Significance Statement
Routine incorporation of pragmatic trials into clinical care delivery has potential to generate answers to important questions, such as optimal approaches for fundamental components of maintenance hemodialysis. The Time to Reduce Mortality in ESRD (TiME) trial was a large pragmatic trial demonstration project designed to determine if a hemodialysis session duration longer than many patients in the United States currently receive improves clinical outcomes. Although the trial accomplished many of its demonstration project objectives, including rapid enrollment of >7000 patients, use of an opt-out consent approach, and complete reliance on clinically acquired data, uptake of the intervention was insufficient to determine whether longer sessions improve outcomes. Embedding trials into hemodialysis clinical care will require more effective strategies for engaging clinical personnel and patients.
Keywords: TiME Trial, dialysis session duration, learning health system, NIH Health Care Systems Research Collaboratory, opt-out consent, consent waiver
Visual Abstract
Abstract
Background
Data from clinical trials to inform practice in maintenance hemodialysis are limited. Incorporating randomized trials into dialysis clinical care delivery should help generate practice-guiding evidence, but the feasibility of this approach has not been established.
Methods
To develop approaches for embedding trials into routine delivery of maintenance hemodialysis, we performed a cluster-randomized, pragmatic trial demonstration project, the Time to Reduce Mortality in ESRD (TiME) trial, evaluating effects of session duration on mortality (primary outcome) and hospitalization rate. Dialysis facilities randomized to the intervention adopted a default session duration ≥4.25 hours (255 minutes) for incident patients; those randomized to usual care had no trial-driven approach to session duration. Implementation was highly centralized, with no on-site research personnel and complete reliance on clinically acquired data. We used multiple strategies to engage facility personnel and participating patients.
Results
The trial enrolled 7035 incident patients from 266 dialysis units. We discontinued the trial at a median follow-up of 1.1 years because of an inadequate between-group difference in session duration. For the primary analysis population (participants with estimated body water ≤42.5 L), mean session duration was 216 minutes for the intervention group and 207 minutes for the usual care group. We found no reduction in mortality or hospitalization rate for the intervention versus usual care.
Conclusions
Although a highly pragmatic design allowed efficient enrollment, data acquisition, and monitoring, intervention uptake was insufficient to determine whether longer hemodialysis sessions improve outcomes. More effective strategies for engaging clinical personnel and patients are likely required to evaluate clinical trial interventions that are fully embedded in care delivery.
Although maintenance hemodialysis has been used for more than 50 years, there is uncertainty about the best approaches for many of the fundamental components of treatment and there are limited data from clinical trials to inform practice.1–4 Embedding pragmatic trials into clinical care delivery has potential for efficiently producing evidence that is highly generalizable to the nonresearch setting.5 However, experience conducting large pragmatic trials in health care settings, in general and in dialysis, specifically, is limited, and feasibility has not been established.
In the United States, most patients with ESRD are treated with thrice-weekly hemodialysis in outpatient dialysis facilities. Hemodialysis session durations are shorter in the United States (average 3.5 hours) compared with most developed countries,6 in part, because of a greater emphasis in this country on dialytic clearance of small solutes such as urea, than on other metrics such as the rate of fluid removal.7 Several observational studies found improved patient survival with hemodialysis sessions that are longer than is typically needed to achieve accepted levels of small-solute removal, but there have been no large, randomized trials evaluating the effect of session duration on clinical outcomes.8–15
The Time to Reduce Mortality in ESRD (TiME) trial was a pragmatic trial designed to (1) develop approaches for embedding large, randomized trials into the routine delivery of clinical care; and (2) determine whether outcomes are improved with hemodialysis session durations that are longer than many patients in the United States currently receive. TiME is one of the pragmatic trial demonstration projects of the National Institutes of Health, Health Care Systems Research Collaboratory, an initiative to “increase the national capacity to implement cost-effective large-scale research efforts that engage health care systems as research partners.”16,17 The TiME trial was conducted through a partnership between academic investigators and two large dialysis provider organizations, using a highly pragmatic design that fully incorporated intervention delivery and data acquisition into routine clinical care. This report provides the trial results and insights that should inform future pragmatic trials embedded in clinical care.
Methods
Study Oversight
A Steering Committee comprising academic investigators, researchers at the dialysis provider organizations, and project scientists from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) developed the protocol and provided oversight for trial implementation and data analysis. The data coordinating center (DCC) was based at the University of Pennsylvania. An external Data and Safety Monitoring Board (DSMB) appointed by the NIDDK reviewed study progress, outcome event rates, and routinely performed laboratory tests as indicators of safety (Supplemental Appendix 1). The trial was approved by the University of Pennsylvania Institutional Review Board (IRB) (protocol #817911), which served as the IRB of record under authorization agreements with the dialysis provider organizations. The trial was registered at Clinicaltrials.gov (identifier NCT02019225; submission date October 30, 2013).
Study Design and Facility Randomization
The protocol is provided in the Supplemental Appendix 2 and the demonstration project objectives are provided in Supplemental Table 1. The trial was a multicenter, cluster-randomized, parallel group trial embedded into the delivery of clinical care at United States outpatient dialysis units operated by two provider organizations, DaVita and Fresenius Medical Care. Dialysis facilities were randomized 1:1 to the intervention or the usual care group, using a permuted block randomization procedure with stratification by dialysis provider organization, and by factors known to be associated with mortality: racial composition (≤50% or >50% black patients) and use of central venous catheters for hemodialysis vascular access (≤20% or >20% of patients).9,18,19 Randomization at the facility level rather than patient level was used to facilitate implementation of the intervention and minimize contamination. The allocation sequence was generated by the DCC and not known by the research teams involved in facility enrollment. The randomized assignment was provided electronically by the DCC to the research teams at the dialysis provider organizations. Neither the participating facilities nor the patients were masked to the assignment.
Eligibility Criteria and Consent Approach
Eligibility criteria for dialysis units were (1) willingness by the facility’s medical and administrative leadership and nephrologists to implement the intervention, (2) operational capacity for longer sessions, and (3) facility use of the dialysis provider organization’s electronic data system. Although not a formal inclusion criterion, facilities with mean session durations that historically were ≤210 minutes were prioritized for enrollment. Inclusion criteria for patients were (1) age ≥18 years, (2) treatment with thrice-weekly in-center hemodialysis, and (3) initiation of dialysis within the previous 120 days. Exclusion criteria for patients were (1) use of a health care proxy to provide consent for dialysis treatment and (2) unwillingness to have clinical data included in the trial dataset.
The trial was conducted under a waiver of the requirement for informed consent on the basis of criteria specified in the Common Rule [45 CFR Part 46.116(c)].20 Patients in both intervention and usual care facilities were given written information about the trial that included the facility’s randomized assignment. Patients were provided with telephone access to the research teams at the dialysis provider organizations to obtain additional information and/or to opt out of having their clinical data included in the trial dataset. Patients meeting the eligibility criteria were enrolled in the trial unless they opted out of data sharing.
Intervention
Dialysis facilities randomized to the intervention adopted a default session duration of ≥4.25 hours (255 minutes) for patients initiating maintenance hemodialysis. If the treating nephrologist felt that the ≥4.25 hour duration was not appropriate for an individual patient, shorter treatments could be prescribed with the goal of achieving session durations as close to 4.25 hours as possible. Dialysis facilities randomized to usual care had no trial-driven approach to session duration. Using historical data, we anticipated an average session duration of 3.5 hours (210 minutes) in usual care facilities. For both intervention and usual care, clinical targets for urea removal were not modified.
Engagement of Facility Personnel and Patients
Because there were no on-site research staff and the intervention was implemented by clinicians rather than researchers, multiple approaches were used to engage facility personnel and participating patients before and throughout the duration of the trial (Supplemental Table 2). Before the enrollment of individual facilities, the research teams at the dialysis provider organizations and the DCC provided facility personnel with information about the trial via teleconferences and web-based presentations. Willingness to adopt the trial intervention was documented by the facility administrator and medical director before randomization. After facility randomization, additional education tailored to the group assignment was provided to the facility staff and clinicians both before starting patient enrollment and during the trial. Mechanisms to promote adherence by the facilities randomized to the intervention were modeled after approaches already in use by the respective dialysis provider organizations for quality improvement. For example, standardized facility-specific reports of overall and individual patient-level adherence were distributed every 2 months to intervention facilities for review at their routine monthly multidisciplinary assessment and process improvement meetings that typically included facility administrative, nursing, and medical leadership, dietitians, and social workers. In addition, the research teams at the dialysis provider organizations had monthly telephone contacts with the nursing and/or administrative leadership at intervention facilities, and investigators reviewed performance via teleconference with the medical directors and other facility personnel to understand barriers to intervention uptake and suggest strategies for improvement. Brief, web-based presentations about the trial purpose and progress were provided for facility personnel, and newsletters created by the dialysis providers, unique to each organization, were distributed to staff and patients three to four times per year. The information provided to intervention facilities emphasized that the rationale for longer session durations was to reduce the rate of fluid removal rather than to increase small-solute clearance.
Data Acquisition
All of the data elements, including outcomes, were obtained from the electronic health and administrative records of the dialysis provider organizations, and were generated through routine clinical care. Prespecified data elements were transmitted each month from the dialysis provider clinical data warehouses to the DCC after removal of all participant identifiers other than dates of treatments, laboratory studies, and outcomes. Data included demographic characteristics, comorbid conditions, vascular access type, laboratory values, and treatment parameters for every dialysis session.
Outcomes
The primary outcome was death. Secondary outcomes were hospitalization rate, predialysis BP, postdialysis hypotension, interdialytic weight gain, fluid removal rate, missed dialysis sessions, and change in quality of life as assessed by the Kidney Disease Quality of Life Short Form-36, which was administered routinely by both dialysis providers in accordance with requirements of the Centers for Medicare and Medicaid Services.21,22 Adverse event reporting was not performed. Prespecified demonstration project milestones or metrics included use of a single, academic IRB of record for oversight of all participating facilities, acceptability by stakeholders of an opt-out approach to informed consent, harmonization of clinical data from multiple providers, attainment of a high degree of data completeness, implementation of the trial with no on-site research staff, centralized monitoring of fidelity to the intervention, assessment of safety without ascertainment of individual adverse events, and attainment of enrollment targets.
Sample Size and Statistical Analyses
The protocol incorporated two prespecified analysis populations, a primary analysis population and a full analysis population. Because patients with large body size usually require dialysis sessions of at least 4 hours (i.e., a session duration similar to the intervention) to achieve adequate small-solute clearance, the primary analysis population excluded patients who had, at baseline, an estimated body water volume >42.5 L as determined by the Watson formula.23 The full analysis population included all enrolled patients regardless of body size.
The initial sample size target for the full analysis population was 6432 participants, using the following assumptions: (1) 402 enrolled facilities, (2) 63% of patients in the primary analysis population, (3) loss to follow-up of 5% of patients per year, (4) usual care mortality rate of 18% per year, (5) median follow-up of 2.5 years, (6) an intracluster correlation coefficient (ICC) for mortality of 0.03, and (7) a 30-minute difference in session duration between the treatment groups. Under these assumptions, the trial would have 80% power with a two-sided α of 0.05 to detect a relative reduction in mortality risk of 15%. The sample size was increased to 6880 in June 2016 to allow for a smaller number of participating facilities and a greater variability in the number of new patients per facility. The revised sample size incorporated an ICC of 0.01 on the basis of the observed ICC at that time.
A decision to enroll fewer facilities than originally planned was made after the trial was underway when it became evident that the target for participant enrollment could be met with approximately 250 facilities and that there would be an overall benefit to the trial if greater effort were focused on trial implementation rather than on recruiting and onboarding additional facilities. The assumptions that were incorporated into the sample size determinations and the actual values observed at the end of the trial are shown in Supplemental Table 3.
The statistical analysis plan is provided in Supplemental Appendix 3. Clustering by facility was accounted for in all analyses. Mean session durations were estimated using per-participant averages weighted by the number of dialysis sessions to account for the unequal number of dialysis sessions among participants. Cox proportional hazards regression was used to compare mortality risk for the intervention and usual care groups with incorporation of the randomization stratification variables.24 Participants within the same facility were modeled using a random component for the hazard function (i.e., frailty models). An intention-to-treat approach was used with participants included in the analysis regardless of the dialysis session duration received. For each outcome, the primary analyses included patients with estimated body water volume ≤42.5 L. For events that could occur repeatedly, rates were analyzed using generalized estimating equations, assuming a Poisson distribution with an offset term to account for length of follow-up and an independent correlation structure to account for participants within the same facility. Continuous measures were compared using linear mixed-effects models to account for both participants within the same facility and repeated measurements within the same participant. In secondary analyses, all outcomes were evaluated using the full analysis population of all patients. A prespecified interim efficacy analysis, and a conditional power analysis on the basis of the observed estimated treatment effect were provided to the DSMB when approximately 50% of the information time, according to patient years of follow-up, had accrued. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and survival, geepack, and lem4 packages in R version 3.4.1 (https://www.r-project.org).
Results
Study Population
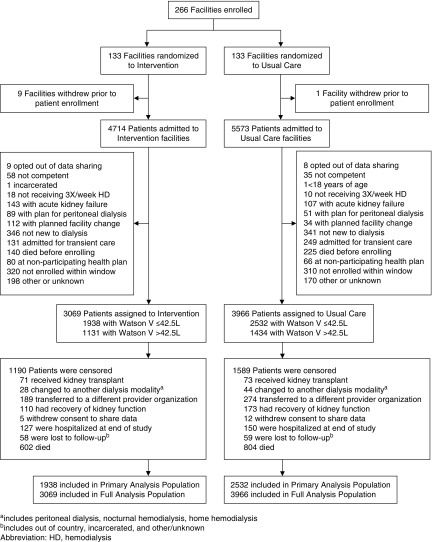
Between November 18, 2013 and November 17, 2015, 266 dialysis facilities were randomized to intervention (n=133) or usual care (n=133) (Figure 1). Ten facilities (nine in intervention and one in usual care) withdrew from participation after randomization but before enrollment of patients. Between December 18, 2013 and September 30, 2016, 7035 patients were enrolled: 3069 in intervention and 3966 in usual care facilities. Seventeen patients (nine from intervention and eight from usual care facilities) were not enrolled because they opted out of data sharing.
Figure 1.
Flow for dialysis facilities and patients. HD, hemodialysis; V, volume.
The baseline characteristics of the participants are shown in Table 1. Characteristics were balanced across treatment groups. Consistent with the pretrial assumptions, the primary analysis population comprised 63.5% of the full cohort. The mean age of participants in the primary analysis population was 66.6 years and 41.2% were men. The difference in participant number between the two treatment groups was driven by the withdrawal before participant enrollment of nine intervention facilities compared with one usual care facility, a greater number of patients initiating treatment at usual care facilities than at intervention facilities, and a modestly larger percentage of patients excluded from participation in intervention facilities than in usual care facilities (Figure 1).
Table 1.
Baseline characteristics of the participants
| Characteristic | Primary Analysis Population, n=4470 | Full Analysis Population, n=7035 | ||
|---|---|---|---|---|
| Intervention, n=1938 | Usual Care, n=2532 | Intervention, n=3069 | Usual Care, n=3966 | |
| Age, yr | 66.7 (14.4) | 66.5 (14.8) | 64.1 (14.5) | 64.1 (14.7) |
| Men | 849 (43.8) | 993 (39.2) | 1832 (59.7) | 2234 (56.3) |
| Race/Ethnicity | ||||
| Non-Hispanic black | 455 (23.7) | 616 (24.7) | 758 (24.9) | 983 (25.1) |
| Non-Hispanic white | 1069 (55.7) | 1408 (56.4) | 1750 (57.5) | 2316 (59.1) |
| Asian | 87 (4.8) | 104 (4.5) | 95 (3.2) | 120 (3.3) |
| Other | 28 (1.5) | 41 (1.6) | 52 (1.7) | 60 (1.5) |
| Hispanic | 279 (14.5) | 328 (13.1) | 388 (12.8) | 440 (11.2) |
| Missing | 20 | 36 | 27 | 48 |
| Weight postdialysis, kg | 71.6 (14.4) | 71.7 (14.3) | 84.8 (24.6) | 84.4 (24.7) |
| Watson volumea, L | 36.0 (4.7) | 34.8 (4.7) | 40.8 (9.5) | 40.5 (9.5) |
| Diabetes mellitus | 771 (39.8) | 1029 (40.6) | 1333 (43.2) | 1762 (44.4) |
| Cardiac diseaseb | 438 (22.6) | 527 (20.8) | 744 (24.1) | 858 (21.6) |
| Cancer | 99 (5.1) | 126 (5.0) | 102 (5.3) | 130 (5.1) |
| Systolic BP, mm Hg | 144.5 (26.1) | 142.5 (25.8) | 144.4 (25.9) | 143.2 (26.1) |
| Diastolic BP, mm Hg | 74.2 (14.8) | 73.6 (14.7) | 75.3 (15.1) | 74.8 (15.3) |
| Vascular access type | ||||
| Arteriovenous fistula | 313 (16.3) | 352 (14.0) | 535 (17.5) | 594 (15.1) |
| Arteriovenous graft | 71 (3.7) | 88 (3.5) | 89 (2.9) | 117 (3) |
| Central venous catheter | 1539 (80) | 2068 (82.5) | 2426 (79.5) | 3219 (81.9) |
| Missing | 15 | 24 | 19 | 36 |
| Hemoglobin, g/dl | 9.52 (1.20) | 9.50 (1.27) | 9.50 (1.27) | 9.51 (1.29) |
| Albumin, g/dl | 3.33 (0.53) | 3.35 (0.54) | 3.34 (0.53) | 3.37 (0.53) |
| BUN, mg/dl | 50.1 (21.1) | 49.8 (21.3) | 51.7 (21.2) | 51.5 (21.4) |
| Creatinine, mg/dl | 5.44 (2.20) | 5.41 (2.28) | 5.75 (2.40) | 5.71 (2.44) |
| Phosphorus, mg/dl | 4.60 (1.46) | 4.59 (1.47) | 4.71 (1.47) | 4.70 (1.49) |
Data are displayed as mean (SD) for continuous variables and n (%) for categorical variables.
Watson total body water volume in liters determined using the following equations. For men: 2.47− (0.09516×age in yr)+(0.1074×height in cm)+(0.3362×weight in kg); for women: −2.097+(0.1069×height in cm)+(0.2455×weight in kg).
Cardiac disease includes coronary artery disease, myocardial infarction, valvular disease, arrhythmias, congestive heart failure, endocarditis, myocarditis, pericarditis, cardiomyopathy, and congenital heart disease.
Trial Termination
Participant follow-up ended on January 31, 2017 on the basis of the recommendation by the DSMB to terminate the trial because of a lower than anticipated difference in session duration between the intervention and usual care groups, and an interim analysis indicating a conditional power close to 0% to detect a hazard ratio (HR) of 0.85 for mortality. At the time the trial ended, the median follow-up was 1.1 (interquartile range, 0.5–1.7) years.
Hemodialysis Session Duration
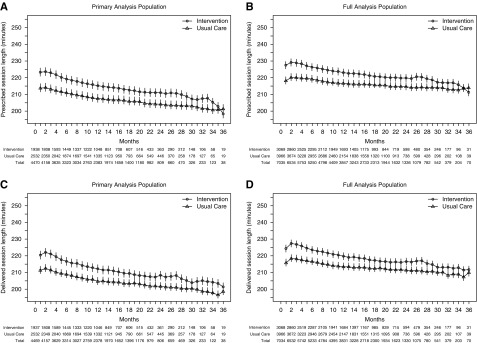
The per-patient mean prescribed and delivered session durations for the primary analysis population and the full analysis populations are shown in Figure 2, A–D. For the primary analysis population, the estimated mean prescribed session duration was 219 (95% confidence interval [95% CI], 217 to 222) minutes in the intervention group and 210 (95% CI, 209 to 213) minutes in the usual care group. The estimated mean delivered session durations in the primary analysis population were 216 (95% CI, 214 to 219) minutes and 207 (95% CI, 206 to 211) minutes in intervention and usual care, respectively. As expected, session durations were longer in both treatment groups for the full analysis population. For both treatment groups, dialytic urea removal was above the clinically acceptable threshold (Supplemental Figure 1).
Figure 2.
The difference in hemodialysis session durations between the intervention and usual care groups was less than targeted and decreased over follow-up time. The value shown at each month represents the per-participant mean value and 95% CIs over the preceding 30 days. Estimated session durations and their months were calculated using linear mixed effects models to account for both participants within the same facility and repeated measurements within the same participant. (A) Prescribed session duration for the primary analysis population (patients with Watson volume ≤42.5 L); slope −0.72 for intervention group; slope −0.50 for usual care group (P=0.07 for difference). (B) Prescribed session duration for the full analysis population (all patients); slope −0.41 for intervention group; slope −0.12 for usual care group (P<0.001 for difference). (C) Delivered session duration for the primary analysis population (patients with Watson volume ≤42.5 L); slope −0.87 for intervention group; slope −0.68 for usual care group (P=0.02 for difference). (D) Delivered session duration for the full analysis population (all patients); slope −0.59 for intervention group; slope −0.39 for usual care group (P<0.001 for difference).
Primary Outcome
For the primary analysis population, there were 425 deaths among 1938 participants (21.9%) in the intervention group and 565 deaths among 2532 participants (22.3%) in the usual care group, corresponding to event rates of 19.2 deaths per 100 patient-years and 19.7 deaths per 100 patient-years in the intervention and usual care groups, respectively (HR, 0.97; 95% CI, 0.84 to 1.12; P=0.69). For the full analysis population the rates were 16.8 and 17.4 per patient-year in the intervention and usual care groups, respectively (HR, 0.97; 95% CI, 0.85 to 1.12; P=0.70) (Figure 3, Table 2). Mortality risk with adjustment for baseline characteristics is shown in Supplemental Table 4. The observed ICC for mortality was 0.008.
Figure 3.
Kaplan-Meier survival curves indicate that mortality did not differ between the intervention and usual care groups. (A) Primary analysis population (patients with Watson volume ≤42.5 L). (B) Full analysis population (all patients).
Table 2.
Primary and secondary outcomes
| Outcome | Primary Analysis Population, n=4470 | Full Analysis Population, n=7035 | ||
|---|---|---|---|---|
| Intervention, n=1938 | Usual Care, n=2532 | Intervention, n=3069 | Usual Care, n=3966 | |
| Primary outcome | ||||
| Death | ||||
| n (%) | 425 (21.9) | 565 (22.3) | 602 (19.6) | 804 (20.3) |
| Events per 100 patient-yra | 19.2 (17.4 to 21.0) | 19.7 (18.1 to 21.4) | 16.9 (15.5 to 18.2) | 17.4 (16.2 to 18.6) |
| HR (95% CI)a | 0.97 (0.84 to 1.12) | 0.97 (0.85 to 1.12) | ||
| P value for HRa | 0.69 | 0.70 | ||
| Secondary outcomes | ||||
| Hospitalizations | ||||
| n (%) with event | 1364 (70.4) | 1792 (70.8) | 2116 (68.9) | 2751 (69.4) |
| HR (95% CI)a | 0.95 (0.87 to 1.05) | 0.98 (0.90 to 1.06) | ||
| P value for HRa | 0.32 | 0.56 | ||
| Events per 100 patient-yrb | 204.5 (186.9 to 223.7) | 214.1 (202.5 to 226.3) | 196.0 (181.5 to 211.7) | 204.6 (194.5 to 215.2) |
| Rate ratio (95% CI)b | 0.96 (0.86 to 1.06) | 0.97 (0.87 to 1.05) | ||
| P value for rate ratiob | 0.40 | 0.36 | ||
| Predialysis systolic BPc | 143.3 (19.2) | 143.0 (19.1) | 143.0 (19.0) | 143.4 (19.1) |
| P valued | 0.88 | 0.26 | ||
| Predialysis diastolic BPc | 73.9 (11.4) | 73.5 (11.0) | 74.8 (11.5) | 74.6 (11.3) |
| P valued | 0.80 | 0.63 | ||
| Postdialysis hypotensione | ||||
| n (%) with event | 325 (16.8) | 475 (18.8) | 539 (17.6) | 774 (19.5) |
| Events per 100 patient-yrb | 75.5 (47.6 to 119.9) | 74.0 (51.8 to 105.8) | 75.2 (51.2 to 110.4) | 68.1 (51.8 to 89.6) |
| Rate ratio (95% CI)b | 1.02 (0.57 to 1.83) | 1.11 (0.69 to 1.77) | ||
| P value for rate ratiob | 0.95 | 0.68 | ||
| Ultrafiltration rate, ml/h per kg | 7.37 (2.77) | 7.51 (3.02) | 7.01 (2.60) | 7.14 (2.82) |
| P valued | 0.42 | 0.29 | ||
| Interdialytic weight gain, kg | 1.69 (0.81) | 1.65 (0.84) | 1.93 (0.98) | 1.88 (1.00) |
| P valued | 0.25 | 0.28 | ||
| Missed dialysis sessions | ||||
| n (%) with event | 1584 (81.7) | 2103 (83.1) | 2526 (82.3) | 3305 (83.3) |
| Events per 100 patient-yrb | 1246 (1103 to 1407) | 1253 (1133 to 1386) | 1285 (1169 to 1413) | 1351 (1237 to 1475) |
| Rate ratio (95% CI)b | 0.99 (0.85 to 1.16) | 0.95 (0.84 to 1.08) | ||
| P value for rate ratiob | 0.94 | 0.45 | ||
Data are displayed as mean (SD) for continuous variables or event rate (95% CI) unless otherwise indicated.
Generated from proportional hazards models with facility as a frailty. Event rates and 95% CIs are unadjusted.
Generated from generalized estimating equation Poisson regression models with facility as a correlation structure.
BP was measured at every dialysis session.
Generated from linear mixed-effects models with facility as a random effect.
Hypotension is defined as systolic BP <90 mm Hg.
Secondary Outcomes and Laboratory-Based Safety Parameters
Hospitalization rates were 204.0 and 212.6 per 100 patient-years in the intervention and usual care groups, respectively, for the primary analysis population (P=0.44), and 195.4 and 203.4 per 100 patient-years in intervention and usual care for the full analysis population (P=0.39). There were no differences between treatment groups in other secondary outcomes or laboratory-based safety parameters (Tables 2–4).
Table 4.
Laboratory safety parameters
| Parameter | Primary Analysis Population, n=4470 | Full Analysis Population, n=7035 | ||
|---|---|---|---|---|
| Intervention, n=1938 | Usual Care, n=2532 | Intervention, n=3069 | Usual Care, n=3966 | |
| Hypokalemiaa (potassium <3.6 mEq/L) | ||||
| n (%) with event | 615 (31.7) | 888 (35.1) | 915 (29.8) | 1291 (32.6) |
| Events per 100 patient-yr (95% CI)b | 71.4 | 81.1 | 64.3 | 74.1 |
| (62.6 to 81.5) | (73.0 to 90.2) | (57.6 to 71.7) | (63.3 to 82.9) | |
| P value for rate ratiob | 0.14 | 0.07 | ||
| Hypophosphatemiaa (phosphorus <3.0 mg/dl) | ||||
| n (%) with event | 753 (38.9) | 979 (38.7) | 1092 (35.6) | 1375 (34.7) |
| Events per 100 patient-yr (95% CI)b | 81.9 | 72.6 | 69.7 | 61.2 |
| (72.8 to 92.2) | (65.8 to 80.2) | (62.8 to 77.3) | (56.4 to 66.5) | |
| P value for rate ratiob | 0.12 | 0.06 | ||
| Hyperbicarbonatemiaa (CO2>26 mmol/L) | ||||
| n (%) with event | 1249 (64.4) | 1548 (61.1) | 1959 (63.8) | 2397 (60.4) |
| Events per 100 patient-yr (95% CI)b | 205.9 | 188.3 | 202.7 | 177.0 |
| (180.2 to 235.3) | (167.4 to 211.7) | (177.4 to 231.6) | (157.6 to 198.8) | |
| P value for rate ratiob | 0.32 | 0.13 | ||
| Hypoalbuminemiaa (albumin <3.2 g/dl) | ||||
| n (%) with event | 1036 (53.5) | 1349 (53.3) | 1572 (51.2) | 1984 (50.0) |
| Events per 100 patient-yr (95% CI)b | 174.0 | 173.4 | 159.1 | 156.5 |
| (156.9 to 192.9) | (158.3 to 190.9) | (145.1 to 174.6) | (145.0 to 169.0) | |
| P value for rate ratiob | 0.96 | 0.79 | ||
Testing was typically performed once per month on blood collected before the dialysis session.
Generated from generalized estimating equation Poisson regression models with facility as a correlation structure. SI conversion factors: to convert potassium to mmol/L, multiply by 1.0; phosphorus to mmol/L, multiply by 0.323; albumin to g/L, multiply by 10.
Table 3.
Health-related quality of life
| Instrument | Primary Analysis Population, n=4470a | Full Analysis Population, n=7035 | ||
|---|---|---|---|---|
| Intervention, n=1938 | Usual Care, n=2532 | Intervention, n=3069 | Usual Care, n=3966 | |
| SF36-SF PCS | ||||
| N | 531 | 698 | 915 | 1166 |
| Baseline | 37.7 (10.9) | 37.9 (10.6) | 37.4 (10.8) | 38.0 (11.1) |
| Follow-up | 37.9 (10.4) | 37.8 (10.9) | 37.6 (10.7) | 37.9 (11.1) |
| P value for changeb | 0.63 | 0.53 | ||
| SF36-SF MCS | ||||
| N | 531 | 698 | 915 | 1166 |
| Baseline | 49.9 (12.0) | 51.5 (10.9) | 50.0 (11.8) | 51.0 (11.2) |
| Follow-up | 51.0 (11.1) | 51.5 (10.6) | 50.8 (11.2) | 51.4 (10.6) |
| P value for changeb | 0.16 | 0.36 | ||
| SF36-SF effect | ||||
| N | 532 | 696 | 919 | 1167 |
| Baseline | 74.4 (22.8) | 78.0 (20.7) | 74.2 (22.2) | 76.6 (21.0) |
| Follow-up | 78.4 (19.6) | 79.7 (19.3) | 76.4 (21.0) | 77.5 (20.2) |
| P value for changeb | 0.07 | 0.22 | ||
| SF36-SF burden | ||||
| N | 533 | 698 | 920 | 1168 |
| Baseline | 51.2 (29.3) | 54.0 (29.2) | 50.5 (28.7) | 52.9 (28.8) |
| Follow-up | 53.0 (28.7) | 54.9 (29.0) | 51.9 (28.3) | 53.2 (29.1) |
| P value for changeb | 0.62 | 0.48 | ||
| SF36-SF symptoms | ||||
| N | 533 | 696 | 920 | 1166 |
| Baseline | 79.0 (16.0) | 80.9 (14.8) | 78.9 (15.7) | 80.5 (15.3) |
| Follow-up | 80.2 (14.6) | 81.0 (14.0) | 79.5 (14.6) | 80.5 (14.8) |
| P value for changeb | 0.48 | 0.52 | ||
SF36-SF, Kidney Disease Quality of Life Short Form-36; PCS, physical component score; MCS, mental component score; effect, effect of kidney disease scale; burden, burden of kidney disease scale; symptoms, symptoms and problems scale.
As part of routine clinical care, patients are asked to complete the SF36-SF within 4 months after initiation of dialysis and annually thereafter.
Generated from linear-effects models with facility as a random effect.
Intervention Fidelity
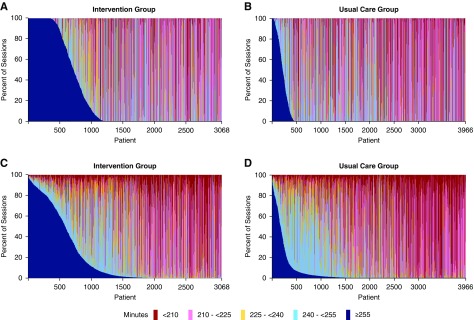
The distributions of session durations by patient and by facility shown in Figure 4 and Supplemental Figure 2, respectively, indicate that the ≥4.25 hour treatment was implemented for the majority of dialysis sessions by a small proportion of participants and facilities. The slopes of the per-patient mean session durations suggest that for individual participants in both groups, session durations decreased as months of dialysis care increased, but this effect was greater for intervention than for usual care patients (Figure 2). Similar patterns were evident for session durations over calendar time (Supplemental Figure 3). An analysis of the relative contributions of session, patient, facility, and dialysis provider organization to the variance of session durations within five different session duration categories (<210, 201 to <225, 225 to <240, 240 to <255, and ≥255 minutes) indicates that, in the intervention group, the facility was an important contributor to variability in implementing the ≥4.25 hour (≥255 minutes) session duration target but not to variability in session durations of <4.25 hours (Supplemental Table 5, A and B).25 Discussions with facility staff and medical directors during the course of the trial indicated that the major reasons for poor uptake of the intervention were unwillingness by patients to have longer dialysis treatments, perception by the treating nephrologists that longer dialysis was not needed because of adequate solute clearance, and perception by the treating nephrologists that longer session durations were not in the best interest of a patient because of older age and/or frailty (Supplemental Tables 6 and 7).
Figure 4.
The distribution of session durations by patient for the full analysis population (all patients) indicates that the target session duration of ≥ than 4.25 hours was implemented for the majority of sessions by a small proportion of patients. Patients are ordered along the x-axis according to the percentage of sessions ≥4.25 hours throughout follow-up. (A) Prescribed session durations for the intervention group. (B) Prescribed session durations for the usual care group. (C) Delivered session durations for the intervention group. (D) Delivered session durations for the usual care group.
Discussion
This study demonstrates that a trial embedded into clinical care delivery with no on-site research personnel can (1) efficiently enroll a large number of participants using an opt-out approach to informed consent, (2) obtain highly granular treatment and outcomes data from hundreds of medical facilities, and (3) monitor trial conduct and safety using a centralized approach. However, although the trial achieved many of its prespecified goals as a demonstration project, the uptake of the intervention was insufficient to determine whether treatment with longer hemodialysis sessions improves clinical outcomes.
There is tremendous interest in pragmatic trials that leverage existing clinical infrastructures because of their potential to produce results that are highly generalizable to the nonresearch setting and reduce the time and resources required for trial conduct.5 Several important successes of the TiME trial provide a foundation for future pragmatic trials in dialysis as well as in other health care settings. The trial enrolled 7035 patients new to dialysis in <3 years, making it not only the largest trial, to date, of incident patients, but also the largest in dialysis overall. The opt-out consent approach was an important contributor to the rapid enrollment and, together with the nonrestrictive eligibility criteria, the opt-out approach generated a trial cohort highly representative of the United States dialysis population. The mean age of the TiME participants was nearly identical to that of the overall United States dialysis population rather than approximately 10 years younger, as has been typical of large dialysis trials.8,26–28 Similarly, the distribution of sex and race, the proportion with diabetes mellitus, and the baseline central venous catheter use, hemoglobin concentration, and serum albumin concentration each mirrored that of the United States incident dialysis population.29,30 Participating facilities were located in 38 states, ensuring broad geographic representation. Fewer than 1% of those otherwise eligible for participation opted out of data-sharing, indicating a high degree of willingness among patients receiving maintenance in-center dialysis to share health data for research purposes. Loss of privacy is one of the major theoretical barriers to acceptability of embedded research.31,32 Although we do not know whether the comfort with sharing data observed in the TiME trial is also present among patients with other medical conditions, or among healthy individuals, the TiME trial experience suggests that concern about sharing personal health data may not be as great as some have anticipated.31,32 The enrollment rate of >99% of eligible patients illustrates the potential for pragmatic trials to efficiently address study questions, particularly if simplified approaches to informed consent can be used.33
The TiME trial successfully harmonized data from more than 250 individual facilities by leveraging the infrastructure of the dialysis provider organizations and the relatively standardized approach to clinical monitoring that characterizes dialysis care. The use of a single IRB of record greatly facilitated oversight of the large number of centers but required willingness by an academic IRB to assume this responsibility, as well as willingness by the two dialysis providers to cede the responsibility. Additionally, the trial developed an approach to monitoring safety that did not require documentation and review of individual adverse events. In contrast to many trials embedded in health care delivery, loss to follow-up was infrequent34; this is likely because of the reliance of patients on dialysis for continued survival, and the low tendency for patients to change dialysis facilities once their care is established. The collaboration between academic investigators and the leadership teams of two health care providers required a legal framework that allowed for the sharing of trial data, including outcomes, with investigators and a DCC outside of the purview of the health systems and, ultimately, with the broader research community. Although there are established processes to prevent sharing of personal health information for individuals participating in research, even when informed consent is waived, a more ad hoc approach is required to ensure analogous protections for providers and health systems participating in research.35
The difficulty implementing the TiME trial intervention exemplifies one of the important challenges for large, pragmatic trials embedded in clinical care delivery. In the TiME trial, inadequate uptake of the intervention occurred despite several protocol elements that were anticipated to reduce barriers to adherence. The trial evaluated a target session duration that is within the standard range of treatment times at many dialysis facilities in the United States, and shorter than the treatments received by most patients in many other countries.6 Trial enrollment was restricted to patients who were new to dialysis to avoid the need to increase session durations that were already established and to keep the intervention operationally feasible for facilities. The medical leaders at the dialysis provider organizations were interested in the trial question, and before facility enrollment and randomization, the medical directors, nurse managers, and administrators for the individual facilities confirmed their willingness to implement the longer sessions. Nonetheless, many facilities found it challenging to implement the intervention. Among the reasons identified by facility personnel for low adherence to the intervention, reluctance by patients to undergo treatments longer than those of many other patients at the same dialysis unit seemed to be the most important. Interestingly, in a qualitative study at dialysis facilities not participating in the TiME trial, patients generally expressed willingness in a hypothetical context to have their facility assigned to longer dialysis session durations for the purpose of research36; however, this was discordant with the findings of the TiME trial.
A second contributor to low uptake of the intervention was the longstanding reliance by United States nephrologists on removal of small solutes (urea, in particular) as the major determinant of session duration. For many patients, urea removal that is considered sufficient can be achieved with hemodialysis treatments that are shorter than the TiME trial intervention.8,15 Despite an appreciation by participating nephrologists for the major hypothesized benefit of longer sessions (reducing hemodynamic alterations from rapid removal of fluid), changing an ingrained practice for the purposes of research proved to be difficult. Engaging clinicians in research when they are not directly involved as investigators is a challenge that should be anticipated by researchers planning future pragmatic trials.37–39 Our analysis of the variance within session duration categories suggests that for intervention facilities, the extent of intervention uptake was driven in large part by facility-level rather than patient-level factors. More work is needed to identify the relevant facility factors, which might include facility size, number of nephrologists, leadership approaches, staffing ratios and stability, and competing initiatives.
The TiME trial difficulties achieving uptake of the intervention should inform future pragmatic trials embedded in clinical care delivery. Recognition of the benefits of partnering with patients to design and conduct research has increased markedly during the past several years and such engagement is becoming more routine.40,41 For the TiME trial, directly involving patients, as members of the research team, in the planning and conduct of the trial might have led to modifications in the design, intervention, and communication strategies that potentially would have resulted in better adherence. Piloting the intervention using an implementation approach similar to that used for the trial also might have led to modifications that would have improved adherence to the intervention. Although we did not conduct a separate pilot study, we did assess the early performance of the first ten facilities enrolled to inform the approach with subsequent facilities. Our finding that adherence to the 4.25 hour sessions during the initial monitoring period was excellent (Supplemental Table 2 footnote) perhaps falsely reassured us that performance would be similar after scaling up the trial implementation. More widespread use of physician and nurse champions also might have improved uptake of the intervention by the on-the-ground clinicians.42 The trial illustrates the importance of assessing fidelity to the intervention, something that often is not possible in pragmatic trials, and is viewed by some as inconsistent with a pragmatic design.43 If dialysis session data had not been available for the TiME trial, the similar outcomes for the two treatment groups might have been misinterpreted as a demonstration that longer sessions durations do not have clinical benefits.
Use of an opt-out approach on the basis of unwillingness to have longer treatment time rather than only on unwillingness to share data might have increased separation between the intervention and usual care groups. However, implementation of this form of opt-out would have been problematic. If the opt-out were provided after facility assignment were known to participants, it would likely have resulted in important imbalances in participant characteristics across treatment groups because the intervention group would be enriched for individuals willing to accept the burden of longer treatment time in the interest of furthering research or potentially improving their health. Although this strategy might have increased the separation in treatment times between groups, there is significant risk that it would have introduced bias, likely favoring the intervention, and potentially resulting in an inappropriate conclusion that longer treatment time improves outcomes. Alternatively, implementing the opt-out on the basis of unwillingness to have longer treatment time before providing potential participants with information about the facility’s randomized assignment might have been perceived by patients or dialysis unit clinicians as deceptive. Additionally, given the high degree of patient–patient and patient–staff interaction that is typical of dialysis facilities, it is likely that some potential participants would know their facility's assignment, resulting in a similar risk of bias as the first approach.
In conclusion, this large pragmatic trial did not answer the question of whether hemodialysis sessions that are longer than many patients in the United States currently receive has clinical benefits, but it did achieve many of its objectives as a demonstration project, and it provides a foundation for future pragmatic trials in dialysis. Developing strategies for effective engagement of clinicians and patients is a critical component of research embedded in routine clinical care delivery.
Disclosures
L.M. Dember receives compensation from the National Kidney Foundation as a Deputy Editor for the American Journal of Kidney Diseases, is a member of a Data Monitoring Committee for Proteon Therapeutics, and is a consultant to GlaxoSmithKline. E. Lacson, Jr. is currently employed by Dialysis Clinic, Inc., a not-for-profit dialysis provider, and was employed by Fresenius Medical Care North America during the planning and much of the conduct of the trial. S.M. Brunelli is employed by DaVita and his wife is employed by AstraZeneca. J.Y. Hsu receives compensation from the National Kidney Foundation as an editor for the American Journal of Kidney Diseases. A.K. Cheung is a consultant for Boehringer-Ingelheim and contributor to UptoDate. C.P. Kovesdy is a recipient of funding for a US Renal Data System Special Study (U01DK102163). D.C. Misulin receives salary support for research activities from Dialysis Clinic, Inc. R.I. Thadhani is a consultant to Fresenius Medical Care North America. A. Young is employed by DaVita. M. Angeletti is employed by Fresenius Medical Care. R.L. Wingard is employed by and receives salary and stock options from Fresenius Medical Care. C. Kahn is employed by and owns stock in Fresenius Medical Care. A.R. Nissenson is employed by and receives salary and stock options from DaVita. F.W. Maddux is employed by and has equity shares in Fresenius Medical Care, and serves on the following Board of Directors: Goldfinch Bio, Vifor Fresenius Medical Care Renal Pharma, Pacific Renal Care Foundation, and American National Bank & Trust (NASDAQ:AMNB). K.C. Abbott has equity in General Electric. The remaining authors have nothing to declare.
Supplementary Material
Acknowledgments
The authors are grateful to the participating patients and the clinical staff, administrators, and nephrologists at the dialysis facilities for their important contributions to the trial. We thank the members of the research teams, informational technology divisions, operations groups, and medical leadership at DaVita and Fresenius Medical Care for embracing a new approach to clinical trial implementation. In particular, we wish to acknowledge the efforts of Shawn Ballard, Mary Burgess, Shalali Cassim, Wendy Gedanken, Elizabeth Glass, Cristin Kwiterovich, Lynn Taylor, and Ann Tierney. Throughout the planning and implementation phases of this work we benefited tremendously from our interactions with the other members of the National Institutes of Health (NIH) Health Care Systems Research Collaboratory.
Individual participant data that underlie the results reported in this article will be available from the Data Repository of the National Institute of Diabetes and Digestive and Kidney Disease 1 year after publication. The data will be deidentified and aggregated across provider organizations. A data use agreement will be required for use of the trial data.
L.M. Dember, E.Lacson, Jr., S.M. Brunelli, J.Y. Hsu A.K. Cheung, J.T. Daugirdas, T. Greene, C.P. Kovesdy, D.C. Miskulin, R.I. Thadhani, W. Winkelmayer, S.S. Ellenberg, A.R. Nissenson, F.W. Maddux, and J.R. Landis contributed to the concept and design of the trial. K.C. Abbott provided administrative, technical, and material support. All authors were involved in acquisition, analysis, or interpretation of data. L.M. Dember and J.Y. Hsu drafted the manuscript. All authors participated in critical revision of the manuscript for important intellectual content. J.Y. Hsu and J.R. Landis performed the statistical analyses. L.M. Dember, E. Lacson, Jr., S.M. Brunelli, J.Y. Hsu, A.K. Cheung, J.T. Daugirdas, T. Greene, C.P. Kovesky, D.C. Miskulin, R.I. Thadhani, W. Winkelmayer, S.S. Ellenberg and J.R. Landis obtained funding. D. Cifelli, R. Madigan, A. Young, M. Angeletti, R.L. Wingard, C. Kahn, A.R. Nissenson, and F.W. Maddux provided administrative, technical, or material support. L.M. Dember, E. Lacson, Jr., S.M. Brunelli, A.R. Nissenson, and F.W. Maddux provided supervision. L.M. Dember had access to the trial data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work was funded by the NIH Common Fund, through the Office of Strategic Coordination within the Office of the NIH Director, and by the National Institute of Diabetes and Digestive and Kidney Diseases, through cooperative agreements UH2 AT007797 and UH3 DK102384 to L.M. Dember.
The data were presented in part as an oral presentation at the American Society of Nephrology Kidney Week in New Orleans, Louisiana, October 31 to November 5, 2017.
The funders worked collaboratively with the investigators on the design of the trial, interpretation of the data, revision and approval of the manuscript, and the decision to submit the manuscript for publication.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018090945/-/DCSupplemental.
Supplemental Appendix 1. Data and Safety Monitoring Board.
Supplemental Appendix 2. Protocol.
Supplemental Appendix 3. Statistical analysis plan.
Supplemental Table 1. Demonstration project objectives.
Supplemental Table 2. Approaches to assessing facility suitability before enrollment and engaging clinical personnel and patients before and during the trial.
Supplemental Table 3. Sample size determination.
Supplemental Table 4. Mortality risk adjusted for baseline characteristics.
Supplemental Table 5. (A) Relative contributions of sources of variation to total variance in prescribed session duration. (B) Relative contributions of sources of variation to total variance in delivered session duration.
Supplemental Table 6. Facility-reported reasons for not implementing intervention session duration.
Supplemental Table 7. Facility-reported challenges implementing the intervention.
Supplemental Figure 1. Dialysis session single-pool Kt/V as an indicator of dialytic urea clearance.
Supplemental Figure 2. Distribution of session durations by facility.
Supplemental Figure 3. Hemodialysis session duration over calendar time.
References
- 1.Inrig JK, Califf RM, Tasneem A, Vegunta RK, Molina C, Stanifer JW, et al.: The landscape of clinical trials in nephrology: A systematic review of Clinicaltrials.gov. Am J Kidney Dis 63: 771–780, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin A, Lancashire W, Fassett RG: Targets, trends, excesses, and deficiencies: Refocusing clinical investigation to improve patient outcomes. Kidney Int 83: 1001–1009, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Dember LM, Archdeacon P, Krishnan M, Lacson E Jr., Ling SM, Roy-Chaudhury P, et al.: Pragmatic trials in maintenance dialysis: Perspectives from the kidney health initiative. J Am Soc Nephrol 27: 2955–2963, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baigent C, Herrington WG, Coresh J, Landray MJ, Levin A, Perkovic V, et al.: KDIGO Controversies Conference on Challenges in the Conduct of Clinical Trials in Nephrology Conference Participants : Challenges in conducting clinical trials in nephrology: Conclusions from a Kidney Disease-Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 92: 297–305, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Califf RM, Robb MA, Bindman AB, Briggs JP, Collins FS, Conway PH, et al.: Transforming evidence generation to support health and health care decisions. N Engl J Med 375: 2395–2400, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Robinson BM, Akizawa T, Jager KJ, Kerr PG, Saran R, Pisoni RL: Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: Differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 388: 294–306, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perl J, Dember LM, Bargman JM, Browne T, Charytan DM, Flythe JE, et al.: American Society of Nephrology Dialysis Advisory Group : The use of a multidimensional measure of dialysis adequacy-moving beyond small solute kinetics. Clin J Am Soc Nephrol 12: 839–847, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, et al.: Hemodialysis (HEMO) Study Group : Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Saran R, Bragg-Gresham JL, Levin NW, Twardowski ZJ, Wizemann V, Saito A, et al.: Longer treatment time and slower ultrafiltration in hemodialysis: Associations with reduced mortality in the DOPPS. Kidney Int 69: 1222–1228, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Miller JE, Kovesdy CP, Nissenson AR, Mehrotra R, Streja E, Van Wyck D, et al.: Association of hemodialysis treatment time and dose with mortality and the role of race and sex. Am J Kidney Dis 55: 100–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall MR, Byrne BG, Kerr PG, McDonald SP: Associations of hemodialysis dose and session length with mortality risk in Australian and New Zealand patients. Kidney Int 69: 1229–1236, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Tentori F, Zhang J, Li Y, Karaboyas A, Kerr P, Saran R, et al.: Longer dialysis session length is associated with better intermediate outcomes and survival among patients on in-center three times per week hemodialysis: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 27: 4180–4188, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flythe JE, Curhan GC, Brunelli SM: Shorter length dialysis sessions are associated with increased mortality, independent of body weight. Kidney Int 83: 104–113, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunelli SM, Chertow GM, Ankers ED, Lowrie EG, Thadhani R: Shorter dialysis times are associated with higher mortality among incident hemodialysis patients. Kidney Int 77: 630–636, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Kidney Foundation : KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis 66: 884–930, 2015 [DOI] [PubMed] [Google Scholar]
- 16.NIH Health Care Systems Research Collaboratory: UH3 Project: Time to Reduce Mortality in End-Stage Renal Disease (TiME). Available at: http://rethinkingclinicaltrials.org/demonstration-projects/uh3-project-time-to-reduce-mortality-in-end-stage-renal-disease-time/. Accessed July 17, 2018
- 17.HCS Research Collaboratory. Available at: https://commonfund.nih.gov/hcscollaboratory. Accessed July 17, 2018
- 18.Agodoa L, Eggers P: Racial and ethnic disparities in end-stage kidney failure-survival paradoxes in African-Americans. Semin Dial 20: 577–585, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Lok CE, Foley R: Vascular access morbidity and mortality: Trends of the last decade. Clin J Am Soc Nephrol 8: 1213–1219, 2013 [DOI] [PubMed] [Google Scholar]
- 20.US Department of Health and Human Services: Code of Federal Regulations - Title 45 Public Welfare CFR 46, 2016. Available at: https://www.govinfo.gov/content/pkg/CFR-2016-title45-vol1/pdf/CFR-2016-title45-vol1-part46.pdf. Accessed December 25, 2018
- 21.RAND Health Care: Kidney Disease Quality of Life Instrument (KDQOL). https://www.rand.org/health-care/surveys_tools/kdqol.html. Accessed July 17, 2018
- 22.Centers for Medicare & Medicaid Services (CMS); Department of Health and Human Services : Medicare and medicaid programs; Conditions for coverage for end-stage renal disease facilities; Final rule. Fed Regist 73: 20369–20484, 2008. [PubMed] [Google Scholar]
- 23.Watson PE, Watson ID, Batt RD: Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr 33: 27–39, 1980 [DOI] [PubMed] [Google Scholar]
- 24.Cox DR: Regression models and life-tables. J R Stat Soc B 34: 187–220, 1972 [Google Scholar]
- 25.Landis JR, King TS, Choi JW, Chinchilli VM, Koch GG: Measures of agreement and concordance with clinical research applications. Stat Biopharm Res 3: 185–209, 2011 [Google Scholar]
- 26.Fishbane S, Schiller B, Locatelli F, Covic AC, Provenzano R, Wiecek A, et al.: EMERALD Study Groups : Peginesatide in patients with anemia undergoing hemodialysis. N Engl J Med 368: 307–319, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, et al.: Dialysis Access Consortium Study Group : Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA 299: 2164–2171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dixon BS, Beck GJ, Vazquez MA, Greenberg A, Delmez JA, Allon M, et al.: DAC Study Group : Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med 360: 2191–2201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saran R, Robinson B, Abbott KC, Agodoa LY, Albertus P, Ayanian J, et al.: US Renal data system 2016 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 69[Suppl 1]: A7–A8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Renal Data System : 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018 [Google Scholar]
- 31.Morain SR, Kass NE: Ethics issues arising in the transition to learning health care systems: Results from interviews with leaders from 25 health systems. EGEMS (Wash DC) 4: 1212, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali J, Califf R, Sugarman J: Anticipated ethics and regulatory challenges in PCORnet: The National Patient-Centered Clinical Research Network. Account Res 23: 79–96, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Sugarman J, Califf RM: Ethics and regulatory complexities for pragmatic clinical trials. JAMA 311: 2381–2382, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Haneuse S, Bogart A, Jazic I, Westbrook EO, Boudreau D, Theis MK, et al.: Learning about missing data mechanisms in electronic health records-based research: A survey-based approach. Epidemiology 27: 82–90, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon GE, Coronado G, DeBar LL, Dember LM, Green BB, Huang SS, et al.: Data sharing and embedded research. Ann Intern Med 167: 668–670, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraybill A, Dember LM, Joffe S, Karlawish J, Ellenberg SS, Madden V, et al.: Patient and physician views about protocolized dialysis treatment in randomized trials and clinical care. AJOB Empir Bioeth 7: 106–115, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.PCORnet: The National Patient-Centered Clinical Research Network. Available at: http://www.pcornet.org. Accessed July 17, 2018
- 38.Weinfurt KP, Hernandez AF, Coronado GD, DeBar LL, Dember LM, Green BB, et al.: Pragmatic clinical trials embedded in healthcare systems: Generalizable lessons from the NIH collaboratory. BMC Med Res Methodol 17: 144, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Topazian R, Bollinger J, Weinfurt KP, Dvoskin R, Mathews D, Brelsford K, et al.: Physicians’ perspectives regarding pragmatic clinical trials. J Comp Eff Res 5: 499–506, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domecq JP, Prutsky G, Elraiyah T, Wang Z, Nabhan M, Shippee N, et al.: Patient engagement in research: A systematic review. BMC Health Serv Res 14: 89, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sacristán JA, Aguarón A, Avendaño-Solá C, Garrido P, Carrión J, Gutiérrez A, et al.: Patient involvement in clinical research: Why, when, and how. Patient Prefer Adherence 10: 631–640, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson KE, Tachibana C, Coronado GD, Dember LM, Glasgow RE, Huang SS, et al.: A guide to research partnerships for pragmatic clinical trials. BMJ 349: g6826, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al.: A pragmatic-explanatory continuum indicator summary (PRECIS): A tool to help trial designers. J Clin Epidemiol 62: 464–475, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.