Abstract.
Schistosomiasis is traditionally classified into an acute and a chronic phase, although a precise temporal distinction between the two phases has not been established. Lung involvement can be observed in both phases. We previously reported seven cases of pulmonary lesions due to chronic schistosomiasis in African immigrants. All cases were documented with CT scans and demonstrated complete resolution after treatment with praziquantel. Moreover, another case showed spontaneous disappearance of the nodule before treatment with praziquantel. These findings are similar to those observed in the acute phase of schistosomiasis, with well-defined or ground glass nodules that resolve spontaneously. According to these findings, we postulate the presence of an “intermediate” phase of schistosomiasis involving the lungs that can be defined as an “early chronic phase,” and presents analogies to the acute phase. We also hypothesize that in the “early chronic phase,” the female worms transit through the lungs where they may lay eggs. These passages not only cause transient, but also radiologically visible alterations. The pathophysiology of lung lesions in the late chronic phase is probably different: the adult worms settled in the mesenteric plexuses produce eggs for years. The eggs repeatedly migrate to the perialveolar capillary beds via portal-caval shunting. Thus, in this case it is the eggs and not the adult worms that reach the lungs in a scattered way. Based on our findings, we suggest the alternative hypothesis that the pulmonary involvement is a phase of the natural evolution of the infection, both from Schistosoma mansoni and Schistosoma haematobium.
INTRODUCTION
Schistosomiasis is a neglected tropical disease that affects an estimated 180 million people in sub-Saharan Africa.1 The disease mainly affects the intestinal (Schistosoma mansoni) and the urinary systems (Schistosoma haematobium). Schistosomiasis is traditionally classified into an acute and a chronic phase, although a precise temporal distinction between the two phases has not been established. Lung involvement in schistosomiasis can be observed in both phases. In the acute phase, a syndrome characterized by fever, urticaria, eosinophilia, myalgia, malaise, fatigue, and nonproductive cough (Katayama syndrome) has been described in travelers.2 Most patients spontaneously recover after 2–10 weeks.2 An interstitial infiltrate may be visible in conventional X-rays with or without nodules,3,4 while with CT scans further characteristics of macro and micro nodules can be observed.5,6 These image findings can be present even in patients without any respiratory symptoms5,6 and are due to blood circulating schistosomula and adult worms, laying eggs in different tissues, including the lungs.2 This acute phase is virtually unknown in the endemic population, most probably because the symptoms are not specific and therefore they are not attributed to schistosomiasis.2,7 The disease then evolves progressively toward a chronic phase that is characterized by hepatosplenic schistosomiasis with or without pulmonary hypertension in the case of S. mansoni, and by a urinary pathology, without lung involvement, in the case of S. haematobium infection.8
In the last decades, Europe, and particularly Italy, has experienced an unprecedented immigration flow from African countries.9 The number of people necessitating screening and treatment for the so-called tropical diseases is increasing in our continent. European doctors are often insufficiently trained to deal with those diseases. On the other hand, clinicians in endemic areas are experienced with the usual clinical aspects but usually lack the sophisticated technology that may be necessary to diagnose the less common clinical presentations.
The use of modern imaging techniques can not only improve the diagnosis, but also contribute to a better understanding of some aspects of the pathophysiology of chronic schistosomiasis that are not yet completely understood. The aim of this perspective article is to discuss and revise the staging of established pulmonary schistosomiasis based on imaging and clinical observation of a series of patients.
CLINICAL OBSERVATIONS
In a previous descriptive series we reported seven cases of pulmonary lesions due to chronic schistosomiasis in African immigrants.10 All cases were documented with CT scans and demonstrated complete resolution after treatment with praziquantel. In five cases the diagnosis was made with pulmonary biopsy, whereas the others were treated presumptively. Since then, six further cases came to our attention; all observed in patients from sub-Saharan Africa diagnosed with either S. mansoni, S. haematobium or mixed Schistosoma spp. infection. All patients had been exposed to multiple reinfections and had not received any treatment for at least 3 years before presenting to our clinic. In all patients, the CT scan detected ground glass lesions (Figure 1) and/or nodules in the lungs. Moreover, two of the cases presented other peculiar aspects that prompted further considerations. One patient had positive microscopy for S. mansoni and S. haematobium ova, and showed a single nodule on the CT scan (Figure 2). He did not present to the scheduled visit for treatment of schistosomiasis, and when he came again to the clinic, nine months later, a CT scan was repeated, showing the spontaneous disappearance of the nodule before treatment with praziquantel. Another patient presented for treatment for S. haematobium infection; he had performed a CT scan 6 months before that was negative. When the CT scan was repeated, a new single nodule had appeared. The patient did not return to an endemic region. He received a treatment with praziquantel and 2 months later the CT scan demonstrated the disappearance of the nodule.
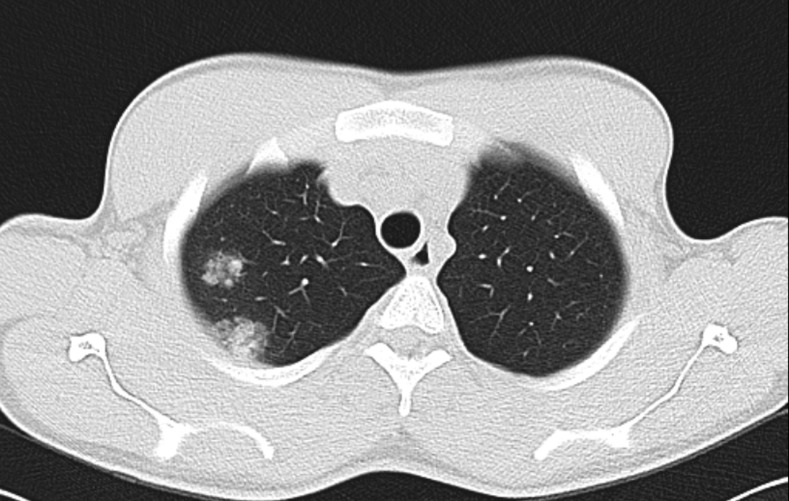
Figure 1.
CT scan showing two peri-nodular halo signs (diameters 21 and 14 mm) on the upper lobe of the right lung.
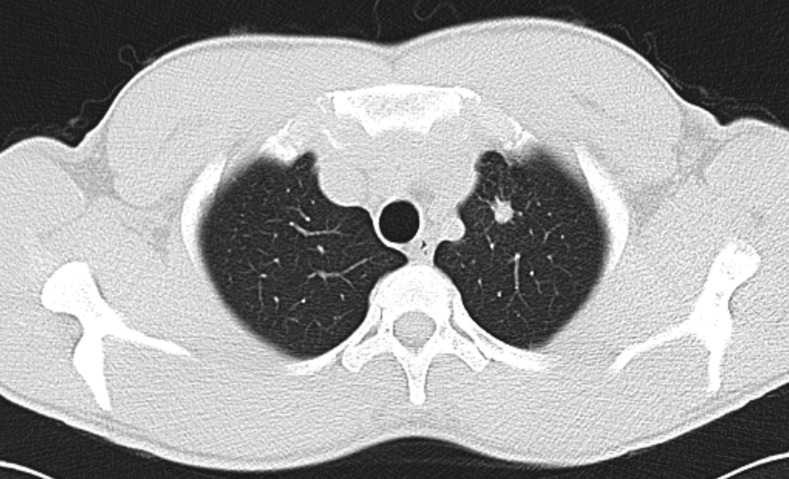
Figure 2.
CT scan showing a single nodule (diameter 10 mm) on the upper lobe of the left lung.
DISCUSSION
One of these two cases demonstrated a transient nodule that appeared and then rapidly disappeared after treatment, and the other case showed a well-defined lung nodule that disappeared without treatment. The lesion of the latter patient was very similar to the lesions described previously,10 were schistosome eggs were found in the biopsies, so we now think that in the previous cases10 praziquantel only sped up the clearance of the lungs lesions. These findings are similar to those observed in the acute phase of schistosomiasis, with well-defined or ground glass nodules that resolve spontaneously11; only, we did not observe the pulmonary infiltrates that are also common in the acute phase. In view of these findings, we postulate the presence of an “intermediate” phase of schistosomiasis occurring in the lungs that can be defined as an “early chronic phase,” and presents analogies to the acute phase. We hypothesize that also in the “early chronic phase,” the female worms transit through the lungs where they may lay eggs. Kane et al.12 in 1948 stated that “it is difficult to account for nests of eggs without the close proximity of a parturient female worm.” Moreover, in the literature, in four of 16 cases of lungs lesions due to chronic schistosomiasis, adult worms were found in situ.10 Hence, the migration of adult worms has been documented. These passages not only cause transient, but also radiologically visible alterations. Although in our patients this early chronic phase was followed by a complete resolution of the pulmonary nodules, in well-established cases of late chronic pulmonary schistosomiasis with pulmonary hypertension the treatment is ineffective. The pathophysiology of lung lesions in the late chronic phase is probably different: the adult worms settled in the mesenteric plexuses produce eggs for years. The eggs repeatedly migrate to the perialveolar capillary beds via portal-caval shunting. Thus, in this case it is the eggs and not the adult worms that reach the lungs. Expectedly, they are homogeneously dispersed and do not organize in nodules, but cause vasculitis, granulomas, and microvascular thrombosis, eventually inducing fibrosis13,14 and pulmonary hypertension. The pulmonary impairment in the late phase is not reversible, and not clearly visible at CT scan.
Although ectopic sites of schistosomiasis infection have been defined as specific local reactions to the worms or their eggs occurring outside the portal-caval venous circulation,15 our findings rather suggest that lung involvement might be, instead, a component of the natural evolution of the disease.
Last but not least, although we cannot claim any evidence so far that the same “early” phenomenon as in the lungs occurs elsewhere, this cannot be of course ruled out. For example, reports from travel medicine suggest that, in acute schistosomiasis, symptoms typical of the early “acute” phase almost overlap with those of the “chronic” phase, also including particularly severe and invalidating complications such as ectopic medullar involvement.16 A practical implication would be rethinking the timing of the treatment of the acute phase and in particular the interval required (if any) between corticosteroid usage and the inception of the causal treatment, and/or the need to administer both treatments concurrently.16 Moreover, an ongoing TropNet study (Gobbi F, unpublished data) shows that there is an extreme variety of opinions among travel medicine experts on this particular topic.
CONCLUSION
Some of the old postulates of Tropical Medicine probably need to be rediscussed. An example is the definition of pulmonary schistosomiasis as an ectopic localization of the infection. Based on our findings, we suggest the alternative hypothesis that the pulmonary involvement is a phase of the natural evolution of the infection, both from S. mansoni and S. haematobium. In endemic areas, after repeated infections (most of which remain untreated), an intermediate (“early chronic”) phase of pulmonary schistosomiasis gets established. This early chronic phase of pulmonary schistosomiasis can be defined radiologically (but not clinically) by the presence of nodules or ground glass lesions without an interstitial infiltrate. This phase tends to resolve spontaneously or after treatment. Presumably, the pathophysiology of the late, irreversible pulmonary involvement is completely different, being due to the repeated, ectopic dissemination of eggs from adults residing in their usual sites.
In endemic areas, reports on clinical and radiological features of the primary infection would be invaluable and so would imaging studies on the (young) adult local population in premises where CT scans are available.
Acknowledgments:
We are grateful to Manuel Corachan for his inspiring work and support. We warmly thank Andrea De Lama and Daniela Fait who referred one of the cases described here to our attention.
REFERENCES
- 1.WHO , 2017. Schistosomiasis and soil-transmitted helminthiases: number of people treated in 2016. Wkly Epidemiol Rec 49: 749–760. [PubMed] [Google Scholar]
- 2.Ross AG, Vickers D, Olds GR, Shah SM, McManus DP, 2007. Katayama syndrome. Lancet Infect Dis 7: 218–224. [DOI] [PubMed] [Google Scholar]
- 3.Rocha MO, Rocha RL, Pedroso ER, Greco DB, Ferreira CS, Lambertucci JR, Katz N, Rocha RS, Rezende DF, Neves J, 1995. Pulmonary manifestations in the initial phase of schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo 37: 311–318. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz E, Rozenman J, Perelman M, 2000. Pulmonary manifestations of early schistosome infection among nonimmune travelers. Am J Med 15: 718–722. [DOI] [PubMed] [Google Scholar]
- 5.Lambertucci JR, 2010. Acute schistosomiasis mansoni: revisited and reconsidered. Mem Inst Oswaldo Cruz 105: 422–435. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen LQ, Estrella J, Jett EA, Grunvald EL, Nicholson L, Levin DL, 2006. Acute schistosomiasis in nonimmune travelers: chest CT findings in 10 patients. AJR Am J Roentgenology 186: 1300–1303. [DOI] [PubMed] [Google Scholar]
- 7.Osakunor DNM, Woolhouse MEJ, Mutapi F, 2018. Paediatric schistosomiasis: what we know and what we need to know. PLoS Negl Trop Dis 12: e0006144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colley DG, Bustinduy AL, Secor WE, King CH, 2014. Human schistosomiasis. Lancet 383: 2253–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Italian Senate , 2018. Immigrazione. Elementi introduttivi. [Immigration. Introductory elements]. Rome, Italy: Italian Senate. Italian. Available at: http://www.senato.it/japp/bgt/showdoc/17/DOSSIER/1000651/index.html?part=dossier_dossier1& parse=si. Accessed April 17, 2018.
- 10.Gobbi F, et al. 2017. Pulmonary nodules in African migrants caused by chronic schistosomiasis. Lancet Infect Dis 17: e159–e165. [DOI] [PubMed] [Google Scholar]
- 11.Coron N, Le Govic Y, Kettani S, Pihet M, Hemery S, de Gentile L, Chabasse D, 2016. Early detection of schistosoma egg-induced pulmonary granulomas in a returning traveler. Am J Trop Med Hyg 94: 611–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kane CA, Most H, 1948. Schistosomiasis of the central nervous system; experiences in World War II and a review of the literature. Arch Neurol Psychiatry 59: 141–183. [DOI] [PubMed] [Google Scholar]
- 13.Papamatheakis DG, Mocumbi AO, Kim NH, Mandel J, 2014. Schistosomiasis-associated pulmonary hypertension. Pulm Circ 4: 596–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrade ZA, Andrade SG, 1970. Pathogenesis of schistosomal pulmonary arteritis. Am J Trop Med Hyg 19: 305–310. [DOI] [PubMed] [Google Scholar]
- 15.Faust EC, 1948. An inquiry into the ectopic lesions in schistosomiasis. Am J Trop Med Hyg 28: 175–199. [DOI] [PubMed] [Google Scholar]
- 16.Bonnefond, et al. 2019. Early complicated schistosomiasis in a returning traveler: key contribution of new molecular diagnostic methods CMI. Int J Infect Dis 79: 72–74. [DOI] [PubMed] [Google Scholar]