release by 5‐HT1A receptor activation. Eur J Pharmacol 1999;380:R1–R3. [DOI] [PubMed] [Google Scholar]
- 67. Ichikawa J, Dai J, Meltzer HY. Lithium differs from anticonvulsant mood stabilizers in prefrontal cortical and accumbal dopamine release: Role of 5‐HT1A receptor agonism. Brain Res 2005;1049:182–190. [DOI] [PubMed] [Google Scholar]
- 68. Wędzony K, Maćkowiak M, Fijal K, Golembiowska K. Ipsapirone enhances the dopamine outflow via 5‐HT1A receptors in the rat prefrontal cortex. Eur J Pharmacol 1996;305:73–78. [DOI] [PubMed] [Google Scholar]
- 69. Nakayama K, Sakurai T, Katsu H. Mirtazapine increases dopamine release in prefrontal cortex by 5‐HT1A receptor activation. Brain Res Bull 2004;63:237–241. [DOI] [PubMed] [Google Scholar]
- 70. Sakaue M, Somboonthum P, Nishihara B, Koyama Y, Hashimoto H, Baba A, Matsuda T. Postsynaptic 5‐hydroxytryptamine1A receptor activation increases in vivo dopamine release in rat prefrontal cortex. Br J Pharmacol 2000;129:1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gobert A, Rivet JM, Cistarelli L, Melon C, Millan MJ. Buspirone modulates basal and fluoxetine‐stimulated dialysate levels of dopamine, noradrenaline and serotonin in the frontal cortex of freely moving rats: Activation of serotonin1A receptors and blockade of alpha2‐adrenergic receptors underlie its actions. Neuroscience 1999;93:1251–1262. [DOI] [PubMed] [Google Scholar]
- 72. Zhang W, Perry KW, Wong DT, Potts BD, Bao J, Tollefson GD, Bymaster FP. Synergistic effects of olanzapine and other antipsychotic agents in combination with fluoxetine on norepinephrine and dopamine release in rat prefrontal cortex. Neuropsychopharmacology 2000;23:250–262. [DOI] [PubMed] [Google Scholar]
- 73. Gobert A, Millan MJ. Serotonin (5‐HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5‐HT, in the frontal cortex of freely‐moving rats. Neuropharmacology 1999;38:315–317. [DOI] [PubMed] [Google Scholar]
- 74. Amargós‐Bosch M, Artigas F, Adell A. Effects of acute olanzapine after sustained fluoxetine on extracellular monoamine levels in the rat medial prefrontal cortex. Eur J Pharmacol 2005;516:235–238. [DOI] [PubMed] [Google Scholar]
- 75. Koch S, Perry KW, Bymaster FP. Brain region and dose effects of an olanzapine/fluoxetine combination on extracellular monoamine concentrations in the rat. Neuropharmacology 2004;46:232–242. [DOI] [PubMed] [Google Scholar]
- 76. Gobert A, Rivet JM, Cistarelli L, Millan MJ. Buspirone enhances duloxatine‐ and fluoxetine‐induced increases in dialysate levels of dopamine and noradrenaline, but not serotonin, in the frontal cortex of freely moving rats. J Neurochem 1997;68:1326–1329. [DOI] [PubMed] [Google Scholar]
- 77. Yoshino T, Nisijima K, Katoh S, Yui K, Nakamura M. Tandospirone potentiates the fluoxetine‐induced increases in extracellular dopamine via 5‐HT1A receptors in the rat frontal cortex. Neurochem Int 2002;40:355–360. [DOI] [PubMed] [Google Scholar]
- 78. Yoshino T, Nisijima K, Shioda K, Yui K, Katoh S. Perospirone, a novel atypical antipsychotic drug, potentiates fluoxetine‐induced increases in dopamine levels via multireceptor actions in the rat medial prefrontal cortex. Neurosci Lett 2004;364:16–21. [DOI] [PubMed] [Google Scholar]
- 79. Denys D, Klompmakers AA, Westenberg HG. Synergistic dopamine increase in the rat prefrontal cortex with the combination of quetiapine and fluvoxamine. Psychopharmacology 2004;176:195–203. [DOI] [PubMed] [Google Scholar]
- 80. Ago Y, Nakamura S, Baba A, Matsuda T. Sulpiride in combination with fluvoxamine increases in vivo dopamine release selectively in rat prefrontal cortex. Neuropsychopharmacology 2005;1:43–51. [DOI] [PubMed] [Google Scholar]
- 81. Huang M, Ichiwaka J, Li Z, Dai J, Meltzer HY. Augmentation by citalopram of risperidone‐induced monoamine release in rat prefrontal cortex. Psychopharmacology 2006;185:274–281. [DOI] [PubMed] [Google Scholar]
- 82. Bortolozzi A, Diaz‐Mataix L, Toth M, Celada P, Artigas F. In vivo actions of aripiprazole on serotonergic and dopaminergic systems in rodent brain. Psychopharmacology 2007;191:745–758. [DOI] [PubMed] [Google Scholar]
- 83. Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: A review and reconceptualization. Am J Psychiat 1991;148:1474–1486. [DOI] [PubMed] [Google Scholar]
- 84. Svensson TH, Nomikos GG, Andersson JL. Modulation of dopaminergic neurotransmission by 5‐HT2 antagonism In: Vanhouette PM, Saxena PR, Paoletti R, Brunello N, Jackson AS, editors. Serotonin: From cell biology to pharmacology and therapeutics. Dordrecht : Kluwer Academic Publishers, 1993;263–270. [Google Scholar]
- 85. Svensson TH, Mathe JM, Andersson JL, Nomikos GG, Hildebrand BE, Marcus M. Mode of action of atypical neuroleptics in relation to the phencyclidine model of schizophrenia: Role of 5‐HT2 receptor and alpha 1‐adrenoceptor antagonism. J Clin Psychopharmacol 1995;15:11S–18S. [DOI] [PubMed] [Google Scholar]
- 86. Arnsten AF, Cai JX, Murphy BL, Goldman‐Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology 1994;116:143–151. [DOI] [PubMed] [Google Scholar]
- 87. Sawaguchi T, Goldman‐Rakic PS. The role of D1 dopamine receptor in working memory: Local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed‐response task. J Neurophysiol 1994;71:515–528. [DOI] [PubMed] [Google Scholar]
- 88. Meltzer HY, Nash JF. VII. Effects of antipsychotic drugs on serotonin receptors. Pharmacol Rev 1991;43:587–604. [PubMed] [Google Scholar]
- 89. Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O’Laughlin IA, Meltzer HY. 5‐HT2A and D2 receptor blockade increases cortical DA release via 5‐HT1A receptor activation: A possible mechanism of atypical antipsychotic‐induced cortical dopamine release. J Neurochem 2001;76:1521–1531. [DOI] [PubMed] [Google Scholar]
- 90. Moghaddam B, Bunney BS. Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: An in vivo microdialysis study. J Neurochem 1990;54:1755–1760. [DOI] [PubMed] [Google Scholar]
- 91. Nomikos GG, Iurlo M, Andersson JL, Kimura K, Svensson TH. Systemic administration of amperozide, a new atypical antipsychotic drug, preferentially increases dopamine release in the rat medial prefrontal cortex. Psychopharmacology 1994;115:147–156. [DOI] [PubMed] [Google Scholar]
- 92. Hertel P, Nomikos GG, Iurlo M, Swensson TH. Risperidone: Regional effects in vivo on release and metabolism of dopamine and serotonin in the rat brain. Psychopharmacology 1996;124:74–186. [DOI] [PubMed] [Google Scholar]
- 93. Volonté M, Monferini E, Cerutti M, Fodritto F, Borsini F. BIMG 80, a novel potential antipsychotic drug: Evidence for multireceptor actions and preferential release of dopamine in prefrontal cortex. J Neurochem 1997;69:182–190. [DOI] [PubMed] [Google Scholar]
- 94. Kuroki T, Meltzer HY, Ichikawa J. Effects of antipsychotic drugs on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens. J Pharmacol Exp Ther 1999;288:774–781. [PubMed] [Google Scholar]
- 95. Westerink BHC, Kawahara Y, De Boer P, et al Antipsychotic drugs classified by their effects on the release of dopamine and noradrenaline in the prefrontal cortex and striatum. Eur J Pharmacol 2001;412:127–138. [DOI] [PubMed] [Google Scholar]
- 96. Li Z, Ichikawa J, Dai J, Meltzer HY. Aripiprazole, a novel antipsychotic drug, preferentially increases dopamine release in the prefrontal cortex and hippocampus in rat brain. Eur J Pharmacol 2004;493:75–83. [DOI] [PubMed] [Google Scholar]
- 97. Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology 1999;21:106S–115S. [DOI] [PubMed] [Google Scholar]
- 98. Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: Their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2003;27:1159–1172. [DOI] [PubMed] [Google Scholar]
- 99. Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D1, D2 and serotonin2 pKi values. J Pharmacol Exp Ther 1989;251:238–246. [PubMed] [Google Scholar]
- 100. Bonaccorso S, Meltzer HY, Li Z, Dai J, Alboszta AR, Ichikawa J. SR46349‐B, a 5‐HT2A /2C receptor antagonist, potentiates haloperidol‐induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Neuropsychopharmacology 2002;27:430–441. [DOI] [PubMed] [Google Scholar]
- 101. Liégeois JF, Ichikawa J, Meltzer HY. 5‐HT2A receptor antagonism potentiates haloperidol‐induced dopamine release in rat medial prefrontal cortex and inhibits that in the nucleus accumbens in a dose‐dependent manner. Brain Res 2002;947:157–165. [DOI] [PubMed] [Google Scholar]
- 102. Ichikawa J, Meltzer HY. Amperozide, a novel antipsychotic drug, inhibits the ability of D‐amphetamine to increase dopamine release in vivo in rat striatum and nucleus accumbens. J Neurochem 1992;58:2285–2291. [DOI] [PubMed] [Google Scholar]
- 103. Ichikawa J, Kuroki T, Kitchen MT, Meltzer HY. R(+)‐8‐OH‐DPAT, a 5‐HT1A receptor agonist, inhibits amphetamine‐induced dopamine release in rat striatum and nucleus accumbens. Eur J Pharmacol 1995;287:179–184. [DOI] [PubMed] [Google Scholar]
- 104. Assié M‐B, Ravailhe V, Faucillon V, Newman‐Tancredi A. Contrasting contribution of 5‐hydroxytryptamine 1A receptor activation to neurochemical profile of novel antipsychotics: Frontocortical dopamine and hippocampal serotonin release in rat brain. J Pharmacol Exp Ther 2005;315:265–272. [DOI] [PubMed] [Google Scholar]
- 105. Claustre Y, De Peretti D, Brun P, et al SSR181507, a dopamine D2 receptor antagonist and 5‐HT1A receptor agonist. Part I: Neurochemical and electrophysiological profile. Neuropsychopharmacology 2003;28:2064–2076. [DOI] [PubMed] [Google Scholar]
- 106. McCreary AC, Glennon JC, Ashby CR, et al SLV313 (1‐(2,3‐dihydro‐benzo [1,4]dioxin‐5‐yl)‐4‐[5‐(4‐fluoro‐phenyl)‐pyridin‐3‐ylmethyl]‐piperazine monohydrochloride): A novel dopamine D2 receptor antagonist and 5‐HT1A receptor agonist potential antipsychotic drug. Neuropsychopharmacology 2007;32:78–94. [DOI] [PubMed] [Google Scholar]
- 107. Assié MB, Mnie‐Filali O, Ravailhe V, et al F15063, a potential antipsychotic with dopamine D2/D3 receptor antagonist, 5‐HT1A receptor agonist and dopamine D4 receptor partial agonist properties: Influence on neuronal firing and neurotransmitter release. Eur J Pharmacol 2009;607:74–83. [DOI] [PubMed] [Google Scholar]
- 108. Millan MJ, Dekene A, Gobert A. Serotonin (5‐HT)2C receptors tonically inhibit dopamine (DA) and noradrenaline (NA), but not 5‐HT release in the frontal cortex in vivo . Neuropharmacology 1998;37:953–955. [DOI] [PubMed] [Google Scholar]
- 109. Hutson PH, Barton CL, Jay M, Blurton P, Burkamp F, Clarkson R, Bristow LJ. Activation of mesolimbic dopamine function by phencyclidine is enhanced by 5‐HT2C/2B receptor antagonists: Neurochemical and behavioural studies. Neuropharmacology 2000;39:2318–2328. [DOI] [PubMed] [Google Scholar]
- 110. Navailles S, De Deurwaerdère PD, Spampinato U. Clozapine and haloperidol differentially alter the constitutive activity of central serotonin2C receptors in vivo . Biol Psychiatry 2006;59:568–575. [DOI] [PubMed] [Google Scholar]
- 111. Herrick‐Davis K, Grinde E, Teitler M. Inverse agonist activity of atypical antipsychotic drugs at human 5‐hydroxytryptamine2C receptors. J Pharmacol Exp Ther 2000;295:226–232. [PubMed] [Google Scholar]
- 112. Rauser L, Savage JE, Meltzer HY, Roth BL. Inverse agonist actions of typical and atypical antipsychotic drugs at the uman 5‐hydroxytryptamine2C receptor. J Pharmacol Exp Ther 2001;299:83–89. [PubMed] [Google Scholar]
- 113. Reavill C, Kettle A, Holland V, Riley G, Blackburn TP. Attenuation of haloperidol‐induced catalepsy by a 5‐HT2C receptor antagonist. Br J Pharmacol 1999;126:572–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Marquis KL, Sabb AL, Logue SF, et al WAY‐163909 [(7bR,10aR)‐1,2,3,4,8,9,10,10a‐Octahydro‐7bH‐cyclopenta‐[b][1,4]diazepino[6,7,1hi]indole]a novel 5‐hydroxytryptamine 2C receptor‐selective agonist with preclinical antipsychotic‐like activity. J Pharmacol Exp Ther 2007;320:486–496. [DOI] [PubMed] [Google Scholar]
- 115. Jones BJ, Blackburn TP. The medical benefit of 5‐HT research. Pharmacol Biochem Behav 2002;71:555–568. [DOI] [PubMed] [Google Scholar]
- 116. Andersson JL, Nomikos GG, Marcus M, Hertel P, Mathé JM, Svensson TH. Ritanserin potentiates the stimulatory effects of raclopride on neuronal activity and dopamine release selectively in the mesolimbic DA‐ergic system. Naunyn-Schmiedeberg's Arch Phatmacol 1995;352:374–385. [DOI] [PubMed] [Google Scholar]
- 117. Li Z, Ichikawa J, Huang M, Prus AJ, Dai J, Meltzer HY. ACP‐103, a 5‐HT2A /2C inverse agonist, potentiates haloperidol‐induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Psychopharmacology 2005;183:144–153. [DOI] [PubMed] [Google Scholar]
- 118. Ehringer H, Hornykiewicz O. Distribution of noradrenaline and dopamine (3‐hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Klin Wochenschr 1960;38:1236–1239. [DOI] [PubMed] [Google Scholar]
- 119. Nicholson SL, Brotchie JM. 5‐hydroxytryptamine (5‐HT, serotonin) and Parkinson's disease—opportunities for novel therapeutics to reduce the problems of levodopa therapy. Eur J Neurol 2002;9:1–6. [DOI] [PubMed] [Google Scholar]
- 120. Utter AA, Basso MA. The basal ganglia: An overview of circuits and function. Neurosci Biobehav Rev 2008;32:333–342. [DOI] [PubMed] [Google Scholar]
- 121. Albin R, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 1989;12:366–375. [DOI] [PubMed] [Google Scholar]
- 122. Hagan JJ, Middlemiss DN, Sharp PC, Poste GH. Parkinson's disease: Prospects for improved drug therapy. Trends Pharmacol Sci 1997;18:156–163. [DOI] [PubMed] [Google Scholar]
- 123. Agid Y. Levodopa: Is toxicity a myth? Neurology 1998;50:858–863. [DOI] [PubMed] [Google Scholar]
- 124. Rodriguez MC, Obeso JA, Olanow CW. Subthalamic nucleus‐mediated excitoxicity in Parkinson's disease: A target for neuroprotection. Ann Neurol 1998;44(Suppl):S175–S188. [DOI] [PubMed] [Google Scholar]
- 125. Di Giovanni G, Di Matteo V, Pierucci M, Benigno A, Esposito E. Serotonin involvement in the basal ganglia pathophysiology: Could the 5‐HT2C receptor be a new target for therapeutic strategies? Curr Med Chem 2006;13:3069–3081. [DOI] [PubMed] [Google Scholar]
- 126. Knobelman DA, Kung HF, Lucky I. Regulation of extracellular concentrations of 5‐hydroxytryptamine (5‐HT) in mouse striatum by 5‐HT1A and 5‐HT1B receptors. J Pharmacol Exp Ther 2000;292:1111–1117. [PubMed] [Google Scholar]
- 127. Fox SH, Brotchie JM. A role for 5‐HT2C receptor antagonists in the treatment of Parkinson's disease? Drugs News Perspect 1999;12:477–483. [Google Scholar]
- 128. Pasqualetti M, Ori M, Castagna M, Marazziti D, Cassano GB, Nardi I. Distribution and cellular localization of the serotonin type 2C receptor messenger RNA in the human brain. Neuroscience 1999;92:601–611. [DOI] [PubMed] [Google Scholar]
- 129. Radja F, Descarrier L, Dewar KM, Reader TA. Serotonin 5‐HT1 and 5‐HT2 receptors in adult rat brain after destruction of nigrostriatal dopamine neurons: A quantitative autoradiographic study. Brain Res 1993;606:273–285. [DOI] [PubMed] [Google Scholar]
- 130. Fox SH, Brotchie JM. 5‐HT2C receptor binding is increased in the substantia nigra pars reticulata in Parkinson's disease. Mov Disord 2000;15:1064–1069. [DOI] [PubMed] [Google Scholar]
- 131. Fox SH, Brotchie JM. 5‐HT2C receptor antagonists enhance the behavioural response to dopamine D1 receptor agonists in the 6‐hydroxydopamine‐lesioned rat. Eur J Pharmacol 2000;398:59–64. [DOI] [PubMed] [Google Scholar]
- 132. Fox SH, Moser B, Brotchie JM. Behavioural effects of 5‐HT2C receptor antagonism in the substantia nigra zona reticulata of the 6‐hydroxydopamine‐lesioned rat model of Parkinson's disease. Exp Neurol 1998;151:35–49. [DOI] [PubMed] [Google Scholar]
- 133. Spanagel R, Weiss F. The dopamine hypothesis of reward: Past and current status. Trends Neurosci 1999;22:521–527. [DOI] [PubMed] [Google Scholar]
- 134. Carboni E, Acquas E, Frau R, Di Chiara G. Differential inhibitory effects of a 5‐HT3 antagonist on drug‐induced stimulation of dopamine release. Eur J Pharmacol 1989;164:515–519. [DOI] [PubMed] [Google Scholar]
- 135. Pei Q, Zetterstrom T, Leslie R, Grahame‐Smith D. 5‐HT3 receptor antagonists inhibit morphine‐induced stimulation of mesolimbic dopamine release and function in the rat. Eur J Pharmacol 1993;230:63–68. [DOI] [PubMed] [Google Scholar]
- 136. Kankaanpää A, Meririnne E, Seppälä T. 5‐HT3 receptor antagonist MDL 72222 attenuates cocaine‐ and mazindol‐, but not methylphenidate‐induced neurochemical and behavioral effects in the rat. Psychopharmacology 2002;159:341–350. [DOI] [PubMed] [Google Scholar]
- 137. De Deurwaerdère P, Moison D, Navailles S, Porras G, Spampinato U. Regionally and functionally distinct serotonin3 receptors control in vivo dopamine outflow in the rat nucleus accumbens. J Neurochem 2005;94:140–149. [DOI] [PubMed] [Google Scholar]
- 138. McNeish CS, Svingos AL, Hitzemann R, Strecker RE. The 5‐HT3 antagonist zacopride attenuates cocaine‐induced increases in extracellular dopamine in rat nucleus accumbens. Pharmacol Biochem Behav 1993;45:759–763. [DOI] [PubMed] [Google Scholar]
- 139. Kankaanpää A, Lillsunde P, Ruotsalainen M, Ahtee L, Seppälä T. 5‐HT3 receptor antagonist MDL 72222 dose‐dependently attenuates cocaine‐ and amphetamine‐induced elevations of extracellular dopamine in the nucleus accumbens and the dorsal striatum. Pharmacol Toxicol 1996;78:317–321. [DOI] [PubMed] [Google Scholar]
- 140. Imperato A, Angelucci L. 5‐HT3 receptors control dopamine release in the nucleus accumbens of freely moving rats. Neurosci Lett 1989;101:214–217. [DOI] [PubMed] [Google Scholar]
- 141. Wozniak KM, Pert A, Linnoila M. Antagonism of 5‐HT3 receptors attenuates the effects of ethanol on extracellular dopamine. Eur J Pharmacol 1990;187:287–289. [DOI] [PubMed] [Google Scholar]
- 142. Campbell A, Kohl R, McBride W. Serotonin‐3 receptor and ethanol‐stimulated somatodendritic dopamine release. Alcohol 1996;13:569–574. [DOI] [PubMed] [Google Scholar]
- 143. Campbell W, McBride WJ. Serotonin‐3 receptor and ethanol‐stimulated dopamine release in the nucleus accumbens. Pharmacol Biochem Behav 1995;51:835–842. [DOI] [PubMed] [Google Scholar]
- 144. Yoshimoto K, Yayama K, Sorimachi Y, et al Possibility of 5‐HT3 receptor involvement in alcohol dependance: A microdialysis study of nucleus accumbens dopamine and serotonin release in rats with chronic alcohol consumption. Alcohol Clin Exp Res 1996;20:311A–319A. [PubMed] [Google Scholar]
- 145. Invernizzi R, Pozzi L, Samanin R. Selective reduction of extracellular dopamine in the rat nucleus accumbens following chronic treatment with DAU6215, a 5‐HT3 receptor antagonist. Neuropharmacology 1995;34:211–215. [DOI] [PubMed] [Google Scholar]
- 146. Porras G, De Deurwaerdère P, Moison D, Spampinato U. Conditional involvement of striatal serotonin3 receptors in the control of in vivo dopamine outflow in the rat striatum. Eur J Neurosci 2003;17:771–781. [DOI] [PubMed] [Google Scholar]
- 147. Cervo L, Pozzi L, Samanin R. 5‐HT3 receptor antagonists do not modify cocaine place conditioning or the rise in extracellular dopamine in the nucleus accumbens of rats. Pharmacol Biochem Behav 1996;55:33–37. [DOI] [PubMed] [Google Scholar]
- 148. Parsons L, Koob GF, Weiss F. RU24969, a 5‐HT1B /1A receptor agonist, potentiates cocaine‐induced increases in nucleus accumbens dopamine. Synapse 1999;32:132–135. [DOI] [PubMed] [Google Scholar]
- 149. O’Dell L, Parsons L. Serotonin1B receptors in the ventral tegmental area modulate cocaine‐induced increases in nucleus accumbens dopamine levels. J Pharmacol Exp Ther 2004;311:711–719. [DOI] [PubMed] [Google Scholar]
- 150. Yan QS, Zheng SZ, Feng MJ, Yan SE. Involvement of 5‐HT1B receptors within the ventral tegmental area in ethanol‐induced increases in mesolimbic dopaminergic transmission. Brain Res 2005;1060:126–137. [DOI] [PubMed] [Google Scholar]
- 151. Maurel‐Remy S, Bervoets K, Millan MJ. Blockade of phencyclidine‐induced hyperlocomotion by clozapine and MDL 100,907 in rats reflects antagonism of 5‐HT2A receptors. Eur J Pharmacol 1995;280:R9–R11. [DOI] [PubMed] [Google Scholar]
- 152. Schmidt CJ, Fadayel GM, Sullivan CK, Taylor VL. 5‐HT2 receptors exert a state‐dependent regulation of dopaminergic function: Studies with MDL 100,907 and the amphetamine analogue, 3,4‐methylenedioxymethamphetamine. Eur J Pharmacol 1992;223:65–74. [DOI] [PubMed] [Google Scholar]
- 153. Kehne JH, Ketteler HJ, McCloskey TC, Sullivan CK, Dudley MW, Schmidt CJ. Effects of the selective 5‐HT2A receptor antagonist MDL 100,907 on MDMA‐induced locomotor stimulation in rats. Neuropsychopharmacology 1996;15:116–124. [DOI] [PubMed] [Google Scholar]
- 154. Bankson GM, Cunningham KA. Pharmacological studies of the acute effects of (+)‐3,4‐Methylenedioxymethamphetamine on locomotor activity: Role of 5‐HT1B /1D and 5‐HT2 receptors. Neuropsychopharmacology 2002;26:40–52. [DOI] [PubMed] [Google Scholar]
- 155. Fletcher PJ, Korth KM, Robinson SR, Baker GB. Multiple 5‐HT receptors are involved in the effects of acute MDMA treatment: Studies on locomotor activity and responding for conditioned reinforcement. Psychopharmacology 2002;162:282–291. [DOI] [PubMed] [Google Scholar]
- 156. Porras G, Di Matteo V, Fracasso C, et al 5‐HT2A and 5‐HT2C /2B receptor subtypes modulate dopamine release induced in vivo by amphetamine and morphine in both the rat nucleus accumbens and striatum. Neuropsychopharmacology 2002;26:311–324. [DOI] [PubMed] [Google Scholar]
- 157. Willins DL, Meltzer HY. Serotonin 5‐HT2C agonists selectively inhibit morphine‐induced dopamine efflux in the nucleus accumbens. Brain Res 1998;781:291–299. [DOI] [PubMed] [Google Scholar]
- 158. McCreary AC, Cunningham KA. Effects of the 5‐HT2C /2B antagonist SB 206553 on hyperactivity induced by cocaine. Neuropsychopharmacology 1999;20:556–564. [DOI] [PubMed] [Google Scholar]
- 159. O’Neill MF, Heron‐Maxwell CL, Shaw G. 5‐HT2 receptor antagonism reduces hyperactivity induced by amphetamine, cocaine, and MK‐801 but not D1 agonist C‐APB. Pharmacol Biochem Behav 1999;63:237–243. [DOI] [PubMed] [Google Scholar]
- 160. McMahon LR, Cunningham KA. Antagonism of 5‐hydroxytryptamine2A receptors attenuates the behavioral effects of cocaine in rats. J Pharmacol Exp Ther 2001;297:357–363. [PubMed] [Google Scholar]
- 161. Fletcher PJ, Phil D, Grottick AJ, Higgins GA. Differential effects of the 5‐HT2A receptor antagonist M100,907 and the 5‐HT2C receptor antagonist SB242,084 on cocaine‐induced locomotor activity, cocaine self‐administration and cocaine‐induced reinstatement of responding. Neuropsychopharmacology 2002;27:576–586. [DOI] [PubMed] [Google Scholar]
- 162. Rocha BA, Goulding EH, O’Dell LE, Mead AN, Coufal NG, Parsons LH, Tecott LH. Enhanced locomotor, reinforcing, and neurochemical effects of cocaine in serotonin 5‐hydroxytryptamine 2C receptor mutant mice. J Neurosci 2002;22:10039–10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Grottick AJ, Fletcher PJ, Higgins GA. Studies to investigate the role of 5‐HT2C receptors on cocaine‐ and food‐maintained behavior. J Pharmacol Exp Ther 2000;295:1183–1191. [PubMed] [Google Scholar]
- 164. Tomkins DM, Joharchi N, Tampakeras M, Martin JR, Wichmann J, Higgins GA. An investigation of the role of 5‐HT2C receptors in modifying ethanol self‐administration behaviour. Pharmacol Biochem Behav 2002;71:735–744. [DOI] [PubMed] [Google Scholar]
- 165. Grottick AJ, Corrigall WA, Higgins GA. Activation of 5‐HT2C receptors reduces the locomotor and rewarding effects of nicotine. Psychopharmacology 2001;157:292–298. [DOI] [PubMed] [Google Scholar]
- 166. Di Matteo V, Pierucci M, Esposito E. Selective stimulation of serotonin2C receptors blocks the enhancement of striatal and accumbal dopamine release induced by nicotine administration. J Neurochem 2004;89:418–429. [DOI] [PubMed] [Google Scholar]
- 167. Pierucci M, Di Matteo V, Esposito E. Stimulation of serotonin2C receptors blocks the hyperactivation of midbrain dopamine neurons induced by nicotine administration. J Pharmacol Exp Ther 2004;309:109–118. [DOI] [PubMed] [Google Scholar]
- 168. Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 2004;303:2040–2042. [DOI] [PubMed] [Google Scholar]
- 169. Kelland MD, Freeman AS, Chiodo LA. Chloral hydrate anaesthesia alters the responsiveness of identified midbrain dopamine neurons to dopamine agonist administration. Synapse 1989;3:30–37. [DOI] [PubMed] [Google Scholar]


