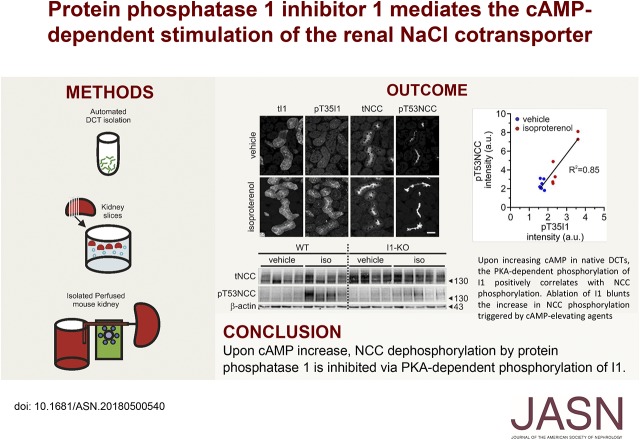
Significance Statement
Stimuli that elevate cAMP, including β-adrenergic agonists and parathyroid hormone, increase phosphorylation (and hence activity) of the thiazide-sensitive NaCl cotransporter (NCC) in the distal convoluted tubule. The protein phosphatase 1 (PP1) modulates NCC phosphorylation, but its role and the mechanism regulating its function are obscure. The authors used in vitro and ex vivo approaches to demonstrate that a PP1 inhibitor, protein phosphatase 1 inhibitor–1 (I1), mediates the effects of cAMP-elevating hormones on NCC. They propose a novel signaling pathway in which protein kinase A–dependent phosphorylation of I1 inhibits the PP1-dependent dephosphorylation of NCC. Given NCC’s critical role in renal control of ion homeostasis and BP, this pathway may contribute to the physiologic regulation of NCC and the development of arterial hypertension in the context of abnormal hormonal stimulation.
Keywords: Cell & Transport Physiology, cyclic AMP, distal tubule, Na transport, NaCl cotransporter, signaling
Visual Abstract
Abstract
Background
A number of cAMP-elevating hormones stimulate phosphorylation (and hence activity) of the NaCl cotransporter (NCC) in the distal convoluted tubule (DCT). Evidence suggests that protein phosphatase 1 (PP1) and other protein phosphatases modulate NCC phosphorylation, but little is known about PP1’s role and the mechanism regulating its function in the DCT.
Methods
We used ex vivo mouse kidney preparations to test whether a DCT-enriched inhibitor of PP1, protein phosphatase 1 inhibitor–1 (I1), mediates cAMP’s effects on NCC, and conducted yeast two-hybrid and coimmunoprecipitation experiments in NCC-expressing MDCK cells to explore protein interactions.
Results
Treating isolated DCTs with forskolin and IBMX increased NCC phosphorylation via a protein kinase A (PKA)–dependent pathway. Ex vivo incubation of mouse kidney slices with isoproterenol, norepinephrine, and parathyroid hormone similarly increased NCC phosphorylation. The cAMP-induced stimulation of NCC phosphorylation strongly correlated with the phosphorylation of I1 at its PKA consensus phosphorylation site (a threonine residue in position 35). We also found an interaction between NCC and the I1-target PP1. Moreover, PP1 dephosphorylated NCC in vitro, and the PP1 inhibitor calyculin A increased NCC phosphorylation. Studies in kidney slices and isolated perfused kidneys of control and I1-KO mice demonstrated that I1 participates in the cAMP-induced stimulation of NCC.
Conclusions
Our data suggest a complete signal transduction pathway by which cAMP increases NCC phosphorylation via a PKA-dependent phosphorylation of I1 and subsequent inhibition of PP1. This pathway might be relevant for the physiologic regulation of renal sodium handling by cAMP-elevating hormones, and may contribute to salt-sensitive hypertension in patients with endocrine disorders or sympathetic hyperactivity.
The thiazide-sensitive NaCl cotransporter (NCC) in the renal distal convoluted tubule (DCT) is crucial for the fine-tuning of renal sodium (Na+) reabsorption and hence for the control of BP. NCC and the DCT are also critically involved in the renal control of potassium (K+), magnesium (Mg2+), calcium (Ca2+), and acid/base homeostasis.1 The crucial role of NCC is evidenced by genetic diseases in which loss-of-function mutations of NCC cause Gitelman syndrome featuring hypokalemic alkalosis, hypomagnesemia, hypocalciuria, and lowered arterial BP.2 Conversely, enhanced NCC activity due to mutations in its regulating kinases, namely the with no lysine (K) (WNK) WNK1 and WNK4, causes familial hyperkalemic hypertension with hypermagnesemia and hypercalciuria.3 Moreover, mutations in ubiquitin-ligase complex proteins such as kelch-like-3 (KLHL3) and cullin-3 (CUL3), which control WNK4 stability, are also causative of familial hyperkalemic hypertension.4,5 The WNK kinases control NCC activity via the STE20/SPS 1–related proline- and alanine-rich kinase (SPAK) and the oxidative stress–response kinase 1 (OSR1). SPAK and OSR1 directly phosphorylate NCC at several serine and threonine residues located within the N-terminal tail of the cotransporter.6,7
The activity of the WNK-SPAK kinase pathway and NCC is regulated by various factors including the renin-angiotensin-aldosterone system.8 Although the DCT expresses the cognate receptors for angiotensin II and aldosterone, recent work suggests that the effect of these hormones on NCC is indirectly mediated via changes in plasma K+ concentration ([K+]).9,10 Plasma [K+] is proposed to modulate WNK4 activity through changes in DCT membrane voltage and intracellular Cl− concentration.11 Other NCC stimulators such as the β-adrenergic agonist isoproterenol as well as the parathyroid hormone (PTH) are thought to mediate their effects via intracellular cAMP.12–14 Recently, the cAMP-dependent protein kinase (protein kinase A [PKA]) was implicated in the regulation of WNK4, suggesting that cAMP may also act via the WNK/SPAK kinase pathway.13 However, these studies were mainly performed in heterologous expression systems and it remained unclear whether this and/or additional pathways contribute to the cAMP-dependent regulation of NCC in the native DCT. Some studies suggested that the kinase OSR1 and the extracellular signal–regulated kinase (ERK)1/2 mitogen-activated protein kinase (MAPK) are also involved in the activation of NCC by catecholamines15 and PTH,16 respectively.
Despite the progress on the elucidation of the role and regulation of the WNK/SPAK/OSR1 kinase pathway, little is known about the phosphatases that counterbalance the action of these kinases. As yet, three protein phosphatases are suggested to modulate NCC phosphorylation: protein phosphatase–1 (PP1), PP3 (calcineurin), and PP4. In Xenopus laevis oocytes, heterologous coexpression of NCC with PP4 lowered NCC phosphorylation.17 Likewise, pharmacologic inhibition of PP1 with calyculin A 18 and of PP3 with tacrolimus19,20 increased NCC phosphorylation in various experimental settings. The stimulatory effect of PP3 inhibition on NCC may have important clinical implications. In fact, a common side effect of calcineurin-inhibitor treatment is renal Na+ retention and arterial hypertension, which correlates with an enhanced urinary excretion of phosphorylated NCC.18,21 Nevertheless, the physiologic role of the different phosphatases in the DCT and the underlying mechanism regulating their function are unclear.
Interestingly, both the catalytic activity and the substrate specificity of phosphatases are often modulated by the interaction with specific regulatory subunits. We recently found that the endogenous inhibitor 1 (I1) of PP1 is highly expressed in the DCT with strong effects on NCC phosphorylation and arterial BP.22 I1 is a small, 171–amino acid cytosolic protein encoded by the Ppp1r1a gene.23 It is expressed in many organs including the brain, skeletal muscle, and the heart, where it is thought to contribute to neuronal plasticity,24 muscle glycogen metabolism,25 and cardiac contractility and excitability.23,26 Moreover, I1 was implicated in the control of the activity of the Na-K-ATPase in the heart,27 whereas PP1 was found to modulate the inhibitory effect of WNK4 on ROMK in the kidney.28 PKA phosphorylates I1 at a threonine residue in position 35 (T35), which activates I1 and makes it a strong and very specific inhibitor of PP1 with an IC50 value of 1 nM.29 Dephosphorylation of T35 by phosphatases such as PP2A and calcineurin (PP3) terminates the inhibitory action of I1.26 Interestingly, I1 is critically involved in β-adrenergic and cAMP-dependent signaling in skeletal and heart muscle,23,26 and I1-deficient mice are partially protected from isoprenaline-induced cardiac remodeling and arrhythmia.30
Here, we tested the hypothesis that I1 is also critically involved in the cAMP/PKA-dependent stimulation of NCC phosphorylation. Using a variety of ex vivo approaches, we propose a novel signal transduction pathway in which cAMP-dependent phosphorylation and activation of I1 mediates the effect of cAMP-elevating hormones on NCC phosphorylation and hence activity.
Methods
Reagents, Cells, and Antibodies
Unless otherwise stated, reagents were purchased from Sigma Aldrich (Buchs, Switzerland). Calyculin A was purchased from Cell Signaling Technologies (Danvers, MA). 8-Br-cAMP; PKI 14–22 amide, myristoylated; and H-89 were purchased from Tocris Bioscience (Bristol, UK). Endothall was purchased from EMD Millipore (Billerica, MA).
tNCC, pT53NCC, pT58NCC, and pS71NCC antibodies were previously described.21,22,31 I1 antibody was purchased from Epitomics (catalog no. 1747–1; Burlingame, CA.). The phosphosite-specific antibody recognizing pT35I1 was obtained via affinity purification of serum from rabbits immunized with the phospho-peptide NH2-CRRRP(pT)PATL-CONH2 corresponding to mouse I1 (Pineda, Berlin, Germany). The specificity of the antibody was confirmed by immunohistochemistry (Supplemental Figure 1). β-actin antibody was purchased from Sigma (catalog no. A5316; Buchs, Switzerland). Rabbit anti-FLAG antibody was purchased from GenScript (catalog no. A01868; Piscataway, NJ). Rabbit anti-AQP1 antibody was previously described.32 tNCC and pT58NCC antibodies used to detect calyculin A and endothall stimulation of NCC in MDCK type I cells were previously described (33,34 respectively). PP1c antibody was purchased from Abcam (catalog no. 53315; Cambridge, UK). Phospho-PKA substrate antibody was purchased from Cell Signaling Technologies (catalog no. 9624; Danvers, MA). OSR1 antibody was purchased from Abcam (catalog no. 125468; Cambridge, UK).
MDCK type I cells with tetracycline-inducible FLAG-tagged NCC were previously characterized.35
Animals
All animal experiments were conducted according to Swiss Laws and approved by the veterinary administration of the Canton of Zurich, Switzerland (license numbers: 213/2015 and 185/2017). Experiments were conducted in male and female I1-deficient mice (I1-KO)24 or wild type (WT). For automated DCT isolation, mice expressing the enhanced green fluorescent protein (EGFP) in the early segment of the DCT (DCT1) under the parvalbumin promoter (PV-EGFP) were used.22 Both transgenic lines and WT animals were kept in a homogenetic C57Bl/6 background. Mice were maintained in a 12-hour light/dark cycle and had access to standard chow type 3430 purchased from Provimi-Kliba (Kaiseraugst, Switzerland) and water ad libitum. Animals were age, weight, and sex matched for each experimental series.
Kidney Slices
Sex-, age-, and weight-matched mice were used for the preparation of kidney slices as described previously.18 To avoid confounding effects on NCC phosphorylation due to unequal dietary intake of K+, all mice were food deprived 16 hours before the experiment. Slices 280 μm thick were incubated in Ringer-type solution for 30 minutes at 30.5°C for equilibration. The [K+] of the buffer was always 3 mmol/L. Stock solutions of isoproterenol; 3-isobutyl-1-methylxanthine (IBMX); calyculin A; PKI 14–22 amide, myristoylated; and forskolin (FSK) were prepared in DMSO. Stock solutions of 8-Bromo-cAMP, Na+ salt, PTH, NE, and H-89 were prepared in H2O. Equal volumes of either DMSO or H2O were added as control vehicle when needed. After 30-minutes incubation with the drugs or vehicle solutions, slices were snap frozen in liquid nitrogen or immersion fixed with 3% PFA and processed for immunoblotting and histology, respectively. Electron microscopy confirmed that the structural integrity of DCT cells was preserved under the ex vivo incubation of the tissue slices (Supplemental Figure 2). Further experimental details can be found in the Supplemental Material and elsewhere.18
Immunoblotting
Immunoblotting was performed as previously described.18
Statistics
Unpaired Student's t-test was used to compare two groups. For multiple comparison, one-way or two-way ANOVA followed by Tukey’s multiple comparison post-test was performed.
Experimental details of the following methods: yeast two-hybrid, immunoprecipitation, immunofluorescence staining and fluorescence quantification, isolated perfused mouse kidney, electron microscopy, and automated DCT isolation are included in the Supplemental Material for this manuscript.
Results
cAMP-Dependent Stimulation of NCC Phosphorylation Is Mediated by PKA
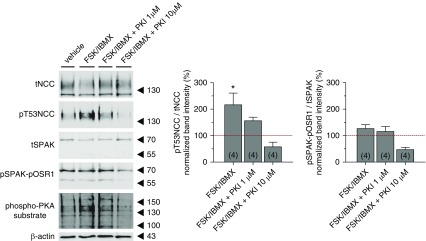
First, we investigated whether an increase in intracellular cAMP levels stimulates NCC phosphorylation in native DCTs via a PKA-dependent pathway. We isolated EGFP-positive early DCT fragments (DCT1) from transgenic mice expressing EGFP under the control of the parvalbumin promoter (PV-EGFP).22 The isolated DCTs were incubated with a cocktail of the adenylate cyclase stimulator FSK (10 μmol/L) and the phosphodiesterase inhibitor IBMX (100 μmol/L) in the presence or absence of the PKA inhibitor PKI 14–22. Stimulation with FSK and IBMX triggered a significant increase in the phosphorylation of NCC accompanied with a mild increase in pSPAK-pOSR1 and a marked activation of PKA as monitored using a phospho-PKA substrate antibody (Figure 1). NCC phosphorylation and the anti–phospho-PKA substrate signal substantially diminished upon coincubation with the PKA inhibitor PKI 14–22 (1 μmol/L). Under these conditions, the phosphorylation of SPAK and OSR1 remained almost unchanged compared with FSK/IBMX stimulation alone. Incubation of DCTs with 10 μM PKI 14–22 abolished the phosphorylation of NCC and of the SPAK-OSR1 kinases, indicating an overinhibition of the kinase pathway. Similar to PKI 14–22, the PKA inhibitor (H-89) blocked the stimulatory effect of FSK/IBMX on NCC phosphorylation. Surprisingly, and for unclear reasons, the effect of H-89 was even more pronounced in cAMP-stimulated DCTs than in unstimulated DCTs (Supplemental Figure 3).
Figure 1.
PKA activation stimulates NCC phosphorylation in native DCTs. Left panel represents a typical immunoblot of isolated DCTs treated with vehicle, 10 μmol/L FSK+100 μmol/L IBMX, FSK/IBMX+1 μmol/L PKI 14–22, and FSK/IBMX+10 μmol/L PKI 14–22. The activity of PKA was monitored using a phospho-PKA substrate antibody. Each lane corresponds to 400 DCT fragments. On the right, the densitometric analysis of pT53NCC/tNCC and pSPAK-pOSR1/tSPAK from four independent experiments (n in brackets) normalized to control vehicle group (red line) is represented. Error bars represent the SEM. *P<0.05 compared with control vehicle condition and assessed by one-way ANOVA followed by Tukey’s multiple comparisons test.
Genetic Ablation of I1 Attenuates the cAMP-Dependent Stimulation of NCC Phosphorylation
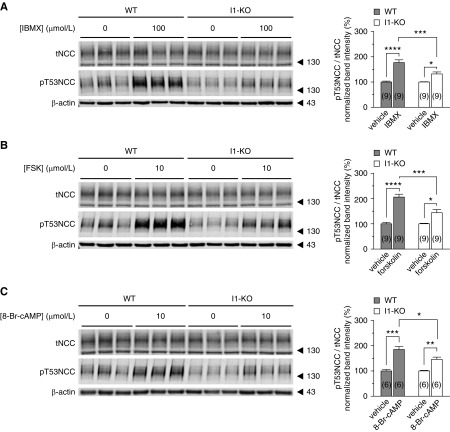
PKA phosphorylates I1 at position T35 in vitro, which renders I1 a potent and highly selective PP1 inhibitor.29,36 To test whether I1 is critical for the cAMP-dependent stimulation of NCC, we analyzed kidney slices from WT and I1-KO mice. Slices were incubated with FSK, IBMX, or the PKA-specific activator 8-Br-cAMP. All agonists strongly increased NCC phosphorylation at T53 (Figure 2, A–C) and at T58 and S71 (Supplemental Figure 4) in kidney slices from WT mice. In contrast, FSK, IBMX, and 8-Br-cAMP had only a small effect on NCC phosphorylation in kidney slices from I1-KO mice (Figure 2, A–C, Supplemental Figure 4).
Figure 2.
PKA stimulation of NCC phosphorylation is impaired in I1-KO mice. Representative immunoblots showing the effect of (A) IBMX (100 μmol/L), (B) FSK (10 μmol/L), and (C) 8-Br-cAMP (10 μmol/L) on NCC phosphorylation at position T53 in WT and I1-KO kidney slices. Bar charts represent the densitometric quantification of pT53NCC/tNCC normalized to control vehicle of each genotype from 6–9 slices (n in brackets) from 2–3 mice. Error bars represent the SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 assessed by two-way ANOVA followed by Tukey’s multiple comparison post-test.
PP1 Interacts with and Dephosphorylates NCC
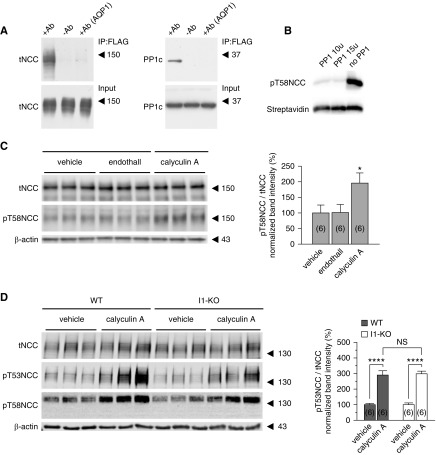
Previous studies by us and others showed that all isoforms of the catalytic subunit of PP1 (Ppp1ca, Ppp1cb, and Ppp1cc) are highly expressed in mouse22 and rat37 DCTs. Using a yeast two-hybrid screen on a mouse total kidney library, we found that an NCC fragment comprising the first 133 amino acids of rat NCC interacts with PP1 (Ppp1cb, GI number 161484667) in addition to other known interacting partners (e.g., OSR1,38 SPAK,39 and Hsp4040). To further confirm that PP1 interacts with NCC, coimmunoprecipitation experiments were performed using lysates from MDCK type I cells stably transfected with a tetracycline-inducible FLAG-tagged NCC.35 As shown in Figure 3A, endogenous PP1 was detected in samples immunoprecipitated with an anti-FLAG antibody but not in samples immunoprecipitated with an anti-AQP1 antibody or in the absence of antibody. Moreover, in vitro experiments showed that the PP1 catalytic subunit α is able to dephosphorylate a synthetic peptide corresponding to the N-terminal tail of mouse NCC with a phosphorylated threonine at the position 58 (Figure 3B).
Figure 3.
PP1 interacts with NCC and dephosphorylates it. (A) FLAG IP pull down of NCC (left) and the catalytic subunit of PP1 (PPIc) (right). Unrelated anti-AQP1 antibody as well as no antibody were used as negative control. (B) In vitro dephosphorylation of biotinylated pT58-mNCC peptide by PP1. (C) Changes in NCC phosphorylation at T58 in MDCK type I cells with tetracycline-inducible FLAG-tagged NCC expression upon treatment with calyculin A (left panel) or specific PP2A inhibitor endothall (right panel). Graph represents the densitometric quantification of immunoblots from two independent experiments. *P<0.05 by one-way ANOVA followed by Tukey’s multiple comparisons test. (D) Changes in NCC phosphorylation upon treatment of WT and I1-KO mouse kidney slices with calyculin A (20 nmol/L). Bar charts represent the densitometric quantification of pT53NCC/tNCC from six slices (in brackets) normalized to control vehicle of each genotype (two mice per group). Error bars represent the SEM. ****P<0.0001 assessed by two-way ANOVA followed by Tukey’s multiple comparison post-test.
To confirm the functional relevance of PP1 for the regulation of NCC, NCC-expressing MDCK cells were also treated with PP1 and PP2A inhibitors. Although the inhibition of PP1 with calyculin A profoundly stimulated NCC phosphorylation, the specific inhibition of PP2A with endothall did not change the phosphorylation of NCC (Figure 3C). Likewise, calyculin A increased NCC phosphorylation in kidney slices from both WT and I1-KO mice to the same extent (Figure 3D), indicating that the effect of calyculin A is downstream of the regulatory action of I1.
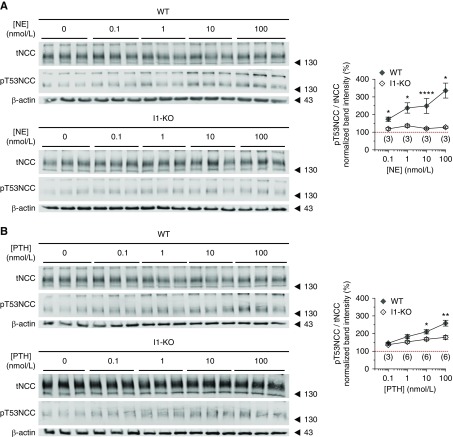
NE and PTH Stimulate NCC Phosphorylation in a Dose- and I1- Dependent Manner
It has been previously proposed that NE stimulates the phosphorylation of NCC via a PKA-dependent mechanism.41 Moreover, PTH was shown to activate the adenylate cyclase in the human and rat DCT promoting a strong increase in intracellular cAMP.12,42 Using kidney slices from WT and I1-KO mice, we tested whether these two hormones directly stimulate the phosphorylation of NCC in native DCTs and whether this effect depends on I1. As shown in Figure 4, A and B, both hormones promote a dose-dependent increase in the phosphorylation of NCC in kidney slices from WT animals. The effect of NE on NCC phosphorylation was completely abolished in I1-KO mice (Figure 4A). On the other hand, PTH still triggered a residual phosphorylation of NCC in kidney slices from I1-KO animals, although substantially weaker than in WT slices (Figure 4B).
Figure 4.
I1 mediates the effect of cAMP-increasing hormones on NCC phosphorylation. Dose-response effect of (A) NE and (B) PTH on NCC phosphorylation (T53) in WT and I1-KO mouse kidney slices. Graphs represent the densitometric quantification of pT53NCC/tNCC normalized to control vehicle of each genotype (red line). The numbers of slices assayed from one (NE) or two (PTH) mice per genotype are in brackets. Error bars represent the SEM. Stars denote statistically significant differences (*P<0.05, **P<0.01, ****P<0.0001) between the two genotypes for the same hormone concentration assessed by unpaired t test.
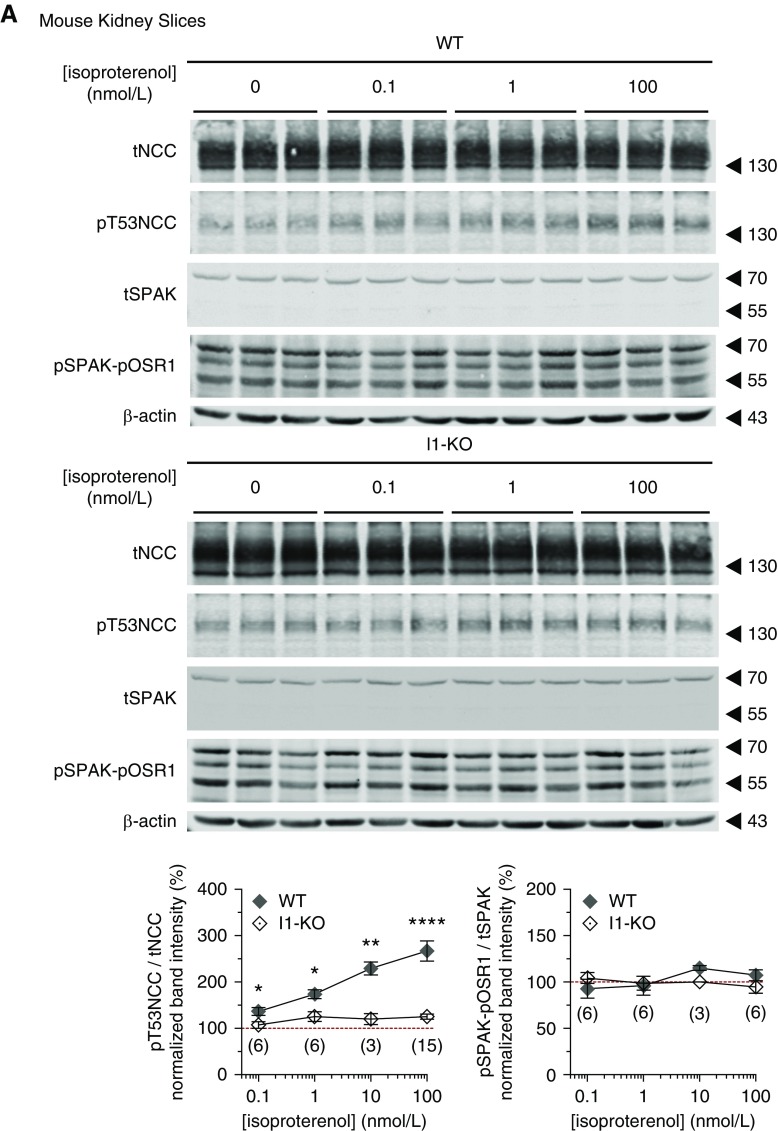
The β-Adrenergic Stimulation of NCC Phosphorylation Is Mediated by I1
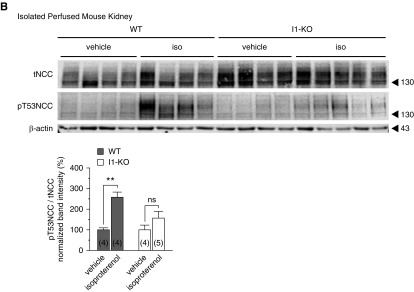
Terker and coworkers suggested that β-adrenergic receptors are instrumental for the stimulation of NCC phosphorylation by catecholamines.15 We hypothesized that the β-adrenergic stimulation of NCC phosphorylation is mediated via I1. To test this hypothesis, kidney slices from WT and I1-KO mice were incubated with the β-adrenergic agonist isoproterenol. Isoproterenol caused a significant rise in NCC phosphorylation in kidney slices from WT mice (Figure 5A) in agreement with previous observations by us18 and others.15 However, this stimulatory effect was blunted in kidney slices from I1-KO mice, confirming the results with NE stimulation. Similar results were obtained using another ex vivo model, namely the isolated perfused mouse kidney (Figure 5B).
Figure 5.
The β-adrenergic stimulation of NCC phosphorylation is impaired in I1-KO mice. (A) Dose-response effect of β-adrenergic agonist isoproterenol on the phosphorylation of NCC (T53), SPAK, and OSR1 in WT and I1-KO kidney slices. Graphs represent the densitometric quantification of the phosphorylation of pT53NCC/tNCC and pSPAK-pOSR1/tSPAK normalized to vehicle control of each genotype from 3–15 slices (in brackets) (1–5 mice). Stars represent statistical significance (*P<0.05, **P<0.01, ****P<0.0001) of the comparison between the two genotypes for the same concentration of isoproterenol assessed by unpaired t test. (B) β-adrenergic stimulation of NCC phosphorylation in isolated perfuse mouse kidneys. Bar charts represent the densitometric quantification of pT53NCC/tNCC normalized to control vehicle of each genotype in 4–5 mice per experiment (n in brackets). **P<0.01 assessed by two-way ANOVA followed by Tukey’s multiple comparison post-test. Error bars represent the SEM.
Surprisingly, we did not observe any significant increase in the phosphorylation of SPAK-OSR1 upon stimulation with isoproterenol (Figure 5A). Moreover, when SPAK phosphorylation and activity were clamped at high levels by incubating the kidney slices in a low Cl− solution (5 mmol/L),18 the stimulatory effect of isoproterenol on NCC was preserved and clearly additive to the effect of low Cl− (Supplemental Figure 5). Moreover, neither the expression of SPAK (Figure 5A) nor of OSR1 (Supplemental Figure 6) shows any difference between WT and I1-KO kidneys. These findings suggest that, at least in our experimental settings, the β-adrenergic stimulation of NCC is largely independent from an activation of the WNK/SPAK-OSR1 pathway.
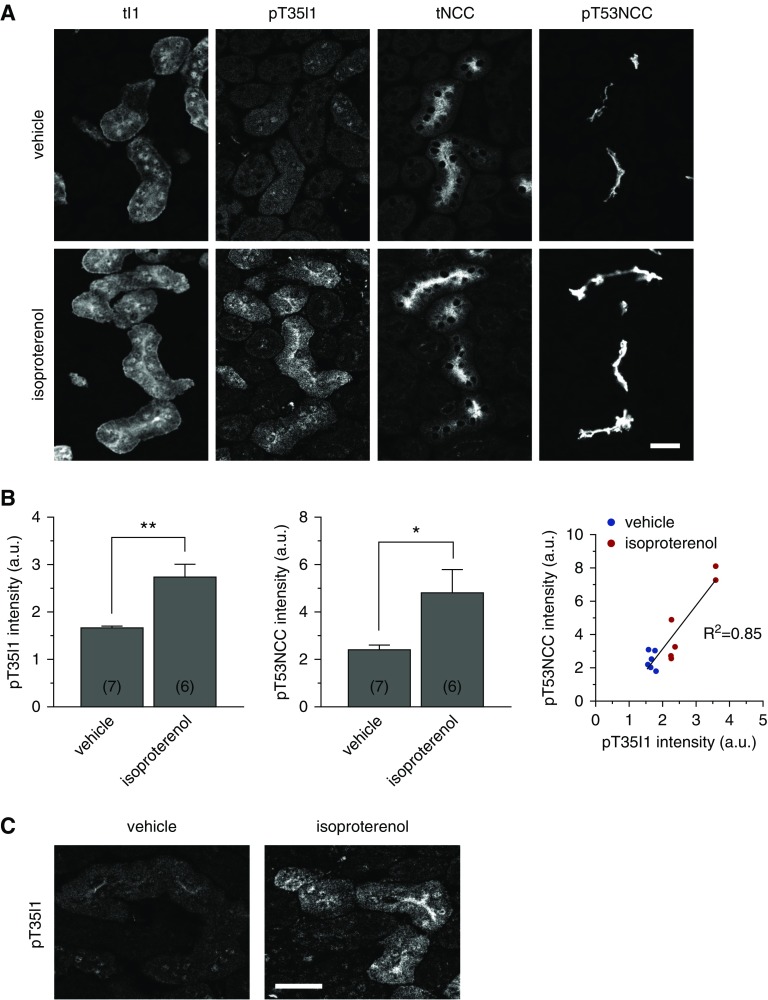
cAMP Promotes I1 Phosphorylation at T35 in Native DCTs
To assess whether the activation of PKA promotes I1 phosphorylation in native DCTs, we developed a phosphoform-specific antibody against the PKA-phosphorylation site. This new pT35I1 antibody recognizes specifically the phosphorylated form of I1 as demonstrated by peptide competition experiments in immunofluorescent studies (Supplemental Figure 1). Unfortunately, the antibody works only for immunofluorescent studies. Therefore, we assessed the phosphorylation levels of I1 by immunohistochemistry. Consecutive cryosections obtained from kidney slices incubated ex vivo either with vehicle or isoproterenol were stained with antibodies against total I1 (tI1), pT35I1, tNCC, and pT53NCC (Figure 6), and the staining intensities in DCTs were then quantified using ImageJ software as described in the Methods section. As previously reported,22 I1 protein was found to be highly abundant in DCTs and in thick ascending limbs of Henle’s loop (Figure 6A). In contrast to total I1, pT35I1 was barely detectable in DCTs in vehicle-treated kidney slices. However, the signal for pT35I1 and also pT53NCC significantly increased in DCTs in kidney slices stimulated with isoproterenol (Figure 6, A and B). Strikingly, the phosphorylation of I1 and NCC showed a strong linear correlation (Figure 6B). Of note, both the total and the phosphorylated form of I1 were mainly seen at the apical cell surface of DCTs and hence in proximity to NCC (Figure 6, A and C).
Figure 6.
β-adrenergic stimulation promotes the phosphorylation of I1 at T35 in native DCTs. (A) Representative immunofluorescence stainings of tI1, pT35I1, tNCC, and pT53NCC in consecutive sections of kidney slices from WT mice are shown. Slices were treated with isoproterenol 100 nmol/L or vehicle (scale bar, 25 μm). (B) The graph represents the relative mean intensity of pT35I1 staining (left panel) or pT53NCC (middle panel) in 6–7 slices (in brackets) from three mice (see Supplemental Material for details). Error bars represent the SEM. *P<0.05, **P<0.01 assessed by unpaired t test. Right panel represents the linear correlation between I1 (pT35) and NCC (pT53) phosphorylation. (C) Higher magnification of typical pT35I1 staining in kidney slices treated with vehicle (left panel) and isoproterenol (right panel), highlighting its marked apical accumulation (scale bar, 25 μm).
Discussion
The DCT is the target for several cAMP-elevating hormones including β-adrenergic agonists, PTH, and vasopressin.12 These hormones are known to activate the DCT-specific NCC but the involved signal transduction pathways remained poorly defined. In this study, we used a set of ex vivo approaches to reveal a complete signal transduction pathway by which cAMP-elevating hormones, via PKA, I1, and PP1, control NCC phosphorylation and hence activity.
In our studies, we tested the effects of two physiologically relevant hormones, namely NE and PTH. Both hormones strongly stimulated NCC phosphorylation in a dose-dependent manner in a range from 0.1 to 100 nmol/L. For NE, this range matches well with the reference range of normal plasma NE concentrations in humans (i.e., 0.83–10 nmol/L).43 For PTH, this range is above physiologic levels (20–65 ng/L, approximately 2–6.5 pmol/L).44 However, the PTH applied to native tissue elicited effects on NCC already at concentrations that were far below those reported in the literature (100 nmol/L) to activate NCC and TRPV516,45 and to downregulate NaPi2a46 in cell systems and tissue slices, respectively. It is also important to consider that PTH is a peptide hormone that likely penetrates less efficiently into the tissue slices than the much smaller catecholamines. Moreover, the high levels of peptidases in the kidney slices (e.g., in the brush border of proximal tubules) may rapidly degrade PTH to inactive metabolites. Independent from these possible technical hurdles, the data clearly indicate that both hormones are able to elicit a graded response of NCC phosphorylation. At least for NE, this graded response occurs in the range of physiologic and pathophysiologic plasma NE variations and may hence contribute to altered renal Na+ reabsorption in response to changed sympathetic tone.
Several studies have already suggested that cAMP-dependent activation of PKA contributes to the hormonal regulation of NCC.13,15,41,47–49 Nevertheless, as pointed out by Mutig et al.,48 direct experimental support for the role of cAMP and PKA for the control of NCC activity in native DCTs had been limited, probably due to the difficulty to establish readily available and suitable DCT cell models. Now, using isolated mouse DCTs and kidney slices incubated ex vivo with FSK and IBMX in the absence or presence of the PKA inhibitors PKI 14–22 and H-89, we provide compelling evidence that cAMP and PKA modify NCC activity in the native DCT. I1 is a direct target for PKA, which phosphorylates I1 at a threonine in position 53 (pT35I1) and converts it into a potent and selective inhibitor of PP1.29 Consistent with an activation of the cAMP/PKA pathway in native DCTs, we observed a profound stimulation of I1 phosphorylation at the consensus PKA site T35 in response to isoproterenol stimulation. The I1 phosphorylation strongly correlated with the level of NCC phosphorylation, suggesting that they are functionally linked. Interestingly, I1 appears to be barely phosphorylated at T35 in DCTs of vehicle-treated kidney slices, suggesting that I1-dependent NCC regulation plays a significant role in response to hormonal stimuli but not under resting conditions.
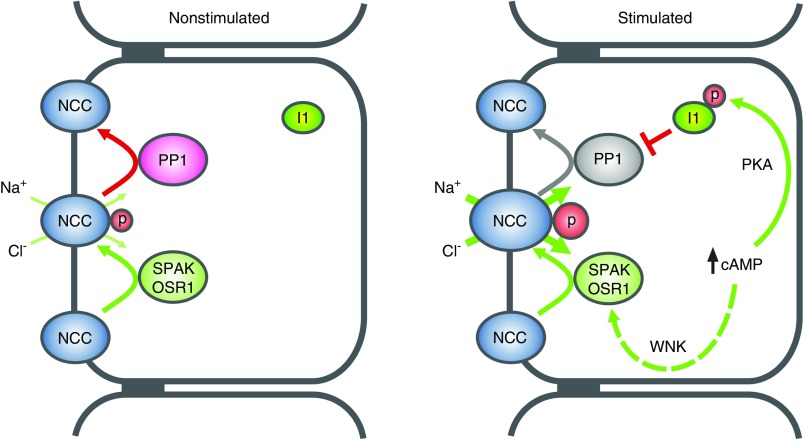
In vitro kinase assays and experiments in heterologous expression systems suggested that PKA exerts its effects on NCC via the classic KLHL3-WNK4-SPAK pathway. PKA-mediated phosphorylation of KLHL3 at S433 decreases KLHL3-dependent ubiquitination and degradation of WNK4.47 Likewise, PKA phosphorylates WNK4 at multiple sites including S64 and S1169, which finally promotes WNK4-dependent phosphorylation and activation of SPAK.13 PP1 was shown to modulate the phosphorylation levels of both WNK428 and SPAK.50 Studies on the regulation of the Na-K-2Cl cotransporter NKCC1, which is structurally related to NCC, suggested that PP1 binds to the N-terminal tail of NKCC1 in direct proximity to SPAK to dephosphorylate both SPAK and NKCC1.50 NCC lacks the amino acid motif mediating the binding of PP1 to NKCC1.50 Nevertheless, our yeast two-hybrid and coimmunoprecipitation data suggest that PP1 and NCC do also interact and might be linked in a signaling complex that may also involve WNK4 and SPAK/OSR1. Therefore, it is conceivable that I1 and PP1 may control NCC activity directly via NCC dephosphorylation and/or indirectly via controlling WNK4 and SPAK/OSR1 phosphorylation. However, neither in this nor in our previous studies did we observe significant evidence for an involvement of I1 in SPAK/OSR1 regulation. The abundance and subcellular localization of total-SPAK, total-OSR1 (this study), and pSPAK/pOSR1 were similar in the kidneys and DCTs of WT and I1-KO mice.22 Moreover, FSK/IBMX and isoproterenol had no significant effects on SPAK/OSR1 phosphorylation in isolated DCTs (Figure 1) and in kidney slices (Figure 5), respectively. Likewise, incubation of the kidney slices in a low-Cl− solution, which clamps SPAK/OSR1 activity at high levels,18 did not block the stimulatory effect of isoproterenol on NCC phosphorylation (Supplemental Figure 5). Nevertheless, these negative results do not formally exclude some activation of the WNK4/SPAK/OSR1 pathway. In fact, we consistently observed slight, but never statistically significant, increases in pSPAK/OSR1 immunoreactivities in tissue samples treated with FSK/IBMX and isoproterenol. Moreover, previous studies on heterologous expression systems linked SPAK to the cAMP/PKA-dependent NCC regulation,13 whereas studies on knockout mouse models implicated OSR1 (but not SPAK) in the catecholamine-dependent control of NCC.15 Parts of these discrepancies may reflect the different experimental settings (e.g., in vitro, ex vivo, in vivo) and compounds used, but they may also indicate some redundancies in the signaling pathways by which cAMP-elevating hormones stimulate NCC activity. In line with this view, we consistently observed some residual cAMP-dependent stimulation of NCC phosphorylation in kidneys of I1-KO mice in response to FSK/IBMX and in particular in response to PTH. In contrast, the effect of catecholamines (isoproterenol and NE) on NCC phosphorylation appeared to depend almost exclusively on the presence of I1. A possible explanation for these differences might be a compartmentalization of the cAMP signaling pathways. In fact, cAMP reporter assays provided evidence for a spatial and temporal control of cAMP dynamics in local microdomains with proteins producing and degrading cAMP,51 which allow circumscribed effects independent from cAMP levels outside of these microdomains.52 Thus, the hormone-induced cAMP/PKA-dependent activation of NCC may involve several redundant pathways including the previously characterized WNK4, SPAK, and OSR1 kinase pathways.15,35,48,53 This study adds I1 as an important additional regulator and suggests that, in addition to the NCC-controlling kinases, also the phosphatases are tightly regulated. Figure 7 shows the proposed signaling model that we believe best explains the current observations in the context of prior knowledge.
Figure 7.
Model of the stimulation of NCC phosphorylation by cAMP-increasing hormones. The cAMP-dependent activation of the WNK/SPAK/OSR1 pathway was shown in previous studies.13,15,53 The cAMP-dependent activation of the I1/PP1 pathway is presented in this study.
Aside from these novel insights into the molecular mechanism controlling NCC function, our findings may have some clinical implications. Inappropriately high sympathetic activity is thought to contribute to cardiovascular diseases including cardiac arrhythmia, cardiac failure, and arterial hypertension.54 Previous studies already implicated I1 in the β-adrenergic response of the heart modulating cardiac contractility,55 excitibality,22 and remodeling.23 This study extends these observations to the kidney and shows that I1 is also critically involved in the renal response to catecholamines. Our data indicate that catecholamines increase the phosphorylation of I1 at the PKA consensus site T35, which converts I1 into a potent inhibitor of PP1-dependent NCC dephosphorylation. This finally increases NCC phosphorylation as observed in this and several other,15,41,56 but not all,57 previous studies and may contribute to NE-induced salt-sensitive hypertension.58 Interestingly, the pT35 site of I1 is dephosphorylated by PP3 (calcineurin).23 Immunosuppressive therapy with calcineurin inhibitors such as tacrolimus and cyclosporine A is often complicated by the development of arterial hypertension, which was suggested to be linked to renal Na+ retention due to an activation of NCC.19 It is tempting to speculate that at least parts of these effects are also mediated via I1. Future in vivo studies will have to address the relevance of renal I1 for catecholamine- and calcineurin-induced arterial hypertension.
In summary, this study identified the I1 of PP1 as a central regulatory element in the signal transduction cascade that mediates the stimulatory effects of cAMP on the thiazide-sensitive NCC. I1 may represent an interesting point of convergence for different kinase and phosphatase pathways in the DCT contributing to the regulation of NCC in health and disease. Given its relevance for both the cardiac and renal response to β-adrenergic stimulation, I1 might also be an interesting drug target for the treatment of cardiovascular diseases, including arterial hypertension.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Monique Carrel, Michèle Heidemeyer, Shunmugam Nagarajan, and Tina Drejer for their excellent technical assistance and Adisa Trnjanin-Hadzic and Erich Brunner for their help with immunoprecipitations and biosorting, respectively.
The authors thank the Center of Microscopy and Image Analysis (ZMB) from the University of Zurich for excellent technical support. Dr. Hannah Monyer and Dr. Paul Greengard provided the PV-EGFP transgenic and the I1-deficient mice, respectively.
D. Penton is a fellow of the Program on Integrative Kidney Physiology and Pathophysiology (IKPP2) funded by the European Union’s Seventh Framework Program for research, technologic development, and demonstration under grant agreement no. 608847. J. Loffing is supported by research funds from the Swiss National Centre for Competence in Research “Kidney.CH,” and by project grants from the Swiss National Science Foundation (310030_143929/1). Funding to R.A. Fenton is provided by the Danish Medical Research Foundation, the Lundbeck Foundation, the Novo Nordisk Foundation, and Aarhus University Research Foundation.
D. Penton, R.A. Fenton, D. Loffing-Cueni, and J. Loffing conceived and designed the study. D. Penton, A. Wengi, S. Moser, J. Czogalla, L.L. Rosenbaek, F. Rigendinger, J.R. Martins, N. Faresse, R.A. Fenton, and D. Loffing-Cueni collected, analyzed, and interpreted the data. D. Penton, R.A. Fenton, and J. Loffing wrote the manuscript. All authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material Table of Content
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018050540/-/DCSupplemental
Detailed methods
Supplemental Figure 1. Assessment of the specificity of the pT35I1 antibody by immunohistochemistry.
Supplemental Figure 2. Assessment of DCT integrity in kidney slices via electron microscopy.
Supplemental Figure 3. Inhibition of cAMP-dependent phosphorylation of NCC by the PKA inhibitor H-89.
Supplemental Figure 4. Stimulation of NCC phosphorylation at T58 and S71 with cAMP-elevating agents.
Supplemental Figure 5. Effect of low Cl− on the stimulation of NCC and SPAK-OSR1 phosphorylation by isoproterenol in kidney slices of WT mouse.
Supplemental Figure 6. Expression of OSR1 in WT and I1-KO mice.
References
- 1.Subramanya AR, Ellison DH: Distal convoluted tubule. Clin J Am Soc Nephrol 9: 2147–2163, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riveira-Munoz E, Chang Q, Bindels RJ, Devuyst O: Gitelman’s syndrome: Towards genotype-phenotype correlations? Pediatr Nephrol 22: 326–332, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Murthy M, Kurz T, O’Shaughnessy KM: WNK signalling pathways in blood pressure regulation. Cell Mol Life Sci 74: 1261–1280, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP: Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci U S A 110: 7838–7843, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, et al.: Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482: 98–102, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang S-S, Fang Y-W, Tseng M-H, Chu P-Y, Yu I-S, Wu H-C, et al.: Phosphorylation regulates NCC stability and transporter activity in vivo. J Am Soc Nephrol 24: 1587–1597, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadchouel J, Ellison DH, Gamba G: Regulation of renal electrolyte transport by WNK and SPAK-OSR1 kinases. Annu Rev Physiol 78: 367–389, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Rojas-Vega L, Gamba G: Mini-review: Regulation of the renal NaCl cotransporter by hormones. Am J Physiol Renal Physiol 310: F10–F14, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Terker AS, Yarbrough B, Ferdaus MZ, Lazelle RA, Erspamer KJ, Meermeier NP, et al.: Direct and indirect mineralocorticoid effects determine distal salt transport. J Am Soc Nephrol 27: 2436–2445, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czogalla J, Vohra T, Penton D, Kirschmann M, Craigie E, Loffing J: The mineralocorticoid receptor (MR) regulates ENaC but not NCC in mice with random MR deletion. Pflugers Arch 468: 849–858, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, et al.: Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morel F: Sites of hormone action in the mammalian nephron. Am J Physiol 240: F159–F164, 1981 [DOI] [PubMed] [Google Scholar]
- 13.Castañeda-Bueno M, Arroyo JP, Zhang J, Puthumana J, Yarborough O 3rd, Shibata S, et al.: Phosphorylation by PKC and PKA regulate the kinase activity and downstream signaling of WNK4. Proc Natl Acad Sci U S A 114: E879–E886, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng L, Wu Q, Kortenoeven MLA, Pisitkun T, Fenton RA: A systems level analysis of vasopressin-mediated signaling networks in kidney distal convoluted tubule cells. Sci Rep 5: 12829, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terker AS, Yang C-L, McCormick JA, Meermeier NP, Rogers SL, Grossmann S, et al.: Sympathetic stimulation of thiazide-sensitive sodium chloride cotransport in the generation of salt-sensitive hypertension. Hypertension 64: 178–184, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko B, Cooke LL, Hoover RS: Parathyroid hormone (PTH) regulates the sodium chloride cotransporter via Ras guanyl releasing protein 1 (Ras-GRP1) and extracellular signal-regulated kinase (ERK)1/2 mitogen-activated protein kinase (MAPK) pathway. Transl Res 158: 282–289, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamba G: Regulation of the renal Na+-Cl− cotransporter by phosphorylation and ubiquitylation. Am J Physiol Renal Physiol 303: F1573–F1583, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penton D, Czogalla J, Wengi A, Himmerkus N, Loffing-Cueni D, Carrel M, et al.: Extracellular K+ rapidly controls NaCl cotransporter phosphorylation in the native distal convoluted tubule by Cl- -dependent and independent mechanisms. J Physiol 594: 6319–6331, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoorn EJ, Walsh SB, McCormick JA, Fürstenberg A, Yang C-L, Roeschel T, et al.: The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med 17: 1304–1309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shoda W, Nomura N, Ando F, Mori Y, Mori T, Sohara E, et al.: Calcineurin inhibitors block sodium-chloride cotransporter dephosphorylation in response to high potassium intake. Kidney Int 91: 402–411, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, et al.: Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Picard N, Trompf K, Yang C-L, Miller RL, Carrel M, Loffing-Cueni D, et al.: Protein phosphatase 1 inhibitor-1 deficiency reduces phosphorylation of renal NaCl cotransporter and causes arterial hypotension. J Am Soc Nephrol 25: 511–522, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wittköpper K, Dobrev D, Eschenhagen T, El-Armouche A: Phosphatase-1 inhibitor-1 in physiological and pathological β-adrenoceptor signalling. Cardiovasc Res 91: 392–401, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Allen PB, Hvalby O, Jensen V, Errington ML, Ramsay M, Chaudhry FA, et al.: Protein phosphatase-1 regulation in the induction of long-term potentiation: Heterogeneous molecular mechanisms. J Neurosci 20: 3537–3543, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nimmo GA, Cohen P: The regulation of glycogen metabolism. Purification and characterisation of protein phosphatase inhibitor-1 from rabbit skeletal muscle. Eur J Biochem 87: 341–351, 1978 [DOI] [PubMed] [Google Scholar]
- 26.Nicolaou P, Hajjar RJ, Kranias EG: Role of protein phosphatase-1 inhibitor-1 in cardiac physiology and pathophysiology. J Mol Cell Cardiol 47: 365–371, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Armouche A, Wittköpper K, Fuller W, Howie J, Shattock MJ, Pavlovic D: Phospholemman-dependent regulation of the cardiac Na/K-ATPase activity is modulated by inhibitor-1 sensitive type-1 phosphatase. FASEB J 25: 4467–4475, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Lin D-H, Yue P, Rinehart J, Sun P, Wang Z, Lifton R, et al.: Protein phosphatase 1 modulates the inhibitory effect of With-no-Lysine kinase 4 on ROMK channels. Am J Physiol Renal Physiol 303: F110–F119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endo S, Zhou X, Connor J, Wang B, Shenolikar S: Multiple structural elements define the specificity of recombinant human inhibitor-1 as a protein phosphatase-1 inhibitor. Biochemistry 35: 5220–5228, 1996 [DOI] [PubMed] [Google Scholar]
- 30.El-Armouche A, Wittköpper K, Degenhardt F, Weinberger F, Didié M, Melnychenko I, et al.: Phosphatase inhibitor-1-deficient mice are protected from catecholamine-induced arrhythmias and myocardial hypertrophy. Cardiovasc Res 80: 396–406, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Wagner CA, Loffing-Cueni D, Yan Q, Schulz N, Fakitsas P, Carrel M, et al.: Mouse model of type II Bartter’s syndrome. II. Altered expression of renal sodium- and water-transporting proteins. Am J Physiol Renal Physiol 294: F1373–F1380, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Terris J, Ecelbarger CA, Nielsen S, Knepper MA: Long-term regulation of four renal aquaporins in rats. Am J Physiol 271: F414–F422, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA: The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci U S A 95: 14552–14557, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedersen NB, Hofmeister MV, Rosenbaek LL, Nielsen J, Fenton RA: Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int 78: 160–169, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Rosenbaek LL, Kortenoeven ML, Aroankins TS, Fenton RA: Phosphorylation decreases ubiquitylation of the thiazide-sensitive cotransporter NCC and subsequent clathrin-mediated endocytosis. J Biol Chem 289: 13347–13361, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang FL, Glinsmann WH: Separation and characterization of two phosphorylase phosphatase inhibitors from rabbit skeletal muscle. Eur J Biochem 70: 419–426, 1976 [DOI] [PubMed] [Google Scholar]
- 37.Lee JW, Chou C-L, Knepper MA: Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, et al.: Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci 121: 675–684, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Piechotta K, Lu J, Delpire E: Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J Biol Chem 277: 50812–50819, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Donnelly BF, Needham PG, Snyder AC, Roy A, Khadem S, Brodsky JL, et al.: Hsp70 and Hsp90 multichaperone complexes sequentially regulate thiazide-sensitive cotransporter endoplasmic reticulum-associated degradation and biogenesis. J Biol Chem 288: 13124–13135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mu S, Shimosawa T, Ogura S, Wang H, Uetake Y, Kawakami-Mori F, et al.: Epigenetic modulation of the renal β-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat Med 17: 573–580, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Chabardès D, Gagnan-Brunette M, Imbert-Teboul M, Gontcharevskaia O, Montégut M, Clique A, et al.: Adenylate cyclase responsiveness to hormones in various portions of the human nephron. J Clin Invest 65: 439–448, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duncan MW, Compton P, Lazarus L, Smythe GA: Measurement of norepinephrine and 3,4-dihydroxyphenylglycol in urine and plasma for the diagnosis of pheochromocytoma. N Engl J Med 319: 136–142, 1988 [DOI] [PubMed] [Google Scholar]
- 44.Insogna KL: Primary hyperparathyroidism. N Engl J Med 379: 1050–1059, 2018 [DOI] [PubMed] [Google Scholar]
- 45.Hoover RS, Tomilin V, Hanson LN, Pochynyuk O, Ko B: PTH modulation of NCC activity regulates TRPV5 Ca2+ reabsorption. Am J Physiol Renal Physiol 310: F144–F151, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bacic D, Schulz N, Biber J, Kaissling B, Murer H, Wagner CA: Involvement of the MAPK-kinase pathway in the PTH-mediated regulation of the proximal tubule type IIa Na+/Pi cotransporter in mouse kidney. Pflugers Arch 446: 52–60, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Yoshizaki Y, Mori Y, Tsuzaki Y, Mori T, Nomura N, Wakabayashi M, et al.: Impaired degradation of WNK by Akt and PKA phosphorylation of KLHL3. Biochem Biophys Res Commun 467: 229–234, 2015 [DOI] [PubMed] [Google Scholar]
- 48.Mutig K, Saritas T, Uchida S, Kahl T, Borowski T, Paliege A, et al.: Short-term stimulation of the thiazide-sensitive Na+-Cl− cotransporter by vasopressin involves phosphorylation and membrane translocation. Am J Physiol Renal Physiol 298: F502–F509, 2010 [DOI] [PubMed] [Google Scholar]
- 49.González-Núñez D, Morales-Ruiz M, Leivas A, Hebert SC, Poch E: In vitro characterization of aldosterone and cAMP effects in mouse distal convoluted tubule cells. Am J Physiol Renal Physiol 286: F936–F944, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Gagnon KB, Delpire E: Multiple pathways for protein phosphatase 1 (PP1) regulation of Na-K-2Cl cotransporter (NKCC1) function: The N-terminal tail of the Na-K-2Cl cotransporter serves as a regulatory scaffold for Ste20-related proline/alanine-rich kinase (SPAK) AND PP1. J Biol Chem 285: 14115–14121, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baillie GS: Compartmentalized signalling: Spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J 276: 1790–1799, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Averaimo S, Assali A, Ros O, Couvet S, Zagar Y, Genescu I, et al.: A plasma membrane microdomain compartmentalizes ephrin-generated cAMP signals to prune developing retinal axon arbors. Nat Commun 7: 12896, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saritas T, Borschewski A, McCormick JA, Paliege A, Dathe C, Uchida S, et al.: SPAK differentially mediates vasopressin effects on sodium cotransporters. J Am Soc Nephrol 24: 407–418, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manolis AJ, Poulimenos LE, Kallistratos MS, Gavras I, Gavras H: Sympathetic overactivity in hypertension and cardiovascular disease. Curr Vasc Pharmacol 12: 4–15, 2014 [DOI] [PubMed] [Google Scholar]
- 55.Carr AN, Schmidt AG, Suzuki Y, del Monte F, Sato Y, Lanner C, et al.: Type 1 phosphatase, a negative regulator of cardiac function. Mol Cell Biol 22: 4124–4135, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walsh KR, Kuwabara JT, Shim JW, Wainford RD: Norepinephrine-evoked salt-sensitive hypertension requires impaired renal sodium chloride cotransporter activity in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 310: R115–R124, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uchida S, Chiga M, Sohara E, Rai T, Sasaki S: Does a β2-adrenergic receptor-WNK4-Na-Cl co-transporter signal cascade exist in the in vivo kidney? Nat Med 18: 1324–1325, author reply 1325–1327, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Fujita T: Mechanism of salt-sensitive hypertension: Focus on adrenal and sympathetic nervous systems. J Am Soc Nephrol 25: 1148–1155, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.