Abstract
Alpha‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazole‐propionic acid (AMPA) type glutamate receptors are critical for synaptic plasticity and induction of long‐term potentiation (LTP), considered as one of the synaptic mechanisms underlying learning and memory. Positive allosteric modulators of AMPA receptors could provide a therapeutic approach to the treatment of cognitive disorders resulting from aging and/or neurodegenerative diseases, such as Alzheimer disease (AD). Several AMPA potentiators have been described in the last decade, but for the moment their clinical efficacy has not been demonstrated due to the complexity of the target, AMPA receptors, and the difficulty in studying cognition in animals and humans. A better understanding of the mechanism of action of this type of drug remains an important issue, if knowledge of these compounds is to be increased and if this novel therapeutic approach is to be an interesting research area. Among the AMPA potentiators, S 18986 is emerging as a new selective positive allosteric modulator of AMPA‐type glutamate receptors. S 18986, as with other positive AMPA receptor modulators, increased induction and maintenance of LTP in the hippocampus as well as the expression of brain‐derived neurotrophic factor (BDNF) both in vitro and in vivo. Its cognitive‐enhancing properties have been demonstrated in various behavioral models (procedural, spatial, “episodic,” working, and relational/declarative memory) in young‐adult and aged rodents. It is interesting to note that memory‐enhancing effects appeared more robust in middle‐aged animals compared with aged ones and in “episodic” and spatial memory tasks. From these results, S 18986 is expected to treat memory deficits associated with early cerebral aging and neurological diseases in elderly people.
Keywords: AMPA modulator, Cerebral aging, Memory enhancer, Neuroprotection, S 18986
Introduction
With an increasing aging population, cognitive decline is becoming an important area for the development of therapeutics. Cholinergic deficit in Alzheimer disease (AD) patients can explain only some of the clinical symptoms. Drugs for cognitive disorders, such as acetylcholinesterase inhibitors, showed efficacy in numerous randomized controlled trials, but the magnitude of effects is limited and restricted to a certain population of patients, considered as “Responders”[1]. In an attempt to develop new therapeutic approaches to memory disorders associated with neurodegenerative disease, a glutamate‐based therapy has recently emerged from preclinical experiments [2, 3, 4]. Glutamate is the brain's major transmitter and is involved in cognitive functions [4, 5, 6]. Glutamate via ionotropic receptors plays a decisive role in the induction of LTP, a process involved in learning and memory [7, 8]. A dysfunction of glutamate transmission has been described in AD patients as a consequence of reduced glutamate release and reduced glutamate uptake [9, 10, 11, 12].
The α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazole‐propionic acid (AMPA) receptors are probably the most abundant subtype of glutamate receptors in the brain. They mediate the majority of fast excitatory amino acid transmission in the central nervous system. Its activation, associated with membrane trafficking and phosphorylation, plays an important role in synaptic plasticity, which is essential for learning and memory [13, 14]. AMPA receptor modulation may be a therapeutic means of enhancing memory in the treatment of neurological disease [2, 15, 16, 17, 18].
Several positive modulators of AMPA receptors have been described in the last decades [18]. These agents bind to allosteric sites on AMPA receptors and enhance signaling through a slow desensitization and/or deactivation of the receptor [17, 19]. By boosting Ca2+ influx, these biophysical parameters increase amplitude and/or duration of excitatory postsynaptic potentials (EPSPs) and thus enhance synaptic responses. Some AMPA receptor potentiators have been shown to facilitate memory in a number of behavioral studies and reverse age‐associated memory impairment in animals. Compounds, such as Ampakine CX516, have shown cognitive‐enhancing activity in the delayed nonmatch‐to‐sample task, a model of short‐term memory, improved olfactory learning, and reversed age‐associated memory deficit in rats [20, 21, 22]. IDRA‐21 showed positive effects on delayed matching performance in young and aged monkeys [23].
AMPA potentiators also exhibit neurotrophic and neuroprotective properties. Molecules such as CX‐546, LY392098, and LY404187 increased brain‐derived neurotrophic factor (BDNF) levels in vitro and in vivo[24, 25, 26], and the compound LY503430 exerted neuroprotective actions in three rodent models of Parkinson disease [27]. The latter results suggested that AMPA potentiators would be suitable molecules to reduce or halt human degenerative disease or for use in other processes as depression or schizophrenia [28, 29, 30]. The relevance of this type of drug in a therapeutic approach has to be investigated, since little information is available about its clinical efficacy. The development of AMPA receptor potentiators as neuroprotective and cognitive‐enhancing agents is a promising strategy. S 18986 was recently selected as a new positive modulator of AMPA receptors [31] and has been proposed as a new candidate for the treatment of memory impairment in neurodegenerative disorders, such as AD. This article reviews preclinical findings obtained with S 18986, which include its pharmacokinetic properties, its memory‐enhancing effects observed in a variety of memory tasks in young adult, middle‐aged, and aged rodents, as well as data from studies assessing its mechanism of action.
Chemistry
The chemical structure of S 18986 is 3a,10‐dihydro‐5,5‐dioxo‐4H‐(S)‐pyrrolidino[1,2‐c][1,2,4]benzothiadiazine (Figure 1). There is one asymmetric carbon atom in the molecule, and S 18986 corresponds to the S enantiomer, which is dextrogyral at 589 nm in ethanol [31]. The R‐isomer is named S 19024 and the racemate S 17951. S 18986 is practically insoluble in water (15 μg/mL). The S 18986 molecular formula is C10H12N2O2S and its molecular weight is 224.1. The melting point of the compound is 221°C. S 18986 is a stable off‐white powder.
Figure 1.
The chemical structure of S 18986—3a, 10‐dihydro‐5,5‐dioxo‐4H‐(S)‐pyrrolidino [1,2‐c][1, 2, 4] benzothiadiazine.
Animal Pharmacokinetics
Absorption
Following single oral administration, absorption of S 18986 was rapid and complete with a tmax at between 0.5 and 1 h in rats and between 0.5 and 3 h in monkeys. Absolute bioavailability was high in rats (from approximately 50% at the lower dose to approximately 90% at the higher dose) and low in monkeys (at around 5%).
Distribution
In vitro, S 18986 was moderately bound to plasma proteins. Free fraction was in the range of 28–40% in rat plasma, 30–38% in mouse plasma, 38–47% in monkey plasma, and 38–45% in rabbit plasma. The blood‐to‐plasma ratio of S 18986 was approximately 1.0 in rats and monkeys.
In vivo, S 18986 was characterized by a low distribution volume (Vss) of 0.83 L/kg in rats and 1.4 L/kg in monkeys. Following oral administration of [14C]‐S 18986 to pigmented rats (Lister‐Hooded strain), radioactivity distributed rapidly throughout the animals' bodies, with maximum concentrations in the majority of tissues occurring at 0.5‐h postdose. Concentrations of radioactivity declined rapidly, with tissue levels being close to the limit of quantification, by 24‐h postdose. At 168 h, most tissue levels of radioactivity were below quantifiable levels.
Bourasset [32] has quantified the blood‐brain uptake and the neuropharmacokinetics of S 18986 and examined its brain partitioning (Table 1).
Table 1.
Main pharmacokinetic parameters, calculated by a noncompartmental approach
| Tmin min | t1/2 min | Cl F–1 mL min‐t | Vd F–1 mL | AUCo‐. ng. mL–1. min | Ratio AUC (Brain/Plasma, unbound) | |
|---|---|---|---|---|---|---|
| Plasma, total | 8 | 60 | 4.2 | 362 | 59,986 | |
| Plasma, unbound | 8 | 60 | 12.67 | 1101 | 19,735 | |
| bECF. frontal cortex | 30 | 46 | 4670 | 0.24 | ||
| bECF. hippocampus | 30 | 57 | 4879 | 0.25 | ||
| CSF | 30 | 61 | 8287 | 0.4 | ||
| bICF, frontal cortex | 30 | 55 | 18,911 | 1 | ||
| bICF, hippocampus | 30 | 52 | 30,385 | 1.5 |
t1/2terminal half‐life of S 18986; Vd F–1, apparent volume of distribution of S 18986 in the central compartment; Cl F‐1, apparent total clearance of S 18986.
bICF and bECF represent the brain intra‐ and extracellular Fluid, respectively.
(From Bourasset et al. (2005) [32] with permission)
First, using the in situ brain perfusion technique, authors showed that in each brain area studied, that is, the frontal cortex, hippocampus, and the rest of the brain, brain uptake clearance (Clup) was constant within the range of concentration tested (1.23–12.27 μM) and high, at around 20 μL S−1 g−1. These results suggested that S 18986 intensively crosses the blood‐brain barrier. These data were in agreement with in vitro results showing that S 18986 was highly permeable across Caco‐2 cells and was not a substrate of P‐glycoprotein (C.O'Connell, R. Weaver, and T. Shepard, unpublished data). It is likely that in the range of concentrations tested, brain uptake clearance of S 18986 took place via passive diffusion.
Second, in order to determine brain extracellular fluid concentrations of S 18986, Bourasset [32] has performed microdialysis in two brain areas of interest, that is, the frontal cortex and the dorsal hippocampus regions, where AMPA‐type receptors are mainly localized [33]. She also measured pharmacokinetic parameters on plasma, brain homogenate, and cerebrospinal fluid (CSF). Terminal half‐lives of S 18986 were similar in plasma and in the brain, at around 1 h, and the product was mainly distributed into hippocampal‐brain intracellular fluid. These results indicate that S 18986 mainly penetrated the brain cells or bound to their plasma membranes, and that S 18986 accumulated more in the hippocampus than in the cortex.
This strongly supports that the hippocampus, a crucial brain region involved in memory processing [8, 34], would be the main effectors site of S 18986.
Mechanism of Action
To counteract age‐related cognitive deficits, a new therapeutic approach is facilitation of central glutamatergic neurotransmission. Among ionotropic glutamate receptor subtypes (NMDA, AMPA, and kainate), the AMPA receptors have a low implication in neurotoxicity and are main mediators of fast excitatory neurotransmission in the hippocampus. A number of experimental in vitro and in vivo studies have established the major role of these receptors in the mechanism of action of S 18986.
S 18986, a Selective Positive Allosteric Modulator of AMPA‐Type Glutamate Receptors
In Xenopus laevis oocytes injected with rat cortex poly(A+) mRNA, (S)‐AMPA was bath‐applied for 30 s and AMPA‐evoked inward currents were recorded at Vh=−60 mV, using a standard two‐electrode voltage‐clamp system. S 18986 was bath‐applied at successive increasing concentrations (3–1000 μM) for 45s before, 30 s during, and 30 s after the application of AMPA. S 18986 significantly potentiated AMPA‐induced inward currents in a concentration‐dependent manner (EC2x= 25 ± 3 μM, concentration of S 18986 responsible for a 2‐fold increase in the amplitude of AMPA‐evoked response; EC50= 130 ± 8 μM; Figure 2A). This potentiation was not observed when S 18986 was applied alone, which therefore indicates that S 18986 is an allosteric modulator and not an agonist.
Figure 2.
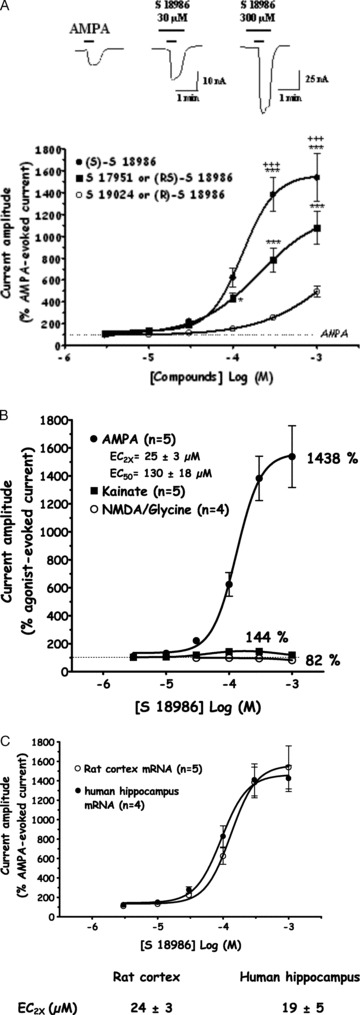
(A) Stereoselectivity of S 18986 on AMPA‐induced current in Xenopus laevis oocytes injected with rat cortex mRNA. Amplitude of the AMPA‐evoked current in the presence of the drug is normalized as a percentage of that induced in the absence of drug on the same oocyte. Two‐way ANOVA (Drug × Dose) followed by Newman–Keuls test. *P≤ 0.05; ***P≤ 0.001vs S 19024 versus (R)‐S 18986; ***P≤ 0.001S versus 17951, racemate. Mean ± SEM (n = 5). (B) Selectivity of S 18986 for AMPA receptors. Dose effects of S 18986 on AMPA‐, kainate‐, and NMDA‐evoked currents in Xenopus laevis oocytes injected with rat cortex mRNA. Amplitude of the agonist‐evoked current in the presence of the drug is normalized as a percentage of that induced in the absence of drug on the same oocyte. The results are shown as mean ± SEM (n = 4/5). (C) Effects of S 18986 on AMPA‐induced current in Xenopus laevis oocytes injected either with human hippocampus mRNA or rat cortex mRNA. Amplitude of the AMPA‐evoked current in the presence of the drug is expressed as a percentage of that induced in the absence of drug in the same oocyte. The results are shown as mean ± SEM (n = 4/5). EC2X corresponds to the concentration of S18986 responsible for a 2‐fold increase in the amplitude of AMPA‐evoked response.
S 18986 was selected as the most potent isomer as the drug was significantly more active than the racemate S 17951 and the R‐isomer S 19024.
Under the same experimental conditions, EC2x of S 18986 has been compared with other positive allosteric AMPA receptor modulators: LY404187 (EC2x= 0.3 μM) > CX614 (EC2x= 1.1 μM) > cyclothiazide (EC2x= 1.7 μM) > S 18986 (EC2x= 25 μM) > IDRA‐21 (EC2x= 134 μM) > aniracetam and CX516 (EC2x > 3000 μM) (data not shown).
In order to study the selectivity of S 18986 toward AMPA‐type receptors, the effects of the compound were investigated on kainate‐ and NMDA‐evoked currents. At a high concentration (300 μM), S 18986 induced a slight increase of 1mM kainate‐induced current (+44% relative to the amplitude of the current evoked by kainate alone) and no significant modification of NMDA (300 μM)/glycine (3 μM)‐induced current at concentrations up to 1 mM (Figure 2B). These results showed the absence of interactions of S 18986 on the two other glutamate receptors.
It is interesting to note that S 18986 increased AMPA‐evoked currents on rat cortex and human hippocampus mRNA with comparable EC2x values (19 ± 5 and 24 ± 3 μM, respectively; Figure 2C).
In order to investigate the selectivity of S 18986 toward other targets, the following interactions were evaluated:
Receptors
IC50 values higher than 10−5M were obtained for all the receptors and subtypes tested:
Adenosine (A1, A2), adrenergic (α1, α2A, α2B, α2C, β1, β2, and NE uptake), angiotensin II (AT1, AT2), benzodiazepine, cannabinoid, cholecystokinin, CGRP, dopamine (D1, D2, D3, D4, D5, and DA uptake), endothelin, GABA, glutamate (AMPA, kainate, NMDA), histamine (H1, H2), muscarinic (M1, M2, M3, M4 and ACh uptake), nicotinic, neurokinin (NK1, NK2), neuropeptide Y, opiate (delta, kappa, mu), prostanoid (TXA2/PGH2), serotonin (5HT1A, 5HT1B, 5HT2C, 5HT3, 5HT4, and 5HT uptake), sigma, steroids.
Ion‐Channels
IC50 values higher than 10−5 M were obtained for the Ca2+‐channel (dihydropyridine site), K+‐channels (voltage and Ca2+‐dependent), and Na+‐channel (batrachotoxin site).
Enzymes
IC50 values higher than 10−5 M were obtained for acetylcholinesterase, adenylyl cyclase, nitric oxide synthase, monoamine oxydase A and B, guanyl cyclase, cyclooxygenase 1, 5‐lipooxygenase, phospholipase C, phosphodiesterase I, sodium‐potassium ATPase.
The results showed an absence of interactions of S 18986 on these other receptors, ion channels and enzymes.
Further electrophysiological data have confirmed the selectivity of S 18986 for AMPA‐receptor types. Transverse hippocampal slices from male Wistar rats were prepared and transferred to a recording chamber, continuously superfused with artificial CSF solution. Extracellular excitatory postsynaptic field potentials (EPSfP) were recorded in the dendritic field of the CA1 region, following stimulation of the Schaeffer collateral. S 18986 was bath‐applied for 10 min at successive increasing concentrations (10–300 μM). S 18986 increased both the amplitude and duration of AMPA‐mediated EPSfP in the CA1 field of the hippocampus compared with vehicle (Figure 3). This effect was concentration‐dependent, with a significant effect obtained from 100 μM. In the same experimental paradigm, S 18986 at 300 μM did not have significant effects on NMDA‐mediated EPSfP in the CA1 region. These findings confirm the selectivity of S 18986 for AMPA receptors relative to NMDA receptors.
Figure 3.
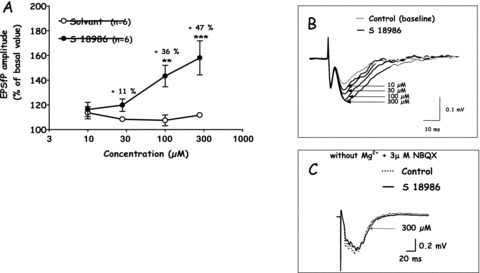
Effects of S 18986 on AMPA‐and NMDA‐mediated excitatory postsynaptic Field potentials evoked in the CA1 region of hippocampal slices. (A) The amplitude of the AMPA‐mediated EPSfP in the presence of each concentration of S 18986–1 or the vehicle was expressed as a percentage of that recorded before the application (basal value). The percentage of change of the amplitude of the synaptic response is plotted as a function of the concentration. The results are expressed as mean ± SEM, two‐way ANOVA (Drug × Dose) followed by Newman–Keuls test to compare drugs effects. The results are shown as mean ± SEM (n = 6). **P≤ 0.01; ***P≤ 0.001 versus vehicle. (B) Representative examples of AMPA‐mediated EPSfPs evoked in the hippocampal CAI area following stimulation of the Schaeffer Collaterals. Application of S 18986 induced a dose‐dependent increase of both the amplitude and the duration of the AMPA‐mediated EPSP. (C) Representative examples of NMDA‐ mediated EPSfPs evoked in the hippocampal. CAI area following stimulation of the Schaeffer Collaterals in the presence of NBQX, an antagonist of AMPA receptors and without Mg2*. Application of S 18986 (300 μM) did not affected the amplitude of the NMDA‐mediated EPSfP.
Effects on Long‐Term Potentiation in the Hippocampus
Glutamate receptors play an essential role in the modulation of synaptic plasticity in the central nervous system [7, 8]. The forms best to study are LTP and long‐term depression of excitatory synaptic transmission, experimental models for investigating the synaptic basis of learning, and memory in the hippocampus.
On anaesthetized adult male Wistar rats, EPSfP were evoked in the dendritic field of the dentate gyrus, following stimulation of the perforant path. Four or 20 bursts of high frequent stimulation or tetanus (400 Hz, 20 ms, given at 0.1 Hz) were applied in order to induce either a short‐ or a long‐term potentiation (STP or LTP, respectively) of the postsynaptic response. S 18986 (5 and 50 mg/kg) or its vehicle was i.p. injected 60 min before the tetanus. S 18986 significantly enhanced the amplitude of the EPSfP recorded before the tetanic stimulation at 50 mg/kg compared with control vehicle rats (Figure 4). Furthermore, S 18986 (5–50 mg/kg) significantly increased both the induction and the maintenance of 4 and 20 bursts tetanus‐evoked potentiation. With four bursts of tetanus, the EPSfP potentiation lasted 108 min and had a magnitude of 169% at the highest dose of S 18986, whereas it only lasted 42 min and reached 130% in control rats (Figure 4B). With 20 bursts of tetanus, S 18986 was able to significantly increase the duration of the LTP to over 3 h (Figure 4A).
Figure 4.
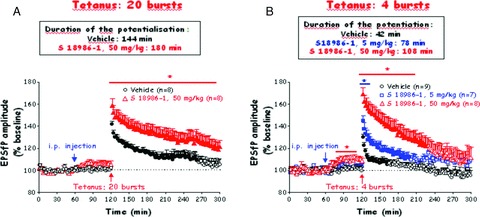
Effects of S 18986 on long‐term potentiation induced in the dentate gyrus of the hippocampus on anaesthetized Wistar rats. The amplitude of the EPSfP was averaged over four stimuli and was expressed as a percentage of the amplitude of EPSfPs averaged during a 1 h of the baseline period preceding the intraperitoneal injection (control value taken as 100%). S 18986 significantly enhanced the amplitude of the EPSfP recorded before the tetanic stimulation at 50 mg/kg compared with control vehicle rats. *P <0.05 S 18986 versus vehicle, two‐way ANOVA followed by Dunnett test. (A) Effect on long‐term potentiation evoked by a 20 bursts tetanus. S 18986 significantly increased the duration of the long term potentiation compared to the vehicle. ns, Two‐way ANOVA; * Treatment's effect: P < 0.05; *** Time's effect P < 0.001. Closed symbols: P < 0.05 versus the average amplitude of EPSPs obtained during a 6‐min‐period preceding the tetanus; one‐way ANOVA followed by Dunnett test. (B) Effect on short‐term potentiation evoked by a four bursts tetanus. S 18986 significantly increased both the induction and duration of the short‐term potentiation compared with the vehicle. *P < 0.05 S 18986 versus vehicle, two‐way ANOVA followed by Dunnett test. Closed symbols: P < 0.05 versus the average amplitude of EPSPs obtained during a 6‐min‐period preceding the tetanus, One way ANOVA followed by Dunnett’ test.
Collectively, these experimental findings demonstrate that S 18986 is a selective positive allosteric modulator of AMPA receptors. The drug increases both the induction and maintenance of LTP in the hippocampus. These effects are suggestive of a role of S 18986 in memory enhancing processes.
Effects on Neurotransmitters Release
In addition to their effects on LTP, cognitive‐enhancing properties of AMPA allosteric modulators could reside in their ability to control the release of several neurotransmitters [35, 36, 37, 38, 39, 40]. We hereupon present a set of evidence that S 18986 can modulate different neurotransmitter systems in the circumscribed brain (regions such as the hippocampus and the prefrontal cortex; Table 2).
Table 2.
Effect of S 18986 on different neurotransmitter systems in the brain
| Ach | NA | DA | GABA | Glutamate | |
|---|---|---|---|---|---|
| Baseline | No effect on PFC | No effect on CA and PFC | No effect on PFC No effect | No effect on CA | No effect on CA |
| Baseline |
 in CA (young and aged rats)a in CA (young and aged rats)a
|
on accumbens nucleus | |||
| Application of (s) AMPA | No effect on PFC |
 in CA and FPCb in CA and FPCb
|
 in FPCc in FPCc
|
ND | ND |
aeffect of S 18986 at 3 and 10 mg/kg i.p.; CA, hippocampus
beffect of S 18986 at 30–1000 μM; PFC, prefrontal; cortex
ceffect of S 18986 100 and 300 μM;  , increase release
, increase release
ACh, acetylcholine; NA, noradrenaline; DA, dopamine; ND, not done
Acetylcholine
Using microdialysis in the prefrontal cortex of freely moving young male Wistar rats, S 18986 (0.3–3–10 mg/kg, i.p.) showed no effect on basal acetylcholine (ACh) release. S 18986 also did not affect cortical ACh release induced by the local application of (S)‐AMPA.
In the hippocampus of young animals, S 18986 increased ACh release at 10 mg/kg i.p. (P < 0.05 vs. respective basal samples) [41]. ACh release was reduced by about 50% in aged (22‐month‐old) compared with young (3‐month‐old) rats. In aged rats, S 18986 induced a long‐lasting increase in ACh release at both 3 and 10 mg/kg i.p. (P < 0.05 vs. respective basal samples). Aged rats were more sensitive to S 18986, which was active from the 3 mg/kg dose. Although basal ACh levels were lower in aged than in young rats, the peak of ACh level induced by S 18986 was similar in amplitude between the two groups. These findings indicate that S 18986 can restore the functional deficit of cholinergic system, which gradually takes place in aged rats.
The increase in ACh extracellular levels induced by S 18986 could be a consequence of its positive AMPA‐receptor modulation, as other AMPA modulators, such as aniracetam, stimulate ACh release in the hippocampus [35]. The activation of ACh may contribute to its memory‐enhancing properties, as the cholinergic system plays a pivotal role in cognitive functions including attention [42].
GABA/Glutamate
It is interesting to note that S 18986 did not affect GABA or glutamate levels in the hippocampus of freely moving young rats [41]. The lack of effect of S 18986 on glutamate level might be related to the absence of agonist activity of the drug on this receptor type.
Noradrenaline
The study of Lockhart [43] described the effect of S 18986 on (S)‐AMPA‐induced [3H]noradrenaline release in rat hippocampus and frontal cortex slices. In baseline conditions, S 18986 did not modulate the [3H]noradrenaline level in the hippocampus and frontal cortex. On the other hand, S 18986 (30–1000 μM) potentiates (+200%) AMPA‐induced release of [3H]noradrenaline in both regions (P < 0.001).
It is interesting to note that S 18986 (250 μM) failed to potentiate either kainate or NMDA‐receptor‐mediated [3H]noradrenaline release at equivalent agonist concentrations.
These findings show that S 18986 enhances AMPA‐induced noradrenaline release in circumscribed regions. As noradrenaline exerts a positive role on both cognitive and attentional processes [44]; the cognitive‐enhancing effects of S 18986 could be in part related to its capacity to enhance AMPA receptor‐mediated noradrenaline release in the rat brain. The clinical relevance of increased noradrenaline release after rather high concentrations of S 18986 remains to be established.
Dopamine
(S)‐AMPA (30 μM–1 mM) significantly increased [3H]dopamine release in rat frontal cortex slices compared with basal values. S 18986 (3–300 μM) alone did not significantly affect [3H]dopamine release under similar conditions. AMPA‐mediated release of [3H]dopamine was dose‐dependently, significantly increased by S 18986 (100 and 300 μM).
When using microdialysis in the nucleus accumbens in rats, S 18986 (1–10 mg/kg, i.p.) showed no effect on dopamine release.
To summarize, S 18986 potentiated AMPA‐evoked noradrenaline release in both hippocampal and frontal cortex slices and dopamine release in frontal cortex slices. In the hippocampus, S 18986 had no effect on GABA or glutamate but increased ACh release in young and aged freely moving rats. These actions of S 18986 on different central neurotransmitter systems could contribute to its cognition‐enhancing properties.
Memory‐Enhancing Properties
Memory enhancing effects of S 18986 have been tested in various memory models in young adult, middle‐aged and aged animals. All data are summarized in Table 3.
Table 3.
Cognitive effects of S 18986 in young adult, middle‐aged and aged rodents
| Test | Species | Active doses (mg/kg) | Route | Effect* | Study reference | Respective figure |
|---|---|---|---|---|---|---|
| Young adult rodent | ||||||
| Passive avoidance/scopolamine | Rats | 3–10 | i.p. | ++ | [41] | Figure 5 |
| Y‐maze/scopolamine | Mice | 0.3 | i.p. | + | ||
| Social recognition | Rats | 0.3 – 1–3 | i.p. | +++ | Personal data | Figure 6 |
| Object recognition | Rats | 0.3 – 1–3 | p.o. | +++ | [48, 49] | Figure 7A, Figure 7B, Figure 7C |
| Morris Water Maze | Rats | 1–3 | p.o. | + | Personal data | Figure 8 |
| Middle‐aged rodent | ||||||
| Y‐maze | Rats | 0.1–1 | p.o. | +++ | [55] | |
| Contextual memory | Mice | 0.1 | p.o. | +++ | [53] | Figure 9 |
| Morris Water Maze | Rats | 1–10 | p.o. | +++ | Bredy TW, Personal Communication; [54] | |
| Aged rodent | ||||||
| Relational/declarative memory (radial maze) | Mice | 0.1 | p.o. | +++ | [57] | Figure 10 |
| Working memory (radial maze) | Mice | 0.1 | s.c. | + | [57] | Figure 11 |
| Morris Water Maze | Rats | 1 | p.o. | + | [54] | Figure 12 |
*This subjective effect evaluation is based on the effect size and/or the dose–response curve aspect.
Effects in Young Adult Rodents
Procedural Memory and Spatial Memory Test/Scopolamine
Scopolamine amnesia is one of the behavioral tests most commonly used to evaluate cognitive‐enhancing properties of drugs, as similarities exist between age‐related memory impairment and scopolamine amnesia in humans [45]. As the nonselective muscarinic receptor antagonist scopolamine is known to induce amnesia by blocking the cholinergic transmission, it became of interest to examine, in this model, the effect of a compound that increases ACh release.
S 18986 was first assessed in a spatial discrimination test (open Y‐maze) in order to explore spatial memory in mice. The test consisted in learning always the same arm (baited with food) out of three. Scopolamine (0.3 mg/kg i.p.), administered 30 min prior to the three sessions of the test (10 daily trials over three consecutive days), induced a significant decrease in the percentage of correct responses compared with vehicle‐treated mice (P≤ 0.01), demonstrating a learning impairment. Oral administration of S 18986 (0.3 mg/kg) 30 min before the scopolamine treatment significantly alleviated scopolamine‐induced learning impairment (P≤ 0.05; data not shown).
S 18986 was then examined in a model of procedural memory: the passive avoidance test. Treatment with scopolamine hydrobromide (1 mg/kg i.p.) administered 30 min before the acquisition session resulted in a profound amnesia, as indicated by a significantly reduced latency to enter into the dark compartment compared with the vehicle‐injected rats. Treatment with S 18986 (1–3–10 mg/kg) prior to the two sessions dose‐dependently increased the latency of scopolamine‐treated animals at 3 and 10 mg/kg (Figure 5) [41].
Figure 5.
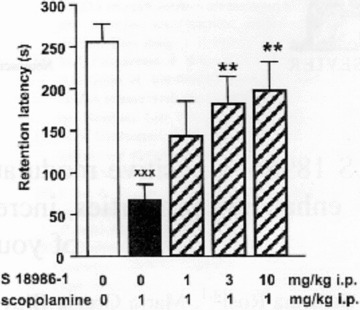
Effect of S 18986 on scopolamine‐induced amnesia in a passive avoidance conditioned response. The bars represent the retention latencies in second (mean ± SEM), n = 10 in each group, Open: saline; grey: scopolamine 1 mg/kg i.p.; stippled: scopolamine in rats pretreated with S 18986 (1, 3, and 10 mg/kg i.p.). *P > 0.05, one‐way ANOVA and Fisher test LSD postcomparison. Reprinted from Neuroscience Letters, Volume 361, Rosi et al., S 18986, a positive modulator of AMPA receptors with cognition‐enhancing properties, increases Ach release in the hippocampus of young and aged rat, pages 120‐3, 2004, with permission from Elsevier [41].
Collectively, these results show that S 18986, given orally, counteracts scopolamine‐induced memory impairment in young adult rodents and are therefore suggestive of an antiamnesic effect of S 18986. Interestingly, these effects are obtained at the same dose range (in the passive avoidance test) as the one that activates the cholinergic system in the hippocampus of aged rats (3 and 10 mg/kg). In the mouse species (Y‐maze), the effect of S 18986 is obtained at a lower dose (0.3 mg/kg). It can also be noticed that the dose of scopolamine used to induce amnesia in mice is lower (0.3 mg/kg vs. 1 mg/kg in the first model) than in the rat model.
Episodic Memory Tests
S 18986 has also been assessed in the social and object recognition tasks, a nonrewarded paradigm based on spontaneous exploratory behavior of rats. These tasks have been developed as a model of short‐term and long‐term episodic memory [46, 47].
In the social recognition test, a rat was exposed to a juvenile rat in two trials (5 min each) separated by an intertrial interval of 2 h. S 18986 (0.3–1–3 mg/kg) administered 30 min prior to the first presentation, and not after, significantly decreased the time of investigation of the juvenile in the second trial in a dose‐dependent manner (Figure 6). These results suggest that in the social recognition test, S 18986 exerted cognitive‐enhancing activity rather than improve the information processing. The effect observed with S 18986 (3 mg/kg i.p.) was related to an improvement of memory and not to nonspecific effects, as no significant effect was observed when a different juvenile was introduced for the second trial (data not shown).
Figure 6.
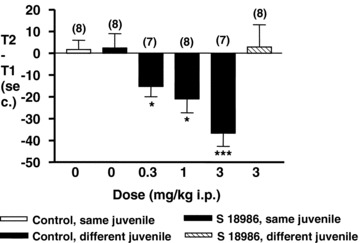
Effects intraperitoneal treatment with S 18986 in a social recognition task in Wistar rats. S 18986 was administered 30 min before the first presentation. The results are shown as mean ± SEM; n = 7 or 8 animals; *P≤ 0.05 and ***P≤ 0.001 versus control.
In the object recognition test [48], recognition is measured by the time spent by rats in exploring two different objects, one familiar and one unfamiliar. Acute oral administration of S 18986 (0.03–100 mg/kg) in adult Wistar rats 60 min before the three test sessions significantly increased the object recognition from 0.3 to 3 mg/kg (P < 0.05 and P < 0.01) and from 30 to 100 mg/kg (P < 0.01 and P < 0.001, respectively; Figure 7A). S 18986 remained active with subchronic (7 days') treatment before the start of the test at the doses of 0.3, 1, 3, and 10 mg/kg/day, with a maximal effect obtained at 1 mg/kg [48]. S 18986 (S‐isomer) was more active than the racemate and the R‐isomer (Figure 7B), confirming the result of the electrophysiological experiments (see Mechanism of Action, above) and neurotransmitter release studies [43]. Surprisingly, despite its short terminal half‐life (around 1 h) in plasma and in the brain, S 18986 remained active up to 4 h postadministration using this paradigm (Figure 7C) [48]. Moreover, multiple administrations (0.3 mg/kg t.i.d.) were not required to obtain maximal efficacy in this model because a single administration 1 mg/kg once a day (o.d.) led to a powerful procognitive activity (P < 0.01; Figure 7D) [49].
Figure 7.
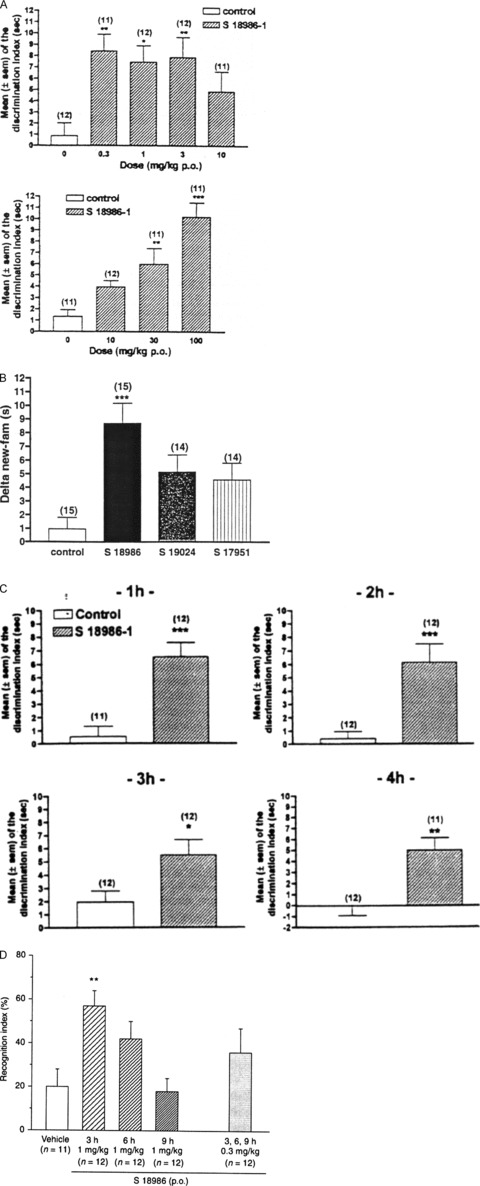
(A) Effects of oral treatment with S 18986 in the object recognition test in rats. S 18986 was administered 60 min before each session with low doses (0.3, 1, 3, 10 mg/kg) and higher doses (10, 30, 100 mg/kg). The discrimination index was the difference between the exploration times of the novel and familiar objects on the second trial (T2), which occurred 24 h after the first trial. Values are means ± SEM with the number of animals/group in parentheses. *P≤ 0.05; **P≤ 0.01; ***P≤ 0.001: versus control. ANOVA and Dunnett t‐test. Reprinted from European Journal of Pharmacology, Vol. 401, Lebrun et al., Effects of S 18986‐1, a novel cognitive enhancer, on memory performances in an object recognition task in rats, pages 205‐12, 2000, with permission from Elsevier [48]. (B) Comparisons between S 18986 (S‐isomer), S 17951 (racemic), or S 19024 (R‐isomer) in the object recognition test in rats. Delta is the difference between the exploration times of the novel and familiar objects on the second trial (T2) which occurred 24 h after the first trial. The results are shown as mean ± SEM, n = 14 or 15 animals. ***P≤ 0.001: value significantly different from control. (C) Effects of delayed treatments with S 18986 in the object recognition test in rats S 18986 was administered to the rats at the dose of 1 mg/kg by oral route. The administrations were made 1, 2, 3, or 4 h before each session of the test. The discrimination index was the difference between the exploration times of the novel and familiar objects on the second trial, which occurred 24 h after the first trial. Values are the means (± SEM) with the number of animald/group in parentheses. *P≤ 0.05; **P≤ 0.01; ***P≤ 0.001 versus control. Reprinted from European Journal of Pharmacology, Vol. 401, Lebrun et al., Effects of S 18986‐1, a novel cognitive enhancer, on memory performances in an object recognition task in rats, pages 205‐12, 2000, with permission from Elsevier [48]. (D) Effects of S 18986 on memory performance (expressed as recognition index) of Sprague‐Dawley rats in the object recognition task. S 18986 was administered orally as a sinde daily administration (1 × 1 mg/kg) or as three daily distinct administrations (3 × 1 mg/kg) 9, 6, or 3 h before each session during three consecutive days. Results are expressed as mean ± SEM; **P≤ 0.01 versus control. (From Bertaina‐Anglade et al. (2007) [49] with permission from John Wiley and Sons).
These results show that in this model of episodic memory, S 18986 administered prior to the recognition trial improves the retention of familiar information in adult rats. S 18986 exerts its procognitive activity by facilitating retrieval, and not acquisition, of the memory information. The promnesiant effects of S 18986 were observed with doses ranging from 0.3 to 100 mg/kg and with a biphasic effect. The hypothesis that could be drawn is that, at lower doses, S 18986 positively modulates postsynaptic currents generated by AMPA receptors and consequently improves cognitive function. At higher doses, the cognitive‐enhancing effect observed with S 18986 could be related to the modulatory activity of the drug on the release of other neurotransmitters, such as noradrenaline [43].
S 18986 exerts a long‐lasting memory effect in the object recognition task. The procognitive effect of S 18986 does not necessarily correlate with the peak of blood level but may be triggered by a cascade of electrophysiological and/or neurochemical adaptative events. Indeed, both events modulated by S 18986, that is, the enhancement of LTP and/or the regulation of neurotrophic factors (see Other Pharmacological Properties), can support the maintenance of its promnesiant effect. As for S 18986, Buccafusco et al. (2004) have reported a robust and long‐lasting (over 48 h) improvement in Delayed Match to Sample task (DMTS) memory performance of adult rhesus monkeys following a single administration of IDRA‐21 [23].
Spatial Memory Test
The Morris Water Maze is a recognized model for the determination of spatial promnesiant properties of drugs. In this model, S 18986 administered orally to adult rats at the doses of 0.3–1–3 mg/kg, had no effect on the learning acquisition process (data not shown). However, when trained rats were submitted to a retention test in the maze following a period of 7 days without training, S 18986 (1 or 3 mg/kg), given both during learning and before the retention test improved performance of the task (Figure 8A). Rats treated with S 18986 (1 mg/kg) during both learning and retention or only before the learning sessions exhibited improved performances. S 18986 was inactive when given only before the retention performance (Figure 8B).
Figure 8.
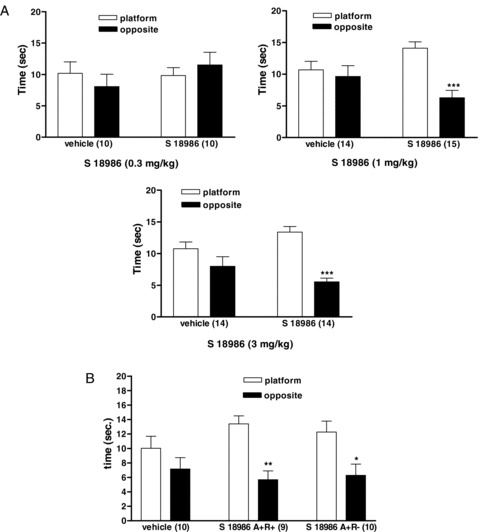
(A) Effect of oral treatment with S 18986 (0.3–1–3 mg/kg) prior to the acquisition and the long term retention test on memory performances in the retention test of the Morris Water Maze. The results are shown as mean ± SEM, the number of animals/group is indicated in parentheses. ***P≤ 0.001: significantly different from platform quadrant. (B) Effect of oral treatment with S 18986 (1 mg/kg) prior to the learning sessions of the Morris Water Maze test on memory performances in the retention test. A+R+: S18986 administered before the acquisition sessions and then before the retention session. A+R‐: S18986 administered only before the acquisition sessions. The results are shown as mean ± SEM, n = 9 or 10 animals. *P≤ 0.05, P≤ 0.01: significantly different from platform quadrant.
These results indicate that S 18986 does not modify the learning process in a spatial reference memory task but increases the long‐term retention of information only when S 18986 treatment takes place prior to the acquisition process.
S 18986 shows memory‐enhancing properties in young adult rats in episodic and spatial memory models with pharmacological doses ranging from 0.3 to 3 mg/kg. S 18986 is mostly active in memory retention processes.
Effects in Middle‐Aged Rodents
Age‐related memory deficits have been described in middle‐aged rodents [50, 51, 52] and seems to be a suitable model for an early pharmacological intervention.
S 18986 has been investigated in three models of age‐related memory disorders in middle‐aged rodents.
Episodic Contextual Memory Test
The contextual serial discrimination was developed to test simultaneously different forms of memory in mice (episodic‐like vs. semantic‐like forms). Using this task, middle‐aged (14–15‐months‐old) mice exhibited a severe memory deficit in remembering the first discrimination but not the second one, which was due to an increase in interference, compared with young adult mice (5–6‐months‐old; P < 0.001). Chronic treatment of mice (9 days) with 0.1 mg/kg of S 18986 reversed the deficit of middle‐aged mice with a significant increase of correct response (P < 0.01) and a tendency to reduce interfering responses (Figure 9) [53].
Figure 9.
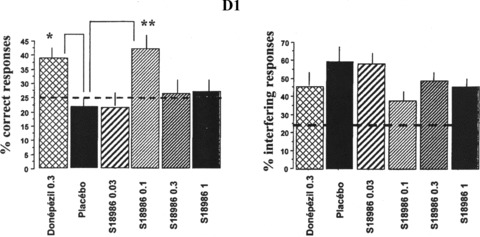
Effects of S 18986 in the episodic contextual memory test in middle‐aged mice: comparison with donepezil. Left panel: Percentage of correct responses (head dips into the hole baited on the same floor at the acquisition phase) at the D1 test session.Right panel: Percentage of interfering responses (head dips into the hole baited on the other floor at the acquisition phase) at the D1 test session. Results are mean ± SEM. *P≤ 0.05; **P≤ 0.01 versus placebo treated middle‐aged mice. Dotted lines chance level (25%). With kind permission from Springer Science + Business Media: Psychopharmacology, Improvement of episodic contextual memory by S 18986 in middle‐aged mice: comparison with donepezil, Vol. 193, 2007, pages 63‐73, Beracochea et al. [53].
S 18986 emerges as having a beneficial impact on contextual memory processes in middle‐aged mice. The promnesiant effect of S 18986 was comparable to the effect of donepezil (0.3 mg/kg), the main cholinesterase inhibitor used in mild to moderate AD.
Spatial Memory Tests
Middle‐aged rats (16‐months‐old) were tested for their learning performances in the Morris Water Maze task following chronic treatment (4 months) with S 18986 (0.3, 1, and 10 mg/kg). In vehicle controls, spatial memory performances were significantly affected in aged animals. Chronic treatment with S 18986 (1 or 10 mg/kg) alleviated the age‐related difference (Bredy TW, personal Communication) [54], and the latency to find the hidden platform decreased in all groups of animals over time.
These data provide evidence of S 18986 action as a counteracting agent of age‐related deficits in the learning process of middle‐aged rodents.
Rats were tested in a Y‐maze apparatus following chronic drug treatment in order to determine locomotor and spatial memory performance [55]. This task was chosen because it has been previously reported as sensitive to aging [56]. For total arms entered during the novel arm trial (locomotor measure), there was a slight increase in arm entries between controls (2.5‐months‐old) and vehicle‐treated aged rats. Results showed an increase of arm entries for both 1 and 0.1 mg/kg S 18986 treated rats, compared with vehicle treated aged rats (18‐months‐old; 80%; P < 0.005). Regarding the percentage of time spent exploring the novel arm (cognitive measure), young adult control rats spent approximately 46% of the total trial in the novel arm, compared with aged ones (P < 0.005). Aged rats treated with S 18986 spent more time exploring the novel arm (165 ± 42% for 1 mg/kg [P < 0.01] and 141 ± 37% for 0.1 mg/kg [P < 0.05]) compared with vehicle rats.
Chronic oral treatment with S 18986 increased locomotor activity and improved performance in a spatial memory task in middle‐aged rodents. Both exploratory behavior and cognitive processes participate in memory performance [56]. It cannot be ruled out that motor activity has some impact on the mnemonic component of the task and needs to be explored further with S 18986.
S 18986 shows antiamnesic properties in middle‐aged rodents in contextual and spatial memory models with pharmacological doses ranging from 0.1 to 10 mg/kg. The low‐dose efficacy of the drug observed with aged animals compared with active doses obtained in their young adult counterparts (0.3–100 mg/kg) suggests a higher sensitivity of middle‐aged rodents to S 18986 treatment.
Effects in Aged Rodents
The memory‐enhancing profile of S 18986 was investigated in two mice models of age‐related memory deficits, using the eight‐arm radial maze paradigm and the Morris Water Maze.
Relational/Declarative Memory Test
The first model was specifically designed to assess relational/declarative memory (flexibility). The results showed that the number of sessions necessary to reach the discrimination criteria did not differ between the young adult (4–6‐months‐old) and the aged vehicle groups (20–24‐months old). By contrast, in stage 2 (the discriminative test), old animals exhibited a deficit with respect to young adult ones (mean% of correct responses on the two test sessions [choices of a baited arm] in the two‐choice trials: 60.3 ± 3.6% vs. 78.1 ± 3.0%; P < 0.01 and P < 0001, respectively). The treatment of aged animals with S 18986 (0.01, 0.1, and 0.3 mg/kg) increased performances significantly at 0.1 mg/kg (73.9 ± 3.1% correct responses; P < 0.05), for which the performance level approached that of the young adult group (Figure 10).
Figure 10.
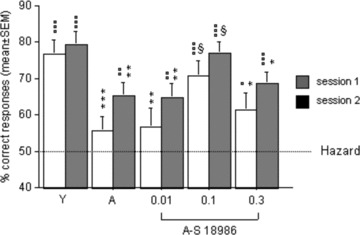
Effect of S18986 on relational/declarative mernory test (stage 2). Stage 2: Percentage of correct responses recorded in the 1st and 2nd session. Performances (mean ± SEM) in stage 2 (discrimination by pairs) of the five groups (Young Aged, A‐0.01, A‐0.1, A‐0.3). *(Statistically different from young vehicle group) P > 0.05; **P < 0.01; ***P < 0.001. §(Statistically different from aged vehicle group) P < 0.05. °(Statistically different from hazard) °P < 0.05; °°P < 0.01; °°°P < 0.001. With permission from Marighetto et al., The AMPA modulator S 18986 improves declarative and working memory performances in aged mice, Behavioural Pharmacology, Vol. 19, issue 3, pages 235‐44 [57].
All in all, S 18986 at the dose of 0.1 mg/kg restored the selective deficit of aged mice observed in the critical test of long‐term/declarative memory flexibility without modifying other aspects of memory performance measured in this paradigm.
Working Memory Test
The second task was similar to the previous one (six adjacent arms grouped into three pairs), but it consisted in “concurrent serial alternations.” Aged mice (20–24‐months old) displayed a severe and long‐lasting deficit compared with young adult mice (4–6‐months old) (P < 0.001). The performance of the aged mice remained at chance level during the training period (maximum 56.4 ± 2.2% correct responses), while the young adult group increased the mean performance (from 65.0 ± 2.1% in the first session blocks vs. 76.5 ± 1.7% in the third session blocks; Figure 11A). S 18986‐treated (0.1 and 1 mg/kg) aged mice exhibited improved performances in the final two blocks of sessions. A maximum of 64.3 ± 4.2% of correct responses was obtained at 0.1 mg/kg, and more than 60% of correct responses was obtained at 1 mg/kg, compared with 52.1 ± 2.8% in the placebo group (Figure 11B) [57].
Figure 11.
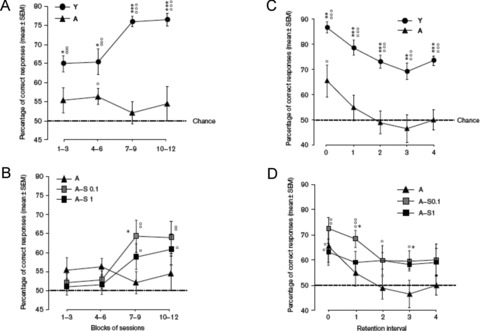
Effect of S 18986 on short‐term/working memory test. Y, young vehicle mice; A, aged vehicle mice; A‐S, aged mice treated with S 18986 (0.1 and 1 mg/kg). (A) and (B) Working memory by block performance over the 12 sessions of training for each group. °P < 0.05, °°P < 0.01, °°°P < 0.001 versus chance; *P < 0.05, ***P < 0.001 versus A. (C) and (D) Working memory performance as a function of retention interval for each group. °P < 0.05, °°P < 0.01, °°°P < 0.001, versus chance; *P < 0.05, **P < 0.01, ***P < 0.001 versus A. With permission from Marighetto et al., The AMPA modulator S 18986 improves declarative and working memory performances in aged mice, Behavioural Pharmacology, Vol. 19, issue 3, pages 235‐44 [57].
When performances were modulated by the retention interval (number of trials interposed; five levels of retention interval were tested from zero “no interposed pair” to level 4), young adult mice showed diminished performances with an increasing retention interval but remained significantly above chance level for all intervals. In aged mice, performance was above chance only when the retention interval was minimal (0 interposed trial), and even in this latter, condition aged mice were impaired with respect to their younger counterparts (Figure 11C). Treatment with S 18986 revealed a significant effect, compared with placebo, at 0.1 mg/kg when one or three trials were interposed (P < 0.05) and a trend for 1 mg/kg when three trials were interposed (P= 0.053) (Figure 11D) [57].
Using the eight‐arm radial maze paradigm, S 18986 at the same low dose of 0.1 mg/kg selectively improved aged‐mice performance in the test of relational/declarative memory and showed a moderate beneficial effect in short‐term retention of successive arm‐visits in the working memory test.
These two models are very complex and highly discriminative for drug screening. These two forms of memory expressions (flexibility and organizational demand) investigated in these models are selective and specific of age‐related memory impairment. These tasks would critically depend on the plasticity of the hippocampus and its connectivity with cortical areas [58], both of which are target areas for S 18986. It can be hypothesized that the compound might restore a synaptic function in aged mice, through modulatory effects on LTP and/or neurotransmitters (see Mechanism of Action), which could contribute to minimizing ordinary memory deterioration induced by ageing.
Spatial Memory Test
Aged rats (24‐months old) were tested in the learning of the Morris Water Maze task following chronic treatment (12 months) with S 18986 (0.3, 1, and 10 mg/kg p.o.). There was a significant effect of age, such that 24‐month‐old control animals performed significantly less than young adult controls (5‐months old) over Days 2–5 of testing. Chronic treatment with S 18986 at the 1.0 mg/kg dose significantly improved the performance of the aged rats on Days 4 and 5 of testing (Figure 12) [54].
Figure 12.
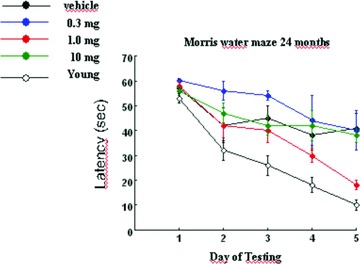
Chronic treatment (12 months for rats at 24‐month‐old) with S 18986 (0.3, 1, and 10 mg/kg p.o.) in the learning of the Morris Water Maze task. Results are mean ± SEM latency (seconds) to locate and climb into the submerged platform. The number of animals was 8 per group. Testing was completed over five consecutive days with three trials per day. *P > 0.05: significant effect of treatment at 1mg/kg on Day 4 and 5 of testing versus vehicle aged controls.
Chronic oral treatment with S 18986 (1 mg/kg) counteracts age‐related deficits in the learning process in a spatial reference memory task in aged rodents.
S 18986 shows antiamnesic properties in aged rodents in relational, working, and spatial memory models with a narrow range of pharmacological doses (at 0.1 and 1 mg/kg) compared with middle‐aged rodent memory models (0.1–10 mg/kg). S 18986 produces a more powerful memory‐enhancing effect in middle‐aged rodents than in aged ones. S 18986 seems to be therefore a promising drug candidate for the treatment of early forms of age‐related memory deficit, probably due to its effects on hippocampal plasticity and synaptic transmission. Other specific properties of S 18986, such as modulatory effects on neurotrophic factors and neuroprotective properties, may also contribute to the procognitive actions of this compound.
Other Pharmacological Properties
There is some evidence that neuronal plasticity is directly regulated by several neurotrophic factors, of which, one is BDNF. BDNF is essential for long‐term hippocampal plasticity and for restoring LTP in the middle‐aged hippocampus [51, 59]. Allosteric modulators of AMPA receptors have been shown to increase BDNF expression in vitro and in vivo[24, 25, 26]. Positive AMPA receptor modulators may also exert their effects on learning and memory through indirect increases in the expression of BDNF, and we have tested this hypothesis by investigating the capacity of S 18986 to modulate in vitro and in vivo expression of BDNF.
Neurotrophic Properties
Lockhart [60] has tested the effects of S 18986 on (S)‐AMPA‐mediated increases in BDNF mRNA and protein expression in rat primary cortical neuronal cultures. Compared with untreated cultures, AMPA (0.01–300 μM) induced a concentration‐dependent increase in BDNF mRNA and protein expression. The maximal increases were observed between 5 and 12 h for mRNAs and at 24 h for protein expression. S 18986 (≤300 μM) alone failed to increase basal BDNF expression, but the drug (300 μM) enhanced AMPA‐induced expression of BDNF mRNA 2–3‐fold, and the protein levels 3–5‐fold.
In vivo, BDNF expression has been examined in middle‐aged (16‐months‐old) and aged (24‐months‐old) rats following chronic oral treatment (4 and 12 months, respectively) with S 18986 at 0.3, 1, and 10 mg/kg p.o. (Bredy TW, personal communicstion) [54]. BDNF was measured, once animals had been tested in the Morris Water Maze task. Compared with young adult controls, BDNF mRNA levels in the hippocampus of vehicle‐treated aged rats were significantly reduced by approximately 40%. BDNF mRNA levels were significantly increased in middle‐aged animals treated with S 18986 (1 and 10 mg/kg) compared with vehicle‐treated aged rats (P < 0.05). In aged animals, S 18986 had no effect on BDNF expression.
In vivo, in middle‐aged rats, increase of hippocampal BDNF expression and promnesiant effect measured in the Morris Water Maze were obtained at similar doses of S 18986 (1 and 10 mg/kg). The enhanced BDNF expression induced by S 18986 occurs in the same region where the drug increased LTP. The promnesiant effects of S 18986 were more robust in middle‐aged animals than in aged animals.
Collectively, these findings suggest that chronic S 18986 treatment could prevent the progression of early age‐related memory deficits, and that increased BDNF expression may be one of the events involved in sustaining this action.
Neuroprotective Properties
Although high concentrations of glutamate are toxic to most neurons, lower concentrations of glutamate or glutamate agonists have been reported to increase cell survival. Enhancement of glutamatergic transmission by infusion of glutamate or AMPA has been shown to increase neuronal survival, attenuate apoptosis, and to prevent synaptic deterioration produced by deafferentation [61, 62, 63]. Normal aging is associated with increased neuronal vulnerability, with impairment of neurotransmitter release and/or levels and increased inflammatory markers [64, 65, 66]. In addition, AMPA modulators have demonstrated neuroprotective effects on dopamine cell loss in animal models of Parkinson disease [27]. We therefore have examined whether S 18986 elicits protection in acute or chronic administration in two models, in a lesion‐induced model and in a model of normal aging.
In a model of lesion induced by a NMDA agonist (ibotenate) in the cortex and periventricular white matter in the neonatal mouse brain, Dicou [67] has shown that the acute administration of S 18986 (10 mg/kg i.p.) exerted a neuroprotective effect in cortical and white matter regions (P < 0.05 and P < 0.01). It was hypothesized that this outcome may be achieved through the MAPK pathway, as an MAPK inhibitor was able to block the neuroprotective effect [67]. In the same study and as previously described [68], MK‐801 i.p. injected prior to ibotenate injection completely suppressed excitotoxic lesions. These results point strongly in favor of a key role of NMDA receptors in the present model. Moreover, BDNF was shown to be neuroprotective in ibotenate‐induced brain lesions in the P5 mouse [69]. The increase of BDNF expression, via activation of MAP kinase, may therefore be an important part of the neuroprotective profile of AMPA modulators.
More recently, Bloss [55] has described that chronic treatment (4 months) of middle‐aged rats (14‐months old at the beginning of the experiment) with S 18986 (0.1 and 1 mg/kg) retarded the decline of forebrain cholinergic neurons and midbrain dopaminergic neurons during aging and attenuated the age‐related increase of microglial markers in the hippocampus (CA3 stratum oriens). No effect of the drug was observed in other regions of the hippocampus or in the substantia nigra pars compacta.
The effect of S 18986 on the preservation of ACh and dopaminergic neurones with aging could be directly linked to its enhancing effect on both neurotransmitters [41] (see Mechanism of Action). These findings obtained with S 18986 are in agreement with those of several studies showing that other AMPA modulators are neuroprotective agents and reduce dopaminergic cell loss in animal models of Parkinson disease [27, 30].
Summary and Conclusion
The present review focuses on the selective positive allosteric AMPA modulator S 18986, a drug that is emerging as a favourable candidate in the prevention of cognitive decline associated with cerebral aging and/or neurological diseases. Ex vivo, this drug increases the amplitude of extracellular excitatory field potentials in the CA1 of the hippocampus; in vivo it enhances LTP. The two brain target areas of S 18986 are the frontal cortex and dorsal hippocampus, two regions classically involved in memory processes. Effects of S 18986 on hippocampal plasticity and synaptic transmission are believed to support its memory‐enhancing properties. The latter have been consistently demonstrated in a number of memory tasks exploring different types of memory, including working, procedural, contextual, relational/declarative, and episodic memory in both young adult and aged animals. In addition, S 18986 presents neurotrophic and neuroprotective effects, which may be involved in its prevention effects on age‐related cognitive deficits.
An interesting attribute of S 18986 is its particularly robust memory‐enhancing effects in middle‐aged animals compared with aged ones. Indeed, keeping in mind that cognitive impairment in AD patients have been hypothesized to result from the downscaling of postsynaptic AMPA receptor function at the onset of the pathology [70], a treatment such as S 18986 that targets AMPA receptors would obviously provide more valuable benefits when initiated early. S 18986 could therefore be proposed in the symptomatic treatment of prodromal forms of AD, such as mild cognitive impairment (MCI).
To date, the current knowledge about the effects of AMPA potentiators on human memory remains rather, limited and the available evidence does not suggest a clear benefit in healthy elderly participants and AD patients [71, 72, 73, 74]. Among compounds that have progressed to clinical level, the AMPA potentiator CX717 has produced mixed results. Promising findings were reported in a model of sleep deprivation in monkeys where CX717 restored memory performance to normal [75]. However, in a trial in healthy volunteers using a stimulated night shift work paradigm, no positive effects on performance and alertness were observed with CX717 treatment [76]. The compound is now being tested in phase II clinical trials in AD patients. Preliminary data with AMPA potentiators appear promising, but clinical translation in patients and a better understanding of its effects on human cognition will be necessary to validate the therapeutic approach.
It remains that several lines of evidence have established the importance of AMPA receptors in synaptic targets for AD. A direct association between AMPA receptors and Aß has been demonstrated and could result in modulation of channel properties. Thus, Aß‐induced downregulation of AMPA receptors has been demonstrated in postsynaptic membranes [77, 78], and Aß may also influence the proteins involved in insertion and stabilization of postsynaptic AMPA receptors [79].
Clinical studies are now warranted to determine whether it is possible to translate the promising preclinical properties of AMPA potentiators into a potential clinical interest, namely that of delaying the progression from MCI to Alzheimer disease.
Conflict of Interest
None.
Acknowledgments
The authors would like to acknowledge Corinne Dumicic for her secretarial assistance and Pierre‐Alain Boyer for reviewing the manuscript.
This paper is dedicated to the memory of Philippe Morain.
References
- 1. Lanctot KL, Herrmann N, Kenneth KY, et al Efficacy and safety of cholinesterase inhibitors in Alzheimer's disease: A meta‐analysis. CMAJ 2003;169:557–564. [PMC free article] [PubMed] [Google Scholar]
- 2. Lynch G. Glutamate‐based therapeutic approaches: Ampakines. Curr Opin Pharmacol 2006;6:82–88. [DOI] [PubMed] [Google Scholar]
- 3. Morrow JA, Maclean JKF, Jamieson C. Recent advances in positive allosteric modulators of the AMPA receptor. Curr Opin Drug Discov Devel 2006;9:571–579. [PubMed] [Google Scholar]
- 4. Robbins TW, Murphy ER. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol Sci 2006;27:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Advokat C, Pellegrin AI. Excitatory amino acids and memory: Evidence from research on Alzheimer's disease and behavioral pharmacology. Spring 1992;16:13–24. [DOI] [PubMed] [Google Scholar]
- 6. Danysz W, Zajackowski W, Parsons CG. Modulation of learning processes by ionotropic glutamate receptor ligands. Behav Pharmacol 1995;6:455–474. [PubMed] [Google Scholar]
- 7. Bliss TV, Collingridge GL. A synaptic model of memory: Long‐term potentiation in the hippocampus. Nature 1993;361:31–39. [DOI] [PubMed] [Google Scholar]
- 8. Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long‐term potentiation in the hippocampus. Science 2006;313:1093–1097. [DOI] [PubMed] [Google Scholar]
- 9. Francis PT, Sims NR, Procter AW, Bowen DM. Cortical pyramidal neurone loss may cause glutamatergic hypoactivity and cognitive impairment in Alzheimer's disease: Investigative and therapeutic perspectives. J Neurochem 1993;60:1589–1604. [DOI] [PubMed] [Google Scholar]
- 10. Ulas J, Cotman CW. Decreased expression of N‐methyl‐D‐aspartate receptor 1 messenger RNA in select regions of Alzheimer brain. Neuroscience 1997;79:973–982. [DOI] [PubMed] [Google Scholar]
- 11. Lauderback CM, Hackett JM, Huang FF, et al The glial glutamate transporter, GLT‐1, is oxidatively modified by 4‐hydroxy‐2‐noner the Alzheimer's disease brain: The role of Abeta1–42. J Neurochem 2001;78:413–416. [DOI] [PubMed] [Google Scholar]
- 12. Westphalen RI, Scott HL, Dodd PR. Synaptic vesicle transport and synaptic membrane transporter sites in excitatory amino acid nerve terminals in Alzheimer disease. J Neural Transm 2003;110:1013,27. [DOI] [PubMed] [Google Scholar]
- 13. Lee HK, Takamiya K, Han JS, et al Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 2003;112:631–643. [DOI] [PubMed] [Google Scholar]
- 14. Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci 2007;8:101–113. [DOI] [PubMed] [Google Scholar]
- 15. Staubli U, Rogers G, Lynch G. Facilitation of glutamate receptors enhances memory. Proc Natl Acad Sci USA 1994;91:777–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamada KA. Therapeutic potential of positive AMPA receptor modulators in the treatment of neurological disease. Expert Opin Investig Drugs 2000;9:765–777. [DOI] [PubMed] [Google Scholar]
- 17. Lynch G, Gall CH. Ampakines and the threefold path to cognitive enhancement. Trends Neurosci 2006;29:554–562. [DOI] [PubMed] [Google Scholar]
- 18. O'Neill MJ, Dix S. AMPA receptor potentiators as cognitive enhancers. IDrugs 2007;10:185–192. [PubMed] [Google Scholar]
- 19. Granger R, Staubli U, Davis M, et al A drug that facilitates glutamatergic transmission reduces exploratory activity and improves performance in a learning‐dependent task. Synapse 1993;15:326–329. [DOI] [PubMed] [Google Scholar]
- 20. Larson J, Lieu T, Petchpradub V, et al Facilitation of olfactory learning by a modulator of AMPA receptors. J Neurosci 1995;15:8023–8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Granger R, Deadwyler S, Davis M, et al Facilitation of glutamate receptors reverses an age‐associated memory impairment in rats. Synapse 1996;22:332–337. [DOI] [PubMed] [Google Scholar]
- 22. Hampson RE, Rogers G, Lynch G, Deadwyler SA. Facilitative effects of the ampakine CX516 on short‐term memory in rats: Enhancement of delayed‐nonmatch‐to‐sample performance. J Neurosci 1998;18:2740–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buccafusco JJ, Weiser T, Winter K, Klinder K, Terry AV Jr. The effects of IDRA 21, a positive modulator of the AMPA receptor, on delayed matching performance by young and aged rhesus monkeys. Neuropharmacology 2004;46:10–22. [DOI] [PubMed] [Google Scholar]
- 24. Lauterborn J, Lynch G, Vanderklish P, Arai A, Gall C. Positive modulation of AMPA receptors increase neurotrophin expression by hippocampal and cortical neurons. J Neurosci 2000;20:8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Legutko B, Li X, Skolnick P. Regulation of BDNF expression in primary neuron culture by LY392098, a novel AMPA receptor potentiator. Neuropharmacology 2001;40:1019–1027. [DOI] [PubMed] [Google Scholar]
- 26. Mackowiak M, O'Neill MJ, Hicks CA, Bleakman D, Skolnick P. An AMPA receptor potentiator modulates hippocampal expression of BDNF: An in vivo study. Neuropharmacology 2002;43:1–10. [DOI] [PubMed] [Google Scholar]
- 27. Murray TK, Whalley K, Robinson CS, et al LY503430, a novel α‐Amino‐3‐ hydroxy‐5‐methylisoxazole‐4‐propionic acid receptor potentiator with functional, neuroprotective and neurotrophic effects in rodent models of Parkinson's disease. J Pharmacol Exp Ther 2003;306:752–762. [DOI] [PubMed] [Google Scholar]
- 28. Johnson SA, Luu NT, Herbst TA, et al Synergistic interactions between ampakines and antipsychotic drugs. J Pharmacol Exp Ther 1999;289:392–397. [PubMed] [Google Scholar]
- 29. Marenco S, Weinberger DR. Therapeutic potential of positive AMPA receptor modulators in the treatment of neuropsychiatric disorders. CNS Drugs 2006;20:173–185. [DOI] [PubMed] [Google Scholar]
- 30. O'Neill MJ, Witkin JM. AMPA receptor potentiators: Application for depression and Parkinson's disease. Curr Drug Targets 2007;8:603–620. [DOI] [PubMed] [Google Scholar]
- 31. Desos P, Serkiz B, Morain P, Lepagnol J, Cordi A. Enantioselective synthesis of a pyrrolo‐benzothiadiazine derivate S 18986–1, a new AMPA receptor positive modulator. Bioorg Med Chem Lett 1996;6:3003–3008. [Google Scholar]
- 32. Bourasset F, Bernard K, Munoz C, Genissel P, Scherrmann JM. Neuropharmacokinetics of a new α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazole propionic acid (AMPA) modulator, S 18986 [(S)‐2,3‐Dihydro‐[3,4]Cyclopentano‐1,2,4‐Benzothiadiazine‐1,1‐Dioxide], in the rat. Drug Metab Dispos 2005;33:1137–1143. [DOI] [PubMed] [Google Scholar]
- 33. Stensbol TB, Slok FA, Trometer J, et al Characterization of a new AMPA receptor radioligand, [3H]2‐amino‐3‐ (3‐carboxy‐5‐methyl‐4‐isoxazolyl) propionic acid. Eur J Pharmacol 1999;373:251–262. [DOI] [PubMed] [Google Scholar]
- 34. Lorenzini CA, Baldi E, Bucherelli C, Sachetti B, Tassoni G. Role of dorsal hippocampus in acquisition, consolidation and retrieval of rat's passive avaidance response: A tetrodotoxin functional inactivation study. Brain Res 1996;730:32–39. [DOI] [PubMed] [Google Scholar]
- 35. Giovannini MG, Rodino P, Mutolo M, Pepeu G. Oxiracetam and aniracetam increase acetylcholine release from the rat hippocampus in vivo . Drug Dev Res 1993;28:503–509. [Google Scholar]
- 36. Desai MA, Burnett JP, Schoepp DD. Cyclothiazide selectively potentiates AMPA‐ and kainate‐induced [3H] norepinephrine release from rat hippocampal slices. J Neurochem 1994;63:231–237. [DOI] [PubMed] [Google Scholar]
- 37. Desai MA, Burnett JP, Ornstein PL, Schoepp DD. Cyclothiazide acts at a site on the alpha‐amino‐3‐hydroxy‐ 5‐methyl‐4‐isoxazole propionic acid receptor complex that does not recognize competitive or noncompetitive AMPA receptor antagonist. J Pharmacol Exp Ther 1995;272:38–43. [PubMed] [Google Scholar]
- 38. Jin S. AMPA and kainite receptors differentially mediate excitatory amino acid‐induced dopamine and acetylcholine release from rat striatal slices. Neuropharmacology 1997;26:1503–1510. [DOI] [PubMed] [Google Scholar]
- 39. Cowen MS, Beart PM. Cyclothiazide and AMPA receptor desensitization: analyses from studies of AMPA‐induced release of [3H]‐noradrenaline from hippocampal slices. Br J Pharmacol 1998;123:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pittaluga A, Bonfanti A, Arvigo D, Raiteri M. Aniracetam, 1‐BCP and cyclothiazide differentially modulate the function of NMDA and AMPA receptors mediating enhancement of noradrenaline release in rat hippocampal slices. Naunyn Schmiedebergs Arch Pharmacol 1999;359:272–279. [DOI] [PubMed] [Google Scholar]
- 41. Rosi S, Giovannini MG, Lestage PJ, Munoz C, Corte LD, Pepeu G. S 18986, a positive modulator of AMPA receptors with cognition‐enhancing properties, increases ACh release in the hippocampus of young and aged rat. Neurosci Lett 2004;361:120–123. [DOI] [PubMed] [Google Scholar]
- 42. Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: What does it mean for learning and memory. Neurobiol Lear Mem 2003;80:245–256. [DOI] [PubMed] [Google Scholar]
- 43. Lockhart B, Iop F, Closier M, Lestage P. (S)‐2,3‐dihydro‐[3,4]cyclopentano‐1,2,4‐benzothiadiazine‐1,1‐dioxide: (S 18986–1) a positive modulator of AMPA receptors enhances (S)‐AMPA‐mediated [3H]noradrenaline release from rat hippocampal and frontal cortex slices. Eur J Pharmacol 2000;401:145–153. [DOI] [PubMed] [Google Scholar]
- 44. Harley C. Noradrenergic and locus coreuleus modulation of the perforant path‐evoked potential in rat dentate gyrus supports a role of the locus coeruleus in attentional and memorial processes. Prog Brain Res 1991;88:307–321. [DOI] [PubMed] [Google Scholar]
- 45. Drachman DA, Leavitt J. Human memory and the cholinergic system: A relationship to ageing? Arch Neurol 1974;30:113–121. [DOI] [PubMed] [Google Scholar]
- 46. Dantzer R, Bluthe RM, Koob GF, Le Moal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology (Berl) 1987;91:363–368. [DOI] [PubMed] [Google Scholar]
- 47. Ennaceur A, Delacour J. A new one‐trial test for neurobiological studies of memory in rats, 1: Behavioral data. Behav Brain Res 1988;31:47–59. [DOI] [PubMed] [Google Scholar]
- 48. Lebrun C, Pilliere E, Lestage P. Effects of S 18986–1, a novel cognitive enhancer, on memory performances in an object recognition task in rats. Eur J Pharmacol 2000;401:205–212. [DOI] [PubMed] [Google Scholar]
- 49. Bertaina‐Anglade V, La Rochelle CD, Munoz C, Morain P, Bernard K. Comparison of single vs. multiple administrations of the AMPA receptors modulator S 18986 in the object recognition task in rats. Fundam Clin Pharmacol 2007;21:349–354. [DOI] [PubMed] [Google Scholar]
- 50. Ward MT, Oler JA, Markus EJ. Hippocampal dysfunction during aging, I: Deficits in memory consolidation. Neurobiol Aging 1999;20:363–372. [DOI] [PubMed] [Google Scholar]
- 51. Rex CS, Kramar EA, Colgin LL, Lin B, Gall CM, Lynch G. Long‐term potentiation is impaired in middle‐aged rats: regional specificity and reversal by adenosine receptor antagonists. J Neurosci 2005;25:5956–5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Francia N, Cirulli F, Chiarotti F, Antonelli A, Aloe L, Alleva E. Spatial memory deficits in middle‐aged mice correlate with lower exploratory activity and a subordinate status: Role of hippocampal neurotrophins. Eur J Neurosci 2006;23:711–728. [DOI] [PubMed] [Google Scholar]
- 53. Beracochea D, Philippin JN, Meunier S, Morain P, Bernard K. Improvement of episodic contextual memory by S 18986 in middle‐aged mice: Comparison with donepezil. Psychopharmacology 2007;193:63–73. [DOI] [PubMed] [Google Scholar]
- 54. Munoz C, Meaney M, Bredy TW, Bernard K. S 18986, a positive modulator of AMPA receptors, prevents progression of age‐related memory deficits and increases BDNF expression in middle aged rodents. 34th Annual Meeting of the American Society for Neuroscience, Washington , 2004.
- 55. Bloss EB, Hunter RG, Waters EM, Munoz C, Bernard K, McEwen BS. Behavioral and biological effects of chronic S 18986, a positive AMPA receptor modulator, during aging. Exp Neurol 2008;210:109–117. [DOI] [PubMed] [Google Scholar]
- 56. Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H. A two‐trial memory task with automated recording: Study in young and aged rats. Brain Res 1992;588:132–139. [DOI] [PubMed] [Google Scholar]
- 57. Marighetto A, Valerio S, Jaffard R, et al The AMPA modulator S 18986 improves declarative and working memory performances in aged mice. Behav Pharmacol 2008;19:235–244. [DOI] [PubMed] [Google Scholar]
- 58. Etchamendy N, Desmedt A, Cortes‐Torrea C, Marighetto A, Jaffard R. Hippocampal lesions and discrimination performance of mice in the radial maze: Sparing or impairment depending on the representational demands of the task. Hippocampus 2003;13:197–211. [DOI] [PubMed] [Google Scholar]
- 59. Pang PT, Teng HK, Zaitsev E, et al Cleavage of proBDNF by tPA/plasmin is essential for long‐term hippocampal plasticity. Science 2004;306:487–491. [DOI] [PubMed] [Google Scholar]
- 60. Lockhart BP, Rodriguez M, Mourvelat S, et al S 18986 : A positive modulator of AMPA‐receptors enhances (S)‐AMPA‐mediated BDNF mRNA and protein expression in rat primary cortical neuronal cultures. Eur J Pharmacol 2007;561:23–31. [DOI] [PubMed] [Google Scholar]
- 61. Bambrick LL, Yarowsky PJ, Krueger BK. Glutamate as a hippocampal neuron survival factor: An inherited defect in the trisomy 16 mouse. Proc Natl Acad Sci USA 1995;92:9692–9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci 1999;2:44–49. [DOI] [PubMed] [Google Scholar]
- 63. Limatola C, Ciotti MT, Mercanti D, et al The chemokine growth‐related gene product beta protects rat cerebellar granule cells from apoptotic cell death through alpha‐amino‐3‐hydroxy‐ 5‐methyl‐4‐isoxazolepropionate receptors. Proc Natl Acad Sci USA 2000;97:6197–6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Backman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci Biobehav Rev 2006;30: 791–807. [DOI] [PubMed] [Google Scholar]
- 65. Sarter M, Bruno JP, Parikh V. Abnormal neurotransmitter release underlying behavioral and cognitive disorders: Towards concepts of dynamic and function‐specific dysregulation. Neuropsychopharmacol 2007;32:1452–1461. [DOI] [PubMed] [Google Scholar]
- 66. Sierra A, Gottfried‐Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 2007;55:412–424. [DOI] [PubMed] [Google Scholar]
- 67. Dicou E, Rangon CM, Guimiot F, Spedding M, Gresens P. Positive allosteric modulators of AMPA receptors are neuroprotective against lesions induced by an NMDA agonist in neonatal mouse brain. Brain Res 2003;970:221–225. [DOI] [PubMed] [Google Scholar]
- 68. Dommergues MA, Patkai J, Renauld JC, Evrard P, Gressens P. Pro‐inflammatory cytokines and IL‐9 exacerbate excitotoxic lesions of the newborn murine neopallium. Ann Neurol 2000;47:54–63. [PubMed] [Google Scholar]
- 69. Husson I, Kosofsky BE, Gressens P. BDNF‐induced protection of cortical neurons against an excitotoxic challenge is maturation stage‐dependent. Pediatr Res 2002;51:447A. [Google Scholar]
- 70. Chang EH, Savage MJ, Flood DG, et al AMPA receptor downscaling at the onset of Alzheimer's disease pathology in double knockin mice. PNAS 2006;103:3410–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lynch G, Granger R, Ambros‐Ingerson J, Davis CM, Kessler M, Schehr R. Evidence that a positive modulator of AMPA‐type glutamate receptors improves delayed recall in aged humans. Exp Neurol 1997;145:89–92. [DOI] [PubMed] [Google Scholar]
- 72. Johnson SA, Simmon VF. Randomized, double‐blind, placebo‐controlled international clinical trial of the AMPAKINE® CX516 in elderly participants with mild cognitive impairment. J Mol Neurosci 2002;19:197–200. [DOI] [PubMed] [Google Scholar]
- 73. Chappell AS, Gonzales C, Williams J, Witte MM, Mohs RC, Sperling R. AMPA potentiator treatment of cognitive deficits in Alzheimer disease. Neurology 2007;68:1008–1012. [DOI] [PubMed] [Google Scholar]
- 74. Wezenberg E, Verkes RJ, Ruigt Ge SF, Hulstijn W, Sabbe B. Acute effects of the ampakine farampator on memory and information processing in healthy elderly volunteers. Neuropsychopharmacology 2007;32:1272–1283. [DOI] [PubMed] [Google Scholar]
- 75. Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol 2005;3:e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wesensten NJ, Reichardt RM, Balkin TJ. Ampakine (CX717) effects on performance and alertness during simulated night shift work. Aviat Space Environ Med 2007;78:937–943. [DOI] [PubMed] [Google Scholar]
- 77. Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid beta‐protein on hippocampal synaptic plasticity: A potent role for trimers. J Physiol 2006;572:477–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Parameshwaran K, Sims C, Kanju P, et al Amyloid beta‐peptide Abeta(1–42) but not Abeta(1–40) attenuates synaptic AMPA receptor function. Synapse 2007;61:367–374. [DOI] [PubMed] [Google Scholar]
- 79. Roselli F, Tirard M, Lu J, Hutzler P, Lamberti P, Livrea P, et al Soluble beta‐amyloid1–40 induces NMDA‐dependent degradation of postsynaptic density‐95 at glutamatergic synapses. J Neurosci 2005;25:11061–11070. [DOI] [PMC free article] [PubMed] [Google Scholar]


