Abstract
Background: Many subjects with bipolar disorder experience significant cognitive dysfunction, even when euthymic, but few studies assess biological correlates of or treatment strategies for cognitive dysfunction.
Method: Nineteen subjects with bipolar disorder in remission, who reported subjective cognitive deficits, were treated with open‐label galantamine‐ER 8–24 mg/day for 4 months. Ten healthy volunteers matched for age and gender were also assessed. Mood and subjective cognitive questionnaires were administered monthly. At the beginning and the end of the trial all subjects were administered neuropsychological tests, including tests of attention (Conners CPT) and episodic memory (CVLT). Bipolar subjects underwent proton magnetic resonance spectroscopy (1H‐MRS) measurements before and after treatment, healthy volunteers completed baseline 1H‐MRS. We acquired 1H‐MRS data at 4.0 T from voxels centered on the left and right hippocampus to measure hippocampal N‐acetyl aspartate (NAA, a measure of neuronal viability) and choline containing compounds (Cho, a marker of lipid metabolism and membrane turn‐over).
Results: Compared to healthy volunteers, bipolar subjects had higher baseline subjective cognitive deficits and lower scores on objective tests of attention (Conner's CPT) and verbal episodic memory (CVLT). After treatment, bipolar subjects experienced significant improvement of subjective cognitive scores and on objective tests of attention (Conner's CPT) and verbal episodic memory (CVLT). In the left hippocampus NAA increased and choline (Cho) decreased in bipolar subjects during treatment.
Conclusion: Bipolar subjects had cognitive dysfunction; treatment with Galantamine‐ER was associated with improved cognition and with increases in neuronal viability and normalization of lipid membrane metabolism in the left hippocampus.
This study was registered on ClinicalTrials.gov (NCT00181636).
Keywords: Acetylcholinesterase inhibitors, Bipolar disorder, Cognitive dysfunction, Galantamine, Magnetic resonance spectroscopy
Introduction
Numerous studies have demonstrated the presence of neuropsychological deficits in subjects with bipolar disorder. Initially such impairments were considered to be related to acute or subsyndromal mood episodes. Deficits of verbal and visual memory as well as executive function have been described in acutely depressed patients [1, 2, 3]. Other researchers have reported attention and executive dysfunction in mania [4, 5].
However, there is now substantial evidence that subjects with bipolar disorder exhibit cognitive impairments even when they are euthymic [1, 6, 7, 8, 9, 10, 11]. Multiple studies reported euthymic bipolar subjects experience deficits in abstract reasoning and visuomotor skills [12], verbal memory [13, 14, 15], nonverbal memory [16], verbal fluency [17], visuospatial ability [18], and general cognitive function [19, 20, 21]. While impairment of executive function was reported by some [9] but not all studies [13, 18], deficits of attention and verbal memory have been reported by a majority of researchers. The severity of neuropsychological deficits increases with both the number of affective episodes and the overall duration of illness [14, 22, 23, 24].
Significant psychosocial functional impairment (e.g., limited ability to hold a job) is also present in patients with bipolar disorder, including during remission [25]. In a review of studies published until 2000, MacQueen et al. [26] reported that 30–60% of individuals with bipolar disorder fail to regain full functioning in occupational and social domains after remission of mood episodes. Zarate et al. [27] postulated that functional impairment in euthymic subjects with bipolar disorder may be related to persistent neuropsychological deficits. Indeed, the severity of cognitive deficits in asymptomatic patients with bipolar disorder has been associated with poor psychosocial functioning, such as inability to hold a job [28, 29].
There is, however, very limited information on potential treatments for cognitive dysfunction in bipolar disorder. One set of potential candidates is represented by acetylcholinesterase inhibitors (AChEIs), a class of drugs approved for the treatment of cognitive dysfunction in subjects with dementia. Galantamine, an AChEI, is an allosteric potentiator of presynaptic nicotinic receptors and a cholinergic agonist, which was shown to improve significantly the declarative memory functions in patients with Alzheimer disorder [30]. Galantamine has a dual mechanism of action. It inhibits acetylcholinesterase, but it also appears to act directly on the brain nicotinic receptors. The modulation of nicotinic receptors results in the release of more acetylcholine, which may be the mechanism of enhanced positive effects on memory functions [31].
AChEIs and in particular galantamine have been used successfully for the treatment of cognitive dysfunction in psychiatric disorders. Treatment with galantamine was reported beneficial for neuropsychological deficits in schizophrenia [32], which may have both symptoms and mechanisms in common with cognitive deficits in bipolar disorder. Small open studies have also suggested AChEIs may be effective for cognitive deficits in affective disorders. In an open study donepezil (Aricept) for psychotropic induced memory loss in nondemented subjects with unipolar and bipolar affective illness, Jacobsen et al. [33] reported 19 of 21 (90%) subjects of those improved on subjective ratings of memory functions. Galantamine was also beneficial for cognitive deficits in two of four bipolar subjects reported by Schrauwen and Ghaemi [34].
In this study we tested whether open treatment with galantamine ER, an AChEIs, would be associated with improvements in specific measures of cognitive dysfunction, previously found to be altered in patients with bipolar disorder. We also measured subjective cognitive improvement. We hypothesized that bipolar subjects will experience deficits of attention and verbal memory, and that such deficits will improve after treatment. In parallel we tested the neuroprotective effects of galantamine using proton magnetic resonance spectroscopy (1H‐MRS) acquired from the left and right hippocampus to measure N‐acetylaspartate (NAA), a marker of neuronal viability and choline containing compounds (Cho), a key component of phospholipid membrane metabolism. We hypothesized that bipolar subjects will have low NAA and higher choline levels at baseline compared with healthy volunteers, and that NAA levels will increase while Cho levels will decrease after treatment.
Methods
Participants
We enrolled twenty subjects with bipolar disorder and ten matched healthy volunteers for a study conducted in the Bipolar Clinic and Research Program at MGH between 2003 and 2006. Written informed consent was obtained from all study participants. This study was registered on ClinicalTrials.gov (NCT00181636).
Bipolar Subjects
The inclusion criteria for this study were: men or women aged 18–65; meeting DSM‐IV diagnostic criteria for bipolar disorder (diagnosed with the use of the Affective Disorder Evaluation, [35]); no acute episodes of depression or mania for the previous 12 weeks, a score of ≤10 on the 17‐item Hamilton Rating Scale for Depression (Ham‐D‐17) and on the Young Mania Rating Scale (YMRS) at the screen visit, and reporting subjective cognitive deficits.
The exclusion criteria for this study were: subjects with suicidal ideation where outpatient treatment is determined unsafe by the study clinician; pregnant women or women of childbearing potential; serious or unstable medical illness, including cardiovascular, hepatic, renal, respiratory, endocrine, neurologic or hematologic disease; history of seizure disorder, brain injury, any history of known neurological disease (multiple sclerosis, degenerative disease, such as ALS, Parkinson disease and any movement disorders, etc.); history or current diagnosis of the following DSM‐IV psychiatric illness: organic mental disorder, schizophrenia, schizoaffective disorder, delusional disorder, psychotic disorders not otherwise specified, major depressive disorder, patients with mood congruent or mood incongruent psychotic features, patients with substance dependence disorders, including alcohol, active within the last 12 months; history of multiple adverse drug reactions; subjects who are active smokers or who stopped smoking less than 3 months prior to enrollment; clinical or laboratory evidence of hypothyroidism; patients who have had an episode of acute depression or mania during the 12 weeks prior to enrollment; patients who have had electroconvulsive therapy (ECT) within the 6 months preceding enrollment; no significant abnormalities on screening laboratory tests (which included complete blood count; urinalysis; comprehensive metabolic panel (CMP) (serum concentrations of electrolytes, BUN, creatinine, SGOT, SGPT, CPK, alkaline phosphatase, total bilirubin, albumin, total protein, and glucose); TSH and electrocardiogram (EKG); subjects with physical contraindications to magnetic resonance spectroscopy (ferrous surgical clips, cardiac pacemaker, ferrous prosthesis), which was determined by a clinical pre‐MRI questionnaire; patients taking any of the following medications: other cholinesterase inhibitors, succinylcholine, neuromuscular blocking agents, cholinergic agonists (e.g., betanechol), cimetidine, ketokonazole, erythromycin, fluoxetine, and fluvoxamine.
We screened 23 bipolar subjects, 20 subjects met inclusion criteria. One subject discontinued the study before starting medications (moved for a new job). Nineteen subjects started study medication. One subject discontinued between weeks 0 and 4 (acute depression); three subjects discontinued between weeks 4 and 8 (2 = adverse reactions; 1 = relocation), two subjects discontinued between weeks 8 and 12 (1 = acute mania; 1 = depression), two subjects discontinued between weeks 12 and 16 (2 = lack of efficacy). Eleven bipolar subjects completed the 16‐week study. The retention rate at endpoint (week 16) was 11/19 = 58%.
Concomitant medications: Out of 23 bipolar subjects screened for this study, 6 (26%) were treated with monotherapy: 4 (17%) on lithium and 2 (9%) on valproate. Of the 17 patients (74%) treated with combination therapies, 4 (17%) were on lithium and 5 (22%) were on valproate. Other medications used for the treatment of bipolar disorder in those subjects were lamotrigine [6], carbamazepine [2], oxcarbazepine [2], quetiapine [4], risperidone [2], ziprasidone [2], aripiprazole [1], buproprion [6], escitalopram [3], sertraline [2], and paroxetine [1].
Healthy Volunteers
We also recruited through advertisements 10 healthy volunteers, matched for age and gender with the first 10 bipolar subjects completing the study. Healthy volunteers underwent the physician administered Affective Disorder Evaluation [36] to rule out any Axis I psychopathology. All subjects were unmedicated. No healthy volunteer subject had a lifetime history of major neurological, medical, psychiatric disorder, or head injury.
Treatment
Bipolar subjects eligible for the study returned for their baseline visit after 2 weeks, during which they continued their existing psychotropic medication. Patients with a Ham‐D‐17 or YMRS score >10 at the baseline visit were excluded from the study. Those patients still eligible at the baseline visit started a 16‐week open treatment with flexible doses of galantamine‐ER 8–24 mg/day. After baseline patients had clinic visits every 4 weeks, during which we assessed Ham‐D, YMRS and side‐effects. Patients started galantamine‐ER 8 mg/day for the first 4 weeks. During weeks 5–8 the dose was increased to 16 mg/day, if the previous dose was well tolerated. During weeks 9–16 the dose was increased to the maximum of 24 mg/day, if tolerated. The presence of adverse events (AEs) was documented by study psychiatrists at every visit by recording all spontaneously reported AEs, which were classified as mild, moderate, or severe. At every point during the study the patients experiencing significant AEs were given the option to reduce the galantamine‐ER dose to 8 mg/day. Patients who could not tolerate galantamine‐ER at 8 mg/day were discontinued from the study.
Assessments of Cognitive Functions
Before the onset of galantamine treatment and after 16 weeks of treatment we administered a battery of neuropsychological tests. The tests were chosen from three cognitive domains, based on their previously demonstrated sensitivity in subjects with bipolar disorder. This includes tests of attention (Conners Continuous Performance Test [36]); verbal episodic memory (California Verbal Learning Test [37]); executive functioning: Wisconsin Card Sorting Test (WCST [38]). On the basis of previous studies in bipolar subjects [15], for each of these neuropsychological tests we pre‐selected the following scales as primary outcomes for this study: Conner's CPT commission errors, CVLT Trial 1, CVLT Trial 1–5, WCST Total errors, WCST Failure to maintain set. Healthy volunteer subjects also had the same neuropsychological tests administered at baseline and at 16‐week follow‐up.
At every study visit we have also administered a self‐report measure of cognitive function (The MGH Cognitive and Physical Function Questionnaire, CPFQ, 39)
Spectroscopy Measurements
We acquired proton magnetic resonance spectroscopy (1H‐MRS) data from all bipolar subjects (before and after treatment) and healthy volunteer subjects (baseline only) on a 4.0 T Varian Unity/Inova whole body MR scanner (Varian NMR Instruments, Palo Alto, CA) equipped with a proton volumetric head coil (MR Instruments, Minneapolis, MN).
The proton spectrum were acquired from two separate voxels localized on the left and right hippocampus (1.5 × 1.5 × 1.5 cm) using the PRESS spectroscopy sequence. The hippocampus voxel was placed to maximize the hippocampus cross‐section in the voxel in the superior/inferior and right/left directions, whilst staying clear of intercranial spaces (Fig. 2b). PRESS parameters include a repetition time (TR) = 2 s; echo time (TE) = 30 ms and averages = 256. The total PRESS acquisition time for each voxel is less than 10 min.
Figure 2.
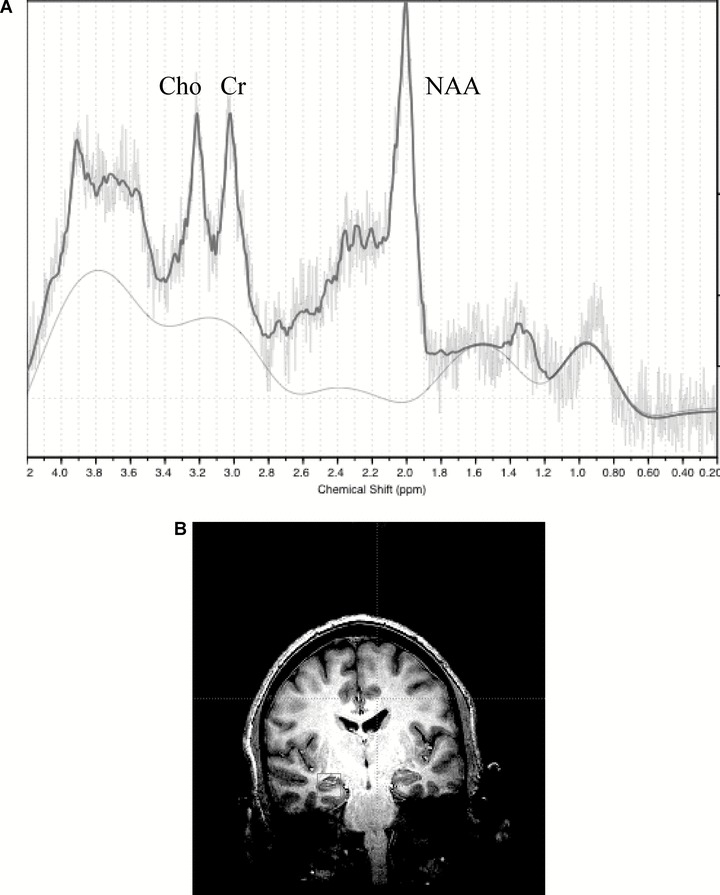
Typical proton spectroscopy (1H‐MRS) spectrum from a subject with bipolar disorder (A) and positioning of the spectroscopy voxel in the left hippocampus (B).
Following data acquisition the proton spectra were transferred to a Sun Ultra 60 (SUN, Mountain View, CA) Workstation and fit using LCModel [40] and a simulated basis‐set. Following spectral fitting, LCModel produces a table and figure (Fig. 2a). The table includes absolute fits (in institutional units) and standard deviations (SD%). The standard deviation is a measure of the reliability of the fit. A standard deviation of 100% means the metabolite would need to double in order for a change to be seen. Spectra with metabolite (NAA, Cr, Cho) SD greater than 15% were excluded from analysis. This criterion was intended to prevent “noisy” spectra from reducing one's power to detect meaningful changes in metabolite levels.
Data Analysis
Group differences in demographic and clinical variables involving continuous data were computed using unpaired t‐tests (age, HAM‐D scores); we used chi‐square for categorical data (gender). We have pre‐specified the following six measures of cognitive function Conner's CPT commission errors, CVLT Trial 1, CVLT Trial 1–5, WCST Total errors, WCST Failure to maintain set and MGH CPFQ total score. We used unpaired t‐tests to compare baseline cognitive scores and changes from baseline to endpoint in cognitive scores between bipolar subjects and healthy volunteers. We used the nonparametric Wilcoxon rank‐sum (Mann‐Whitney U) test to compare baseline levels of MRS metabolites (NAA, Cho) between bipolar subjects and healthy volunteers. Statistical significance was defined at the 0.05 level, two‐tailed.
Results
Demographic characteristics of subjects with bipolar disorder (BD) and healthy volunteers are listed in Table 1. No statistically significant differences in age, gender, and YMRS scores were noted between BD and healthy subjects (p > 0.05 for all analyses). Baseline depression scores (Ham‐D) were higher in bipolar subjects although still in the remission range (3.79 ± 2.92 vs. 1.60 ± 1.58, p= 0.037). Compared to healthy volunteers, bipolar subjects had more baseline subjective cognitive deficits (CPFQ, 21.05 ± 6.43 vs. 13.91 ± 2.47, p < 0.01) and lower scores on objective tests of attention (Conner's CPT, 11.82 ± 5.44 vs. 7.50 ± 4.93, p < 0.05) and verbal episodic memory (CVLT learning 1–5, 50.63 ± 10.58 vs. 60.18 ± 8.01, p < 0.02). There were no significant differences in executive function scores between bipolar subjects and healthy volunteers (Table 1).
Table 1.
Demographic data and neuropsychological scores at baseline in bipolar subjects and healthy volunteers, and changes in the same neuropsychological tests during the 16‐week study (mean ± SD)
| Baseline Data | Statistics | Changes over 16 weeks | Statistics | ||||
|---|---|---|---|---|---|---|---|
| Bipolar Subjects (N = 20) | Healthy volunteers (N = 10) | Bipolar Subjects Completers (N = 11) | Healthy volunteers (N = 10) | ||||
| Age | 40.7 ± 11.9 | 38.9 ± 10.1 | df = 28, t= 0.43, P= 0.67 | ||||
| Gender (female) | 6 (30%) | 3 (30%) | χ2= 0.001, df = 1, P= 0.97 | ||||
| Diagnosis | Bipolar 1 | 15 (75%) | 8 (73%) | ||||
| Bipolar 2 | 5 (25%) | 3 (27%) | |||||
| HAM‐D‐17 | 3.79 ± 2.92 | 1.60 ± 1.58 | df = 28, t=–2.2, P= 0.037 | 2.92 ± 4.12 | 0.88 ± 1.73 | df = 18, t= 1.32, P= 0.20 | |
| YMRS | 1.85 ± 2.32 | 0.70 ± 1.25 | df = 28, t=–1.46, P= 0.16 | 2.09 ± 4.44 | 1.63 ± 4.27 | df = 18, t= 0.23, P= 0.82 | |
| WCST | Tot errors | 16.82 ± 9.90 | 14.06 ± 9.48 | df = 25, t=–0.73, P= 0.47 | ‐3.00 ± 4.92 | ‐0.72 ± 8.34 | df = 18, t= 0.72, P= 0.48 |
| Fail maintain set | 0.31 ± 0.23 | 0.73 ± 0.79 | df = 25, t= 1.71, P= 0.10 | ‐0.58 ± 0.67 | 0.22 ± 0.83 | df = 19, t= 2.46, P= 0.023* | |
| Conner CPT | Commission errors | 12.41 ± 4.82 | 7.50 ± 4.93 | df = 25, t= 2.54, P= 0.018* | ‐6.40 ± 3.78 | ‐0.63 ± 4.34 | df = 18, t= 2.34, P= 0.031* |
| CVLT | Learning 1–5 | 50.80 ± 10.33 | 60.18 ± 8.01 | df = 28, t=–2.60, P= 0.014* | 7.0 ± 7.4 | 0.78 ± 3.23 | df = 18, t= 2.34, P= 0.031* |
| CVLT | Recall trial 1 | 6.95 ± 2.35 | 8.73 ± 1.68 | df = 28, t=–2.21, P= 0.035* | 1.27 ± 0.91 | 0.33 ± 0.87 | df = 18, t= 2.36, P= 0.030* |
| MGH CPFQ (subjective) | 21.56 ± 6.46 | 13.30 ± 1.49 | df = 26, t= 3.95, P= 0.005* | 6.8 ± 6.0 | ‐2.1 ± 3.8 | df = 23, t= 3.85, P= 0.0008* | |
*P < 0.05.
Eighteen of the 19 bipolar subjects and N = 10 healthy volunteers had 1H‐MRS scans of adequate quality for metabolite measurements. At baseline subjects with bipolar disorder had higher choline (Cho) levels in both left and right hippocampus, compared to healthy volunteers. Numerically NAA levels were increased in bipolar subjects; the difference was statistically significant in the right but not in the left hippocampus (Table 2).
Table 2.
Baseline levels of N‐acetyl aspartate (NAA), choline compounds (Cho) and creatine in bipolar subjects and healthy volunteers, and changes in the same 1H‐MRS metabolite levels in bipolar subjects who completed the 16‐week treatment (Institutional Units, mean ± SD)
| Metabolite | Hippocampus | Bipolar subjects (N = 18) | Healthy volunteers (N = 10) | Statistics | Bipolar completers—baseline (N = 8) | Bipolar completers—endpoint (N = 8) | Statistics |
|---|---|---|---|---|---|---|---|
| NAA | Left | 5.26 ± 3.63 | 3.32 ± 3.68 | df = 26, t= 1.35, P= 0.19 | 4.87 ± 1.78 | 8.15 ± 4.74 | df = 7, t= 2.63, P= 0.039* |
| Right | 6.41 ± 2.11 | 4.44 ± 2.01 | df = 26, t= 2.38, P= 0.025* | 6.11 ± 1.49 | 5.38 ± 2.72 | df = 7, t= 1.37, P= 0.21 | |
| Cho | Left | 2.12 ± 1.08 | 0.90 ± 0.96 | df = 26, t= 2.99, P= 0.006* | 2.41 ± 0.93 | 1.30 ± 0.84 | df = 7, t= 2.52, P= 0.040* |
| Right | 1.88 ± 0.66 | 1.24 ± 0.50 | df = 25, t= 2.66, P= 0.014* | 1.88 ± 0.62 | 1.48 ± 0.67 | df = 7, t= 0.94, P= 0.38 | |
| Creatine | Left | 5.71 ± 2.90 | 2.97 ± 2.99 | df = 26, t= 2.36, P= 0.026* | 6.17 ± 3.16 | 6.04 ± 3.45 | df = 7, t= 0.49, P= 0.64 |
| Right | 5.16 ± 1.86 | 3.58 ± 1.48 | df = 26, t= 2.29, P= 0.031* | 5.90 ± 2.00 | 4.76 ± 2.03 | df = 7, t= 0.89, P= 0.40 |
*P < 0.05.
After treatment, bipolar subjects experienced significant improvement of subjective cognitive scores (p < 0.01) and on objective tests of attention (CPT, p < 0.04) and verbal episodic memory (CVLT, p < 0.03) (Table 1). The subjective improvement in cognitive scores was gradual during the 16‐week study; most of the benefit was achieved in the first 12 weeks of the treatment (Fig. 1).
Figure 1.
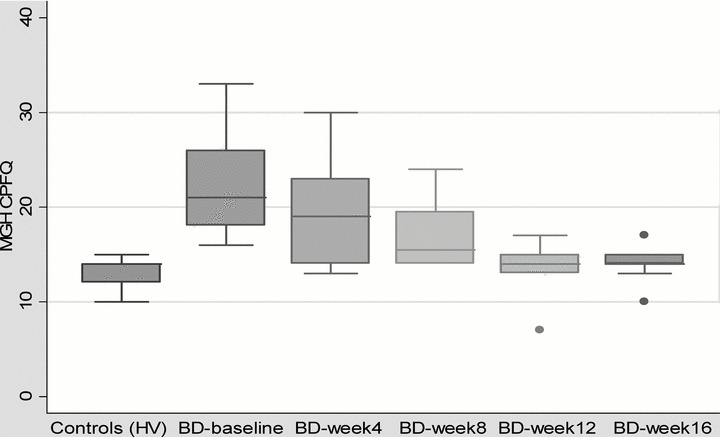
Changes in subjective cognitive scores (CPFQ) in bipolar subjects (BD) during treatment and comparison with healthy volunteers (HV).
Only bipolar subjects had repeat MRS testing. From the N = 11 bipolar subjects who completed the study, only eight had adequate pre‐ and posttreatment 1H‐MRS scans to allow the comparison of metabolite levels. Cho levels decreased from baseline to week 16 in the left hippocampus; the end‐of‐study levels were close to the levels measured in healthy volunteers. The numerical decrease in Cho in the right hippocampus was not statistically significant. NAA levels increased in the left but not in the right hippocampus during the 16‐week treatment with galantamine ER (Table 2).
Treatment with galantamine ER was relatively well tolerated. Two subjects (10%) discontinued the study due to side effects (nausea = 1, diarrhea and migraines = 1). Three subjects (16%) experienced new mood episodes (1 = mania, 2 = depression); this rate of new episodes in bipolar subjects is consistent with larger samples where relapse of bipolar disorder is common [42, 43].
Discussion
This is to our knowledge the largest study published to date on the treatment of cognitive dysfunction in bipolar disorder. In our study, bipolar subjects treated with galantamine ER for 16 weeks experienced significant improvement in their cognitive functions, especially in attention (Conner's CPT) and verbal memory (CVLT), as well as in their subjective cognitive scores (CPFQ). It is unlikely that the observed improvements in cognitive functions (attention, memory) could be explained by improvements in mood, since subjects in our study were euthymic at baseline.
At baseline bipolar subjects had more subjective and objective cognitive dysfunction, compared to healthy volunteers. This is consistent with multiple previous studies suggesting bipolar subjects experience cognitive dysfunction during periods of euthymia [1, 6, 7, 8, 9, 10, 11]. Savitz et al. [41] reviewed 40 studies examining neurocognitive function in euthymic individuals with bipolar disorder. Only three of those 40 studies failed to detect cognitive impairment in euthymic bipolar patients, suggesting that cognitive dysfunction may represent a permanent trait in this population and not only related to acute mood episodes. This could be an underlying genetic trait, as first‐degree relatives of patients with bipolar disorder exhibit impaired attention set shifting [42], but can also be a progressive phenomenon as neuropsychological deficits are associated with both the number of affective episodes and the overall duration of illness [22, 23, 24].
In the current study, we are not able to assess the effect of concomitant bipolar medications on the cognitive dysfunction experienced by bipolar subjects at baseline. However, since other bipolar medications were continued unchanged during the study, it is likely that the cognitive improvement observed his related to treatment with galantamine. In general, the relation between cognitive deficits and the psychotropic medications used to treat the bipolar disorder cannot explain the majority of cognitive deficits in this population [43]. Lithium has been reported to have adverse effects on memory and psychomotor functioning [10, 44, 45], while valproate and carbemazepine may cause attentional difficulties [46]. Antipsychotics have been associated with deficits in sustained attention [47] and executive function [23]. However, longitudinal studies failed to detect evidence of cognitive decline in bipolar patients treated with lithium [48]. In the same study, comparison of long‐ and short‐term lithium treatment groups also failed to show significant differences in memory scores. Previous studies have also linked cognitive deficits in bipolar disorder with subsyndromal mood symptoms and to comorbid substance abuse. However, those factors do not play a significant role in our study since all bipolar subjects were euthymic and substance abuse was an exclusionary criterion.
We found that bipolar subjects at baseline had higher choline (Cho) levels compared to healthy volunteers. Our finding is consistent with multiple MRS studies in bipolar disorder [49, 50, 51]. The choline (Cho) signal consists primarily (approximately 80%) of phosphocholine (PC) and glycerophosphocholine (GPC) [52, 53]. Choline is required for the synthesis of both the neurotransmitter acetylcholine and the phospholipid phosphatidylcholine. While acetylcholine is produced only by cholinergic neurons, phosphatidylcholine is produced in all cells as a major membrane constituent [52]. Consequently, changes in the Cho signal are primarily associated with alterations in membrane synthesis and composition [52]. Significant increases in the Cho signal have been observed in neurodegenerative disorders such as Alzheimer's disease and multiple sclerosis (MS), as well as cases of ischemia and head trauma, probably related to the release of Cho‐containing compounds during membrane breakdown [53].
Contrary to our hypothesis, NAA levels were numerically higher at baseline in bipolar subjects compared to healthy volunteers (and the difference was statistically significant for the right hippocampus). NAA is located primarily within neurons and a reduction of NAA levels has been interpreted as reflecting neuronal loss or damage [54]. The increased NAA levels in our euthymic bipolar subjects may be the result of their existing treatments with potential role in increasing neuroprotection. Valproate and lithium have been previously associated with increased NAA levels in bipolar subjects [55, 56].
During the 16‐week treatment NAA levels increased and Cho levels decreased in the left hippocampus in bipolar subjects, which is consistent with a neuroprotective effect of galantamine. Our results are in agreement with previous studies in healthy volunteers and in neurological disorders where measures of cognitive function have been correlated with brain NAA and Cho levels as measured by 1H‐MRS [57, 58, 59, 60, 61]. Moreover, in some studies NAA levels appear to increase (i.e., renormalize) in parallel with performance on neuropsychological testing [62] which is also consistent with the results reported here.
Our data is consistent with previous reports suggesting the mechanism by which AChEI would be effective for cognitive dysfunction may be mediated by neuroprotective effects. Preclinical studies suggest that AChEI protect neurons from death in cell culture models of neurodegenerative disease [63, 64]. The activity of galantamine on nicotinic receptors may be associated with additional neuroprotective mechanisms [65]. Neurogenesis in the hippocampus is another important mechanism for enhancing the resilience of neuronal systems in stressful conditions, and galantamine (and other AChEIs) have also been reported to enhance neurogenesis in vitro and in vivo[66]. However, other mechanisms such as the role of galantamine in increasing prefrontal dopaminergic activity may also explain its cognitive benefits [67]
Our study has several limitations, including small sample size and open design with no placebo comparator, which makes it difficult to assess the true efficacy of galantamine‐ER. Subjects in our study were taking multiple treatments for bipolar disorder it is difficult to assess the effect of concomitant treatments on the cognitive dysfunction and on the improvement of symptoms. Another limitation is that because we administered objective neuropsychological tests only twice during the study to minimize learning effects, therefore we can test the efficacy of galantamine only in study completers. Only subjective cognitive improvements can be estimated in subjects who dropped out during the study. Similarly, improvements in hippocampal NAA and Cho can only be assessed in subjects completing the 16‐week study. Because this was an open study, we cannot differentiate between the cognitive and neuroprotective effects of galantamine ER and those related to placebo (i.e., the on‐going healing which may occur in euthymic bipolar subjects). No correction for multiple comparisons was made in this small pilot study; larger studies will be needed to confirm our results.
Despite these methodological limitations our study suggests that Galantamine‐ER can improve objective and subjective cognitive deficits in subjects with bipolar disorder (especially attention and verbal memory deficits). In our study treatment with Galantamine was also associated with increases in neuronal viability and lipid membrane metabolism in the left hippocampus. Larger studies, using a placebo controlled design will be needed to establish the efficacy of galantamine for cognitive dysfunction in bipolar disorder and to assess the eventual improvements in functional status triggered by the improvement in cognition.
Funding
This study was supported by a grant from Ortho‐McNeil Neurologics, Inc (Dr. Iosifescu).
Conflict of Interest
Dr. Iosifescu has received research support from Aspect Medical Systems, Forest Laboratories and Ortho‐McNeil Neurologics; he has been a consultant for Forest Laboratories, Gerson Lehrman Group and Pfizer, Inc., and he has been a speaker for Eli Lilly & Co., Forest Laboratories, Pfizer, Inc and Reed‐Elsevier. Drs. Moore, Deckersbach and Ms. Tilley report no potential conflicts. Dr. Ostacher has received research support from Pfizer, and honoraria, Speaker Bureau or travel support from AstraZeneca, Bristol Myers‐Squibb, Concordant Rater Systems, Eli Lilly, Glaxo SmithKline. Dr. Nierenberg has received research support from Bristol‐Myers Squibb, Cederroth, Cyberonics, Forest Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, Lichtwer Pharma, Eli Lilly, Pfizer and Wyeth‐Ayerst; he has been a consultant for Abbott Laboratories, BrainCells Inc., Bristol‐Myers Squibb, Genaissance, GlaxoSmithKline, Innapharma, Janssen Pharmaceutica, Eli Lilly, Novartis, Pfizer, Sepracor, Shire and Somerset, and he has been a speaker for Bristol‐Myers Squibb, Cyberonics, Forest Pharmaceuticals, GlaxoSmithKline, Eli Lilly, and Wyeth‐Ayerst.
References
- 1. Malhi GS, Ivanovski B, Hadzi‐Pavlovic D, Mitchell PB, Vieta E, Sachdev P. Neuropsychological deficits and functional impairment in bipolar depression, hypomania and euthymia. Bipolar Disord 2007;9:114–125. [DOI] [PubMed] [Google Scholar]
- 2. Cohen RM, Weingartner H, Smallberg SA, Pickar D, Murphy DL. Effort and cognition in depression. Arch Gen Psychiatry 1982;39:593–597. [DOI] [PubMed] [Google Scholar]
- 3. Elliot R, Sahakian BJ, McKay AP, et al Neuropsychological impairments in unipolar depression: The influence of perceived failure on subsequent performance. Psychol Med 1996;26:975–989. [DOI] [PubMed] [Google Scholar]
- 4. Clark LD, Iversen SD, Goodwin G. A neuropsychological investigation of prefrontal cortex involvement in acute mania. Am J Psychiatry 2001;158:1605–1611. [DOI] [PubMed] [Google Scholar]
- 5. Martinez‐Aran A, Vieta E, Reinares M, et al Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry 2004;161:262–270. [DOI] [PubMed] [Google Scholar]
- 6. Malhi GS, Lagopoulos J, Owen AM, Ivanovski B, Shnier R, Sachdev P. Reduced activation to implicit affect induction in euthymic bipolar patients: An fMRI study. J Affect Disord 2007b;97:109–122. [DOI] [PubMed] [Google Scholar]
- 7. Ozdel O, Karadag F, Atesci FC, Oguzhanoglu NK, Cabuk T. Cognitive functions in euthymic patients with bipolar disorder. Ann Saudi Med 2007;27:273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goswami U, Sharma A, Khastigir U, Ferrier IN, Young AH, Gallagher P, Thompson JM, Moore PB. Neuropsychological dysfunction, soft neurological signs and social disability in euthymic patients with bipolar disorder. Br J Psychiatry 2006;188:366–373. [DOI] [PubMed] [Google Scholar]
- 9. Ferrier IN, Stanton BR, Kelly TP, Scott J. Neuropsychological function in euthymic patients with bipolar disorder. Br J Psychiatry 1999;175:246–251. [DOI] [PubMed] [Google Scholar]
- 10. Honig A, Arts BM, Ponds RW, Riedel WJ. Lithium induced cognitive side‐effects in bipolar disorder: A qualitative analysis and implications for daily practice. Int Clin Psychopharmacol 1999;14:167–171. [PubMed] [Google Scholar]
- 11. McKay AP, Tarbuck AF, Shapleske J, McKenna PJ. Neuropsychological function in manic‐depressive psychosis. Evidence for persistent deficits in patients with chronic, severe illness. Br J Psychiatry 1995;167:51–57. [DOI] [PubMed] [Google Scholar]
- 12. Ali SO, Denicoff KD, Altshuler LL, et al A preliminary study of the relation of neuropsychological performance to neuroanatomic structures in bipolar disorder. Neuropsychiatry Neuropsychol Behav Neurol 2000;13:20–28. [PubMed] [Google Scholar]
- 13. Van Gorp WG, Altshuler L, Theberge DC, Mintz J. Declarative and procedural memory in bipolar disorder. Biol Psychiatry 1999;46:525–531. [DOI] [PubMed] [Google Scholar]
- 14. Cavanagh JTO, Van Beck M, Muir W, Blackwood DHR. Case‐control study of neurocognitive function in euthymic patients with bipolar disorder: An association with mania. Br J Psychiatry 2002;180:320–326. [DOI] [PubMed] [Google Scholar]
- 15. Deckersbach T, Savage CR, Reilly‐Harrington N, Clark L, Sachs G, Rauch SL. Episodic memory impairment in bipolar disorder and obsessive‐compulsive disorder: The role of memory strategies. Bipolar Disord 2004;6:233–244. [DOI] [PubMed] [Google Scholar]
- 16. Deckersbach T, McMurrich S, Ogutha J, Savage CR, Sachs G, Rauch SL. Characteristics of non‐verbal memory impairment in bipolar disorder: The role of encoding strategies. Psychol Med 2004b;34:823–832. [DOI] [PubMed] [Google Scholar]
- 17. Lebowitz BK, Shear PK, Steed MA, Strakowski SM. Verbal fluency in mania: Relationship to number of manic episodes Neuropsychiatry Neuropsychol Behav Neurol 2001;14:177–182. [PubMed] [Google Scholar]
- 18. Rubinsztein JS, Michael A, Paykel ES, Sahakian BJ. Cognitive impairment in remission in bipolar affective disorder. Psychol Med 2000;30:1025–1036. [DOI] [PubMed] [Google Scholar]
- 19. Friedman L, Findling RL, Kenny JT, et al An MRI study of adolescent patients with either schizophrenia or bipolar disorder as compared to healthy control subjects. Biol Psychiatry 1999;46:78–88. [DOI] [PubMed] [Google Scholar]
- 20. Kessing LV. Cognitive impairment in the euthymic phase of affective disorder. Psychol Med 1998;28:1027–1038. [DOI] [PubMed] [Google Scholar]
- 21. Krabbendam L, Honig A, Wiersma J, et al Cognitive dysfunctions and white matter lesions in patients with bipolar disorder in remission. Acta Psychiatr Scand 2000;101:274–280. [PubMed] [Google Scholar]
- 22. Clark L, Iversen SD, Goodwin GM. Sustained attention deficit in bipolar disorder. Br J Psychiatry 2002;180:313–319. [DOI] [PubMed] [Google Scholar]
- 23. Zubieta JK, Huguelet P, O’Neil RL, Giordani BJ. Cognitive function in euthymic bipolar I disorder. Psychiatry Res 2001;102:9–20. [DOI] [PubMed] [Google Scholar]
- 24. Denicoff KD, Ali SO, Mirsky AF et al Relationship between prior course of illness and neuro‐psychological functioning in patients with bipolar disorder. J Affect Disord 1999;56:67–73. [DOI] [PubMed] [Google Scholar]
- 25. Fagiolini A, Kupfer DJ, Masalehdan A, Scott JA, Houck PR, Frank E. Functional impairment in the remission phase of bipolar disorder. Bipolar Disord 2005;7:281–285. [DOI] [PubMed] [Google Scholar]
- 26. MacQueen GM, Young LT, Joffe RT. A review of psychosocial outcome in patients with bipolar disorder. Acta Psychiatr Scand 2001;103:163–170. [DOI] [PubMed] [Google Scholar]
- 27. Zarate CA Jr, Tohen M, Land M, Cavanagh S. Functional impairment and cognition in bipolar disorder. Psychiatr Q 2000;71:309–329. [DOI] [PubMed] [Google Scholar]
- 28. Atre‐Vaidya N, Taylor MA, Seidenberg M, Reed R, Perrine A, Glick‐Oberwise F. Cognitive deficits, psychopathology, and psychosocial functioning in bipolar mood disorder. Neuropsychiatry Neuropsychol Behav Neurol 1998;11:120–126. [PubMed] [Google Scholar]
- 29. Torrent C, Martínez‐Arán A, Daban C, Sánchez‐Moreno J, Comes M, Goikolea JM, Salamero M, Vieta E. Cognitive impairment in bipolar II disorder. Br J Psychiatry 2006;189:254–259. [DOI] [PubMed] [Google Scholar]
- 30. Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C. A 5‐month, randomized, placebo‐controlled trial of galantamine in AD. The Galantamine USA‐10 Study Group. Neurology 2000;54:2269–2276. [DOI] [PubMed] [Google Scholar]
- 31. Maelicke A, Schrattenholz A, Samochocki M, Radina M, Albuquerque EX. Allosterically potentiating ligands of nicotinic receptors as a treatment strategy for Alzheimer's disease. Behav Brain Res 2000;113:199–206. [DOI] [PubMed] [Google Scholar]
- 32. Schubert MH, Young KA, Hicks PB. Galantamine improves cognition in schizophrenic patients stabilized on risperidone. Biol Psychiatry 2006;60:530–533. [DOI] [PubMed] [Google Scholar]
- 33. Jacobsen FM, Comas‐Diaz L. Donepezil for psychotropic‐induced memory loss. J Clin Psychiatry 1999;60:698–704. [DOI] [PubMed] [Google Scholar]
- 34. Schrauwen E, Ghaemi SN. Galantamine treatment of cognitive impairment in bipolar disorder: Four cases. Bipolar Disord 2006;8:196–199. [DOI] [PubMed] [Google Scholar]
- 35. Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, Lavori P, Lebowitz B, Rudorfer M, Frank E, Nierenberg AA, Fava M, Bowden C, Ketter T, Marangell L, Calabrese J, Kupfer D, Rosenbaum JF. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP‐BD). Biol Psychiatry 2003;53:1028–1042. [DOI] [PubMed] [Google Scholar]
- 36. Conners CK. Conners’ Continuous Performance Test. Toronto : Multi‐Health Systems; 1995. [Google Scholar]
- 37. Delis DC, Freeland J, Kramer JH, Kaplan E. Integrating clinical assessment with cognitive neuroscience: Construct validation of the California Verbal Learning Test. J Consult Clin Psychol 1988;56:123–130. [DOI] [PubMed] [Google Scholar]
- 38. Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test manual: Revised and expanded, Psychological Assessment Resources, Odessa, FL, 1993.
- 39. Fava M, Iosifescu DV, Pedrelli P, Baer L. Reliability And Validity Of The MGH Cognitive And Physical Functioning Questionnaire (CPFQ). Psychother Psychosomat 2009;78:91–97. [DOI] [PubMed] [Google Scholar]
- 40. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 2001;14:260–264. [DOI] [PubMed] [Google Scholar]
- 41. Savitz J, Solms M, Ramesar R. Neuropsychological dysfunction in bipolar affective disorder: A critical opinion. Bipolar Disord 2005;7:216–35. [DOI] [PubMed] [Google Scholar]
- 42. Clark L, Sarna A, Goodwin GM. Impairment of executive function but not memory in first‐degree relatives of patients with bipolar I disorder and in euthymic patients with unipolar depression. Am J Psychiatry 2005;162:1980–1982. [DOI] [PubMed] [Google Scholar]
- 43. Donaldson S, Goldstein LH, Landau S, Raymont V, Frangou S. The Maudsley Bipolar Disorder Project: The effect of medication, family history, and duration of illness on IQ and memory in bipolar I disorder. J Clin Psychiatry 2003;64:86–93. [PubMed] [Google Scholar]
- 44. Squire LR, Judd LL, Janowsky DS, et al Effects of lithium carbonate on memory and other cognitive functions. Am J Psychiatry 1980;137:1042–1046. [DOI] [PubMed] [Google Scholar]
- 45. Kocsis JH, Shaw ED, Stokes PE, et al Neuropsychologic effects of lithium discontinuation. J Clin Psychopharmacol 1993;13:268–275. [PubMed] [Google Scholar]
- 46. Thompson PJ, Trimble MR. Anticonvulsant drugs and cognitive functions. Epilepsia 1982;23:531–544. [DOI] [PubMed] [Google Scholar]
- 47. King DJ. Psychomotor impairment and cognitive disturbance induced by neuroleptics. Acta Psychiatr Scand 1994;89:S53–S58. [DOI] [PubMed] [Google Scholar]
- 48. Engelsmann F, Katz J, Ghadirian AM, Schachter D. Lithium and memory: A long‐term follow‐up study. J Clin Psychopharmacol 1988;8:207–211. [PubMed] [Google Scholar]
- 49. Kato T, Hamakawa H, Shioiri T, Murashita J, Takahashi Y, Takahashi S, Inubushi T. Choline‐containing compounds detected by proton magnetic resonance spectroscopy in the basal ganglia in bipolar disorder. J Psychiatry Neurosci 1996;21:248–254. [PMC free article] [PubMed] [Google Scholar]
- 50. Hamakawa H, Kato T, Murashita J, Kato N. Quantitative proton magnetic resonance spectroscopy of the basal ganglia in patients with affective disorders. Eur Arch Psychiatry Clin Neurosci 1998;248:53–58. [DOI] [PubMed] [Google Scholar]
- 51. Moore CM, Breeze JL, Gruber SA, Babb SM, Frederick BB, Villafuerte RA, Stoll AL, Hennen J, Yurgelun‐Todd DA, Cohen BM, Renshaw PF. Choline, myo‐inositol and mood in bipolar disorder: A proton magnetic resonance spectroscopic imaging study of the anterior cingulate cortex. Bipolar Disord 2000;2(3 Pt 2):207–216. [DOI] [PubMed] [Google Scholar]
- 52. Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed 2000;13:129–153. [DOI] [PubMed] [Google Scholar]
- 53. Rudkin TM, Arnold DL. Proton magnetic resonance spectroscopy for the diagnosis and management of cerebral disorders. Arch Neurol 1999;56:919–926. [DOI] [PubMed] [Google Scholar]
- 54. Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci 1993;13:981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moore CM, Wardrop M, DeB Frederick B, Renshaw PF. Topiramate raises anterior cingulate cortex glutamine levels in healthy men; a 4.0 T magnetic resonance spectroscopy study. Psychopharmacology (Berl) 2006;188:236–243. [DOI] [PubMed] [Google Scholar]
- 56. Moore CM, Frazier JA, Glod CA, Breeze JL, Dieterich M, Finn CT, Frederick B, Renshaw PF. Glutamine and glutamate levels in children and adolescents with bipolar disorder: A 4.0‐T proton magnetic resonance spectroscopy study of the anterior cingulate cortex. J Am Acad Child Adolesc Psychiatry 2007;46:524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ferguson KJ, MacLullich AM, Marshall I, Deary IJ, Starr JM, Seckl JR, Wardlaw JM. Magnetic resonance spectroscopy and cognitive function in healthy elderly men. Brain 2002;125(Pt 12):2743–2749. [DOI] [PubMed] [Google Scholar]
- 58. Ferrier CH, Alarcon G, Glover A, Koutroumanidis M, Morris RG, Simmons A, Elwes RD, Cox T, Binnie CD, Polkey CE. N‐Acetylaspartate and creatine levels measured by (1)H MRS relate to recognition memory. Neurology 2000;55:1874–1883. [DOI] [PubMed] [Google Scholar]
- 59. Martin RC, Sawrie S, Hugg J, Gilliam F, Faught E, Kuzniecky R. Cognitive correlates of 1H MRSI‐detected hippocampal abnormalities in temporal lobe epilepsy. Neurology 1999;53:2052–2058. [DOI] [PubMed] [Google Scholar]
- 60. Pan JW, Krupp LB, Elkins LE, Coyle PK. Cognitive dysfunction lateralizes with NAA in multiple sclerosis. Appl Neuropsychol 2001;8:155–160. [DOI] [PubMed] [Google Scholar]
- 61. Valenzuela MJ, Sachdev PS, Wen W, Shnier R, Brodaty H, Gillies D. Dual voxel proton magnetic resonance spectroscopy in the healthy elderly: Subcortical‐frontal axonal N‐acetylaspartate levels are correlated with fluid cognitive abilities independent of structural brain changes. Neuroimage 2000;12:747–756. [DOI] [PubMed] [Google Scholar]
- 62. Bendszus M, Weijers HG, Wiesbeck G, Warmuth‐Metz M, Bartsch AJ, Engels S, Boning J, Solymosi L. Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: Correlation with clinical and neuropsychological data. AJNR Am J Neuroradiol 2001;22:1926–1932. [PMC free article] [PubMed] [Google Scholar]
- 63. Akasofu S, Kosasa T, Kimura M, Kubota A. Protective effect of donepezil in a primary culture of rat cortical neurons exposed to oxygen‐glucose deprivation. Eur J Pharmacol 2003;472:57–63. [DOI] [PubMed] [Google Scholar]
- 64. Sobrado M, Roda JM, Lopez MG, Egea J, Garcia AG. Galantamine and memantine produce different degrees of neuroprotection in rat hippocampal slices subjected to oxygen‐glucose deprivation. Neurosci Lett 2004;365:132–136. [DOI] [PubMed] [Google Scholar]
- 65. Arias E, Ales E, Gabilan NH, Cano‐Abad MF, Villarroya M, Garcia AG, Lopez MG. Galantamine prevents apoptosis induced by beta‐amyloid and thapsigargin: Involvement of nicotinic acetylcholine receptors. Neuropharmacology 2004;46:103–114. [DOI] [PubMed] [Google Scholar]
- 66. Jin K, Xie L, Mao XO, Greenberg DA. Alzheimer's disease drugs promote neurogenesis. Brain Res 2006;1085:183–188. [DOI] [PubMed] [Google Scholar]
- 67. Wang D, Noda Y, Zhou Y, Nitta A, Furukawa H, Nabeshima T. Synergistic effect of combined treatment with risperidone and galantamine on phencyclidine‐induced impairment of latent visuospatial learning and memory: Role of nAChR activation‐dependent increase of dopamine D1 receptor‐mediated neurotransmission. Neuropharmacology 2007;53:379–389. [DOI] [PubMed] [Google Scholar]


