Nephrogenic syndrome of inappropriate antidiuresis (NSIAD) is the mirror image of nephrogenic diabetes insipidus (NDI): in NDI, the kidneys cannot concentrate the urine, whereas in NSIAD, urinary dilution is impaired, independent of the presence or absence of vasopressin. Consequently, patients with NDI are at risk of hypernatremic dehydration, whereas hyponatremia is a typical manifestation of NSIAD, mimicking the syndrome of inappropriate antidiuresis.1
The diagnostic pathways also mirror. In NDI, an agonist for the vasopressin V2 receptor (AVPR2), such as d-amino d-arginine vasopressin, is given to assess the ability of the kidneys to concentrate the urinary. Conversely, administration of an AVPR2 antagonist, such as tolvaptan, provides an assessment of urinary dilution capacity in patients suspected of NSIAD. In patients who did not present with dysnatremia but are suspected of having an underlying defect in urinary concentration, water deprivation (NDI) or water load (NSIAD) challenges the kidneys for an appropriate response. Lastly, vasopressin levels, measured either directly or indirectly via copeptin,2 can help distinguish nephrogenic disorders of urinary concentration from those of disturbed vasopressin secretion.
Genetic studies identified AVPR2, a G protein–coupled receptor (GPCR) expressed on the basolateral aspect of the principal cells in the collecting duct, as a key molecule in both disorders. This provided an elegant explanation for the clinical observations: recessive loss-of-function mutations in AVPR2 cause NDI, whereas NSIAD is due to dominant gain-of-function mutations. Two recent studies have identified mutations in the stimulatory Gα-protein GNAS as another cause of NSIAD. The association of GNAS with NSIAD provides further insight into the complex function of GNAS but fits perfectly with our current understanding of the physiology of urinary concentration.
In this issue of the Journal of the American Society of Nephrology, Miyado et al.3 report two families with a dominantly inherited form of NSIAD segregating with the GNAS variants p.F68_G70 del and p.M255V. The Gαs mutation p.F376V was reported in two unrelated patients with hyponatremia, and it was associated with additional clinical symptoms, suggesting gain-of-function not only of AVPR2 but also, of other GPCR, including the lutropin (LHGR) and parathyroid hormone (PTH1R) receptors.4 The severity of the phenotype in the patients with the dominantly inherited mutations was quite variable: some patients presented with seizures in early childhood associated with euvolemic hyponatremia, inappropriately elevated urine osmolality, and suppressed vasopressin levels. Other patients had no apparent symptoms and were normonatremic when investigated, but they had elevated urine osmolality despite suppressed vasopressin levels and a history of spontaneously low fluid intake. Symptomatic family members were treated with fluid restriction with normalization of hyponatremia.
The patients with the spontaneous mutation both presented with hyponatremia in the neonatal period.4 One of them was investigated in more detail at the age of 3 years old. Treatment until then had consisted of salt supplementation, which was associated with hypertension, consistent with the concept that hyponatremia was due to water overload rather than salt deficiency. When the salt supplements were stopped at the age of 3 years old, BP normalized without further antihypertensive medication, and the patient remained normonatremic. This is consistent with the idea of early childhood as the “vulnerable” period for NSIAD due to the coupling of fluid and caloric intake. At the age of 3 years old, the presumably suppressed thirst was sufficient to maintain normonatremia. Spontaneous urine osmolalities were consistently elevated (800–1000 mOsm/kg) despite suppressed copeptin levels, and urine osmolality did not decrease after tolvaptan, suggesting that urinary concentration was independent of AVPR2 activation by vasopressin. Together, these data clearly establish the diagnosis of NSIAD.
Heterotrimeric G proteins function as molecular switches in signal transduction pathways (Figure 1). Each G protein is composed of an α-, β-, and γ-subunit (Figure 2A) encoded by separate genes, and it is defined by its α-subunit, which binds guanine nucleotides and interacts with specific receptors and effectors.
Figure 1.
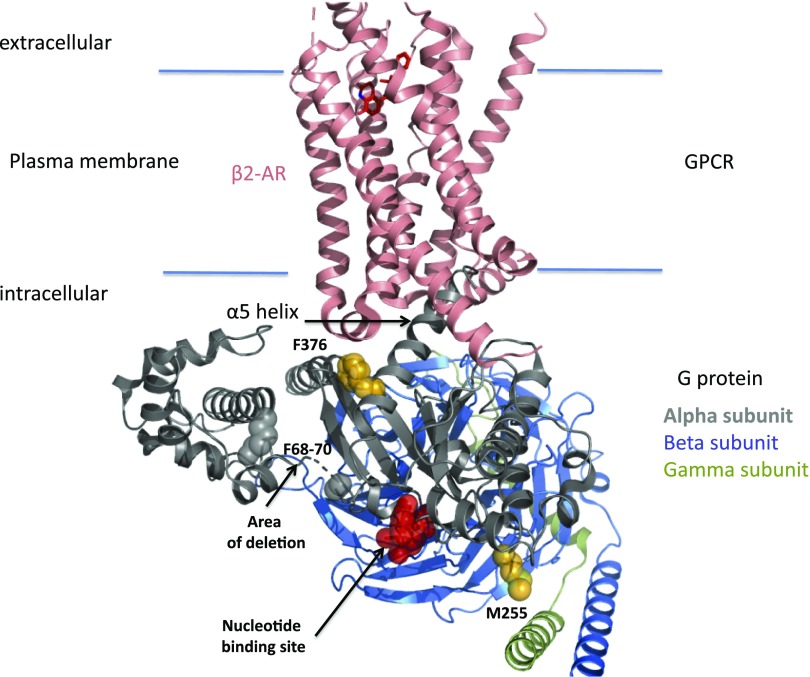
Structural view of a receptor G-protein signaling complex highlighting the position of amino acids mutated in GNAS variants. Schematic representation of the structure of the β2-adrenoreceptor (β2-AR)–Gs complex using the 3SN6 pdb file6 and localization of GNAS mutations. F376 belongs to the α5 helix of the Gα-subunit, which is critical for the receptor-induced release of the nucleotide from the G protein. M255 belongs to the rat sarcoma domain of the α-subunit, and F68–F70 belongs to the helical domain. GPCR, G protein–coupled receptor.
Figure 2.
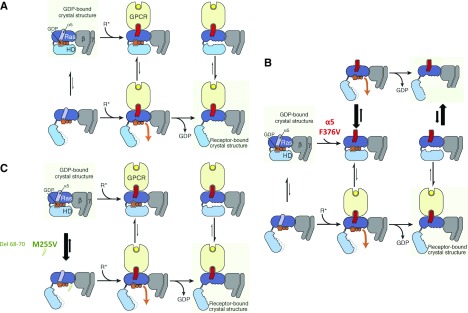
Schematic view of the G protein activation process for the wild type and GNAS variants. (A) Proposed mechanism of receptor-catalyzed nucleotide release (from ref. 7). (Left panels) The rat sarcoma (Ras) and helical domains separate frequently, even in the absence of a receptor, but such separation does not usually lead to guanosine diphosphate (GDP) release. (Upper left panel) This rapid (relative to overall GDP release) equilibrium favors the closed conformation. (Center panels) Binding of an activated receptor (R*) favors a Ras domain conformational change—displacement of α5 away from GDP—that weakens interactions between GDP and the Ras domain, allowing (lower center panel) GDP to escape when the Gα-domains happen to spontaneously separate. (Right panels) Loss of GDP shifts the equilibrium toward Gα-conformations with widely separated domains (lower right panel). Reprinted from ref. 7, with permission. (B and C) Proposed mechanism of gain of function with Gαs mutation F376V, M255V, or del 68–70 (using the same format as in A). (B) The F376V α5 mutation leads to the destabilization of the C-terminal helix (middle panels; red), thus mimicking the binding of an activated receptor (i.e., favoring a Ras domain conformational change—displacement of α5 away from GDP—that weakens interactions between GDP and the Ras domain, allowing GDP to escape when the Gα-domains happen to spontaneously separate) (top panels). An activated receptor can also bind to the mutated Gs protein (bottom panels). (Right panels) Loss of GDP shifts the equilibrium toward Gα-conformations with widely separated domains in the absence (right top panel) or the presence of an activated receptor (right bottom panel). The absence of effects of tolvaptan could be explained by the presence of Gs activated independently from the vasopressin V2 receptor (right top panel). (C) The M255V (or del 68–70) mutations favor the open conformation (unlike the wild-type Gs), thus shifting the equilibrium toward Gα-conformations with widely separated domains (lower panels), and thus, favor the receptor–Gα-protein complex with widely separated domains, resulting in higher G-protein activity on loss of GDP (right panels). GPCR, G protein–coupled receptor.
Because of its interactions with multiple different GPCR, GNAS has been associated with a whole spectrum of different diseases. Online Mendelian Inheritance in Man currently lists eight different phenotypes under the GNAS entry 139320, not yet including the two recent reports with NSIAD discussed here. The variability in phenotype partly reflects the nature of mutations (gain versus loss of function) but also, the fact that GNAS is highly imprinted so that, in various tissues, either the maternal or the paternal allele can be predominantly expressed.5
Existing structural models of GNAS homologs provide some insight into the potential disease mechanisms (Figure 2). The Gαs subunit is composed of a rat sarcoma–like GTPase domain and an α-helical domain (Figure 2A). The guanosine diphosphate binding site occupies the interface between these two domains. F376 belongs to the α5 helix of the Gα-subunit (Figure 1), which is critical for the receptor-induced release of the nucleotide from the G protein. The F376V mutation most likely induces a conformational change in the G protein that, in wild-type protein, is induced by activation of the associated receptor, explaining the receptor-independent signaling that is reflected in the high urinary concentration of the patient even when the receptor is blocked with tolvaptan (Figure 2B). The resistance of hyponatremia to tolvaptan is consistent with Gs activity independent from AVPR2 (illustrated in the upper panel of Figure 2B). Tolvapan inhibition of Gs (activated by arginine vasopressin as illustrated in the lower panel of Figure 2B) is not visible in vivo probably because of the signal amplification from Gs to adenylyl cyclase to protein kinase A to aquaporins. The differences observed in the basal activation of F376V between conditions in which different GPCRs were coexpressed in vitro could come from coupling properties of each receptor as well as from the possibility that they also couple to Gi protein (modification of cAMP accumulation). In this case, in vitro data are hard to interpret.
M255 localizes to the rat sarcoma domain of the α-subunit but interacts with N167 in the helical domain, suggesting that the M255V mutation destabilizes the closed conformation, enhancing nucleotide release. In contrast, F68–F70 are part of the helical domain, but the deletion mutation presumably also destabilize the closed conformation (Figure 2C).
Deciphering rare cases of hereditary hyponatremia in patients is a rich source for understanding GPCR signaling and human physiology.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “Germline-Derived Gain-of-Function Variants of Gsα-Coding GNAS Gene Identified in Nephrogenic Syndrome of Inappropriate Antidiuresis,” on pages 877–889.
References
- 1.Bockenhauer D, Penney MD, Hampton D, van’t Hoff W, Gullett A, Sailesh S, et al. : A family with hyponatremia and the nephrogenic syndrome of inappropriate antidiuresis. Am J Kidney Dis 59: 566–568, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Timper K, Fenske W, Kühn F, Frech N, Arici B, Rutishauser J, et al. : Diagnostic accuracy of copeptin in the differential diagnosis of the polyuria-polydipsia syndrome: A prospective multicenter study. J Clin Endocrinol Metab 100: 2268–2274, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Miyado M, Fukami M, Takada S, Terao M, Nakabayashi K, Hata K, et al. : Germline-derived gain-of-function variants 1 of Gsα-coding GNAS gene identified in nephrogenic syndrome of inappropriate antidiuresis. J Am Soc Nephrol 30: 877–889, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biebermann H, Kleinau G, Schnabel D, Bockenhauer D, Wilson LC, Tully I, et al. : A new multisystem disorder caused by the Gαs mutation p.F376V. J Clin Endocrinol Metab 104: 1079–1089, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linglart A, Maupetit-Méhouas S, Silve C: GNAS -related loss-of-function disorders and the role of imprinting. Horm Res Paediatr 79: 119–129, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, et al. : Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477: 549–555, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dror RO, Mildorf TJ, Hilger D, Manglik A, Borhani DW, Arlow DH, et al. : SIGNAL TRANSDUCTION. Structural basis for nucleotide exchange in heterotrimeric G proteins. Science 348: 1361–1365, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]