Motion Sickness Index: Appropriate Evaluation Criteria in Mice
Motion sickness is discomfort felt by individuals caused by repetitive angular and linear acceleration and deceleration [1]. Rodents have been used to model motion sickness, but are of limited value because rodents do not vomit [2, 3]. Pica is thought to be the equivalent of vomiting in rats and is widely used to model motion sickness [4]. However, increasing evidence indicated that pica is not a sensitive assay of motion sickness [5].
In a previous study from this laboratory, a set of criteria based on fecal and urinal incontinence was used to quantify the degree of motion sickness induced by horizontal rotation in rats and mice [5]. Whether this set of criteria is suitable to examine the core features of motion sickness (summation, variability, and habituation) is not clear. In this study, we attempted to validate this evaluation criteria in mice with different stimulation patterns, double labyrinthectomized and antimotion sickness drugs.
Male mice weighing 18–22 g were purchased from Sino‐British SIPPR/BK Lab Animal Ltd (Shanghai, China). All animals received humane care and experimental procedures were in compliance with institutional animal care guidelines. Motion sickness was simulated using a DSL‐1 minitype animal centrifuge unit (Peace Medical Equipment Factory, Beijing, China). Symptoms were monitored for a period of 5 min after the rotation. Motion sickness index (MSI) was calculated on the basis of a set of evaluation criteria previously published [5]. Data are expressed as mean ± SD, and analyzed with unpaired Student t‐test. Statistical significance was set at P < 0.05.
Similar to a previous report [6], we showed that MSI increase was much greater after dual‐axis rotation than after single‐axis rotation (5.14 ± 1.37 vs. 3.06 ± 1.07, P < 0.05; Figure 1A). MSI was highest with 240°/s peak speed and 40°/s2 angular acceleration (Figure 1B). MSI also correlated with the duration of the rotation, and was higher after 40‐min rotation than after 10 or 20 min rotation (Figure 1C). MSI after 60‐min rotation was lower than that for 40‐min rotation, indicating development of adaption. Alternatively, and the limit of defecation and urination might have been reached. Taken together, these summation observed demonstrated that this method is a suitable criterion for motion sickness.
Figure 1.
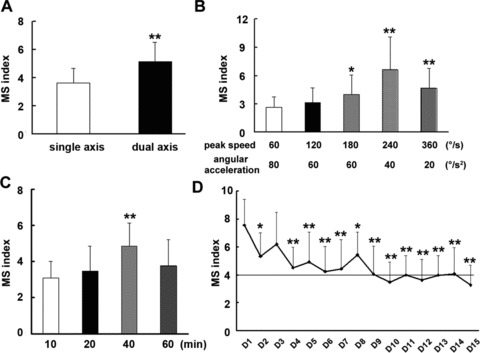
Motion sickness index in mice stimulated by different rotary pattern and time. Each value represents as mean ± SD of all three experiments (n = 8 in each group). *P < 0.05, **P < 0.01 versus control group or the first day.
Habituation is a common characteristic of motion sickness. Mitchell et al. showed that kaolin consumption increases initially upon rotation but gradually decreases as the trials progressed [7]. In this study, MSI gradually decreased to a stable level upon daily exposure to rotation over a period of 10–14 days (Figure 1D). The apparent habituation observed in our study support the use of this criteria for motion sickness.
The vestibular system is critical in motion sickness. In this study, MSI was decreased by intratympanic injection of gentamycin (2.00 ± 0.98 vs. 4.9 ± 1.48, P < 0.05; Figure 2A). Histological results showed that hair cells and sertoli cells were practically absent in mice receiving gentamycin (Figure 2B). These results demonstrated that similar to the sensitivity of pica to vestibular system damage [8], our criteria is sensitive to bilateral labyrinthectomy.
Figure 2.
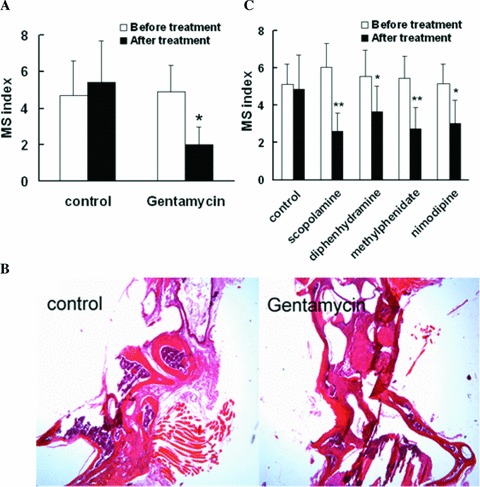
Double labyrinthectomized and antimotion sickness drugs on motion sickness index in mice. Each value represents mean ± SD (n = 8 in each group). * P < 0.05, **P < 0.01 versus before treatment.
Scopolamine, methylphenidate, diphenhydramine, and nimodipine decreased MSI in mice by 56.7%, 49.8%, 34.0%, and 40.8%, respectively (Figure 2C). The result demonstrated that our evaluation criteria are suitable to evaluated antimotion sickness drugs. However, it is well known that the variability is the other one of the most reliable characteristic of motion sickness. The greater variability of MSI in mice was due to fundamental variability of individual susceptibility to motion sickness per se as well as variability of fecal and urinal incontinence‐based evaluation criteria. As a result, animal subjects need to be screened simultaneously under the same condition for drug screening.
Our criteria differed from the kaolin consumption or spontaneous activities in two important respects: (1) the assay is easily quantifiable; the fecal granules of mice and rats could be easily scored; urinal incontinence, piloerection and tremble could also be easily measured, and (2) the assay is reliable and valid. Jia et al. have compared kaolin consumption, MSI and spontaneous activities in a set of repeated experiments. Their results showed that MSI has better accuracy and reproducibility under the same experimental conditions [9].
In conclusion, this work demonstrated that the fecal and urinal incontinence‐based evaluation criteria are suitable for evaluating the severity of motion sickness and MSI is appropriate in mice.
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (30971427) and a Key Discipline Grant of Shanghai Pudong New Area Health System (PWZXK2010‐11).
References
- 1. Bowins B. Motion sickness: A negative reinforcement model. Brain Res Bull 2010;81:7–11. [DOI] [PubMed] [Google Scholar]
- 2. Yates BJ, Miller AD, Lucot JB. Physiological basis and pharmacology of motion sickness: An update. Brain Res Bull 1998;47:395–406. [DOI] [PubMed] [Google Scholar]
- 3. Mitchell D, Laycock JD, Stephens WF. Motion sickness‐induced pica in the rat. Am J Clin Nutr 1977;30:147–150. [DOI] [PubMed] [Google Scholar]
- 4. Zajonc TP, Roland PS. Vertigo and motion sickness. Part II: Pharmacologic treatment. ENT-Ear, Nose Throat J 2006;85:25–35. [PubMed] [Google Scholar]
- 5. Yu XH, Cai GJ, Liu AJ, et al A novel animal model for motion sickness and its first application in rodents. Physiol Behav 2007;92:702–707. [DOI] [PubMed] [Google Scholar]
- 6. Lackner JR, DiZio P. Adaptation in a rotating artificial gravity environment. Brain Res Brain Res Rev 1998;28:194–202. [DOI] [PubMed] [Google Scholar]
- 7. Mitchell D, Krusemark ML, Hafner E. Pica: A species relevant behavioral assay of motion sickness in the rat. Physiol Behav 1977;18:125–130. [DOI] [PubMed] [Google Scholar]
- 8. Morita M, Takeda N, Kubo T, Matsunaga T. Pica as an index of motion sickness in rats. ORL J Otorhinolaryngol Relat Spec 1988;50:188–192. [DOI] [PubMed] [Google Scholar]
- 9. Jia L, Zhou LM, Wang WF, et al Comparative study of three commonly used indices of motion sickness in rats. Chin J Naut Med Hyperbar Med 2010;17:110–112. [Google Scholar]


