Significance Statement
Among adults with membranous nephropathy, about 3%–5% have autoantibodies directed against thrombospondin type 1 domain–containing 7A (THSD7A), a podocyte-expressed transmembrane protein. To better understand the effects of THSD7A autoantibodies in this disease, the authors investigated THSD7A’s temporal expression, spatial expression, and biological function in podocytes. They found that embryonic THSD7A expression begins on glomerular vascularization and slit diaphragm formation and that THSD7A and THSD7A autoantibodies localize to the slit diaphragm domain of foot processes. Expression of THSD7A in cultured human podocytes was associated with enhanced adhesion and decreased ability to migrate, suggesting that THSD7A may be involved in stabilizing the slit diaphragm and that autoantibodies to THSD7A might structurally and functionally alter the slit diaphragm’s permeability to protein.
Keywords: THSD7A, podocyte, membranous nephropathy, slit diaphragm
Visual Abstract
Abstract
Background
About 3%–5% of adults with membranous nephropathy have autoantibodies directed against thrombospondin type 1 domain–containing 7A (THSD7A), a podocyte-expressed transmembrane protein. However, the temporal and spatial expression of THSD7A and its biologic function for podocytes are unknown, information that is needed to understand the effects of THSD7A autoantibodies in this disease.
Methods
Using a variety of microscopic techniques, we analyzed THSD7A localization in postnatal, adult, and autoantibody-injected mice as well as in human podocytes. We also analyzed THSD7A function in human podocytes using confocal microscopy; Western blotting; and adhesion and migration assays.
Results
We found that THSD7A expression begins on glomerular vascularization with slit diaphragm formation in development. THSD7A localizes to the basal aspect of foot processes, closely following the meanders of the slit diaphragm in human and mice. Autoantibodies binding to THSD7A localize to the slit diaphragm. In human podocytes, THSD7A expression is accentuated at filopodia and thin arborized protrusions, an expression pattern associated with decreased membrane activity of cytoskeletal regulators. We also found that, phenotypically, THSD7A expression in human podocytes is associated not only with increases in cell size, enhanced adhesion, and reduced detachment from collagen type IV–coated plates but also, with decreased ability to migrate.
Conclusions
Our findings suggest that THSD7A functions as a foot process protein involved in the stabilization of the slit diaphragm of mature podocytes and that autoantibodies to THSD7A, on the basis of their localization, might structurally and functionally alter the slit diaphragm’s permeability to protein.
Glomerular epithelial cells (GECs), termed podocytes, are terminally differentiated cells of the glomerular filtration barrier. Podocytes participate in the formation of a three-layered size- and charge-selective filtration barrier by embracing the glomerular capillaries with their complex three-dimensional network of primary processes and foot processes (FPs). In between the fenestrated endothelial cells of the glomerular capillaries and the FPs of podocytes is the glomerular basement membrane (GBM).1 Podocyte FPs adhere to the glomerular filtration barrier via adhesion proteins, such as integrins.2 Specialized cell-cell contacts, termed slit diaphragms, connect interdigitating podocytes and are thought to form a flexible3 and mechanic-sensitive4,5 barrier to protein.
In membranous nephropathy (MN), patients develop autoantibodies, which bind to podocyte antigens and subsequently, injure podocytes. The result of this autoantibody binding to podocyte antigens is the development of a nephrotic syndrome, which is characterized by moderate to heavy proteinuria, edema, hypoalbuminemia, and hyperlipidemia. The morphologic hallmarks of MN are the subepithelial deposition of human IgG (mostly IgG4) and complement visible by immunohistochemistry and the occurrence of subepithelial electron dense deposits with podocyte FP effacement by electron microscopy.6 The name “membranous” nephropathy relates to the typical thickening of the GBM as a result of incorporation of electron dense deposits and new synthesis of GBM components.7,8 Three podocyte-expressed antigens have been identified in patients with MN so far: neutral endopeptidase in neonates and the phospholipase A2 receptor 1 and thrombospondin type 1 domain–containing 7A (THSD7A) in adults.9–11 THSD7A is a glycosylated 250-kD type 1 transmembrane protein composed of a large extracellular region, a single transmembrane domain, and a short intracellular tail.11 The overall domain structure of THSD7A is highly conserved in vertebrate evolution. It is found in all extant teleost and cartilaginous fish, including the elephant shark (Callorhinchus milii), the slowest evolving of all known vertebrates.12 THSD7A expression has been documented in human tumors,13 and an etiological link between THSD7A-expressing tumors and THSD7A autoantibody generation is suspected in MN.14 Studies performed in human umbilical vascular endothelial cells (HUVECs) and zebrafish suggest that THSD7A is involved in directed endothelial cell migration during embryonic angiogenesis.15,16 In the kidney, THSD7A expression has been shown in human,11 rodent,17,18 and zebrafish19 podocytes. Despite its high conservation and prominent expression in podocytes, the temporal and spatial expression of THSD7A is yet unknown. This knowledge would, however, provide insights into the potential function of THSD7A for normal podocyte biology and the effects that autoantibodies to THSD7A might exert in MN.
Methods
Generation and Culture of THSD7A-Expressing Podocytes
Murine immortalized podocytes (a gift from Peter Mundel, Boston, MA) were transfected with pCMV6 plasmid containing flag-tagged murine THSD7A full-length cDNA20 or an empty backbone vector for the generation of mock control cells using JetPEI (Polyplus-transfection). Stable cell lines were generated using G418 sulfate (Gibco) as a selection agent. Murine podocytes were cultured as described26 under permissive conditions: 32°C and 5% CO2; RPMI 1640 (Gibco) supplemented with 10% FCS (Gibco), 15 mmol/L HEPES Buffer solution (Gibco), 1 mmol/L sodium pyruvate (Gibco), 10 U/ml IFN-γ (PeproTech), and 0.5 mg/ml G418 sulfate (Gibco) in vented 25-mm2 tissue culture flasks for sensitive adherent cells (Sarstedt). For differentiation, podocytes were cultured for 13 days under nonpermissive conditions (38°C, 5% CO2; RPMI 1640 supplemented with 10% FCS, 15 mmol/L HEPES Buffer solution, 1 mmol/L sodiumpyruvate, and 0.5 mg/ml G418 sulfate). Cell density was kept below 80%–90% to allow for process development.
For generation of stable human THSD7A-expressing human podocytes, lentiviral particles expressing flag-tagged human full-length THSD7A or an empty backbone vector for the generation of mock control cells under EF1a promoter were produced by transfection of HEK293T cells using transfer plasmid LeGO-EF1α hTHSD7A-FLAG-iPur2 (derivative of Addgene plasmid 27341; LeGO-iG2 after exchanging SFFV promoter for EF1a promoter and GFP for puromycin acetyltransferase gene) and packaging plasmids psPAX2 (Addgene Plasmid 12260) and pMD2.G (Addgene Plasmid 12259). VSV-G–pseudotyped viral particles were concentrated by ultracentrifugation, resuspended in DPBS, and then, used to transduce human immortalized podocytes (a gift from Moin Saleem, University of Bristol). Hexadimethrine bromide (Polybrene; Sigma-Aldrich) was used to increase transduction efficiency. Finally, stable cell lines were generated by using puromycin (Sigma-Aldrich) as a selection agent. Human podocytes were cultured under permissive conditions: 32°C, 5% CO2; RPMI 1640 supplemented with 10% FCS, 1× insulin, transferrin selenium (ITS; PAN Biotech), and 1 μg/ml Puromycin (Sigma-Aldrich) in uncoated, vented tissue culture flasks (Sarstedt) as described.22 For differentiation, podocytes were cultured for 10 days under nonpermissive conditions (38°C, 5% CO2; RPMI 1640 supplemented with 10% FCS and 1× ITS) on either uncoated or coated (collagen type IV; Sigma-Aldrich), vented 25- or 75-mm2 tissue culture flasks; 100-mm tissue culture dishes for sensitive adherent cells; six-well tissue culture or 96-well High Binding ELISA plates (all Sarstedt); 35-mm2 glass-bottomed dishes (Ibidi); or collagen type IV–coated six-well silicone plates (Flexcell International Corporation). Cell density was kept below 80%–90% to allow for process development. DNA was extracted from cell pellets and controlled for the absence of mycoplasma infections on a 2- to 3-month basis according to the manufacturer’s instructions (Minerva Biolabs); cellular passages below 45 were used for experiments.
Glomerular Epithelial Cell Culture and Immunofluorescence
For the isolation of glomeruli, mice were perfused with Dynabeads (Dynal) as previously described.23,24 Decapsulated glomeruli were plated on collagen type IV–coated 10-cm culture plates (Biocoat) in RPMI 1640 supplemented with 10% FCS, 15 mmol/L HEPES, 1 mmol/L sodium pyruvate, 100 U/ml penicillin, and 100 mg/ml streptomycin at 38°C and 5% CO2. Four days after initial glomerular isolation, primary culture GECs were fixed with 4% PFA (EMScience) for 8 minutes at RT and processed for immunofluorescence analyses of THSD7A and nephrin expression. Primary antibodies used were human anti-THSD7A (1:40020) and guinea pig anti-nephrin (1:200; Acris). Staining was evaluated using an LSM510 meta confocal microscope (Zeiss) and processed with the LSM software (Zeiss).
Cultured Podocyte Immunofluorescence
For immunofluorescence analyses, human and murine podocytes were differentiated for 10 days on collagen type IV–coated silicone membranes (Flexcell International Corporation) and fixed with 37°C 4% PFA in PBS for 10 minutes at RT or ice-cold MeOH:acetone (1:1) at −20°C for 20 minutes. Unspecific binding was blocked with 5% horse serum (Vector) in 0.05% Triton X-100 (Sigma-Aldrich) in PBS for 30 minutes at RT. Primary antibodies were diluted in blocking buffer and incubated overnight at 4°C. Primary antibodies used were human anti-THSD7A (1:40020), rabbit anti-THSD7A (1:40019), mouse anti–α-tubulin (1:500; Sigma-Aldrich), globular actin (G-actin; 1:100; deoxyribonuclease I, AF488; Molecular Probes), rhodamin-wheat germ agglutinin (1:400; Vector), rabbit anti-phospho (Y118) paxillin (1:200; Life Technologies), rabbit anti–β1-integrin (1:400; GenTex), goat anti–β3-integrin (1:200; R&D Systems), rabbit anti-phospho (Ser19) myosin light chain (MLC; (1:50; Cell Signaling), or AF568- or AF647-Phalloidin (all 1:400; Molecular Probes). Stainings were evaluated using an LSM510 meta confocal microscope or an ELYRA super-resolution microscope and processed with LSM or Zen Blue software (all Zeiss). The ratio of G-actin to filamentous actin (F-actin) and the mean intensity of fluorescence of p-paxillin and p-MLC per podocyte were measured using FIJI. For G-actin to F-actin ratio, p-MLC, and p-paxillin mean intensity of fluorescence, all podocytes seen on ten micrographs per condition at ×20 or ×100 magnification in two to four independent experiments were analyzed in a blinded manner.
Immunofluorescence of Paraffin Sections
Three-micrometer-thick paraffin-embedded kidney sections of Balb/C adult, C57BL/6 adult, Balb/C postnatal day 5, Balb/C mice injected with patient serum containing anti-THSD7A autoantibodies or control human serum as described,20 or Balb/C mice injected with purified rabbit anti-THSD7A containing IgG or control rabbit IgG as described19 were used for staining. The detailed protocol of rabbit anti-THSD7A antibody generation is in the work by Tomas et al.19 Paraffin sections were deparaffinized and rehydrated to water. Antigen retrieval was performed using citrate buffer (pH 6.1), antigen retrieval buffer (pH 9; DAKO) for 30 minutes at 98°C in a steam cooker, or protease 24 digestion (5 μg/ml; Sigma-Aldrich) for 15 minutes at 37°C. Unspecific binding was blocked with 5% horse serum in 0.05% Triton X-100 (Sigma-Aldrich) in PBS for 30 minutes at RT. Primary antibodies were diluted in blocking buffer and incubated overnight at 4°C. Primary antibodies used were goat anti-THSD7A (1:200; Santa Cruz), rabbit anti-THSD7A (1:200; Atlas; Sigma-Aldrich), guinea pig anti-nephrin (1:200), rabbit anti–β1-integrin (1:400), goat anti-collagen type IV (1:400; Southern Biotechnologies), Cy2-donkey anti-human IgG (1:200; Jackson Immunoresearch Laboratories), Cy2-donkey anti-rabbit IgG (1:200; Jackson Immunoresearch Laboratories), and FITC-Lotus Tetragonolobus lectin (1:400; FITC-LTA; Vector). Secondary antibody incubation was performed for 30 minutes at RT diluted in blocking buffer. Secondary antibodies used were donkey anti-goat AF647, donkey anti-rabbit AF488, and donkey anti-guinea pig AF586 for confocal microscopy (all 1:400; Jackson Immunoresearch Laboratories) or AF594 rabbit anti-guinea pig (1:100; Cohesion Biosciences) and Star Red goat anti-rabbit (1:100; Abberior). DNA was visualized using Hoechst (1:1000; Molecular Probes) or DRAQ5 (1:1000; Molecular Probes). Stainings were visualized using an LSM510 (Zeiss) for conventional confocal microscopy, an LSM800 with Airyscan (Zeiss) for high-resolution confocal microscopy, an ELYRA microscope (Zeiss) for SR-SIM microscopy, or a four-channel stimulated emission depletion (STED) microscope (Abberior) and processed using the LSM (Zeiss), ZEN Blue software (Zeiss), FIJI software, or Bitplane Imaris 3D software.
Immunogold Electron Microscopy
For fixation for immunogold labeling, the fixation procedure and preparation of Bouin solution for the immunogold staining were modified according to Somogyi and Takagi.25 Animals were transcardially perfused with 0.1 M phosphate buffer (PB; pH 7.4) at 4°C. The fixative consisted of 500 ml 0.2 M PB (pH 7.4), 150 ml saturated picric acid (Bouin solution: 0.044 M picric acid, 5% [vol/vol] acidic acid, and 10% [vol/vol] formaldehyde in aqua dest.), 346.8 ml of 4% paraformaldehyde solution, and 3.2 ml of 25% glutaraldehyde at a final pH 7.4. The final concentrations were 4% paraformaldehyde, 0.08% glutaraldehyde, and 0.15% picric acid. Kidneys were removed after the perfusion procedure and postfixed in 100 ml 0.1 M PB including 4% paraformaldehyde and 0.15% picric acid for 24 hours at 4°C. Vibratome sections (LEICA VT 1000S) of fixed tissue were made with a thickness of 60 μm of areas of interest, and the slices were placed into cryoprotection solution containing 0.1 M PB (pH 7.4), 30% sucrose, 1% polyvinylpyrrolidone, and 30% ethylenglycol. For the pre-embedding immunogold protocol, to remove cryoprotection solution, the 60-μm thin slices were washed twice in 0.1 M sodium PB (pH 7.4; PBS) for 20 minutes followed by washing of the slices in 0.1 M PBS with 10% sucrose for 30 minutes and 0.1 M PBS with 20% sucrose for another 30 minutes. Tissue was transferred to a drop of PBS with 20% sucrose and frozen briefly with nitrogen, and then, it was thawed to room temperature. Subsequently, the tissue was washed three times in PBS (pH 7.4) for 10 minutes. Blocking reagent solution (BRS) was prepared with 100 ml PBS, 800 mg BSA (A7638–5G; Sigma-Aldrich), 250 μl 40% cold water fish skin gelatin (CWFSG 900.033; AURION), and 162.5 mg sodium acid. To increase the specificity of huIgG reaction, BRS supplemented with 2% normal goat serum (Sigma-Aldrich) was used for 1 hour for the blocking reaction. The 1.4 nm Nanogold-Fab goat anti-rabbit IgG (H+L; Nanoprobes) or the 12 nm Gold-AffiniPure goat anti-human IgG (H+L) antibody (Jackson ImmunoResearch) was solved in BRS overnight at 4°C. After 24 hours, the tissue was washed three times in PBS (pH 7.4) for 5 minutes each followed by 1% glutaraldehyde solution in 0.1 M PB for 10 minutes. The tissue was washed in distilled water three times followed by incubation with HQ Silver Enhancement solution (Nanoprobes) for 5 minutes. The tissue was solved in 1% OsO4 in distilled water at 4°C for 10 minutes followed by dehydration in an ethanol series. Subsequently, the probes were transferred twice into propylene oxide for 5 minutes and embedded in a mixture of epon and propylene oxide (4:1) overnight. Tissue samples were cut with an ultrathin (Reichert Jung Ultracut) microtome, and thin sections were inspected in Phillips CM 100 and Zeiss EM 910 electron microscopes. Pictures were taken assisted by iTEM 5.2 software (Quemesa; Olympus) as well as ImageSP 1.2.8.111 by TRS (Slow-scan CCD Camera; TRS).
Adhesion Assay/Detachment Assay
For adhesion assays, 4×105 human podocytes per 75-mm2 uncoated tissue culture flask were cultured for 10 days under nonpermissive conditions. To avoid extracellular protein domains being cleaved off, cells were detached for 5 minutes using 10 mM EDTA (stock solution sterilely filtered; Sigma-Aldrich) in fresh medium and knocking against the flask three times after every minute. The cell suspension was centrifuged at 1800 rpm and 4°C for 5 minutes, washed with DPBS (Gibco), and centrifuged again as described. Cell pellets were resuspended in fresh medium, and 5×103 cells per well were plated onto collagen type IV–coated High Binding 96-well ELISA plates. After 30, 60, and 180 minutes of cultivation under nonpermissive conditions, the medium of eight wells per point of time was changed. Finally, after 360 minutes, all cells were fixed with ice-cold 100% methanol at −20°C for 20 minutes.
For detachment assays, 6×103 human podocytes per well were cultured on a collagen type IV–coated High Binding 96-well ELISA plates for 10 days under nonpermissive conditions. Eight wells per time point were then treated with 10 mM EDTA in fresh medium for 2.5, 5, 7.5, or 10 minutes. After each time point, the plate was carefully moved up and down three times on the working surface, and EDTA medium of the corresponding condition was replaced by normal medium. Last, all cells were fixed with ice-cold 100% methanol at −20°C for 20 minutes.
To analyze the cell quantity in both assays, unspecific binding was blocked with 5% horse serum in 0.05% Triton X-100 in PBS for 30 minutes at RT, and cells were incubated with mouse anti–β-actin (1:400; Sigma-Aldrich) in blocking buffer o/n at 4°C. Next, podocytes were incubated with horseradish peroxidase (HRP)–conjugated goat anti-mouse IgG (1:10,000; Jackson ImmunoResearch Laboratories) in blocking buffer for 1 hour at RT. Samples were washed three times for 5 minutes in PBS after incubation with primary or secondary antibodies. Finally, a standard TMB ELISA measurement at 450 nm was performed using 5.7% phosphoric acid (Sigma-Aldrich; TMB Aviva Systems Biology) as a stop solution.
Migration Assay
In total, 4–6×104 human podocytes per well were plated onto collagen type IV–coated six-well tissue culture plates. Cells were cultured under permissive conditions until 90% confluence was reached. After 10 days of differentiation under nonpermissive conditions, the medium was removed, and two scratches per well were made using 1000-μl pipette tips (Sarstedt). Controls were immediately fixed with 37°C 4% PFA in DPBS for 10 minutes at RT. After rinsing with medium followed by a medium change, samples were cultured until differences in migration were seen under nonpermissive conditions and then, fixed as described above. Phase contrast images were taken with a Zeiss Axio Observer.A1 microscope and analyzed with AxioVision software (Zeiss). All scratches seen on four micrographs per condition at a ×2.5 magnification in three independent experiments were analyzed in a blinded manner.
Immunoblotting
Protein samples (10–15 μg) of 3×105 lysed cultured murine or human podocytes were prepared for Western blot analysis by addition of 5× Laemmli buffer (1.5 M Tris-HCl [pH 6.8], 50% glycerol, 10% SDS, and 1% bromophenol blue) and subsequent heating to 95°C for 10 minutes. If reducing conditions were desired, 20% β-mercaptoethanol was added to the 5× loading buffer. Proteins were separated by electrophoresis in precast gradient gels (4%–15%; Bio-Rad) containing SDS in an electrophoresis chamber (Bio-Rad) in the presence of a migration buffer (25 mM Tris, 192 mM glycine, and 0.1% SDS; Amresco). Proteins were then transferred to methanol-soaked PVDF membranes (Millipore) under semidry conditions in the presence of 25 mM Tris (pH 8.5), 192 mM glycine, and ethanol 20% using Transblot Turbo (Bio-Rad) at 25 V constant for 35 minutes. Membranes were blocked for 2 hours at RT with 5% powdered milk in PBS and then, incubated with primary antibodies overnight at 4°C and secondary antibodies for 2 hours at RT. Primary antibodies were diluted in SuperBlock Blocking Buffer (Thermo Scientific) if not otherwise specified, and HRP-conjugated secondary antibodies were diluted in PBS alone. Membranes were washed three times for 5 minutes in PBS-Tween 0.05% after incubation with primary or secondary antibodies. Primary antibodies used were rabbit anti-m/hTHSD7A (1:1000 in 0.5% powdered milk in PBS19), mouse anti–β-actin (1:3000), rabbit anti–α-actinin-4 (1:1000; Immunoglobe), rat anti–UCH-L1 (1:250 in 3% powdered milk in PBS26), rabbit anti-LMP7 (β5i; 1:1000; Abcam), rabbit anti-β5 (1:5000; a gift from Xuejun Wang, University of South Dakota), rabbit anti-LC3B (1:5000; Sigma-Aldrich), rabbit anti-Limp2 (1:2000; a gift from Paul Saftig, Kiel, Germany), and rabbit anti-Lamp2 (1:1000; Sigma-Aldrich). Secondary antibodies were HRP-conjugated goat anti-mouse IgG (1:20,000), goat anti-rabbit IgG (1:10,000), and rabbit anti-rat IgG + IgM (1:15,000; all Jackson ImmunoResearch Laboratories). Pictures were developed with an Amersham Imager 600 (GE) using SuperSignal West Substrates (Pico and Femto; Thermo Scientific) and analyzed with FIJI.
Statistical Analyses
Data are expressed as median or mean±SEM. For statistical analyses, we performed the nonparametric Mann–Whitney U test to enable robust conclusions on effect significance in case of departures from normality associated with small sample sizes or the two-tailed t test in parametric datasets. For comparison of more than two conditions, nonparametric one-way ANOVA was performed with Dunnett or Bonferroni multiple comparisons post-test. Replicates used were biologic replicates, which were measured using different samples derived from distinct culture plates. All culture plates were blindly assigned to the experimental groups.
Results
THSD7A Expression Is a Characteristic of Mature Podocytes
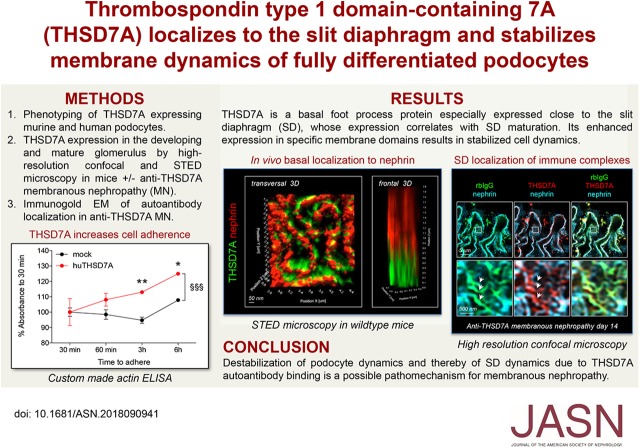
THSD7A is one of the most abundant proteins of the murine glomerular proteome and has recently come into focus of attention due to its involvement in MN development in a small subset of patients. In the developing murine kidney, THSD7A expression becomes apparent in late stages of glomerular/podocyte development (Figure 1A). Although nephrin expression has been reported to start around E18.527 and can be appreciated in the comma and S-shaped bodies in a mostly vesicular intracellular pattern, THSD7A coexpression in podocytes starts with glomerular vascularization during the capillary loop stage and is fully visible in podocytes of mature glomeruli. In line, primary culture GECs lose THSD7A expression with the distance migrated away from the seeded decapsulated glomerular tufts as shown after 4 days of culture on collagen type IV–coated plates (Figure 1B, Supplemental Figure 1). Of note, nephrin immunoreactivity is preserved in GECs that have migrated away from the glomerular tuft, suggesting that a downregulation of THSD7A is required for GEC migration from the tuft. In line, THSD7A immunoreactivity is prominent in the remaining cells within the glomerular tuft, with only faint membranous and cell process expression of THSD7A remaining in cells having explanted farther from the glomerular tuft. Analysis of THSD7A levels in immortalized cultured murine podocytes supported the observation that THSD7A expression was a feature of mature podocytes. Thereby, Western blot analyses exhibited a faint THSD7A expression on differentiation, which however, did not corroborate with a membrane expression of THSD7A protein by immunofluorescence (Figure 1, C and D). Re-establishment of THSD7A protein expression by stable transfection of murine podocytes with murine THSD7A resulted in a prominent membranous expression pattern of THSD7A. Podocytes exhibited elaborate membrane morphology with fine membrane protrusions and filopodia in contrast to mock-transfected control podocytes (Figure 1D).
Figure 1.
Thrombospondin type 1 domain–containing 7A (THSD7A) expression is a characteristic of mature podocytes. (A) Representative confocal micrographs of THSD7A (green) expression in the postnatal day 5 kidney of a Balb/C mouse using the goat anti-THSD7A antibody. Nephrin (red) was used to visualize premature to mature podocytes, Lotus tetragonolobus lectin (LTA; light blue) marks the glycocalyx of proximal tubuli brush border, DNA is in blue. (B) Decapsulated murine glomeruli were isolated and plated onto collagen type IV–coated plates for 4 days. Primary glomerular epithelial cells (GECs) that have grown out of the tuft were stained for THSD7A (green) and nephrin (red). The asterisk labels the glomerular tuft from which the GECs have grown out. Note the decrease in THSD7A expression in cells that have migrated farther away from the glomerular tuft (arrows), whereas nephrin immunoreactivity is maintained. (C) Western blot analyses of murine podocytes cultured under permissive (P; 32°C) and nonpermissive (NP; 38°C) conditions against murine THSD7A. β-actin of the same samples run under reducing WB conditions was used to control for equal loading. The graph exhibits densitometric analyses of three independent experiments; values are expressed as median, and they are calculated as percentage change to permissive mock levels (P-mock). ***P<0.001 (one-way ANOVA, Dunnett multiple comparison test). (D) Murine immortalized podocytes were transduced with murine thrombospondin type 1 domain–containing 7A (mTHSD7A) or control transduced (mock) with empty vector. Representative confocal micrographs of THSD7A (green) expression in relation to filamentous actin (F-actin; red) are shown. Note the membranous expression of THSD7A, which is also in thin actin free processes (arrows).
THSD7A Localizes to Podocyte Foot Processes in Close Proximity to the Slit Diaphragm
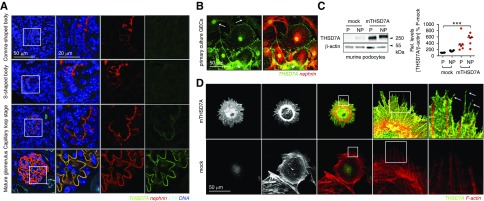
THSD7A was found to localize to podocyte FPs by confocal microscopy17 and immunogold electron microscopy.18 Knowledge of the precise foot process localization of proteins can give hints towards their function. A localization at the basal domain of FP can indicate an involvement in FP adhesion to the GBM, or a localization at the slit diaphragm an involvement in filtration. We therefore thought at precisely localizing THSDA using high resolution confocal microscopy and STED microscopy. From its extracellular domain structure, THSD7A contains a series of 21 thrombospondin type 1 domains, which can be further classified as either thrombospondin 1 (THBS1) like or complement component 6 (C6)-like.28 THBS1 itself acts as an adhesive glycoprotein that can interact with components of the extracellular matrix29–31 among others. Furthermore, on the basis of a homology model, the extracellular domain of THSD7A is predicted to bind heparan sulfates.32 Therefore, we suspected that THSD7A would be expressed at FP domains in which podocyte adhesion proteins, such as integrins, are found. High-resolution confocal microscopy in paraffin samples of naïve C57BL/6 wild-type mice demonstrated that THSD7A (when stained with a commercial goat anti-THSD7A antibody) was indeed expressed at the basal aspect of FP at the level of β1-integrin (Supplemental Figure 2) below the slit diaphragm spanning protein nephrin in the frontal confocal plane (Supplemental Figure 3). However, to our surprise, in samples stained for THSD7A, β1-integrin, and nephrin simultaneously, THSD7A localization was closer to nephrin than to β1-integrin–containing FP domains in the frontal and especially, transversal confocal planes (Figure 2A). Indeed, THSD7A was found to form a meandering staining pattern very similar to the one observed and described before for nephrin33 when the glomerular filtration barrier was imaged in the transversal plane. To further enhance resolution, we, therefore, performed STED microscopy of murine and human kidney samples stained for THSD7A and nephrin using a commercial rabbit anti-THSD7A antibody (Figure 2, Supplemental Figure 4). Again, rather than localizing where cell adhesion proteins are to be found, namely in between the meanders formed by nephrin at the slit diaphragm, the expression pattern of THSD7A was in a comparable meander pattern to nephrin in both mouse and human glomeruli. In some areas, nephrin and THSD7A expression was within one plane, suggesting specific areas of close proximity of both proteins at the slit diaphragm (Figure 2, B and D). Thereby, 3D reconstructions of STED Z stacks demonstrated that THSD7A localized basally to nephrin at the frontal and transversal levels of the slit diaphragm (Figure 2, C and E), a localization that is schematized in Figure 2F.
Figure 2.
Thrombospondin type 1 domain–containing 7A (THSD7A) localizes to podocyte foot process domains close to the slit diaphragm. (A) Three-micrometer-thick paraffin sections of a naive C57BL/6 mouse were stained for THSD7A (green), β1-integrin (red), and nephrin (light blue). The glomerular filtration barrier (GFB) is visualized in the enlarged boxes in the frontal plane or the transversal plane as indicated. Note the localization of THSD7A side by side in an alternating pattern with β1-integrin (white arrows) at the basal aspect of foot processes (FPs). Also note the localization of THSD7A basally of nephrin in the frontal plane (red arrows) but in the same FP domain when visualized in the transversal plane. c, capillary side of GFB; u, urinary side of GFB. (B) Z stacks of THSD7A (green) and nephrin (red) localization visualized by stimulated emission depletion (STED) microscopy of a naive C57BL/6 mouse paraffin section. (C) A 3D reconstruction of THSD7A (green) and nephrin (red) localization in the same FP domain of the slit diaphragm but with THSD7A located basally from nephrin as visualized by STED microscopy of a naive C57BL/6 mouse paraffin section. (D) Z stacks of THSD7A (green) and nephrin (red) localization visualized by STED microscopy of a human frozen section derived from the healthy part of a tumor nephrectomy. (E) A 3D reconstruction of THSD7A (green) and nephrin (red) localization in the same FP domain of the slit diaphragm but with THSD7A located basally from nephrin as visualized by STED microscopy of a human frozen section derived from the healthy part of a tumor nephrectomy. (F) A 3D scheme of GFB indicating the localization of the frontal versus the transversal optical plane (lower left panel) and the proposed localization of THSD7A in relation to β1-integrin and nephrin.
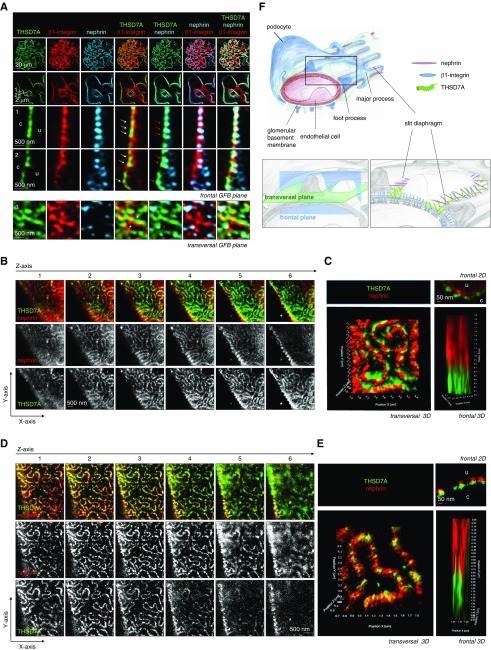
Because our data suggest THSD7A localization in close conjunction to the slit diaphragm, we next evaluated whether the autoantibodies targeting THSD7A were also found in the curvilinear network representing the slit diaphragm. Therefore, we analyzed the localization of bound rabbit (on day 14) or human anti-THSD7A autoantibodies (on week 18) after injection into Balb/C in relation to the antigen by immunofluorescence (Figure 3, Supplemental Figures 5 and 6). Interestingly, the human IgG-THSD7A antigen/antibody complexes were still persistent 18 weeks after a single injection of human anti-THSD7A IgG–containing serum at the subepithelial space in relation to the GBM protein collagen type IV (Supplemental Figure 5). Human IgG staining appeared granular (as expected for MN) when the glomerular filtration barrier was imaged in the frontal plane. In areas in which the glomerular filtration barrier was visualized transversally, a meandering pattern of human IgG deposition was appreciated (Supplemental Figure 5A). Similar observations were made for bound rabbit IgG in relation to collagen type IV in mice 14 days after injection of rabbit anti-THSD7A antibodies (Supplemental Figure 5B), suggesting a preferential THSD7A/autoantibody localization at the slit diaphragm domain of podocyte FPs as an early and persistent event in subepithelial immune deposit formation in MN. Mice injected with control rabbit IgG or control human serum exhibited mesangial IgG deposition but no subepithelial IgG deposition (Supplemental Figure 6). High-resolution confocal microscopy of paraffin sections simultaneously stained for rabbit IgG (Figure 3A) or human IgG (Figure 3B) in conjunction with THSD7A, and nephrin showed a specific localization of IgG and THSD7A in FP domains containing nephrin in the transversal plane. THSD7A staining intensity was slightly decreased in areas of additional IgG binding, a finding possibly related to steric interference of the injected IgG with the detecting IgG for the same antigen. These findings were confirmed by immunogold electron microscopy against rabbit IgG and human IgG (Figure 3, C and D, Supplemental Figures 7 and 8). Thereby, 1 day after injection into Balb/C mice, rabbit IgG was detected in the subepithelial space with an accentuated localization at the podocyte FP membrane domain underlying the slit diaphragm in mice injected with anti-THSD7A antibody–positive rabbit IgG and was not detected in mice receiving control rabbit IgG (Figure 3C, Supplemental Figure 7); 18 weeks after injection into a Balb/C mouse, human IgG of anti-THSD7A autoantibody-containing patient serum was still bound to the podocyte FP domains underlying the slit diaphragm. Of note, human IgG was also found bound to the more apical podocyte FP membrane domain overlying the slit diaphragm, suggesting a displacement of THSD7A/anti-THSD7A human IgG at the FP membrane after prolonged autoantibody binding to THSD7A (Figure 3D, Supplemental Figure 8).
Figure 3.
Injected antibodies to thrombospondin type 1 domain–containing 7A (THSD7A) localize to the slit diaphragm. (A) A Balb/C mouse was injected once with 150 μl purified rabbit anti-THSD7A IgG, and kidneys were harvested on day 14 after injection. The 3-μm paraffin sections were stained for rabbit IgG (green), THSD7A (red), and nephrin (light blue). The arrows exemplarily depict areas of rabbit IgG/THSD7A localization at the nephrin-positive slit diaphragm. (B) A Balb/C mouse was injected once with 600 μl human anti-THSD7A–positive serum, and kidneys were harvested on week 18 after injection; 3-μm paraffin sections were stained for human IgG (green), THSD7A (red), and nephrin (light blue). The arrows exemplarily depict areas of human IgG/THSD7A localization at the nephrin-positive slit diaphragm. GFB, glomerular filtration barrier. (C) Balb/c mice were injected once with 180 μl purified rabbit IgG with and without specific anti-THSD7A antibodies. One day later, bound rabbit IgG was specifically localized to podocyte foot processes (FPs) by immunogold electron microscopy (EM) only in the mouse treated with anti-THSD7A–positive serum. Note the preferential localization of rabbit IgG at the FP domain underlying the slit diaphragm (red arrows). (D) Balb/C mice were injected once with 600 μl human serum of a control patient or a patient with THSD7A-associated membranous nephropathy; 18 weeks later, bound human IgG was localized to podocyte FP (arrows) by immunogold EM in the mouse treated with anti-THSD7A–positive serum only. Note the prominent binding of human IgG to the podocyte FP domain neighboring the slit diaphragm (red arrows). Prominent human IgG binding is also present in more apical FP membrane domains (black arrows).
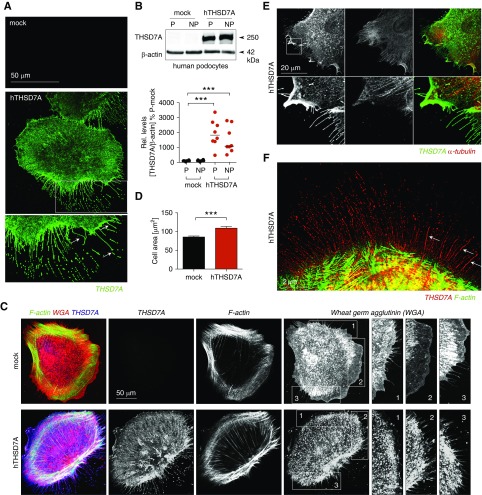
THSD7A Localizes to Thin Arborized Protrusions and Filopodia in Human Podocytes
To investigate the biologic function of THSD7A in podocytes, we generated a human immortalized podocyte cell line that stably expressed human THSD7A (Figure 4, A and B), because human podocytes in culture normally lose their THSD7A expression. We first established that hTHSD7A expression did not stress human podocytes, because activation levels of the ubiquitin proteasomal or autophagosomal lysosomal degradation system were comparable with those of mock-transfected control cells (Supplemental Figure 9). Similar to mTHSD7A expression in murine podocytes, hTHSD7A expression was prominent at the cell membrane, and human podocytes exhibited an elaborate morphology with fine membrane protrusions in comparison with mock-transfected control cells (Figure 4A). Compared with mock-transfected control podocytes, hTHSD7A-expressing podocytes also exhibited abundant fine membrane protrusions on surface membrane labeling with the lectin wheat germ agglutinin (Figure 4C) and were larger than mock control cells (Figure 4D, Supplemental Figure 10). Thorough analyses of the membrane localization of hTHSD7A demonstrated that hTHSD7A expression was accentuated at α-tubulin–containing filopodia and in thin α-tubulin–free membrane processes (Figure 4E). Structured illumination (SR-SIM) high-resolution microscopy demonstrated that the THSD7A-expressing thin membrane processes, so-called thin arborized protrusions (TAPs), were also free of F-actin (Figure 4F). Because the morphology of hTHSD7A-expressing podocytes appeared elaborate, we assessed whether the expression pattern of the two typical podocyte-specific proteins podocin and α-actinin-4 differed (Supplemental Figures 11 and 12). Both proteins exhibited comparable cellular levels and distribution in hTHSD7A-expressing podocytes and mock control cells.
Figure 4.
Thrombospondin type 1 domain–containing 7A (THSD7A) localizes to thin actin free processes and filopodia in human podocytes. (A) Representative confocal images of THSD7A (green) immunofluorescence in differentiated human podocytes transduced with human thrombospondin type 1 domain–containing 7A (hTHSD7A). Note the THSD7A expression in long thin processes (arrows). Mock-transduced cells show no immunofluorescence. (B) Western blot analyses of human podocytes cultured under permissive (P; 32°C) and nonpermissive (NP; 38°C) conditions against THSD7A under native conditions. β-actin of the same membrane was used to control for equal loading. Graphs exhibit densitometric analyses of five independent experiments with one to two biologic replicates; values are expressed as median, and they are calculated as percentage change to permissive mock levels (P-mock). ***P<0.001 (one-way ANOVA, Dunnett multiple comparison test). (C) Representative confocal images of the wheat germ agglutinin (WGA; red)–labeled membrane surface of differentiated mock or hTHSD7A-transduced human podocytes. Note the scarce number of WGA-labeled thin membrane protrusions in the mock podocyte; THSD7A is in blue, and filamentous actin (F-actin) is in green. (D) Measurement of podocyte cell area using ImageJ. Values are expressed as mean±SEM. ***P<0.001 (two-tailed t test). (E) Representative confocal images of THSD7A (green) and α-tubulin (red) immunofluorescence in differentiated human podocytes transduced with hTHSD7A. Note the prominent THSD7A expression in α-tubulin–containing filopodia and thin α-tubulin–free processes. (F) Structured illumination (SR-SIM) high-resolution microscopy exhibits thin α-tubulin–free THSD7A (red)-expressing processes that are also F-actin (green) free.
THSD7A Expression in Podocytes Dampens Activity of Cytoskeletal Regulatory Proteins in Differentiated Cultured Human Podocytes
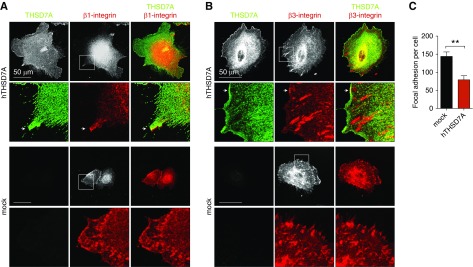
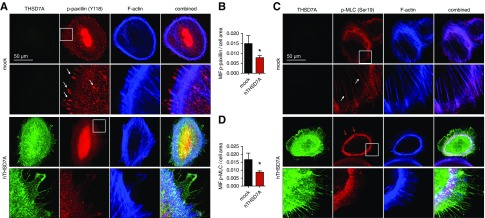
In human umbilical vein endothelial cells, a strict colocalization of THSD7A with αv/β3-integrin and paxillin at focal adhesions has been described,16 and soluble THSD7A fragments have been implied in regulating focal adhesion assembly.34 Thus, we carefully assessed whether THSD7A colocalized with focal adhesions in human podocytes. Immunofluorescence staining for β1- and β3-integrin, however, failed to exhibit a marked colocalization of THSD7A with β1- and β3-integrin at focal adhesions in human podocytes. A slight colocalization of THSD7A with β1- or β3-integrin was only apparent at the leading membrane domain of filopodia (Figure 5, A and B). Of note, THSD7A-expressing human podocytes exhibited fewer focal adhesions per cell than mock-transduced control cells (Figure 5B), suggesting decreased membrane dynamics. In line, TAPs are thought to be a special form of filopodia, which indicate slower membrane dynamics35 in podocytes. Because THSD7A strongly localized to TAPs and THSD7A-expressing podocytes exhibited fewer focal adhesions than mock control cells, we assessed whether human podocytes overexpressing hTHSD7A showed signs of altered activation of proteins involved in cytoskeletal regulation at the membrane. We first assessed the phosphorylation status of paxillin, which is involved in integrin-mediated cytoskeletal reorganization. The amount of phospho-paxillin-positive focal adhesions was strongly decreased in hTHSD7A-expressing podocytes (Figure 6, A and B). Concordantly, levels of phosphorylated MLC were also decreased at actin fibers of hTHSD7A-expressing podocytes (Figure 6, C and D). MLC is required for the formation of crossbridges to actin for contraction. To assess whether hTHSD7A expression altered actin dynamics in podocytes, we assessed the ratio of G-actin to F-actin. However, no significant difference in the G-actin–to–F-actin ratio was detected (Supplemental Figure 13). Taken together, these data suggest that hTHSD7A stabilizes/slows membrane dynamics of human podocytes.
Figure 5.
Thrombospondin type 1 domain–containing 7A (THSD7A) does not colocalize to focal adhesions in human podocytes. (A) Representative confocal micrographs of THSD7A (green) and β1-integrin (red) in human podocytes. The arrows point toward a slight colocalization of THSD7A with β1-integrin at the leading edge of a filopodium. (B) Representative confocal micrographs of THSD7A (green) and β3-integrin (red) in human podocytes. Note the absence of THSD7A localization at β3-integrin–positive focal adhesions. The arrows point toward a slight colocalization of THSD7A with β3-integrin at the leading edge of a filopodium. (C) Quantification of β3-integrin containing focal adhesions per cell. Values are expressed as mean±SEM of ten cells per condition of three representative experiments. hTHSD7A, human thrombospondin type 1 domain–containing 7A. **P<0.01 (Mann–Whitney U test).
Figure 6.
Thrombospondin type 1 domain–containing 7A (THSD7A) expression in podocytes dampens activity of cytoskeletal regulatory proteins in differentiated cultured human podocytes. (A) Representative confocal micrographs of THSD7A (green), the activated (Y118-phosphorylated) form of the focal adhesion protein paxillin (which is involved in integrin-mediated cytoskeletal reorganization), and filamentous actin (F-actin; blue). Note the prominent localization of p-paxillin at focal adhesions (arrows) in the mock-transduced control podocyte, which is nearly absent in the THSD7A-expressing podocyte. (B) Quantification of mean intensity of fluorescence (MIF) of p-paxillin per cell normalized to cell area in n=10 cells of three independent experiments; values are depicted as the mean±SEM. *P<0.05 (unpaired t test). (C) Representative confocal micrographs of THSD7A (green), the activated (Ser19-phosphorylated) form of myosin light chain (MLC; which is required for the formation of crossbridges to actin for contraction), and F-actin (blue). Note the presence of p-MLC at actin fibers in the mock podocyte (arrows) and the prominent cortical p-MLC ring (red arrows) in the THSD7A-expressing podocyte. (D) Quantification of MIF of p-MLC per cell normalized to cell area in n=5–8 cells of three pooled independent experiments; values are depicted as the mean±SEM. hTHSD7A, human thrombospondin type 1 domain–containing 7A. *P<0.05 (unpaired t test).
THSD7A Expression Augments Podocyte Adhesion and Slows Motility
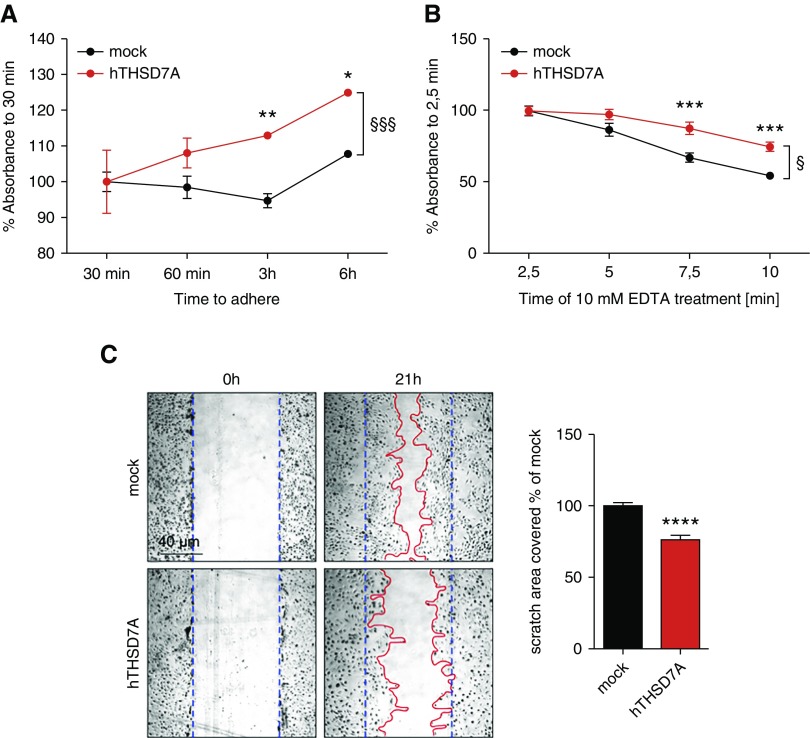
Because THSD7A is expressed in close proximity to the FP slit diaphragm of mature podocytes and because THSD7A expression in culture promotes an increase in cell size as well as the formation of TAPs with decreased activity of cytoskeletal regulators, we investigated whether THSD7A altered adhesive and migratory properties of podocytes in culture. Human THSD7A-expressing podocytes exhibited enhanced adhesion to collagen type IV–coated plates when replated after detachment from the original culture flask with 10 mM EDTA (Figure 7A). Detachment of cells with trypsin was avoided, because extracellular domains of membrane proteins, such as THSD7A, would have been cleaved. Further corroborating this finding, detachment of differentiated hTHSD7A-expressing podocytes from collagen type IV–coated plates was also slowed under 10 mM EDTA treatment in comparison with mock control cells (Figure 7B). Of note, the measured differences in adhesive properties were, albeit significant, mild in comparison with mock-transfected control cells. Next, we assessed whether the capability to migrate was altered with THSD7A expression. Indeed, performing the classic scratch (wound) assay revealed a slower closure of the scratch if podocytes expressed hTHSD7A in comparison with mock-transfected control cells (Figure 7C).
Figure 7.
Thrombospondin type 1 domain–containing 7A (THSD7A) expression augments podocyte adhesion and slows motility. (A) Adhesion assay of THSD7A-expressing human podocytes in comparison with mock-transduced control cells to collagen type IV–coated 96-well plates. Adhering cells were quantified by a custom-made ELISA to β-actin. Values were calculated as percentage absorbance to the 30-minute timepoint, and they are depicted as the mean±SEM of pooled data from seven independent experiments with eight biologic replicates. *P>0.05 (between the time courses; two-way ANOVA, Bonferroni multiple comparison); **P>0.01 (between the time courses; two-way ANOVA, Bonferroni multiple comparison); §§P<0.01 (between the time courses; two-way ANOVA, Bonferroni multiple comparison). (B) Detachment assay of THSD7A-expressing human podocytes in comparison with mock-transduced control cells differentiated for 10 days on collagen type IV–coated 96-well plates. Podocyte detachment was obtained by incubation with 10 mM EDTA and quantified by a custom-made ELISA to β-actin. Values were calculated as percentage absorbance to 2.5 minutes of EDTA incubation (at which time no cell detachment has occurred yet), and they are depicted as the mean±SEM of pooled data from five independent experiments with eight biologic replicates. ***P<0.001 (between the time courses; two-way ANOVA, Bonferroni multiple comparison); §P<0.01 (between the time courses; two-way ANOVA, Bonferroni multiple comparison). (C) Migration assay of THSD7A-expressing and mock control human podocytes differentiated on collagen type IV–coated six-well plates for 10 days after the placement of a scratch at 0 hours (demarcated by the blue dotted line). After 21 hours, cells were fixed, and phase contrast micrographs were taken. The area of cells that had migrated into the wound (demarcated by the red line) was analyzed by ImageJ. The graph exhibits the filled area of scratch in percentage of mock. hTHSD7A, human thrombospondin type 1 domain–containing 7A. ****P<0.001 (two-tailed t test).
Discussion
THSD7A is a highly conserved protein, which shares a 90.525% sequence homology between human and mouse. THSD7A was initially cloned as a gene that was relatively overexpressed in venous versus arterial endothelial cells,16 and it was later implicated in the angiogenic sprouting of vessels from dorsal aorta in zebrafish.15,34 Only with its identification as a target antigen in a small subset of patients with MN11 has THSD7A become known as a podocyte-expressed protein but with unknown temporal and spatial expression and unknown biologic functions for podocytes. So far, THSD7A has been localized to the very basal surface of podocytes in humans and rodents,11,17 and immunogold EM images showed that THSD7A is present in FPs near slit diaphragms.18 New studies unraveled the structure of the slit diaphragm, with single NEPH1 molecules forming the lower part of the junction, whereas single-nephrin molecules form an adjacent junction more apically.3 We could now deepen our knowledge of podocyte THSD7A localization to the most basal aspect of the podocyte FP domain in close proximity to the slit diaphragm and not in the FP domain, in which the majority of adhesion proteins are expressed. This finding was highly reproducible with two different commercial antibodies to the extracellular domain of THSD7A in frozen paraformaldehyde- or acetone-fixed sections as well as in paraffin-embedded tissue in human and murine samples. Intriguingly, THSD7A expression commenced with glomerular vascularization. Although in the comma-shaped body, podocytes represent a classic columnar epithelium interconnected by tight junctions. With the intrusion of endothelial cells and unfolding of capillary loops, podocytes start to expand their apical surface and translocate their apical cell-junctional belt toward the basal side. This apical membrane expansion is accompanied by a sequential change of cell-junctional protein expression and results in a change of vesicular nephrin expression toward a linear basal nephrin expression within a modified adherens junction-like contact, the slit diaphragm.36,37 On the basis of structural findings, the slit diaphragm is thought to represent a highly dynamic cell-cell contact that forms an adjustable nonclogging barrier within the renal filtration apparatus.3 Therefore, it is intriguing to speculate that THSD7A might function in stabilizing mature slit diaphragm dynamics, potentially by interaction with extracellular matrix proteins of the GBM. As such, the extracellular part of THSD7A contains a series of 21 thrombospondin type 1 domains, which on the basis of their amino acid sequence, can be further classified as either THBS1 like or complement component 6 like.28 Because THBS1 itself acts as an adhesive glycoprotein that can interact with components of the extracellular matrix,29–31,38 cell receptors,39–45 and proteases,46,47 we hypothesized that THSD7A could mediate podocyte FP adhesion to the GBM. Furthermore, THSD7A is predicted to bind heparan sulfates on the basis of a homology model of the extracellular THSD7A protein domain.32 In line, our experiments revealed an increase in human podocyte adhesion to collagen type IV–coated tissue culture plates in the presence of THSD7A. However, the increase in adhesive properties mediated by THSD7A was not as strong as described for other podocyte adhesion proteins, such as the FERM protein EPB41L5,48 or α3/β1-integrin,49 suggesting that promoting FP adhesion to the GBM was not the main function of THSD7A. This finding is also supported by the fact that, at the basal FP membrane domain, THSD7A is preferentially expressed close to the slit diaphragm rather than the basal FP domain, where focal adhesion components, such as the adhesion proteins EPB41L5 and α3/β1-integrin, localize.48
A strong line of evidences suggests that THSD7A could be involved in the regulation of cell dynamics. In human umbilical endothelial cells, THSD7A inhibits cell migration and tube formation by both gain- and loss-of-function approaches.16 THSD7A contains an extracellular RGD motif in close proximity to the transmembrane domain. RGD motifs are found in extracellular matrix proteins, such as fibronectin, vitronectin, and laminin,21,50 and they are recognized and bound by specific subtypes of integrins, such as αvβ3, α5β1, and αIIbβ3, to mediate cell-substratum and cell-cell interactions.51 THSD7A was described to affect cytoskeletal rearrangement in human umbilical vein endothelial cells by means of focal adhesion rearrangement and colocalization with αv/β3-integrin and paxillin.16 Furthermore, soluble THSD7A was shown to affect the distribution patterns of vinculin and phosphorylated focal adhesion kinase in HUVECs, implying a role for THSD7A in focal adhesion assembly.34 We, therefore, carefully assessed whether, in human podocytes, THSD7A colocalized with focal adhesions, but we could not find a strict colocalization of THSD7A with either β1- or β3-integrin at focal adhesions. Rather, THSD7A-expressing podocytes exhibited fewer focal adhesions than control cells, and THSD7A expression was accentuated at filopodia, which sense the microenvironment and drive directed cell migration. Interestingly, at the leading edge of filopodia, a distinct colocalization of THSD7A with β1- and β3-integrin was detected in human podocytes, which agrees with findings in HUVECs.16 Ongoing studies by us are currently assessing the significance of this finding.
The most striking feature of THSD7A-expressing podocytes was the development of TAPs. TAPs are actin-free membrane processes typical for podocytes, which are distinct from filopodia due to the absence of focal adhesion proteins, such as zyxin.35 The function of TAPs is unknown; nevertheless, they are indicative of slow membrane dynamics. TAPs develop in cultured podocytes on expression of the tetraspanins CD15135 and CD9,52 which play an essential role in proliferation, cell dynamics, and adhesion. Similar to THSD7A, CD151 is expressed at low levels in developing podocytes and high levels in mature podocytes,53 and CD151 stabilizes cells.54 Acknowledging these similarities, THSD7A could be required for the stabilization of specific FP domain dynamics, such as the slit diaphragm. In conjunction, our study demonstrated fewer focal adhesions and decreased activity levels of cytoskeletal regulators, such as paxillin, at focal adhesions and MLC at actin fibers in podocytes. Our finding that expression of full-length THSD7A reduced podocyte cell migration further supported this idea. Interestingly, HUVECs that overexpress a C-terminal fragment of THSD7A also exhibit decreased migration,16 whereas treatment of HUVECs with a soluble extracellular fragment of THSD7A enhances HUVEC migration without affecting HUVEC adhesion to collagen type 1 or poly-l-lysine–coated coverslips.34 These opposing results demonstrate that the effects that THSD7A mediates on cellular dynamics depend on the cellular system and the THSD7A form studied.
Although it was not the focus of this study to evaluate the effects of THSD7A autoantibody binding on THSD7A function and localization, it is noteworthy to state that we observed a persistent localization of bound IgG to the basal FP domain closest to the slit diaphragm at 2 weeks, which was still apparent at 18 weeks after injection of anti-THSD7A antibodies into mice. To our knowledge, this is the first time that antigen/antibody complexes have been observed specifically at the slit diaphragm in MN. This could be related to the fact that this antibody binding pattern was only visible in transversal optical planes of very fresh paraffin or frozen sections, which needed to be >3 μm. THSD7A staining pattern was granular in the subepithelial space in the frontal optical plane (which represents the typical IgG deposition pattern described for MN), when the staining was performed in thin paraffin section or in paraffin sections originating from older paraffin-blocks.
In summary, our study establishes THSD7A as a basal FP protein preferentially expressed at the membrane domains closest to the slit diaphragm in human and mice, the expression of which commences on slit diaphragm maturation in glomerular development. Physiologically, THSD7A expression is accentuated at distinct membrane domains, such as TAPs and filopodia, resulting in stabilized podocyte cell dynamics. Transferring these findings to the in vivo situation, THSD7A might be involved in regulating slit diaphragm dynamics of the glomerular filtration barrier. Thereby, it is intriguing to speculate that autoantibodies to THSD7A disrupt the glomerular filtration barrier either by mechanical alteration of the slit diaphragm and/or by altering the biologic function of THSD7A. Both could result in a destabilization of FP cell dynamics and thereby, the slit diaphragm dynamics, resulting in the typical scenario of FP effacement and proteinuria in MN.
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to thank Nicole Endlich (Department of Anatomy, University of Greifswald) for help in Super resolution structured illumination microscopy (SR-SIM) microscopy. We also thank Ingke Braren (University Medical Center Eppendorf Vector Facility) for the generation of lentiviral particles and Maximilian Ruffer (Department of Chemistry, University Hamburg) for expert help in using Photoshop.
This study was supported by the Deutsche Forschungsgesellschaft as part of the Sonderforschungsbereich 1192, grant to project B3 (to Prof. Meyer-Schwesinger) and to the Integrated Research Training Group (to J. Herwig and S. Skuza).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018090941/-/DCSupplemental.
Supplemental Figure 1. THSD7A expression decreases on outgrowth from isolated glomeruli.
Supplemental Figure 2. THSD7A is in the confocal plane of podocyte β1-integrin but does not follow the β1-integrin staining pattern at the base of foot processes.
Supplemental Figure 3. THSD7A is localized basally from nephrin partially following the slit diaphragm meanders.
Supplemental Figure 4. Overview of a capillary loop imaged for THSD7A (green) and nephrin (red) by STED microscopy.
Supplemental Figure 5. Patterns of anti-THSD7A IgG deposition differ in frontal and transversal high-resolution confocal planes.
Supplemental Figure 6. Anti-THSD7A IgG binding in murine glomeruli.
Supplemental Figure 7. Immunogold EM of rabbit IgG binding on day 1.
Supplemental Figure 8. Immunogold EM of human IgG binding at week 18.
Supplemental Figure 9. Overexpression of human THSD7A (hTHSD7A) in cultured immortalized human podocytes does not affect protein degradation pathways.
Supplemental Figure 10. Overexpression of human THSD7A (hTHSD7A) in cultured immortalized human podocytes affects cell size and morphology.
Supplemental Figure 11. Overexpression of human THSD7A (hTHSD7A) in cultured immortalized human podocytes does not affect podocin expression.
Supplemental Figure 12. Overexpression of human THSD7A (hTHSD7A) in cultured immortalized human podocytes does not affect α-actinin-4 expression.
Supplemental Figure 13. Overexpression of human THSD7A (hTHSD7A) in cultured immortalized human podocytes does not affect actin polymerization.
References
- 1.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Baraldi A, Zambruno G, Furci L, Manca V, Vaschieri C, Lusvarghi E: Beta-1 integrins in the normal human glomerular capillary wall: An immunoelectron microscopy study. Nephron 66: 295–301, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Grahammer F, Wigge C, Schell C, Kretz O, Patrakka J, Schneider S, et al.: A flexible, multilayered protein scaffold maintains the slit in between glomerular podocytes. JCI Insight 1: pii: 86177, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huber TB, Schermer B, Benzing T: Podocin organizes ion channel-lipid supercomplexes: Implications for mechanosensation at the slit diaphragm. Nephron Exp Nephrol 106: e27–e31, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Huber TB, Schermer B, Müller RU, Höhne M, Bartram M, Calixto A, et al.: Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci U S A 103: 17079–17086, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fogo AB, Lusco MA, Najafian B, Alpers CE: AJKD atlas of renal pathology: Membranous nephropathy. Am J Kidney Dis 66: e15–e17, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Beck LH Jr, Salant DJ: Membranous nephropathy: Recent travels and new roads ahead. Kidney Int 77: 765–770, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Ronco P, Debiec H, Imai H: Circulating antipodocyte antibodies in membranous nephropathy: Pathophysiologic and clinical relevance. Am J Kidney Dis 62: 16–19, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann JP, Bensman A, et al.: Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med 346: 2053–2060, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al.: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomas NM, Beck LH Jr, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, et al.: Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371: 2277–2287, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkatesh B, Lee AP, Ravi V, Maurya AK, Lian MM, Swann JB, et al.: Elephant shark genome provides unique insights into gnathostome evolution. Nature 505: 174–179, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stahl PR, Hoxha E, Wiech T, Schröder C, Simon R, Stahl RA: THSD7A expression in human cancer. Genes Chromosomes Cancer 56: 314–327, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Hoxha E, Wiech T, Stahl PR, Zahner G, Tomas NM, Meyer-Schwesinger C, et al.: A mechanism for cancer-associated membranous nephropathy. N Engl J Med 374: 1995–1996, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Wang CH, Chen IH, Kuo MW, Su PT, Lai ZY, Wang CH, et al.: Zebrafish Thsd7a is a neural protein required for angiogenic patterning during development. Dev Dyn 240: 1412–1421, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Wang CH, Su PT, Du XY, Kuo MW, Lin CY, Yang CC, et al.: Thrombospondin type I domain containing 7A (THSD7A) mediates endothelial cell migration and tube formation. J Cell Physiol 222: 685–694, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Meyer-Schwesinger C, Lambeau G, Stahl RA: Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 372: 1074–1075, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Gödel M, Grahammer F, Huber TB: Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 372: 1073, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Tomas NM, Meyer-Schwesinger C, von Spiegel H, Kotb AM, Zahner G, Hoxha E, et al.: A heterologous model of thrombospondin type 1 domain-containing 7A-associated membranous nephropathy. J Am Soc Nephrol 28: 3262–3277, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomas NM, Hoxha E, Reinicke AT, Fester L, Helmchen U, Gerth J, et al.: Autoantibodies against thrombospondin type 1 domain-containing 7A induce membranous nephropathy. J Clin Invest 126: 2519–2532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felding-Habermann B, Cheresh DA: Vitronectin and its receptors. Curr Opin Cell Biol 5: 864–868, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, et al.: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, et al.: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Fan Q, Yang G, Liu N, Chen D, Jiang Y, et al.: Isolating glomeruli from mice: A practical approach for beginners. Exp Ther Med 5: 1322–1326, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somogyi P, Takagi H: A note on the use of picric acid-paraformaldehyde-glutaraldehyde fixative for correlated light and electron microscopic immunocytochemistry. Neuroscience 7: 1779–1783, 1982 [DOI] [PubMed] [Google Scholar]
- 26.Sosna J, Voigt S, Mathieu S, Kabelitz D, Trad A, Janssen O, et al.: The proteases HtrA2/Omi and UCH-L1 regulate TNF-induced necroptosis. Cell Commun Signal 11: 76, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong MA, Cui S, Quaggin SE: Identification and characterization of a glomerular-specific promoter from the human nephrin gene. Am J Physiol Renal Physiol 279: F1027–F1032, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Seifert L, Hoxha E, Eichhoff AM, Zahner G, Dehde S, Reinhard L, et al.: The most N-terminal region of THSD7A is the predominant target for autoimmunity in THSD7A-associated membranous nephropathy. J Am Soc Nephrol 29: 1536–1548, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galvin NJ, Vance PM, Dixit VM, Fink B, Frazier WA: Interaction of human thrombospondin with types I-V collagen: Direct binding and electron microscopy. J Cell Biol 104: 1413–1422, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sercu S, Lambeir AM, Steenackers E, El Ghalbzouri A, Geentjens K, Sasaki T, et al.: ECM1 interacts with fibulin-3 and the beta 3 chain of laminin 332 through its serum albumin subdomain-like 2 domain. Matrix Biol 28: 160–169, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Dardik R, Lahav J: Multiple domains are involved in the interaction of endothelial cell thrombospondin with fibronectin. Eur J Biochem 185: 581–588, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Stoddard SV, Welsh CL, Palopoli MM, Stoddard SD, Aramandla MP, Patel RM, et al.: Structure and function insights garnered from in silico modeling of the thrombospondin type-1 domain-containing 7A antigen. Proteins 87: 136–145, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegerist F, Ribback S, Dombrowski F, Amann K, Zimmermann U, Endlich K, et al.: Structured illumination microscopy and automatized image processing as a rapid diagnostic tool for podocyte effacement. Sci Rep 7: 11473, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuo MW, Wang CH, Wu HC, Chang SJ, Chuang YJ: Soluble THSD7A is an N-glycoprotein that promotes endothelial cell migration and tube formation in angiogenesis. PLoS One 6: e29000, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blumenthal A, Giebel J, Ummanni R, Schlüter R, Endlich K, Endlich N: Morphology and migration of podocytes are affected by CD151 levels. Am J Physiol Renal Physiol 302: F1265–F1277, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Ruotsalainen V, Patrakka J, Tissari P, Reponen P, Hess M, Kestilä M, et al.: Role of nephrin in cell junction formation in human nephrogenesis. Am J Pathol 157: 1905–1916, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schell C, Wanner N, Huber TB: Glomerular development--shaping the multi-cellular filtration unit. Semin Cell Dev Biol 36: 39–49, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Herndon ME, Stipp CS, Lander AD: Interactions of neural glycosaminoglycans and proteoglycans with protein ligands: Assessment of selectivity, heterogeneity and the participation of core proteins in binding. Glycobiology 9: 143–155, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Asch AS, Silbiger S, Heimer E, Nachman RL: Thrombospondin sequence motif (CSVTCG) is responsible for CD36 binding. Biochem Biophys Res Commun 182: 1208–1217, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Gao AG, Lindberg FP, Dimitry JM, Brown EJ, Frazier WA: Thrombospondin modulates alpha v beta 3 function through integrin-associated protein. J Cell Biol 135: 533–544, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA: Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem 271: 21–24, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, et al.: Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem 284: 1116–1125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calzada MJ, Annis DS, Zeng B, Marcinkiewicz C, Banas B, Lawler J, et al.: Identification of novel beta1 integrin binding sites in the type 1 and type 2 repeats of thrombospondin-1. J Biol Chem 279: 41734–41743, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Calzada MJ, Sipes JM, Krutzsch HC, Yurchenco PD, Annis DS, Mosher DF, et al.: Recognition of the N-terminal modules of thrombospondin-1 and thrombospondin-2 by alpha6beta1 integrin. J Biol Chem 278: 40679–40687, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Lawler J, Hynes RO: An integrin receptor on normal and thrombasthenic platelets that binds thrombospondin. Blood 74: 2022–2027, 1989 [PubMed] [Google Scholar]
- 46.Yang Z, Strickland DK, Bornstein P: Extracellular matrix metalloproteinase 2 levels are regulated by the low density lipoprotein-related scavenger receptor and thrombospondin 2. J Biol Chem 276: 8403–8408, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Bein K, Simons M: Thrombospondin type 1 repeats interact with matrix metalloproteinase 2. Regulation of metalloproteinase activity. J Biol Chem 275: 32167–32173, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Schell C, Rogg M, Suhm M, Helmstädter M, Sellung D, Yasuda-Yamahara M, et al.: The FERM protein EPB41L5 regulates actomyosin contractility and focal adhesion formation to maintain the kidney filtration barrier. Proc Natl Acad Sci U S A 114: E4621–E4630, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cybulsky AV, Carbonetto S, Huang Q, McTavish AJ, Cyr MD: Adhesion of rat glomerular epithelial cells to extracellular matrices: Role of beta 1 integrins. Kidney Int 42: 1099–1106, 1992 [DOI] [PubMed] [Google Scholar]
- 50.Ruoslahti E: Fibronectin and its receptors. Annu Rev Biochem 57: 375–413, 1988 [DOI] [PubMed] [Google Scholar]
- 51.Van Agthoven JF, Xiong JP, Alonso JL, Rui X, Adair BD, Goodman SL, et al.: Structural basis for pure antagonism of integrin αVβ3 by a high-affinity form of fibronectin. Nat Struct Mol Biol 21: 383–388, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blumenthal A, Giebel J, Warsow G, Li L, Ummanni R, Schordan S, et al.: Mechanical stress enhances CD9 expression in cultured podocytes. Am J Physiol Renal Physiol 308: F602–F613, 2015 [DOI] [PubMed] [Google Scholar]
- 53.McMahon AP, Aronow BJ, Davidson DR, Davies JA, Gaido KW, Grimmond S, et al.: GUDMAP Project : GUDMAP: The genitourinary developmental molecular anatomy project. J Am Soc Nephrol 19: 667–671, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Hasegawa M, Furuya M, Kasuya Y, Nishiyama M, Sugiura T, Nikaido T, et al.: CD151 dynamics in carcinoma-stroma interaction: Integrin expression, adhesion strength and proteolytic activity. Lab Invest 87: 882–892, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.