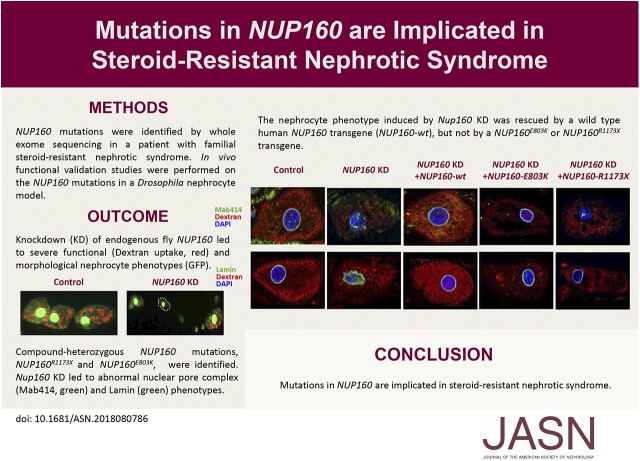
Significance Statement
Mutations in >50 genes can lead to monogenic steroid-resistant nephrotic syndrome (SRNS). The authors found that a young patient with familial SRNS and FSGS carried novel compound-heterozygous mutations in NUP160; this gene encodes nucleoporin 160 kD, one of the protein components of the nuclear pore complex. Using an in vivo renal cell assay on the basis of Drosophila nephrocytes (an experimental podocyte model previously used to validate candidate renal disease genes and specific patient-derived mutant alleles), they validated the NUP160 gene variants as factors implicated in kidney pathology. The findings indicate that NUP160 should be included in the SRNS diagnostic gene panel to identify additional patients with SRNS carrying homozygous or compound-heterozygous NUP160 mutations.
Keywords: nephrocyte, Drosophila, nephrotic syndrome, NUP160, genetic renal disease, human genetics
Visual Abstract
Abstract
Background
Studies have identified mutations in >50 genes that can lead to monogenic steroid-resistant nephrotic syndrome (SRNS). The NUP160 gene, which encodes one of the protein components of the nuclear pore complex nucleoporin 160 kD (Nup160), is expressed in both human and mouse kidney cells. Knockdown of NUP160 impairs mouse podocytes in cell culture. Recently, siblings with SRNS and proteinuria in a nonconsanguineous family were found to carry compound-heterozygous mutations in NUP160.
Methods
We identified NUP160 mutations by whole-exome and Sanger sequencing of genomic DNA from a young girl with familial SRNS and FSGS who did not carry mutations in other genes known to be associated with SRNS. We performed in vivo functional validation studies on the NUP160 mutations using a Drosophila model.
Results
We identified two compound-heterozygous NUP160 mutations, NUP160R1173× and NUP160E803K. We showed that silencing of Drosophila NUP160 specifically in nephrocytes (fly renal cells) led to functional abnormalities, reduced cell size and nuclear volume, and disorganized nuclear membrane structure. These defects were completely rescued by expression of the wild-type human NUP160 gene in nephrocytes. By contrast, expression of the NUP160 mutant allele NUP160R1173× completely failed to rescue nephrocyte phenotypes, and mutant allele NUP160E803K rescued only nuclear pore complex and nuclear lamin localization defects.
Conclusions
Mutations in NUP160 are implicated in SRNS. Our findings indicate that NUP160 should be included in the SRNS diagnostic gene panel to identify additional patients with SRNS and homozygous or compound-heterozygous NUP160 mutations and further strengthen the evidence that NUP160 mutations can cause SRNS.
Nephrotic syndrome is a renal disease caused by disruption of the glomerular filtration barrier, resulting in massive proteinuria, hypoalbuminemia, hyperlipidemia, and edema.1 With respect to responsiveness to standard steroid therapy, nephrotic syndrome is classified into steroid-sensitive nephrotic syndrome and steroid-resistant nephrotic syndrome (SRNS). SRNS can have either an immunologic or genetic etiology.2 The contributions of genetic factors are increasingly emphasized in the growing understanding of SRNS pathogenesis. To date, >50 monogenic genes have been identified that cause SRNS when mutated.3 These include genes encoding components of the slit diaphragm, such as NPHS1, NPHS2, and CD2AP; genes encoding actin cytoskeleton proteins, such as ACTN4, INF2, and MYO1E; genes encoding actin-regulating small GTPases of the Rho/Rac/Cdc42 family, including ARHGDIA, ARHGAP24, and KANK; and genes encoding nucleoporins (Nups), including NUP93, NUP107, and NUP205. Mutations in NPHS1, NPHS2, and CD2AP disrupt the integrity of the slit diaphragm and lead to congenital nephrotic syndrome, early-onset autosomal recessive SRNS, and early-onset FSGS, respectively.4–6 Mutations in ACTN4 change the cytoskeletal dynamics of podocytes and lead to adult-onset autosomal dominant FSGS.7 Mutations in ARHGDIA alter podocyte migration capabilities and lead to early-onset SRNS.8 Mutations in NUP93 inhibit podocyte proliferation, promote podocyte apoptosis, and lead to early childhood-onset SRNS.9 Mutations in NUP107 cause hypoplastic glomerular structures and abnormal podocyte foot processes and lead to early childhood-onset SRNS.10 Recently, Braun et al.11 described mutations in NUP107, NUP85, NUP133, and NUP160 in 13 families with SRNS.
The NUP160 gene (mapping to chromosome 11p) encodes Nup160, which is a component of the Nup107–160 complex required for early stages of nuclear pore complex (NPC) assembly.12,13 It is expressed in both human and mouse kidney cells. Knockdown of the NUP160 gene damages mouse podocytes cultured in vitro.14 Within a nonconsanguineous Chinese family two siblings, a brother with SRNS and a sister with proteinuria, were both found to carry NUP160E803K and NUP160R910× compound-heterozygous mutations.11
We now describe two compound-heterozygous mutations in NUP160, NUP160R1173× and NUP160E803K, identified in a young girl with familial SRNS and FSGS. This patient did not carry mutations in genes previously associated with SRNS. Furthermore, we functionally validated the NUP160 mutations in vivo using a Drosophila model.15–21 The Drosophila nephrocyte system has been previously proven to be very useful for validating candidate gene mutations for involvement in monogenic SRNS and investigating molecular disease mechanisms underlying podocyte cellular pathologies.22–25 We first showed that nephrocyte-specific silencing of the conserved endogenous fly Nup160 gene induced severe renal cell defects, thereby experimentally validating NUP160 as a novel candidate renal disease gene. We then showed complete rescue of nephrocyte phenotypes by expression of a wild-type human NUP160 transgene but not by either patient-derived mutant allele, providing in vivo evidence to implicate these NUP160 mutations as pathogenic.
Methods
Study Participants
Written informed consent from index family members and control subjects was obtained under a protocol approved by the institutional review boards of Shanghai Ruijin Hospital and Fuzhou Dongfang Hospital of China. DNA samples from 520 healthy persons were used as controls. The nonconsanguineous Chinese index family included the proband, unaffected parents, and five siblings, two of whom died from SRNS in the 1990s (Figure 1C, Supplemental Table 1). DNA samples of the index family were available from the proband (II6), a healthy sibling (II5), and both parents (I1 and I2). Nephrotic syndrome was diagnosed on the basis of urinary protein excretion >50 mg/kg per day with hypoalbuminemia <25 g/L. Steroid resistance was defined as a failure of induction of complete remission after 4 weeks of standard therapy with prednisone (2 mg/kg per day given in three divided doses; maximum 60 mg/d). ESRD was defined as a GFR<10 ml/min per 1.73 m2 or the necessity of any RRT. Tissue biopsies were evaluated by renal pathologists. We excluded the possibility that the proband carried mutations in known genes associated with SRNS (Supplemental Table 2).
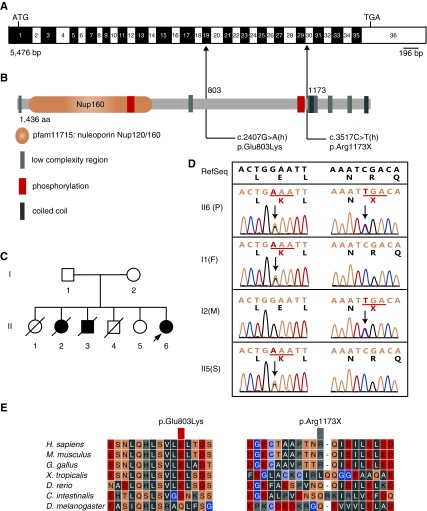
Figure 1.
Compound-heterozygous mutations in NUP160, NUP160Glu803Lys and NUP160Arg1173X, were identified in an autosomal recessive family with steroid-resistant nephrotic syndrome. (A) Human NUP160 cDNA (NM_015231.2; 5476 bp) showing exons (numbered; alternating black and white), positions of start (ATG) and stop (TGA) codons, and mutation sites (arrows). (B) Human Nup160 protein (NP_056046.1; 1436 amino acids) structural domains, phosphorylation sites, and locations of missense (amino acid 803) and truncation (amino acid 1173) lesions. (C) A pedigree of the affected family (family members diagnosed with kidney disease are indicated in black). The arrow indicates the proband. (D) Compound-heterozygous missense and truncating NUP160 mutations identified from proband (II6; patient [P]) sequencing. Nucleotide and corresponding amino acid sequences are shown for wild-type (upper panel; in black) and mutant (lower panel; in green) alleles. Codons are underlined (green), and altered nucleotides and amino acids are highlighted (red). Arrows indicate positions of mutations in relation to exons and protein motifs (in A and B, respectively). Relevant sequences from surviving family members (I1, father [F]; I2, mother [M]; II5, sister [S]) are also shown. (E) Phylogenetic comparisons of amino acid sequences of Nup160 protein regions affected by the identified mutations.
Whole-Exome Sequencing
Genomic DNA was purified from blood samples collected from the proband (II6), her surviving sister (II5), and her parents (I1 and I2) using the DNeasy Blood & Tissue Kit (Qiagen) using standard procedures (Figure 1C). Whole-exome capture and sequencing were performed on a SureSelect platform (Agilent) with 3 μg of genomic DNA from each individual using SureSelect Exome Capture System V4 (5M). The resulting libraries were sequenced on a HiSeq 2000 Multiplexed Sequencing platform (Illumina) according to the manufacturer’s instructions for paired end 100-bp reads. Reads were aligned to the human genome reference sequence (GRCh37/hg19; build of the UCSC Genome Browser; http://genome.ucsc.edu) using the Burrows–Wheeler Alignment (version 0.5.9), and possible duplicate paired end reads were marked using Picard version 1.35 (http://broadinstitute.github.io/picard/). The Genome Analysis Toolkit version 2.6–4 was used for base quality score recalibration, indel realignment, and variant discovery (single-nucleotide variants and indels). Read mapping and variant calling were used for data analysis and interpretation. Variants were annotated with SeattleSeq SNP Annotation 150 (http://snp.gs.washington.edu/SeattleSeqAnnotation150/). Variants present at a frequency of >0.01 in the database of single-nucleotide polymorphisms (dbSNP; dbSNP 151: http://www.ncbi.nlm.nih.gov/SNP/) and the NHLBI GO Exome Sequencing Project or present from local exomes of unaffected individuals were excluded. Variants were filtered with the in-house Variant Analysis and Filtration Tool software. Candidate genetic variants in the NUP160 gene were confirmed by Sanger sequencing on an ABI 3730XL DNA Analyzer (Shanghai Invitrogen Biotechnology Co.).
Variant Filtering
The following criteria were used for variant filtering: (1) rare variants with <0.01 allele frequency in control databases, including dbSNP 151 and Exome Variant Server; (2) homozygous or compound-heterozygous variants; (3) nonsynonymous variants affecting the coding sequence, obligatory splice acceptor and donor site mutations, and in-frame coding deletion/insertions; (4) pathogenic or likely pathogenic revealed by web-based mutation analysis prediction tools, including PolyPhen-2, SIFT, PROVEAN, and MutationTaster; (5) variants outside the above criteria discarded; and (6) mutations of interest confirmed by Sanger sequencing in the proband, the healthy sibling, and the parents.
For additional annotative information about potential genes of interest, we also examined genomic, proteomic, genetic, and functional information on candidate genes using databases, including GeneCards (http://www.genecards.org), Uniprot (http://www.uniprot.org), Ensembl (http://ensembl.org), and the Human Protein Atlas (http://www.proteinatlas.org), as well as literature searches to help decide potential pathogenicity. Mouse Genome Informatics (http://www.informatics.jax.org) was used for establishing whether SRNS disease phenotypes might be found in related mouse models.
Fly Strains
Flies were reared on standard food at room temperature or 29°C for UAS-GAL4 strains. The Dot-Gal4 strain23 was obtained from the Bloomington Drosophila Stock Center (BDSC). Transgenic RNA interference (RNAi) lines were obtained from the BDSC or the Vienna Drosophila Resource Center. The Hand-GFP26 and MHC-ANF-RFP23 strains were also used in this study as readouts for nephrocyte morphology and function.
cDNA Cloning and Generation of NUP160 Transgenic Fly Strains
A wild-type human NUP160 cDNA encoding the common 1437-amino acid NUP160 isoform (NCBI reference sequence NP_056046.1) was obtained from OriGene (catalog no. RC218855). NUP160E803K and NUP160R1173× cDNAs were generated from the wild-type allele using the QuikChange Site-Directed Mutagenesis Kit (Clontech) and sequenced to confirm that no other mutations were induced except for the intended variation at glutamic acid 803 and the truncation at arginine 1173, respectively. The cDNAs were individually cloned into the pUAST-attB vector, and the transgenes were introduced into a fixed chromosomal docking site by germ-line transformation to ensure that the wild-type NUP160 allele and the two variant alleles were expressed at the exact same level in the nephrocytes.
RFP Uptake Assay
Flies from the MHC-ANF-RFP, Hand-GFP, and Dot-Gal4 transgenic lines were crossed with flies from the UAS-Nup160-RNAi transgenic lines at 25°C.23 One day after egg laying, embryos were shifted to 29°C. RFP uptake by pericardial nephrocytes was assessed in second instar larvae or adult flies 1 day postemergence by dissecting heart tissue into Schneider Drosophila Medium (ThermoFisher) and examining cells using fluorescence microscopy. For quantification, ≥20 nephrocytes were analyzed from each of three female flies per genotype. The results are presented as mean±SD. A paired t test was used to analyze the data. Statistical significance was defined as P<0.05.
Dextran Uptake Assay
Flies of the appropriate genotypes were allowed to lay eggs at 25°C. One day after egg laying, embryos were shifted to 29°C. Dextran uptake by pericardial nephrocytes was assessed ex vivo in third instar larvae or adult flies 1 day postemergence by dissecting heart tissue into Schneider Drosophila Medium and examining the cells by fluorescence microscopy after 20 minutes incubation with AlexaFluo568-Dextran (10 kD; 0.05 mg/ml). For quantification, ≥20 nephrocytes were analyzed from each of three female flies per genotype. The results are presented as mean±SD. A paired t test was used to analyze the data. Statistical significance was defined as P<0.05.
Silver Nitrate Uptake Assay
Flies of the appropriate genotype were allowed to lay eggs on standard apple juice plates for 24 hours. Freshly emerged first instar larvae were transferred to agar-only plates supplemented with regular yeast paste containing silver nitrate (AgNO3; 2.0 g yeast in 3.5 ml 0.0005% AgNO3 solution) and allowed to develop at 29°C until adulthood.23 AgNO3 uptake by pericardial nephrocytes was assessed in third instar larvae or adult flies 1 day postemergence by dissecting heart tissues into Schneider Drosophila Medium and examining nephrocytes by phase contrast microscopy. For quantification, >20 nephrocytes were analyzed from each of three female flies per genotype. The results are presented as mean±SD. A paired t test was used to analyze the data. Statistical significance was defined as P<0.05.
Immunofluorescence Imaging and Confocal Microscopy
Larvae and adult flies were dissected and fixed for 10 minutes in 4% paraformaldehyde in PBS. Mab 414 (ab24609; Abcam) and anti-Lamin (ADL84.12; Developmental Studies Hybridoma Bank) primary antibodies were used at 1:500 dilution followed by Cy5-conjugated secondary antibodies (Jackson Lab). Confocal imaging was performed with a Zeiss ApoTome.2 microscope using a 20× Plan-Apochromat 0.8 N.A. air objective. To quantitatively compare fluorescence intensities, common settings were chosen to avoid oversaturation. ImageJ Software version 1.49 was used for image processing.
Results
Patient Clinical Features
A 10-year-old girl (II6) was admitted to the Department of Nephrology, Shanghai Ruijin Hospital for persistent proteinuria that began at age 7 years old. The proband (II6) presented normal BP and 3+ urinary albumin; 24-hour urinary protein excretion was 8.34 g, and serum albumin was 24 g/L. Serum cholesterol was 4.55 mmol/L, serum creatine was 53 μmol/L, and serum urea nitrogen was 4.2 mmol/L. Audiometry was normal, and a skin test showed continuous collagen IV.
Histopathology
A renal biopsy was performed for the first time in 1999 when the proband (II6) was age 10 years old. Light microcopy revealed 14 glomeruli with global sclerosis (in one glomerulus), segmental sclerosis (in two glomeruli), vessels with spheroidal hyaline degeneration (in two glomeruli), mesangial enlargement (in nine glomeruli), focal tubular atrophy, segmental fibrous proliferation, and inflammatory cell infiltrates in the tubulointerstitium but no lesions of small vessels. Immunofluorescence (IF) showed scattered mesangial deposits of both IgA (++) and IgM (+) but without deposits of IgG, C3, C4, C1q, Fibrin, κ, and λ in five glomeruli.
A renal biopsy was performed for the second time in 2002. Light microscopy revealed nine glomeruli with segmental sclerosis (in one glomerulus), mild segmental mesangial proliferation (in eight glomeruli), renal tubular atrophy, focal fibrous proliferation, and inflammatory cell infiltrates in the tubulointerstitium but no lesions in small vessels (Figure 2C). IF showed scattered mesangial deposits of IgA (+++) but without deposit of IgM, IgG, C3, C4, C1q, Fibrin, κ, and λ in five glomeruli. Electron microcopy showed effacement of podocyte foot processes, partial thickened and sclerosing glomerular basement membrane, mesangial hypercellularity, and subendothelial deposition of an electron dense material (Figure 2D).
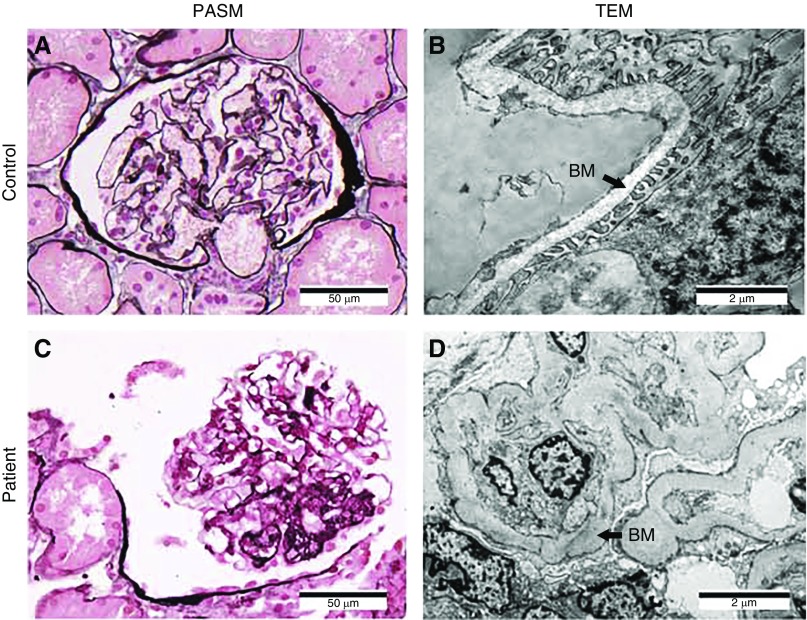
Figure 2.
The proband's renal tissue histology and cell ultrastructure revealed FSGS. (A and B) Control normal glomerulus tissue samples were obtained from a patient with a kidney tumor. (C and D) Patient tissues were obtained from the proband (patient II6) (Figure 1C). (A and C) Light microscopic examination of periodic acid–silver metheramine (PASM)–stained glomerulus showing focal segmental glomerular hyaline degeneration and sclerosis in the patient sample. (B and D) Transmission electron microscopy (TEM) revealed effacement of podocyte foot processes and partial thick and sclerosing glomerular basement membrane (BM; arrows) in the patient sample. Magnification, ×400 in A and C; ×12,000 in B and D.
Treatment Outcomes
Treatments with prednisone, cyclophosphamide, and tripterygium wilfordii glycosides all failed. The proband (II6) progressed to ESRD at age 15 years old, received a kidney transplant at age 16.4 years old, and is alive at this time. The parents (I1 and I2) and surviving sister (II5) are healthy. One sister (II1) and one brother (II4) died of unknown causes in early childhood. The other two older siblings (II2 and II3) were affected with SRNS, and both died of ESRD at age 17 years old (Figure 1C, Supplemental Table 1).
NUP160 Variants
Whole-exome sequencing of family members revealed 56,387 single-nucleotide variants and 6383 small insertions and deletions with mapping statistics (Supplemental Table 3). According to the criteria for variant filtering, most remaining variants were excluded. Mutations in known genes associated with SRNS were also excluded (Supplemental Table 2). Six variant genes remained (TTC12, DCANP1, KIAA1549, NUP160, DNAH5, and HYDIN) (Supplemental Table 4).
The index family pedigree (Figure 1C) displayed characteristics typical of an autosomal recessive disease. According to the clinical phenotype, all of the patients (II2, II3, and II6) could be diagnosed as having nonsyndromic kidney disease. On the basis of kidney phenotype, number of homozygotes, and expression of genes in human glomerular cells, we excluded five of the remaining genes (TTC12, DCANP1, KIAA1549, DNAH5, and HYDIN) (Supplemental Table 4).
Compound-heterozygous variants E803K and R1173× of NUP160 (RefSeq accession number NM_015231.2) were in trans configuration (Figure 1). Variant E803K was detected in the father (I1) and the sister (II5), and variant R1173× was detected in the mother (I2). The healthy surviving individuals (I1, I2, and II5) are heterozygotes carrying a single mutated allele of either E803K or R1173×, but the proband (II6) carries two mutant alleles of NUP160 (Figure 1D). Two siblings (II2 and II3) with SRNS died earlier, and DNA samples were not available for analysis (Figure 1C, Supplemental Table 1). NUP160 was the only candidate gene in which the variants were precisely cotransmitted with the underlying recessive disease status and in addition, were not detected in 520 control subjects. R1173× results in premature termination of translation and production of a truncated protein. E803K is predicted to be disease-causing by MutationTaster and neutral by Polyphen, SIFT, and PROVEAN (Supplemental Table 4), and it was also identified in another unrelated Chinese family.11 To provide functional evidence to validate the NUP160 mutations as pathogenic, we used a Drosophila cardiac nephrocyte experimental model to study the effects of NUP160 gene deficiency in vivo.
Drosophila Nup160 Gene Silencing
NUP160 is highly conserved from flies to humans (Figure 1E), and it is constitutively expressed in all cell types. To study NUP160 renal phenotypes in Drosophila, we first used RNAi-based gene silencing to lower expression of the endogenous fly Nup160 gene specifically in nephrocytes. Nup160 gene silencing significantly reduced the lifespan of adult flies (Figure 3A). In larval stage, animals’ total numbers of nephrocytes were significantly reduced compared with controls. Adult flies were found to lack nephrocytes entirely (Figure 3B). These observations indicated that Nup160 was essential for nephrocyte survival during immature stages of development through adult emergence and that it was required for maintenance of normal adult lifespan.
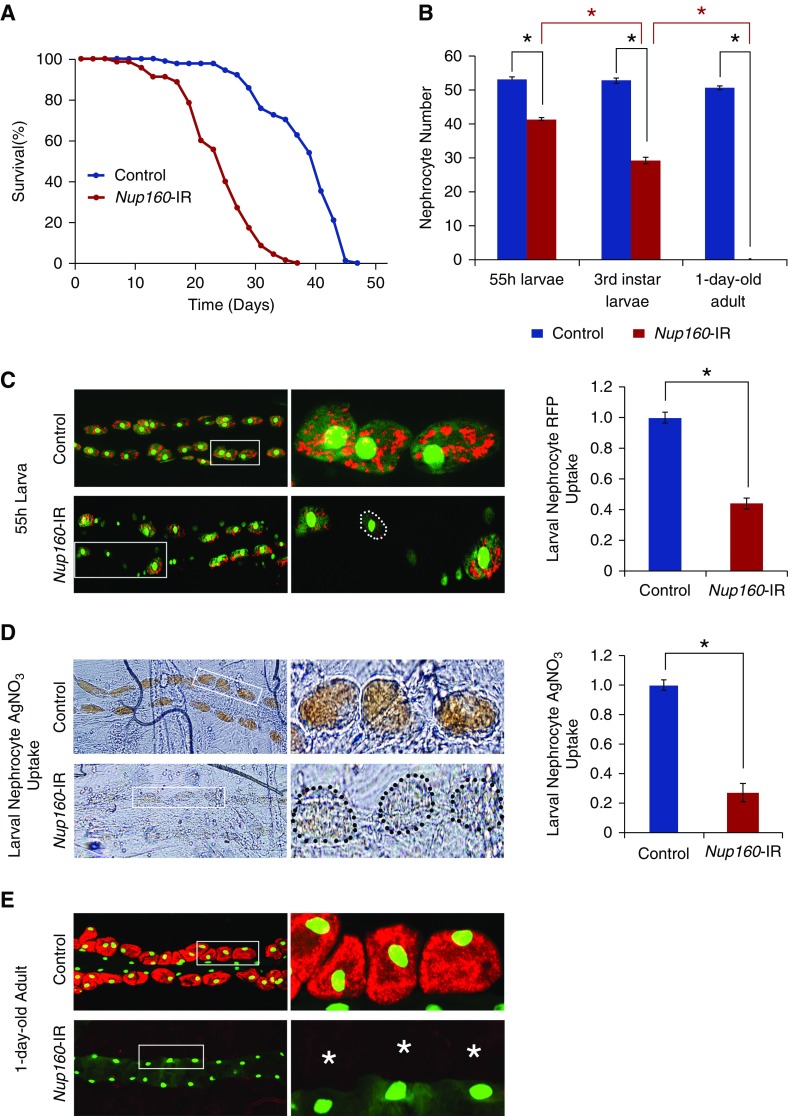
Figure 3.
Nephrocyte-specific silencing of endogenous Nup160 expression led to shortened adult fly lifespan, fewer nephrocytes, and reduced nephrocyte function. Nup160-IR flies carry a UAS-Nup160-RNAi transgene plus a nephrocyte-specific Dot-GAL4 driver that together silence endogenous fly Nup160 expression. Control flies carry only the Dot-GAL4 driver. (A) Survival curves for Nup160-IR and control adult flies (newly emerged on day 0). Nephrocyte-specific silencing of endogenous Nup160 expression was associated with reduced lifespan: Nup160-IR flies experienced 50% mortality at day 25 versus day 40 for control flies. (B) Nephrocyte-specific silencing of Nup160 induced progressive loss of nephrocytes during larval- to adult-stage development. Nephrocytes were counted in second instar (55-hour larvae) and third instar larval stages and adult flies within 24 hours postemergence. Progressively fewer nephrocytes were observed during larval-stage development, and nephrocytes were entirely absent in adult flies. For quantification, nephrocytes were counted from each of five larvae or adult flies of each genotype. The results are presented as mean±SD. *Statistical significance was defined as P<0.05. (C) Nephrocyte-specific silencing of Nup160 led to reduced uptake of fluorescently labeled marker protein. All larvae carry an MHC-ANF-RFP transgene expressing ANF-RFP marker protein in muscle cells, which is secreted into the fly hemolymph; it is normally taken up by and accumulated in nephrocytes (red fluorescence) before protein breakdown and recycling of amino acids. In addition, all flies carry a Hand-GFP transgene expressing a nuclear-localized GFP marker (green; predominantly nuclear fluorescence) in both pericardial nephrocytes (larger nuclei with faint cytoplasmic fluorescence) and cardiomyocytes (smaller nuclei). Fluorescence microscopy revealed that, in control 55-hour larvae, almost every nephrocyte took up and accumulated abundant ANF-RFP marker protein from the larval hemolymph (left panels; center panels show the boxed areas). By contrast, nephrocytes of Nup160-IR larvae expressing the Nup160-RNAi transgene showed reduced levels of ANF-RFP overall, although a minority of nephrocytes displayed essentially normal levels of RFP. Quantification of ANF-RFP uptake by control versus Nup160-IR nephrocytes is shown in right panel. Nephrocyte RFP uptake was reduced approximately 2.5-fold as a result of Nup160 gene silencing; ≥20 nephrocytes were analyzed from each of five larvae per genotype. The results are presented as mean±SD. *Statistical significance was defined as P<0.05. (D) Ingested silver nitrate (AgNO3) taken up and sequestered by third instar larval nephrocytes revealed by phase contrast microscopy. Upper left and upper center panels show abundant AgNO3 within control nephrocytes, whereas lower left and lower center panels show very reduced levels in Nup160-IR nephrocytes delineated by the dotted outline in left panels (center panels show higher-magnification views of boxed nephrocytes). Quantification of AgNO3 in control versus Nup160-IR nephrocytes is shown in right panel. Nephrocyte AgNO3 was reduced nearly fivefold as a result of Nup160 gene silencing; ≥20 nephrocytes were analyzed from each of five larvae per genotype. The results are presented as mean±SD. *Statistical significance was defined as P<0.05. (E) Nup160 gene silencing led to complete absence of adult fly nephrocytes. All flies express MHC-ANF-RFP and Hand-GFP transgenes. Fluorescence microscopy showed control adult nephrocytes with abundant cytoplasmic ANF-RFP (red) and nuclear GFP (green) as well as cardiomyocytes (smaller green nuclei; no RFP fluorescence). By contrast, no nephrocytes were observed in Nup160-IR flies expressing a UAS-Nup160-RNAi driven by Dot-Gal4, although cardiomyocytes (GFP-positive nuclei) were normal. Right panels show higher magnification views of boxed areas. *Normal locations of nephrocytes relative to cardiomyocytes.
To study nephrocyte physiologic functional deficits induced by Nup160 gene silencing, we examined in vivo uptake of RFP from the hemolymph in nephrocytes of larvae (Figure 3C) and adult flies (Figure 3E). In larval nephrocytes, Nup160 gene silencing was associated with approximately 2.5-fold less RFP uptake than observed in control larvae. In adult flies, fluorescence microscopy confirmed complete absence of nephrocytes and consequently, no RFP uptake (Figure 3E). Functional phenotypic analysis was extended to a nephrocyte sequestration assay in which larvae are raised on a diet supplemented with AgNO3. In normal flies, ingested toxic AgNO3 accumulated and was sequestered in cardiac nephrocytes (Figure 3D). When nephrocyte Nup160 gene expression was silenced, sequestered AgNO3 was reduced by approximately fivefold in larvae (Figure 3D). Drosophila Nup160 gene silencing in nephrocytes, therefore, led to significant functional deficits in vivo and was associated with reduced adult fly survival.
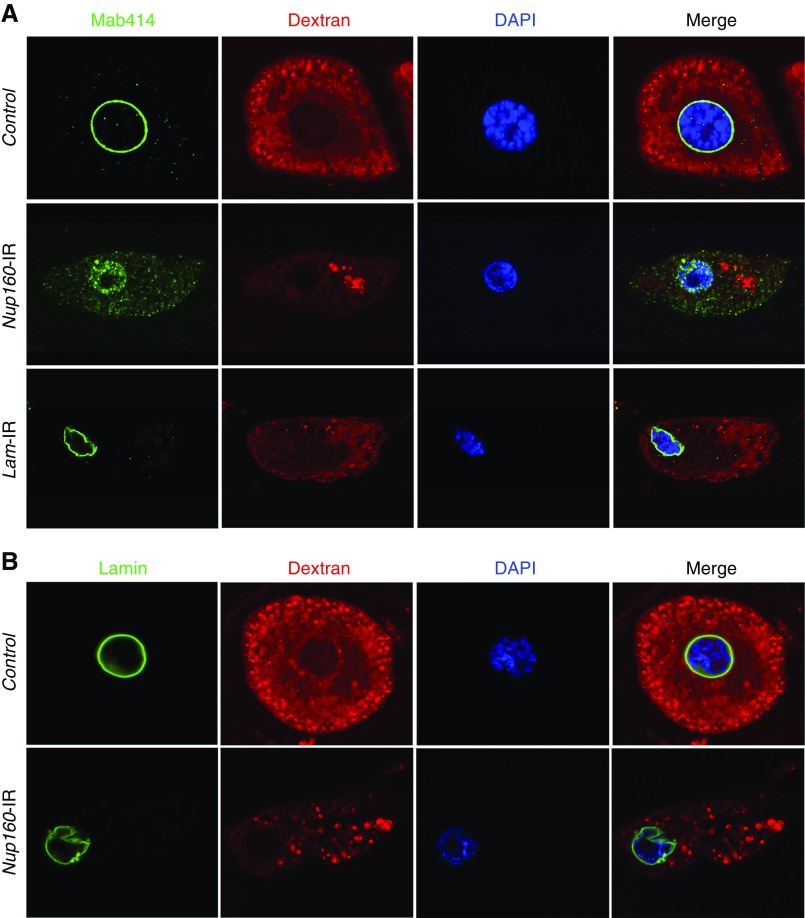
To better understand the effects of Nup160 silencing on renal cells, we examined larval nephrocytes by fluorescence microscopy using structural and functional markers (Figure 4). Freshly dissected nephrocytes were functionally assayed ex vivo for uptake of FITC-labeled 10-kD Dextran beads. Control nephrocytes readily took up the beads, resulting in abundant distinct pericytoplasmic vesicular fluorescence. NUP160 silencing severely reduced Dextran uptake, and limited fluorescence was mostly concentrated in the nephrocyte cell interior in clumped aggregates; 4′,6-diamidino-2-phenylindole staining showed that Nup160 silencing induced reduction in nuclear volume. The profound effect of Nup160 silencing on the nephrocyte nucleus was clearly shown by Mab414 IF labeling of the NPC. In normal nephrocytes, Mab414 distinctly and concisely labeled the nuclear circumference. Nup160 silencing, by contrast, led to Mab414 labeling NPC components dispersed within the shrunken nuclear compartment (defined by 4′,6-diamidino-2-phenylindole colabeling) (Figure 4A), presumably the result of failed NPC assembly. The defective nuclear structure induced by Nup160 silencing was further confirmed by anti-Lamin antibody immunolabeling in which the normal (control) circumferential nuclear lamin localization appeared instead very irregular (Figure 4B). Drosophila Lamin gene silencing induced similar nephrocyte morphologic changes (i.e., reduced nucleus) and functional deficits (reduced Dextran uptake). Lamin silencing, however, did not lead to aberrantly localized Mab414 IF (Figure 4A). Nephrocyte functional defects induced by Nup160 gene silencing were, therefore, associated with striking changes in nephrocyte cellular morphology, presumably the result of severe breakdown of normal nucleocytoplasmic trafficking due to disrupted NPC localization caused by failed complex assembly in the absence of sufficient Nup160 protein.
Figure 4.
Nup160 gene silencing induced nuclear pore complex (NPC) and lamin abnormalities in Drosophila nephrocytes. (A) Mab414 (green) immunolabeled components of the NPC, which in control nephrocytes, generated a sharp and highly regular ring around the nucleus (indicated by 4′,6-diamidino-2-phenylindole [DAPI] staining; blue). In nephrocytes of larvae in which Nup160 gene expression was silenced (Nup160-IR), by contrast, Mab414 immunolabeling was dispersed across the nucleus, and it was more abundant in the cytoplasm, indicating abnormal localization of NPC components. Normal ex vivo uptake of FITC-labeled 10-kD Dextran particles (red) was significantly affected by Nup160 gene silencing, indicating functional nephrocyte defects linked to abnormal NPC localization. Silencing of the Drosophila Lamin gene by nephrocyte-specific expression of a UAS-Lam-RNAi transgene driven by Dot-Gal4 (Lam-IR) led to marked abnormalities in nuclear size and shape and a severe functional deficit, but NPC localization appeared normal. (B) Mab ADL67.10 labels all isoforms of Drosophila Sf9 Lamin but does not label Lamin C. In control nephrocytes, Lamin (green) appeared as a sharp regular ring around the nucleus. Nup160 gene silencing (Nup160-IR), by contrast, did not disrupt circumferential nuclear Lamin localization (although nuclear morphology was altered as indicated by DAPI staining), but labeling was less concise and uniform than in control nephrocytes.
Overexpression of Wild-Type or Mutant Human NUP160 Transgenes Did Not Induce Nephrocyte Abnormalities
Nephrocyte phenotypes induced by human NUP160 transgene expression were analyzed. Nephrocyte-specific overexpression of human NUP160WT cDNA did not affect RFP uptake, and nephrocyte morphology was unchanged compared with nephrocytes of control flies (Supplemental Figure 1). Overexpression of NUP160E803K or NUP160R1173× cDNA transgenes in nephrocytes did not lead to significant changes in nephrocyte function. These results indicate that neither of these two mutant alleles encodes a gain-of-function or dominant negative NUP160 mutation.
Rescue of Endogenous NUP160 Deficiency by “Replacement” Human Transgene Expression
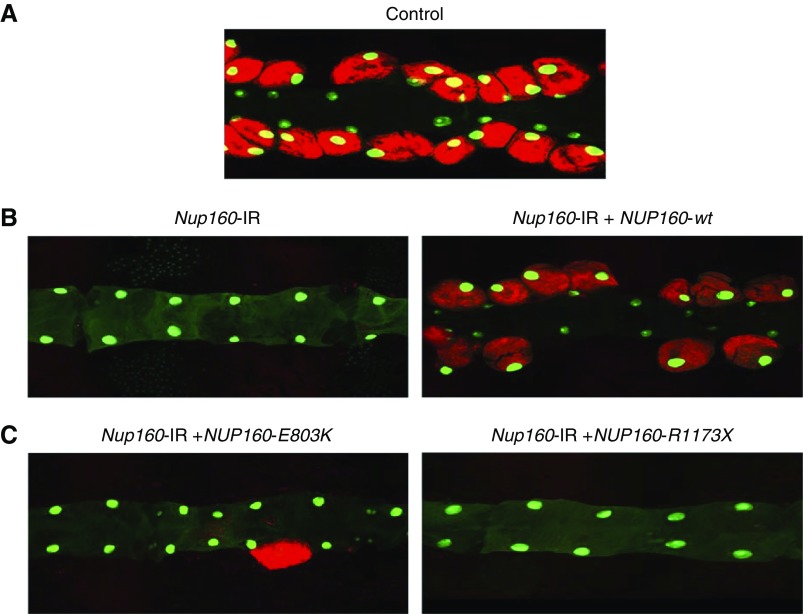
The Drosophila model allows for a highly efficient “gene replacement” approach to assess the relative abilities of wild-type and mutant versions of human genes to rescue the nephrocyte phenotypes induced by silencing of endogenous fly gene homologs. In this way, specific patient-derived mutant alleles identified through genome sequencing can be functionally implicated (i.e., validated) in renal cell dysfunction.27 We first coexpressed a wild-type human NUP160 cDNA transgene specifically in nephrocytes while simultaneously silencing endogenous fly Nup160 expression. As shown in Figure 5B, wild-type human NUP160 expression completely rescued Nup160-IR–induced absence of adult nephrocytes, and the rescued nephrocytes were fully functional. By contrast, neither the patient-derived NUP160 mutant allele E803K nor R1173× was capable of rescuing the adult nephrocyte phenotype (Figure 5C).
Figure 5.
Adult nephrocyte phenotypes induced by Nup160-IR were rescued by wild type human NUP160 transgene, but not by NUP160E803K or NUP160R1173X. (A) Fluorescence micrograph shows nephrocytes of adult flies 1 day postemergence. ANF-RFP fluorescence (red) is merged with GFP (green; mostly nuclear). A GFP transgene is expressed under the control of a Hand gene enhancer (Hand-GFP) to confirm pericardial nephrocyte cell identity. All flies are transgenic for Hand-GFP. Control flies carry the Dot-Gal4 driver but no RNA interference construct. (B, left panel) Nephrocyte-specific Nup160-IR transgene expression silenced endogenous fly Nup160, leading to complete absence of adult nephrocytes. Unaffected cardiomyocytes exhibited GFP fluorescence. (B, right panel) Nephrocyte-specific expression of a wild-type human NUP160 transgene (NUP160-wt) rescued the adult nephrocyte phenotype induced by Nup160-IR. (C, left panel) Nephrocyte-specific expression of a mutant E803K NUP160 transgene (NUP160-E803K) failed to rescue the adult nephrocyte phenotype induced by Nup160-IR. (C, right panel) Nephrocyte-specific expression of a mutant R1173× NUP160 transgene (NUP160-R1173×) failed to rescue the adult nephrocyte phenotype induced by Nup160-IR.
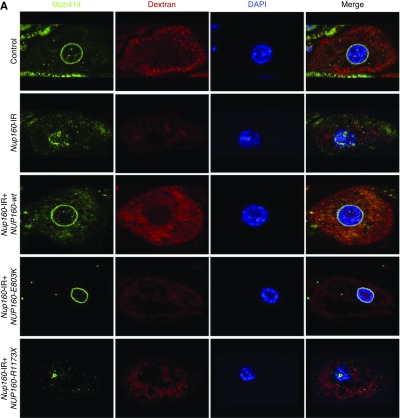
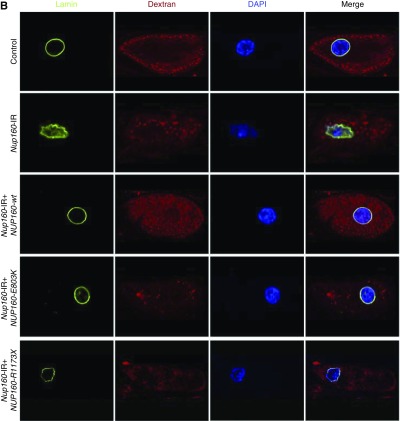
In third instar larvae, wild-type human NUP160 expression completely rescued the fly gene silencing–induced defects in nuclear volume, Dextran uptake, NPC localization, and nuclear lamin morphology (Figure 6). By contrast, neither the patient-derived NUP160 mutant allele E803K nor R1173× was capable of rescuing defective nuclear volume or Dextran uptake. The NUP160E803K mutant transgene did rescue NPC localization and nuclear lamin morphology, whereas the NUP160R1173× transgene did not (Figure 6). This result suggests that the E803K missense allele produces an Nup160 protein that can promote NPC assembly and support nuclear lamin and potentially, nuclear membrane morphology but that still results in defective nucleocytoplasmic transport, with severe consequences for nephrocyte cellular structure and function. The truncated R1173× allele, by contrast, is essentially null.
Figure 6.
Third instar larval nephrocyte phenotypes induced by Nup160-IR were rescued by wild type human NUP160, but not by NUP160E803K or NUP160R1173X. (A) Mab414 (green) immunolabeled components of the nuclear pore complex (NPC), fluorescent Dextran uptake indicated nephrocyte function (Figure 4), and 4′,6-diamidino-2-phenylindole (DAPI) staining revealed nuclear position and overall morphology. (B) Mab ADL67.10 labels all isoforms of Drosophila Sf9 Lamin (green) but does not label Lamin C. Nephrocyte-specific expression of a wild-type human NUP160 transgene (NUP160-wt) rescued all larval nephrocyte phenotypes. An NUP160E803K mutant transgene (NUP160-E803K) rescued NPC localization and Lamin morphology but not Dextran uptake. A NUP160R1173× mutant transgene (NUP160-R1173×) failed to rescue any larval nephrocyte phenotypes.
Discussion
The findings in this study indicate that compound-heterozygous mutations, NUP160R1173× and NUP160E803K, in the proband with familial SRNS are likely pathogenic, which implies that NUP160 mutations are associated with SRNS.
The pedigree of this study delineated an autosomal recessive family with SRNS (Figure 1C), which consisted of healthy parents (I1 and I2), the proband (II6), two siblings (II2 and II3) affected with SRNS, two siblings (II1 and II4) dying of unknown causes, and one healthy sibling (II5). The proband’s renal specimen revealed FSGS (Figure 2), and IF staining showed nonspecific scattered mesangial deposits of IgA and IgM. The proband (II6) progressed to ESRD and underwent renal transplantation. Until now, a 13-year follow-up of the graft kidney shows normal renal function and no post-transplant reoccurrence of nephrotic syndrome.
Here, on the basis of genomic sequencing, we excluded the possibility that the proband carried mutations in genes already known to be associated with SRNS (Supplemental Table 2). We systematically reduced the candidate gene list to six variant genes through bioinformatics-based analysis of minor allele frequency, mutation type, clinical characteristics, and web-based pathogenic prediction (Supplemental Table 4). We ultimately focused on compound-heterozygous mutations NUP160R1173× and NUP160E803K on the basis of the proband having only renal manifestations without syndromic disorder associated with extrarenal manifestations. The residues affected in these NUP160 mutant alleles are largely conserved. NUP160R1173× changes an arginine codon to a stop codon. The NUP160E803K mutation is predicted to be disease causing by MutationTaster. Finally, we functionally validated the NUP160 mutations in vivo as potentially implicated in SRNS pathogenicity.
We used the Drosophila nephrocyte as an experimental podocyte model. The nephrocyte has previously been shown to share striking molecular, cellular, structural, and functional homologies with mammalian podocytes, and it has been used previously to validate candidate renal disease genes as well as specific patient-derived mutant alleles.8,22,25,27,28 Here, we showed that Nup160 is essential for normal nephrocyte cell maintenance/survival through adult-stage development and that it is furthermore required for normal adult fly lifespan. Nephrocyte function as assayed by uptake of protein, AgNO3, and Dextran particles was severely impaired as a result of Nup160 gene silencing. Affected nephrocytes exhibited reduced nuclear volume, NPC components were dispersed, and nuclear lamin localization was irregular. These observations indicate that Nup160 is essential for NPC assembly and nuclear structure to support nucleocytoplasmic trafficking required for normal cell morphology and function.
The nephrocyte model further allowed us to examine differential phenotypic rescue potential of human NUP160WT, NUP160E803K, and NUP160R1173× alleles using a “gene replacement” approach. NUP160WT was shown to completely rescue the entire spectrum of nephrocyte phenotypes induced by endogenous fly Nup160 gene silencing. NUP160R1173×, by contrast, exhibited no phenotype rescue, consistent with this being a null allele. The missense NUP160E803K mutant allele did rescue NPC component localization and lamin morphology defects but not defects in other phenotypes, suggesting that this mutation can promote NPC assembly and localization but still severely impair nucleocytoplasmic transport and other NPC-associated functions required for normal nephrocyte physiology. Nephrocyte-specific overexpression of missense and truncated mutant human alleles did not induce functional defects, suggesting that these two mutant alleles are not gain-of-function or dominant negative mutations. Together, the experimental observations indicate that NUP160E803K and NUP160R1173× mutations are pathogenic mutations in the patient with SRNS and FSGS described in this study.
We recently found that knockdown of NUP160 inhibited cell proliferation; induced apoptosis, autophagy, and cell migration of mouse podocytes cultured in vitro; and altered the expression and localization of podocyte-associated molecules, including nephrin, podocin, CD2AP, and α-actinin-4.14 Mutations in NPHS1, NPHS2, and CD2AP disrupt the integrity of the slit diaphragm and lead to congenital nephrotic syndrome, early-onset autosomal recessive SRNS, and early-onset FSGS, respectively.4–6 Mutations in ACTN4 change the cytoskeletal dynamics of podocytes and lead to adult-onset autosomal dominant FSGS.7 Moreover, in vertebrates, transmembrane Nups anchor the NPCs within the nuclear envelope through interactions with two major scaffold modules: the symmetrically localized Nup107–160 complex and the central Nup93 complex. Nup107–160 complexes are composed of nine distinct subunits: Nup160/Nup120, Nup133, Nup107, Nup96, Nup85/Nup75, Nup43, Nup37, Seh1, and Sec13. In contrast to dynamic peripheral Nups, the components of the Nup107–160 complex are very stable and anchored within the NPCs during interphase.12,13 Significantly, previous reports have provided functional evidence from model system experiments that NPC-related mutations in NUP85,11 NUP93,9 NUP107,10,11 NUP133,11 and NUP2059 genes are causal for SRNS. Together, these data suggest that two compound-heterozygous mutations, NUP160E803K and NUP160R1173×, caused familial SRNS in the patient analyzed in this study.
Two limitations of this study should be noted. First, renal biopsy tissues taken from the patient (II6) 16 years ago were not analyzed for expression of NUP160. Second, only one patient with SRNS was identified with compound-heterozygous mutations in NUP160. Recently, however, Braun et al.11 reported compound-heterozygous potentially pathogenic mutations NUP160E803K and NUP160R910× in two siblings with SRNS and proteinuria from another nonconsanguineous Chinese family. The older brother presented at age 16 years old with nephrotic syndrome that was resistant to therapy with steroids or other immunosuppressants. His biopsy revealed FSGS, and his renal function corresponded to stage 3 CKD. The sister presented at age 7 years old with proteinuria. Neither sibling exhibited extrarenal symptoms. Interestingly, the missense mutation NUP160E803K found in the Chinese family in the work by Braun et al.11 is the same as that in the family in our study, whereas these two Chinese families with SRNS are not closely related.
In conclusion, considered in conjunction with findings from previous studies, our results implicate NUP160 mutations as causal in SRNS. Our findings indicate that NUP160 should be included in the SRNS diagnostic gene panel to identify additional patients with SRNS with homozygous or compound-heterozygous NUP160 mutations.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the patients and their families for participating in this study. We thank Prof. Jie Ding for advice on study design and facilitating collaborations. We also thank the Bloomington Drosophila Stock Center and the Vienna Drosophila Resource Center for fly stocks and the Developmental Studies Hybridoma Bank at the University of Iowa for antibodies.
Dr. Zhao is supported by Nature Science Foundation of Fujian Province of China grant 2015J01407. Dr. Yu is supported by National Nature Science Foundation of China grant 81270766, Major Project of Nanjing Military Command grant 14ZX27, and Key Project of Social Development of Fujian Province of China grant 2013Y0072. Dr. Han is supported by US National Institutes of Health grants DK098410 and HL134940.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Filling the Gap: Drosophila Nephrocytes as Model System in Kidney Research,” on pages 719–720.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018080786/-/DCSupplemental.
Supplemental Figure 1. Overexpression of wild-type and mutant human NUP160 transgenes.
Supplemental Table 1. Clinical features of three patients in a nonconsanguineous Chinese family with steroid-resistant nephrotic syndrome.
Supplemental Table 2. A list of known genes associated with steroid-resistant nephrotic syndrome.
Supplemental Table 3. Mapping statistics of whole-exome sequencing.
Supplemental Table 4. Rare variants found in patient II6.
References
- 1.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Trautmann A, Bodria M, Ozaltin F, Gheisari A, Melk A, Azocar M, et al. PodoNet Consortium : Spectrum of steroid-resistant and congenital nephrotic syndrome in children: The PodoNet registry cohort. Clin J Am Soc Nephrol 10: 592–600, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preston R, Stuart HM, Lennon R: Genetic testing in steroid-resistant nephrotic syndrome: Why, who, when and how? Pediatr Nephrol 34: 195–210, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, et al.: Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, et al.: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Löwik MM, Groenen PJ, Pronk I, Lilien MR, Goldschmeding R, Dijkman HB, et al.: Focal segmental glomerulosclerosis in a patient homozygous for a CD2AP mutation. Kidney Int 72: 1198–1203, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, et al.: Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24: 251–256, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Gee HY, Saisawat P, Ashraf S, Hurd TW, Vega-Warner V, Fang H, et al.: ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J Clin Invest 123: 3243–3253, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun DA, Sadowski CE, Kohl S, Lovric S, Astrinidis SA, Pabst WL, et al.: Mutations in nuclear pore genes NUP93, NUP205 and XPO5 cause steroid-resistant nephrotic syndrome. Nat Genet 48: 457–465, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyake N, Tsukaguchi H, Koshimizu E, Shono A, Matsunaga S, Shiina M, et al.: Biallelic mutations in nuclear pore complex subunit NUP107 cause early-childhood-onset steroid-resistant nephrotic syndrome. Am J Hum Genet 97: 555–566, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun DA, Lovric S, Schapiro D, Schneider R, Marquez J, Asif M, et al.: Mutations in multiple components of the nuclear pore complex cause nephrotic syndrome. J Clin Invest 128: 4313–4328, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morchoisne-Bolhy S, Geoffroy MC, Bouhlel IB, Alves A, Audugé N, Baudin X, et al.: Intranuclear dynamics of the Nup107-160 complex. Mol Biol Cell 26: 2343–2356, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vollmer B, Lorenz M, Moreno-Andrés D, Bodenhöfer M, De Magistris P, Astrinidis SA, et al.: Nup153 recruits the Nup107-160 complex to the inner nuclear membrane for interphasic nuclear pore complex assembly. Dev Cell 33: 717–728, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Wang P, Zhao F, Nie X, Liu J, Yu Z: Knockdown of NUP160 inhibits cell proliferation, induces apoptosis, autophagy and cell migration, and alters the expression and localization of podocyte associated molecules in mouse podocytes. Gene 664: 12–21, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Bier E: Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet 6: 9–23, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Chien S, Reiter LT, Bier E, Gribskov M: Homophila: Human disease gene cognates in Drosophila. Nucleic Acids Res 30: 149–151, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umbhauer M: [Nephrocytes and podocytes, even fight?]. Med Sci (Paris) 25: 27, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Cagan RL: The Drosophila nephrocyte. Curr Opin Nephrol Hypertens 20: 409–415, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Hermle T, Braun DA, Helmstädter M, Huber TB, Hildebrandt F: Modeling monogenic human nephrotic syndrome in the Drosophila garland cell nephrocyte. J Am Soc Nephrol 28: 1521–1533, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weavers H, Prieto-Sánchez S, Grawe F, Garcia-López A, Artero R, Wilsch-Bräuninger M, et al.: The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457: 322–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuang S, Shao H, Guo F, Trimble R, Pearce E, Abmayr SM: Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development 136: 2335–2344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashraf S, Gee HY, Woerner S, Xie LX, Vega-Warner V, Lovric S, et al.: ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J Clin Invest 123: 5179–5189, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang F, Zhao Y, Han Z: An in vivo functional analysis system for renal gene discovery in Drosophila pericardial nephrocytes. J Am Soc Nephrol 24: 191–197, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang F, Zhao Y, Chao Y, Muir K, Han Z: Cubilin and amnionless mediate protein reabsorption in Drosophila nephrocytes. J Am Soc Nephrol 24: 209–216, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu Y, Zhu JY, Richman A, Zhao Z, Zhang F, Ray PE, et al.: A Drosophila model system to assess the function of human monogenic podocyte mutations that cause nephrotic syndrome. Hum Mol Genet 26: 768–780, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han Z, Olson EN: Hand is a direct target of Tinman and GATA factors during Drosophila cardiogenesis and hematopoiesis. Development 132: 3525–3536, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Zhu JY, Fu Y, Richman A, Zhao Z, Ray PE, Han Z: A personalized model of COQ2 Nephropathy rescued by the wild-type COQ2 allele or dietary coenzyme Q10 supplementation. J Am Soc Nephrol 28: 2607–2617, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gee HY, Zhang F, Ashraf S, Kohl S, Sadowski CE, Vega-Warner V, et al.: KANK deficiency leads to podocyte dysfunction and nephrotic syndrome. J Clin Invest 125: 2375–2384, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.