Significance Statement
IgA nephropathy (IgAN) has a clinical course that varies from asymptomatic nonprogressive to aggressive disease. However, few studies have investigated mortality in IgAN, and most of those that have done so lacked matched controls, did not report absolute risks, and had limited generalizability. In this nationwide cohort study in Sweden, the authors compared 3622 patients with IgAN with 18,041 matched general population controls, finding a 53% relative increase in mortality and a modest increase in absolute death rate, with one extra death per 310 person-years. On average, patients with IgAN died 6 years earlier than people without the disease. Excess mortality appeared to be limited to individuals with IgAN that had progressed to ESRD. These findings may have relevance to patient communication and policy development.
Keywords: mortality risk, IgA nephropathy, end-stage renal disease, Epidemiology and outcomes
Abstract
Background
The clinical course of IgA nephropathy (IgAN) varies from asymptomatic nonprogressive to aggressive disease, with up to one in four patients manifesting ESRD within 20 years of diagnosis. Although some studies have suggested that mortality appears to be increased in IgAN, such studies lacked matched controls and did not report absolute risk.
Methods
We conducted a population-based cohort study in Sweden, involving patients with biopsy-verified IgAN diagnosed in 1974–2011; main outcome measures were death and ESRD. Using data from three national registers, we linked 3622 patients with IgAN with 18,041 matched controls; we also conducted a sibling analysis using 2773 patients with IgAN with 6210 siblings and a spousal analysis that included 2234 pairs.
Results
During a median follow-up of 13.6 years, 577 (1.1%) patients with IgAN died (10.67 per 1000 person-years) compared with 2066 deaths (0.7%) in the reference population during a median follow-up of 14.1 years (7.45 per 1000 person-years). This corresponded to a 1.53-fold increased risk and an absolute excess mortality of 3.23 per 1000 person-years (equaling one extra death per 310 person-years) and a 6-year reduction in median life expectancy. Similar increases in risk were seen in comparisons with siblings and spouses. IgAN was associated with one extra case of ESRD per 54 person-years. Mortality preceding ESRD was not significantly increased compared with controls, spouses, or siblings. Overall mortality did not differ significantly between patients with IgAN-associated ESRD and patients with ESRD from other causes.
Conclusions
Patients with IgAN have an increased mortality compared with matched controls, with one extra death per 310 person-years and a 6-year reduction in life expectancy.
IgA nephropathy (IgAN) is the most common primary GN worldwide.1,2 The incidence rate has been estimated at 2–10 per 100,000 person-years.1,3–6 A renal biopsy with mesangial deposition of IgA immune complex is required for diagnosis. Disease course ranges from asymptomatic nonprogressive to aggressive, with up to one in four patients suffering from ESRD within 20 years from diagnosis, requiring RRT.7,8
Although several studies have sought to identify risk markers for disease progression,9–11 few have investigated mortality in IgAN. Furthermore, most of these studies have been limited to single centers,12 certain IgAN subgroups,13,14 or restricted to patients undergoing RRT,15,16 all of which limit generalizability. One Norwegian population-based study of 663 IgAN individuals showed an almost two-fold increased mortality, although excess mortality was only seen in those with ESRD or those at high risk of ESRD at the time of IgAN diagnosis.17
We examined overall and cause-specific mortality as well as risk of ESRD in a nationwide population-based cohort of 4125 patients with a biopsy-verified diagnosis of IgAN and a median follow-up >13 years.18,19 To minimize potential confounding, we compared patients with IgAN not only with general population controls, but also with secondary controls (siblings and spouses). We hypothesized that the patients with IgAN would be at increased risk of death compared with population controls as well as secondary controls.
Methods
Through unique personal identity numbers,20 we linked data on biopsy-verified IgAN from Swedish pathology departments with the Total Population Register (TPR), the National Patient Register (NPR), and the Cause of Death Register (CDR). See online Supplemental Appendix for register descriptions.
Definition of IgAN
We defined IgAN as having a computerized biopsy record of IgAN (diagnosed in 1974–2011) at any of the four pathology departments that evaluate all renal biopsy specimens in Sweden (Stockholm, Gothenburg, Linköping and Malmö/Lund). Patients were ascertained by the local computer departments that searched biopsy records for the IgAN Systematized Nomenclature of Medicine Clinical Term (SnoMed) code D67300,21 except for in Malmö/Lund, where renal biopsy reports were reviewed manually (for a detailed description, see Welander et al.18). As ESRD was one of our main outcomes, we excluded all patients with ESRD before inclusion date (see below for definition of ESRD). This exclusion criterion should exclude virtually all transplant biopsy specimens.
The IgAN diagnoses were previously validated through manual review of patient charts and biopsy reports of a random subset of 127 individuals, indicating a correct histopathologic diagnosis in 95% (95% confidence interval [95% CI], 92% to 99%) of patients.19 In this subset, the most common cause for diagnostic work-up was macroscopic hematuria (29%), with screening (26%; in hypertension evaluation and dipstick in occupational or pregnancy health service or accidental finding when evaluating other symptoms; dipstick screening was not universal in schools or occupational medicine during the study period) and purpura (11%) constituting other important factors for initial work-up. Some 91% of the patients with IgAN had a record of hematuria at some stage, although some patient chart records were incomplete, which likely explains the <100%. Almost half of the patients had hypertension. In patients with available data the median creatinine level was 104 µmol/L and 89% had C3 deposits. None had a record of HIV infection or chronic liver cirrhosis. Chronic viral hepatitis was noted in two men (one type B, one type C), and one woman had a resolved hepatitis B, all of whom had a typical presentation of IgAN with intermittent macroscopic hematuria.
Matched Reference Individuals (Controls)
The government agency Statistics Sweden identified up to five controls for each individual with IgAN. Controls, selected from the TPR, were matched for age, sex, calendar year, and county of residence at the time of renal biopsy.22
Identification of First-Degree Relatives and Spouses
First-degree relatives were identified through the Multi-Generation Register, which is a component of the TPR.22 The Multi-Generation Register contains data on siblings and parents to all individuals born in Sweden since 1932, who were still alive in 1961. We specifically identified those biologic relatives (parents, siblings, and children) who had IgAN or an ESRD code in the NPR (definitions in Supplemental Tables 1 and 2). Spouses were defined as the first person any patient with IgAN was married to and identified through the TPR.
Secondary Comparison Groups
For sensitivity analyses and to attenuate the effect of genetic, environmental, and behavioral factors, patients with IgAN were compared with their siblings and spouses. Relatives with a biopsy report of IgAN were excluded. Patients with IgAN without siblings or who were never married were excluded from the secondary analyses.
Medication
We used the Swedish Prescribed Drug Register (PDR) to identify drugs commonly used in patients with IgAN that may correlate with prognosis: corticosteroids, other immunosuppressant drugs, renin-angiotensin-aldosterone system (RAAS) inhibitors, and other antihypertensive drugs and statins (relevant ATC codes in Supplemental Table 3). The PDR, which started on July 1, 2005, has a high sensitivity. Patient identity data are available in about 99.7% of all records in the register.23 Over-the-counter medications or hospital-administered drugs are not included.
Additional Covariates
Data on education (as a proxy for socioeconomic status) were obtained from the TPR. Patients with IgAN and controls were categorized according to length of education (≤9, 10–12, and ≥13 years). Country of birth was divided into Nordic countries (including Sweden) versus non-Nordic countries.
Follow-Up
We defined the date of IgAN diagnosis as the day of the first renal biopsy demonstrating IgAN. Follow-up began at IgAN diagnosis and the corresponding date in the controls, and ended with death, emigration, or May 31, 2017 (December 31, 2015 in ESRD analyses and cause-specific mortality), whichever occurred first.
Overall and Cause-Specific Death
The CDR24 began in 1952 but became complete in 1961. Causes of death are reported in about 99.5% of the deceased participants (the remaining 0.5% are assigned the code R99.9). In this study, we began by examining overall mortality and then analyzed cause-specific mortality (three groups: cardiovascular disease, cancer, and other diseases; relevant International Classification of Diseases [ICD] codes in Supplemental Table 4).
Definition of ESRD
ESRD was defined as having a diagnosis of CKD stage 5, chronic renal dialysis, or kidney transplantation (corresponding ICD codes are listed in Supplemental Table 2), either as the primary or secondary cause of death in the CDR, or as a diagnosis in the NPR. To increase specificity, we required three or more records of ESRD diagnoses, with a minimum of 4 months between the first and last diagnosis.
Characterization of Patients with IgAN
The NPR was used to stratify for certain diagnoses that might affect mortality or ESRD development in patients with IgAN. The register includes hospital admissions since 1964, with national coverage from 1987, and nonprimary health care outpatient visits since 2001.25
Statistical Analyses
For survival analysis, we used Cox regression with internal stratification (each IgAN individual is compared with his or her matched controls) to calculate hazard ratios (HRs) for overall mortality, cause-specific mortality, ESRD, and a composite outcome (death or ESRD). We also examined ESRD mortality separately in the pre-ESRD period (ESRD as a censoring event) and in the post-ESRD period (first ESRD diagnosis as starting point and corresponding date in controls). As proportionality of hazards over time was not statistically verified, HRs were considered as estimates of the geometric means of the true changing HRs.26 Results were visualized using Cox regression survival curves. Additionally, we calculated median age at death for patients with IgAN and controls using the Kaplan–Meier method.
In the main analyses we adjusted for educational level, heredity for IgAN, heredity for ESRD, and the presence at baseline of cardiovascular comorbidity, cancer, diabetes mellitus, and other systemic inflammatory diseases. In sibling and spousal analyses we also adjusted for sex and age, but not for heredity. We then calculated mortality rates per 1000 person-years in patients with IgAN and controls and presented absolute excess rates. Throughout the analyses, we exclusively report adjusted HRs.
HRs for death and ESRD were also stratified on the basis of the presence of cardiovascular disease, cancer, diabetes mellitus (type 1 or 2), extrarenal IgA vasculitis (IgAV; Henoch–Schönlein purpura), other systemic inflammatory diseases (corresponding ICD codes in Supplemental Table 1), heredity for IgAN (defined as above), or ESRD, in patients with IgAN (not in controls), with the same adjustments as in the main analysis.
Furthermore, we calculated the HR for death in patients with IgAN with a certain medication compared with those without such medication. Medication was entered as a time-dependent covariate in Cox models, with left truncation on July 1, 2005 (this was when the PDR started). A patient was considered on treatment from the first date that the drug was dispensed until death or end of follow-up.
Because a number of individuals may have been diagnosed with IgAN as part of a work-up for the terminally ill, we investigated mortality in compliance with time since IgAN diagnosis (<1 year of follow-up, 1–4.99, ≥5, ≥10, ≥20, and ≥30 years). Stratified HRs were calculated as specified by age at the first diagnosis of IgAN (0–17, 18–39, 40–59, and ≥60 years). We also examined mortality as per sex, age, educational level, calendar year at diagnosis, and comorbidities.
To compare mortality in IgAN-related ESRD and ESRD from other causes, all patients with IgAN and reference individuals with ESRD were selected for a secondary analysis. Survival from first date of ESRD diagnosis was analyzed using nonstratified Cox regression with cluster robust SEM, with additional adjustment for age at ESRD.27
We used Stata Statistical Software, release 13 (StataCorp LP, College Station, TX) for the statistical calculations. P values <0.05 were considered statistically significant.
Ethics
The study was approved by the Stockholm Ethics Review Board (January 22, 2014; approval number: 2013/2095–31/2). Because this was a strictly register-based study, informed consent was waived by the Board.28
Results
Background Results
We identified 4125 individuals with IgAN. After exclusion of 504 (12.2%: one because of a redundant personal identity number and 503 because of ESRD before the diagnosis of IgAN), there remained 3622 for the final analyses (Table 1), including 227 (6.3%) with extrarenal manifestations of IgAV (Henoch–Schönlein purpura). Those 3622 patients were matched with 18,041 controls (99.6% of intended). Our sibling analyses included 2773 patients with IgAN with 6210 siblings, whereas the spousal analyses included 2234 pairs.
Table 1.
Baseline characteristics of IgAN diagnosed in Sweden in 1974–2011
| Characteristics | IgAN | General Population Controls | IgAN | Siblings | IgAN | Spouses |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Total | 3622 | 18,041 | 2773 | 6210 | 2234 | 2234 |
| Matched variables | ||||||
| Sex | ||||||
| Women | 1084 (29.9%) | 5408 (30.0%) | 802 (28.9%) | 3036 (48.9%) | 664 (29.7%) | 1591 (71.2%) |
| Men | 2538 (70.1%) | 12,633 (70.0%) | 1971 (71.1%) | 3174 (51.1%) | 1570 (70.3%) | 643 (28.8%) |
| Age | ||||||
| Age, mean (SD) | 39.6 (17.1) | 39.6 (17.1) | 35.8 (15.2) | 35.5 (16.9) | 44.1 (14.9) | 43.5 (15.2) |
| Age, median (IQR) | 38.8 (26.0–52.0) | 38.7 (26.0–52.0) | 34.9 (23.3–47.0) | 35.8 (22.9–48.4) | 43.7 (32.6–54.8) | 43.3 (32.3–54.3) |
| ≤17 yr | 370 (10.2%) | 1843 (10.2%) | 351 (12.7%) | 1025 (16.5%) | 62 (2.8%) | 90 (4.0%) |
| 18–39 yr | 1548 (42.7%) | 7754 (43.0%) | 1346 (48.5%) | 2601 (41.9%) | 864 (38.7%) | 864 (38.7%) |
| 40–59 yr | 1214 (33.5%) | 6010 (33.3%) | 887 (32.0%) | 2115 (34.1%) | 949 (42.5%) | 949 (42.5%) |
| ≥60 yr | 490 (13.5%) | 2434 (13.5%) | 189 (6.8%) | 469 (7.6%) | 359 (16.1%) | 326 (14.6%) |
| Unknown | 0 (0.0%) | 5 (0.2%) | ||||
| Calendar year | ||||||
| Entry year, median (IQR) | 2001 (1994–2007) | 2001 (1994–2007) | 2001 (1994–2008) | 2001 (1994–2007) | 2000 (1992–2006) | 2000 (1992–2006) |
| 1974–1988 | 428 (11.8%) | 2135 (11.8%) | 311 (11.2%) | 712 (11.5%) | 317 (14.2%) | 317 (14.2%) |
| 1989–2001 | 1448 (40.0%) | 7214 (40.0%) | 1086 (39.2%) | 2447 (39.4%) | 974 (43.6%) | 974 (43.6%) |
| 2002–2015 | 1746 (48.2%) | 8692 (48.2%) | 1376 (49.6%) | 3051 (49.1%) | 943 (42.2%) | 943 (42.2%) |
| Unmatched variables | ||||||
| Follow-up | ||||||
| Mean (SD) | 14.9 (8.61) | 15.4 (8.66) | 15.5 (8.58) | 16 (8.62) | 16 (8.83) | 16.8 (8.79) |
| Median (IQR) | 13.6 (7.99–20.8) | 14.1 (8.32–21.5) | 14.3 (8.46–21.4) | 14.8 (9.01–22.2) | 15.3 (8.93–22.4) | 16 (9.57–23.3) |
| <1 yr | 49 (1.4%) | 152 (0.8%) | 15 (0.5%) | 30 (0.5%) | 29 (1.3%) | 15 (0.7%) |
| 1–4 yr | 366 (10.1%) | 1690 (9.4%) | 238 (8.6%) | 469 (7.6%) | 197 (8.8%) | 147 (6.6%) |
| ≥5 yr | 3207 (88.5%) | 16,199 (89.8%) | 2520 (90.9%) | 5711 (92.0%) | 2008 (89.9%) | 2072 (92.7%) |
| Reason for ending the follow-up | ||||||
| Death | 577 (15.9%) | 2066 (11.5%) | 257 (9.3%) | 362 (5.8%) | 400 (17.9%) | 245 (11.0%) |
| Emigration | 101 (2.8%) | 664 (3.7%) | 76 (2.7%) | 152 (2.4%) | 47 (2.1%) | 39 (1.7%) |
| May 31, 2017 | 2944 (81.3%) | 15,311 (84.9%) | 2440 (88.0%) | 5696 (91.7%) | 1787 (80.0%) | 1950 (87.3%) |
| Person-years | 54,058 | 277,463 | 43,106 | 99,467 | 35,774 | 37,566 |
| Country of origin | ||||||
| Nordic | 3317 (91.6%) | 16,277 (90.2%) | 2715 (97.9%) | 6069 (97.7%) | 2008 (89.9%) | 1963 (87.9%) |
| Non-Nordic | 304 (8.4%) | 1762 (9.8%) | 58 (2.1%) | 141 (2.3%) | 226 (10.1%) | 211 (9.4%) |
| Unknown | 1 (<1%) | 2 (<1%) | 0 (0.0%) | 60 (2.7%) | ||
| Educational level | ||||||
| Compulsory school, ≤9 yr | 770 (21.3%) | 3823 (21.2%) | 468 (16.9%) | 1140 (18.4%) | 518 (23.2%) | 435 (19.5%) |
| Upper secondary school, 1–3 yr | 1611 (44.5%) | 8016 (44.4%) | 1300 (46.9%) | 2941 (47.4%) | 955 (42.7%) | 984 (44.0%) |
| University level | 1148 (31.7%) | 5745 (31.8%) | 958 (34.5%) | 1908 (30.7%) | 734 (32.9%) | 732 (32.8%) |
| Unknown | 93 (2.6%) | 457 (2.5%) | 47 (1.7%) | 221 (3.6%) | 27 (1.2%) | 83 (3.7%) |
| Henoch–Schönlein purpuraa | ||||||
| Yes | 227 (6.3%) | 26 (0.1%) | 191 (6.9%) | 18 (0.3%) | 93 (4.2%) | 4 (0.2%) |
| Medical historyb | ||||||
| Cardiovascular disease | 1283 (35.4%) | 1595 (8.8%) | 867 (31.3%) | 455 (7.3%) | 863 (38.6%) | 252 (11.3%) |
| Cancer | 226 (6.2%) | 973 (5.4%) | 150 (5.4%) | 339 (5.5%) | 148 (6.6%) | 141 (6.3%) |
| Diabetes, type 1 or 2 | 157 (4.3%) | 344 (1.9%) | 87 (3.1%) | 108 (1.7%) | 100 (4.5%) | 59 (2.6%) |
| Other systemic inflammatory disease | 292 (8.1%) | 434 (2.4%) | 205 (7.4%) | 198 (3.2%) | 189 (8.5%) | 74 (3.3%) |
| First-degree relative with renal disease | ||||||
| IgA nephropathyc | 35 (1.0%) | 31 (0.2%) | ||||
| ESRDd | 74 (2.0%) | 118 (0.7%) | ||||
Within 1 year of first renal biopsy indicating IgAN.
Before study entry (date of biopsy and corresponding date in controls). For definitions, see Supplemental Table 1.
Same definition as in the IgAN cohort (biopsy report of IgAN 1974–2011).
For relevant ICD and procedure codes, see Supplemental Table 2.
The median age at the time of IgAN diagnosis was 34.9 years (interquartile range [IQR], 26–52). The male-to-female ratio was 7:3. Median follow-up time was 13.6 years (IQR, 8.0–20.8) for patients with IgAN and 14.1 years (IQR, 8.3–21.5) for controls in the main analyses, 14.3 (IQR, 8.5–21.4) versus 14.8 years (IQR, 9.0–22.2) in the sibling analyses, and 15.3 (IQR, 8.9–22.4) versus 16.0 years (IQR, 9.6–23.3) in the spousal analyses. In this study, 994 patients with IgAN were followed for >20 years and 234 for >30 years. With the exception that patients with IgAN were more often of Nordic origin, patients and controls did not differ in other demographic characteristics, as a result of matching. Patients with IgAN had more cardiovascular disease, diabetes, and systemic inflammatory disease than controls, but not more cancer. Among the patients with IgAN, 1.0% had a first-degree relative with IgAN compared with 0.2% of the controls. ESRD in a first-degree relative was three times more common in patients with IgAN.
Overall Mortality
During a follow-up of 54,058 person-years, 577 (1.1%) patients with IgAN died (10.7 per 1000 person-years) (Figures 1 and 2, Supplemental Table 5). This figure can be compared with 2066 deaths (0.7%) in the reference population during a follow-up of 277,463 person-years (7.4 per 1000 person-years). The absolute excess mortality was 3.23 per 1000 person-years, which is equal to one extra death per 310 person-years. The corresponding HR was 1.53 (95% CI, 1.37 to 1.72). Median age at death was 77.0 years (95% CI, 75.9 to 78.0) in patients with IgAN, compared with 83.0 years (95% CI, 82.4 to 83.5) in controls, representing a reduction in median life expectancy of 6.0 years. Unadjusted HR was 1.83 (95% CI, 1.65 to 2.02); adjusting for all covariates except cardiovascular comorbidity changed this estimate only marginally (HR, 1.74; 95% CI, 1.57 to 1.94).
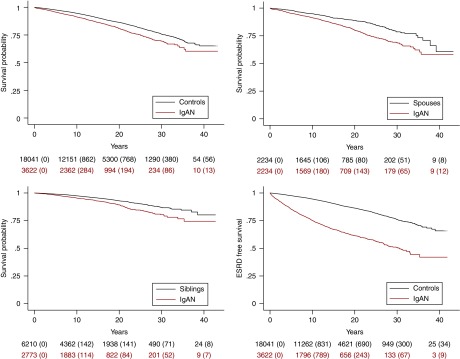
Figure 1.
Reduced patient and renal survival in IgAN patients compared to reference groups, visualized visualized through standardized Cox survival curves. Top left: patient survival of patients with IgAN compared with matched controls from the general population (TPR); P<0.001. Bottom left: patient survival of patients with IgAN compared with their siblings (Multi-Generation Register); P<0.001. Top right: patient survival of patients with IgAN compared with their spouses (the first person an IgAN patient was married to, according to the TPR); P<0.001. Bottom right: renal survival in patients with IgAN compared with matched controls (TPR); ESRD as outcome variable, death as a censoring event; P<0.001. Emigration and end of follow-up are censoring events in all analyses. Small figures indicate individuals at risk (instances of the outcome of interest within parentheses).
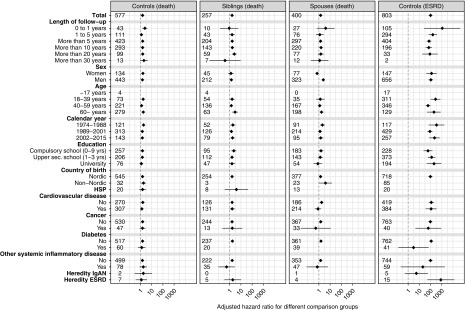
Figure 2.
Adjusted HRs for death and ESRD in patients with IgAN with subgroup stratification, showing increased mortality in IgAN compared to all reference groups, and increased ESRD compared to matched general population controls. First column: HRs for death of patients with IgAN compared with matched controls from the general population (TPR). Second column: HRs for death in of patients with IgAN compared with their siblings (Multi-Generation Register). Third column: HRs for death of patients with IgAN compared with their spouses (the first person an IgAN patient was married to, according to the TPR). Fourth column: HRs for ESRD in patients with IgAN compared with matched controls (TPR). Numbers represent absolute numbers of events in patients with IgAN. Whiskers represent 95% CIs. HSP, Henoch-Schönlein purpura.
The HR for mortality was similar in men and women. The highest mortality was found in the first year after diagnosis (HR, 2.47; 95% CI, 1.50 to 4.06), followed by a nonsignificant increase during the 1–4 years postdiagnosis (HR, 1.21; 95% CI, 0.93 to 1.58) and a 1.58-fold increased death hazard ≥5 years after diagnosis (95% CI, 1.39 to 1.80). Respective 10-, 20- and 30-year patient survival rates were 91.3% (95% CI, 90.3% to 92.2%), 80.7% (95% CI, 79.0% to 82.3%), and 69.6% (95% CI, 66.7% to 72.3%). Mortality was increased in all strata (HRs, 1.21–1.88), and did not differ by age (P for interaction =0.13) or calendar period (P for interaction =0.24). Adjustment for country of birth (Nordic versus not Nordic) did not influence the risk estimates (data not shown).
Risk estimates in the patients with IgAN were generally very similar in comparison with their siblings (HR, 1.62; 95% CI, 1.29 to 2.03) (Figures 1 and 2, Supplemental Table 6) and spouses (HR, 1.42; 95% CI, 1.11 to 1.83) (Figures 1 and 2, Supplemental Table 7), except that women with IgAN demonstrated lower mortality than their spouses (HR, 0.68; 95% CI not available because the estimation of the variance-covariance matrix did not converge).
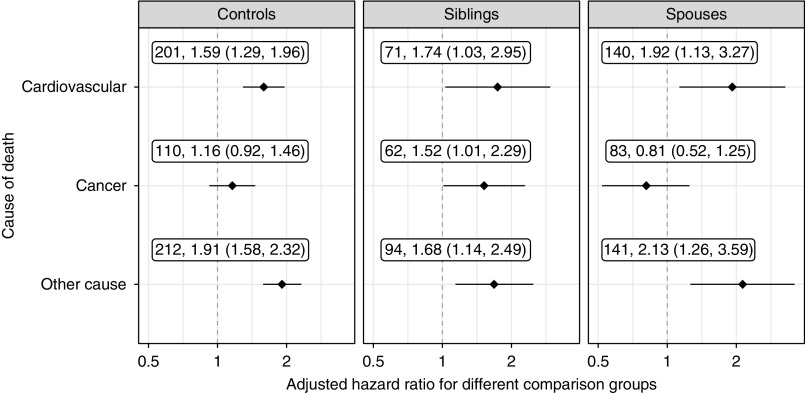
An increase in death from cardiovascular disease (HR, 1.59; 95% CI, 1.29 to 1.96) and from other causes was observed (HR, 1.91; 95% CI, 1.58 to 2.32) in patients with IgAN compared with the general population. However, patients with IgAN were not at an increased risk for death from cancer, except for in the sibling analysis (HR, 1.52; 95% CI, 1.01 to 2.29) (Figure 3).
Figure 3.
Mortality from cardiovascular disease and other causes was increased in IgAN patients compared with primary and secondary comparison groups, but mortality from cancer was only increased compared with siblings. Figures refer to number of deaths and adjusted HRs (diamonds) with 95% CIs (whiskers), with the respective comparison group in column heading. For definition of cardiovascular disease and cancer, please see Supplemental Table 4. Other cause is death from any cause other than cardiovascular disease or cancer.
Mortality preceding ESRD was not significantly increased compared with the controls (HR, 1.11; 95% CI, 0.97 to 1.28), spouses (HR, 1.23; 95% CI, 0.91 to 1.65), or siblings (HR, 1.15; 95% CI, 0.87 to 1.52). Contrastingly, a marked increase in mortality was seen in patients with IgAN after ESRD, compared with matched controls (HR, 3.14; 95% CI, 2.61 to 3.78), with an absolute excess death rate of 18.95 per 1000 person-years equal to one excess death in 53 person-years. When restricting data to patients with IgAN and controls with ESRD, IgAN was not associated with mortality (HR, 0.71; 95% CI, 0.44 to 1.16).
Both use of steroids (HR, 1.95; 95% CI, 1.53 to 2.50) and of other immunosuppressants (HR, 1.33; 95% CI, 1.02 to 1.74) was associated with increased death rates in IgAN. The use of antihypertensives other than RAAS inhibitors was also linked to an increased mortality (HR, 1.84; 95% CI, 1.19 to 2.85), whereas RAAS inhibitors (HR, 0.80; 95% CI, 0.61 to 1.04) and statins (HR, 0.83; 95% CI, 0.67 to 1.04) were not (Supplemental Table 8).
ESRD
During a follow-up of 42,569 person-years, 803 (22.2%) patients with IgAN reached ESRD. This figure can be compared with only 67 (0.4%) cases of ESRD during 255,365 person-years in controls. The corresponding incidence rates were 18.86 in patients with IgAN versus 0.26 cases per 1000 person-years in controls (Figures 1 and 2, Supplemental Table 9). Accordingly, the absolute excess risk for ESRD was 18.60 per 1000 person-years, i.e., one extra case of ESRD per 54 person-years, corresponding to an HR of 100 (95% CI, 67.7 to 148).
The absolute risk for ESRD was highest in the first year of follow-up (30 per 1000 person-years; HR, 1109; 95% CI, 27.1 to 45,400), declining to 1.9 per 1000 person-years (HR, 87.4; 95% CI, 17.9 to 426) after >20 years of follow-up. ESRD-free survival at 10 years after diagnosis was 75.2% (95% CI, 73.6% to 76.7%), 61.4% (95% CI, 59.3% to 63.4%) after 20 years, and 50.7% (95% CI, 47.7% to 53.7%) after 30 years.
Discussion
Principal Findings
This population-based cohort study of more than 3600 individuals with biopsy-verified IgAN found a 53% increase in all-cause mortality compared with matched controls. The absolute death rate was moderately increased, with one extra death per 310 person-years, corresponding to a 6-year reduction in life expectancy. Mortality was still increased after stratification by sex, age, calendar period, and educational level. Patients with IgAN also had an increased mortality compared with their siblings or spouses, except that women with IgAN had a 31% lower mortality than their male spouses, possibly implicating that the excess risk from IgAN was superseded by the generally longer life expectancy in women. Relative mortality did not seem to decrease with calendar period, and also among patients diagnosed in 2002–2015 we saw a 48% increase in mortality. This is, on the one hand, surprising (for instance both RAAS inhibitors and steroids are likely to have increased), but on the other hand, mortality in the comparator group (the general population) is likely to have improved in the same period.
Comparison with Previous Studies
Consistent with our findings (overall mortality: HR, 1.53), a Korean group following 1364 patients with IgAN in a single tertiary center for a median of 8 years reported a 43% increase in mortality rate.12 Although they found a higher standardized mortality ratio (SMR) in females (2.17) than in males (1.22), HRs in our study did not differ by sex. Our 10- and 20-year patient survival rates were substantially lower than those in the Korean study.12 One reason for this difference may be the older age (median 39 versus 33 years) and a higher proportion of men (70% versus 50%) in our study. However, we included four times as many patients with IgAN with more than 20 years of follow-up. Our cohort also had a slightly higher reported prevalence of hypertension and a much higher reported prevalence of diabetes mellitus and cancer than the Korean study population, although different methods for identifying comorbidities make a direct comparison precarious. Conversely, ESRD-free survival rates at 10 and 20 years were moderately lower in our study.
Our finding that mortality is not increased in the pre-ESRD period may be comforting to patients with stable or slowly progressing disease. This finding might be surprising, given that IgAN is associated with a high risk of hypertension and vascular disease,29 and contradicts the dominating concept that the majority of CKD die before progression to ESRD.30,31 However, our results agree with previous studies restricted to primary GN.32 A Norwegian national register-based study of 633 individuals with IgAN and a median follow-up of 11.8 years found an almost two-fold death rate (SMR, 1.9) and no excess mortality before ESRD (SMR, 1.2; 95% CI, 1.0 to 1.7).17 Our study confirms these findings, but markedly reduces residual confounding through our comparisons with siblings and spouses. Furthermore, with more than five times as many patients with IgAN and a longer follow-up, we were able to calculate long-term survival with better precision. We also present data on absolute death rates, which help patients and health care providers gain a better understanding of the prognosis of IgAN. A recent American study of 251 adult patients with IgAN (mean follow-up 19.3 years) found a 10-year ESRD-free survival at a rate of 60% (substantially lower than our 75%) and a 10-year reduction of life expectancy.33 The difference in mortality might be explained by a worse baseline disease severity (more proteinuria and a lower GFR) in the American study.
As has been demonstrated in other studies of primary renal disease,17,32 we found that the association between IgAN and ESRD was much stronger than between IgAN and death, with one excess case of ESRD in 54 person-years. ESRD-free 10- and 20-year survival rates were 75.2% and 61.4%, respectively. This observation is in line with larger multi-center studies.34–36 Older studies present very diverse ESRD incidence, which might reflect variation between studies in lead-time bias because of different renal biopsy praxis, as well as genetic or environmental diversity.37,38 Although not statistically significant, mortality after ESRD was lower in patients with IgAN than in ESRD from other causes. This is in line with an Australia and New Zealand study, showing favorable renal and patient survival in patients with IgAN with ESRD compared with ESRD from other causes.16,39
In contrast to some earlier studies,12,33 we included patients with extrarenal IgAV, thus 6.3% had a diagnosis of IgAV before or within 1 year after biopsy. This is lower than the 11% with IgAV in our validation study,19 possibly reflecting that IgAV is a rare cause for inpatient care in Sweden (the NPR did not register outpatient visits before 2001). Our data show similar mortality rates in the IgAV subgroup as in all patients with IgAN.
Taking advantage of the Swedish PDR, we found an increased mortality in IgAN-patients treated with corticosteroids, with other immunosuppressants and with non-RAAS inhibitor antihypertensives. This might indicate a more severe disease course rather than an effect of the medication. Interestingly, RAAS inhibitor or statin prescription was not associated with an increased mortality, although indication bias could be expected with these drugs. A renoprotective effect of RAAS inhibitors in IgAN has been established in several randomized, controlled studies, summarized in two larger meta-analyses.40,41 A beneficial effect of statins has been proposed by a small study with short follow-up,42 but has yet to be confirmed by larger randomized, controlled studies.
Strengths and Limitations
A major strength of our study is the nationwide population-based design. Our inclusion of all biopsy-verified cases of IgAN in Sweden should minimize the risk of a biased selection of patients with more complicated disease, which might be an issue in studies restricted to tertiary centers. In addition, Swedish national registers have an extremely high coverage and virtually no loss to follow-up. Another strength is the large number of patients and the long follow-up period. For instance, we followed 994 patients for >20 years and 234 patients for >30 years. Furthermore, we included a reference population matched not only for age and sex, but also for the year of diagnosis, enabling us to perform internally stratified Cox regression analyses and identify possible confounders.
To minimize intrafamilial confounding, we compared patients with IgAN with their siblings and spouses. These comparisons may compensate for shared lifestyle and health-seeking behavior within families. Our finding of very similar HRs in these comparisons strengthens the assumption that the increased mortality seen in patients with IgAN is related to the disease and not to shared risk factors.
One limitation of our study is the lack of information on BP, GFR, proteinuria, and histologic findings (all known predictors of outcome in IgAN). Thus, we could not present any separate risk estimates on the basis of such patient characteristics. However, our cohort has been characterized in a diagnosis validation study,19 where we displayed clinical and laboratory characteristics of a subset of 127 patients showing a high concordance with previous studies.43,44 We also did not have data on smoking. Yet, although smoking is a strong risk factor for death, a recent study found identical smoking habits in patients with IgAN and matched controls,43 suggesting that adjustment for smoking is unlikely to have affected our risk estimates. Ethnical differences in mortality were not examined because there are no data on race or ethnicity in Swedish registers. Using biopsy-derived data for the diagnosis of IgAN is likely to increase the validity of our exposure, but we cannot rule out that the lack of nonbiopsy-confirmed IgAN has influenced our risk estimates. Furthermore, as follow-up starts with biopsy date, we are unable to study the effect of IgAN before this date. As delay from disease onset to biopsy may be long, there is a risk of bias toward more advanced stages. Still, mortality was not increased before ESRD, indicating a truly low mortality risk in early disease stages. Occasional reference individuals with undiagnosed IgAN may have been included in the study. As IgAN is a rare disease, this is unlikely to affect our results. Also, individuals with other CKD might have been included in the reference population. This might have driven our results toward the null. Our data were adjusted for cardiovascular disease present before biopsy date. It could not be excluded that undiagnosed IgAN before this date could have contributed to cardiovascular morbidity, in which case this adjustment might cause bias. On the other hand, the higher estimate (HR, 1.74) given from analyses unadjusted for cardiovascular disease is probably exaggerated. Because of the limited power of the trial, we included both prevalent (diagnosed before July 2005) and incident IgAN (diagnosed after July 2005) in our drug analyses. We cannot rule out that this introduced slight bias in these analyses because our requirement that patients with IgAN lived beyond July 2005 may have selected individuals with less severe IgAN than the average. In conclusion, this population-based study shows a 53% increased mortality in patients with IgAN compared with matched controls siblings and spouses. The absolute excess risk is one extra death in 310 person-years, with a 6-year reduction in life expectancy.
Disclosures
Dr. Ludvigsson coordinates a study on behalf of the Swedish IBD quality register (SWIBREG). This study has received funding from Janssen corporation.
Supplementary Material
Acknowledgments
Dr. S. Jarrick and J.F. Ludvigsson conceived and designed the study with input from the other authors. Dr. S. Jarrick and J.F. Ludvigsson wrote the first draft of the paper. J.F. Ludvigsson supervised the project. J.F. Ludvigsson obtained funding for data collection and register-based linkages. J. Höijer and M. Bottai analyzed the data. All authors interpreted the data and contributed to the writing of the paper. All authors revised and approved the final version. J.F. Ludvigsson had full access to all of the data and takes responsibility for the integrity of the data and the accuracy of the data analyses. Other researchers can be granted individual access to our data through the Swedish National Board of Health and Welfare.
This study was supported by grants from the Research Committee of Örebro County Council.
This manuscript represents the views of the authors. The principal author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. A STROBE statement's checklist is provided in online (Supplemental Appendix 2).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Mortality Risk in IgA Nephropathy,” on pages 720–722.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi: 10.1681/ASN.2018101017/-/DCSupplemental.
Supplemental Appendix 1. Description of national registers included in the paper.
Supplemental Appendix 2. Strobe statement.
Supplemental Table 1. ICD codes for renal disease, cardiovascular disease, diabetes, cancer, Henoch–Schönlein purpura, and other systemic inflammatory diseases.
Supplemental Table 2. Definition of renal end points.
Supplemental Table 3. Anatomical therapeutic chemical (ATC) codes for medications.
Supplemental Table 4. ICD codes for cause specific death.
Supplemental Table 5. Adjusted HRs for death compared with the general reference population (corresponding to column 1 in Figure 3).
Supplemental Table 6. Adjusted HRs for death compared with siblings (corresponding to column 2 in Figure 3).
Supplemental Table 7. Adjusted HRs for death compared with spouses (corresponding to column 3 in Figure 3).
Supplemental Table 8. Adjusted HRs for death, with different medications as time-dependent covariates in a regular Cox model.
Supplemental Table 9. Adjusted HRs for ESRD compared with the general reference population (corresponding to column 4 in Figure 3).
References
- 1.Simon P, Ramee MP, Boulahrouz R, Stanescu C, Charasse C, Ang KS, et al.: Epidemiologic data of primary glomerular diseases in western France. Kidney Int 66: 905–908, 2004 [DOI] [PubMed] [Google Scholar]
- 2.McGrogan A, Franssen CF, de Vries CS: The incidence of primary glomerulonephritis worldwide: A systematic review of the literature. Nephrol Dial Transplant 26: 414–430, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Donadio JV, Grande JP: IgA nephropathy. N Engl J Med 347: 738–748, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Utsunomiya Y, Koda T, Kado T, Okada S, Hayashi A, Kanzaki S, et al.: Incidence of pediatric IgA nephropathy. Pediatr Nephrol 18: 511–515, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Shibano T, Takagi N, Maekawa K, Mae H, Hattori M, Takeshima Y, et al.: Epidemiological survey and clinical investigation of pediatric IgA nephropathy. Clin Exp Nephrol 20: 111–117, 2016 [DOI] [PubMed] [Google Scholar]
- 6.McQuarrie EP, Mackinnon B, McNeice V, Fox JG, Geddes CC: The incidence of biopsy-proven IgA nephropathy is associated with multiple socioeconomic deprivation. Kidney Int 85: 198–203, 2014 [DOI] [PubMed] [Google Scholar]
- 7.D’Amico G: Natural history of idiopathic IgA nephropathy: Role of clinical and histological prognostic factors. Am J Kidney Dis 36: 227–237, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Barratt J, Feehally J: Treatment of IgA nephropathy. Kidney Int 69: 1934–1938, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, et al.:Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Bjørneklett R, Vikse BE, Bostad L, Leivestad T, Iversen BM: Long-term risk of ESRD in IgAN; validation of Japanese prognostic model in a Norwegian cohort. Nephrol Dial Transplant 27: 1485–1491, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Goto M, Wakai K, Kawamura T, Ando M, Endoh M, Tomino Y: A scoring system to predict renal outcome in IgA nephropathy: A nationwide 10-year prospective cohort study. Nephrol Dial Transplant 24: 3068–3074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H, Kim DK, Oh KH, Joo KW, Kim YS, Chae DW, et al.: Mortality of IgA nephropathy patients: A single center experience over 30 years. PLoS One 7: e51225, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan M, Li W, Zou G, Zhang C, Fang J: Clinicopathological features and outcomes of IgA nephropathy with hematuria and/or minimal proteinuria. Kidney Blood Press Res 40: 200–206, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Gutiérrez E, Zamora I, Ballarín JA, Arce Y, Jiménez S, Quereda C, et al.:Grupo de Estudio de Enfermedades Glomerulares de la Sociedad Española de Nefrología (GLOSEN) : Long-term outcomes of IgA nephropathy presenting with minimal or no proteinuria. J Am Soc Nephrol 23: 1753–1760, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komatsu H, Kikuchi M, Nakagawa H, Fukuda A, Iwakiri T, Toida T, et al.: Long-term survival of patients with IgA nephropathy after dialysis therapy. Kidney Blood Press Res 37: 649–656, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Liu X, Pascoe EM, Badve SV, Boudville NC, Clayton PA, et al.: Long-term outcomes of end-stage kidney disease for patients with IgA nephropathy: A multi-centre registry study. Nephrology (Carlton) 21: 387–396, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Knoop T, Vikse BE, Svarstad E, Leh S, Reisæter AV, Bjørneklett R: Mortality in patients with IgA nephropathy. Am J Kidney Dis 62: 883–890, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Welander A, Sundelin B, Fored M, Ludvigsson JF: Increased risk of IgA nephropathy among individuals with celiac disease. J Clin Gastroenterol 47: 678–683, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Jarrick S, Lundberg S, Welander A, Fored CM, Ludvigsson JF: Clinical validation of immunoglobulin A nephropathy diagnosis in Swedish biopsy registers. Clin Epidemiol 9: 67–73, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A: The Swedish personal identity number: Possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 24: 659–667, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SNOMED International: SNOMED CT, Swedish Edition v1.37.3, 2018. Available at: http://browser.ihtsdotools.org/index-ie.html?perspective=full&conceptId1=404684003&edition=se-edition&release=v20180531&server=https://prod-browser-exten.ihtsdotools.org/api/snomed&langRefset=46011000052107. Accessed May 31, 2010
- 22.Ludvigsson JF, Almqvist C, Bonamy AK, Ljung R, Michaëlsson K, Neovius M, et al.: Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 31: 125–136, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, et al.: The new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 16: 726–735, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Brooke HL, Talbäck M, Hörnblad J, Johansson LA, Ludvigsson JF, Druid H, et al.: The Swedish cause of death register. Eur J Epidemiol 32: 765–773, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al.: External review and validation of the Swedish national inpatient register. BMC Public Health 11: 450, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persson I, Khamis H: Bias of the Cox model hazard ratio. J Mod Appl Stat Methods 4: 90–99, 2005 [Google Scholar]
- 27.Huber PJ: The behavior of maximum likelihood estimates under nonstandard conditions. Proceedings of the fifth Berkeley symposium on mathematical statistics and probability, University of California Press, Berkeley, CA, 1967. pp 221–233 [Google Scholar]
- 28.Ludvigsson JF, Håberg SE, Knudsen GP, Lafolie P, Zoega H, Sarkkola C, et al.: Ethical aspects of registry-based research in the Nordic countries. Clin Epidemiol 7: 491–508, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myllymäki J, Syrjänen J, Helin H, Pasternack A, Kattainen A, Mustonen J: Vascular diseases and their risk factors in IgA nephropathy. Nephrol Dial Transplant 21: 1876–1882, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Johnson ES, Thorp ML, Yang X, Charansonney OL, Smith DH: Predicting renal replacement therapy and mortality in CKD. Am J Kidney Dis 50: 559–565, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Menon V, Wang X, Sarnak MJ, Hunsicker LH, Madero M, Beck GJ, et al.: Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int 73: 1310–1315, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Hastings MC, Bursac Z, Julian BA, Villa Baca E, Featherston J, Woodford SY, et al.: Life expectancy for patients from the Southeastern United States with IgA nephropathy. Kidney Int Rep 3: 99–104, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le W, Liang S, Hu Y, Deng K, Bao H, Zeng C, et al.: Long-term renal survival and related risk factors in patients with IgA nephropathy: Results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant 27: 1479–1485, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Moriyama T, Tanaka K, Iwasaki C, Oshima Y, Ochi A, Kataoka H, et al.: Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One 9: e91756, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asaba K, Tojo A, Onozato ML, Kinugasa S, Miyazaki H, Miyashita K, et al.: Long-term renal prognosis of IgA nephropathy with therapeutic trend shifts. Intern Med 48: 883–890, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Geddes CC, Rauta V, Gronhagen-Riska C, Bartosik LP, Jardine AG, Ibels LS, et al.: A tricontinental view of IgA nephropathy. Nephrol Dial Transplant 18: 1541–1548, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Coppo R, D’Amico G: Factors predicting progression of IgA nephropathies. J Nephrol 18: 503–512, 2005 [PubMed] [Google Scholar]
- 39.Lee H, Kim DK, Oh K-H, Joo KW, Kim YS, Chae D-W, et al.: Mortality and renal outcome of primary glomerulonephritis in Korea: Observation in 1,943 biopsied cases. Am J Nephrol 37: 74–83, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Reid S, Cawthon PM, Craig JC, Samuels JA, Molony DA, Strippoli GF: Non-immunosuppressive treatment for IgA nephropathy. Cochrane Database Syst Rev 3: CD003962, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Cheng J, Zhang W, Zhang XH, He Q, Tao XJ, Chen JH: ACEI/ARB therapy for IgA nephropathy: A meta analysis of randomised controlled trials. Int J Clin Pract 63: 880–888, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Moriyama T, Oshima Y, Tanaka K, Iwasaki C, Ochi A, Itabashi M, et al.: Statins stabilize the renal function of IgA nephropathy. Ren Fail 36: 356–360, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Lundberg S, Gunnarsson I, Jacobson SH: Impact of the apolipoprotein B/apolipoprotein A-I ratio on renal outcome in immunoglobulin A nephropathy. Scand J Urol Nephrol 46: 148–155, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J, et al.: VALIGA study of the ERA-EDTA Immunonephrology Working Group : Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int 86: 828–836, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.