Abstract.
Maternal rectovaginal colonization is the major risk factor for early-onset neonatal sepsis due to Group B Streptococcus (GBS), a major cause of early life morbidity and mortality. Transmission generally occurs perinatally from colonized mothers to infants. Vaccines targeting a subset of GBS serotypes are under development, but GBS epidemiology remains poorly understood in many African nations. We performed a cross-sectional study of GBS colonization among pregnant women at two sites in Botswana, a country with minimal prior GBS carriage data. We found a rectovaginal colonization rate of 19%, comparable with studies in other regions; however, we also noted a striking predominance of serotype V (> 45% of strains). Although further studies are required to delineate the burden of invasive GBS disease in Botswana and the generalizability of type V epidemiology, these data provide a useful baseline for understanding the potential local impact of GBS prevention strategies, including vaccines.
Neonatal sepsis is a primary driver of early life mortality, and even nonfatal cases may lead to substantial lifelong morbidity. Group B Streptococcus (GBS; Streptococcus agalactiae) emerged as a major cause of neonatal sepsis in the 1970s, possibly driven by selection for virulent clones because of widespread use of tetracycline antibiotics in humans and animals.1 The major risk factor for early-onset (EO) (occurring in the first week of life) GBS disease is colonization of the maternal vaginal and/or gastrointestinal mucosal surfaces in late pregnancy. Screening for rectovaginal colonization at 35–37 weeks of gestation and the use of intrapartum antibiotic prophylaxis are the mainstay of GBS prevention in high-income countries, including the United States.2 Such strategies have helped decrease the rate of EO GBS infections but have not significantly altered rates of late-onset disease.2 In addition, screen-and-treat programs are both labor-intensive and costly, factors that may decrease their feasibility in low- and middle-income countries (LMIC).3
Based on prior work suggesting that sufficient levels of maternal GBS capsule–specific IgG likely confers protection against neonatal disease due to transplacental antibody transfer in late pregnancy, polysaccharide–protein conjugate vaccines are under development.4 There are 10 known GBS serotypes (Ia, Ib, and II–IX), of which types Ia, III, and V are generally the most common in studies of colonization and neonatal disease.5,6 Current candidate vaccines each cover a limited number of serotypes. There can be significant geographic variability in serotype prevalence, and understanding local serotype distribution is crucial to predicting the potential impact of conjugate vaccine programs.7 Notably, data regarding serotype distribution are sparse among many African nations, including Botswana.
Ethical approval was obtained from Botswana Ministry of Health and Wellness’ Research and Development Committee, Bamalete Lutheran Hospital (BLH), Princess Marina Hospital (PMH), the University of Botswana, New York University, and the University of Pennsylvania. We performed a prospective, cross-sectional study of women in late pregnancy (gestational age ≥ 35 weeks, 0 days) from October 2015 until March 2017. Women were enrolled at two hospitals in Botswana: PMH and BLH. Princess Marina Hospital is an urban, tertiary care hospital in the capital city of Gaborone, and BLH is a semirural primary hospital in Ramotswa, southern Botswana. Following written informed consent, each study subject underwent collection of rectovaginal swab specimens by a trained research nurse, according to the Centers for Disease Control and Prevention algorithm.2 Study participants did not receive compensation for enrollment. The swabs were placed into transport media (Becton–Dickinson ESwab Transport and Collection System; Becton-Dickenson, Franklin Lakes, NJ) and stored in an insulated cooler with cold packs until transport to the National Health Laboratory in Gaborone (< 6 hours), where they are assessed for GBS by culture and frozen at −70°C for shipment. Group B Streptococcus status was confirmed by growth in Lim Broth, followed by DNA extraction and polymerase chain reaction (PCR) targeting the sip gene with consensus sequence–derived primers, as previously reported.8 Serotype was determined by real-time PCR, and, when possible, cases of co-colonization were confirmed by isolation of each individual serotype and confirmation by latex agglutination or real-time PCR.9
We performed descriptive statistics and univariate analysis based on age quartile, gravity quartile, nulliparity, enrollment site, HIV status, comorbidities, and smoking status as potential predictors of GBS status. In a subgroup analysis of GBS-positive women, we examined potential relationships between cohort characteristics and the presence of serotype V GBS. Statistical analyses were performed using IBM SPSS (version 25; International Business Machines, Armonk, NY).
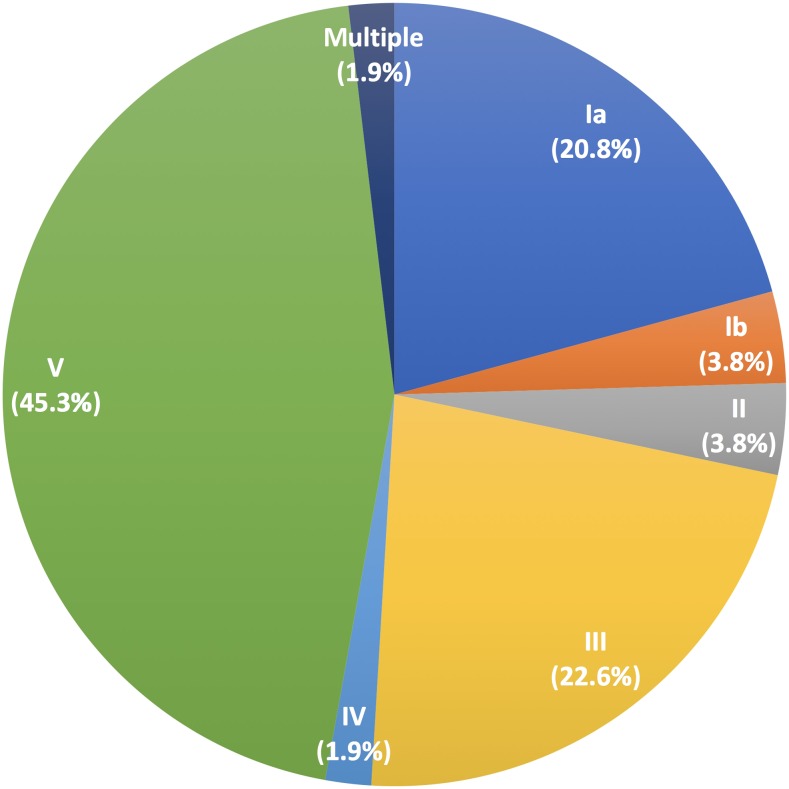
Of 275 women enrolled, samples were available for analysis for 274 (one sample was lost during processing, and that subject was omitted from analysis). Cohort characteristics are presented in Table 1. There were no significant differences by chi-square statistics between GBS-negative and GBS-positive women based on any of the subject characteristics studied, including prior antibiotic receipt, HIV status, and enrollment site. As reported, 53/274 (19%) samples were positive for GBS by sip PCR. The distribution of capsular serotypes of the GBS-positive samples is presented in Figure 1. One sample contained two serotypes (Ia and III) detected by real-time PCR and confirmed by culture and latex agglutination, consistent with prior descriptions of simultaneous colonization with multiple GBS types.10 Because of the high rate of serotype V GBS compared with prior studies, we examined subject characteristics within the GBS-positive cohort that might be associated with type V carriage. As with the full cohort analysis, no statistically significant predictors of serotype V carriage were determined.
Table 1.
Characteristics of study cohort (N = 274) and univariate analysis
| Median (range) | ||
|---|---|---|
| Age (years) | 31 (19–43) | |
| Gravidity | 2 (1–8) | |
| Parity | 1 (0–5) | |
| N (%) | ||
| Enrollment site | Princess Marina Hospital | 203 (74) |
| Bamalete Lutheran Hospital | 71 (26) | |
| HIV status | Positive | 67 (24) |
| Negative | 204 (74) | |
| Unknown | 3 (1) | |
| Prior antibiotics (past 9 months) | Yes | 102 (37) |
| No | 160 (58) | |
| Unknown | 12 (4) | |
| Comorbid condition | Yes | 6 (2) |
| No | 268 (98) | |
| GBS status | Positive | 53 (19) |
| Negative | 221 (81) |
GBS = group B Streptococcus. Comorbid conditions included pulmonary tuberculosis (N = 1), “precancer” (N = 1), hypertension (N = 2), and unknown condition (N = 2). All factors had a nonsignificant (P > 0.05) relationship with GBS status.
Figure 1.
Serotype distribution among Group B Streptococcus-positive rectovaginal samples from the cohort (N = 53). Only serotypes I–V were identified in this cohort. One sample (“multiple”) contained both serotypes Ia and III. This figure appears in color at www.ajtmh.org.
Despite the importance of GBS as an agent of neonatal sepsis and, increasingly, a cause of invasive disease in adult populations, the global epidemiology of GBS colonization and disease remains incompletely understood. Recent systematic reviews and meta-analyses5,6 suggest substantial differences in disease rates and serotype distribution across geographic areas. This work is limited as well, given the paucity of studies from LMIC, particularly in sub-Saharan Africa.5,11 In this assessment of carriage rates and serotype distribution among pregnant women from two sites in Botswana, we found that the GBS rectovaginal colonization rate (19%) was consistent with findings from other geographic areas, suggesting that screening of pregnant women for GBS colonization may be of benefit if the substantial logistical barriers to implementation of intrapartum antibiotic prophylaxis can be overcome. Baseline and ongoing collection of data regarding neonatal sepsis rates and etiologies in Botswana would be crucial to understanding the impact of such interventions.
We were encouraged to find that the GBS serotypes represented in this sample mirrored those found in many other areas. We did not identify study subjects colonized with the “higher number” serotypes (VI–IX) that are not represented in candidate vaccines. A candidate GBS polysaccharide–protein conjugate vaccine containing serotypes Ia, Ib, and III has been evaluated in phase I and II studies12 and, based on the serotype distribution that we noted, would be expected to provide protection for 49% of colonized individuals (including the co-colonized subject) identified in this Botswana cohort. Candidate vaccines with an increased number of serotypes are also in early clinical trials.13 Given our findings, inclusion of serotype V may be an important component of conjugate vaccine development if candidates are to be efficacious in a wide range of settings. Notably, some studies from other African nations have also noted higher rates of type V GBS carriage in pregnancy than those described outside of the region.14–16 Serotype V can cause invasive neonatal disease and has emerged as an important cause of disease among nonpregnant adults.17,18 Thus, ongoing surveillance of GBS serotype distribution and expanded studies of invasive disease in Botswana and other LMIC are indicated.
Acknowledgments:
We thank the women who volunteered their time to participate in the study, study nurses (Banno Moorad and Relebogile Thipe) for their expertise in enrolling study subjects, and the laboratory staff who assisted with sample processing in Botswana.
REFERENCES
- 1.Da Cunha V, et al. 2014. Streptococcus agalactiae clones infecting humans were selected and fixed through the extensive use of tetracycline. Nat Commun 5: 4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verani JR, McGee L, Schrag SJ, 2010. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep 59: 1–36. [PubMed] [Google Scholar]
- 3.Ginsberg GM, Eidelman AI, Shinwell E, Anis E, Peyser R, Lotan Y, 2013. Should Israel screen all mothers-to-be to prevent early-onset of neonatal group B streptococcal disease? A cost-utility analysis. Isr J Health Policy Res 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi M, Vekemans J, Baker CJ, Ratner AJ, Le Doare K, Schrag SJ, 2016. Group B Streptococcus vaccine development: present status and future considerations, with emphasis on perspectives for low and middle income countries. F1000Res 5: 2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madrid L, et al. 2017. Infant group B streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses. Clin Infect Dis 65: S160–S172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell NJ, et al. 2017. Maternal colonization with group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin Infect Dis 65: S100–S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dzanibe S, Madhi SA, 2018. Systematic review of the clinical development of group B Streptococcus serotype-specific capsular polysaccharide-based vaccines. Expert Rev Vaccines 17: 635–651. [DOI] [PubMed] [Google Scholar]
- 8.Khatami A, Randis TM, Chamby A, Hooven TA, Gegick M, Suzman E, A’Hearn-Thomas B, Steenhoff AP, Ratner AJ, 2018. Improving the sensitivity of real-time PCR detection of group B Streptococcus using consensus sequence-derived oligonucleotides. Open Forum Infect Dis 5: ofy164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breeding KM, Ragipani B, Lee KD, Malik M, Randis TM, Ratner AJ, 2016. Real-time PCR-based serotyping of Streptococcus agalactiae. Sci Rep 6: 38523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrieri P, Hillier SL, Krohn MA, Moore D, Paoletti LC, Flores AE, 2004. Characterization of vaginal & rectal colonization with multiple serotypes of group B streptococci using multiple colony picks. Indian J Med Res 119 (Suppl): 208–212. [PubMed] [Google Scholar]
- 11.Kwatra G, Cunnington MC, Merrall E, Adrian PV, Ip M, Klugman KP, Tam WH, Madhi SA, 2016. Prevalence of maternal colonisation with group B Streptococcus: a systematic review and meta-analysis. Lancet Infect Dis 16: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 12.Madhi SA, et al. 2016. Safety and immunogenicity of an investigational maternal trivalent group B Streptococcus vaccine in healthy women and their infants: a randomised phase 1b/2 trial. Lancet Infect Dis 16: 923–934. [DOI] [PubMed] [Google Scholar]
- 13. Anderson AL, et al. 2017. Updates on the Development of a Multivalent Group B Streptococcal Vaccine. International Society for Vaccines Annual Congress, Abstract O1.3.
- 14.Brochet M, Couve E, Bercion R, Sire JM, Glaser P, 2009. Population structure of human isolates of Streptococcus agalactiae from Dakar and Bangui. J Clin Microbiol 47: 800–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moyo SR, Maeland JA, Bergh K, 2002. Typing of human isolates of Streptococcus agalactiae (group B Streptococcus, GBS) strains from Zimbabwe. J Med Microbiol 51: 595–600. [DOI] [PubMed] [Google Scholar]
- 16.Suara RO, Adegbola RA, Baker CJ, Secka O, Mulholland EK, Greenwood BM, 1994. Carriage of group B Streptococci in pregnant Gambian mothers and their infants. J Infect Dis 170: 1316–1319. [DOI] [PubMed] [Google Scholar]
- 17.Blumberg HM, Stephens DS, Modansky M, Erwin M, Elliot J, Facklam RR, Schuchat A, Baughman W, Farley MM, 1996. Invasive group B streptococcal disease: the emergence of serotype V. J Infect Dis 173: 365–373. [DOI] [PubMed] [Google Scholar]
- 18.Skoff TH, et al. 2009. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clin Infect Dis 49: 85–92. [DOI] [PubMed] [Google Scholar]