Abstract.
This study aimed to identify recurrent acute rheumatic fever (ARF) episodes which occurred despite adherence to prophylactic benzathine penicillin G (BPG). Data from Australia’s Northern Territory were analyzed; ARF recurrences between 2012 and 2017 diagnosed while the person was prescribed BPG were identified. Days at risk (DAR)—median and interquartile range—preceding ARF onset were calculated. The timing of BPG doses was examined for individuals with no DAR. One hundred sixty-nine ARF recurrences were analyzed; median DAR in the previous 8 weeks before ARF onset was 29. Most recurrences occurred following > 7 DAR (87%). Eight recurrences (5%) occurred despite no DAR; all were aged less than 16 years at the time of their recurrence/s. Recurrent ARF most commonly occurs after delayed BPG doses, but in some cases, receiving every prescribed BPG dose on time did not prevent recurrent ARF. A method to identify high-risk individuals before recurrent ARF is needed.
Acute rheumatic fever (ARF) and rheumatic heart disease (RHD) cause significant burdens in developing countries and disadvantaged populations in developed countries, including Australia. Aboriginal and Torres Strait Islander people living in the Northern Territory (NT) of Australia suffer from some of the highest documented rates of ARF and RHD in the world.1 Acute rheumatic fever results from an autoimmune response to Group A streptococcal (GAS) infection and variably affects the joints, skin, brain, and heart. Damage to cardiac valves can be permanent, leading to RHD. In Australia, ARF and RHD control relies heavily on secondary prophylaxis (SP) with regular antibiotics to prevent ARF recurrences. The standard regimen of 4-weekly intramuscular injections of benzathine penicillin G (BPG) has proven efficacy,2 although pharmacokinetic studies show that protective penicillin levels wane by 14–21 days.3 Low penicillin levels after day 21 may result in GAS infections occurring before the due date of the next dose; however, BPG can treat existing GAS infections if administered shortly after infection, which may explain the effectiveness of 4-weekly BPG. There are limited data on the pharmacokinetics of BPG for Aboriginal and Torres Strait Islander populations in Australia,4 but one study is currently underway.
A recent study using case–control and case-crossover designs found that increased adherence to BPG injections reduces the likelihood of recurrent ARF5; however, there are anecdotal reports of cases of recurrent ARF in the NT that have occurred despite excellent adherence. This retrospective cohort study was designed to identify whether individuals with very high adherence to prophylactic BPG have experienced recurrent ARF.
Ethical approval was obtained from the Human Research Ethics Committee of the NT Department of Health and Menzies School of Health Research (2016-2630). Deidentified data were provided, and individual participant consent was not sought.
Episodes of definite recurrent ARF diagnosed between January 1, 2012, and December 31, 2017, which occurred at least 8 weeks after the individual was prescribed BPG were eligible for inclusion. Definite cases required positive streptococcal serology; however, data on timing of preceding streptococcal infections were unavailable. Cases without a documented onset date or residing outside the NT at the time of data extraction were excluded. Acute rheumatic fever episodes characterized by Sydenham’s chorea were excluded because the interval between streptococcal infection and symptom onset is longer and less well defined than in other ARF manifestations. Deidentified data on patient demographics, diagnostic details, and dates of BPG doses were provided by the NT RHD Control Program. Actual dose of penicillin was not recorded, but guidelines recommend 900 mg for people > 20 kg and 450 mg for children < 20 kg. In the NT, the standard measure of adherence for SP is percent adherence (number of BPG doses given/number of doses prescribed × 100). A limitation of percent adherence is that it does not capture timing of doses: the correct number of prescribed doses may be administered—resulting in 100% percent adherence—but the interval/s between some doses may be longer than 28 days, meaning potential vulnerability to GAS infection for a period of time. Therefore, for this study, “days at risk” (DAR) were used to describe adherence. If a dose of BPG was not given by the 28th day after the previous dose, each day late was counted as a DAR. For individual ARF episodes, the interval between streptococcal infection and ARF symptom onset is thought to be 2–4 weeks for most ARF manifestations,6 but diagnosis of ARF can take days to weeks; therefore, the total DAR in the 8 weeks preceding ARF episodes were calculated.
For cases with no DAR in the 8 weeks before an ARF recurrence, the timing of BPG doses was examined to identify when each person may have had GAS infection. For these cases, we evaluated possible windows of risk, based on a more conservative estimate of the protection afforded by a dose of BPG (21 days instead of 28 days).
At the commencement of the study, approximately 1,360 people were prescribed BPG. During the 6-year period, 189 people were diagnosed with a total of 241 recurrences at least 8 weeks after being prescribed BPG. Approximately 14% of people prescribed BPG had a recurrence. Seventy-two cases were excluded (39 were not definite ARF, six had no documented ARF recurrence onset date, 25 cases included chorea, and two had a documented onset date after the diagnosis date). One hundred sixty-nine definite recurrences (142 individuals) were included; 56% of individuals were female and the median age at ARF recurrence was 16.5 years (range: 5–55 years). All were Aboriginal or Torres Strait Islander Australians.
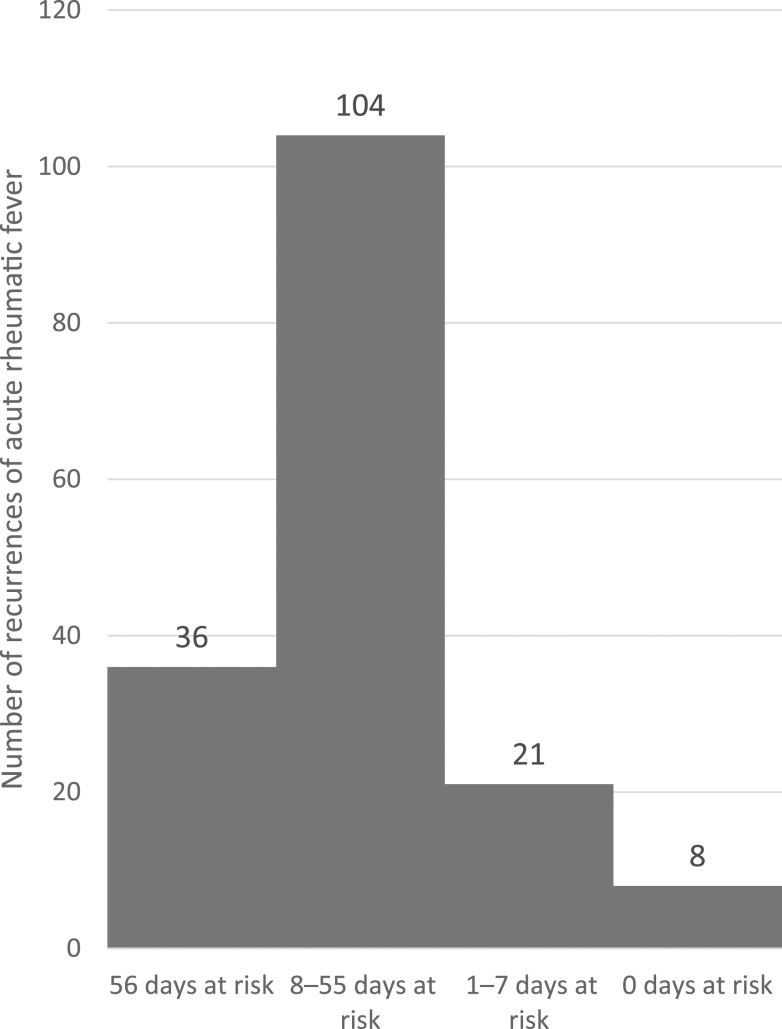
The median number of DAR in the 8 weeks preceding ARF recurrence was 29 (interquartile range: 15–52). Thirty-six cases (21%) occurred in individuals who had no documented BPG doses in the relevant period before their recurrence. One hundred four cases (62%) had 8–55 DAR, 21 cases (12%) had 1–7 DAR, and eight cases (7 individuals) (5%) had no DAR in the 8 weeks preceding ARF onset (Figure 1).
Figure 1.
Number of recurrences of acute rheumatic fever according to number of days at risk (days more than 28 days after the last benzathine penicillin G dose).
Of the seven individuals with no DAR preceding ARF recurrence, four were male, all were aged between 9 and 16 years at the time of their recurrence, and all had been prescribed SP for at least 6 months before their ARF recurrence.
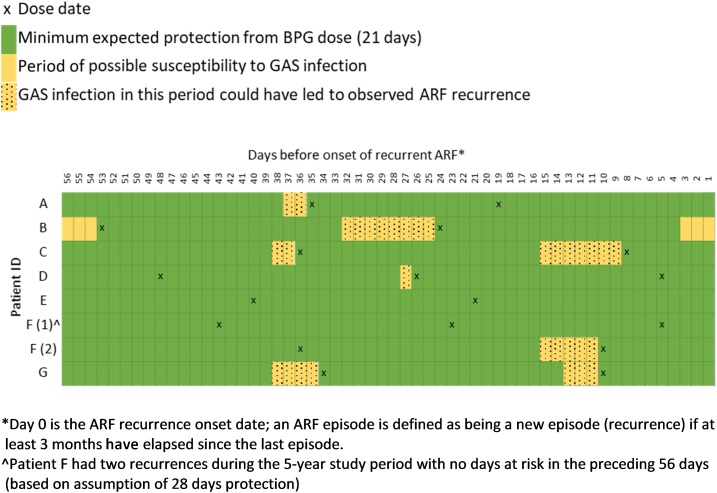
Based on the conservative estimate of protection (21 days instead of 28 days) in the 8 weeks before ARF recurrence, six cases had periods when they were vulnerable to GAS infection, and two did not (Figure 2).
Figure 2.
Timing of benzathine penicillin G (BPG) doses and periods of potential vulnerability to Group A streptococcal (GAS) infection before acute rheumatic fever (ARF) recurrence in those receiving every prescribed BPG dose.
This study shows that in our setting, most ARF recurrences (95%) occur in individuals who have not received BPG doses every 28 days in the 8-week period before onset. Given the high burden of GAS in the NT, receiving BPG doses as prescribed and ensuring all individuals at risk of ARF recurrence are prescribed BPG during their highest risk years are paramount. Surprisingly though, some individuals experience recurrences despite perfect adherence to 4-weekly BPG doses, and two people in this cohort received BPG every 3 weeks and still experienced a recurrence.
Those who had recurrences despite receiving every prescribed BPG dose were all children. These children may process BPG more quickly than others, which means they had inadequate protection against GAS between doses. Children are also more frequently exposed to GAS infection than adults.7 There are several other proposed explanations for penicillin failure which may be relevant, such as the presence of beta-lactamase–secreting pathogens, which reduce the effectiveness of penicillin or the limited effectiveness of antibiotics when the bacterial load is particularly high.8
Limitations included that data on patient weight and penicillin dose were unavailable. Based on age, all seven individuals with breakthrough recurrences should have been > 20 kg and, therefore, receiving full-dose (900 mg) BPG; it is possible that underdosing could have contributed to ARF recurrences. Days at risk are not currently widely used to measure adherence to SP, and there is not yet consensus on the method of calculation or its application to the 3-weekly regimen. It is possible that the window of adherence examined (8 weeks preceding ARF onset) did not cover the entire period during which GAS infection could have led to an episode of ARF, especially given potential delays in recognizing ARF episodes.
Repeated episodes of ARF contribute to progression of RHD, which can lead to significant morbidity and premature mortality. This study reinforces the finding that most ARF recurrences occur in the context of “DAR” due to missed or late penicillin doses. However, the study in addition shows that a small number of individuals experience ARF recurrence despite minimal or no DAR. These individuals then need to be prescribed a 21-day BPG regimen. Currently available data have not identified ways of predicting which individuals are most at risk. Factors responsible for “breakthrough” ARF despite adherence to a 28-day regimen likely include environmental factors (providing opportunities for GAS infection to occur), host immune factors (such that ARF is likely to follow GAS infection), and penicillin dosing and pharmacokinetic considerations. Broader prevention strategies to reduce GAS infection risk and ARF recurrence are needed. These strategies include renewed focus on decreasing household crowding, ensuring functioning health hardware, and promoting early attendance at primary health centers for treatment of sore throats and skin sores. Improved understanding of the pharmacokinetics of BPG in our population is also needed to appropriately tailor SP regimens.
REFERENCES
- 1.Australian Health Ministers’ Advisory Council , 2017. Aboriginal and Torres Strait Islander Health Performance Framework 2017 Report. Canberra, Australia: AHMAC; Available at: https://www.pmc.gov.au/resource-centre/indigenous-affairs/health-performance-framework-2017-report. Accessed February 24, 2019. [Google Scholar]
- 2.Manyemba J, Mayosi BM, 2002. Penicillin for secondary prevention of rheumatic fever. Cochrane Database Syst Rev 3: CD002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broderick MP, Hansen CJ, Faix DJ, 2012. Factors associated with loss of penicillin G concentrations in serum after intramuscular benzathine penicillin G injection: a meta-analysis. Pediatr Infect Dis J 31: 722–725. [DOI] [PubMed] [Google Scholar]
- 4.Currie BJ, Burt T, Kaplan EL, 1994. Penicillin concentrations after increased doses of benzathine penicillin G for prevention of secondary rheumatic fever. Antimicrob Agents Chemother 38: 1203–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Dassel JL, de Klerk N, Carapetis JR, Ralph AP, 2018. How many doses make a difference? An analysis of secondary prevention of rheumatic fever and rheumatic heart disease. J Am Heart Assoc 7: e010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catanzaro FJ, Stetson CA, Morris AJ, Chamovitz R, Rammelkamp CH, Jr., Stolzer BL, Perry WD, 1954. The role of the streptococcus in the pathogenesis of rheumatic fever. Am J Med 17: 749–756. [DOI] [PubMed] [Google Scholar]
- 7.Spellerberg B, Brandt C, 2016. Laboratory diagnosis of Streptococcus pyogenes (group A streptococci). Ferretti J, Stevens D, Fischetti V, eds. Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]. Oklahoma City, OK: University of Oklahoma Health Sciences Center. [PubMed] [Google Scholar]
- 8.Brook I, 2017. Treatment challenges of group A beta-hemolytic streptococcal pharyngo-tonsillitis. Int Arch Otorhinolaryngol 21: 286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]