SUMMARY
Objective: To evaluate the efficacy and tolerability of topiramate in patients with posttraumatic stress disorder (PTSD). Method: We conducted a 12‐week double‐blind, randomized, placebo‐controlled study comparing topiramate to placebo. Men and women aged 18–62 years with diagnosis of PTSD according to DSM‐IV were recruited from the outpatient clinic of the violence program of Federal University of São Paulo Hospital (Prove‐UNIFESP), São Paulo City, between April 2006 and December 2009. Subjects were assessed for the Clinician‐Administered Posttraumatic Stress Scale (CAPS), Clinical Global Impression, and Beck Depression Inventory (BDI). After 1‐week period of washout, 35 patients were randomized to either group. The primary outcome measure was the CAPS total score changes from baseline to the endpoint. Results: 82.35% of patients in the topiramate group exhibited improvements in PTSD symptoms. The efficacy analysis demonstrated that patients in the topiramate group exhibited significant improvements in reexperiencing symptoms: flashbacks, intrusive memories, and nightmares of the trauma (CAPS‐B; P= 0.04) and in avoidance/numbing symptoms associated with the trauma, social isolation, and emotional numbing (CAPS‐C; P= 0.0001). Furthermore, the experimental group demonstrated a significant difference in decrease in CAPS total score (topiramate −57.78; placebo −32.41; P= 0.0076). Mean topiramate dose was 102.94 mg/d. Topiramate was generally well tolerated. Conclusion: Topiramate was effective in improving reexperiencing and avoidance/numbing symptom clusters in patients with PTSD. This study supports the use of anticonvulsants for the improvement of symptoms of PTSD.
Keywords: Clinician‐administered PTSD scale, Posttraumatic stress disorder, Randomized controlled clinical trial, Topiramate
Introduction
Posttraumatic stress disorder (PTSD) has been officially recognized as a formal psychiatric disorder in 1980 in the Diagnostic and Statistical Manual of Mental Disorders (DSM‐III) [1]. Since then, several studies on the epidemiology, neurobiology, course, and treatment have been developed to provide a better knowledge of PTSD. For instance, the National Comorbidity Survey in the United States estimated a lifetime prevalence rate of 7.8% (10.4% for women and 5% for men) [2]. Moreover, it is widely known that more than one‐third of persons fail to remit symptoms after several years of the occurrence of the traumatic event, showing that PTSD may run as a chronic disorder [2].
Stein et al. [3] conducted a systematic review of 35 short‐term randomized controlled medication trials for PTSD (4597 participants). In 13 trials, response to medication occurred in 59.1% of patients (644 participants), while response to placebo was seen in 38.5% of patients (628 participants). Significant reductions in symptom severity were observed for patients who received medications in 17 trials. Evidence of treatment efficacy was most convincing for the selective serotonin reuptake inhibitors (SSRIs). Although the SSRIs are the first‐line drugs for treating PTSD, many patients do not have a satisfactory response to these medications [3, 4]. The unsatisfactory response to available medicines and a high prevalence of the disorder, justify the need to further test other pharmacologic agents in the treatment of PTSD.
Anticonvulsants have been evaluated for their potential efficacy for the treatment of PTSD. The hypotheses on the etiology of PTSD have suggested that after exposure to traumatic events; the limbic nuclei may become kindled or sensitized, resulting in increased susceptibility to physiologic arousal [5]. The implication of the limbic kindling‐like phenomenon in the pathophysiology of PTSD has stimulated clinical research in antiepileptic drugs in the treatment of PTSD.
Topiramate is an anticonvulsant with inhibitory activity in animal kindling models and with multiple mechanisms of action: inhibition of carbonic anhydrase, blockade of Na+ channels, inhibition of some high‐voltage‐activated Ca2+, negative modulating effect on the kainate/AMPA subtype of glutamate receptors, ability to modulate NMDA glutamate receptor, and enhancement of GABAergic activity at GABA2 receptors [6, 7].
Besides the antikindling effect, topiramate may be beneficial in the treatment of PTSD due to its action in the glutamate and GABA neurotransmitters, which are involved in controlling fear response. Topiramate enhances brain GABAergic neurotransmission and receptor responsivity through GABAA receptors activation and it inhibits glutamatergic neurotransmission by inhibition of AMPA and kainate instead of NMDA receptors [8].
Although mechanisms involved in PTSD hippocampal degeneration are not completely understood, cortisol and glucocorticoid receptor may play a role in the pathophysiology of this disorder. Cortisol regulates the hypothalamus and pituitary through a negative feedback mechanism [9]. The chronic activation of the stress system leads to hypothalamus pituitary adrenal (HPA) axis dysfunction. In PTSD, low cortisol levels may cause the upregulation of glucocorticoid receptors, which enhances a negative feedback regulation of the HPA axis [10]. Glucocorticoid levels chronically elevated have been associated with loss of neurons in the hippocampi of mammals [11].
Topiramate can affect the activity of glucocorticoid receptor by potentiating the inhibitory activity of GABAergic neurotransmission and antagonizing the kainate/AMPA glutamate receptor. The blockage or the stabilization of the AMPA (α‐amino‐3‐hydroxy‐5 methyl‐4 isoxazole propionic acid) receptor may be a possible mechanism responsible for the increase in the threshold for flashbacks and nightmares that may explain the treatment response to topiramate in PTSD [12].
Clinical studies with topiramate suggest that it is effective as monotherapy or adjunctive therapy in PTSD. The aim of this study was therefore to test the efficacy and tolerability of topiramate in civilian‐related PTSD, by means of a double‐blind randomized controlled trial.
Material and Methods
This 12‐week, randomized, double‐blind, placebo‐controlled study was conducted at the outpatient clinic from the violence program of Federal University of São Paulo Hospital (Prove‐UNIFESP), São Paulo City, between April 2006 and December 2009. The inclusion criteria comprised men and women aged 18–62 years with a diagnosis of PTSD according to Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM‐IV), confirmed by the use of the Structured Clinical Interview for DSM‐IV Axis I and Axis II (SCID‐I and SCID II, respectively) [13, 14]. Women of childbearing potential had to be practicing reliable contraception and were advised not be pregnant or breastfeeding during the study. Participants with lifetime history of bipolar, psychotic, borderline personality disorder, substance dependence or abuse (excluding nicotine and caffeine) in the previous 6 months, serious or unstable concurrent illness, history of nephrolithiasis, use of psychotropic medications for the previous 2 weeks (6 weeks for fluoxetine), body mass index below 20, current suicidal ideation or psychotic symptoms, were excluded from the study.
Two sources of referrals were performed in the study: (1) consecutive patients searching for treatment in the violence program of the Federal University of São Paulo (Prove); and (2) an epidemiological survey of a random sample of 3000 participants living in the city of Sao Paulo was carried out to assess the relationship between exposure to violence and the prevalence of PTSD (study protocol: Violence and PTSD in Sao Paulo and Rio de Janeiro, Brazil: the protocol for an epidemiological and genetic survey) [15]. Cases identified as PTSD were referred to the Prove program of the university.
The study was approved by UNIFESP Institutional Review Board (IRB), following the Helsinki Declaration, and local ethical laws. Patients were included only after reading and signing the informed consent (Ethical Committee: 196/96).
Participants who fulfilled inclusion criteria provided a medical and psychiatric history, and were submitted to an electrocardiogram and laboratory tests. Trained interviewers administered the Clinician‐Administered Posttraumatic Stress Scale (CAPS) [16], and the patients completed the Beck Depression Inventory (BDI) [17] at baseline and 12th week. The psychiatrist in charge of the case applied the Clinical Global Impression (CGI) [18] scale. During the washout phase, patients received placebo pills and were reevaluated by the clinician after 1 week. Those having a significant improvement of the symptoms according to the CGI‐I score (less than 3) were excluded (avoiding premature placebo effects). Participants were then randomly assigned to receive either placebo or topiramate, in a 1:1 ratio using computer‐generated code. The participants, interviewers, and the practitioners in charge of the cases were all blind to the study experimental and control groups.
Study medication started at 25 mg/day once daily, at night, and increased in 25 mg weekly, as tolerated, until complete or nearly complete efficacy was achieved or until maximum dose allowed was reached (200 mg/day).
Study follow‐up visits were conducted at baseline, and weeks 1, 2, 3, 4, 6, 8, and 12. Symptoms and side effects were assessed in every attendance. Participants showing worsening of symptoms when receiving drug treatment, characterized by a 1‐point decrease at CGI–I, or presenting serious adverse effects related to medication, were withdrawn from the study. No other psychotropic medications were allowed during the study, except for zolpidem (10 mg/day), if needed, for insomnia. Compliance was assessed by counting the amount of pills left in the bottle of medicine in every visit. Tolerability of topiramate was assessed throughout the study by patient report of adverse events. Adverse events reported by participants, 10% of patients in each treatment group, were recorded.
Statistical Analysis
Participants who had both a baseline and at least 1 postbaseline CAPS assessment were included in an intent‐to‐treat analysis. Response assessment used the last‐observation‐carried‐forward (LOCF) method. The primary outcome endpoint was defined as the change from baseline to final visit in the CAPS total score. The hypothesis was that topiramate group would show a significant improvement in CAPS total score compared with the placebo group at week 12. Secondary efficacy included change from baseline in PTSD symptom clusters of reexperiencing (CAPS‐B), avoidance/numbing (CAPS‐C), and hyperarousal (CAPS‐D), and BDI. Randomized patients who received at least 1 dose of study medication were included in the safety analysis.
The efficacy analysis for the change in CAPS total score, CAPS‐B, CAPS‐C, and CAPS‐D subscale and BDI were conducted using a nonparametric test for repeated ordinal measures (similar to ANOVA) [19] comparing each scales from baseline and 12 weeks and between treatment groups. Response was defined as ≥30% improvement on CAPS and CAPS subscales and remission was defined as CAPS total score <20. Response and remission rates between treatment groups were compared using chi‐square test. All statistical testing was 2‐sided, and treatment groups’ comparisons were performed at significance level of 0.05.
Results
Thirty‐six civilian cases with a confirmed diagnosis of PTSD agreed to participate in the study and provided a signed informed consent. One patient showed remarkable improvement in the washout phase, being excluded from the study. Thus, 35 patients were randomized, but 4 subjects failed to return to the first visit and were not considered in the evaluation. Thirty‐one eligible cases were then enrolled in the study, and were considered for the efficacy analysis (n = 17 topiramate, and n = 14 placebo). Twenty‐six subjects completed the entire 12‐week study: 2 patients were withdrawn for worsening of symptoms characterized by 1‐point decrease at CGI and 3 discontinued treatment (1 due to worsening of symptoms, 1 due to an adverse event, and 1 dropout treatment), as described in the Consort flowchart (Figure 1).
Figure 1.
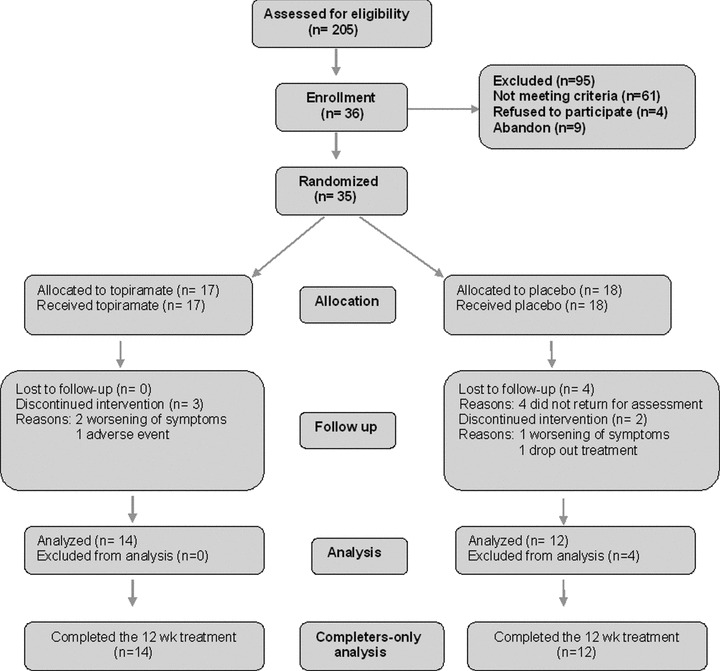
The Consort flowchart of the topiramate for PTSD randomized controlled trial.
The clinical and demographic characteristics of the sample are displayed in Table 1. As it can be seen in Table 1 there were no significant differences between groups according to age, gender, marital status, duration of illness, and baseline levels of CAPS and BDI. The mean CAPS total score at final visit was 30.41 (SD = 30.90) for topiramate and 35.78 (SD = 33.76) for placebo. The intention to treat (ITT) analysis showed that the experimental group had more severe symptomatology at baseline and a greater reduction of symptoms compared to placebo, but it was not statistically significant (CAPS total: topiramate =–48.35, placebo =–30.35, P= 0.49, f = 0.46).
Table 1.
Clinical and demographic characteristics
| Characteristic | Topiramate (n = 17) | Placebo (n = 14) |
|---|---|---|
| Age (y), mean (SD) | ||
| 43.7 (13.44) | 36.5 (7.97) | |
| Sex, N (%) | ||
| Male | 5 (29.41) | 5 (35.71) |
| Female | 12 (70.58) | 9 (64.28) |
| Marital status, N (%) | ||
| Single | 7 (50) | 5 (29.41) |
| Married | 4 (28.57) | 8 (47.05) |
| Divorced | 2 (14.28) | 3 (17.64) |
| Widower | 1 (7.14) | 1 (5.88) |
| PTSD durationa | ||
| Months, mean (SD) | ||
| 48.52 (66.21) | 37.92 (45.62) | |
P > 0.05 not statistically significant difference.
aOne patient in each group treatment was excluded from PTSD duration analysis due to childhood trauma exposure and could not be calculated.
The mean baseline and endpoint scores of CAPS‐B (reexperiencing symptoms: flashbacks, intrusive memories, and nightmares of the trauma), CAPS‐C (avoidance/numbing symptoms: avoidance of stimuli associated with the trauma, social isolation, and emotional numbing), CAPS‐D (hyperarousal symptoms: irritability, anxiety, vigilance, impaired concentration, poor sleep), and BDI‐II are described in Table 2. As showed in Table 2, most of the differences were in favor of the experimental group, but the comparisons were not statistically significant, except for the avoidance/numbing symptoms (CAPS‐C: topiramate = 12.17, SD = 13.30; placebo = 18.35, SD = 17.54, P < 0.05, f = 8.74).
Table 2.
PTSD and depressive symptom severity at baseline and at endpoint according to CAPS and BDI scores: effectiveness analysis
| Outcome | Baseline Mean (SD) | Endpoint Mean (SD) | P | f |
|---|---|---|---|---|
| CAPS TOTAL | 0.49 | 0.46 | ||
| Topiramate (n = 17) | 78.76 (12.64) | 30.41 (30.90) | ||
| Placebo (n = 14) | 66.14 (22.63) | 35.78 (33.76) | ||
| Reexperiencing symptoms (CAPS‐B) | 0.64 | 0.21 | ||
| Topiramate (n = 17) | 23.05 (5.88) | 6.29 (9.07) | ||
| Placebo (n = 14) | 21.21 (7.15) | 9.28 (9.26) | ||
| Avoidance/numbing symptoms (CAPS‐C)* | 0.003 | 8.74 | ||
| Topiramate (n = 17) | 31.58 (7.13) | 12.17 (13.30) | ||
| Placebo (n = 14) | 24.78 (11.30) | 18.35 (17.54) | ||
| Hyperarousal symptoms (CAPS‐D) | 0.26 | 1.26 | ||
| Topiramate (n = 17) | 24.11 (5.69) | 11.29 (10.76) | ||
| Placebo (n = 14) | 20.14 (8.29) | 9 (9.42) | ||
| CGI | 0.71 | 0.13 | ||
| Topiramate (n = 17) | 4.82 (0.63) | 2.11 (1.69) | ||
| Placebo (n = 14) | 4.64 (0.74) | 2.71 (2.05) | ||
| BDI | 0.72 | 0.12 | ||
| Topiramate (n = 17) | 22.29 (9.47) | 13.81 (10.29) | ||
| Placebo (n = 14) | 22.0 (11.80) | 18.14 (14.77) | ||
*P < 0.05 statistically significant difference.
CAPS, Clinician‐Administered Posttraumatic Stress Scale; CGI, Clinical Global Impression; BDI, Beck Depression Inventory
In addition, the groups did not differ in terms of response (82.35% for topiramate; 64.28% for placebo) to treatment (P= 0.37).
Patients who received the full treatment, that is, the efficacy analysis (N = 14 for topiramate; N = 12 for placebo) described in Table 3, revealed that the reduction in the CAPS scale (–57.78), was higher for topiramate group than that observed in the placebo group (–32.41), this difference being statistically significant (P < 0.05, f = 7.11).
Table 3.
PTSD and depressive symptom severity at baseline and at endpoint according to CAPS and BDI scores: efficacy analysis
| Outcome | Baseline mean (SD) | Endpoint mean (SD) | P | f |
|---|---|---|---|---|
| CAPS TOTAL* | 0.007 | 7.11 | ||
| Topiramate (n = 17) | 79.64 (12.03) | 21.85 (21.38) | ||
| Placebo (n = 14) | 64.33 (22) | 31.91 (28.22) | ||
| Reexperiencing symptoms (CAPS‐B)* | 0.04 | 4.08 | ||
| Topiramate (n = 17) | 24 (5.65) | 4.5 (5.27) | ||
| Placebo (n = 14) | 21.08 (6.76) | 7.91 (6.59) | ||
| Avoidance/numbing symptoms (CAPS‐C)* | 0.0001 | 15.46 | ||
| Topiramate (n = 17) | 32 (5.76) | 8.5 (9.81) | ||
| Placebo (n = 14) | 23.25 (11.25) | 16.25 (15.28) | ||
| Hyperarousal symptoms (CAPS‐D) | 0.47 | 0.52 | ||
| Topiramate (n = 17) | 23.64 (6.15) | 8.85 (8.61) | ||
| Placebo (n = 14) | 20 (8.33) | 8.75 (9.19) | ||
| CGI | 0.22 | 1.45 | ||
| Topiramate (n = 17) | 4.78 (0.57) | 1.5 (0.85) | ||
| Placebo (n = 14) | 4.75 (0.62) | 2.41 (1.78) | ||
| BDI | 0.45 | 0.55 | ||
| Topiramate (n = 17) | 21.28 (9.83) | 11.76 (9.86) | ||
| Placebo (n = 14) | 22.91 (11.5) | 17.75 (14.75) | ||
*P < 0.05 statistically significant difference.
CAPS, Clinician‐Administered Posttraumatic Stress Scale; CGI, Clinical Global Impression; BDI, Beck Depression Inventory.
Indeed the efficacy analysis showed that patients in the topiramate group exhibited significant reduction in comparison with the placebo group in reexperiencing (CAPS‐B: topiramate—19.5, SD = 7.25; placebo—13.16, SD = 6.02; P= 0.04, f = 4.08) and in avoidance/numbing (CAPS‐C: topiramate—23.5, SD = 11.37; placebo—7, SD = 9.91; P < 0.05, f = 15.46).
Mean topiramate dose was 102.94 mg/day (range 50–200 mg/day). Topiramate was generally well tolerated. The most commonly reported adverse events in patients receiving topiramate included somnolence (23%), insomnia (23%), paresthesia (17%), headache (11%), irritability (11%), dyspepsia (17%), and difficulty with concentration (11%) and the most commonly reported adverse events in patients receiving placebo included somnolence (35%), headache (21%), and insomnia (7%).
There were no meaningful differences between topiramate and placebo group in laboratory tests and vital sign levels during the study.
Discussion
This randomized, placebo‐controlled study suggests that topiramate may be effective in the treatment of PTSD. In general, topiramate was well tolerated and did not cause any serious adverse event.
Open‐label studies demonstrated topiramate to be effective and to have a rapid onset of action. Berlant and van Kammen [20] have reported that topiramate decreased nightmares in 79% and flashbacks in 86% of civilian patients with chronic PTSD with improvement of nightmares in 50% and of intrusions in 54% of patients with these symptoms. Response was seen in 91% of full responders at a dosage of 100 mg/day or less. In a prospective open‐label study [21], 33 civilians with chronic PTSD were treated with topiramate as monotherapy or augmentation therapy. The PTSD Checklist‐Civilian Version (PCL‐C) total symptoms declined by 49% at 4th week, with similar subscale reductions for reexperiencing, avoidance/numbing, and hyperarousal symptoms. Topiramate suppressed nightmares in 79% and decreased symptoms of intrusions in 94% in these patients. Topiramate has also been assessed in double‐blind, placebo‐controlled studies. Akuchekian and Amanat [22] conducted a 12‐week double‐blind, randomized, placebo‐controlled, add‐on trial. Sixty‐seven Iranian combat veterans with chronic PTSD treated with topiramate demonstrated statistically significant effects on most measures of CAPS‐Scale, especially on criterion B (reexperiencing symptoms) including, intrusive memory, nightmares, flashbacks, and some of criterion D (hyperarousal symptoms) including sleep problem, irritability, and anger. In a placebo‐controlled trial of monotherapy topiramate, Tucker et al. [23] evaluated 38 civilian PTSD patients with topiramate or placebo. There was a reduction in total CAPS score from baseline (topiramate =–52.7; placebo =–42.0), however the difference was not statistically significant (P= 0.232). Nevertheless, the active agent group exhibited significant reductions in reexperiencing symptoms (CAPS cluster B: topiramate = 74.9%; placebo = 50.2%; P= 0.038) and reductions approaching statistical significance in mean total CGI‐Improvement Scale scores (topiramate = 1.9 ± 1.2; placebo = 2.6 ± 1.1; P= 0.055).
But not all studies found positive results, Lindley et al. [24] in a double‐blind, placebo‐controlled study using topiramate as an augmentation therapy in 40 male veterans with PTSD did not find significant treatment effects versus placebo. However, there was a high dropout rate from the study with 11 (55%) topiramate and 5 (25%) placebo subjects not completing the 7‐week treatment, with 40% of topiramate and 10% of placebo dropping because of adverse effects. Patients in the topiramate group that completed the study had a significant improvement in the reexperiencing subscale of the CAPS, suggesting efficacy for the patients that continued the medication. Although there are limited clinical studies with topiramate in the treatment of PTSD, the findings of prior topiramate studies, particularly, the positive controlled trial in Iran's combat veterans suggest that this anticonvulsant may be an effective adjunctive therapy with antidepressants or other psychiatric agents in the management of refractory PTSD.
In our study, the finding of a significant improvement for topiramate‐treated patients who completed the study in the total CAPS, reexperiencing and avoidance/numbing symptoms (CAPS‐B and CAPS‐C, respectively) may be due to the fact that 3 (2 from topiramate and 1 from placebo groups) out of the 5 participants who did not complete the study were treatment resistant since they had already tried other medications (including SSRIs) and did not respond to previous treatments, suggesting a subgroup of medication resistant patients.
This study has several limitations. The small sample size results in low statistical power. Subjects taking placebo had a response rate of 64.28%. Although a reduction in CAPS total score was observed in topiramate‐treated patients, this difference did not reach statistical significance may be due to the high placebo response rate. This high placebo response rate is comparable to rates reported in other placebo‐controlled studies in PTSD [25, 26]. Compliance may have been variable in our participants since therapeutic serum levels were not measured. In addition to the limitations described above, the presence of comorbid depression may be a confounding factor. In our study, 4 patients met lifetime criteria for major depression. PTSD symptoms, especially criterion C (avoidance) and criterion D (hyperarousal) symptoms may overlap with major depression symptoms: social withdrawal, diminished interest, difficulty concentrating, and insomnia [27]. Therefore, patients with primary major depression may result in an underestimating of the role of topiramate in PTSD symptoms.
In conclusion, the results of this study suggest that topiramate can be effective for reexperiencing and avoidance/numbing symptoms of PTSD. However, the generalizability of our findings is limited to this sample of civilian population with PTSD. Further adequately powered controlled studies of topiramate for the treatment of PTSD are necessary, since most of the existing research were with small sample size, which may affect analysis of treatment responses.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
MSLY is the principal investigator and writer of this manuscript. JJM and MFM developed the design of the randomized clinical trial and participated in writing the article. MCPC was the study coordinator. RAB and SBA participated on the study design. All authors have read and approved the final manuscript.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
The authors give special thanks to Victor Fossaluza, who provided supervision in the statistics carried out in the article. This study was partly funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (grant: 2004/15039‐0); MCPC received a scholarship from the Ministry of Education (CAPES grant: 27909024886). JJM is a CNPq Level I Researcher, and MFM, RAB, and SBA are CNPq level II researchers.
References
- 1. American Psychiatric Association . Diagnostic and statistical manual of mental disorders, 3rd ed. Washington DC : APA, 1980. [Google Scholar]
- 2. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995;52:1048–1060. [DOI] [PubMed] [Google Scholar]
- 3. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for posttraumatic stress disorder. Cochrane Database Syst Rev 2006;25:CD 002795. [Google Scholar]
- 4. American Psychiatric Association . Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Washington , DC : APA, 2004. [PubMed] [Google Scholar]
- 5. Post RM, Weiss SR, Smith M, Li H, McCann U. Kindling versus quenching. Implications for the evolution and treatment of posttraumatic stress disorder. Ann N Y Acad Sci 1997;821:285–295. [DOI] [PubMed] [Google Scholar]
- 6. Shank RP, Joseph FG, Streeter AJ, Maryanoff BE. An overview of the preclinical aspects of topiramate: Pharmacology, pharmacokinetics, and mechanism of action. Epilepsia 2000;41(Suppl 1):S3–S9. [PubMed] [Google Scholar]
- 7. Wauquier A, Zhou S. Topiramate: A potent anticonvulsant in the amygdala‐kindled rat. Epilepsy Res 1996;24:73–77. [DOI] [PubMed] [Google Scholar]
- 8. Berlant JL. Antiepileptic treatment of posttraumatic stress disorder. Prim Psychiatry 2003;10:41–49. [Google Scholar]
- 9. Stephan JC. Corticotropin‐releasing hormone (CRH) in psychiatry: From stress to psychopathology. Ann Med 2004;36:50–61. [DOI] [PubMed] [Google Scholar]
- 10. Yehuda R, Boisoneau D, Mason JW, Giller EL. Glucocorticoid receptor number and cortisol excretion in mood, anxiety, and psychotic disorders. Biol Psychiatry 1993;34:18–25. [DOI] [PubMed] [Google Scholar]
- 11. Sapolsky RM. Glucocorticoids, hippocampal damage and the glutamaterigoc synapse. Prog Brain Res 1990;86:13–23. [DOI] [PubMed] [Google Scholar]
- 12. Zullino DF, Krenz S, Besson J. AMPA blockade may be the mechanism underlying the efficacy of topiramate in PTSD. J Clin Psychiatry 2003;64:219–220. [DOI] [PubMed] [Google Scholar]
- 13. Spitzer RL, Williams JB, Gibbon M, First MB. The structured clinical interview for DSM‐III‐R (SCID). I: History, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629. [DOI] [PubMed] [Google Scholar]
- 14. First M, Spitzer RL, Gibbon M, Williams JBW. The structured clinical interview for DSM‐III‐R personality disorders (SCID‐II). Part I: Description. J Pers Disord 1995;9:83–91. [Google Scholar]
- 15. Andreoli SB, Ribeiro WS, Quintana MI, et al Violence and post‐traumatic stress disorder in Sao Paulo and Rio de Janeiro, Brazil: The protocol for an epidemiological and genetic survey. BMC Psychiatry 2009;9:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM: The development of a Clinician‐Administered PTSD Scale. J Trauma Stress 1995;8:75–90. [DOI] [PubMed] [Google Scholar]
- 17. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–571. [DOI] [PubMed] [Google Scholar]
- 18. Guy W. Clinical global impression . ECDEU assessment manual for psychopharmacology, revised. Rockville , MD : National Institute of Mental Health , 1976. [Google Scholar]
- 19. Singer JM, Polero FZ, Rosa P. Parametric and nonparametric analyses of repeated ordinal categorical data. Biom J 2004;46:460–473. [Google Scholar]
- 20. Berlant J, van Kammen DP. Open‐label topiramate as primary or adjunctive therapy in chronic civilian posttraumatic stress disorder: A preliminary report. J Clin Psychiatry 2002;63:15–20. [DOI] [PubMed] [Google Scholar]
- 21. Berlant J. Prospective open‐label study of add‐on and monotherapy topiramate in civilians with chronic nonhallucinatory posttraumatic stress disorder. BMC Psychiatry 2004;4:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akuchekian Sh, Amanat S. The comparison of topiramate and placebo in the treatment of posttraumatic stress disorder: A randomized, double‐blind study. J Res Med Sciences 2004;5:240–244. [Google Scholar]
- 23. Tucker P, Trautmann RP, Wyatt DB, Thompson J, Wu SC, Capece JA, Rosenthal NR. Efficacy and safety of topiramate monotherapy in civilian posttraumatic stress disorder: A randomized, double‐blind, placebocontrolled study. J Clin Psychiatry 2007;68:201–206. [DOI] [PubMed] [Google Scholar]
- 24. Lindley SE, Carlson EB, Hill K. A randomized, double‐blind, placebo‐controlled trial of augmentation topiramate for chronic combat‐related posttraumatic stress disorder. J Clin Psychopharmacol 2007;27:677–681. [DOI] [PubMed] [Google Scholar]
- 25. Davidson JRT, Brady K, Mellman TA, Stein MB, Pollack MH. The efficacy and tolerability of tiagabine in adult patients with post‐traumatic stress disorder. J Clin Psychopharmacol 2007;27:85–88. [DOI] [PubMed] [Google Scholar]
- 26. Connor KM, Sutherland SM, Tupler LA, Malik ML, Davidson JR. Fluoxetine in post‐traumatic stress disorder: Randomized, double‐blind study. BR J Psychiatry 1999;175:17–22. [DOI] [PubMed] [Google Scholar]
- 27. Berlant JL. Topiramate as a therapy for chronic PTSD. Psychiatry 2006;3:40–45. [PMC free article] [PubMed] [Google Scholar]


