Abstract
Medication nonadherence, especially in psychiatric disorders, has been associated with treatment failure and other negative outcomes. Orally disintegrating formulations have been developed as an alternative to improve medication adherence. This report reviews the properties, efficacy, and safety profile of olanzapine as an orally disintegrating tablet, and explores their association with medication compliance compared with standard oral formulation. Medical literature, published on orally disintegrating formulation of olanzapine identified using Pubmed and EMBASE, was used. Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug. Studies evaluating the biostability, biodisposability, pharmacokinetics, efficacy, and safety of orally disintegrating olanzapine as treatment of patients with psychiatric disorders were reviewed. Measurement tools included the Positive and Negative Syndrome Scale (PANSS), Clinical Global Impressions‐Severity (CGI‐S), Simpson‐Angus Scale (SAS), Abnormal Involuntary Movement Scale, and Nursing Assessment of Medication Acceptance (NAMA). Orally disintegrating olanzapine, an effective atypical antipsychotic with an acceptable safety profile, can facilitate the burden of treatment on patients and caregivers due to its ease of administration. This is especially important in diseases such as schizophrenia and bipolar disorder, which can be chronic and require long‐term treatment.
Keywords: Compliance, Efficacy, Olanzapine orally disintegrating tablet, Schizophrenia
Introduction
Olanzapine (Zyprexa®), derived from thieno‐benzodiazepine, is well established as an effective, orally and intramuscular administered, atypical antipsychotic agent. Oral olanzapine is indicated for the treatment of schizophrenia and acute mixed or manic episodes in patients with bipolar I disorder, and for maintenance therapy to prevent recurrence in responders. The intramuscular formulation is indicated for the treatment of agitation associated with schizophrenia and bipolar I mania. Comprehensive reviews of both oral [1, 2] and intramuscular [3] olanzapine in the treatment of schizophrenia and bipolar I disorder have been published. In recent years, a new orally disintegration formulation became available for clinical use in this patient population. The biological properties, risks and benefits, and medication adherence relative to this route of administration are the focus of this review.
Treatment Adherence in Psychiatric Disorders
Concern about medication compliance has existed as long as the use of medication itself. There are many definitions of compliance, but generally it can be interpreted as the degree to which a patient's behavior is consistent with medical advice [4]. Medication adherence is defined as the degree to which a patient is consistent with an agreed‐upon mode of treatment [5]. Conversely, medication nonadherence is the number of doses not taken or taken incorrectly that jeopardize therapeutic outcome, including: not filling a prescription, taking an incorrect dose, taking a medication at the wrong time, forgetting to take a prescribed dose, or stopping therapy too soon [6]. Medication nonadherence is a significant concern that is closely linked to treatment failure, suboptimal clinical response, and high rates of recurrence (especially in chronic disease) [7]. The medical literature reports the medication noncompliance rate at around 50% in patients treated for diseases including arthritis, seizure disorders, or hypertension [8].
In psychiatric disorders, the problem of treatment adherence problem is also affected by the special idiosyncrasy of the disease itself, in which cognition and motivation are affected directly by the illness process. The data show that about one‐third of patients receiving treatment of schizophrenia are declared partially compliant, meaning they will either reduce the prescribed drug dose or fail to take the medication from time to time, and another one‐third do not follow prescription instructions at all [9].
Consequences of nonadherence include nonremitting symptoms, relapse, or recurring or fluctuating adverse effects. Some studies have reported that up to 55% of relapses in patients with schizophrenia were due to treatment noncompliance [10]. Patients who do not comply with their treatment plan experience a relapse rate of 11% per month versus 3.5% among compliant patients [11]. Relapse is considered the largest part of inpatient care and in consequence the main contribution to the cost of schizophrenia [12]. Added to that, repeated episodes may cause patients, family, and caregivers to become increasingly discouraged and pessimistic about the course of illness [13], which may produce secondary consequences of nonadherence: neurological deterioration [14], comorbid illness progression [15], substance use [16], jail [17], suicide attempts [18], re‐hospitalization [11, 19], or homelessness [20]. Because improving medication compliance may substantially reduce the relapse rate [21], even costly interventions may produce savings [12].
Predictive factors of medication nonadherence have been classified in four big groups as follows [12, 22, 23, 24].
-
•
Patient‐ and/or disease‐related factors. This group includes disease‐independent factors such as age, sex, or physical comorbidities, and factors related to the nature of the illness such as lack of insight, psychopathology, or cognitive impairment. Apart from the primary illness, comorbid alcohol or substance abuse is a strong predictor of noncompliance.
-
•
Environment‐related factors. Family and social support and assistance, as well as positive attitudes in social environments toward psychiatric treatment contribute to increased treatment compliance.
-
•
Physician‐related factors. The relationship between a clinician and patient is critical to treatment compliance. Physicians' level of interest and amount of time they dedicate to each patient, their attitude toward both patient and treatment, and the exchange of information they share with patients contribute to create a therapeutic alliance that facilitates medication compliance.
-
•
Treatment‐related factors. This group includes positive, in terms of delay in perceived benefits (i.e., delayed onset of action and delayed relapse immediately after discontinuation) and negative (i.e., potential adverse events, particularly extra‐pyramidal symptoms and treatment‐emergent dysphoria that are clearly identifiable), effects of treatment. An improved safety profile such as of second‐generation antipsychotics may contribute to increase in patient adherence, despite other adverse events such as sexual dysfunction or metabolic issues [25]. The complexity of dose regimen and dose also influence adherence. Patients with complicated drug schedules and polypharmacy have more problems. Patients treated with less than recommended doses may not improve while patients treated with higher than recommended doses may have a greater risk of certain treatment‐emergent adverse events [8, 26].
The route of administration also plays an important role in patient medication compliance. In the acute setting, intramuscular administration guarantees drug delivery, but may be perceived as a violation of the therapeutic alliance between patient and physician [27]. Depot neuroleptic formulations may confer a compliance advantages compared with oral neuroleptics formulations. Nevertheless, nonadherent patients can also be irregular in receiving their injections so depot drugs may not be effective for some patients [24].
For these reasons, orally disintegrating tablets have been widely investigated as an alternative administration route aimed at improving medication adherence among patients with schizophrenia and other psychiatric disorders.
Orally Disintegrating Tablets Potential Contribution to Medication Adherence
Over the past three decades, orally disintegrating tablets have gained much attention as a preferred alternative to conventional oral medications including tablets and capsules. The U.S. Food and Drug Administration (FDA) defines an orally disintegrating tablet (ODT) as, “A solid dosage form containing medicinal substances which disintegrates rapidly, usually within a matter of seconds, when placed upon the tongue”[28]. These tablets are distinguished from conventional sublingual tablets, which require more than a minute to dissolve in the mouth.
Recent studies indicate that more than half of surveyed patients prefer orally disintegrating tablets to other dosage forms [29], and most patients would ask their doctors for (70%) or purchase (70%) orally disintegrating tablets, or prefer them to regular tablets or liquids (>80%) [30]. These responses may reflect known advantages associated with orally disintegrating tablets, such as ease of administration, ease of swallowing, pleasant taste, and choice of several flavors [31].
Orally disintegrating tablets are especially useful to treat patients who have difficulty swallowing tablets and hard gelatin capsules, those who are bedridden, or active working and traveling populations [31, 32]. This route of administration offers additional advantage for treatment of patients with psychiatric disorders [33, 34]. Willfully noncompliant psychiatric patients may refuse medication or develop surreptitious behaviors such as failure to swallow pills (i.e., cheeking) and then expulsion of pills (i.e., spitting).
In order to facilitate compliance, an orally disintegrating formulation has been developed for olanzapine using the ZYDIS technology [31]. In this review, we analyze the biodisposability, pharmacokinetics, efficacy, and safety of the orally disintegrating formulation of olanzapine.
Pharmacokinetics Profile/Bioequivalence
The bioequivalence of orally disintegrating olanzapine formulation was compared to the standard oral tablet in phase I clinical trials of healthy subjects. Results showed that the two formulations were bioequivalent [35, 36]. The mean plasma concentration‐time curves were essentially identical, with similar Cmax values, areas under the curve, and terminal elimination phase slopes.
Nevertheless, evaluation of plasma concentrations over the first hour after administration revealed significantly more subjects taking the orally disintegrating tablet formulation had measurable concentrations of olanzapine at earlier time points compared with those taking the standard tablets (at a dosage of 5 mg, 79% vs. 0%, respectively, at 15 min). Significantly, more subjects receiving orally disintegrating tablets compared to standard tablets had higher plasma concentrations (≥1 ng/mL) over the first hour (63% vs. 11%). These small early concentration differences became indistinguishable before reaching Cmax (Fig. 1) [35]. The clinical relevance of early onset of absorption to clinical treatment has not been tested.
Figure 1.
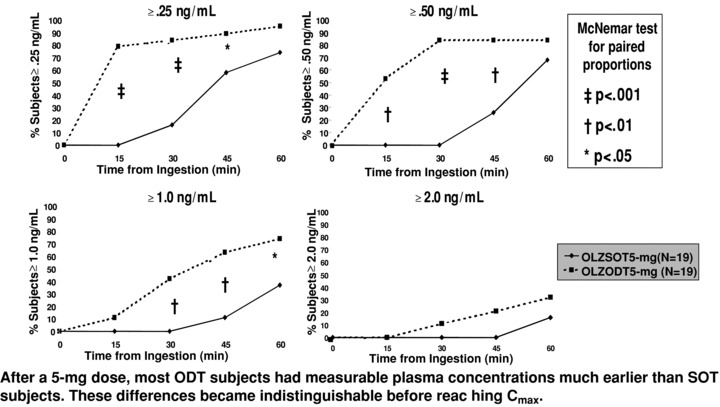
Concentration time curve following 5 mg dose of olanzapine. Abbreviations: cmax= maximum observed drug concentration; ODT = orally disintegrating tablet; SOT = standard oral tablet. Dashed line = olanzapine ODT 5 mg (n = 19); solid line = olanzapine SOT 5 mg (n = 19).
In a recently published 3‐way crossover, randomized, open‐label study, Markowitz et al. [37] examined not only the bioequivalence of both formulations but sought to establish if the orally disintegrating tablet had any sublingual additional adsorption. The authors confirmed previous reports that the orally disintegrating administration resulted in more measurable early plasma concentrations relative to standard olanzapine tablets, but that there were no statistically significant differences for observed pharmacokinetic parameters (maximum observed drug concentration [Cmax], time of maximum observed drug concentration [Tmax], or area under the concentration versus time curve from zero to 8 h [AUC(0–8 h)]. They also observed no difference in these parameters between orally disintegrating tablet administered in normal fashion on top of the tongue or the sublingual administration.
Biodisposability
To assess the dissolution, tolerability, and acceptability of daily orally disintegrating olanzapine tablets, Chue et al. [38] designed a 7‐day, open label pilot study in which 11 patients diagnosed with schizophrenia that were stable on oral olanzapine standard tablets, were switched to the same dose of the orally disintegrating tablet. Time to initial and complete disintegration of tablet was assessed. Adverse events were graded at each visit, and patients completed an acceptance questionnaire. The mean time to initial tablet disintegration was 15.78 seconds, and the mean time to complete disappearance was 0.97 min. There were no serious adverse events. Only two adverse events (asthenia and insomnia) were reported as new during the study. All 11 patients reported that the orally disintegrating tablet was an acceptable way of taking medication; subjective comments were all positive.
Dosage and Administration
Proven its equivalence with the conventional olanzapine tablet, dosage recommended for treatment of schizophrenia, manic episode, and recurrence prevention in bipolar disorder, are within the range 5–20 mg/day as reported in the European Union summary of product characteristics [39] and the FDA label [40]. Oral olanzapine should be administered on a once‐a‐day schedule without regard to meals as absorption is not affected by food.
Nevertheless, due to the nature of schizophrenia and bipolar disorder, patients may refuse to take medication when dispensed in solid oral dosage forms. It is a generally accepted standard of practice in institutional settings for health care professionals to compound extemporaneous preparations to assist patients with medication compliance. To date only one study has been published that discusses the preparation of a liquid olanzapine preparation [41]. It should be noted that the prescribing information for olanzapine tablets or olanzapine orally disintegrating tablets does not include instructions for administration of the drug in extemporaneously prepared mixtures. Nevertheless, extemporaneous solutions or suspensions are usually prepared by crushing or dispersing tablets into beverages such as fruit juice, milk, coffee, cola, and water, and served to patients.
The orally disintegrating tablets may be placed in the mouth or dispersed in water or other suitable beverage for administration (data on file: Eli Lilly and Company). Stability of the orally dispersible tablet formulation was tested in water, apple juice, orange juice, coffee, milk, and cola beverages, and it was found that a stable suspension formed with each of these beverages except cola. The suspension with apple juice, orange juice, coffee, and water is stable at room temperature for 6 h. All of these suspensions are also stable under refrigeration for 24 and 48 h. Coffee was not tested under refrigeration at any time period. The suspension in milk is stable for 6, 24, and 48 h under refrigeration. A precipitate forms when the orally disintegrating tablet is mixed with cola; therefore it is not recommended that orally disintegrating olanzapine tablets be mixed with cola beverages. At the time of this report, evaluation of the efficacy and safety of extemporaneous preparation administration has not been conducted.
Clinical Studies
Efficacy
The efficacy and compliance with olanzapine orally disintegrating tablets was evaluated by Kinon et al. [42] in a open label study with 85 acutely ill patients who met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) criteria for schizophrenia or schizoaffective disorder and who met the criteria for medication noncompliance: active or passive refusal of prescribed antipsychotic medication, direct evidence or suspicion of cheeking or spitting medication, or a claim that medication cannot be swallowed despite the absence of any physical limitation. Study participants received olanzapine orally disintegrating tablets (10–20 mg/day) for up to 6 weeks. During the first week, they received study medication within a supervised medication program, after which they could be released from supervised care. The primary efficacy measure was the Positive and Negative Symptom Scale (PANSS) total score [43]. Other efficacy measures included the Clinical Global Impression (CGI) Severity and Improvement Scales [44].
Sixty‐four patients (75.3%) completed the study. Patients showed a significant improvement in overall psychopathology measured as a reduction of 24.41 (SD: ± 22.61) in the PANSS total score P < 0.001. This improvement was observed as early as the first week after initiating treatment, when 32% of patients showed a 20% or greater reduction in the PANSS total score (improvement of 20% or greater in the PANSS total score was set a priori as a measure of clinical response). By week 6, 60% of patients were considered responders. This significant improvement was also evident in the CGI scale, with improvement occurring as early as day 2. The authors concluded that the orally disintegrating tablet formulation of olanzapine was effective in rapidly reducing psychopathology measure by PANSS scale.
These results were confirmed in a recent published study in which 512 patients with DSM‐IV schizophrenia or schizoaffective disorder, 43.9% of whom were previously treated with antipsychotics, received orally disintegrating olanzapine tablets [45]. Among those previously treated patients, 62.4% were considered to have been poorly compliant or noncompliant with their therapy. Regarding severity of illness, patients were assessed as markedly (45.5%) or severely ill (42.0%) on the CGI scale; mean PANSS total score was 107 (standard deviation [SD]= 19.9). The orally disintegrating tablet was prescribed for a first psychotic episode in 24.8% of patients and as a substitution of another antipsychotic treatment in 56.1% of patients. Main reasons for choosing orally disintegrating olanzapine tablets were ambivalence/refusal of care (74.6%) and poor or noncompliance to previous treatment (51%). At the end of week 1, the mean daily dose of olanzapine was 19.1 mg (SD = 10.5). At the end of the study, improvement according to the CGI criteria was “much” or “very much” in 47.9% and 22.0% of patients, respectively, and the mean change in the PANSS total score was −40.1 (SD = 23.6). Nurses considered that their work was “very” or “totally” facilitated by the use of orally disintegrating formulation of olanzapine for 74.2% of patients. Authors concluded that this olanzapine formulation was effective in reducing psychotic symptoms. Moreover, patient, physician, and nurse questionnaires confirmed that this formulation is particularly adapted for the treatment of acute and noncompliant schizophrenic patients.
As was suggested in the recent Cochrane review on intramuscular and orally disintegrating olanzapine [46] another potential use for orally disintegrating tablets of olanzapine may be treatment of patients who are acutely disturbed and/or suffer from agitated psychosis or manic psychosis. Notably, they pointed out the need for well designed and executed randomized studies of this formulation.
Although the orally disintegrating formulation dissolves rapidly in the mouth, its adsorption occurs via the gastric mucosa, so the onset of action is equivalent to the tablet form [47]. Nevertheless, some published case reports suggest that the use of orally disintegrating olanzapine as alternative to the injection of antipsychotic drugs may provide significant advantages in certain cases, specially related to ease of administration [48].
In a naturalistic, open‐label study of 80 acutely agitated psychotic patients, participants received either 20 mg olanzapine as an orally disintegrating tablet or other pharmacological treatment (haloperidol, N = 30; benzodiazepine, N = 7; or haloperidol plus benzodiazepine, N = 3) depending on the attending psychiatrist's preference. Both groups showed a significant reduction in mean the Excitement Component of the PANSS score (PANSS‐EC) (mean reductions of –12.9 in patients treated with orally disintegrating olanzapine tablet and –13.6 in patients treated with other pharmacological treatment). There were statistically significant differences in the PANSS‐EC scale (P= 0.007) between the two groups 1 h after treatment. There were also statistically significant differences between groups regarding the need for a second pharmacological intervention, 30% versus 50%, respectively, of patients in the orally disintegrating treatment versus other pharmacological treatment group (P= 0.04). The authors concluded that orally disintegrating olanzapine formulation is an effective, fast‐acting, and safe treatment option in agitated psychotic patients [49].
Another naturalistic study in patients with acute schizophrenia [50] the effectiveness, efficacy, safety and medication acceptance of the standard olanzapine tablets and olanzapine disintegrating tablets were evaluated. The choice of the therapy, as well as the switching between formulations, was at the physician' discretion. Both olanzapine formulations, orally disintegrating tablets (N = 247) and standard formulation (N = 207), showed similar effectiveness after 2 weeks. CGI‐I improved in 92.1% of patients (91.8% in patients treated with orally disintegrating olanzapine tablet and 92.3% in patients' standard olanzapine tablets). In patients receiving both formulations suicidal ideations, measured by MADRS item 10 were reduced (in the disintegrating formulation improvements went from 53.9% to 20.6% and in the standard one from 51.2% to 22.7%). According to the results, the orally disintegrating olanzapine formulation was preferably given to severely ill (64.4% vs. 49.8%) and aggressive patients (37.7% vs. 16.4%). Authors concluded that the outcomes with both olanzapine formulations were comparable.
Another study in acutely agitated psychotic patients analyzed the efficacy and tolerability of risperidone oral solution and olanzapine orally disintegrating tablet [51]. Patients with a PANSS‐EC score ≥ 15 were assigned to one of those treatments (34 to the orally disintegrating olanzapine and 53 to risperidone oral solution) and assessed every 15 min. Repeated‐measures analysis of variance revealed only a significant main effect of time course on PANSS‐EC (F = 82.2, P < 0.0001) but not significant main effect on treatment. Results showed that both drugs yielded similar improvements in terms of efficacy (scales and physiological measurements) and tolerability (no differences in rate of extrapyramidal symptoms) neither on patient satisfaction with assigned treatment were found. However patients in the orally disintegrating olanzapine group recovered significantly more from tachycardia than those in the risperidone group (P= 0.03). Authors conclude on both drugs are similar in acutely agitated patients who accepted oral medication.
Safety/Tolerability
In addition to its demonstrated bioequivalence, the rapidly disintegrating tablet formulation shares the well‐known safety profile of the conventional olanzapine tablet as reported in the European Union summary of product characteristics [38] and the FDA label [39].
In the Kinon et al. study [42], safety was evaluated using the modified Simpson–Angus Scale, the Barnes Akathisia Scale, and the Abnormal Involuntary Movement Scale. Vital signs, weight, and treatment‐emergent adverse events (TEAEs) were also collected. There was no significant increase in extrapyramidal symptoms during treatment; in fact, the modified Simpson–Angus Scale showed a significant decrease of −1.14 from baseline to endpoint (P < 0.001). During the study, TEAEs were reported in fewer than 10% of patients. The most frequent adverse events reported during the study were agitation, anxiety, dry mouth, headache, insomnia, somnolence, and weight gain. No clinically significant changes from baseline were seen in laboratory analyses or vital signs, other than a mean weight gain of 2.96 ± 3.62 kg.
In the Pascual et al. study [49] of acutely agitated psychotic patients, treatment with orally disintegrating olanzapine tablets was well tolerated and no serious TEAEs were observed. There were no differences between the orally disintegrating olanzapine tablet group and other pharmacological treatment groups. Three olanzapine patients experienced hypotension, and one nonclinically significant bradycardia. No movement disorders were spontaneously reported by patients; three patients had akinesia and mild tremor.
In the Czekalla et al. [50] study of acute psychotic patients, TEAEs were reported for 6.5% of patients treated with the orally disintegrating formulation and 2.9% of patients treated with the standard ones. According to the authors, this difference was possibly caused by the characteristics of patients receiving the orally disintegrating formulation.
Potential Effects on Treatment Emergent Weight Gain
One of the most well‐known adverse events during treatment with olanzapine is the weight gain, which can itself contribute to treatment noncompliance. Methods oriented to decrease or reverse the treatment‐emergent weight gain are of interest. In the literature, there have been reported some anecdotal case reports [52, 53] and one pilot study [54], which suggest that during treatment with orally disintegrating olanzapine tablets there is an effect of weight reduction. In the pilot study [54], authors compared weight in patients whose treatment was switched from olanzapine conventional tablets to orally disintegrating tablets (N = 9) versus those who continued on conventional tablet formulation (N = 9). They report substantial weight reduction after switching from conventional olanzapine to the orally disintegrating formulation (mean weight lost 6.6 kg; mean BMI [body mass index] lost 2.1 kg/m2) when compared with continuous treatment with conventional olanzapine (mean weight gain 3.7 kg; mean BMI gain 1.1 kg/m2), (P < 0.001).
A larger naturalistic study evaluated weight change in 33 patients who had received treatment with conventional olanzapine tablets for a mean of 43.3 months before switching to orally disintegrating olanzapine tablets [55]. Four months after receiving the first disintegrating dose, 60% (20 patients) lost or did not gain any weight and 30% (10 patients) gained weight. The mechanism by which orally disintegrating tablet may result in less weight gain relative to conventional olanzapine is unknown. The authors hypothesize that this finding may be associated with peripheral serotonin receptors (specifically 5‐HT2C). Studies in the rat indicated that peripheral 5‐HT might exert its anorectic action by contracting the pylorus via a 5‐HT2‐like receptor [56]. In human studies the 5‐HT2C receptor gene has been shown to be related with antipsychotic treatment‐emergent weight gain [57]. Because of its fast dissolution, orally disintegrating tablets are absorbed prior to the level of pylorus, before the drug makes contact with the 5‐HT2C receptors that are responsible for mediating satiety. This hypothesis is also pointed out by Markowitz et al. [37] in a recent study of the pharmacokinetics of this formulation.
Nevertheless according to data presented in the General Safety section of the olanzapine depot formulation document presented in February 2008 to the FDA, the mean change in weight was not statistically significantly different for OP Depot‐treated patients compared with oral olanzapine‐treated patients in the Olanzapine‐Controlled Database [58].
A recent post hoc analysis of data from a randomized clinical trial involving antipsychotic treatment of never treated, first‐onset psychotic patients was used to compare weight change after 6 weeks of olanzapine as a standard tablet versus an orally disintegrating formulation [59]. In this study, standard olanzapine tablets were nonrandomly and consecutively prescribed to the first 19 patients and, as the orally disintegrating olanzapine formulation became available, the next 19 patients received it. After 6 weeks, a significantly greater increase in weight was noted in the standard versus orally disintegrating groups (6.3 +1.9 kg vs. 3.3 ± 3.2 kg, respectively; P= 0.009). There was also a significantly higher BMI among patients who received standard tablet compared to orally disintegrating tablet (2.1 kg/m2 vs. 1.1 kg/m2; P= 0.036). Clinically significant weight gain (≥7% increase from baseline weight) was observed in 84.2% and 31.6%, respectively, of patients on standard versus orally disintegrating tablets (P= 0.014).
Although olanzapine use in adolescents population is nowadays under review by regulatory authorities, a recent study compared changes in weight and BMI in 52 hospitalized adolescents treated with 12 weeks of olanzapine standard (N = 10; mean daily dose, 18 mg) versus orally disintegrating tablets (N = 16; mean daily dose, 16.6 mg) versus risperidone (N = 26; mean daily dose, 2.8 mg). Significantly greater increases in mean weight and BMI were observed in patients treated with olanzapine standard tablets (8.9 ± 5.1 kg and 1.9 ± 0.6 kg/m2, respectively) compared to those treated with orally disintegrating tablets (3.0 ± 2.1 kg and 1.1 ± 0.8 kg/m2, respectively). Similarly, olanzapine orally disintegrating treatment was associated with significantly greater increases in weight and BMI compared to risperidone (1.0 ± 1.8 kg and 0.4 ± 0.7 kg/m2, respectively). These findings suggest that adolescents may gain less weight when treated with olanzapine orally disintegrating tablets than those treated with the standard tablets [60].
An open label prospective study developed by Chawla et al. [61] investigated the long weight loss outcomes during usual clinical practice after switching 26 patients with schizophrenia who were clinically stable on olanzapine standard tablets to orally disintegrating olanzapine. Patients included in the study were on olanzapine standard tablets for a minimum of 1 year and had a BMI ≥ 25 kg/m2. All other aspects of treatment remained constant. Weight was recorded at 3, 6, and 12 months. The average baseline weight and baseline BMI were 96.2 ± 3.6 kg and 32.4 ± 1.2 kg/m2, respectively. Mean duration of olanzapine treatment previous the switching to the orally disintegrating formulation was 5.5 years (SD = 2.2). Results showed that patients incurred an average weight loss of 2.7 ± 0.7 kg (P= 0.001) after switching patients from olanzapine standard tablets to the orally disintegrating formulation at 12 months. Peak weight loss was observed at 6 months (2.9 ± 0.9 kg, P= 0.003); however, significant weight loss was achieved as early as 3 months: 86% of the patients' total weight loss occurred during the first 3 months. The majority (81.9%) of patients lost weight, with an average weight change at 12 months of –3.8 ± 0.6 kg, while 18.1% had no weight change or weight gain. BMI significantly decreased by 1.0 ± 0.3 kg/m2 (P= 0.001). Interestingly, patients treated with higher doses of olanzapine (≥20 mg) incurred a greater weight loss of their body weight (5.6%), compared to those treated with lower doses (<20 mg), who lost 1.9% of their body weight (P= 0.04). Authors concluded that in usual clinical practice, switching patients from olanzapine standard tablets to orally disintegrating olanzapine treatment resulted in significant weight loss that was maintained over 12 months.
Karagianis et al. [62], in order to investigate the changes in BMI and weight in patients who are gained weight on olanzapine standard tablet treatment and continued with this formulation as compared who those who switched to olanzapine disintegrating tablet, developed a double‐blind, double dummy, parallel study of outpatients with schizophrenia, schizoaffective disorder, related psychotic disorder, or bipolar disorder in which 149 patients were randomized to received the standard tablet plus a disintegrating placebo (65 patients) or the orally disintegrating olanzapine tablet plus a conventional placebo tablet (84 patients) during 16 weeks. Patients must have a BMI increase of 1 kg/m2 or weight gain ≥ 5 kg during 1–12 months of standard olanzapine tablet previous to the study. Results showed that there were no significant differences in BMI changes during the study. The difference in BMI was 0.20 kg/m2 (P= 0.465) and in weight was 0.65 kg (P= 0.385). Full results are in preparation for publication
The impact of oral disintegrating olanzapine on weight change in olanzapine‐naïve patients is unknown, so further studies are warranted in this area.
Other Results
Effects on Treatment Compliance
During the efficacy study performed by Kinon et al. [42] compliance with olanzapine orally disintegrating tablets was evaluated using various scales: Rating of Medication Influences (ROMI) [63], the Treatment Compliance Interview (TCI) [64], the Nursing Assessment of Medication Acceptance (NAMA), and the Patient Global Impression (PGI) scale (scales described in the original paper) [42]. TCI is a clinical interview, separate versions of which are given to patients, families, and clinicians that assesses the degree of medication adherence in three areas: dosage deviation, level of medication supervision, and willingness to remain on medication [64]. Trough plasma concentrations were collected as a proxy measure of medication compliance, considering adequate ingestion of medication as concentrations above 9 ng/mL [65]. Significant improvement in medication compliance was observed with baseline to endpoint increases in ROMI compliance score (Baseline: 15.86, last observation carried forward [LOCF]: 17.23; P= 0.013) and decreases in the ROMI noncompliance score (Baseline: 16.38, LOCF: 12.84; P < 0.001). Improvements in compliance were detected as early as one week after initiating treatment [42].
In a post hoc analysis, authors examined individual ROMI items and their correlation with clinical psychopathology as measured by PANSS factors. They found that statistically significant mean increases in endorsement of ROMI compliance items (ROMI‐C: “perceived benefit,”“fear of relapse,”“side effect relief”) and decreases in endorsement of noncompliance items (ROMI‐NC: “no benefit,”“unnecessary,”“never was ill,”“interferes with life goals,”“distressed by side effects,” and “outside opposition to taking medication”) occurred by week 1. Significant correlation coefficients at baseline (P < 0.05) involved ROMI‐C items and PANSS factors for “positive,”“negative,”“disorganized,” and “hostile” symptoms. There were also correlations between ROMI‐NC items “no benefit” and “distressed by side effects” with PANSS factors for “hostility” and “depression.” Changes in ROMI item “perceived benefit” was significantly associated with symptom improvement for PANSS “positive symptom” and “disorganized thought” domains. Authors concluded that patient attitude toward medication adherence significantly improved, mainly by perception of medication benefit and improved insight. This suggests that patient attitude toward treatment compliance can be influenced by treatment response, such that noncompliant patients may become compliant with proper and effective treatment [66].
Improvements in medication compliance attitude and nursing care burden were also observed with significant baseline to endpoint reductions on the TCI total score and in each item (“attitude,”“compliance,”“ingestion,”“nursing burden”) as well as in the total NAMA score (P < 0.001). Visitwise comparisons revealed significant improvement in the NAMA rating scale as early as day 2 (P < 0.001). In addition, patient‐rated feelings about medication showed positive acceptance at all measured time points (PGI scores 2.01–2.74). Plasma concentrations were >9 ng/mL throughout the study in 80–90% of patients [41]. Authors concluded that the orally disintegrating tablet formulation is not only effective in rapidly reducing psychopathology, but also in improving medication compliance attitudes and behaviors [66].
This study has two limitations pointed out by the authors. The first one is the open label design, which does not allow for direct comparisons. The other one is the fact that patients were willing to sign inform consent that might suggest the inclusion of relatively “more compliant” patients as was originally suggested by the patients' baseline characteristics.
In the Czekalla study [50], patients' medication acceptance and attitude toward olanzapine medication was assessed by the NAMA questionnaire. At baseline, medication acceptance by the patient was lower in patients treated with the orally disintegrating formulation. After 2 weeks, results showed that medication acceptance improved for both formulations, in particular the orally disintegrating tablet (attitude from 31.6% to 68.4%; ingestion from 48.9% to 83.4%; nursing from 53.9% to 86.2%; and compliance from 75.7% to 93.9%).
Additional Clinical Implications
In addition to noncompliant patients, all patients can benefit from orally disintegrating olanzapine. This formulation can be especially useful for patients who seek convenience because they are active, working and/or going to school, traveling, or who are concerned about calling attention to their illness/medication or who have no access to water at the time of medication administration [67].
This formulation is also useful for patients with an underlying medical condition which impedes taking oral medication and/or those with difficulty swallowing, as the published case of a manic patient with esophageal stricture plus chronic pharyngitis [68], or the case of a terminally ill patient who was receiving parenteral nutrition in which orally disintegrating olanzapine tablets plus alprazolam was used successfully to relieve his anxiety and tension, improving his relationships with his physicians and his daily life [69].
Patient Preference
Patient satisfaction and preliminary preference data obtained from efficacy trials suggest that the orally disintegrating formulation is preferred by patients. In order to compare patient preference for the orally disintegrating formulation versus conventional formulation, a 12‐week open label, randomized, crossover, multinational study was developed [70]. Outpatients with stable schizophrenia (CGI‐S<4) on olanzapine conventional formulation monotherapy for at least 1 month before study inclusion were randomized 1:1 to either orally disintegrating formulation or conventional formulation during 6 weeks and then switched the formulation during another 6 weeks.
Preference was evaluated according to a simple questionnaire after the 12 weeks treatment. From 265 randomized patients, 207 were eligible for the analysis and 175 patients answered the preference question. A total of 106 (61%) patients preferred orally disintegrating formulation versus 48 (27%) preferred olanzapine conventional tablets (P < 0.001 adjusted for treatment sequence); 21 (12%) expressed no preference. The adverse event profiles of both formulations were similar: most common (>1%) adverse events were weight increase, hypertriglyceridemia, and somnolence. According to authors, given the importance of patient's preference as one of the factors for future compliance, olanzapine orally disintegrating formulation could be a good choice.
Conclusions
One of the essential factors associated with compliance in patients with schizophrenia or bipolar disorder is the initial acceptance of drug therapy during the acute illness phase. Patients are more likely to accept treatment if it improves symptoms and is tolerable. Thus, the primary consideration in choosing an antipsychotic drug should be efficacy, which itself is an important contributor to treatment adherence. Second, factors related to safety profile, ease and convenience of administration, and patient/physician and patient/caregiver attitudes toward treatment can positively affect patient adherence and ultimately improve patient outcomes including a decreased risk of relapse.
Generally, patients prefer oral versus intramuscular route of administration, as the latter may be perceived as a violation of their right and ability to choose and may negatively impact the doctor‐patient relationship. Orally disintegrating olanzapine facilitates patients' acceptance of treatment because of its well‐known efficacy and safety profiles and ease of use.
This formulation can also decrease the burden of illness for caregivers such as nurses and families. By increasing patients' acceptance of drug therapy and minimizing symptoms, daily tensions and relationships may be improved.
The choice of the most appropriate antipsychotic drug and its optimal formulation for each patient with psychiatric disorder is a critical question that should take into account, in addition to efficacy, parameters related to tolerability and ease of use that may sustain long‐term treatment adherence. This is especially important because schizophrenia and bipolar disorders are chronic diseases in which patients are likely to require treatment over years or perhaps for their lifetime.
Conflict of Interest
Marta Casillas, Antonio Ciudad, and Inmaculada Gilaberte are full‐time Lilly employees.
Acknowledgments
Authors wish to thank Elena Perrin, Svetlana Domínguez, Virginia Stauffer, and Christine Droste for reviewing this manuscript.
References
- 1. Bhana N, Foster RH, Olney R, Plosker GL. Olanzapine: An updated review of its use in the management of schizophrenia. Drugs 2001;61:111–161. [DOI] [PubMed] [Google Scholar]
- 2. McCormack PL, Wiseman LR. Olanzapine: A review of its use in the management of bipolar I disorder. Drugs 2004;64:2709–2726. [DOI] [PubMed] [Google Scholar]
- 3. Wagstaff AJ, Easton J, Scott LJ. Intramuscular olanzapine: A review of its use in the management of acute agitation. CNS Drugs 2005;19:147–164. [DOI] [PubMed] [Google Scholar]
- 4. Haynes RB, Sackett DL. Compliance in health care. Baltimore , MD : Johns Hopkins Press; 1979. [Google Scholar]
- 5. National Institute of Health. Research on adherence to interventions for mental disorders . Available at http://www.grants.nih.gov/grants/guide/pa‐files/PA‐03‐111.html.
- 6. Nichols‐English G, Poirier S. Optimizing adherence to pharmaceutical care plans. J Am Pharm Assoc 2000;40:475–485. [PubMed] [Google Scholar]
- 7. Miller NH. Compliance with treatment regimens in chronic asymptomatic diseases. Am J Med 1997;102:43–49. [DOI] [PubMed] [Google Scholar]
- 8. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–497. [DOI] [PubMed] [Google Scholar]
- 9. Oehl M, Hummer M, Fleischhacker WW. Compliance with antipsychotic treatment. Acta Psychiatr Scand Suppl 2000;407:83–86. [DOI] [PubMed] [Google Scholar]
- 10. Hogarty GE, Goldberg SC, Schooler NR, Ulrich RF. Drug and sociotherapy in the aftercare of schizophrenic patients II. Two‐year relapse rates. Arch Gen Psychiatry 1974;31:603–608. [DOI] [PubMed] [Google Scholar]
- 11. Weiden PJ, Olfson M. Cost of relapse in schizophrenia. Schizophr Bull 1995;21:419–429. [DOI] [PubMed] [Google Scholar]
- 12. Lindstrom E, Bingefors K. Patient compliance with drug therapy in schizophrenia. Economic and clinical issues. Pharmacoeconomics 2000;18:106–124. [DOI] [PubMed] [Google Scholar]
- 13. Gilmer TP, Dolder CR, Lacro JP, Folsom DP, Lindamer L, Garcia P, Jeste DV. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry 2004;161:692–699. [DOI] [PubMed] [Google Scholar]
- 14. Lieberman J, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D, Bider R. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry 2001;49:487–499. [DOI] [PubMed] [Google Scholar]
- 15. Sokal J, Messias E, Dickerson FB, Kreyenbuhl J, Brown CH, Goldberg RW, Dixon LB. Comorbidity of medical illnesses among adults with serious mental illness who are receiving community psychiatric services. J Nerv Ment Dis 2004;192:421–427. [DOI] [PubMed] [Google Scholar]
- 16. Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 1990;264:2511–2518. [PubMed] [Google Scholar]
- 17. Modestin J, Ammann R. Mental disorder and criminality: Male schizophrenia. National Institutes of Health. Research on adherence to interventions for mental disorders. Schizophr Bull 1996;22:69–82. [DOI] [PubMed] [Google Scholar]
- 18. Herings RM, Erkens JA. Increased suicide attempt rate among patients interrupting use of atypical antipsychotics. Pharmacoepidemiol Drug Saf 2003;12:423–424. [DOI] [PubMed] [Google Scholar]
- 19. Misdrahi D, Llorca PM, Lançon C, Bayle FJ. Compliance in schizophrenia: Predictive factors, therapeutical considerations and research implications. Encephale 2002;28:266–272. [PubMed] [Google Scholar]
- 20. Olfson M, Mechanic D, Hansell S, Boyer CA, Walkup J, Weiden PJ. Prediction of homelessness within three months of discharge among inpatients with schizophrenia. Psychiatr Serv 1999;50:667–673. [DOI] [PubMed] [Google Scholar]
- 21. Schooler NR. Relapse prevention and recovery in the treatment of schizophrenia. J Clin Psychiatry 2006;67(Suppl 5):19–23. [PubMed] [Google Scholar]
- 22. Ascher‐Svanum H, Zhu B, Faries D, Lacro JP, Dolder CR. A prospective study of risk factors for nonadherence with antipsychotic medication in the treatment of schizophrenia. J Clin Psychiatry 2006;67:1114–1123. [DOI] [PubMed] [Google Scholar]
- 23. Fenton WS, Blyler CR, Heinssen RK. Determinants of medication compliance in schizophrenia: Empirical and clinical findings. Schizophr Bull 1997;23:637–651. [DOI] [PubMed] [Google Scholar]
- 24. Fleischhacker WW, Oehl MA, Hummer M. Factors influencing compliance in schizophrenia patients. J Clin Psychiatry 2003;64(Suppl 16):10–13. [PubMed] [Google Scholar]
- 25. Awad AG, Voruganti LN. New antipsychotics, compliance, quality of life, and subjective tolerability–are patients better off? Can J Psychiatry 2004;49:297–302. [DOI] [PubMed] [Google Scholar]
- 26. Donohoe G, Owens N, O'Donnell C, Burke T, Moore L, Tobin A, O'Callaghan E. Predictors of compliance with neuroleptic medication among inpatients with schizophrenia: A discriminant function analysis. Eur Psychiatry 2001;16:293–298. [DOI] [PubMed] [Google Scholar]
- 27. Godleski LS, Goldsmith LJ, Vieweg WV, Zettwoch NC, Stikovac DM, Lewis SJ. Switching from depot antipsychotic drugs to olanzapine in patients with chronic schizophrenia. J Clin Psychiatry 2003;64:119–122. [DOI] [PubMed] [Google Scholar]
- 28. U.S. Food and Drug Administration [online] . Available from URL: http://www.fda.gov/cder/dsm/DRG/drg00201.htm [Accessed June 30, 2008.
- 29. Deepak K. Orally disintegrating tablets. Tables & Capsules 2004;7:30–35. [Google Scholar]
- 30. Brown D. Orally disintegrating tablets: Taste over speed. Drug Deliv Technol 2001;3:58–61. [Google Scholar]
- 31. Seager H. Drug delivery products and the Zydis fast‐dissolving dosage form. J Pharm Pharmacol 1998;50:375–382. [DOI] [PubMed] [Google Scholar]
- 32. DeRoche CC. Consumer preference for orally disintegrating tablets over conventional forms of medication: Evolving methodology for medication intake in dysphagia [lecture]. 12th Annual Meeting of the Dysphagia Research Society Oct 2–4; San Francisco , CA .2003.
- 33. Danileviciūte V, Adomaitiene V, Sveikata A, Maciulaitis R, Kadusevicius E, Volbekas V. Compliance in psychiatry: results of a survey of depressed patients using orally disintegrating tablet. Medicina (Kaunas) 2006;42:1006–1012. [PubMed] [Google Scholar]
- 34. Thyssen A, Remmerie B, D'Hoore P, Kushner S, Mannaert E. Rapidly disintegrating risperidone in subjects with schizophrenia or schizoaffective disorder: A summary of ten phase I clinical trials assessing taste, tablet disintegration time, bioequivalence, and tolerability. Clin Ther 2007;29:290–304. [DOI] [PubMed] [Google Scholar]
- 35. Bergstrom RF, Mitchell M, Witcher J, Huorston JP, Hill AL, Taylor CC, Liu‐Seifert H, Jones B. Rapid onset of absorption with olanzapine orally disintegrating tablets. Schizophr Res 2004;67(suppl 1):160. [Google Scholar]
- 36. Witcher JW, Bergstrom RF, Cerimele BJ, Hatcher BL, Jewell H, Hemingway J, Ratnasingam L Mitchell MI. Pharmacokinetics and bioequivalence of olanzapine rapidly‐disintegrating tablets [abstract no. 3431]. American Association of Pharmacists Annual Meeting; 1998 Nov 15–19; San Francisco ( CA ). 1998;Available at URL: http://www.aapspharmsci.org/abstracts/AM_1998/3431.html [Accessed June 30, 2008.
- 37. Markowitz JS, DeVane CL, Malcolm RJ, Gefroh HA, Wang JS, Zhu HJ, Donovan JL. Pharmacokinetics of olanzapine after single‐dose oral administration of standard tablet versus normal and sublingual administration of an orally disintegrating tablet in normal volunteers. J Clin Pharmacol 2006;46:164–171. [DOI] [PubMed] [Google Scholar]
- 38. Chue P, Jones B, Taylor CC, Dickson R. Dissolution profile, tolerability, and acceptability of the orally disintegrating olanzapine tablet in patients with schizophrenia. Can J Psychiatry 2002;47:771–774. [DOI] [PubMed] [Google Scholar]
- 39. Zyprexa–EU Summary of Product Characteristics . Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/Zyprexa/H‐115‐PI‐en.pdf [Accessed June 30, 2008.
- 40. Zyprexa Zydis (Olanzapine) Orally Disintegrating Tablets Approved Agreed‐Upon Labeling . Available from URL: http://www.fda.gov/cder/foi/label/2007/020592s042s043,021086s022s023,021253s026lbl.pdf [Accessed June 30, 2008.
- 41. Harvey EJ, Flanagan RJ, Taylor DM. The preparation and stability of a liquid olanzapine preparation for oral administration in hospitals. Pharm J 2000;265:275–276. [Google Scholar]
- 42. Kinon BJ, Hill AL, Liu H, Kollack‐Walker S. Olanzapine orally disintegrating tablets in the treatment of acutely ill, non‐compliant patients with schizophrenia. Int J Neuropsychopharmacol 2003;6:97–102. Erratum in: Int J Neuropsychopharmacol 2003; 6(3):313. [DOI] [PubMed] [Google Scholar]
- 43. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 44. Guy W. Clinical Global Impressions ECDEU Assessment Manual for Psychopharmacology, Revised (DHEW Publ. No. ADM 76–338). Rockville , MD : National Institute of Mental Health, 1976;218–222. [Google Scholar]
- 45. Dardennes R, Chartier F, Heurtebize N, Olivier V, Perrin E. Naturalistic use of the orally disintegrating tablet formulation of olanzapine in acute schizophrenic patients: An observational prospective study. Int J Neuropsychopharmacol 2004;7(Suppl. 2):P01.389. [Google Scholar]
- 46. Belgamwar RB, Fenton M. Olanzapine IM or velotab for acutely disturbed/agitated people with suspected serious mental illnesses Cochrane Database of Systematic Reviews 2005, Issue 2, Art. No.: CD003729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simpson JR, Thompson CR, Beckson M. Impact of orally disintegrating olanzapine on use of intramuscular antipsychotics, seclusion, and restraint in an acute inpatient psychiatric setting. J Clin Psychopharmacol 2006;26:333–335. [DOI] [PubMed] [Google Scholar]
- 48. Reeves RR, Wallace KD, Rogers‐Jones C. Orally disintegrating olanzapine: A possible alternative to injection of antipsychotic drugs. J Psychosoc Nurs Ment Health Serv 2004;42:44–48. [DOI] [PubMed] [Google Scholar]
- 49. Pascual JC, Perez V, Martin JL, Safont E, Puigdemont D, Alvarez G. Olanzapine orally‐disintegrating tablet in severe psychotic agitation: A naturalistic study. Actas Esp Psiquiatr 2007;35:47–51. [PubMed] [Google Scholar]
- 50. Czekalla J, Wagner T, Schacht A, Kluge M, Kinon B. Effectiveness and medication acceptance of olanzapine disintegrating tablets compared to standard olanzapine tablets in acutely treated psychiatric patients. Patient Preferences and Adherence 2007;1:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hatta K, Kawabata T, Yoshida K, Hamakawa H, Wakejima T, Furuta K, Nakamura M, Hirata T, Usui C, Nakamura H, Sawa Y. Olanzapine orally disintegrating tablet vs. risperidone oral solution in the treatment of acutely agitated psychotic patients. Gen Hosp Psychiatry 2008;30:367–371. [DOI] [PubMed] [Google Scholar]
- 52. Cohen JA, Perel JM. Adolescent weight loss during treatment with olanzapine. J Child Adolesc Psychopharmacol 2004;14:617–620. [DOI] [PubMed] [Google Scholar]
- 53. Tiedge UA. Schizophrenia: Advantages by changeover from olanzapine film‐coated tablets to disintegrating tablets. Nervenheilkunde 2005;24:232. [Google Scholar]
- 54. De Haan L, Van Amelsvoort T, Rosien K, Linszen D. Weight loss after switching from conventional olanzapine tablets to orally disintegrating olanzapine tablets. Psychopharmacology (Berl) 2004;175(3):389–390. [DOI] [PubMed] [Google Scholar]
- 55. Stip E, Anselmo K, Wolfe M, Lessard C, Landry P. Long‐term treatment with atypical antipsychotics and risk of weight gain. Drug Saf 2006;29:550–552 [Author reply 552 to: Drug Saf 2006; 29(4): 303–19. [DOI] [PubMed] [Google Scholar]
- 56. Eberle‐Wang K, Braun BT, Simansky KJ. Serotonin contracts the isolated rat pylorus via a 5‐HT2‐like receptor. Am J Physiol 1994;266:R284–R291. [DOI] [PubMed] [Google Scholar]
- 57. Templeman LA, Reynolds GP, Arranz B, San L. Polymorphisms of the 5‐HT2C receptor and leptin genes are associated with antipsychotic drug‐induced weight gain in Caucasian subjects with a first‐episode psychosis. Pharmacogenet Genomics 2005;15:195–200. [DOI] [PubMed] [Google Scholar]
- 58. Eli Lilly and Company . Psychopharmacologic Drugs Advisory Committee Briefing Document. Zyprexa® Olanzapine Pamoate (OP) Depot (Olanzapine Long‐Acting Injection) Schizophrenia. Available at: http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008‐4338b1‐03‐Lilly.pdf. Accessed 19 January 2008.
- 59. Arranz B, San L, Dueñas RM, Centeno M, Ramirez N, Salavert J, Del Moral E. Lower weight gain with the orally disintegrating olanzapine than with standard tablets in first‐episode never treated psychotic patients. Hum Psychopharmacol 2007;22:11–15. [DOI] [PubMed] [Google Scholar]
- 60. Crocq MA, Guillon MS, Bailey PE, Provost D. Orally disintegrating olanzapine induces less weight gain in adolescents than standard oral tablets. Eur Psychiatry 2007;22:453–454. [DOI] [PubMed] [Google Scholar]
- 61. Chawla B, Luxton‐Andrew H. Long‐term weight loss observed with olanzapine orally disintegrating tablets in overweight patients with chronic schizophrenia. A 1 year open‐label, prospective trial. Hum Psychopharmacol 2008;23:211–216. [DOI] [PubMed] [Google Scholar]
- 62. Karagianis J, Landry J, Milev R, Grossman L, Monga N, De Hann L, Maguire GA, Hoffmann VP, Lee B. A 16‐week, randomized, doublet blind, double dummy trial of sublingual orally disintegrating olanzapine vs standard olanzapine tablets in patients who gained weight during olanzapine treatment: The PLATYPUS Study. Schizophr Res 2008;102/1–3(Suppl 2):238. [Google Scholar]
- 63. Weiden P, Rapkin B, Mott T, Zygmunt A, Goldman D, Horvitz‐Lennon M, Frances A. Rating of medication influences (ROMI) scale in schizophrenia. Schizophr Bull 1994;20:297–310. [DOI] [PubMed] [Google Scholar]
- 64. Weiden PJ, Mott T, Curcio N. Recognition and management of neuroleptic noncompliance In: Shriqui C, Nasrallah H, eds. Contemporary issues in the treatment of schizophrenia. Washington , DC : American Psychiatric Press, 1995;463–485. [Google Scholar]
- 65. Perry PJ, Sanger T, Beasly C. Olanzapine plasma concentrations and clinical response in acutely ill schizophrenic patients. J Clin Psychopharmacol 1997;17:472–477. [DOI] [PubMed] [Google Scholar]
- 66. Liu‐Seifert H, Houston J, Adams D, Kinon BJ. Association of acute symptoms and compliance attitude in noncompliant patients with schizophrenia. J Clin Psychopharmacol 2007;27:392–394. [DOI] [PubMed] [Google Scholar]
- 67. Tornatore FL. Orally disintegrating antipsychotics may promote compliance and adherence in patients with schizophrenia. J Clin Psychiatry 2005;66:1493–1494 [Author reply to: J Clin Psychiatry 2005; 66(1): 122–133. [DOI] [PubMed] [Google Scholar]
- 68. Shen YC, Lee MY, Lin CC, Chen CH. Orally disintegrating olanzapine for the treatment of a manic patient with esophageal stricture plus chronic pharyngitis. Prog Neuropsychopharmacol Biol Psychiatry 2007;31:541–542. [DOI] [PubMed] [Google Scholar]
- 69. Douzenis A, Michopoulos I, Economopoulos T, Lykouras L, Soldatos CR. Sublingual use of olanzapine in combination with alprazolam to treat agitation in a terminally ill patient receiving parenteral nutrition. Eur J Cancer Care 2007;16:289–290. [DOI] [PubMed] [Google Scholar]
- 70. Ciorabai EM, Oyffe I, Dilbaz N, Lozano S, Ruschel S, Salburg J, Dyachkova Y, Treuer T. Patients preference of olanzapine orodispersible tablet compared with olanzapine classic oral tablet in a multinational, randomized, crossover study. Eur Psychiatry 2008;23(Suppl 2):150–151. [DOI] [PMC free article] [PubMed] [Google Scholar]


