Significance Statement
Dysregulation of vasopressin-induced water reabsorption in the renal collecting duct leads to diabetes insipidus, a congenital or acquired syndrome. Some forms of diabetes insipidus lack effective treatments to prevent the excessive loss of hypotonic urine that characterizes the condition. The authors previously identified the antimycotic drug fluconazole as a potential therapy, acting to modulate the effects of a water channel protein aquaportin-2 (AQP2). In this study, they show in vitro and in vivo that fluconazole induces a vasopressin-independent insertion of AQP2 into the plasma membrane of collecting duct principal cells, thereby lowering urinary output. Hence, fluconazole might have clinical utility in treating certain forms of diabetes insipidus—such as hereditary X-linked nephrogenic diabetes insipidus—in which the kidney responds inappropriately to vasopressin.
Keywords: cyclic AMP, diabetes insipidus, intracellular signal, renal cell biology, vasopressin, water channels
Abstract
Background
Arginine-vasopressin (AVP) binding to vasopressin V2 receptors promotes redistribution of the water channel aquaporin-2 (AQP2) from intracellular vesicles into the plasma membrane of renal collecting duct principal cells. This pathway fine-tunes renal water reabsorption and urinary concentration, and its perturbation is associated with diabetes insipidus. Previously, we identified the antimycotic drug fluconazole as a potential modulator of AQP2 localization.
Methods
We assessed the influence of fluconazole on AQP2 localization in vitro and in vivo as well as the drug's effects on AQP2 phosphorylation and RhoA (a small GTPase, which under resting conditions, maintains F-actin to block AQP2-bearing vesicles from reaching the plasma membrane). We also tested fluconazole's effects on water flow across epithelia of isolated mouse collecting ducts and on urine output in mice treated with tolvaptan, a VR2 blocker that causes a nephrogenic diabetes insipidus–like excessive loss of hypotonic urine.
Results
Fluconazole increased plasma membrane localization of AQP2 in principal cells independent of AVP. It also led to an increased AQP2 abundance associated with alterations in phosphorylation status and ubiquitination as well as inhibition of RhoA. In isolated mouse collecting ducts, fluconazole increased transepithelial water reabsorption. In mice, fluconazole increased collecting duct AQP2 plasma membrane localization and reduced urinary output. Fluconazole also reduced urinary output in tolvaptan-treated mice.
Conclusions
Fluconazole promotes collecting duct AQP2 plasma membrane localization in the absence of AVP. Therefore, it might have utility in treating forms of diabetes insipidus (e.g., X-linked nephrogenic diabetes insipidus) in which the kidney responds inappropriately to AVP.
The binding of antidiuretic hormone (arginine-vasopressin [AVP]) to its cognate V2 receptors (V2Rs) on the surface of renal collecting duct principal cells leads to the redistribution of the water channel aquaporin-2 (AQP2) from intracellular vesicles into the plasma membrane. The plasma membrane insertion increases the osmotic water permeability of the collecting duct and facilitates water reabsorption from primary urine.1–3 AQP2 constitutively recycles between its intracellular and plasma membrane localization. V2R stimulation shifts this equilibrium toward plasma membrane localization.4 This shift is associated with changes in the phosphorylation of serine 256 (S256), S264, and S269 of AQP2 as well as a decrease in the phosphorylation of S261 and a reduction in ubiquitination.5–10
The AVP-induced redistribution of AQP2 is also associated with the inhibition of the small GTPase, RhoA. RhoA maintains F-actin as a physical barrier, preventing AQP2-bearing vesicles from reaching the plasma membrane and thus, avoiding inappropriate water reabsorption under resting conditions. AVP induces the protein kinase A (PKA)–mediated phosphorylation of RhoA at S188 and provides for its inhibition. The inhibition causes depolymerization of F-actin and the removal of the barrier, facilitating the redistribution of AQP2 to the plasma membrane.11–14
Dysregulation of AVP-mediated water reabsorption is associated with or causes disease. When AVP levels are increased, such as in the syndrome of inappropriate antidiuretic hormone secretion, late-stage heart failure, or hepatic cirrhosis, AQP2 is predominantly located in the plasma membrane and causes excessive water retention, leading to hyponatremia. Vice versa, defects of the system cause diabetes insipidus (DI). Patients with DI produce large amounts of hypotonic urine and polydipsia.15–18 If the hormone is absent as a consequence of mutations in the encoding gene, the disease is classified as central DI. When the collecting duct principal cells cannot respond to the hormone due to mutations in the genes encoding the V2R or AQP2, the result is nephrogenic DI. DI can also be acquired, for example, as a consequence of lithium therapy of bipolar disorder. In about 55% of the patients treated with lithium, the AQP2 redistribution is inhibited. Thiazide diuretics and a low-salt diet are prescribed to increase solute and water reabsorption in other nephron segments.17 However, effective treatments are not available for all forms of DI.
In the case of deficient AVP, defects in the V2R or in the presence of lithium, AQP2, and the machinery for its transport to the plasma membrane are intact. To find potential targets and molecules that might exhibit clinical utility in the treatment of such DI forms, we screened 17,700 small molecules in a cell-based assay to identify modulators of the AQP2 localization. In that study, the widely used antimycotic drug, fluconazole, emerged as a candidate.19 Fluconazole is the first-in-line drug for treatment of mucosal and invasive Candida infections.20 Fluconazole is also an integrated component for treating cryptococcal infections.21 The drug belongs to the azole family and serves as an alternative to ketoconazole because of fewer side effects.22 Fluconazole inhibits 11-β-hydroxylase and 17-α-hydroxylase and has been used in treating Cushing syndrome. In that regard, fluconazole was superior to ketoconazole.23
In this study, we investigated the hypothesis that fluconazole can induce trafficking of AQP2 to the plasma membrane, thereby increasing osmotic water transport in the renal collecting duct and reducing water excretion.
Methods
Fluorescence Microscopic Detection of AQP2 F-Aktin in Primary Inner Medullary Collecting Duct Cells and Mouse Kidneys
Primary rat inner medullary collecting duct (IMCD) cells were obtained and cultured, and AQP2 and F-actin were visualized by laser scanning microscopy (LSM780; Zeiss) as previously described.19,24 AQP2 was detected with antibody H27 (1:600) and Cy3-coupled anti-rabbit IgG (#211–165–109, 1:300; Jackson ImmunoResearch Laboratories). F-actin was visualized using Alexa Fluor 647-Phalloidin (#A22287; 1:30; Invitrogen). Nuclei were stained with 4′,6-diamidine-2′-phenylindole dihydrochloride (#10236276001, 1:100; Roche Diagnostics GmbH).
Mouse kidneys were fixed in PBS containing 4% paraformaldehyde (1 hour at 4°C), dehydrated, and embedded in Tissue-Tek O.C.T. Compound (Sakura Finetek) for cryosectioning. Immunofluorescence microscopy was carried out as described.25 In brief, unspecific binding was inhibited by blocking with fish skin gelatin (0.27%) for 60 minutes at 37°C. The sections were incubated with anti-AQP2 antibody H27 (1:600 dilution) overnight at 4°C, washed three times with PBS, and incubated with Cy3-coupled secondary anti-rabbit antibody (1:300; see above). Nuclei were detected with 4′,6-diamidine-2′-phenylindole dihydrochloride and F-actin through incubation with Alexa Fluor 647-Phalloidin for 45 minutes at 37°C. Finally, the sections were washed with PBS three times, and signals were visualized using the LSM780.
Animal Experiments
Wild-type BALB/C6 mice were housed in the animal facility of the Max Delbrück Center for Molecular Medicine according to the recommendations of the Federation of European Laboratory Animal Science Associations in a specific pathogen-free environment. All procedures were carried out in accordance with ethical guidelines and permission of the local authority (Landesamt für Gesundheit und Soziales Berlin and Ministerium für Energiewende, Landwirtschaft, Umwelt und ländliche Räume des Landes Schleswig-Holstein, Kiel).
Seven-week-old C57bl/6N male mice were water deprived for 24 hours, and they were treated with equal volumes of NaCl (0.9% saline; control), fluconazole (dissolved in 0.9% saline; Braun Melsungen), tolvaptan (Samsca), or combinations as indicated in Results and the figures. The animals were treated with intraperitoneal fluconazole injections (80 mg/kg) to achieve a concentration of 15.4 mg/L in the blood or with saline every 24 hours over a period of 96 hours. Tolvaptan was administered for the final 24 hours by intraperitoneal injection. Plasma levels of fluconazole were determined by mass spectrometry by a commercial vendor (Labor 28). Urine was collected in metabolic cages, and osmolality measured using a cryo-osmometer. At the end of the experiments, the mice were euthanized by cervical dislocation, and kidneys were harvested.
Specifically, we conducted three different experiments. The timelines are indicated in the figures and a scheme representing the regimes in Supplemental Figure 1: (1) 4 days fluconazole or saline with 24 hours of water restriction in metabolic cages, (2) 4 days of fluconazole or saline treatment without metabolic cages and water restriction, and (3) 4 days of fluconazole or saline treatment with a single dose of tolvaptan or saline.
Western Blotting, Immunoprecipitation, and Determination of RhoA Activity
Western blotting for the detection of AQP2, the phosphorylated forms of AQP2, RhoA, and RhoA phosphorylated at S188 and Hsp90 and Rhotekin pull downs for the detection of active GTP-bound RhoA were carried out as previously described.8,11–14,19
For determination of RhoA activity, IMCD cells were treated with fluconazole and/or forskolin, subsequently incubated with ice-cold Rhotekin buffer (50 mM Tris, pH 7.2, 1% [wt/vol] Triton X-100, 0.5% sodium deoxycholate, 500 mM NaCl, 10 mm MgCl2, PhosSTOP EASY [Roche Diagnostics], and Complete mini EDTA-free [Roche Diagnostics]) for 10 minutes, and lysed. Lysates (300–400 μg protein) were incubated with 300 μl of Rhotekin beads. Proteins were eluted from beads with Laemmli buffer and analyzed by Western blotting. Active RhoA was related to RhoA in the input fraction and the ratio to the loading control, Hsp90.
Immunoprecipitation of AQP2 from IMCD cells was carried out as described.8 In brief, cells were lysed with ubiquitin lysis buffer (0.15 M NaCl, 25 mM HEPES, 1% Triton X-100, 2.5 mM NEM, 0.5 mM PMSF, 1× PhosSTOP EASY, and 1× Complete mini EDTA-free); cell debris was removed by centrifugation, and the supernatants were incubated with Protein A-Sepharose, preincubated with mouse monoclonal anti-AQP2 antibody E-2 (sc-515770; Santa Cruz; 4 μl/20 mg of Sepharose beads in 1 ml of ubiquitin lysis buffer), and rotated overnight at 4°C. Beads were washed four times with ubiquitin lysis buffer. AQP2 and coprecipitated ubiquitin were detected by Western blotting (anti-AQP2 C-17 antibody; Santa Cruz) and monoclonal mouse antibody against ubiquitin (Cell Signaling).
PKA and Para-Nitrophenylphosphate Phosphatases Activity Assays
PKA activity was monitored using the PepTag Assay (Promega) according to the manufacturer’s instruction. For para-Nitrophenylphosphate–based evaluation, cells were lysed in lysis buffer without phosphatase inhibitors, and aliquots of 100 μg of lysates in triplicates were diluted in Colorimetric Assay Buffer (containing 10 mM para-Nitrophenylphosphate) as described.26 After 30 minutes of incubation at 30°C, the absorbance was determined at 405 nm with an xMarkMicroplate Absorbance Spectrophotometer (Bio-Rad), and phosphatase activity was calculated.
Renal Collecting Duct Water Permeability Measurement
Renal cortical collecting ducts (CCDs) of 12 C57bl/6J mice (male, 8–11 weeks old) were dissected and perfused for luminal fluorescence measurements.27 The CCDs were luminally perfused with solution 150 (osmolality: 150 mosm/kg; 72.5 mM NaCl, 0.2 mM KH2PO4, 0.8 mM K2HPO4, 0.5 mM MgCl2, 0.65 mM Ca-gluconate, and 2.5 mM glucose supplemented with 50 μM 150 kD FITC dextran) while continuously superfused (approximately 5 ml/min) with control solution (osmolality: 300 mosm/kg; 145 mM NaCl, 0.4 mM KH2PO4, 1.6 mM K2HPO4, 1 mM MgCl2, 1.3 mM Ca-gluconate, and 5 mM glucose, pH 7.4). After initial perfusion with fluorescent solution 150, the distal end was occluded with a holding pipette. A constant perfusion pressure at the perfusion side kept the tubule open and at constant volume. The fluorescence emission intensity from the lumen was monitored. On reaching a stable baseline of at least 90 seconds without collapse or clearly visible leakage, forskolin (30 μM), fluconazole (50 μM), or as a control, the solvent of these agents, DMSO (0.1%), was added to the bath (basolateral side of CCD; time point 0), and luminal fluorescence was monitored for another 180 seconds. A transepithelial water flow is indicated by an increment of luminal fluorescence. The increment results from water leaving the lumen along the lumen to bath osmotic gradient and an increase in luminal FITC dextran concentration caused by replenishment through the perfusion pipette. Fluorescence intensity over time was analyzed by Meta-Fluor software and normalized to the intensity at time point 5 seconds before time point 0. Relative fluorescence intensity changes as measures of water permeability were calculated after application of the indicated agents.
Statistical Analyses
Statistical analyses were carried out using GraphPad Prism 7 software and the Mann–Whitney U test, t test, or one-way ANOVA combined with a Bonferroni post hoc comparison test to evaluate statistical significance.
Results
Fluconazole Promotes the AQP2 Plasma Membrane Localization and Decreases Urine Output Independent of AVP
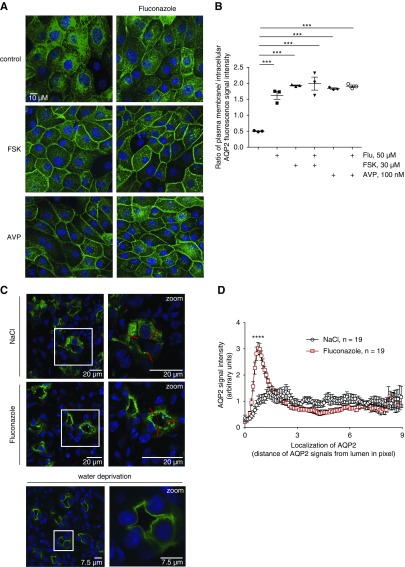
We tested the influence of fluconazole on the AQP2 localization in primary rat IMCD cells (Figure 1). Under resting conditions, AQP2 is located mainly in the perinuclear region of the cells. AVP or direct activation of adenylyl cyclases with forskolin leads to cAMP elevation, activation of PKA, and the redistribution of AQP2 to the plasma membrane.8,24 Fluconazole alone (50 μM) enhanced the membrane localization of AQP2, similar to forskolin and AVP, but had no additive effect with these agents (Figure 1A). Semiquantitative analyses of intracellular and plasma membrane AQP2 fluorescence signal intensities19,28 (Figure 1B) confirmed these observations. The data were surprising, because our preliminary findings had suggested an inhibitory effect of fluconazole on the forskolin-induced redistribution of AQP2.19
Figure 1.
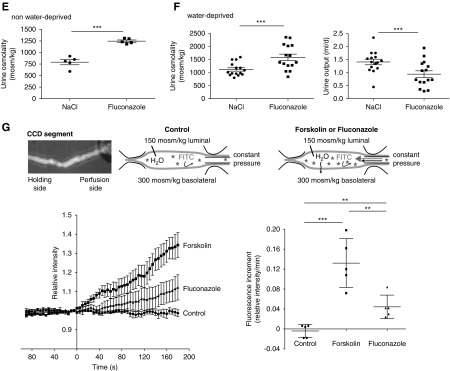
Fluconazole (Flu) promotes a plasma membrane localization of aquaporin-2 (AQP2), and it decreases urine output and increases urine osmolality in mice. (A) Primary inner medullary collecting duct cells were left untreated (control) or stimulated with forskolin (FSK; 30 μM, 30 minutes), arginine-vasopressin (AVP; 100 nM, 30 minutes), or Flu (50 μM, 60 minutes) alone or in combination with FSK or AVP (addition of FSK or AVP for an additional 30 minutes). AQP2 was detected by immunofluorescence microscopy using specific primary (H27) and Cy3-coupled secondary antibodies (green). Nuclei were stained with 4′,6-diamidine-2′-phenylindole dihydrochloride (blue). Shown are representative images from one of three independent experiments. (B) Intensities of intracellular and plasma membrane immunofluorescence signals arising from AQP2 were determined, related to perinuclear signal intensities, and ratios of plasma membrane to intracellular signal intensities were calculated. Ratios less than one indicate a predominant intracellular localization, and ratios greater than one indicate a predominant plasma membrane location of AQP2 (mean±SEM; n=3 independent experiments). ***P<0.001. (C–F) Wild-type C57bl/6N male mice were treated every 24 hours over a period of 96 hours with Flu (intraperitoneal injections; 80 mg/kg body wt) or 0.9% saline (NaCl), or they were water deprived without additional treatment. Schemes for depicting animal treatments are indicated in Supplemental Figure 1. (C) Tissue sections from kidneys were prepared, and AQP2 and nuclei were detected as indicated in A. Shown are representative images from three independent experiments each carried out with five animals per condition. The magnified views of collecting ducts are from the indicated white boxes. (D) The AQP2 localization was semiquantitatively analyzed in three animals (n=19 collecting ducts of each Flu and NaCl treated). Plotted is the AQP2 signal intensity along the indicated red line. The peak in Flu signals corresponds to the apical plasma membrane of collecting duct principal cells (mean±SEM). ****P<0.001. (E) Baseline urine osmolality measurements with mice that had access to water ad libitum were carried out in stock cages using spot urine. (F) Water-deprived animals were kept in metabolic cages during the final 24 hours of the experiment. Urine was collected, and urine osmolality and output were monitored. n=15 animals per water-derived group and n=5 per nonwater-deprived group. Statistically significant differences are indicated (mean±SEM). ***P<0.001. (G) In vitro microperfusion of cortical collecting ducts (CCDs) isolated from wild-type C57Bl/6J mice. (Upper left panel) Combined light microscopy and fluorescence (485-nm) image of a live CCD segment in perfusion with 150 kD FITC dextran in the lumen. The CCD segments were occluded at the distal end with a holding pipette and connected on the opposite side to a perfusion pipette. (Upper center panel) The experimental setting in the control situation in the absence of treatment and on DMSO application. (Upper right panel) In the presence of FSK or Flu, water exits the lumen and is replenished via the perfusion pipette, which leads to a continuous increase of intraluminal FITC dextran. An osmotic gradient is established through the presence of 150 mosm/kg solution within the CCD lumen and 300 mosm/kg solution in the bath (basolateral). The CCDs were perfused at a constant pressure, and continuous monitoring of the fluorescence emission intensity from the lumen was started. On reaching a stable baseline of at least 90 seconds, FSK (30 μM), Flu (50 μM), or DMSO as a control (0.1%) was added (time point: 0 seconds). Fluorescence was measured for another 180 seconds. Results are summarized in the lower left panel. Increased water flow across the epithelia was quantitatively expressed as fluorescence increment values as a direct measure of water permeation velocity (lower right panel). Statistically significant differences are indicated (mean±SEM). For control, n=5 CCDs from five animals. For FSK, n=5 CCDs from five animals, and for Flu, n=5 CCDs from four animals. Each CCD was measured independently. ***P<0.001.
To test whether fluconazole promoted AQP2 redistribution and water reabsorption in vivo, we treated wild-type mice with fluconazole (Figure 1, C–F). The fluconazole dose was chosen to achieve the extracellular fluconazole concentration in patients treated for fungal infections. The patients receive 200–400 mg/d.29 We used 80 mg/kg body wt for 4 days in the mice, and the serum concentrations were 15–50 μM (data not shown). This is also similar to the concentration applied in our cell culture experiments above. Immunofluorescence microscopy (Figure 1C) and semiquantitative analysis of the AQP2 localization (Figure 1D) revealed that the renal collecting duct principal cells of fluconazole-treated mice displayed an accumulation of AQP2 at the apical plasma membrane. This situation resembled the localization of AQP2 after water deprivation, a condition associated with high plasma AVP levels. In control animals treated with equivalent volumes of 0.9% saline, AQP2 was found throughout the cytoplasm.
The fluconazole-induced redistribution of AQP2 to the plasma membrane suggested that the drug promotes water reabsorption in collecting ducts, thereby increasing urinary osmolality. Indeed, compared with mice treated with 0.9% saline, fluconazole treatment increased urine osmolality in mice allowed free access to drinking water (Figure 1E). Under water deprivation fluconazole-treated mice displayed decreased urinary output and increased urinary osmolality compared with 0.9% saline-treated mice (Figure 1F). We calculated creatinine clearance in baseline male mice as a measure for the GFR according to Dunn et al.30 We saw no difference in creatinine clearance between fluconazole- and saline chloride–treated animals. There was also no difference in water or food intake (Supplemental Figure 2).
To test whether the fluconazole-induced redistribution of AQP2 is associated with an increase in osmotic water permeability, we measured water flow across epithelia of freshly isolated mouse CCDs. The CCDs were perfused with a hypotonic solution containing FITC dextran. When added to the bath (basolateral), fluconazole and forskolin induced a transepithelial water flow as indicated by an increment of luminal fluorescence (Figure 1G). Fluconazole and forskolin did not alter the luminal diameter, and without the lumen to bath osmotic gradient, fluconazole did not induce transepithelial water flow (Supplemental Figure 3). Thus, the fluconazole-induced redistribution of AQP2 in renal principal cells caused an increase in water reabsorption.
Fluconazole Modulates AQP2 Phosphorylation and Increases Its Abundance
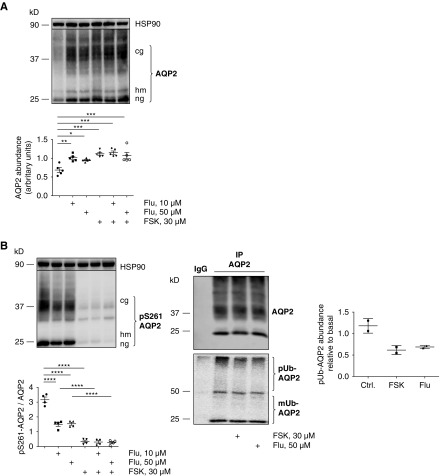
To elucidate the molecular mechanisms underlying the fluconazole-induced AQP2 trafficking, we investigated signaling downstream of the V2R. As previously reported,8 forskolin increased the abundance of AQP2 in IMCD cells within 30 minutes (Figure 2A). Fluconazole in concentrations of 10 and 50 μM alone had a similar effect (Figure 2A). An additive effect of forskolin and fluconazole was not evident.
Figure 2.
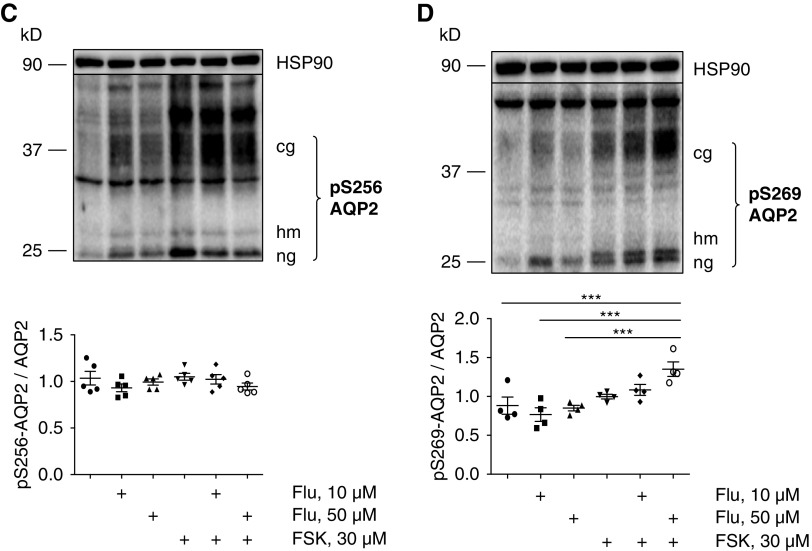
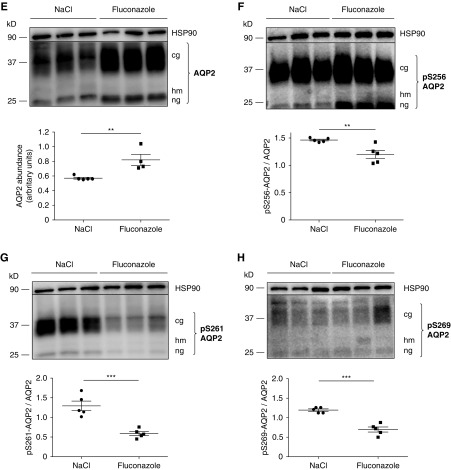
Fluconazole (Flu) increases aquaporin-2 (AQP2) abundance and changes the phosphorylation status of AQP2 in primary inner medullary collecting duct (IMCD) cells and mice. (A–D) IMCD cells were left untreated or stimulated with forskolin (FSK; 30 μM, 30 minutes) or Flu alone or in combination with FSK in the indicated concentrations. If Flu and FSK were combined, FSK was added after 30 minutes of incubation with Flu for another 30 minutes. Lysates were prepared, and AQP2 was detected by Western blotting: (A) glycosylated (cg), high mannose (hm), and nonglycosylated (ng) forms of AQP2 and (B) AQP2 phosphorylated at serine 261 (S261; pS261-AQP2), (C) S256 (pS256-AQP2), and (D) S269 (pS269-AQP2). HSP90 was detected as the loading control. Shown are representative blots from one of four independent experiments. The signals emerging from the cg, hm, and ng forms of AQP2 were semiquantitatively analyzed by densitometry, and the sums were statistically compared. Statistically significant differences are indicated (mean±SEM). *P<0.05; **P<0.01; ***P<0.001; ****P<0.001. (C, right panel) IMCD cells were left untreated or treated with FSK (30 μM, 30 minutes) or Flu (50 μM, 30 minutes). AQP2 was immunoprecipitated (IP), and AQP2 and coimmunoprecipitated ubiquitin were detected by Western blotting (polyubiquitin [pUbi] and monoubiquitin [mUbi]). (E–H) Wild-type C57bl/6N male mice were treated every 24 hours over a period of 96 hours with Flu (intraperitoneal injections; 80 mg/kg) or 0.9% NaCl. The renal inner medullae (n=5 per each condition) were obtained, lysates were prepared, and AQP2 was detected by Western blotting: (E) AQP2, (F) pS256-AQP2, (G) pS261-AQP2, (H) pS269-AQP2, and HSP90 as loading control. Shown are representative Western blots. (Lower panels) The signals emerging from the cg, hm, and ng forms of AQP2 were semiquantitatively analyzed by densitometry, and the sums were statistically compared. Statistically significant differences are indicated (mean±SEM). **P<0.01; ***P<0.001.
We had previously shown that a rise in cAMP and PKA activation in IMCD cells causes inhibition of p38MAPK-mediated phosphorylation of AQP2 at S261.8 This leads to a decrease in AQP2 ubiquitination and thereby, a decrease in the proteolytic degradation of AQP2.8 The result is an increase in AQP2 protein abundance.8 Fluconazole alone also mediated a decrease in the S261 phosphorylation (Figure 2B) that was associated with a decrease in the ubiquitination of AQP2, explaining the increase in AQP2 abundance (Figure 2A). Fluconazole did not enhance the forskolin effect on the S261 phosphorylation. The fluconazole-induced decrease of AQP2 ubiquitination was also observed in MCD4 cells; MCD4 is an immortalized cell line that represents another model system for the AQP2 redistribution (data not shown). Fluconazole did not affect AQP2 mRNA levels in IMCD cells or our mice (Supplemental Figure 4).
V2R stimulation regulates phosphorylation of AQP2 at S256 and S269, and these alterations were previously linked with its endocytosis.7,31 Fluconazole alone did not affect AQP2 S256 or S269 phosphorylation in IMCD cells (Figure 2, C and D). Consistent with our findings in IMCD cells, fluconazole treatment in mice increased the AQP2 abundance and decreased the S261 phosphorylation. The phosphorylation of AQP2 at S256 and S269 was decreased in response to fluconazole (Figure 2, E–H).
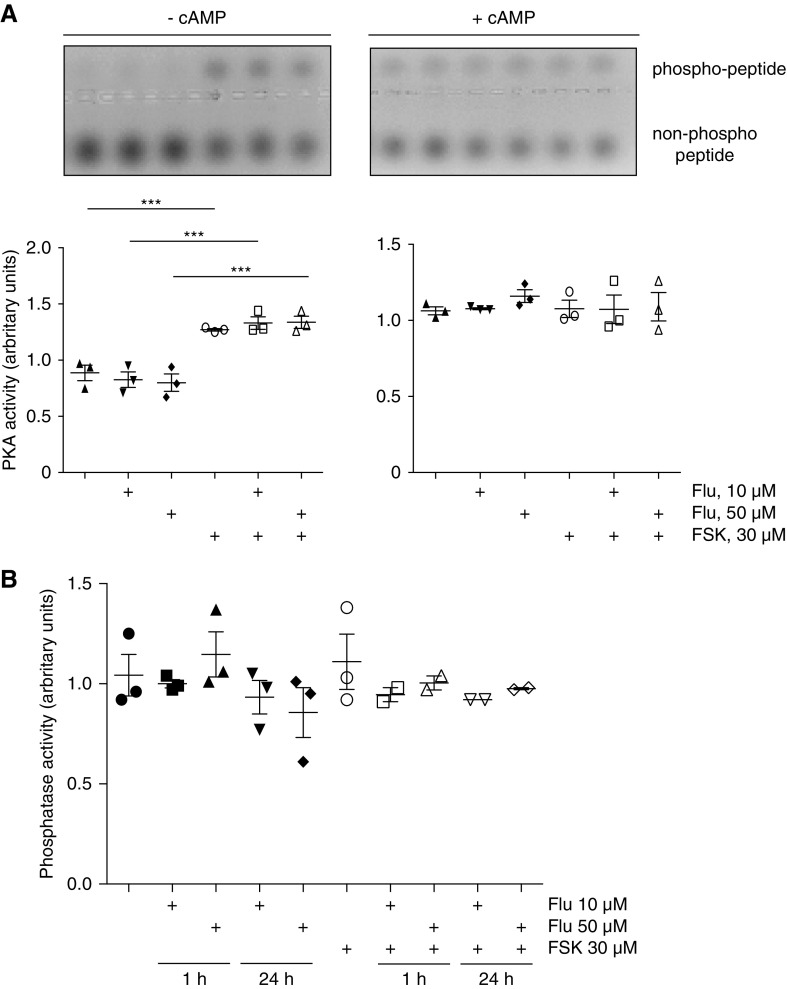
V2R stimulation induces PKA activation. Therefore, we determined the effect of fluconazole on global PKA activity in IMCD cells. Although forskolin increased PKA activity compared with the basal state, fluconazole did not (Figure 3A). The decrease in the phosphorylation of S261 could be due to activation of protein phosphatases. However, fluconazole did not modulate protein phosphatase activity in IMCD cells (Figure 3B). Thus, changes of global PKA or phosphatase activity did not explain the effect of fluconazole on the phosphorylation status of AQP2.
Figure 3.
Fluconazole (Flu) does not affect global protein kinase A (PKA) or protein phosphatase activity in inner medullary collecting duct (IMCD) cells. IMCD cells were left untreated, or they were stimulated with forskolin (FSK; 30 μM, 30 minutes), Flu alone or in combination with FSK for 1 hour in the indicated concentrations (FSK was added for the final 30 minutes). (A) Cell lysates were prepared, and PKA activity was determined by measuring its ability to phosphorylate the substrate peptide PepTag A1. Where indicated, cAMP (1 mM) was added to induce maximal PKA activity. PKA activity is expressed as the ratio of phosphorylated to nonphosphorylated PepTag A1 peptides. (Upper panels) Agarose gels from one representative of three experiments showing PKA-phosphorylated and nonphosphorylated PepTag A1 peptide. (Lower panels) The amounts of phosphorylated and nonphosphorylated PepTag A1 peptides were semiquantitatively analyzed. Statistically significant differences are indicated (mean±SEM). ***P<0.001. (B) IMCD cells were left untreated or stimulated with FSK, Flu alone, Flu in combination with FSK for 1 or 24 hours in the indicated concentrations. Lysates were prepared without phosphatase inhibitors, and amounts of 100 μg of proteins were loaded (each sample in triplicate in 96-well plate). The samples were incubated with substrate solution (10 mM para-Nitrophenylphosphate [pNPP]) for 30 minutes at 37°C, and absorbance was measured at 405 nm. The molar extinction coefficient for pNPP is 18,000 M−1 cm−1. The blank was subtracted to account for any phosphate release occurring in the absence of phosphatase. Shown are results from three independent experiments. There were no statistically significant differences found (mean±SEM).
Fluconazole Decreases RhoA Activity and Depolymerizes F-Actin
We had previously shown that V2R/PKA activation in renal principal cells causes inhibition of RhoA and the depolymerization of F-actin, which could remove a physical barrier to facilitate the transport of AQP2-bearing vesicles to the plasma membrane. Similarly, inhibition of RhoA with toxins was associated with a depolymerization of F-actin and the redistribution of AQP2, indicating that RhoA is an important regulator of the localization of AQP2.11–14
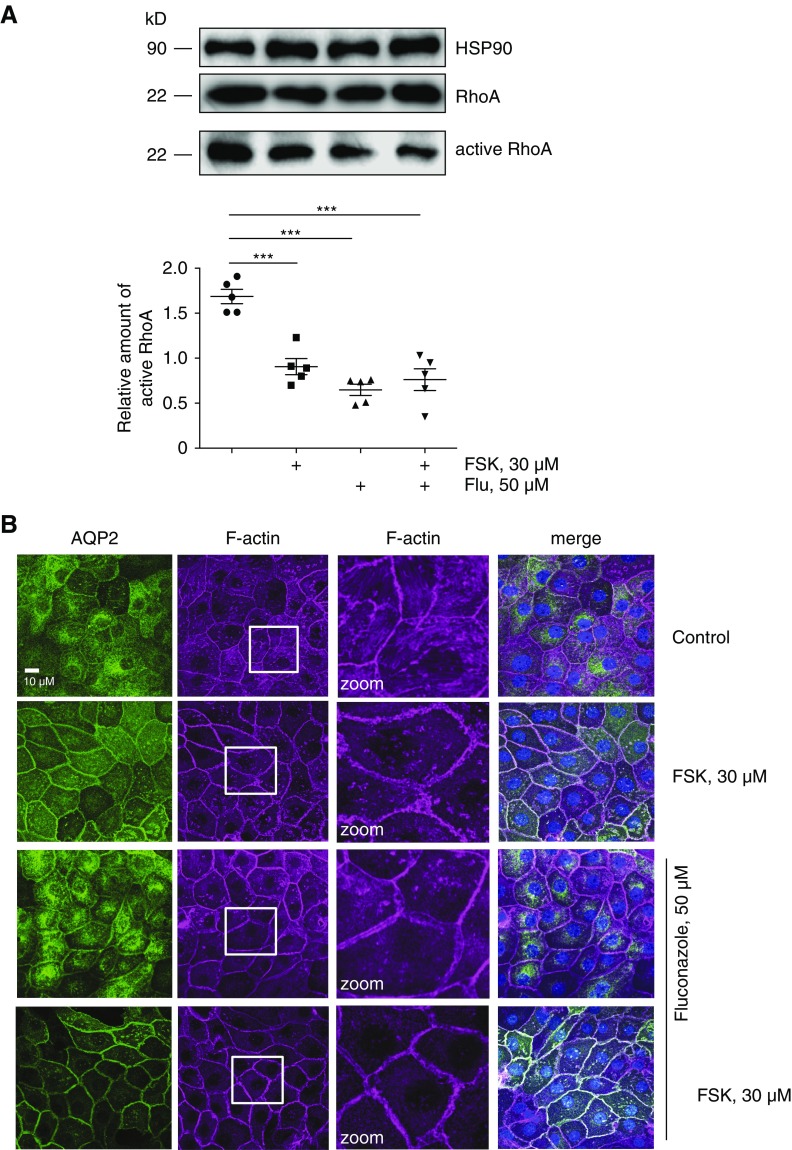
In line with our previous observations,12,13 forskolin reduced RhoA activity in our primary IMCD cells (Figure 4A). Fluconazole alone had a similar effect. The two agents together did not act additively. A decrease of F-actin was evident on forskolin treatment compared with the control condition (Figure 4B). Fluconazole alone decreased F-actin, and when cells were treated with both fluconazole and forskolin, intracellular F-actin almost vanished (Figure 4B).
Figure 4.
Fluconazole (Flu) causes a decrease of RhoA activity and a depolymerization of F-actin in inner medullary collecting duct (IMCD) cells. (A) IMCD cells were left untreated or stimulated with forskolin (FSK), Flu alone, or Flu in combination with FSK for 1 hour in the indicated concentration. Lysates were prepared, and active RhoA was precipitated with Rhotekin beads. Precipitated active RhoA, RhoA in the lysates, and HSP90 as a loading control were detected by Western blotting using specific antibodies. The amount of active RhoA was related to normalized RhoA (RhoA in lysates to HSP90 [input]). Shown are representative blots from one of five independent experiments. Blots were semiquantitatively analyzed and statistically compared. Statistically significant differences are indicated (mean±SEM). ***P<0.001. (B) IMCD cells were left untreated or treated as indicated in A. Aquaporin-2 (AQP2) was detected by immunofluorescence microscopy using specific primary antibodies (H27) and Cy3-coupled anti-rabbit secondary antibodies (green), and F-actin was detected by using Alexa Fluor 647-Phalloidin (red). Nuclei were stained with 4′,6-diamidine-2′-phenylindole dihydrochloride (blue). The magnified views (zoom) of F-actin are from the indicated squares. Shown are representative images from one of three independent experiments.
The effect of fluconazole was similar in mice. Fluconazole reduced RhoA activity in the renal inner medullae compared with treatment with 0.9% saline (Figure 5A). This was associated with an increase in the inactivating phosphorylation of RhoA at S188 (Figure 5B), explaining the reduction of RhoA activity. Consistently, fluconazole caused a marked reduction in F-actin (Figure 5C).
Figure 5.
Fluconazole decreases RhoA activity and causes depolymerization of F-actin in renal inner medullae of mice. Wild-type C57bl/6N male mice were treated with fluconazole intraperitoneal injections (80 mg/kg) or 0.9% NaCl every 24 hours over a period of 96 hours. (A) Lysates were prepared from renal inner medullae (n=5 per each condition), and active RhoA was precipitated with Rhotekin beads. Precipitated active RhoA, RhoA, and HSP90 (loading control) in the lysates were detected using specific antibodies. Shown are representative Western blots. Blots were semiquantitatively analyzed, and signals were statistically compared. The signals arising from active RhoA were related to normalized RhoA (RhoA to HSP90 in lysates). Statistically significant differences are indicated (mean±SEM). **P<0.01. (B) Mice were treated as indicated in A, and lysates from one inner medulla from each animal were prepared (n=5 per condition). Total RhoA, inactive RhoA phosphorylated at serine 188 (S188; pS188-RhoA), and HSP90 as a loading control were detected by Western blotting. Blots were semiquantitatively analyzed and statistically compared. Statistically significant differences are indicated (mean±SEM). Shown are representative Western blot results. *P≤0.05. (C) The second inner medullae of kidneys of each animal were subjected to fluorescence microscopic analysis of aquaporin-2 (AQP2), F-actin (Phalloidin), and nuclei (DAPI). Shown are representative images. Lower panels show magnified views of the areas indicated in upper panels. Scale bars, 10 μm.
Fluconazole Ameliorates the Tolvaptan-Compromised Urinary Concentrating Ability
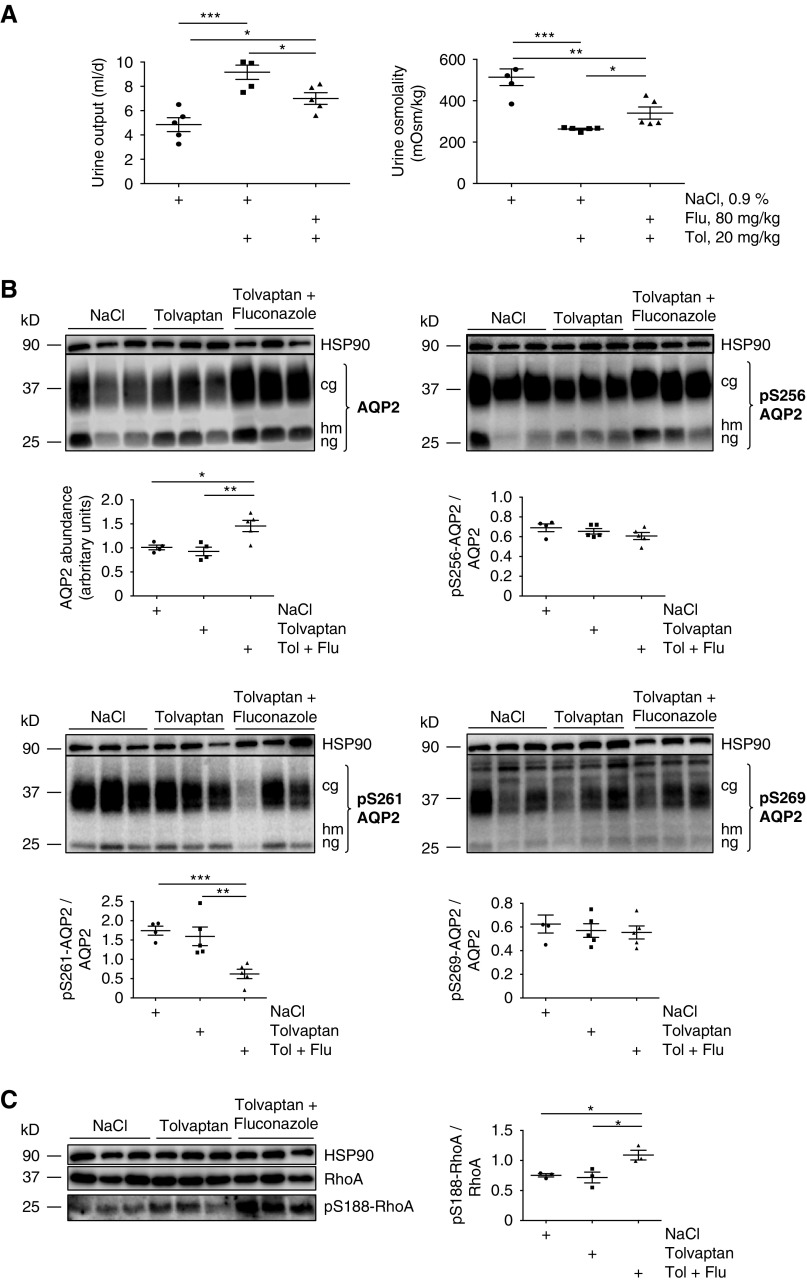
The V2R blocker tolvaptan causes a nephrogenic DI–like excessive loss of hypotonic urine.32,33 To assess whether fluconazole ameliorates the aquaretic effect of tolvaptan and thus, beneficially influences the DI phenotype, we treated mice with fluconazole or saline for 96 hours as indicated above and produced an aquaretic effect through single intraperitoneal injections of 20 mg/kg body wt tolvaptan. Tolvaptan was administered together with the final injection of fluconazole or saline at 72 hours. As expected, in the saline group, the urine output increased and urine osmolality decreased when tolvaptan was administered compared with controls that received only saline (Figure 6A). Compared with cotreatment with saline and tolvaptan, the cotreatment with fluconazole and tolvaptan decreased urine output while increasing urine osmolality. Tolvaptan did not affect AQP2 abundance, the phosphorylation at S256, S261, and S269 (Figure 6B); or RhoA activity as indicated by the unchanged phosphorylation at S188 (Figure 6C). However, fluconazole increased the AQP2 abundance and decreased the phosphorylation of AQP2 at S261 in the tolvaptan-treated mice (Figure 6B). No effect on the AQP2 S256 and S269 phosphorylation was observed. The increased phosphorylation of RhoA at S188 indicated RhoA inhibition (Figure 6C). Thus, fluconazole at least partially compensates for the aquaretic effect of tolvaptan by facilitating AQP2 plasma membrane localization independent of AVP-mediated V2R stimulation.
Figure 6.
Fluconazole (Flu) decreases urine output in tolvaptan (Tol)-treated wild-type mice. Wild-type C57bl/6N male mice were treated every 24 hours over a period of 96 hours with Flu (intraperitoneal injections; 80 mg/kg) or 0.9% NaCl. (A) Twenty-four hours before euthanasia, the mice were placed in metabolic cages, and single intraperitoneal injections (20 mg/kg) of Tol were administered to the 0.9% NaCl– and Flu-treated mice (Supplemental Figure 1); 24-hour urine osmolality and output were monitored (n=5 per each group). Statistically significant differences are indicated (mean±SEM). (B and C) Aquaporin-2 (AQP2), RhoA, and phosphorylation of AQP2 and RhoA were detected by Western blotting. The Western blots were quantified as described in Figures 2, 4, and 5. Shown are results from three mice for each condition. *P≤0.05; **P≤0.01; ***P≤0.001.
Discussion
We report here that fluconazole causes the insertion of AQP2 into the plasma membrane of renal collecting duct principal cells, and in mice, it causes a decrease in urine output and an increase of urine osmolality, indicative of enhanced water reabsorption. This is corroborated by our observation that fluconazole increases water flow across the epithelia of isolated mouse collecting ducts. Because the observed effects are independent of AVP, fluconazole could have therapeutic utility in forms of DI that are resistant to AVP, such as hereditary X-linked DI where V2R is dysfunctional.
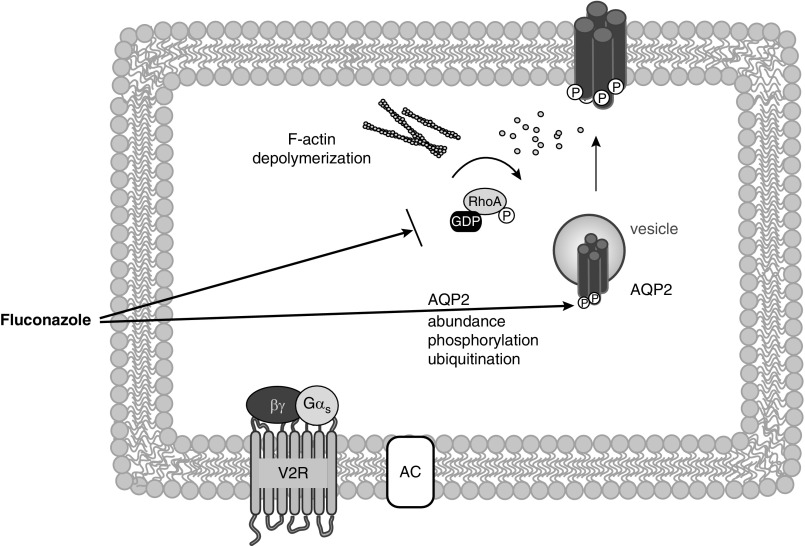
Fluconazole decreases the phosphorylation of AQP2 at S261 and its ubiquitination. Ubiquitination is involved in the endocytic sorting of receptors and channels. AVP stimulation causes the deubiquitination of AQP2, which is associated with its plasma membrane localization in IMCD cells.8 In MDCK cells, ubiquitin-deficient AQP2 is predominantly located in the plasma membrane, and ubiquitination enhances its endocytosis.34 Thus, the fluconazole-induced reduction in ubiquitination is likely to promote the plasma membrane localization of AQP2. Inhibition of RhoA is associated with the plasma membrane localization of AQP2.11–14 Fluconazole mimics the inhibitory effect of AVP on RhoA. Therefore, with the phosphorylation of S261 and ubiquitination of AQP2 and RhoA activity, fluconazole targets critical regulatory factors controlling the AQP2 localization (Figure 7).
Figure 7.
Fluconazole induces the redistribution of aquaporin-2 (AQP2) into the plasma membrane of renal collecting duct principal cells through modulation of AQP2 abundance, its phosphorylation and ubiquitination as well as through RhoA inhibition. Fluconazole induces, independent of arginine-vasopressin (AVP) and V2 receptor (V2R) activation, an increase in the abundance of AQP2 and a decrease in the phosphorylation of AQP2 at serine 261 (S261) that is associated with a decrease in its ubiquitination. S261 phosphorylation and ubiquitination are involved in the control of AQP2 trafficking. Fluconazole also increases the inhibitory phosphorylation of RhoA at S188. Inhibition of RhoA causes depolymerization of F-actin and thereby, removal of this physical barrier that prevents the redistribution of AQP2 to the plasma membrane in the absence of AVP. With targeting the S261 phosphorylation and ubiquitination of AQP2 and RhoA activity, fluconazole promotes the plasma membrane localization of AQP2.
Fluconazole blocks fungal lanosterol 14α-demethylase (CYP51A1) and inhibits ergosterol biosynthesis, thereby causing membrane defects.35 In mammalian cells, CYP51A1 is involved in cholesterol synthesis. CYP51A1 is expressed in primary IMCD cells and rodent kidney (data not shown). In primary IMCD cells and the inner medullae of our mice, fluconazole did not cause any obvious membrane defects. In Candida albicans, fluconazole caused an accumulation of mevalonate, an intermediate in cholesterol synthesis.36 By interfering with mevalonate conversion, fluconazole, similar to statins, most likely interferes with the formation of isoprenoids, such as farnesyl-PP,37 which are important for the membrane tethering and activation of small GTPases.38,39 Thus, in addition to the inhibition of RhoA, a decreased membrane association could contribute to the fluconazole-induced inhibition of RhoA that we observed in renal principal cells.
The azole drug family to which fluconazole belongs has side effects, and our findings may explain some of these better. Azoles modulate actions of other drugs, because they inhibit cytochrome P450 (CYP) enzymes40 and thereby, the extent of the half-lives of drugs that are metabolized by the targeted CYPs. For example, tolvaptan is metabolized by CYP3A4, which in turn, is inhibited by ketoconazole (threefold more potently than by fluconazole). As a result, ketoconazole enhanced the aquaretic effect of tolvaptan.41 However, this enhancing effect of ketoconazole on the tolvaptan action also shows that the antidiuretic effect of fluconazole is apparently not a common property of azoles. Moreover, azoles also differ in other pharmacologic properties. Ketoconazole is extensively metabolized in the liver, increasing the risk of hepatotoxicity.42 Fluconazole is minimally metabolized in the liver, and 80% of it is excreted unmodified with urine.43 Together, the known differences between the azoles and our observations may serve as a guide to new chemical structures mimicking the antidiuretic effect of fluconazole but that have more favorable pharmacologic properties.
Earlier strategies attempted to increase AQP2 surface expression. The PDE5 inhibitor Sildenafil44 and the antidiabetic drug rosiglitazone increase urine osmolality and decrease urine output in DI animal models45 and isolated patient reports.46 Another antidiabetic drug metformin (and metformin-mediated AMP-activated protein kinase activation)47 and inhibition of the EGF receptor with the anticancer drug erlotinib48 promoted the plasma membrane localization of AQP2 and were beneficial in DI animal models. However, findings with regard to effects of AMPK activation are controversial.49 Statins have been tested in mouse models for nephrogenic DI.50,51 We had identified a small molecule, FMP-API-1, that globally activates PKA and uncouples it nonselectively from its interactions with A-kinase anchoring proteins.52 This molecule induced the plasma membrane insertion of AQP2 and was suggested as a starting point for a new treatment strategy for DI.52,53
In conclusion, we elucidate an antidiuretic effect of the approved and widely used drug fluconazole. Fluconazole could be repurposed for treating AVP-resistant forms of DI where AQP2 and its trafficking machinery are intact. Moreover, fluconazole’s chemical structure and pharmacologic properties may serve as a guide to new antidiuretic drugs that target renal principal cells and cause fewer side effects than fluconazole.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Andrea Geelhaar for excellent technical assistance. We also thank F.C. Luft, Experimental and Clinical Research Center/Charité Berlin, and Dr. John Weston for critical reading of the manuscript.
This work was supported by Deutsche Forschungsgemeinschaft (DFG) grants 394046635 – SFB 1365 (to K.M. Schmidt-Ott and E. Klussmann) and KL1415/7-1 (to E. Klussmann), Else Kröner Fresenius Stiftung grant 2013_A145 (to E. Klussmann), German Centre for Cardiovascular Research (DZHK) Partner Site Berlin grant B18-005 SE (to E. Klussmann), Bundesministerium für Bildung und Forschung (BMBF) grant 16GW0179K (to E. Klussmann) and the German Israeli Foundation (GIF) grant I-1452-203.13/2018 (to E. Klussmann). C. Hinze was supported by the Berlin Institute of Health Charité Clinical Scientist Program funded by Charité Universitätsmedizin and the Berlin Institute of Health. K.M. Schmidt-Ott was supported by the Urological Research Foundation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “An Antifungal for Antidiuresis?,” on pages 717–718.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018060668/-/DCSupplemental.
Supplemental Figure 1. Scheme showing animal treatment.
Supplemental Figure 2. Fluconazole does not affect (A) creatinine clearance, (B) water, or (C) food intake of mice.
Supplemental Figure 3. (A) Without transepithelial osmotic gradient, the application of 50 μM fluconazole does not induce transepithelial water flow across epithelia of isolated CCDs; (B) diameter of mouse CCDs during water flux measurements at a given osmotic gradient (300/150 mosm/kg) does not change in response to DMSO, fluconazole (50 μM), or forskolin (30 μM).
Supplemental Figure 4. Fluconazole does not alter AQP2 mRNA abundance in primary IMCD cells and mice.
References
- 1.Klussmann E, Maric K, Rosenthal W: The mechanisms of aquaporin control in the renal collecting duct. Rev Physiol Biochem Pharmacol 141: 33–95, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Klussmann E, Rosenthal W: Role and identification of protein kinase A anchoring proteins in vasopressin-mediated aquaporin-2 translocation. Kidney Int 60: 446–449, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Nedvetsky PI, Tamma G, Beulshausen S, Valenti G, Rosenthal W, Klussmann E: Regulation of aquaporin-2 trafficking. Handb Exp Pharmacol 190:133–157, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Brown D, Breton S, Ausiello DA, Marshansky V: Sensing, signaling and sorting events in kidney epithelial cell physiology. Traffic 10: 275–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishimoto G, Zelenina M, Li D, Yasui M, Aperia A, Nielsen S, et al.: Arginine vasopressin stimulates phosphorylation of aquaporin-2 in rat renal tissue. Am J Physiol 276: F254–F259, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Fenton RA, Moeller HB, Hoffert JD, Yu MJ, Nielsen S, Knepper MA: Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc Natl Acad Sci U S A 105: 3134–3139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, et al.: Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem 283: 24617–24627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nedvetsky PI, Tabor V, Tamma G, Beulshausen S, Skroblin P, Kirschner A, et al.: Reciprocal regulation of aquaporin-2 abundance and degradation by protein kinase A and p38-MAP kinase. J Am Soc Nephrol 21: 1645–1656, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fushimi K, Sasaki S, Marumo F: Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272: 14800–14804, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Isobe K, Jung HJ, Yang CR, Claxton J, Sandoval P, Burg MB, et al.: Systems-level identification of PKA-dependent signaling in epithelial cells. Proc Natl Acad Sci U S A 114: E8875–E8884, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klussmann E, Tamma G, Lorenz D, Wiesner B, Maric K, Hofmann F, et al.: An inhibitory role of Rho in the vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J Biol Chem 276: 20451–20457, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Tamma G, Wiesner B, Furkert J, Hahm D, Oksche A, Schaefer M, et al.: The prostaglandin E2 analogue sulprostone antagonizes vasopressin-induced antidiuresis through activation of Rho. J Cell Sci 116: 3285–3294, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Schrade K, Tröger J, Eldahshan A, Zühlke K, Abdul Azeez KR, Elkins JM, et al.: An AKAP-Lbc-RhoA interaction inhibitor promotes the translocation of aquaporin-2 to the plasma membrane of renal collecting duct principal cells. PLoS One 13: e0191423, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamma G, Klussmann E, Maric K, Aktories K, Svelto M, Rosenthal W, et al.: Rho inhibits cAMP-induced translocation of aquaporin-2 into the apical membrane of renal cells. Am J Physiol Renal Physiol 281: F1092–F1101, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Qureshi S, Galiveeti S, Bichet DG, Roth J: Diabetes insipidus: Celebrating a century of vasopressin therapy. Endocrinology 155: 4605–4621, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Noda Y, Sohara E, Ohta E, Sasaki S: Aquaporins in kidney pathophysiology. Nat Rev Nephrol 6: 168–178, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Vukićević T, Schulz M, Faust D, Klussmann E: The trafficking of the water channel aquaporin-2 in renal principal cells-a potential target for pharmacological intervention in cardiovascular diseases. Front Pharmacol 7: 23, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dayal D, Verma Attri S, Kumar Bhalla A, Kumar R: Response to low dose indomethacin in two children with nephrogenic diabetes insipidus. Pediatr Endocrinol Diabetes Metab 20: 178–181, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Bogum J, Faust D, Zühlke K, Eichhorst J, Moutty MC, Furkert J, et al.: Small-molecule screening identifies modulators of aquaporin-2 trafficking. J Am Soc Nephrol 24: 744–758, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao M, Wang H, Zhu L: Quercetin assists fluconazole to inhibit biofilm formations of fluconazole-resistant Candida albicans in in vitro and in vivo antifungal managements of vulvovaginal candidiasis. Cell Physiol Biochem 40: 727–742, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Srichatrapimuk S, Sungkanuparph S: Integrated therapy for HIV and cryptococcosis. AIDS Res Ther 13: 42, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.García Rodríguez LA, Duque A, Castellsague J, Pérez-Gutthann S, Stricker BH: A cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugs. Br J Clin Pharmacol 48: 847–852, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burns K, Christie-David D, Gunton JE: Fluconazole in the treatment of Cushing’s disease. Endocrinol Diabetes Metab Case Rep 2016: 150115, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faust D, Geelhaar A, Eisermann B, Eichhorst J, Wiesner B, Rosenthal W, et al.: Culturing primary rat inner medullary collecting duct cells [published online ahead of print June 21, 2013]. J Vis Exp 76: e5115, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stefan E, Wiesner B, Baillie GS, Mollajew R, Henn V, Lorenz D, et al.: Compartmentalization of cAMP-dependent signaling by phosphodiesterase-4D is involved in the regulation of vasopressin-mediated water reabsorption in renal principal cells. J Am Soc Nephrol 18: 199–212, 2007 [DOI] [PubMed] [Google Scholar]
- 26.McAvoy T, Nairn AC: Serine/threonine protein phosphatase assays. Curr Protoc Mol Biol Chapter 18: Unit18.18, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong Y, Himmerkus N, Sunq A, Milatz S, Merkel C, Bleich M, et al.: ILDR1 is important for paracellular water transport and urine concentration mechanism. Proc Natl Acad Sci U S A 114: 5271–5276, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klussmann E, Maric K, Wiesner B, Beyermann M, Rosenthal W: Protein kinase A anchoring proteins are required for vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J Biol Chem 274: 4934–4938, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Louie A, Banerjee P, Drusano GL, Shayegani M, Miller MH: Interaction between fluconazole and amphotericin B in mice with systemic infection due to fluconazole-susceptible or -resistant strains of Candida albicans. Antimicrob Agents Chemother 43: 2841–2847, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunn SR, Qi Z, Bottinger EP, Breyer MD, Sharma K: Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int 65: 1959–1967, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Arnspang EC, Login FH, Koffman JS, Sengupta P, Nejsum LN: AQP2 plasma membrane diffusion is altered by the degree of AQP2-S256 phosphorylation. Int J Mol Sci 17: pii:E1804, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miranda CA, Lee JW, Chou CL, Knepper MA: Tolvaptan as a tool in renal physiology. Am J Physiol Renal Physiol 306: F359–F366, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gankam Kengne F, Couturier BS, Soupart A, Decaux G: Urea minimizes brain complications following rapid correction of chronic hyponatremia compared with vasopressin antagonist or hypertonic saline. Kidney Int 87: 323–331, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Kamsteeg EJ, Hendriks G, Boone M, Konings IB, Oorschot V, van der Sluijs P, et al.: Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc Natl Acad Sci U S A 103: 18344–18349, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strushkevich N, Usanov SA, Park HW: Structural basis of human CYP51 inhibition by antifungal azoles. J Mol Biol 397: 1067–1078, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Katragkou A, Alexander EL, Eoh H, Raheem SK, Roilides E, Walsh TJ: Effects of fluconazole on the metabolomic profile of Candida albicans. J Antimicrob Chemother 71: 635–640, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Liao JK, Laufs U: Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 45: 89–118, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li W, Zhang Y, Bouley R, Chen Y, Matsuzaki T, Nunes P, et al.: Simvastatin enhances aquaporin-2 surface expression and urinary concentration in vasopressin-deficient Brattleboro rats through modulation of Rho GTPase. Am J Physiol Renal Physiol 301: F309–F318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wennerberg K, Der CJ: Rho-family GTPases: It’s not only Rac and Rho (and I like it). J Cell Sci 117: 1301–1312, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Niwa T, Shiraga T, Takagi A: Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol Pharm Bull 28: 1805–1808, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Shoaf SE, Bricmont P, Mallikaarjun S: Effects of CYP3A4 inhibition and induction on the pharmacokinetics and pharmacodynamics of tolvaptan, a non-peptide AVP antagonist in healthy subjects. Br J Clin Pharmacol 73: 579–587, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ervine CM, Matthew DE, Brennan B, Houston JB: Comparison of ketoconazole and fluconazole as cytochrome P450 inhibitors. Use of steady-state infusion approach to achieve plasma concentration-response relationships. Drug Metab Dispos 24: 211–215, 1996 [PubMed] [Google Scholar]
- 43.Como JA, Dismukes WE: Oral azole drugs as systemic antifungal therapy. N Engl J Med 330: 263–272, 1994 [DOI] [PubMed] [Google Scholar]
- 44.Sanches TR, Volpini RA, Massola Shimizu MH, Bragança AC, Oshiro-Monreal F, Seguro AC, et al.: Sildenafil reduces polyuria in rats with lithium-induced NDI. Am J Physiol Renal Physiol 302: F216–F225, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Procino G, Gerbino A, Milano S, Nicoletti MC, Mastrofrancesco L, Carmosino M, et al.: Rosiglitazone promotes AQP2 plasma membrane expression in renal cells via a Ca-dependent/cAMP-independent mechanism. Cell Physiol Biochem 35: 1070–1085, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Assadi F, Sharbaf FG: Sildenafil for the treatment of congenital nephrogenic diabetes insipidus. Am J Nephrol 42: 65–69, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Efe O, Klein JD, LaRocque LM, Ren H, Sands JM: Metformin improves urine concentration in rodents with nephrogenic diabetes insipidus. JCI Insight 1: pii: 88409, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheung PW, Nomura N, Nair AV, Pathomthongtaweechai N, Ueberdiek L, Lu HA, et al.: EGF receptor inhibition by erlotinib increases aquaporin 2-mediated renal water reabsorption. J Am Soc Nephrol 27: 3105–3116, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Bataineh MM, Li H, Ohmi K, Gong F, Marciszyn AL, Naveed S, et al.: Activation of the metabolic sensor AMP-activated protein kinase inhibits aquaporin-2 function in kidney principal cells. Am J Physiol Renal Physiol 311: F890–F900, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Procino G, Portincasa P, Mastrofrancesco L, Castorani L, Bonfrate L, Addabbo F, et al.: Simvastatin increases AQP2 urinary excretion in hypercholesterolemic patients: A pleiotropic effect of interest for patients with impaired AQP2 trafficking. Clin Pharmacol Ther 99: 528–537, 2016 [DOI] [PubMed] [Google Scholar]
- 51.Sands JM, Klein JD: Physiological insights into novel therapies for nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 311: F1149–F1152, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christian F, Szaszák M, Friedl S, Drewianka S, Lorenz D, Goncalves A, et al.: Small molecule AKAP-protein kinase A (PKA) interaction disruptors that activate PKA interfere with compartmentalized cAMP signaling in cardiac myocytes. J Biol Chem 286: 9079–9096, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ando F, Mori S, Yui N, Morimoto T, Nomura N, Sohara E, et al.: AKAPs-PKA disruptors increase AQP2 activity independently of vasopressin in a model of nephrogenic diabetes insipidus. Nat Commun 9: 1411, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.