Abstract
Cholinesterase inhibitors constitute one of the mainstays of treatment of Alzheimer disease (AD). Gastrointestinal side effects, difficulty accessing therapeutic doses and poor patient compliance have been identified as barriers to effective treatment with these substances. The rivastigmine transdermal patch provides continuous delivery of drug through the skin into the bloodstream, avoiding the fluctuations in plasma concentration associated with oral administration. This pharmacokinetic profile is associated with reduced side effects, resulting in easier access to expected target doses. These benefits, along with other practical advantages of the transdermal patch, may contribute to enhanced patient compliance. Here, we present a review of the current literature on rivastigmine patch, and offer advice based on our own collective clinical experience. Rivastigmine patch provides an efficient option for managing patients with AD, to be considered among the first line therapies for the disease.
Keywords: Alzheimer's disease, Patch, Rivastigmine, Transdermal, Treatment
Introduction
Conventionally, treatments for the management of Alzheimer's disease (AD) have all been administered orally. Cholinesterase inhibitors, exemplified by donepezil, galantamine, and rivastigmine, form one of the mainstays of AD treatment and have been available since 1997. The N‐methyl d‐aspartate (NMDA)‐receptor antagonist memantine followed in 2006. Oral rivastigmine (capsules or liquid) was also approved in many countries worldwide for the treatment of mild to moderate Parkinson's disease dementia (PDD) in 2006.
Alzheimer's disease is a progressive neurodegenerative condition that predominantly affects the elderly population; as such the management of the disease poses challenges to both the patient and their caregiver. There can be a high medication burden due to concurrent illnesses [1]. The use of multiple drugs every day places AD patients at a greater risk for poor compliance, hence effective and well‐tolerated treatment options that might enhance compliance are welcome [2]. Transdermal patch therapy has the potential to reduce the side effects observed with orally administered drugs allowing easier access to optimal doses and easier administration, benefits which are expected to enhance patient compliance.
Rivastigmine is a cholinesterase inhibitor that inhibits both acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) [3]. The efficacy of oral rivastigmine has been demonstrated in pivotal clinical trials involving more than 3000 AD patients and 500 PDD patients [4, 5, 6, 7, 8]. Rivastigmine is a small molecule (<400 Da), and is both lipophilic and hydrophilic [9]. These properties mean that rivastigmine can pass easily through the skin into the bloodstream as well as through the blood–brain barrier, making it well‐suited to transdermal delivery. Its high potency is also advantageous since it allows a transdermal patch to be small and discreet [9]. These properties, combined with improved patch technology, constituted the basis for the development of rivastigmine transdermal patch, the first transdermal option for the symptomatic treatment of mild to moderate AD [10]. Several primary papers have been published demonstrating the pharmacokinetic profile, efficacy, and safety of rivastigmine capsules and patch. The objective of this article is to provide primary care physicians, specialists, and other professionals working in the field with a concise and comprehensive review of the rivastigmine transdermal patch for treatment of AD.
Rationale for Transdermal Patch Treatment in Dementia
Cholinesterase inhibitors are associated with central cholinergic gastrointestinal side effects, such as nausea and vomiting, particularly during initial dose titration [11, 12]. This is believed to be caused by the rapid increase in acetylcholine levels in the brain resulting from inhibition of the target enzyme(s) [13]. Strategies that prolong the time to reach maximal drug plasma concentrations have been shown to reduce the incidence of gastrointestinal side effects with cholinesterase inhibitors, in particular with rivastigmine. For example, administering rivastigmine with food reduces gastrointestinal side effects by delaying absorption from the gastrointestinal tract [14]. Further, in a 26‐week placebo‐controlled study in which rivastigmine capsules were administered twice daily (BID) or three times daily (TID) in 687 mild to moderate AD patients, the TID dosing regimen was shown to be superior in terms of tolerability [7]. These findings indicated that a more gradual increase in plasma concentration that prolongs the time to reach maximal values, would be expected to lead to reduced side effects. In turn, this would allow easier access to optimal doses, improving patient compliance and potentially efficacy. Transdermal administration delivers a drug directly through the skin into the bloodstream, avoiding the gastrointestinal tract, thus avoiding the first‐pass effect as well as potential interaction with other drugs with regards to absorption. Smooth delivery is also expected to provide a steadier plasma concentration of the drug.
Along with an improved pharmacokinetic profile leading to improved tolerability, transdermal drug delivery may offer other practical benefits. Some AD patients have been shown to exhibit “non‐responsiveness” to cholinesterase inhibitors. It has been proposed that this may be due to genetic factors, ultra‐rapid metabolism or progressive damage to cholinergic neurons; in some cases, however, it may be due to under‐dosing and poor compliance [15]. The simplified dosing regimen provided by a transdermal patch has the potential to improve compliance and provide access to higher doses, particularly for those patients who already have a high medication burden [1]. A transdermal patch also provides visual reassurance to the caregiver that medication is being taken, as well as reducing the risk of accidental over‐dosing. There is an additional advantage for patients that have swallowing difficulties.
Pharmacokinetic Profile of the Rivastigmine Patch
The pharmacokinetic profile of the rivastigmine patch was compared to that of the rivastigmine capsule in an open‐label, ascending dose study of 51 AD patients [16]. Rivastigmine capsule was rapidly absorbed, with a median tmax of 1 h for all doses. In comparison, tmax with rivastigmine patch was reached at approximately 8 h for all patch sizes, reflecting the more gradual increase in plasma concentration with transdermal administration. The mean Cmax with transdermal administration was consistently lower than with capsules, and there was less variation in peak–trough rivastigmine concentrations, demonstrating a smoother, continuous drug delivery [16].
Drug exposure, assessed by measuring the area under the curve, showed that the starting dose 4.6 mg/24 h patch provided comparable drug exposure to 6 mg/day capsules, while the target dose 9.5 mg/24 h patch provided comparable exposure to the highest recommended dose of capsules, 12 mg/day (Figure 1) [17, 18]. The efficacy of rivastigmine is dose‐dependent, but a 6 mg/day dose of rivastigmine capsules has been shown to provide therapeutic drug levels [19]. As the 4.6 mg/24 h patch provides comparable drug exposure to 6 mg/day capsules, transdermal administration of rivastigmine is expected to provide plasma concentrations within the range of effective therapeutic doses from the initiation of treatment.
Figure 1.
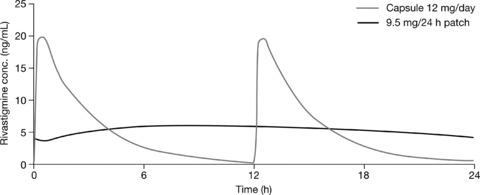
Mean plasma rivastigmine levels following administration of 9.5 mg/24 h patch versus 12 mg/day capsules (adjusted for baseline weight and gender) [17]. Reproduced from Expert Rev Neurotherapeutics 2007;7:1457–1463 with permission from Expert Reviews Ltd [18].
The pharmacokinetic study was also designed to compare inhibition of target enzymes with the rivastigmine patch and capsule [16]. AChE has an extremely low concentration in the plasma, and as such the enzymatic activity is hard to measure. However, as rivastigmine is a dual inhibitor, the enzymatic activity of BuChE in the plasma may be used as a marker of target enzyme inhibition over time [20, 21]. The pharmacodynamics of rivastigmine mirrored the pharmacokinetic profile, with the transdermal patch providing a smooth, continuous reduction in plasma BuChE activity, while two distinct peak–troughs were seen with capsule administration.
A separate study examined the effect of body application site on the pharmacokinetics of rivastigmine patch, and showed that bioavailability was greatest when the patch was applied to the upper back, upper arm, or chest [22]. Adhesiveness was also good at these body sites. The patch may be applied to one of these sites to ensure maximal exposure, although application site rotation (including alternating between left and right sides of the body each day) is an important strategy in optimizing skin tolerability with medical patches.
In order to provide the concentration gradient required to drive the diffusion of rivastigmine through the skin, all rivastigmine transdermal patches are loaded with a greater amount of the drug than will be absorbed into the bloodstream. The average amount of rivastigmine absorbed from a patch over a 24‐h application period is approximately 50% of the total loading dose [16]. Absorption of any remaining rivastigmine following the 24‐h application period occurs very slowly. Although the previous patch should be removed before the new one is administered, patients are at a low risk of toxic exposure should a new patch be mistakenly applied without prior removal of the previous patch [23]. Once the patch is removed, the short elimination half‐life of rivastigmine (∼3.4 h) ensures a rapid reduction of drug levels in the plasma [16]. As a result, even with the continuous delivery provided by the rivastigmine patch, there is little potential for drug accumulation in the body. This can also be an advantage under emergency conditions when a rapid removal of the cholinergic drug is desired.
Efficacy and Safety of the Rivastigmine Patch
The efficacy and safety of the rivastigmine patch was evaluated in a single, 24‐week, international, randomized, double‐blind trial of 1,195 patients with mild to moderate AD [10]. The Investigation of transDermal Exelon in ALzheimer's disease (IDEAL) study enrolled patients with probable AD (Mini‐Mental State Examination [MMSE] scores of 10–20). Patients were randomized to placebo, rivastigmine capsule (12 mg/day), rivastigmine 9.5 mg/24 h patch or rivastigmine 17.4 mg/24 h patch. They were titrated to their target doses in 4‐week intervals over 16 weeks and maintained at their highest well‐tolerated dose for the remaining 8 weeks. Primary outcome measures were the Alzheimer's Disease Assessment Scale‐Cognitive subscale (ADAS‐cog), and the Alzheimer's Disease Cooperative Study‐Clinical Global Impression of Change (ADCS‐CGIC). Secondary efficacy outcomes included the Alzheimer's Disease Cooperative Study‐Activities of Daily Living (ADCS‐ADL), the MMSE, the Neuropsychiatric Inventory (NPI), and the Trail‐making Test part A (TMT‐A).
With regard to the drug exposure, although the target dose in the capsule group was 12 mg/day, the mean oral dose was 9.7 mg/day, with 64.4% of patients achieving the maximal 12 mg/day. In contrast, the mean patch size applied to patients in the 9.5 mg/24 h (10 cm2) patch group was 9.8 cm2, with almost all patients (95.9%) reaching the target 9.5 mg/24 h patch [10, 23].
All rivastigmine treatment groups (patch and capsule) showed significant improvements compared with placebo at Week 24 with respect to the primary outcomes (ADAS‐cog, ADCS‐CGIC), as well as many of the secondary outcomes (ADCS‐ADL, MMSE, TMT‐A) (Table 1) [10]. The 9.5 mg/24 h patch provided similar efficacy to capsules (maximum 12 mg/day) on these outcome measures.
Table 1.
Mean changes from baseline at Week 24 for primary and secondary efficacy measures in the IDEAL study (ITT‐LOCF population) [10]. Reproduced from Expert Rev Neurotherapeutics 2007;7:1457–63 with permission from Expert Reviews Ltd. [18]
| Mean 24‐week change from baseline | |||
|---|---|---|---|
| Rivastigmine 9.5 mg/24 h patch | Rivastigmine 12 mg/day capsules | Placebo | |
| Primary measures | |||
| ADAS‐cog | −0.6** | −0.6** | 1.0 |
| ADCS‐CGIC | 3.9** | 3.9** | 4.2 |
| Secondary measures | |||
| MMSE | 1.1** | 0.8** | 0.0 |
| Trail Making Test part A | −12.3*** | −9.8*** | 7.7 |
| ADCS‐ADL | −0.1** | −0.5* | −2.3 |
| NPI‐12 | −1.7 | −2.2 | −1.7 |
ADAS‐cog, cognitive subscale of the Alzheimer's Disease Assessment Scale; ADCS‐ADL, Alzheimer's Disease Cooperative Study Activities of Daily Living scale; ADCS‐CGIC, Alzheimer's Disease Cooperative Study Clinical Global Impression of Change; MMSE, Mini‐Mental State Examination; NPI‐12, 12‐item Neuropsychiatric Inventory.
Negative change scores on ADAS‐cog, Trail Making Test part A, and NPI‐12 indicate improvement.
Negative change scores on MMSE and ADCS‐ADL indicate deterioration.
ADCS‐CGIC is scored as a judgement of change, with a score of 4 indicating no change, <4 indicating improvement, and >4 indicating deterioration.
*P≤ 0.05, **P≤ 0.01, ***P≤ 0.001 versus placebo.
The most striking findings comparing oral capsules with transdermal patch were observed with regards to adverse events. The most commonly reported adverse events in the IDEAL study tended to be those associated with cholinergic overstimulation in general, including gastrointestinal events, such as nausea, vomiting, and diarrhoea [10]; these were mostly classed as mild or moderate. In the 9.5 mg/24 h patch treatment group, 7% and 6% of patients experienced nausea and vomiting, respectively, compared with 23% and 17% of patients receiving capsule. The incidence of nausea and vomiting in the 9.5 mg/24 h rivastigmine patch group was not significantly different to that observed with placebo. Weight loss was less frequent in patch‐treated patients compared with those who received capsule (3% in the 9.5 mg/24 h patch group, 5% in the capsule group, and 1% in the placebo group experienced a decrease in weight during the 24‐week study).
The majority of patients experienced no, mild, or moderate application site skin reactions, and the incidence of severe skin reactions was low. In total, 7.6% of patients reported moderate or severe erythema with the 9.5 mg/24 h patch, and less than 2.5% of patients discontinued due to adverse skin reactions [10].
Switching to the Rivastigmine Patch
During an open‐label extension of the IDEAL study, in which all patients who entered the extension phase were switched to the 9.5 mg/24 h patch without titration, rivastigmine patch displayed a similar safety and tolerability profile; treatment effects were largely sustained for up to 1 year [24]. Similar to the double‐blind phase, adverse events were predominantly cholinergic in nature and mild to moderate in severity, with nausea and vomiting reported most frequently. A relevant finding was that patients formerly randomized to rivastigmine treatment (capsule or patch) reported fewer adverse events than those formerly randomized to placebo, indicating that treatment with rivastigmine patch should be started with the lower patch size in patients who have not been previously exposed to rivastigmine [24].
The SWitch from Aricept to Patch (SWAP) study was a prospective, 5‐week, open‐label, randomized, parallel group study that evaluated the safety and tolerability of switching from 5 to 10 mg/day donepezil tablets to rivastigmine transdermal patch [25]. Patients were randomized to two groups: those who switched to rivastigmine 4.6 mg/24 h patch immediately, and those who discontinued donepezil treatment 7 days before initiation of patch therapy. In both groups, rivastigmine patch was well‐tolerated, with a low incidence of nausea (<4% in both groups) and vomiting (<2% in both groups). There were no statistically significant differences between the switch paradigm groups with regards to adverse events or to the number of patient discontinuations [25].
Based on these two studies [24, 25], guidelines for switching patients already receiving oral cholinesterase inhibitors to rivastigmine patch have been developed. New (treatment‐naïve) patients should receive the 4.6 mg/24 h patch for 4 weeks and then, tolerability permitting, be titrated to the 9.5 mg/24 h patch. Patients taking ≤6 mg/day rivastigmine orally should switch to the 4.6 mg/24 h patch before titrating to the target dose after 4 weeks. Patients taking >6 mg/day rivastigmine orally may switch directly to 9.5 mg/24 h patch, unless an oral dose of 9 mg/day has not been stable or well‐tolerated, in which case a switch to the 4.6 mg/24 h patch is recommended. These recommendations are based on EU guidelines [26]; regional differences may apply. For example, in the USA, it is recommended that patients receiving an oral dose of less than 6 mg/day are switched to the 4.6 mg/24 h patch, while all patients receiving 6 mg/day or more can be switched directly to the 9.5 mg/24 h patch [27].
Based on our clinical experience, a perceptible loss of efficacy may be observed by caregivers in patients who are switched from the 12 mg/day oral doses to the 4.6 mg/24 h patch, and vice versa, an intolerable “excessive” effect with hyperactivity, restlessness and irritability may appear if patients on oral doses of less than 6 mg/day are put directly on the 9.5 mg/24 h patch.
Patients with AD receiving donepezil tablets may also switch directly to rivastigmine 4.6 mg/24 h patch without a withdrawal period. The first patch should be applied the day after the last oral dose. To date, no clinical studies evaluating the safety and tolerability of switching patients from galantamine to the rivastigmine patch have been published in the primary literature. The choice of patch size to be initially used in patients previously receiving galantamine is therefore based on physician's discretion.
Similarly, there are currently no data available on the use of rivastigmine patch in combination with memantine. However, studies with rivastigmine capsule and memantine in patients with AD did not detect any safety issues with combination therapy [28, 29, 30]. Our clinical experience suggests that combination therapy with rivastigmine patch and memantine is tolerable and may provide added benefits.
Clinical Utility of Rivastigmine Patch
The rivastigmine patch is applied once‐daily. It is important to ensure that the caregiver is aware that the old patch should be removed before a new patch is applied. Caregivers can write on the rivastigmine patch to serve as a reminder, if necessary. Some caregivers write the day of the week onto the patch so that they can be sure that they have applied the patch that day and removed the previous day's patch. Everyday activities, such as bathing, showering, and swimming, can be continued. When the 24‐h period is over, the patch should be removed gently (“ripping”off the patch should be avoided, as this can damage fragile skin). Any residual patch adhesive can be removed using an oil‐based substance such as olive oil or baby oil.
The rivastigmine patch should be applied to gently cleaned, dry, hairless, intact, healthy skin. Recommended application sites are the upper or lower back, chest, or upper arm. It is important to rotate the application site daily (alternating between left and right sides of the body); if possible, the same exact area should not be used again for at least 14 days. This simple rotation of the application site can result in better skin tolerability [27].
When using the patch, some patients may develop skin reactions. These typically take the form of erythema (redness) and pruritus (itching), caused by irritation, and usually do not present a serious medical problem. Nevertheless, simple steps may be taken to minimize these events and enhance patient comfort. Other than rotation of the application site, additional precautions include keeping skin healthy by moisturizing (particularly for elderly patients who tend to have dry, fragile skin), and trimming rather than shaving excess hair at the application site. Should irritation occur, moisturizers may help speed the healing of secondary symptoms such as scaling [31], and antihistamines or zinc oxide/iron (III) oxide suspension can relieve troublesome itching. Allergic dermatitis with rivastigmine patch is rare, and typically manifests as localized redness with swelling, which may “spread” beyond the border of the patch. Topical corticosteroids may be used in cases of severe allergic dermatitis. If the signs and symptoms of dermatitis are not tolerable by the patient at any point, patch treatment should be discontinued. Appropriate management and treatment will help to ensure that any signs and symptoms of skin reactions, should they occur, will likely be transient, mild‐to‐moderate, and cause minimal discomfort. If skin reactions remain, but the patient and caregiver are satisfied with patch treatment, we recommend consulting a dermatologist for advice on overcoming the skin tolerability problems.
Adhesion of the patch to the skin is very good, with caregivers in the IDEAL study reporting that the patch was completely attached or had the edges just lifting off in 96% of the 1,336 adhesion evaluations of the 9.5 mg/24 h patch. This was despite normal activities such as bathing being allowed, and some study centres being located in warm climates where perspiration would be expected [10].
Caregiver preference for patches versus capsules was a prospective outcome measure in the IDEAL study. The results showed that more than 70% of caregivers preferred patches to capsules overall. Patches were preferred to capsules with respect to ease of use and ease of following the schedule, and caregivers indicated greater satisfaction with patch therapy overall, and less interference with daily life [32]. Improved caregiver satisfaction may be expected to lead to better compliance to treatment.
The rivastigmine patch was first approved for the treatment of mild to moderate AD in Europe in 2007. Our clinical experience with the rivastigmine patch supports findings from the clinical studies, confirming that the patch is an effective and valuable treatment option for AD patients. Based on our own cumulative clinical experience, some practical advice and suggestions for physicians prescribing the rivastigmine patch to their patients, and some potential additional features of the patch that we have observed in our clinics, are listed in Table 2.
Table 2.
Practical tips and comments on utility of rivastigmine patch, based on clinical experience
| • May help to improve patient compliance; may be ideal for patients who already have high medication burdens or swallowing problems. |
| • May reduce caregiver stress as caregivers indicated reduced interference with daily life and greater overall satisfaction. |
| • Skin reactions may occur; irritant reactions account for the majority of cases, and are generally harmless and manageable. |
| • The risk of skin irritation can be minimized by: |
| ○ rotating the application site daily |
| ○ removing the patch gently (avoiding “ripping”) |
| ○ removing residual adhesive with oil‐based substances such as olive oil |
| ○ keeping skin healthy by moisturizing |
| ○ trimming rather than shaving any hair at the application site. |
| • Should irritation occur, moisturizer can be used to speed healing, antihistamines, or ointments can relieve troublesome itching. |
| • Ensure that there are no misunderstandings regarding application sites; only one site should be used at a time. Do not cut the patch. The old patch should be removed before a new patch is applied, and the old one should be properly disposed of as it still contains active substance. |
Conclusions
Cholinesterase inhibitors are commonly used to treat the symptoms of AD. However, poor patient compliance due to gastrointestinal side effects and difficulty accessing therapeutic doses with oral administration can be a barrier to effective therapy [1]. Although patients typically have AD for more than 7 years [33], studies have shown that the average treatment duration with the oral cholinesterase inhibitors rivastigmine and donepezil ranges from about 120 to 500 days [34, 35, 36]. The advantages of continuing cholinesterase inhibitor therapy should not be underestimated; patients who manage to stay on therapy for longer have a greater chance of slowing or delaying progression of their symptoms, and a decreased risk of institutionalization [37, 38].
The pharmacokinetic profile of the rivastigmine transdermal patch reduces side effects, allowing patients to more easily reach target doses and to stay on treatment for longer [10, 23]. These benefits, along with the simplified drug regimen, visual reassurance of drug administration and other practical advantages provided by the patch, give transdermal administration the potential to improve treatment compliance in AD. Elderly people may derive particular benefit from transdermal drug administration for a number of reasons. Elderly patients often have a high medication burden, and have to take a number of drugs for different conditions every day. This can lead to poor compliance due to forgetfulness and confusion caused by complicated drug regimens [1]. In addition, a greater number of comorbidities, and difficulties in swallowing, mean that the elderly should be considered as a group with special requirements with regards to drug administration [39]. Transdermal administration of rivastigmine may be an age‐appropriate way to deliver this drug to elderly patients [40].
Cholinesterase inhibitors and memantine are the only currently available treatments for AD. Although these therapies effectively relieve the symptoms of AD, they have a limited effect on the progression of the disease. It is hoped that in the future, development of therapies that directly target the underlying pathology of AD may offer more substantial disease modification. Combinations of these novel disease‐modifying agents with symptomatic treatments like cholinesterase inhibitors may become the standard therapeutic regimen for patients with AD.
In summary, rivastigmine patch provides an efficient option for managing patients with AD, and should be considered a first‐line therapy for mild to moderate AD patients. In the USA, the patch is also approved for patients with PDD, based on the bioequivalence data of rivastigmine patches versus capsules and the proven effect of rivastigmine itself in PDD patients. A study with the rivastigmine patch in patients with PDD is ongoing, primarily focusing on safety.
Conflict of Interest
The authors are all members of the Neurology in EUROpe educational NETwork (NEURONET), a Novartis‐sponsored initiative that provides a regular forum at which European experts debate clinical issues and contribute to an evolving educational programme for practicing clinicians. Dr. Dziadulewicz is an employee of Novartis. All other authors have acted as paid consultants for Novartis, and have received research funding, consultancy fees or speech honoraria from companies involved in the manufacture and marketing of drugs or products for Alzehimer's disease. In addition, Professor Frölich was a Principal Investigator in the Novartis‐sponsored IDEAL study. The authors did not receive remuneration for the production of this manuscript.
Acknowledgments
Alpha‐Plus Medical Communications Ltd (UK) provided medical writing and editorial support in the production of this manuscript; this service was sponsored by Novartis. All authors were involved in drafting and critical review of the manuscript and all authors approved the manuscript for submission.
References
- 1. Small G, Dubois B. A review of compliance to treatment in Alzheimer's disease: Potential benefits of a transdermal patch. Curr Med Res Opin 2007;23:2705–2713. [DOI] [PubMed] [Google Scholar]
- 2. Barat I, Andreasen F, Damsgaard EM. Drug therapy in the elderly: What doctors believe and patients actually do. Br J Clin Pharmacol 2001;51:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinstock M. Selectivity of cholinesterase inhibition. Clinical implications for the treatment of Alzheimer's disease. CNS Drugs 1999;12:307–323. [Google Scholar]
- 4. Rösler M, Anand R, Cicin‐Sain A, et al Efficacy and safety of rivastigmine in patients with Alzheimer's disease: International randomised controlled trial. BMJ 1999;318:633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corey‐Bloom J, Anand R, Veach J. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer's disease. Int J Geriatr Psychopharmacol 1998;1:55–65. [Google Scholar]
- 6. Emre M, Aarsland D, Albanese A, et al Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med 2004;351:2509–2518. [DOI] [PubMed] [Google Scholar]
- 7. Feldman HH, Lane R. Rivastigmine: A placebo‐controlled trial of BID and TID regimens in patients with Alzheimer's disease. J Neurol Neurosurg Psychiatry 2007;78:1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schneider LS, Anand R, Farlow MR. Systematic review of the efficacy of rivastigmine for patients with Alzheimer's disease. Int J Geriatr Psychopharmacol 1998;1(suppl 1):S26–S34. [Google Scholar]
- 9. Winblad B, Machado JC. Use of rivastigmine transdermal patch in the treatment of Alzheimer's disease. Expert Opin Drug Deliv 2008;5:1377–1386. [DOI] [PubMed] [Google Scholar]
- 10. Winblad B, Cummings J, Andreasen N, et al A six‐month double‐blind, randomized, placebo‐controlled study of a transdermal patch in Alzheimer's disease–rivastigmine patch versus capsule. Int J Geriatr Psychiatry 2007;22:456–467. [DOI] [PubMed] [Google Scholar]
- 11. Jhee SS, Shiovitz T, Hartman RD, et al Centrally acting antiemetics mitigate nausea and vomiting in patients with Alzheimer's disease who receive rivastigmine. Clin Neuropharmacol 2002;25:122–123. [DOI] [PubMed] [Google Scholar]
- 12. Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int J Clin Pract Suppl 2002;127:45–63. [PubMed] [Google Scholar]
- 13. Darvesh S, Walsh R, Kumar R, et al Inhibition of human cholinesterases by drugs used to treat Alzheimer disease. Alzheimer Dis Assoc Disord 2003;17:117–126. [DOI] [PubMed] [Google Scholar]
- 14. Spencer CM, Noble S, Rivastigmine. A review of its use in Alzheimer's disease. Drugs Aging 1998;13:391–411. [DOI] [PubMed] [Google Scholar]
- 15. Bellelli G, Lucchi E, Minicuci N, et al Results of a multi‐level therapeutic approach for Alzheimer's disease subjects in the “real world” (CRONOS project): A 36‐week follow‐up study. Aging Clin Exp Res 2005;17:54–61. [DOI] [PubMed] [Google Scholar]
- 16. Lefèvre G, Sędek G, Jhee S, et al Pharmacokinetics and pharmacodynamics of the novel daily rivastigmine transdermal patch compared with twice‐daily capsules in Alzheimer's disease patients. Clin Pharmacol Ther 2008;83:106–114. [DOI] [PubMed] [Google Scholar]
- 17. Mercier F, Lefèvre G, Huang HL, et al Rivastigmine exposure provided by a transdermal patch versus capsules. Curr Med Res Opin 2007;23:3199–3204. [DOI] [PubMed] [Google Scholar]
- 18. Cummings J, Winblad B. A rivastigmine patch for the treatment of Alzheimer's disease and Parkinson's disease dementia. Expert Rev Neurother 2007;7:1457–1463. [DOI] [PubMed] [Google Scholar]
- 19. Anand R, Messina J, Hartman R. Dose‐response effect of rivastigmine in the treatment of Alzheimer's disease. Int J Geriatr Psychopharmacol 2000;2:68–72. [Google Scholar]
- 20. Giacobini E, Spiegel R, Enz A, et al Inhibition of acetyl‐ and butyryl‐cholinesterase in the cerebrospinal fluid of patients with Alzheimer's disease by rivastigmine: Correlation with cognitive benefit. J Neural Transm 2002;109:1053–1065. [DOI] [PubMed] [Google Scholar]
- 21. Darreh‐Shori T, Almkvist O, Guan ZZ, et al Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology 2002;59:563–572. [DOI] [PubMed] [Google Scholar]
- 22. Lefèvre G, Sędek G, Huang HL, et al Pharmacokinetics of a rivastigmine transdermal patch formulation in healthy volunteers: Relative effects of body site application. J Clin Pharmacol 2007;47:471–478. [DOI] [PubMed] [Google Scholar]
- 23. Kurz A, Farlow M, Lefèvre G. Pharmacokinetics of a novel transdermal rivastigmine patch for the treatment of Alzheimer's disease: A review. Int J Clin Pract 2009;63:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grossberg G, Sadowsky C, Förstl H, et al Safety and tolerability of the rivastigmine patch: Results of a 28‐week open‐label extension. Alzheimer Dis Assoc Disord 2009;23:158–164. [DOI] [PubMed] [Google Scholar]
- 25. Sadowsky CH, Dengiz A, Olin JT, et al Switching from donepezil tablets to rivastigmine transdermal patch in Alzheimer's disease. Am J Alzheimers Dis Other Demen 2009;24:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rivastigmine (Exelon®). Summary of product characteristics. Available from: http://www.emea.europa.eu/humandocs/PDFs/EPAR/Exelon/H‐169‐PI‐en.pdf [Assessed July 15 2009.
- 27. Exelon Patch® . US prescribing information. East Hanover , NJ : Novartis Pharmaceuticals Corporation , 2009. [Google Scholar]
- 28. Riepe MW, Adler G, Ibach B, et al Adding memantine to rivastigmine therapy in patients with mild‐to‐moderate Alzheimer's disease: Results of a 12‐week, open‐label pilot study. Prim Care Companion J Clin Psychiatry 2006;8:258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shua‐Haim J, Smith J, Picard F, et al Steady‐state pharmacokinetics of rivastigmine in patients with mild to moderate Alzheimer's disease not affected by co‐administration of memantine: An open‐label, crossover, single‐centre study. Clin Drug Investig 2008;28:361–374. [DOI] [PubMed] [Google Scholar]
- 30. Olin JT, Bhatnagar V, Reyes P, et al Safety and tolerability of rivastigmine capsule with memantine in patients with probable Alzheimer's disease: A 26‐week, open‐label, prospective trial (Study ENA713B US32). Int J Geriatr Psychiatry 2009. [Epub ahead of print]. doi: 10.1002/gps.2355. [DOI] [PubMed] [Google Scholar]
- 31. Ale I, Lachapelle J‐M, Maibach HI. Skin tolerability associated with transdermal drug delivery systems: An overview. Adv Ther 2009;26:920–935. [DOI] [PubMed] [Google Scholar]
- 32. Winblad B, Kawata AK, Beusterien KM, et al Caregiver preference for rivastigmine patch relative to capsules for treatment of probable Alzheimer's disease. Int J Geriatr Psychiatry 2007;22:485–491. [DOI] [PubMed] [Google Scholar]
- 33. Fitzpatrick AL, Kuller LH, Lopez OL, et al Survival following dementia onset: Alzheimer's disease and vascular dementia. J Neurol Sci 2005;229–230:43–49. [DOI] [PubMed] [Google Scholar]
- 34. Singh G, Thomas SK, Arcona S, et al Treatment persistency with rivastigmine and donepezil in a large state medicaid program. J Am Geriatr Soc 2005;53:1269–1270. [DOI] [PubMed] [Google Scholar]
- 35. Sicras‐Mainar A, Vergara J, Leon‐Colombo T, Febrer L, Rejas‐Gutierrez J. Retrospective comparative analysis of antidementia medication persistence patterns in Spanish Alzheimer's disease patients treated with donepezil, rivastigmine, galantamine and memantine [In Spanish]. Rev Neurol 2006;43:449–453. [PubMed] [Google Scholar]
- 36. Mauskopf JA, Paramore C, Lee WC, Snyder EH. Drug persistency patterns for patients treated with rivastimgine or donepezil in usual care settings. J Manag Care Pharm 2005;11:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Small GW, Kaufer D, Mendiondo MS, et al Cognitive performance in Alzheimer's disease patients receiving rivastigmine for up to 5 years. Int J Clin Pract 2005;59:473–477. [DOI] [PubMed] [Google Scholar]
- 38. Becker M, Andel R, Rohrer L, Banks SM. The effect of cholinesterase inhibitors on risk of nursing home placement among medicaid beneficiaries with dementia. Alzheimer Dis Assoc Disord 2006;20:147–152. [DOI] [PubMed] [Google Scholar]
- 39. Breitkreutz J, Boos J. Paediatric and geriatric drug delivery. Expert Opin Drug Deliv 2007;4:37–45. [DOI] [PubMed] [Google Scholar]
- 40. Priano L, Gasco MR, Mauro A. Transdermal treatment options for neurological disorders: Impact on the elderly. Drugs Aging 2006;23:357–375. [DOI] [PubMed] [Google Scholar]


