Abstract.
Hemorrhagic fever with renal syndrome (HFRS) is a febrile disorder caused in Korea by the Hantaan and Seoul viruses. Its characteristic clinical manifestations include fever, hemorrhage, and renal failure, but a primary presentation with acute infectious diarrhea is rare. Owing to decreased urine output and renal function, a 54-year-old patient was transferred to our hospital from a local clinic, where he had been receiving treatment for diarrhea occurring more than 10 times a day. The patient was treated in the Gastroenterology Department at our hospital for acute renal failure secondary to inflammatory diarrhea based on the findings of stool leukocytes. An immunofluorescent antibody assay showed a 4-fold increase in the acute-phase antibody titer to Hantavirus during recovery. A nested reverse transcription polymerase chain reaction (RT-nPCR) assay of plasma yielded negative results, but Hantaan virus positivity was confirmed on an RT-nPCR assay of the buffy coat. Another 60-year-old patient with watery diarrhea was treated conservatively for suspected infectious diarrhea. However, an immunofluorescent antibody assay showed a 4-fold increase in the acute-phase HFRS antibody titer. RT-nPCR using plasma yielded negative results, but Seoul virus was detected on an RT-nPCR buffy coat assay, confirming the diagnosis of HFRS. Hemorrhagic fever with renal syndrome can present with gastrointestinal symptoms such as acute diarrhea alone. This report highlights the importance of considering HFRS in the differential diagnosis of patients with acute diarrhea and the need for additional research on the usefulness of the buffy coat in the PCR diagnosis of HFRS.
INTRODUCTION
Hemorrhagic fever with renal syndrome (HFRS) is caused by various species of viruses in the Hantavirus genus of the Bunyaviridae family, such as Hantaan virus, Seoul virus, Dobrava virus, and Puumala virus.1,2 Hemorrhagic fever with renal syndrome started receiving attention during the Korean War, when it resulted in numerous casualties in the United Nations army. In 1976, Lee et al. discovered the causative antigen for HFRS in a striped field mouse and named it Hantaan virus.3 Hemorrhagic fever with renal syndrome was registered as a nationally notifiable infectious disease in 1977 with 176 reported cases. It occurs primarily between October and December, but a small number of cases occur throughout the year nationally, including in the Jeju area. Since 2000, approximately 400 cases have occurred every year. The causative viruses for HFRS identified in Korea are Hantaan virus and Seoul virus.4
HFRS shows a characteristic progression from a febrile stage to hypotensive, oliguric, polyuric, and recovery stages. It shows a characteristic triad of fever, renal failure, and hemorrhage but can present with variable clinical patterns, from minimal to fatal symptoms.
In the two cases reported here, the patients presented with the primary concern of acute diarrhea and were initially treated for inflammatory diarrhea due to infection. However, an elevated Hantaan virus antibody titer was confirmed in both patients, who showed positive buffy coat test results despite negative plasma polymerase chain reaction (PCR) results, and the diagnosis of HFRS due to Hantaan virus and Seoul virus was confirmed.
We report here these two cases along with a literature review to aid in the differential diagnosis of HFRS, which can present with a solitary gastrointestinal manifestation such as acute diarrhea.
METHODOLOGY AND RESULTS
Case 1.
A 54-year-old patient was transferred to our hospital owing to hypotension during treatment for diarrhea. The patient had gone fishing 2 weeks before presentation and developed fever and myalgia 5 days previously. For 3 days before presentation, the patient developed watery diarrhea occurring more than 10 times per day, which was accompanied by fever. He was admitted to a different hospital 1 day before presentation to our hospital for infectious colitis and received antibiotics and supportive therapy with no improvement of symptoms. He subsequently developed decreased urine output, increased serum creatinine level, and hypotension and was transferred to Chosun University Hospital.
The patient had been diagnosed with hypertension 2 months previously and was being treated with antihypertensives. He had no travel history. Physical examination showed blood pressure of 80/40 mmHg, heart rate of 120 beats/minutes, respiratory rate of 22/minutes, and temperature of 37.5°C. He was alert and oriented but appeared acutely ill. Conjunctival injection was observed, and his chest and abdominal examination was normal. There were no rashes or other dermatological lesions. Blood tests performed 3 days before admission at another hospital showed a white blood cell (WBC) count of 3,990/mm3, hemoglobin (Hb) level of 15.6 g/dL, hematocrit of 45.2%, platelet count of 200,000/mm3, aspartate aminotransferase (AST) of 57 IU/L, alanine aminotransferase (ALT) of 72 IU/L, blood urea nitrogen (BUN) of 9.7 mg/dL, and serum creatinine of 1.0 mg/dL. After transfer, the patient continued to have more than 10 episodes of diarrhea daily. He was admitted under the Department of Gastroenterology owing to a positive stool WBC test. An arterial blood test at the time of admission showed pH 7.33, PCO2 22.8 mmHg, PO2 83.4 mmHg, HCO3 12.0 mmol/L, and oxygen saturation of 95%, consistent with metabolic acidosis. Peripheral blood tests showed hemoconcentration and thrombocytopenia with a WBC count of 10,070/mm3, Hb level of 18.8 g/dL, hematocrit of 52.7%, and platelet count of 36,000/mm3. Biochemical tests showed elevated liver enzymes and decreased renal function with a total protein of 5.74 g/dL, albumin of 3.27 g/dL, AST of 333.2 IU/L, ALT of 139.7 U/L, BUN of 34.8 mg/dL, and serum creatinine of 5.06 mg/dL. His erythrocyte sedimentation rate was 2 mm/hour, C-reactive protein was 8.14 mg/L, and procalcitonin was 5.69 ng/mL. An electrolyte panel showed a serum sodium of 134 mEq/L and potassium of 4.6 mEq/L. A urine dipstick showed protein 3+, a red blood cell (RBC) count of 5–9/high-power field (HPF), and a WBC count of 50–99/HPF. Peripheral blood smear showed a left shift of the WBCs, atypical lymphocytes, and thrombocytopenia without aggregation, and negative result for the antiplatelet antibody assay was obtained.
The patient received fluid resuscitation for hypotension secondary to dehydration due to diarrhea, and his blood pressure increased to 90/50 mmHg. However, his urine output was persistently low at less than 30 mL/hour despite intravenous fluid and diuretic administration with accompanying metabolic acidosis, and the patient underwent hemodialysis. Stools were positive for WBCs. With an initial diagnosis of acute renal failure secondary to infectious colitis, the patient was treated with 9 days of intravenous ceftriaxone. On the third hospital day, the urine output increased to more than 100 mL/hour and increased to 4,000 mL/day by the following day.
Urine and blood cultures on presentation did not show any bacteria. The Hantaan virus antibody titer, according to a immunofluorescence assay (IFA; Green Cross Corp., Yongin, Korea), was 1:80, and an IFA performed in our hospital using a Korea Centers for Disease Control and Prevention (KCDC) method showed an IgM titer of < 1:16 and IgG titer of 1:64. In follow-up tests conducted on the eighth hospital day, the IFA (Green Cross Corp.) showed a Hantaan virus antibody titer of 1:40, and the IFA using a KCDC method showed an IgM titer of < 1:16 and IgG titer of > 1:2,048.
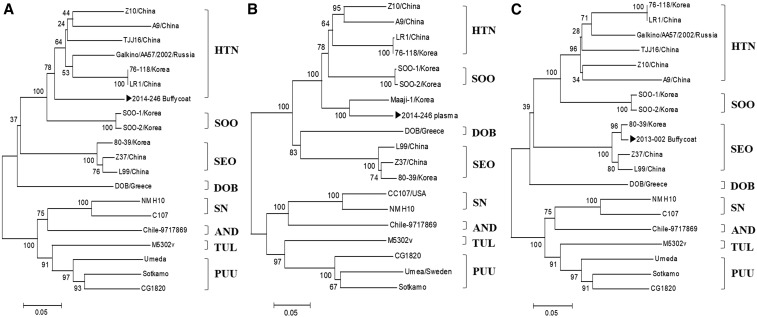
On presentation, viral RNA was extracted from the patient’s plasma (150 µL) and buffy coat (150 µL), using the Viral Gene-spin™ Viral DNA/RNA Extraction Kit (iNtRON, Seongnam, Korea) according to the manufacturer’s protocol. Nested reverse transcription-PCR (RT-nPCR)5 targeting the large (L: encodes viral RNA–dependent RNA polymerase) segment of genus Hantavirus (including Hantaan virus, Seoul virus, Dobrava virus, and Puumala virus) was performed with recombinant complementary DNA produced with SuperScript VILO MasterMix (Invitrogen, Waltham, MA).5 The RT-nPCR using the patient’s plasma yielded negative results for Hantavirus on presentation, but a subsequent buffy coat RT-nPCR yielded positive results. A phylogenetic tree analysis using the 360-bp amplified product of the PCR targeting the Hantavirus L segment6 (Figure 1A) showed 97% homology with the previously reported Hantaan virus isolate CUH15-126 (accession no. MG663537) L segment RNA–dependent RNA polymerase gene, partial coding sequence 347/357 and 84.2% homology with the Hantaan virus isolate Galkino/AA57/2002 (accession no. AB620033). The PCR result was negative on RT-nPCR using the S segment target in the plasma, but the result for the buffy coat was positive for Hantavirus.6 A phylogenetic tree analysis using the 645-bp amplification product of the PCR targeting the S segment (shown in Figure 1B) showed 88.5% homology with the Hantaan virus strain Maaji-1 (accession no. AF321094) and 81.1% homology with the Hantaan virus strain 76–118 (accession no. M14626). However, the PCR targeting the M segment showed negative results in both buffy coat and plasma. Polymerase chain reaction and antibody tests were negative for Leptospira spp., Orientia tsutsugamushi, Anaplasma spp.,5 and severe fever with thrombocytopenia syndrome virus. The patient improved with conservative treatment and was discharged.
Figure 1.
Phylogenetic tree analysis of blood buffy coat specimens in patients presenting with diarrhea using the amplification product of the polymerase chain reaction (PCR) targeting Hantavirus in nested reverse transcription PCR. (A) Results of the phylogenetic tree analysis using the 360-bp amplification product of the PCR targeting the Hantavirus L segment in a 54-year-old male patient. (B) Results of the phylogenetic tree analysis using the 645-bp amplification product of the PCR targeting the Hantavirus S segment in the same patient. (C) Results of the phylogenetic tree analysis using the 360-bp amplification product of the PCR targeting the Hantavirus L segment in a 60-year-old male patient. HTN = Hantaan virus; SEO = Seoul virus; SOO = Soochongvirus; SN = Sin Nombre virus; PUU = Puumala virus; AND = Andes virus; TUL = Tula virus; DOB = Dobrava-Belgrade virus.
Case 2.
A 60-year-old patient with watery diarrhea had been treated at a local clinic 15 days previously for fever, chills, and myalgia with no improvement of symptoms. He continued to experience abdominal discomfort and watery diarrhea with nausea and generalized myalgia and presented at the outpatient clinic at our center. He was on antihypertensives and aspirin for hypertension but had not taken them for 10 days before presentation. The patient worked at a pig farm.
Physical examination on admission showed blood pressure of 90/50 mmHg, heart rate of 86 beats/minutes, respiratory rate of 20/minutes, and temperature of 37.5°C. The patient was alert and oriented but appeared acutely ill. Laboratory investigations showed a WBC count of 4,580/mm3, Hb level of 15 g/dL, hematocrit of 42%, platelet count of 89,000/mm3, AST of 495 U/L, ALT of 331 U/L, sodium of 132 mEq/L, potassium of 3.9 mEq/L, BUN of 20.7 mg/dL, serum creatinine of 1.13 mg/dL, creatine kinase of 1,027 U/L, and a negative stool WBC test. A urine dipstick showed protein+, an RBC count of 1–4/HPF, and a WBC count of 1–4/HPF, with no decrease or increase in urine output. A peripheral blood smear showed a left shift of the WBCs, atypical lymphocytes, and thrombocytopenia without aggregation. The patient was suspected to have acute infectious diarrhea but was treated conservatively owing to the negative stool WBC test. Urine and blood cultures on presentation did not show any bacterial growth, and a stool culture did not isolate a causative pathogen. An IFA (Green Cross Corp.) showed a serum Hantaan virus antibody titer of 1:40, and a Hantaan virus IFA performed at our hospital showed an IgM titer of ≤ 1:16 and IgG titer of < 1:32. A Hantaan virus IFA performed on the third hospital day showed an IgM titer of < 1:16 and IgG titer of 1:128. Plasma RT-nPCR targeting the Hantavirus L segment on presentation yielded negative results, but the buffy coat RT-nPCR tested positive for Hantavirus. A phylogenetic tree analysis6 using the 360-bp amplification product of the PCR targeting the L segment showed 98.3% homology with the Seoul virus isolate 80-39 (accession no. NC 005238) and 95.8% and 95% homology with Seoul virus isolates L99 and Z37 (accession nos. AF288297 and AF285266), respectively. However, PCR tests targeting the M segment and S segment were both negative.
The patient received fluid replacement for hypotension during the hypotensive phase of HFRS and for dehydration due to diarrhea, after which his blood pressure recovered to 120/80 mmHg. He recovered on conservative management and was discharged.
DISCUSSION
In a study by Park et al.,7 the most common symptoms of HFRS in Korea were fever (94.5%), abdominal pain (64.4%), and headache (50.7%). Approximately one-third of patients showed complications involving major organs apart from the kidneys. Among extrarenal complications, pancreatobiliary diseases (11%) and major bleeding (10%) were the most common, and cardiovascular and central nervous system symptoms were observed in a portion of the patients.7
Primary presentation with only severe diarrhea is rarely considered as an early clinical symptom of HFRS. These cases are often challenging for clinicians. Acute abdominal inflammatory symptoms mimicking appendicitis may present as an early manifestation of HFRS. Extrarenal symptoms of HFRS include cholecystitis, pancreatitis, cholangitis, myocarditis, pericarditis, meningitis, cerebritis, cerebral infarction, gastrointestinal hemorrhage, retroperitoneal hemorrhage, intramural hemorrhage, acute respiratory distress syndrome, and hemophagocytic lymphohistiocytosis.8,9 Such extrarenal symptoms may be related to the severity of HFRS and may occur at any stage of HFRS. Hence, one must be aware of the extrarenal presentation of HFRS to be able to make an early diagnosis.
Noh et al.10 reported that although fever was the most common symptom in HFRS patients in Korean tertiary hospitals (occurring in 97.1% of patients), many patients (82.9%) complained of gastrointestinal symptoms, among which nausea (65.7%), diarrhea (45.7%), and vomiting (42.9%) were the main symptoms. A retrospective chart analysis of patients in Poland, who were admitted for renal failure, that compared HFRS-seropositive patients with a control group reported that in patients with suspected HFRS, cough, and diarrhea, which are typically not considered characteristic features of HFRS, were significantly high in frequency.11
The exact pathophysiological mechanism of diarrhea (development of colitis associated with HFRS) in patients with HFRS is not known, but it may occur secondary to increased vascular permeability of the colon.8
The presence of WBCs in stool is known to occur in inflammatory diarrheas such as inflammatory bowel disease (Crohn’s disease and ulcerative colitis), bacterial infection (Shigella, Clostridium difficile, Salmonella, and Campylobacter), and parasitic infection. Stool WBCs are rarely found in viral diarrhea, but 10–15% of cases caused by bacterial pathogens test negative for stool WBCs.12 The presence of WBCs in stool was confirmed in our first patient. However, no study has reported stool WBCs in patients with HFRS, and none have described a confirmed case of HFRS diagnosed during treatment for suspected infectious diarrhea based on a positive stool WBC test. Further research would be necessary to investigate the proportion of patients with HFRS who show positive results on stool WBC testing. It is interesting to note that for both patients, plasma Hantavirus RT-nPCR on presentation yielded negative results, but the subsequent buffy coat RT-nPCR yielded positive results. No study has discussed specimens useful for the diagnosis of HFRS, but plasma is often used for diagnosis. In a patient with HFRS due to Puumala virus, Hantavirus RNA was found in the patient’s saliva in addition to plasma.13 Although we report only two cases, both showed a negative plasma Hantavirus RT-nPCR and a positive buffy coat Hantavirus RT-nPCR result. It is currently hard to determine whether buffy coats become PCR positive because the virus has infected blood cells or whether the buffy coat sample corresponds to so much more original blood as compared with the plasma sample. Hence, further research seems necessary, including the comparative sensitivity of plasma and buffy coat PCR for the diagnosis of HFRS.
In conclusion, Hantavirus infection must be considered in patients presenting with a complaint of diarrhea and suspected to have acute infectious diarrhea in an HFRS-endemic region. This report also proposes the need to compare the usefulness of different clinical specimens such as plasma and buffy coat for PCR diagnosis of HFRS.
REFERENCES
- 1.Jiang H, Du H, Wang LM, Wang PZ, Bai XF, 2016. Hemorrhagic fever with renal syndrome: pathogenesis and clinical picture. Front Cell Infect Microbiol 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meier M, Kramer J, Jabs WJ, Nolte C, Hofmann J, Krüger DH, Lehnert H, Nitschke M, 2018. Proteinuria and the clinical course of Dobrava-Belgrade Hantavirus infection. Nephron Extra 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee HW, Lee PW, Johnson KM, 1978. Isolation of the etiologic agent of Korean hemorrhagic fever. J Infect Dis 137: 298–308. [DOI] [PubMed] [Google Scholar]
- 4.Korea Centers for Disease control and Prevention (KCDC) , 2016. Infectious Disease Surveillance Yearbook, 2016. Available at: http://www.cdc.go.kr/npt/biz/npp/nppMain.do. Accessed November 2018.
- 5.Ahn HJ, Chung JH, Kim DM, Yoon NR, Kim CM, 2018. Hemorrhagic fever with renal syndrome accompanied by panhypopituitarism and central diabetes insipidus: a case report. J Neurovirol 24: 382–387. [DOI] [PubMed] [Google Scholar]
- 6.Baek LJ, et al. 2006. Soochong virus: an antigenically and genetically distinct Hantavirus isolated from Apodemus peninsulae in Korea. J Med Virol 78: 290–297. [DOI] [PubMed] [Google Scholar]
- 7.Park KH, Kang YU, Kang SJ, Jung YS, Jang HC, Jung SI, 2011. Experience with extrarenal manifestations of hemorrhagic fever with renal syndrome in a tertiary care hospital in South Korea. Am J Trop Med Hyg 84: 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong YM, So MS, Kang KP, Kim W, Park SK, Lee S, 2012. A neglected diagnosis in severe diarrhea, thrombocytopenia, and elevated serum creatinine levels. J Clin Virol 55: 1–3. [DOI] [PubMed] [Google Scholar]
- 9.Mustonen J, Mäkelä S, Outinen T, Laine O, Jylhävä J, Arstila PT, Hurme M, Vaheri A, 2013. The pathogenesis of nephropathia epidemica: new knowledge and unanswered questions. Antiviral Res 100: 589–604. [DOI] [PubMed] [Google Scholar]
- 10.Noh JY, Cheong HJ, Song JY, Kim WJ, Song KJ, Klein TA, Lee SH, Yanagihara R, Song JW, 2013. Clinical and molecular epidemiological features of hemorrhagic fever with renal syndrome in Korea over a 10-year period. J Clin Virol 58: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gut AK, Gut R, Pencuła M, Jarosz MJ, 2013. New cases of suspected HFRS (Hantavirus infection) in south-eastern Poland. Ann Agric Environ Med 20: 544–548. [PubMed] [Google Scholar]
- 12.Herbert ME, 2000. Medical myth: measuring white blood cells in the stools is useful in the management of acute diarrhea. West J Med 172: 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pettersson L, Klingström J, Hardestam J, Lundkvist A, Ahlm C, Evander M, 2008. Hantavirus RNA in saliva from patients with hemorrhagic fever with renal syndrome. Emerg Infect Dis 14: 406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]