Abstract
The aryloxy triester phosphoramidate prodrug approach has been used with success in drug discovery. Herein, we describe the first application of this prodrug technology to the monophosphate derivative of the phosphoantigen HMBPP and one of its analogues. Some of these prodrugs exhibited specific and potent activation of Vγ9/Vδ2 T-cells, which were then able to lyse bladder cancer cells in vitro. This work highlights the promise of this prodrug technology in the discovery of novel immunotherapeutics.
Introduction
Present since birth,1 Vγ9/Vδ2 T-cells represent the dominant subtype of human γδ T-cells in adult peripheral blood.2 They expand in response to various infections, including tuberculosis, leprosy, typhoid, malaria, and toxoplasmosis, and studies in primate models have suggested a role in immunity to Mycobacterium tuberculosis.3 Interestingly, they also exhibited an ability to target and lyse a diverse range of cancer cells in vitro.2 Such properties have made the Vγ9/Vδ2 subset a major focus in the therapeutic exploitation of γδ T-cells.4
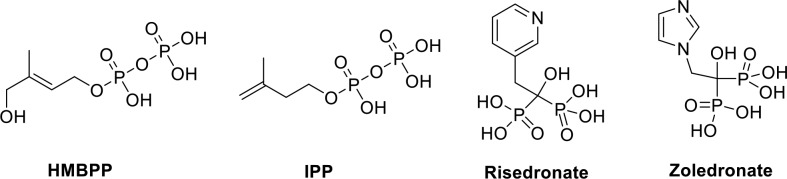
Interestingly, Vγ9/Vδ2 T-cells have been shown to be activated by small molecule phosphoantigens (PAg) such as (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP) and isopentenyl pyrophosphate (IPP) (Figure 1).5,6 Beyond these natural ligands, two synthetic molecules, risedronate and zoledronate, activate Vγ9/Vδ2 T-cells through accumulation of IPP and are currently used in the clinic to treat osteoporosis and some types of cancer (Figure 1).7−9
Figure 1.
Chemical structures of natural phosphoantigens HMBPP and IPP as well as synthetic molecules risedronate and zoledronate, which activate Vγ9/Vδ2 T-cells.
The mechanism by which these small molecule phosphoantigens activate Vγ9/Vδ2 T-cells is understood to be mediated by the type-1 transmembrane protein butyrophilin 3A1.10,11 Although conflicting reports exist as to whether PAgs bind to the extracellular or intracellular domains of this transmembrane protein, there is an increasing body of evidence that supports the notion that these PAgs bind the intracellular B30.2 domain of butyrophilin 3A1.10,12−15
Encouraged by Vγ9/Vδ2 T-cells’ ability to mount immune responses toward pathogens, lyse tumor cells, as well as their amenability to be targeted and modulated by small molecules (PAgs and their synthetic mimics), we embarked on the discovery of small molecules that have the potential to activate Vγ9/Vδ2 T-cells. Given our interest in the discovery and development of phosphorylated molecules and their prodrugs as therapeutics,16−18 we focused our efforts on the natural phosphoantigen HMBPP as it is the most potent activator of Vγ9/Vδ2 T-cells reported to date (Figure 1).19 HMBPP has a pyrophosphate group, which accounts for poor drug-like properties, namely, poor cell membrane permeability, due its charged nature under physiological conditions (pH ≤ 7.4) and limited in vivo stability. These properties have hindered the development of many drugs with unmasked phosphate or pyrophosphate groups. To overcome these drawbacks, numerous phosphate prodrug strategies have been developed and used with success in the discovery of mostly nucleotide monophosphates and monophosphonates.20−22
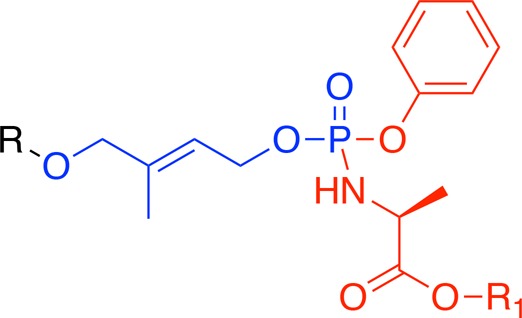
There have been reports in the literature of the application of the bis-pivaloyloxymethyl (bisPOM) phosphate prodrug technology and its derivatives to HMBP and its diphosphonates, which resulted in potent phosphoantigens though these were less potent than the parent phosphoantigen HMBPP.19,23−25 Aiming to discover phosphoantigen prodrugs that are as potent as HMBPP, we focused our work on the aryloxy triester phosphoramidate26 prodrug approach, in which the monophosphate or monophosphonate groups are masked by an aryl motif and an amino acid ester moiety. This prodrug technology is known to be more efficient in delivering monophosphorylated molecules than the bisPOM approach.27 Over the past decade or so, it has led to at least 10 clinical candidates with two being eventually approved for clinical use.28 Notably, this prodrug approach has mostly been used on nucleotide monophosphates and monophosphonates. In this work, we applied this powerful phosphate prodrug technology to the monophosphate derivative of the phosphoantigen HMBPP, (E)-4-hydroxy-3-methylbut-2-enyl phosphate (HMBP). The aryloxy triester phosphoramidate prodrugs of the phosphoantigen HMBP will be referred to as HMBP ProPAgens in this work.
Results and Discussion
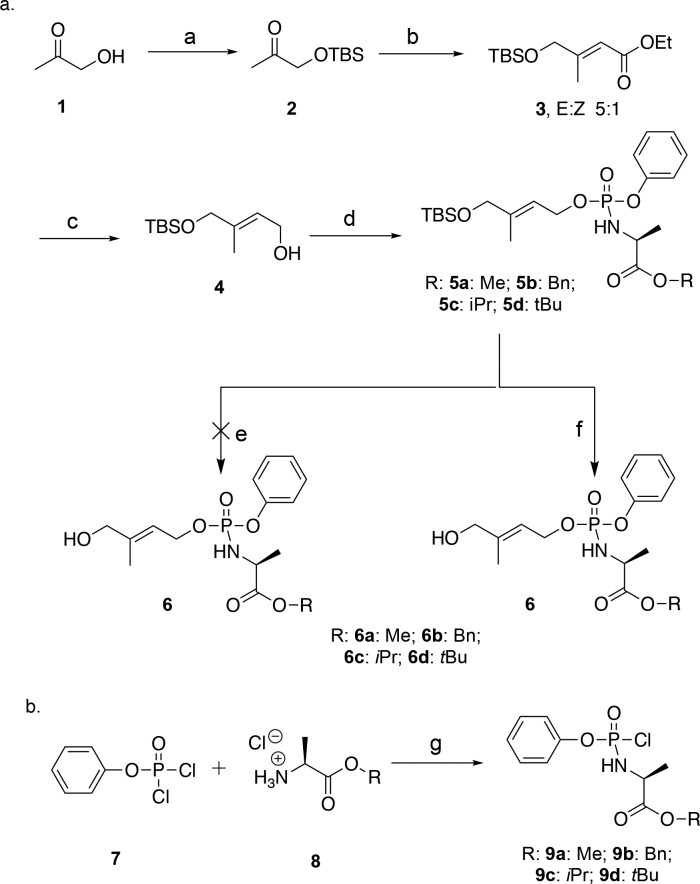
The synthesis of HMBP ProPAgens was similar to that reported by Reichenberg et al.29 Briefly, the hydroxyl group of 1-hydroxypropan-2-one (1) was protected with TBS in the presence of imidazole (Scheme 1a). The ether product 2 was then treated with a Horner–Wadsworth–Emmons reagent, specifically triethyl phosphonoacetate, to create the double bond with E selectivity and yield the ester product 3. This compound, 3, was produced in a mixture of E/Z isomers with a 5:1 ratio similar to what is reported in the literature.5 The E/Z isomers were separated by column chromatography using solvent mixture of 5–7% diethyl ether in hexane. The two products were then evaluated with 1H and 13C NMR utilizing previous work by Hintz et al.5 who performed NOESY experiments to determine the identity of each isomer. The desired E-isomer, 3, was subsequently reduced using lithium aluminum hydride in THF to afford the key intermediate 4. The coupling of compound 4 to phosphorochloridates to afford the desired prodrugs was achieved using standard procedures.17,18 Phosphorochloridates, 9a–d, bearing methyl, isopropyl, tert-butyl, or benzyl esters, were synthesized following the procedure reported by Mehellou et al.17 and as shown in Scheme 1b. These were coupled to 4 in the presence of triethylamine or N-methylimidazole (NMI) to afford the desired HMBP ProPAgens, 5a–d, in modest yields. Notably, NMI was only used in cases when TEA gave very low yields. The removal of the TBS protecting group was initially pursued using TBAF in THF.30 However, this strategy did not work in our hands as the reaction yielded so many products and no traces of the desired HMBP ProPAgens were detected by mass spectroscopy. As an alternative, we used mild acidic conditions, 0.1 equiv of 1.25 M HCl in methanol, and this achieved the TBS deprotection without degrading the phosphate masking moieties to afford the desired HMBP ProPAgens 6a–d in good yields.
Scheme 1. (a) Synthesis of HMBP ProPAgens and (b) Synthesis of Aryl Phosphorochloridates.
Part a, reagents and conditions: (a) TBSCl, imidazole, DCM, rt, yield 95%; (b) triethyl phosphonoacetate, NaH, THF, 0 °C, yield 50%; (c) LiAlH4, THF, 0 °C, yield 40%; (d) 9a–d, TEA or NMI, DCM, yields 36–56%; (e) TBAF, THF; (f) HCl, MeOH, yields 20–74%. Part b, reagents and conditions: (g) Et2O, TEA, −78 °C, yields 54–95%. Me, methyl; iPr, isopropyl; tBu, tert-butyl; Bn, benzyl.
The unprotected HMBP ProPAgens 6a–d, however, exhibited low stability and underwent rapid degradation, which prevented their biological testing (purity <95%). In order to get an insight into the metabolism of ProPAgens 6a–d, compound 6d was incubated with the carboxypeptidase cathepsin A in vitro and the reaction was monitored with 31P NMR. The data show that after 72 h, the major metabolite had a phosphorus peak at ∼1.95 ppm (Supporting Information Figure S1). Mass spectroscopy analysis of these samples showed that the degradation product, which had a 31P NMR peak of ∼1.95 ppm, was the phosphate group masked with the aryl motif and the amino acid ester moiety (Supporting Information Figure S2). This indicated that the P–O bond in HMBP ProPAgens 6a–d was labile and was cleaved off to release the unphopshorylated PAg backbone rather than HMBP. This may explain literature reports pursuing the phosphonates of HMBP where the −O-P- bond was replaced by a −CH2-P- one and lack of reports on the native HMBP phosphate prodrugs.19
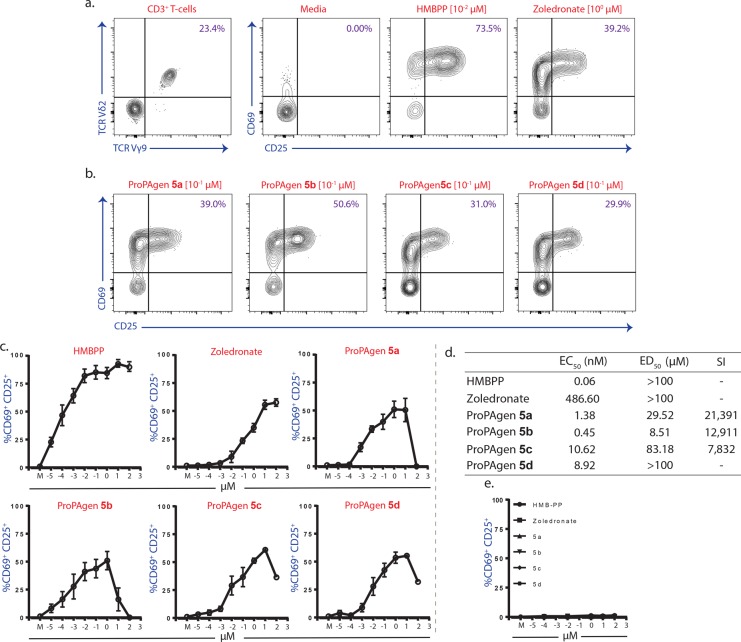
Interestingly, HMBP ProPAgens 5a–d, which had the side chain hydroxyl group protected with a TBS moiety, exhibited better stability than 6a–d, and hence we were able to characterize them fully and obtain a measure of their purity (see Supporting Information). Prodrugs 5a–d were then investigated for their ability to activate Vγ9/Vδ2 T-cells. For this, peripheral blood mononuclear cells (PBMCs) containing Vγ9/Vδ2 T-cells derived from healthy donors were incubated with increasing concentrations of HMBPP, zoledronate, or HMBP ProPAgens 5a–d (up to 100 μM) (Figure 2a and Figure 2b). Peripheral blood γδ T-cells lack appreciable levels of surface CD69 or CD25 under steady state conditions, but T-cell receptor (TCR) stimulation upregulates both T-cell activation markers.31 PAg responsive Vγ9/Vδ2 T-cells were then distinguished by TCR Vγ9 and Vδ2 expression and assessed for the upregulation of CD69 and CD25.
Figure 2.
Activation of human Vγ9/Vδ2+ T cells by HMBP ProPAgens. (a) Human peripheral blood mononuclear cells (PBMC) were incubated with media or indicated concentrations of HMB-PP or zoledronate for 18 h. TCR Vγ9/Vδ2+ T cells were then assessed for the upregulation of cell surface markers, CD69 and CD25. Data representative of n = 5. (b) As in (a), with PBMC incubated with indicated concentrations of HMBP ProPAgens. Data representative of n = 4. (c) As in (a) and (b), with data showing titrations of each of HMB-PP, zoledronate, and HMBP ProPAgens, alongside a medium control (M). Data from n = 4–5 donors. (d) EC50, ED50, and selectivity index (SI) values for each HMBP ProPAgens. Specific cell death, for ED50 values, was calculated after adjusting for nonspecific cell death in medium controls. (e) CD69 and CD25 activation in CD3+ CD8+ αβ T-cells for each compound.
As shown in Figure 2c and Figure 2d, the natural phosphoantigen HMBPP showed significant activation of Vγ9/Vδ2 T-cells, EC50 = 0.06 nM, comparable to its reported potency.19 Additionally, zoledronate showed a moderate activation, EC50 ≈ 500 nM as expected (Figure 2c and Figure 2d). The four HMBP ProPAgens 5a–d exhibited potent Vγ9/Vδ2 T-cell activation with 5b being the most potent, EC50 = 0.45 nM, followed by 5a, EC50 = 1.38 nM. Such potency makes HMBP ProPAgen 5b 1081 times more active than the clinically used agent zoledronate. The other two HMBP ProPAgens also exhibited low nanomolar activation of Vγ9/Vδ2 T-cells, 10.62 nM for 5c and 8.92 nM for 5d (Figure 2c and Figure 2d). Impressively, CD8+ αβ T-cells, which are activated by peptides, showed no reactivity toward HMBP ProPAgens, exemplifying their specificity toward Vγ9/Vδ2 T-cells (Figure 2e). Notably, the unphopshorylated backbone compound 4 did now show any activation of Vγ9/Vδ2 T-cells (data not shown).
Next, we tested the potential for ProPAgens to sensitize the urinary bladder carcinoma cell line T24 for targeted killing by in vitro expanded Vγ9/Vδ2 T cells. Without sensitization, medium pulsed T24 cells were poorly targeted by increasing ratios of Vγ9/Vδ2 T cells, but upon pulsing for 4 h with zoledronate or ProPAgens 5a–d, T24 cells were specifically lysed (Figure 3a). The short pulsing period, poor lipophilicity, and requirement to target a metabolic enzyme resulted in only a marginal increase in specific T24 cell killing by zoledronate (Figure 3b). In contrast, a 1000-fold lower concentration of each ProPAgen mediated a 2- to 4-fold increase in specific lysis of T24 over the same period (Figure 3b).
Figure 3.
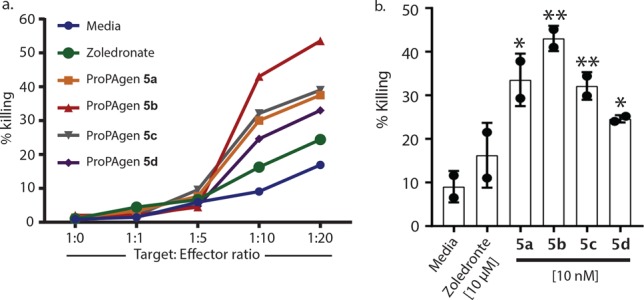
HMBP ProPAgens mediate the specific lysis of bladder cancer cells by Vγ9/Vδ2 T-cells. (a) Human T24 urinary bladder carcinoma cell lines (target) were pulsed for 4 h with 10 nM of the indicated ProPAgens, media, or 10 μM zoledronate, then cocultured for 18 h with expanded Vγ9/Vδ2 T cells (effector) at the indicated target/effector ratios. Specific killing of target cells was measured by amine-reactive dead cell marker staining. Data display the mean of n = 2. (b) As in (a) showing the mean ± SD specific killing of T24 cells at 1:10 target/effector ratio for each indicated treatment, n = 2: (∗) P < 0.05; (∗∗) P < 0.01; determined by one-way ANOVA.
The activity across the different HMBP ProPAgens in both biological assays was in agreement with what is typically observed with aryloxy triester phosphoramidate (ProTide) prodrugs of nucleoside analogues as it correlated with the lipophilicity and rate of degradation. The HMBP ProPAgen with benzyl esters, e.g., 5b, has higher lipophilicity and thus better (passive) cellular uptake. Also, the benzyl group is a better leaving group than the other aliphatic esters, and thus the metabolism of the benzyl ester of 5b proceeds faster than the other ProPAgens, 5a, 5c, and 5d. Together, these explain the superior activity of ProPAgen 5b. HMBP ProPAgen 5a also exhibited relatively potent activation of Vγ9/Vδ2 T-cells with EC50 = 1.38 nM. The fact that ProPAgen 5c, which has an isopropyl ester, exhibited lower potency in activating Vγ9/Vδ2 T-cells as compared to 5a and 5d was surprising as often phosphoramidate prodrugs with this ester exhibit similar activity to those with a methyl ester. However, the less potent activity observed with the HMBP ProPAgen 5d was expected and is in agreement with the literature where phosphoramidate prodrugs with tBu esters show lower biological activity than those with other ester motifs. This is because the hydrolysis of the tBu ester group of the phosphoramidates by esterase enzymes proceeds much slower than those with Me, iPr, and Bn esters.
Conclusion
We reported the application of the aryloxy triester phosphoramidate technology to the phosphoantigen HMBP and one of its synthetic analogues to generate HMBP ProPAgens. Although the ProPAgens of the native HMBP compound (6a–d) were of very low stability, those of the synthetic analogue (5a–d) were more stable. These later ones exhibited potent activation of Vγ9/Vδ2 T-cells. Notably, HMBP ProPAgen 5b was the most potent activator of Vγ9/Vδ2 T-cells, EC50 = 0.45 nM with the other three ProPAgens, 5a, 5c, and 5d, also showing low nanomolar activation. Impressively, Vγ9/Vδ2 T-cells activated by HMBP ProPAgens exhibited potent lysis of urinary bladder carcinoma cancer cells (T24) in vitro. In terms of specificity, HMBP ProPAgens, 5a–d, showed excellent specificity toward the activation of Vγ9/Vδ2 T-cells as they had no effects of other T-cells such as CD8+ αβ T-cells. Efforts aimed at improving the stability of the native HMBP ProPAgens are currently underway and will be reported in the future. In conclusion, the application of the aryloxy triester phosphoramidate prodrug technology to HMBP to generate HMBP ProPAgens yielded potent and specific activators of Vγ9/Vδ2 T-cells. Together, this showcases the promise of this prodrug technology in the discovery of novel immunotherapeutic agents.
Experimental Section
General Information
All of the reactions were carried out under argon atmosphere and were monitored with analytical thin layer chromatography (TLC). NMR data were recorded on a Bruker AV300, AVIII300, AV400, AVIII400, or DRX500 spectrometer in the deuterated solvents indicated, and the spectra were calibrated on residual solvent peaks. Chemical shifts (δ) are quoted in ppm, and J values are quoted in Hz. In reporting spectral data, the following abbreviations were used: s (singlet), d (doublet), t (triplet), q (quartet), dd (doublet of doublets), td (triplet of doublets), and m (multiplet). HPLC was carried out on a DIONEX summit P580 quaternary low-pressure gradient pump with a built-in vacuum degasser using a Summit UVD 170s UV/vis multichannel detector. HPLC grade solvents were used. Chromeleon software was used to visualize and process the obtained chromatograms. Analytical separations used a flow rate of 1 mL/min and preparative used a flow rate of 20 mL/min. The purity of the tested compounds was determined by high-performance liquid chromatography (HPLC) where all of the tested compounds had ≥95% purity.
1-((tert-Butyldimethylsilyl)oxy)propan-2-one, 2
Hydroxyacetone 1 (1.00 g, 13.50 mmol, 1 equiv) and TBSCl (3.05 g, 20.25 mmol, 1.5 equiv) were combined in dry CH2Cl2 (50 mL) under an argon. Imidazole (2.02 g, 29.70, 2.2 equiv) was then added portionwise at 0 °C. The mixture was allowed to warm to room temperature and left to stir at that temperature for 2.5 h. The reaction mixture was then washed with brine (50 mL), extracted with Et2O (50 mL × 3), dried (MgSO4) and the solvents were removed under reduced pressure to give a crude oil, which was purified via column chromatography (10:1 hexane/EtOAc). 2 was obtained as a colorless oil (2.45 g, 95%). δH NMR (300 MHz, CDCl3): 4.15 (s, 2H, CH2), 2.17 (s, 3H, CH3), 0.93 (s, 9H, CH3), 0.09 (s, 6H, CH3). δC NMR (101 MHz, CDCl3): 209.25, 69.57, 25.98, 25.76, 18.29, −5.51. MS-ESI (%): 211.1 (100, M + Na).
Ethyl (E)-4-((tert-Butyldimethylsilyl)oxy)-3-methylbut-2-enoate, 3
To a 0 °C suspension of NaH (0.20 g, 8.38 mmol, 1 equiv) in dry THF (50 mL) under an argon was added diethyl phosphonoacetate (1.88 g, 8.38 mmol, 1 equiv) dropwise. The resulting solution was left to stir for 25 min at 0 °C. Silyl ether 2 (1.5 g, 7.96 mmol, 0.95 equiv) was then added dropwise as a solution in dry THF (25 mL). The mixture was then stirred at 0 °C for a further 30 min, then warmed to room temperature and stirred for a further 2 h or until complete via TLC. A saturated solution of brine (50 mL) was added, dropwise at first, then the organic layer was extracted with Et2O (50 mL × 3), dried (MgSO4) and the solvents were removed to give a mixture of E/Z isomers (5:1 via 1H NMR) as a crude oil. This was purified via column chromatography (10% Et2O in hexane) to give product 3 as a colorless oil (1.04 g, 50%). δH NMR (300 MHz, CDCl3): 5.99 (d, J = 1.4 Hz, 1H, CH), 4.17 (q, J = 7.1 Hz, 2H, CH2), 4.11 (d, J = 1.4 Hz, 2H, CH2), 2.05 (s (broad), 3H, CH3), 1.29 (t, J = 7.1 Hz, 3H, CH3), 0.93 (s, 9H, CH3), 0.09 (s, 6H, CH3). δC NMR (101 MHz, CDCl3): 167.10, 157.16, 113.35, 67.10, 59.59, 25.88, 18.37, 15.44, 14.36, −5.45. MS-ESI (%): 258.2 (8, M), 243.2 (100, M – CH3).
(E)-4-((tert-Butyldimethylsilyl)oxy)-3-methylbut-2-en-1-ol, 4
In a flame-dried Schlenk flask were placed ester 3 (0.5 g, 1.93 mmol, 1 equiv) and 20 mL of dry THF, and the resulting mixture was cooled to 0 °C with stirring. Then LiAlH4 (1 M solution in THF) (1.93 mL, 1.93 mmol, 1 equiv) was added dropwise, and the solution was allowed to warm to room temperature and stirred for 2 h. MeOH (1 mL) was then added cautiously, after which a saturated solution of sodium potassium tartrate (30 mL) was added which formed a white gel. This was stirred overnight at room temperature, and then the phases were allowed to separate. The organic layer was collected and the aqueous layer was washed with Et2O (20 mL × 3), dried (MgSO4) and the solvents were removed under reduced pressure to leave a crude oil. This was then purified by column chromatography using hexane/ethyl acetate (4:1) as an eluant to give product 4 (0.1677 g, 40%) as a colorless oil. δH (300 MHz, CDCl3) 5.72–5.65 (m, 1H, CH), 4.21 (d, J = 6.9 Hz, 2H, CH2), 4.04 (s, 2H, CH2), 1.65 (s (broad), 3H, CH3), 0.92 (s, 9H, 3 × CH3), 0.08 (s, 6H, CH3). δC (101 MHz, CDCl3) 138.32, 122.53, 67.66, 59.14, 25.94, 18.41, 13.50, −5.34. MS (ESI) (%): 239.1 (100, M + Na).
Methyl ((((E)-4-((tert-Butyldimethylsilyl)oxy)-3-methylbut-2-en-1-yl)oxy)(phenoxy)phosphoryl)-l-alaninate, 5a
To a stirring solution of alcohol 4 (0.1301 g, 0.6166 mmol, 1 equiv) in dry CH2Cl2 (10 mL) was added methyl phosphorochloridate 9a (0.2055 g, 0.7400 mmol, 1.2 equiv). The solution was cooled to 0 °C, and NMI (0.10 mL g, 1.2333 mmol, 2 equiv) was added dropwise and the resulting solution was warmed to room temperature and stirred overnight. Brine (10 mL) was then added and the organic layer separated off. The aqueous layer was then extracted with Et2O (20 mL × 3), the organic layers were combined, dried (MgSO4) and the solvents removed under reduced pressure to leave a crude oil. This was then purified by column chromatography (4:1 Hex/EtOAc to 1:1 Hex/EtOAc) to give 5a (0.1228 g, 43%) as a colorless oil. δH NMR (400 MHz, CDCl3): 7.31 (t, J = 7.3 Hz, 2H, 2 × CH), 7.21 (t, J = 7.3 Hz, 2H, 2 × CH), 7.14 (t, J = 7.3 Hz, 1H, CH), 5.75–5.59 (m, 1H, CH), 4.72–4.64 (m, 2H, CH2), 4.10–3.97 (m, 3H, CH2 and CH), 3.70 (d, J = 8.8 Hz, 3H, CH3), 3.53 (dd, J = 18.0, 9.7 Hz, 1H, NH), 1.65 (d, J = 3.5 Hz, 3H, CH3), 1.37 (t, J = 7.4 Hz, 3H, CH3), 0.91 (s, 9H, 3 × CH3), 0.07 (s, 6H, 2 × CH3). δC NMR (101 MHz, CDCl3): 173.96 (d, J = 6.0 Hz), 150.89 (d, J = 5.7 Hz,), 141.03, 129.58, 124.70, 120.22, 117.86 (d, J = 7.1 Hz), 67.28, 63.28 (t, J = 5.0 Hz), 52.43, 50.11 (d, J = 4.1 Hz), 25.90, 21.05 (d, J = 3.3 Hz), 18.37, 13.59, −5.39. δP NMR (121 MHz, CDCl3) 2.48, 2.50. HRMS (ESI): found M + Na, 480.1949, [C21H36NO6SiPNa] requires 480.1947.
Benzyl ((((E)-4-((tert-Butyldimethylsilyl)oxy)-3-methylbut-2-en-1-yl)oxy)(phenoxy)phosphoryl)-l-alaninate, 5b
Prepared under the same procedure as described for 5a using alcohol 4 (0.16 g, 0.77 mmol, 1 equiv), benzyl phosphorchloridate 9b (0.32 g, 0.92 mmol, 1.2 equiv), and dry Et3N (0.22 mL, 1.5496 mmol, 2 equiv) to give a crude yellow oil which was then purified by column chromatography (4:1 Hex/EtOAc to 2:3 Hex/EtOAc) to afford 5b (0.1306 g, 31%) as a colorless oil. δH NMR (400 MHz, CDCl3) δ 7.44–7.06 (m, 10H, Ph), 5.66 (m, 1H, CH), 5.18–5.09 (m, 2H, CH2), 4.71–4.61 (m, 2H, CH2), 4.13–4.04 (m, 1H, CH), 4.02 (d, J = 5.3 Hz, 2H, CH2), 3.59–3.45 (m, 1H, NH), 1.63 (d, J = 5.1 Hz, 3H, CH3), 1.38 (t, J = 6.9 Hz, 3H, CH3), 0.91 (s, 9H, CH3), 0.06 (s, 6H, 2 × CH3). δC NMR (101 MHz, CDCl3): 173.32 (2s), 150.88 (s), 141.06 (2s), 135.28, 129.59 (2s), 128.64 (2s), 128.48 (2s), 128.19 (2s), 124.71 (2s), 120.23 (s (broad)), 117.87 (2s), 67.32 (2s (broad)), 67.18 (2s (broad)), 63.35 (2s), 50.25 (2s), 25.91 (2s), 21.11 (2s), 13.59 (2s), −5.37. δP NMR (121 MHz, CDCl3): 2.38, 2.56. HRMS (ESI): found M + Na, 556.2262, [C27H40NO6PSiNa] requires 556.2260.
Isopropyl ((((E)-4-((tert-Butyldimethylsilyl)oxy)-3-methylbut-2-en-1-yl)oxy)(phenoxy)phosphoryl)-l-alaninate, 5c
Prepared under the same procedure as described for 5a using alcohol 4 (0.23 g, 1.08 mmol, 1 equiv), isopropyl phosphorchloridate 9c (0.39 g, 1.29 mmol, 1.2 equiv), and dry Et3N (0.30 mL, 2.16 mmol, 2 equiv) to give a crude yellow oil which was then purified by column chromatography (4:1 Hex/EtOAc) to afford 5c (0.1914 g, 36%) as a colorless oil. δH NMR (400 MHz, CDCl3) 7.30 (td, J = 8.5, 2.1 Hz, 2H, 2 × CH), 7.20 (td, J = 7.5, 0.9 Hz, 2H, 2 × CH), 7.16–7.08 (m, 1H, CH), 5.66 (q, J = 7.2 Hz, 1H, CH), 5.06–4.92 (m, 1H, CH), 4.75–4.59 (m, 2H, CH2), 4.02 (s, 2H, CH2), 4.00–3.88 (m, 1H, CH), 3.60–3.48 (m, 1H, NH), 1.63 (s, 3H, CH3), 1.38–1.32 (m, 3H, CH3), 1.26–1.17 (m, 6H, 2 × CH3), 0.90 (s, 9H, 3 × CH3), 0.05 (s, J = 4.2 Hz, 6H, 2 × CH3). δC NMR (101 MHz, CDCl3) 173.00 (2d, J = 4.13 Hz), 150.92 (t, J = 6.7 Hz), 140.96, 129.57, 124.68 (s (broad)), 120.23 (t, J = 4.6 Hz), 117.92 (2s), 69.11, 67.30, 63.28 (t, J = 5.1 Hz), 50.30 (2s), 25.90, 21.66 (2s), 21.09 (2s), 18.37, 13.59, −5.39. δP NMR (121 MHz, CDCl3): 2.62, 2.65. HRMS (ESI): found M + Na, 508.2258, [C23H40NO6PSiNa] requires 508.2260.
tert-Butyl ((((E)-4-((tert-Butyldimethylsilyl)oxy)-3-methylbut-2-en-1-yl)oxy)(phenoxy)phosphoryl)-l-alaninate, 5d
Prepared under the same procedure as described for 5a using alcohol 4 (0.14 g, 0.65 mmol, 1 equiv), tert-butyl phosphorchloridate 9d (0.25 g, 0.78 mmol, 1.2 equiv), and dry NMI (0.11 mL, 1.30 mmol, 2 equiv) to give a crude yellow oil which was then purified by column chromatography (4:1 Hex/EtOAc) to afford 5d (0.1832 g, 56%) as a colorless oil. δH NMR (400 MHz, CDCl3): 7.29 (t, J = 7.2 Hz, 2H, 2 × CH), 7.22 (t, J = 7.2, 2H, 2 × CH), 7.12 (t, J = 7.2 Hz, 1H, CH), 5.68 (qd, J = 7.2, 1.3 Hz, 1H, CH), 4.74–4.62 (m, 2H, CH2), 4.02 (s, 2H, CH2), 3.95–3.83 (m, 1H, CH), 3.73–3.62 (m, 1H, NH), 1.64 (s, 3H, CH3), 1.43 (2s, 9H, 3 × CH3), 1.33 (2d, J = 3.1 Hz, 3H, CH3), 0.91 (s, 9H, 3 × CH3), 0.07 (s, 6H, 2 × CH3). δC NMR (101 MHz, CDCl3): 172.64 (2d, J = 4.1 Hz), 150.94 (t, J = 6.2 Hz), 140.76, 129.49, 124.55, 120.21 (t, J = 4.8 Hz), 118.01 (2s (broad)), 81.69, 67.27, 63.15 (t, J = 5.0 Hz), 50.66 (2s), 27.86, 25.87, 21.06 (2s), 18.31, 13.53, −5.41. δP NMR (121 MHz, CDCl3): 2.82 (s (broad)). HRMS (ESI): found M + Na, 522.2415, [C24H42NO6SiPNa] requires 522.2417.
Methyl ((((E)-4-Hydroxy-3-methylbut-2-en-1-yl)oxy)(phenoxy)phosphoryl)-l-alaninate, 6a
The silyl ether 5a (0.12 g, 0.26 mmol, 1 equiv) was placed in a flask containing MeOH (3 mL). HCl (1.25 M in MeOH, 0.0215 mL, 0.0268 mmol, 0.1 equiv) was then added at 0 °C, and the resulting solution was warmed to room temperature while stirring for 40 min, until reaction was complete via TLC, upon which it was neutralized with sodium bicarbonate. The mixture was then filtered and MeOH removed under reduced pressure. Brine (10 mL) and CH2Cl2 (10 mL) were then added. The organic layer was collected, and the aqueous layer was extracted with CH2Cl2 (10 mL × 3). The organic layers were combined, dried (MgSO4) and the solvents removed under reduced pressure to leave a crude brown oil. This was then purified by column chromatography (4:1 Hex/EtOAc) to leave 6a (0.0920 g, 49%) as a colorless oil. δH NMR (400 MHz, CDCl3): 7.32 (t, J = 7.8 Hz, 2H, 2 × CH), 7.25–7.18 (m, 2H, 2 × CH), 7.15 (t, J = 7.3 Hz, 1H, CH), 5.67 (dtd, J = 12.5, 7.0, 1.4 Hz, 1H, CH), 4.78–4.58 (m, 2H, CH2), 4.16–3.93 (m, 3H, CH2 and CH), 3.71 (d, J = 8.4 Hz, 3H, CH3), 3.64–3.43 (m, 1H, NH), 1.70 (s, 3H, CH3), 1.37 (t, J = 7.0 Hz, 3H, CH3). δC NMR (101 MHz, CDCl3): 174.11 (2s), 150.78 (2s), 141.65 (s (broad)), 129.60, 124.76, 120.19 (t, J = 5.5 Hz), 118.48 (t, J = 7.2 Hz), 67.19, 63.49 (t, J = 5.7 Hz), 52.47 (s), 50.14 (2s), 20.95 (t, J = 5.3 Hz), 13.78. δP NMR (121 MHz, CDCl3): 2.42, 2.57. HRMS (ESI): found M + Na, 366.1081, [C15H22NO6PNa] requires 366.1082.
Benzyl ((((E)-4-Hydroxy-3-methylbut-2-en-1-yl)oxy)(phenoxy)phosphoryl)-l-alaninate, 6b
Prepared following the same procedure as described for 6a using silyl ether 5b (0.13 g, 0.24 mmol, 1 equiv) and HCl (1.25 M in MeOH, 0.19 mL, 0.24 mmol, 1 equiv) in dry MeOH (5 mL), to give a crude brown oil after 2 h. This was then purified by column chromatography (1:1 Hex/EtOAc to 1:4 Hex/EtOAc) to afford 6b (0.0207 g, 20%) as a colorless oil. δH NMR (400 MHz, CDCl3): 7.53–7.02 (m, 10H, Ph), 5.64 (dtd, J = 21.5, 7.0, 1.3 Hz, 1H, CH), 5.13 (d, J = 11.7 Hz, 2H, CH2), 4.75–4.57 (m, 2H, CH2), 4.15–4.01 (m, 1H, CH), 3.99 (d, J = 8.9 Hz, 2H, CH2), 3.65–3.50 (m, 1H, NH), 1.97 (s (broad), 1H, OH), 1.68 (d, J = 4.8 Hz, 3H, CH3), 1.37 (t, J = 7.0 Hz, 3H, CH3). δC NMR (100 MHz, CDCl3): 173.45 (2d, J = 3.3 Hz), 150.82 (t, J = 6.1 Hz), 141.44 (s (broad)), 135.23, 129.60, 128.65, 128.51 (2s), 128.20, 124.78, 120.23 (2d, J = 5.0 Hz), 118.81 (2d, J = 6.5 Hz), 67.43, 67.24, 63.40 (t, J = 5.8 Hz), 50.29 (2s), 29.70, 13.81. δP NMR (121 MHz, CDCl3): 2.36, 2.53. HRMS (ESI): found M + Na, 442.1392, [C21H26NO6PNa] requires 442.1395.
Isopropyl ((((E)-4-Hydroxy-3-methylbut-2-en-1-yl)oxy)(phenoxy)phosphoryl)-l-alaninate, 6c
Prepared following the same procedure as described for 6a using silyl ether 5c (0.19 g, 0.39 mmol, 1 equiv) and HCl (1.25 M in MeOH, 0.03 mL, 0.0364 mmol, 0.1 equiv) in dry MeOH (5 mL), to give a crude brown oil after 1.5 h. This was then purified by column chromatography (1:1 Hex/EtOAc) to afford 6c (0.0725 g, 49%) as a colorless oil. δH NMR (400 MHz, CDCl3): 7.34–7.28 (m, 2H, CH), 7.21 (t, J = 7.4 Hz, 2H, 2 × CH), 7.14 (t, J = 7.3 Hz, 1H, CH), 5.73–5.61 (m, 1H, CH), 5.08–4.91 (m, 1H, CH), 4.76–4.57 (m, 2H, CH2), 4.04–3.86 (m, 3H, CH2 & CH), 3.77–3.64 (m, 1H, NH), 2.89 (s, 1H, OH), 1.68 (s, 3H, CH3), 1.34 (t, J = 6.5 Hz, 3H, CH3), 1.22 (ddd, J = 11.7, 7.0, 5.5 Hz, 6H, 2 × CH3). δC NMR (101 MHz, CDCl3): 173.21 (2s), 150.90 (t, J = 6.2 Hz), 141.69 (s, (broad)), 129.66, 124.81, 120.28 (2d, J = 4.8 Hz), 118.63 (t, J = 5.7 Hz), 69.28, 67.26, 63.52 (t, J = 5.1 Hz), 50.37 (2s), 21.71 (2s), 21.19–20.94 (m), 13.85. δP NMR (121 MHz, CDCl3): 2.54, 2.68.
tert-Butyl ((((E)-4-Hydroxy-3-methylbut-2-en-1-yl)oxy)(phenoxy)phosphoryl)-l-alaninate, 6d
Prepared following the same procedure as described for 6a using silyl ether 5d (0.1832 g, 0.3666 mmol, 1 equiv) and HCl (1.25 M in MeOH, 0.0293 mL, 0.0366 mmol, 0.1 equiv) in dry MeOH (3 mL), to give a crude brown oil. This was then purified by column chromatography (2:3 Hex/EtOAc) to afford 6d (0.0983 g, 74%) as a colorless oil. δH NMR (400 MHz, CDCl3): 7.31 (t, J = 7.2 Hz, 2H, 2 × CH), 7.21 (dd, J = 7.6, 6.6 Hz, 2H, 2 × CH), 7.13 (t, J = 7.3 Hz, 1H, CH), 5.73–5.61 (m, 1H, CH), 4.75–4.59 (m, 2H, CH2), 3.99 (s (broad), 2H, CH2), 3.96–3.80 (m, 1H, CH), 3.72–3.61 (m, 1H, NH), 2.96 (s (broad), 1H, OH), 1.68 (s, 3H, CH3), 1.43 (2s, 9H, CH3), 1.36–1.29 (t, J = 6.3 Hz, 3H, CH3). δC NMR (101 MHz, CDCl3): 172.79 (2s), 150.86 (t, J = 6.1 Hz), 141.57, 129.58, 124.69, 120.22 (2d, J = 4.9 Hz), 118.61 (2s), 81.97, 67.19, 63.40 (t, J = 5.1 Hz), 50.70, 27.89, 21.11 (2d, J = 4.6 Hz), 13.79. δP NMR (121 MHz, CDCl3): 2.70, 2.79. HRMS (ESI): found M + Na, 408.1554, [C18H28NO6PNa] requires 408.1554.
Methyl (Chloro(phenoxy)phosphoryl)-l-alaninate, 9a
Phenyl phosphorodichloridate 7 (0.2 g, 0.95 mmol, 1 equiv) and l-alanine methyl ester hydrogen chloride (0.13 g, 0.95 mmol, 1 equiv) were combined in dry CH2CL2 (10 mL) under an argon flow at −78 °C and stirred at −78 °C for 20 min. Dry Et3N (0.26 mL, 1.90 mmol, 2 equiv) was then added dropwise over 15 min and the reaction stirred at −78 °C for a further 30 min. The temperature is then raised to room temperature and the reaction stirred for a further 3.5 h. The solvents are removed under reduced pressure, and the mixture is filtered and washed with Et2O, which is removed under reduced pressure to give a crude oil. This is then purified by column chromatography (7:3 Hex/EtOAc) to give 9a as a colorless oil (0.14 g, 54%), which is stored under argon. δH NMR (300 MHz, CDCl3): 7.40–7.34 (m, 2H, 2 × CH), 7.29–7.23 (m, 3H, 2 × CH), 4.66–4.49 (m, 1H, NH), 4.28–4.12 (m, 1H, CH), 3.78 (2s, 3H, CH3), 1.51 (2 × dd, J = 4.1, 0.5 Hz, 3H, CH3). δC (101 MHz, CDCl3): 173.11 (2d, J = 8.8 Hz), 149.73 (2d, J = 7.14 Hz), 129.90, 125.99, 120.54 (2d, J = 1.4 Hz), 52.80 (2s), 50.39 (2d, J = 1.5 Hz), 20.51 (2d, J = 1.5 Hz). δP NMR (121 MHz, CDCl3): 7.62, 7.94.
Benzyl (Chloro(phenoxy)phosphoryl)-l-alaninate, 9b
Followed the same procedure as described for 9a using 7 (0.35 mL, 3.32 mmol, 1 equiv), Et3N (0.63 mL, 4.64 mmol, 2 equiv), and l-alanine benzyl ester hydrogen chloride (0.5 g, 2.32 mmol, 1 equiv) to give phosphorchloridate 9b (0.505 g, 95%). δH NMR (300 MHz, CDCl3): 7.42–7.32 (m, 7H, Ph), 7.25–7.20 (m, 3H, Ph), 5.21 (2s, 2H, CH2), 4.34–4.15 (m, 2H, CH and N-H), 1.52 (dd, J = 6.6, 2.4 Hz, 3H, CH3). δC NMR (101 MHz, CDCl3): 172.52 (dd, J = 11.6, 8.7 Hz), 149.75 (dd, J = 11.6. 8.7 Hz), 135.05 (d, J = 5.8 Hz, C-10), 129.92, 128.68, 128.59, 128.33, 125.99, 120.57, 67.60, 50.67, 20.47 (t, J = 4.5 Hz). δP NMR (121 MHz, CDCl3): 7.49, 7.85.
Isopropyl (Chloro(phenoxy)phosphoryl)-l-alaninate, 9c
Followedthe same procedure as described for 9a using 7 (0.62 mL, 4.18 mmol, 1 equiv), Et3N (1.13 mL, 8.36 mmol, 2 equiv), and l-alanine isopropyl ester hydrogen chloride (0.700 g, 4.18 mmol, 1 equiv) to give phosphorchloridate 9c (1.185 g, 93%). δH NMR (300 MHz, CDCl3): 7.41–7.34 (m, 2H, 2 × CH), 7.30–7.21 (m, 3H, 3 × CH), 5.15–5.01 (m, 1H, CH), 4.50–4.31 (m, 1H, NH), 4.22–4.04 (m, 1H, CH), 1.50 (dd, J = 7.0, 2.1 Hz, 3H, CH3), 1.34–1.22 (m, 6H, 2 × CH3). δC NMR (101 MHz, CDCl3): 172.15 (dd, J = 13.9, 9.2 Hz), 149.76 (t, J = 7.0 Hz), 129.93, 125.98, 120.56 (d, J = 4.9 Hz), 69.79 (d, J = 10.5 Hz), 50.70 (2s), 21.67 (2s), 20.55 (d, J = 4.4 Hz). δP NMR (121 MHz, CDCl3): 7.76, 8.11.
tert-Butyl (Chloro(phenoxy)phosphoryl)-l-alaninate, 9d
Followed the same procedure as described for 9a using 7 (0.22 mL, 1.5 mmol, 1 equiv), Et3N (0.42 mL, 3 mmol, 2 equiv), and l-alanine tert-butyl ester hydrogen chloride (0.27 g, 1.5 mmol, 1 equiv) to give phosphorchloridate 9d (0.44 g, 90%). δH NMR (400 MHz, CDCl3): 7.37 (t, J = 7.8 Hz, 2H, 2 × CH), 7.30–7.22 (m, 3H, 2 × CH), 4.46–4.24 (m, 1H, NH), 4.16–3.97 (m, 1H, CH), 1.50–1.46 (m, 12H, 4 × CH3). δC NMR (101 MHz, CDCl3): 171.77 (dd, J = 15.8, 9.4 Hz), 149.80, 129.92, 125.96, 120.57 (d, J = 5.0 Hz), 82.70 (2s), 51.09 (2s), 27.93, 20.63. δP NMR (121 MHz, CDCl3): 7.85, 8.24.
Acknowledgments
The work was supported by Wellcome Trust Investigator award funding to B.E.W. (Grant code: 099266/Z/12/Z), supporting M.S.D., and A.T.B, and Wellcome Trust Pathfinder award funding to B.E.W. (Grant code: 200983/Z/16/Z), supporting T.E.T.
Glossary
Abbreviations Used
- Bis-POM
bis-pivaloyloxymethyl
- Bn
benzyl
- HMBP
(E)-4-hydroxy-3-methylbut-2-enyl monophosphate
- HMBPP
(E)-4-hydroxy-3-methylbut-2-enyl pyrophosphate
- IPP
isopentenyl pyrophosphate
- iPr
isopropyl
- Me
methyl
- NMI
N-methylimidazole
- PAg
phosphoantigen
- PBMC
peripheral blood mononuclear cell
- ProPAgen
prodrug of a phosphoantigen
- TBAF
tetrabutylammonium fluoride
- TBS
tert-butyldimethylsilyl
- tBu
tert-butyl
- TCR
T-cell receptor
- THF
tetrahydrofuran
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.7b01824.
Author Contributions
∥ M.S.D., R.M., and R.C.M. contributed equally. R.M. and R.C.M. synthesized all of the reported compounds. M.S.D., A.T.B., and T.E.T. carried out the biological testing of the prodrugs. C.S.L.D. assisted with the NMR and mass spectroscopy studies. B.E.W. and Y.M. designed and supervised the experiments. Y.M. wrote the manuscript, and all of the authors gave approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Dimova T.; Brouwer M.; Gosselin F.; Tassignon J.; Leo O.; Donner C.; Marchant A.; Vermijlen D. Effector Vγ9Vδ2 T cells dominate the human fetal γδ T-cell repertoire. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, E556–565. 10.1073/pnas.1412058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita C. T.; Jin C.; Sarikonda G.; Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol. Rev. 2007, 215, 59–76. 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- Shen Y.; Zhou D.; Qiu L.; Lai X.; Simon M.; Shen L.; Kou Z.; Wang Q.; Jiang L.; Estep J.; Hunt R.; Clagett M.; Sehgal P. K.; Li Y.; Zeng X.; Morita C. T.; Brenner M. B.; Letvin N. L.; Chen Z. W. Adaptive immune response of Vγ2Vδ2+ T cells during mycobacterial infections. Science 2002, 295, 2255–2258. 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. P.; Heuijerjans J.; Yan M.; Gustafsson K.; Anderson J. γδ T cells for cancer immunotherapy: a systematic review of clinical trials. Oncoimmunology 2014, 3, e27572. 10.4161/onci.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintz M.; Reichenberg A.; Altincicek B.; Bahr U.; Gschwind R. M.; Kollas A. K.; Beck E.; Wiesner J.; Eberl M.; Jomaa H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett. 2001, 509, 317–322. 10.1016/S0014-5793(01)03191-X. [DOI] [PubMed] [Google Scholar]
- Tanaka Y.; Morita C. T.; Tanaka Y.; Nieves E.; Brenner M. B.; Bloom B. R. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature 1995, 375, 155–158. 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- Maraka S.; Kennel K. A. Bisphosphonates for the prevention and treatment of osteoporosis. BMJ. 2015, 351, h3783. 10.1136/bmj.h3783. [DOI] [PubMed] [Google Scholar]
- Neville-Webbe H. L.; Holen I.; Coleman R. E. The anti-tumour activity of bisphosphonates. Cancer Treat. Rev. 2002, 28, 305–319. 10.1016/S0305-7372(02)00095-6. [DOI] [PubMed] [Google Scholar]
- Roelofs A. J.; Jauhiainen M.; Monkkonen H.; Rogers M. J.; Monkkonen J.; Thompson K. Peripheral blood monocytes are responsible for γδ T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br. J. Haematol. 2009, 144, 245–250. 10.1111/j.1365-2141.2008.07435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavassori S.; Kumar A.; Wan G. S.; Ramanjaneyulu G. S.; Cavallari M.; El Daker S.; Beddoe T.; Theodossis A.; Williams N. K.; Gostick E.; Price D. A.; Soudamini D. U.; Voon K. K.; Olivo M.; Rossjohn J.; Mori L.; De Libero G. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat. Immunol. 2013, 14, 908–916. 10.1038/ni.2665. [DOI] [PubMed] [Google Scholar]
- Harly C.; Guillaume Y.; Nedellec S.; Peigne C. M.; Monkkonen H.; Monkkonen J.; Li J.; Kuball J.; Adams E. J.; Netzer S.; Dechanet-Merville J.; Leger A.; Herrmann T.; Breathnach R.; Olive D.; Bonneville M.; Scotet E. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood 2012, 120, 2269–2279. 10.1182/blood-2012-05-430470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. A.; Chen H. C.; Price A. J.; Keeble A. H.; Davey M. S.; James L. C.; Eberl M.; Trowsdale J. Activation of human γδ T cells by cytosolic interactions of BTN3A1 with soluble phosphoantigens and the cytoskeletal adaptor periplakin. J. Immunol. 2015, 194, 2390–2398. 10.4049/jimmunol.1401064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom A.; Peigne C. M.; Leger A.; Crooks J. E.; Konczak F.; Gesnel M. C.; Breathnach R.; Bonneville M.; Scotet E.; Adams E. J. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity 2014, 40, 490–500. 10.1016/j.immuni.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Morita C. T. Sensor function for butyrophilin 3A1 in prenyl pyrophosphate stimulation of human Vγ2Vδ2 T Cells. J. Immunol. 2015, 195, 4583–4594. 10.4049/jimmunol.1500314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim M.; Knowles T. J.; Baker A. T.; Davey M. S.; Jeeves M.; Sridhar P.; Wilkie J.; Willcox C. R.; Kadri H.; Taher T. E.; Vantourout P.; Hayday A.; Mehellou Y.; Mohammed F.; Willcox B. E. BTN3A1 discriminates γδ T cell phosphoantigens from nonantigenic small molecules via a conformational sensor in its B30.2 domain. ACS Chem. Biol. 2017, 12, 2631–2643. 10.1021/acschembio.7b00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehellou Y.; McGuigan C.; Brancale A.; Balzarini J. Design, synthesis, and anti-HIV activity of 2′,3′-didehydro-2′,3′-dideoxyuridine (d4U), 2′,3′-dideoxyuridine (ddU) phosphoramidate ’ProTide’ derivatives. Bioorg. Med. Chem. Lett. 2007, 17, 3666–3669. 10.1016/j.bmcl.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Mehellou Y.; Valente R.; Mottram H.; Walsby E.; Mills K. I.; Balzarini J.; McGuigan C. Phosphoramidates of 2′-β-D-arabinouridine (AraU) as phosphate prodrugs; design, synthesis, in vitro activity and metabolism. Bioorg. Med. Chem. 2010, 18, 2439–2446. 10.1016/j.bmc.2010.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osgerby L.; Lai Y. C.; Thornton P. J.; Amalfitano J.; Le Duff C. S.; Jabeen I.; Kadri H.; Miccoli A.; Tucker J. H.; Muqit M. M.; Mehellou Y. Kinetin riboside and its ProTides activate the Parkinson’s disease associated PTEN-induced putative kinase 1 (PINK1) independent of mitochondrial depolarization. J. Med. Chem. 2017, 60, 3518–3524. 10.1021/acs.jmedchem.6b01897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C. H.; Lin X.; Barney R. J.; Shippy R. R.; Li J.; Vinogradova O.; Wiemer D. F.; Wiemer A. J. Synthesis of a phosphoantigen prodrug that potently activates Vγ9Vδ2 T-lymphocytes. Chem. Biol. 2014, 21, 945–954. 10.1016/j.chembiol.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Hecker S. J.; Erion M. D. Prodrugs of phosphates and phosphonates. J. Med. Chem. 2008, 51, 2328–2345. 10.1021/jm701260b. [DOI] [PubMed] [Google Scholar]
- Thornton P. J.; Kadri H.; Miccoli A.; Mehellou Y. Nucleoside phosphate and phosphonate prodrug clinical candidates. J. Med. Chem. 2016, 59, 10400–10410. 10.1021/acs.jmedchem.6b00523. [DOI] [PubMed] [Google Scholar]
- Pradere U.; Garnier-Amblard E. C.; Coats S. J.; Amblard F.; Schinazi R. F. Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem. Rev. 2014, 114, 9154–9218. 10.1021/cr5002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilcollins A. M.; Li J.; Hsiao C. H.; Wiemer A. J. HMBPP analog prodrugs bypass energy-dependent uptake to promote efficient BTN3A1-mediated malignant cell lysis by Vγ9Vδ2 T lymphocyte effectors. J. Immunol. 2016, 197, 419–428. 10.4049/jimmunol.1501833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippy R. R.; Lin X.; Agabiti S. S.; Li J.; Zangari B. M.; Foust B. J.; Poe M. M.; Hsiao C. C.; Vinogradova O.; Wiemer D. F.; Wiemer A. J. Phosphinophosphonates and their tris-pivaloyloxymethyl prodrugs reveal a negatively cooperative butyrophilin activation mechanism. J. Med. Chem. 2017, 60, 2373–2382. 10.1021/acs.jmedchem.6b00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust B. J.; Poe M. M.; Lentini N. A.; Hsiao C. C.; Wiemer A. J.; Wiemer D. F. Mixed aryl phosphonate prodrugs of a butyrophilin ligand. ACS Med. Chem. Lett. 2017, 8, 914–918. 10.1021/acsmedchemlett.7b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehellou Y.; Rattan H. S.; Balzarini J.. The ProTide prodrug technology: from the concept to the clinic. J. Med. Chem. [Online early access]. DOI: 10.1021/acs.jmedchem.7b00734. Published Online: August 9, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E. J.; He G. X.; Lee W. A. Metabolism of GS-7340, a novel phenyl monophosphoramidate intracellular prodrug of PMPA, in blood. Nucleosides, Nucleotides Nucleic Acids 2001, 20, 1091–1098. 10.1081/NCN-100002496. [DOI] [PubMed] [Google Scholar]
- Mehellou Y. The ProTides boom. ChemMedChem 2016, 11, 1114–1116. 10.1002/cmdc.201600156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A.; Hintz M.; Kletschek Y.; Kuhl T.; Haug C.; Engel R.; Moll J.; Ostrovsky D. N.; Jomaa H.; Eberl M. Replacing the pyrophosphate group of HMB-PP by a diphosphonate function abrogates its potential to activate human γδ T cells but does not lead to competitive antagonism. Bioorg. Med. Chem. Lett. 2003, 13, 1257–1260. 10.1016/S0960-894X(03)00138-0. [DOI] [PubMed] [Google Scholar]
- Corey E. J.; Venkateswarlu A. Protection of hydroxyl groups as tert-butyldimethylsilyl derivatives. J. Am. Chem. Soc. 1972, 94, 6190–6191. 10.1021/ja00772a043. [DOI] [Google Scholar]
- Davey M. S.; Lin C. Y.; Roberts G. W.; Heuston S.; Brown A. C.; Chess J. A.; Toleman M. A.; Gahan C. G.; Hill C.; Parish T.; Williams J. D.; Davies S. J.; Johnson D. W.; Topley N.; Moser B.; Eberl M. Human neutrophil clearance of bacterial pathogens triggers anti-microbial γδ T cell responses in early infection. PLoS Pathog. 2011, 7, e1002040. 10.1371/journal.ppat.1002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.