Abstract.
Despite recent large-scale investments, malaria remains a major public health concern. Few studies have examined congenital malaria, defined as the presence of malaria parasitemia within the first 7 days of life, in endemic areas. This study aimed to determine the prevalence, to describe the clinical presentation, and to examine factors associated with congenital malaria in newborns aged up to 7 days attending Tororo General Hospital in Uganda. A total of 261 mother/baby pairs were recruited in this cross-sectional study. Giemsa-stained thick blood smears for malaria parasites and rapid malaria diagnostic tests were performed on capillary blood samples from all newborns and mothers, as well as on placental and cord samples from newborns delivered in the hospital. The prevalence of congenital malaria in the newborns was 16/261 (6.1%). No single clinical feature was associated with congenital malaria. However, there were associations between congenital malaria and maternal parasitemia (P < 0.001), gravidity of one (P = 0.03), maternal age < 19 years (P = 0.01), cord blood parasitemia (P = 0.01), and placental malaria (P = 0.02). In conclusion, congenital malaria is not rare in Uganda and there are no obvious clinical features associated with it in the newborn. Based on these findings, we recommend strengthening malaria prevention during pregnancy to reduce the occurrence of congenital malaria in newborns.
INTRODUCTION
In 2017, the WHO reported that there were 219 million cases of malaria, resulting in 435,000 deaths.1 Most of the cases (80%) and deaths (90%) were in sub-Saharan Africa, and mainly affected children younger than 5 years.2 However, the devastating consequences of malaria start before the child is even born and these children suffer adverse outcomes related to gestational malaria, placental malaria, and congenital malaria.
During pregnancy, the acquired antimalarial immunity of a woman residing in a malaria-endemic area decreases.3 Several studies over the past two decades, especially in Nigeria, have shown that the prevalence of malaria in neonates appears to be increasing with values as high as 25%.4,5 The true burden of congenital malaria, however, might be underestimated because of the nonspecific clinical picture and the absence or the delayed presentation of symptoms. It is reported that it might take 3–4 weeks before congenitally infected infants present symptoms6 and only 34% of parasitemic newborns would present symptoms within 3 days.7 In Burkina Faso, 11.8% of newborns with congenital malaria died and the average amount of time from hospital admission to death was 4.8 days, with 55% of deaths occurring in the first 24 hours after admission.8
In Uganda, malaria is still a major public health problem, accounting for 30–50% of all outpatient consultations and up to 35% of hospital admissions.9 Recent hospital-based studies have examined malaria admissions in various parts of the country, including a 2010 study, that estimated the prevalence of placental malaria to be 74% among women who took 0–1 prophylactic doses of sulfadoxine–pyrimethamine and 60% among women who took 2–3 doses prophylactic doses of sulfadoxine–pyrimethamine.10 However, few studies have reported the prevalence and clinical features of congenital malaria. Our study aimed to address this gap in the literature by 1) determining the prevalence of congenital malaria, 2) describing the clinical features of congenital malaria, and 3) identifying risk factors for congenital malaria in Ugandan newborns in the first 7 days of life.
METHODS
Design.
This cross-sectional study of congenital malaria among newborn babies was conducted at Tororo General Hospital in Uganda from February to April 2014.
Setting.
Tororo is a rural district in southeastern Uganda approximately 230 km east of the capital city, Kampala. The district experiences hyperintense malaria transmission with an estimated entomological inoculation rate of 562 infective bites per person year (approximately 1.5 infectious bites every day).11 The incidence of malaria in this area has a bimodal distribution that follows the rainy seasons of March to May and September to November. Tororo General Hospital is a public 200-bed hospital serving a catchment population of approximately 500,000 people. Between 230 and 330 mothers deliver at the Tororo General Hospital every month.
Participants.
Participants were newborn babies, aged up to 7 days born at or who attended the Tororo General Hospital for other services, for example, for immunization or neonatal illness during the study period, and their mothers. Macerated and fresh still births were excluded.
Procedures.
Recruitment.
Written informed consent was obtained from mother. Participants were then recruited by convenience to achieve the sample size. Mother/baby pairs delivering in the hospital were recruited within the first hour following birth. In addition, newborns babies aged up to 7 days who were admitted to the pediatric ward for any condition, or who were delivered late at night or from elsewhere but attended the immunization unit in the postnatal ward were also selected for enrollment.
Data collection.
Clinical data.
A medical officer was trained as a research assistant to assist in data collection. A standard questionnaire was administered to all study subjects by one of the investigators (M. H.) or the research assistant. The questionnaire including sociodemographic data, gravidity, gestational age (estimated by Naegle’s rule using the self-reported last menstrual date recorded on the antenatal card at the first prenatal visit), use of treated mosquito bed nets, and antimalarial medicines for both treatment and prevention during the pregnancy. The interview was performed face to face with the mothers in either English or in one of the local language in Tororo.
All newborn participants had a full physical examination. The newborn weight (in kilograms) was obtained using an electronic baby scale (SECA, Model, Nagano City, Japan); length (in centimeters) was measured in the dorsal decubitus position using Stadiometer, whereas the head circumference (in centimeters) was determined using a flexible measuring tape. Axillary temperature was determined using a digital thermometer and pyrexia was defined as temperature ≥ 37.5°C. The presence of congenital abnormalities, dysmorphic features, as well as pallor of the mucus membranes, jaundice, enlargement of the liver and spleen, and neonatal reflexes were documented for each participant.
Laboratory testing.
Malaria was diagnosed by microscopic examination of Giemsa-stained blood smears (BSs).
Blood from finger prick samples (mothers) and foot prick samples (newborns) was used to prepare the thick smears. The smears were air-dried and stained for 30 minutes using 2% Giemsa. Two experienced microscopists read the thick smears at ×100 magnification using immersion oil. Positive BSs were defined as parasite density ≥ 1 parasite/μL. Smears were considered negative if no malaria parasites were found after review of 100 high-power fields. Parasite density was also determined. The two microscopists were blinded to each other’s readings, and discrepant readings were settled by a third microscopist.
For babies delivered at Tororo General Hospital, within an hour of delivery, 1 mL of placental blood was also collected in a sterile ethylenediamine tetraacetic acid (EDTA) tube using the incision method.12 This method consisted of making a shallow incision into the maternal side of the placenta with scissors and collecting blood that pooled in the intervillous space. A separate collection was made from the cord. To do so, the cord was cleaned with a 70% alcohol swab to prevent contamination with maternal blood and 1 mL of cord blood was taken from the umbilical cord about 15 cm from the point of attachment to the placenta.
Thick BSs were prepared within 24 hours from blood samples obtained from the cord and placenta and they were examined using the same procedure as peripheral BSs.
In addition, malaria rapid diagnostic tests (RDT) (Malaria Ag P.f HRP-II Rapid Test, Somerset, NJ) were also performed on all blood samples. A positive malaria test was defined as any positive result on either BS or RDT. In addition, hemoglobin levels were measured using a Hemocue Photometer version 3.0.1 (Model 3000-0031-6801, EKF Diagnostic, Barleben, Germany). Anemia was diagnosed if the hemoglobin level was < 15 g/dL.13
HIV testing is routine for all women delivering at Tororo General Hospital with uptakes of up to 100% antenatal HIV testing.14 For women without HIV test results at enrollment, an HIV test was performed using a rapid test (Determine HIV-1/2; Alere Medical co., Ltd., Chiba, Japan) following Ugandan guidelines as in the National HIV testing algorithm. Pre- and posttest counseling by a qualified counselor was performed.
Ethics, consent, and permissions.
Ethical approval for the study was provided by Makerere University School of Medicine Research and Ethics Committee. Written informed consent for participation was obtained from all mothers. Participants who tested positive for malaria were treated with a full course of quinine (newborns) or artemether–lumefantrine (mothers).
Data analysis.
Data entry was performed using Epidata 3.1 (“The EpiData Association,” Odense, Denmark) and exported to SPSS (version 19; IBM SPSS 17, 18 and 19, Chicago, IL) for analysis. The prevalence of congenital malaria was calculated. Normally distributed continuous data were summarized by medians with ranges. Fisher’s exact test was used to compare categorical data to establish whether an association existed between congenital malaria and explanatory variables. A P-value < 0.05 was considered statistically significant.
RESULTS
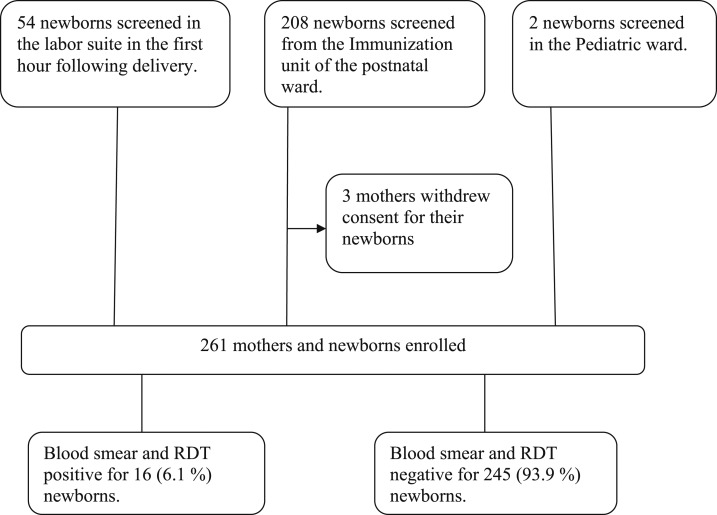
From February 14, 2014 to April 4, 2014, 264 mother/baby pairs were screened. Three mothers denied consent. Thus, a final sample of 261 mother/baby pairs were recruited (see Figure 1).
Figure 1.
Study Profile. Subject recruitment flowchart is depicted using a consecutive sampling technique. Fifty-four newborns and their mothers were recruited from the labor suite, followed by recruitment of 208 newborns and their mothers in the immunization unit, and then by recruitment of two newborns and their mothers in the pediatric ward. Three mothers withdrew consent to make a final sample size of 261 mothers and their newborns. Blood smears and RDT were performed on all 261 newborns. Sixteen were positive, making a prevalence of congenital malaria to be 6.1%. RDT = rapid diagnostic test for malaria.
The prevalence of congenital malaria.
The prevalence of congenital malaria was 16/261 (6.1%). All cases were positive on both microscopy and RDT.
Clinical presentation of the newborns with malaria.
Babies with congenital malaria were similar to those without congenital malaria with regard to gestational age, Apgar scores, birth weight, fetal length, and body temperature. The proportion with jaundice, mucosal pallor, hepatomegaly or splenomegaly, and the hemoglobin levels were also similar (Table 1). The median parasite density in newborns with congenital malaria was 460 parasites/µL (range: 80–1,550 parasites/µL). Overall, no specific clinical feature was associated with congenital malaria. Instead, most newborns with congenital malaria had a normal clinical examination. Only one newborn (6.2%) with congenital malaria had a temperature > 37.5°C and another (6.2%) had a hemoglobin level < 15 g/dL. Newborns with congenital malaria had a median birth weight of 3.2 kg (range: 2.8–3.5 kg), median gestational age of 39 weeks (range: 38–40 weeks), a median fetal length of 50 cm (range: 49–50 cm), a median temperature of 36.4°C (range: 36–36.8°C), and a median hemoglobin level of 18.3 g/dL (range: 16.7–19.9°C).
Table 1.
Clinical presentation of the newborns
| Variables | Outcome | Congenital malaria | OR | 95% CI | P-value | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| N (%) | N (%) | |||||
| Birth weight (kg) | < 2.5 | 0 (0.0) | 11 (4.5) | NA | NA | 0.49 |
| ≥ 2.5 | 16 (100.0) | 234 (95.5) | ||||
| Gestational age (week) | < 37 | 0 (0.0) | 6 (2.4) | NA | NA | 0.68 |
| ≥ 37 | 16 (100.0) | 239 (97.6) | ||||
| Apgar score | 4–6 | 0 (0.0) | 3 (1.2) | NA | NA | 0.83 |
| 7–10 | 16 (100.0) | 242 (98.8) | ||||
| Fetal length (cm) | < 49.5 | 0 (0.0) | 16 (6.5) | NA | NA | 0.32 |
| ≥ 49.5 | 16 (100.0) | 229 (93.5) | ||||
| Temperature (°C) | < 37.5 | 15 (93.8) | 242 (93.8) | 5.38 | 0.53–54.86 | 0.23 |
| ≥ 37.5 | 1 ( 6.2) | 3 (1.2) | ||||
| Jaundice | Yes | 0 (0.0) | 4 (1.6) | NA | NA | 0.78 |
| No | 16 (100.0) | 241 (98.4) | ||||
| Pallor | Yes | 0 (0.0) | 2 (0.8) | NA | NA | 0.88 |
| No | 16 (100.0) | 243 (99.2) | ||||
| Hepatomegaly | Yes | 0 (0.0) | 2 (0.8) | NA | NA | 0.72 |
| No | 16 (100.0) | 243 (99.2) | ||||
| Splenomegaly | Yes | 0 (0.0) | 0 (0.0) | NA | NA | NA |
| No | 16 (100.0) | 245 (100.0) | ||||
| Hemoglobin level (g/dL) | < 15 | 1 (6.2) | 4 (1.6) | 4.02 | 0.42–38.21 | 0.27 |
| ≥ 15 | 15 (93.8) | 241 (98.4) | ||||
Sociodemographic and maternal factors associated with congenital malaria.
The household factors, maternal factors, and other factors (placental malaria and cord blood parasitemia) were analyzed using bivariate analysis. Risk factors associated with congenital malaria included the first pregnancy, teenage pregnancy (maternal age < 19 years), maternal malaria parasitemia at the time of delivery, placental malaria, and cord blood parasitemia. Factors found to be protective against congenital malaria included mothers residing in a brick house, mothers residing in a house with iron sheet roof, and mothers who had malaria and were treated with quinine during pregnancy (Table 2).
Table 2.
Household, maternal, and other characteristics associated with congenital malaria
| Variables | Outcome | Congenital malaria | OR | 95% CI | P-value | ||
|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||
| N (%) | N (%) | ||||||
| Household factors | Wall type | Brick | 1 (6.2) | 76 (29.5) | 0.15 | 0.02–1.14 | 0.03 |
| Mud | 15 (93.8) | 169 (69.0) | |||||
| Roof type | Iron sheet | 7 (43.8) | 179 (73.3) | 0.29 | 0.10–0.80 | 0.02 | |
| Grass | 9 (56.2) | 66 (26.9) | |||||
| Use spray | Yes | 1 (6.2) | 19 (7.8) | 0.79 | 0.10–6.33 | 0.65 | |
| No | 15 (93.8) | 226 (92.2) | |||||
| Use of Net | Non-ITN | 0 (0.0) | 4 (1.6) | NA | NA | 0.76 | |
| ITN | 16 (100.0) | 241 (98.4) | |||||
| Family size | < 5 person | 9 (56.2) | 87 (35.5) | 1.07 | 1.03–1.10 | 0.08 | |
| ≥ 5 person | 7 (43.8) | 158 (64.5) | |||||
| Maternal factors | Malaria treated during pregnancy | Quinine | 4 (100.0) | 24 (32.0) | 0.86 | 0.74–0.10 | 0.01 |
| Other* | 0 (0.0) | 51 (68.0) | |||||
| Chemoprevention during pregnancy | IPT-SP | 15 (93.8) | 239 (97.6) | NA | NA | 0.11 | |
| TS | 1 (6.2) | 0 (0.0) | |||||
| Both | 0 (0.0) | 4 (1.6) | |||||
| None | 0 (0.0) | 2 (0.80) | |||||
| Gravidity | G1 | 9 (56.2) | 74 (30.2) | 2.97 | 1.07–8.28 | 0.03 | |
| ≥ G2 | 7 (43) | 171 (69.8) | |||||
| Maternal age | < 19 years | 8 (50.0) | 46 (18.8) | 4.33 | 1.54–12.13 | 0.01 | |
| ≥ 19 | 8 (50.0) | 199 (81.2) | |||||
| Level of education | Primary | 10 (62.5) | 152 (62.0) | 1.02 | 0.36–2.90 | 0.60 | |
| Post Primary | 6 (37.5) | 93 (38.0) | |||||
| Fever during pregnancy | 1st and 2nd trimester | 6 (60.0) | 71 (81.6) | 0.34 | 0.09–1.34 | 0.12 | |
| 3rd trimester | 4 (40.0) | 16 (18.4) | |||||
| HIV status during pregnancy | Positive | 1 (6.2) | 6 (2.4) | 2.66 | 0.30–23.50 | 0.36 | |
| Negative | 15 (93.8) | 239 (97.6) | |||||
| Maternal malaria at delivery | Yes | 8 (50.0) | 19 (7.8) | 11.90 | 4.02–35.24 | 0.00 | |
| No | 8 (50.0) | 226 (92.2) | |||||
| Other factors | Placental malaria | Yes | 3 (75.0) | 8 (15.4) | 16.50 | 1.52–179.22 | 0.02 |
| No | 1 (25.0) | 44 (84.6) | |||||
| Cord blood parasitemia | Yes | 2 (50.0) | 1 (2.0) | 49.00 | 3.02–794.52 | 0.01 | |
| No | 2 (50.0) | 49 (98.0) | |||||
ITN = insecticide treated net; SP = sulfadoxine/ pyrimethamine; TS = trimethoprim/sulfamethoxazole. A P-value < 0.05 was considered statistically significant. The bold values in table two show association between congenital malaria and those concerned explanatory variables on binary analysis.
* Other antimalarial drugs included Coartem and Fansidar.
General results for RDT and thick smears.
The prevalence of cord blood parasitemia on RDT and thick smear was 3/54 (5.6%) and 1/54 (1.8%), respectively. The prevalence of placental malaria on RDT and thick smear was 9/54 (16.7%) and 9/54 (16.7%), respectively. The prevalence of maternal malaria on RDT and thick smear was 27/261 (10.3%) and 23/261 (8.8%), respectively (Table 3).
Table 3.
General results for RDT and thick smears
| Test | RDT | Thick smear | Total | ||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| Cord | 3 (5.6) | 51 (94.4) | 1 (1.9) | 53 (98.1) | 54 |
| Placenta | 9 (16.7) | 45 (83.3) | 9 (16.7) | 45 (83.3) | 54 |
| Newborns | 16 (6.1) | 245 (93.9) | 16 (6.1) | 245 (93.9) | 261 |
| Maternal | 27 (10.3) | 234 (89.7) | 23 (8.8) | 238 (91.2) | 261 |
RDT = rapid diagnostic test.
DISCUSSION
This study aimed to determine the prevalence of congenital malaria, to describe the clinical features of congenital malaria, and to identify risk factors for congenital malaria in Ugandan newborns in the first seven 7 days of life.
The prevalence of congenital malaria in Tororo General Hospital was 6.1% (16/261; 95% CI: 3.2–9%). This prevalence data are similar to reports in Tanzania, Burkina Faso, and Nigeria,15–17 but higher than those in Burundi and the Calabar area of Nigeria.18,19 Two studies that used polymerase chain reaction (PCR) to detect malaria parasitemia found a higher prevalence of congenital malaria but this is most likely because of the more sensitive detection method used.20,21 Thus, these observed differences in congenital malaria prevalence could be related to varying utilization rates of malaria preventive measures during pregnancy, differences in malaria transmission intensity, or the laboratory operational techniques used in the various studies. In Tororo district, the large-scale distribution of insecticide-treated mosquito nets by the Ministry of Health of Uganda to all households just before the survey could explain the lower than expected prevalence of congenital malaria compared with the prevalence reported in other areas of similar transmission in Africa.
We did not find any clinical characteristics in the newborns that were significantly associated with congenital malaria. In our study, birth weight and anemia were not affected by congenital malaria. In fact, most newborns with congenital malaria were clinically normal. This presents a diagnostic challenge because clinicians may not suspect malaria in these newborns, who are otherwise healthy and have a normal examination. Our observation is similar to that in Burkina Faso,8 Nigeria,22 and Ghana20 where most newborns with congenital malaria were asymptomatic. Similarly, in Tanzania,15 newborn birth weight was not associated with congenital malaria, and, in Lagos, anemia was not associated with congenital malaria.23 On the contrary, Nagaraj et al. in India24 and Mwaniki et al. in Kenya25 found anemia to be one of the features of congenital malaria at birth. In addition, Okechukwu et al. in Abuja, Nigeria,26 found that hepatomegaly, fever, and jaundice were associated with congenital malaria. Low birth weight, anemia, and prematurity were the most common features associated with congenital malaria in Maumere, Indonesia.27 The presence of malaria parasites in asymptomatic newborns is documented to increase the risk of anemia in infancy.28 It has been hypothesized that the effectiveness of the placenta as a barrier, the presence of maternal antibodies, and the protective effect of fetal hemoglobin may all make congenital malaria rare in this population.29 However, reasons for decreased malaria in this young population are generally unknown.
It is also reported that it could take 3–4 weeks before congenitally infected infants present with symptoms6 and only 34% of parasitemic newborns present with symptoms within 3 days.7 The fact that we did not find an association between clinical symptoms and congenital malaria may be because of our selection criteria. In our study, all newborns were 1–7 days of age and most of them were recruited from the labor suite and immunization clinics of the postnatal ward, increasing the likelihood that our newborns were healthy. On the other hand, the study in Abuja26 only included newborns with suspected neonatal sepsis and the study in Indonesia and Kenya25 only included newborns hospitalized for various health conditions, biasing their samples toward sicker newborns. Furthermore, in highly endemic and stable transmission regions, such as our study site, signs and symptoms of active malaria infection in newborns are infrequent.30,31
In this study, maternal malaria parasitemia at delivery, gravidity of one, maternal age less than 19 years, cord blood parasitemia, and placental malaria were associated with congenital malaria. However, factors found to be protective against congenital malaria included mothers residing in brick houses or houses with iron sheet roofs and mothers who had malaria treated with quinine during pregnancy. In Benin, there was no association between maternal sociodemographic factors and congenital malaria.32 However, there was an association between maternal malaria parasitemia and congenital malaria in studies from Benin and Calabar, Nigeria.19,33 Other previous studies have also reported multiple factors associated with congenital malaria, such us placental malaria, cord blood parasitemia, gravidity of one, and maternal age less than 19 years, as was the case in this study.15,21,34–36
We show that mothers who had malaria during pregnancy and who were treated with quinine were less likely to deliver newborns with congenital malaria. Our study also shows that mothers who were sleeping in brick homes and in houses with iron sheet roofs (possibly indicative of a higher socioeconomic status) were less likely to deliver newborns with congenital malaria. An earlier study in Tanzania had found a higher number of mosquitoes in houses with mud walls or grass/thatch roofing than in cement-plastered walls and metal roofing, and these houses also had an increased risk of indoor mosquito bites, resulting in a higher risk of malaria transmission.37 The increased risk of mosquito bites in the mud walls and grass/thatch roofing houses could increase the risk of malaria in pregnant mothers, thereby increasing the risk of congenital malaria in their newborns. This study had some limitations that could have impacted on our findings. First, we did not use PCR for diagnosis of malaria, which is a more sensitive diagnostic approach. Second, we did not follow up the newborns to assess for their long-term outcome and also improve our understanding of the dynamics of malaria transmission and risk in this population in the first 1 week of life. Third, we may have selected healthier newborns, given our recruitment sites at the health facility and, in doing so, potentially underestimated the true burden of congenital malaria in the community.
Despite these limitations, we believe the study findings have important public health implications. These findings suggest that congenital malaria is not rare in Uganda but that there are no obvious clinical signs or symptoms associated with it, thus presenting a diagnostic dilemma for health workers. There was an association between congenital malaria and maternal malaria parasitemia at delivery, maternal age less than 19 years, gravidity of one, placental malaria, and cord blood parasitemia. Based on the findings, we recommended the strengthening of malaria prevention and treatment during pregnancy to improve pregnancy outcomes overall and to reduce the occurrence of congenital malaria in newborns.
Acknowledgments:
We would like to thank the mothers and the newborns who participated in this study; the midwives of Tororo General Hospital (Florence Anyango, Patricia, and Nora Nabudde); the microscopists in Tororo (Oswald Byaruhanga, Peter, and Charles); the research assistants Owini Moses and Abraham Masinda; and the hospital superintendent Ochara who allowed us to conduct the research in this hospital. We also thank our colleagues: Batte, Nabatte, Tongun, Mnzava, Edrako, Apiyo, Atugonza, Tumukunde, Kimera, and Ajilong and Susan Bartels for their support during this work. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1.WHO , 2018. World Malaria Report 2018. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2.WHO , 2013. World Malaria Report. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 3.Alecrim WD, Espinosa FE, Alecrim MG, 2000. Plasmodium falciparum infection in the pregnant patient. Infect Dis Clin North Am 14: 83–95, viii–ix. [DOI] [PubMed] [Google Scholar]
- 4.Mukhtar M, Lesi F, Iroha E, Egri-Okwaji M, Mafe A, 2005. Congenital malaria among inborn babies at a tertiary centre in Lagos, Nigeria. J Trop Pediatr 52: 19–23. [DOI] [PubMed] [Google Scholar]
- 5.Runsewe-Abiodun IT, Ogunfowora OB, Fetuga BM, 2006. Neonatal malaria in Nigeria-a 2 year review. BMC Pediatr 6: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larkin G, Thuma P, 1991. Congenital malaria in a hyperendemic area. Am J Trop Med Hyg 45: 587–592. [DOI] [PubMed] [Google Scholar]
- 7.Falade C, Mokuolu O, Okafor H, Orogade A, Falade A, Adedoyin O, Oguonu T, Aisha M, Hamer DH, Callahan MV, 2007. Epidemiology of congenital malaria in Nigeria: a multi‐centre study. Trop Med Int Health 12: 1279–1287. [DOI] [PubMed] [Google Scholar]
- 8.Nagalo K, Dao F, Minodier P, Sawadogo O, Sanon H, Tall FH, Yé D, 2014. Le paludisme congénital maladie à Plasmodium falciparum: aspects épidémiologiques, cliniques, biologiques, thérapeutiques et pronostiques à Ouagadougou, Burkina Faso. Pan Afr Med J 18: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ndyomugyenyi R, Magnussen P, 2004. Trends in malaria-attributable morbidity and mortality among young children admitted to Ugandan hospitals, for the period 1990–2001. Ann Trop Med Parasitol 98: 315–327. [DOI] [PubMed] [Google Scholar]
- 10.CDC , 2013. Malaria Operational Plan FY 2013. Atlanta, GA: Center for Disease Control and Prevention Researchers for Uganda.
- 11.Okello PE, van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, d’Alessandro U, Coosemans M, 2006. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg 75: 219–225. [PubMed] [Google Scholar]
- 12.Othoro C, Moore JM, Wannemuehler K, Nahlen BL, Otieno J, Slutsker L, Lal AA, Shi YP, 2006. Evaluation of various methods of maternal placental blood collection for immunology studies. Clin Vaccine Immunol 13: 568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newland AC, Evans TG, 1997. ABC of clinical haematology. Haematological disorders at extremes of life. BMJ 314: 1262–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman PM, Wanzira H, Tumwine G, Arinaitwe E, Waldman S, Achan J, Havlir D, Rosenthal PJ, Dorsey G, Clark TD, 2009. Placental malaria among HIV-infected and uninfected women receiving anti-folates in a high transmission area of Uganda. Malar J 8: 1475–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosha TC, Ntarukimana D, John M, 2010. Prevalence of congenital malaria among newborn babies at Morogoro Regional Hospital, Morogoro, Tanzania. Tanzan J Health Res 12: 237–242. [DOI] [PubMed] [Google Scholar]
- 16.Orogade AA, Falade CO, Okafor HU, Mokuolu OA, Mamman AI, Ogbonu TA, Ogunkunle OO, Ernest KS, Callahan MV, Hamer DH, 2008. Clinical and laboratory features of congenital malaria in Nigeria. J Pediatr Infect Dis 3: 181–187. [Google Scholar]
- 17.Natama HM, Ouedraogo DF, Sorgho H, Rovira-Vallbona E, Serra-Casas E, Somé MA, Coulibaly-Traoré M, Mens PF, Kestens L, Tinto H, 2017. Diagnosing congenital malaria in a high-transmission setting: clinical relevance and usefulness of P. falciparum HRP2-based testing. Sci Rep 7: 2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stassijns J, Boogaard W, Pannus P, Nkunzimana A, Rosanas-Urgell A, 2016. Prevalence and diagnostics of congenital malaria in rural Burundi, a cross-sectional study. Malar J 15: 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etim S, Ogbeche J, Uno O, 2016. Retrospective study of congenital malaria in Calabar, south-eastern Nigeria. Int J Infect Trop Dis 3: 7–12. [Google Scholar]
- 20.Enweronu-Laryea CC, Adjei GO, Mensah B, Duah N, Quashie NB, 2013. Prevalence of congenital malaria in high-risk Ghanaian newborns: a cross-sectional study. Malar J 12: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mwangoka GW, Kimera SI, Mboera LE, 2008. Congenital Plasmodium falciparum infection in neonates in Muheza district, Tanzania. Malar J 7: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sotimehin S, Runsewe-Abiodun T, Fetuga M, Adedeji A, Njokanma O, 2009. Clinical profiles of newborns with malaria parasitaemia in Sagamu. Nigerian Hospital Practice 3. [Google Scholar]
- 23.Lesi F, Mukhtar M, Iroha E, Egri-Okwaji M, 2010. Clinical presentation of congenital malaria at the Lagos University Teaching Hospital. Niger J Clin Prac 13: 134–138. [PubMed] [Google Scholar]
- 24.Nagaraj N, Berwal PK, Sharma M, Jevaji P, Swami S, Yadav V, Choudary L, 2017. Congenital and neonatal malaria in Asian Indian population. Int J Commun Med Public Health 2: 639–642. [Google Scholar]
- 25.Mwaniki MK, Talbert AW, Mturi FN, Berkley JA, Kager P, Marsh K, Newton CR, 2010. Congenital and neonatal malaria in a rural Kenyan district hospital: an eight-year analysis. Malar J 9: 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okechukwu A, Olateju E, Olutunde E, 2011. Congenital malaria among newborns admitted for suspected neonatal sepsis in Abuja. Niger J Paediatr 38: 82–89. [Google Scholar]
- 27.Fitri LE, Jahja NE, Huwae IR, Nara MB, Berens-Riha N, 2014. Congenital malaria in newborns selected for low birth-weight, anemia, and other possible symptoms in Maumere, Indonesia. Korean J Parasitol 52: 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashemzadeh A, Heydarian F, 2005. Congenital malaria in a neonate. Arch Iranian Med 8: 226–228. [Google Scholar]
- 29.Menendez C, Mayor A, 2007. Congenital malaria: the least known consequence of malaria in pregnancy. Seminars in Fetal and Neonatal Medicine. Forsgren M, Broliden K, Navér L, eds. Amsterdam, The Netherlands: Elsevier, 207–213. [DOI] [PubMed] [Google Scholar]
- 30.Valecha N, Bhatia S, Mehta S, Biswas S, Dash AP, 2007. Congenital malaria with atypical presentation: a case report from low transmission area in India. Malar J 6: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiwanitkit V, 2006. Congenital malaria in Thailand, an appraisal of previous cases. Pediatr Int 48: 562–565. [DOI] [PubMed] [Google Scholar]
- 32.Alphonse N, Gratien SG, Didier AJ, Joseph A, Achille OA, Saturnin LD, 2017. Frequency and sociodemographic factors associated with congenital malaria at the Borgou Regional University Teaching Hospital (CHUD-B) in Benin in 2015. Open J Pediatr 7: 215. [Google Scholar]
- 33.Sagbo GG, Noudamadjo A, Agossou J, Adedemy JD, Obossou AA, Lokossou DS, 2017. Epidemiological, clinical, biological, therapeutic features and outcome of congenital malaria at the Borgou Regional University Teaching Hospital (CHUD-B) in Benin in 2015. Open J Pediatr 7: 263. [Google Scholar]
- 34.Pineros-Jimenez JG, Alvarez G, Tobon A, Arboleda M, Carrero S, Blair S, 2011. Congenital malaria in Uraba, Colombia. Malar J 10: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okafor U, Oguonu T, Onah H, 2006. Risk factors associated with congenital malaria in Enugu, south eastern Nigeria. J Obstet Gynaecol 26: 612–616. [DOI] [PubMed] [Google Scholar]
- 36.Omalu ICJ, Mgbemena C, Mgbemena A, Ayanwale V, Olayemi IK, Lateef A, Chukwuemeka VI, 2011. Prevalence of congenital malaria in Minna, north central Nigeria. J Trop Med 2012: 274142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lwetoijera DW, Kiware SS, Mageni ZD, Dongus S, Harris C, Devine GJ, Majambere S, 2013. A need for better housing to further reduce indoor malaria transmission in areas with high bed net coverage. Parasit Vectors 6: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]