Abstract
Tolperisone, a piperidine derivative, is assigned to the group of centrally acting muscle relaxants and has been in clinical use now for decades. The review summarizes the known pharmacokinetics, pharmacodynamics, toxicology and side effects in humans and the clinical use of tolperisone. A future perspective for further exploration of this drug is given.
Keywords: Stroke, Neuropsychopharmacology, Neuromuscular disease, Movement disorders, Parkinson's disease, Behavioural neurology, Painful muscle spasm
Introduction
Generally, muscle relaxants are used to achieve reversible relaxation of skeletal muscle. The term “muscle relaxant” refers to drugs belonging to a group of medications that are heterogeneous with respect to their chemical structure as well as their molecular targets. According to their site of action they can be divided into two groups: (1) muscle relaxants acting on spasticity by interaction with upper motor neurons (central acting muscle relaxants) and (2) those treating muscular pain or spasms by action on peripheral musculoskeletal elements (peripherally acting muscle relaxants). Tolperisone, a piperidine derivative, is assigned to the group of centrally acting muscle relaxants and has been in clinical use now for decades in Europe and Asia. The first synthesis of 1‐Piperidino‐2‐Methyl‐3‐(P‐Tolyl)‐Propan‐3‐on (Tolperisone, N‐553, Abbsa, Atmosgen, Arantoick, Besnoline, Isocalm, Kineorl, Menopatol, Metosomin, Minacalm, Mydocalm, Mydeton, Naismeritin, Tolisartine) was achieved, starting from the structure of cocaine and the first pharmacological experiments, indicating a central action of the drug, were performed (Porszasz et al. 1961). Several related compounds exist: eperisone (E‐646, EMPP, Mional, Myonal), lanperisone (NK‐433), inaperisone (HY‐770), and silperisone (RGH‐5002, a nonchiral, tolperisone‐like, organosilicone compound).
Because of a chiral center (marked with asterisks in Fig. 1), stereoisomers of tolperisone and related compounds exist. Generally the racemic mixtures are used for medication. Interesting in this context are several analytical and preparative procedures that have been described, which allow the separation of the racemic mixtures into the pure stereo selective compounds (Armstrong et al. 1991; Haginaka et al. 1999; Matsunaga et al. 2003; Tsukamoto et al. 1997, 1999; Velmurugan et al. 2002; Welch et al. 1997). The current review will focus on the most important substance, on tolperisone, but will also deal with progress on related drugs, except silperisone (silperisone being covered by a recent review (Farkas 2006)).
Figure 1.
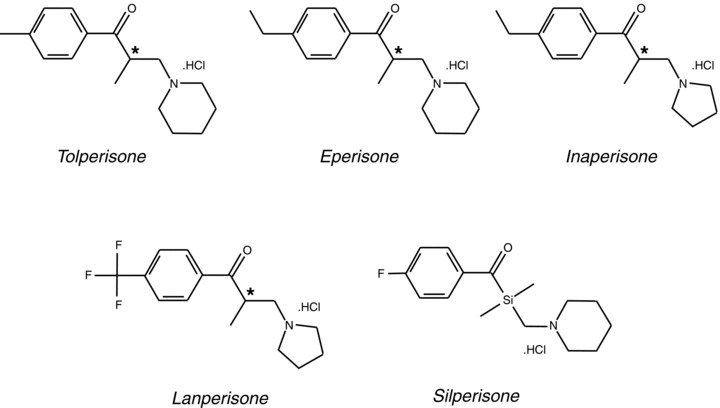
Chemical structure of tolperisone and related compounds.
Pharmacokinetics
Analytical Methods for Tolperisone Quantization
Until 1978, titrimetric and spectrophotometric methods were used for determination of tolperisone in pharmaceutical preparations and for stability assays. Gazdag et al. (1978) described a gas–liquid chromatographic method for separation and quantitative determination of multicomponent pharmaceuticals including tolperisone. Later on, a high‐performance thin layer chromatographic method (HPTLC) for simultaneous determination of tolperisone hydrochloride and lidocaine hydrochloride in pharmaceutical formulations was published by Liawruangrath and Liawruangrath (1999). Another method of determination of tolperisone hydrochloride was the reverse phase high‐performance liquid chromatographic method described by Liawruangrath et al. (2001); Youngvises et al. (2003) developed a simple, fast, and versatile micellar liquid chromatographic (MLC) method for simultaneous determination of lidocaine and tolperisone hydrochloride.
Earlier methods to determine tolperisone in biological fluids, such as plasma, suffered from high variability (Miskolczi et al. 1987; Miyazaki et al. 1975). The first reliable method for HPLC determination of tolperisone in human plasma samples was published by Bae et al. in 2006. The HPLC method for quantization of tolperisone in human plasma is considered as simple, accurate, reproducible, and suitable for pharmacokinetic study of tolperisone. For the determination of the tolperisone‐related substance eperisone in human plasma, Cappiello et al. (1990) reported a capillary gas chromatography‐mass spectrometry. The liquid chromatography‐electrospray ionization‐mass spectrometry method (LC‐ESI‐MS), developed by Ding et al. (2004) provides a simple and rapid assay for detection of eperisone in plasma, suitable for pharmacokinetic studies.
Metabolism and Bioavailability
Animal experiments
The disposition of (+)‐tolperisone as well as of (−)‐tolperisone was assessed in serum of rats, following i.v. administration of the racemic mixture and of the pure stereoisomers (Yokoyama et al. 1992). Stereoselective disposition of tolperisone was studied for up to 30 min after injection in this study and found to be only partially preserved for this rather short time interval tested. Most importantly, interconversion of the two stereoisomeric forms occurred, resulting in the detection of racemic mixture following injection of either pure stereoisomeric form. In another study, where the first‐pass metabolism was investigated in rats, rapid metabolism of eperisone to an ω‐1‐hydroxylated metabolite was observed (Mihara et al. 2001), resulting in very low bioavailability when administered orally. Consequently, transdermal application in rats was found to result in more potent and longer‐lasting muscle relaxation than orally applied eperisone (Yang et al. 2004).
Results on humans
Bioavailability studies after single‐dose applications in humans also exerted fast decay of tolperisone in human plasma (half times of 1.46 h and 2.47 h, for two kinetically distinct processes; Miskolczi et al. 1987). The short live times of tolperisone were opposed by the fact that less than 0,1% of i.v. administered tolperisone are excreted within 24 h in urine and indicate rapid resorption and/or metabolism of the substance. Consequently, the pathways of tolperisone metabolism were investigated in vitro, using human liver microsomes and recombinant enzymes (Dalmadi et al. 2003a, 2003b). Formation of several metabolites was detected and the pharmaceutical synthesis of some of the most important metabolites was described (M1: Balint et al. 2000; M2: Balint et al. 2001; M3, M4 + M5: Balint et al. 2002; see Fig. 2 for an overview). Functional data on the pharmacological effects of the different metabolites are, however, still lacking.
Figure 2.
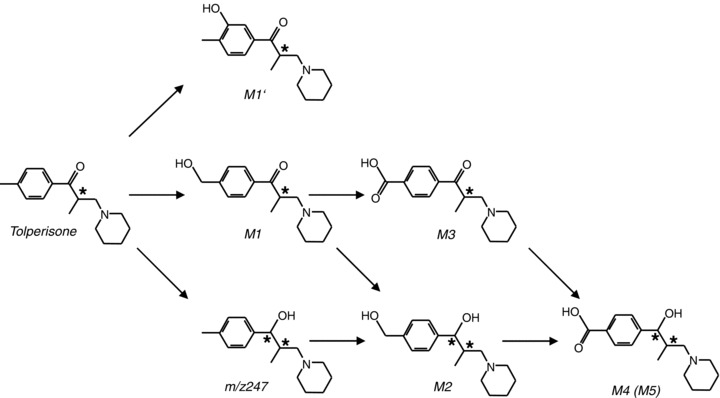
Tolperisone metabolism.
Pharmacodynamics of Tolperisone
Pharmacology in Whole Animals
Although of rather limited value for conclusions on systemic effects on humans, a vast body of work on such effects in animals exists and is worth mentioning: Initial experiments, undertaken in 1961, revealed spasmolytic properties on masseter muscle of rats, on electrically induced convulsions of the fore‐ and hind limbs of the rats and on smooth muscle of guinea pig intestine (Porszasz et al. 1961). In the same study it was shown that tolperisone inhibited the controlateral extensor reflex in cats, accompanied by hypotension. Interestingly, the drop in blood pressure was transient in its nature, whereas the depression of controlateral extensor reflex was persistent. Sedative effects depend on species and occur in rats and mice, but not in cats. In another study Furuta and Yoshikawa showed transient hypotonic action of tolperisone in anesthetized mongrel dogs, but observed another additional, secondary and prolonged hypotension at higher doses (Furuta and Yoshikawa 1976). A general increase in arterial blood flow was reported, but 90× higher doses of tolperisone were required to increase mesenteric arterial, when compared to the dose required to increase femoral arterial flow. Accordingly, at a given dose of the substance, vasodilatation of arteries of skeletal muscle was selectively induced, whereas visceral vessels were only slightly affected. Furthermore, tolperisone reduced the contractile force of isolated dog papillary muscle. Later, Furuta and Ishii described dog trachea dilatation as well as vasodilatation (Furuta and Ishii 1977). An inhibition of the tonic periodontal masseteric reflex in rats was also observed in later studies (Funakoshi and Kawamura 1986; Funakoshi and Nakashima 1982). The central action of tolperisone was addressed in a study by Farkas et al., when the effects on extracellularly recorded spinal root potentials and spinal reflex activities of spinal cats were evaluated (Farkas et al. 1989). Interestingly, the inhibition of mono‐ as well as of polysynaptic reflexes, induced by tolperisone was observed, whereas the much slower occurring dorsal root potentials were virtually unaffected. The marked inhibition of spike generation in the dorsal root was suggested to be the result of a depressant, membrane‐stabilizing mechanism of action of the drug (Ono et al. 1984). Morikawa et al. arrived at similar conclusions, based on less extensive studies (Morikawa et al. 1987). Funakoshi and Nakashima reported a reversible inhibition of the tonic periodontal masseteric reflex in rats upon intraperitoneal injection of tolperisone (Funakoshi and Nakashima 1982). In another study, the effects of tolperisone on the polysynaptic flexor reflex, mediated by group II afferent fibers in decerebrated (spinal) and intact rats were tested (Sakitama 1993). A depression was observed under both conditions, pointing at the spinal cord as important target of tolperisone action. Also in laminectomized mice, single i.v. applications of tolperisone transiently inhibited the amplitude of monosynaptic reflex potentials, indicating that tolperisone may act as a membrane stabilizing drug (Okada et al. 2001). When the effects of lidocaine, eperisone, and tolperisone on acute thermal, as well as acute mechanical nociception in mice were studied it was shown that these agents, using the plantar heating and the tail pressure test in mice, preferentially impaired acute thermal nociception over acute mechanical nociception, whereas morphine impaired both types of nociception approximately equally (Sakaue et al. 2004). In the same study, the local anesthetic action of lidocaine, eperisone, and tolperisone was assessed by showing their ability to block evoked action potentials of isolated mouse ischiatic nerve. In an extensive comparative study by Kocsis et al. the effects of tolperisone, eperisone, inaperisone, lanperisone, and silperisone on different spinal reflexes were analyzed and compared to lidocaine (Kocsis et al. 2005). It was shown that monosynaptic reflex potentials, afferent fiber potentials, and excitatory postsynaptic potential (EPSP) related potentials were significantly attenuated by all these drugs tested in an isolated, hemisected spinal cord preparation from rat in vitro. Furthermore, monosynaptic, disynaptic, and polysynaptic reflex potentials from decerebrated, laminectomized rats, recorded in vivo, were also depressed by these agents. Also motor neuron excitability as well as afferent nerve conduction were studied and seen to be attenuated by the drugs tested. Interestingly, the local anesthetic lidocaine exerted significantly stronger blocking effects on direct electrical excitability of motor neurons and primary afferents than silperisone, whereas the efficiency in inhibiting synaptic transmissions was more or less comparable. Tolperisone and the other tolperisone‐like drugs ranged in‐between lidocaine and silperisone in this respect. The muscle relaxant actions of lanperisone, tolperisone, and eperisone were tested on decerebrated rats and mice (α‐ as well as γ‐rigidity; Sakitama et al. 1995). The drugs were applied either i.v. or orally and generally had identical pharmacological properties, that is, decrease of muscle tone, induced by decerebrate rigidity. In contrast to tolperisone and eperisone, the action of lanperisone was generally longer lasting, indicating a slower metabolism of the latter drug. In the same study, tolperisone reduced the frequency of muscle spindle discharges that were also measured, whereas the other drugs exerted no effect on this parameter. Other studies where tolperisone itself was not studied, but instead at least one of the related substances was investigated also exist. In a study in anesthetized cats, eperisone was applied i.v. or i.p. directly to the lumbal spinal cord and nociceptive potentials from ganglia of the dorsal horn were recorded (Davies 1989). The result was a transient, reversible antinociceptive effect resulting from noxious or mild stimuli that was, however, not observed in all animals tested. In another study on newborn rats, partially in the entire animal or in isolated spinal cord/tail preparations, the antinociceptive effect of eperisone on mono‐ and polysynaptic reflex potentials (resulting from tail‐pinch) could be established (Ishizuki and Yanagisawa 1992). A transient, but clear, inhibition of the stretch reflex in anesthetized cats, produced by an inhibition of motor neurons from the ventral root, substantiates the central action of i.v. applied eperisone (Nakajima and Wada 1989). Renshaw cells are inhibitory interneurons found in the gray matter of the spinal cord, having feed‐forward as well as feed‐back connections with α‐motorneurons and hence are of interest for the investigation of the action of centrally acting muscle relaxants. Using decerebrated spinal cats, it was found that i.v. application of eperisone reduced the early firing rate of Renshaw cells, whereas the late firing rate was increased (Kato and Yang 1990). If and how these effects may contribute to the muscle relaxant and/or pain‐relieving action of eperisone, remains obscured. In another study, the effects of lanperisone and eperisone in different spinal reflex arches of decerebrated and intact cats and rats were tested (Sakitama et al. 1997). Generally, both drugs exerted inhibitory actions on spinal reflexes, regardless whether or not inhibitory interneurons were involved. Interestingly, the action of lanperisone was longer lasting than that of eperisone and seemed to act in addition to the spinal circuits via supraspinal elements. In order to study possible cardiac side effects, eperisone was injected into the sinus node artery of isolated canine atrium (Saegusa et al. 1991). Dose‐related negative chronotropic and inotropic effects were observed.
Although the studies mentioned above differ in scope, amount of data, and experimental design, a vast body of evidence has accumulated that clearly shows the central muscle relaxant and pain‐relieving action of tolperisone and related compounds.
Pharmacological Profile in Tissue and Cell Preparations
A first attempt to study the molecular mechanism underlying the central relaxant action of tolperisone was undertaken by Hinck and Koppenhofer, who studied the effects of tolperisone on ionic currents in the node of Ranvier of sciatic nerves of Xenopus laevis (Hinck and Koppenhofer 1997, 2001), using a modified vaseline gap method (Bohuslavizki et al. 1994). It was found that 100 μmole/L of tolperisone induced a marked and reversible depression of voltage‐dependent sodium currents. At the same concentration of tolperisone Hinck and Koppenhofer (2001) also observed effects on voltage‐dependent potassium currents that were, however, less pronounced (a slight increase of K+ permeability at weak depolarization that turned into a decrease at higher depolarization). The apparent dissociation constant for the effect on sodium channels turned out to be much lower (around 60 μmole/L) than the one for the effect on the potassium conductance (320 μmole/L). In the same study, use‐dependence of tolperisone block of sodium channels could be observed, when action potentials were recorded at different stimulation frequencies in the presence of tolperisone.
Using Nav1.6 and Nav1.8α‐subunits of voltage‐dependent sodium channels, heterologously expressed in oocytes of X. laevis, Quasthoff et al. were able to observe block of the corresponding sodium currents by tolperisone, sustaining the role of tolperisone as a direct blocker of voltage‐dependent sodium channels (Quasthoff et al. 2003). In an extensive, comparative study, Hofer et al. evaluated the effect of tolperisone on seven different isoforms of voltage‐dependent sodium channels (Hofer et al. 2006). These effects were compared to the pharmacological profile of lidocaine. Nav1.2, Nav1.3, Nav1.4, Nav1.5, Nav1.6, Nav1.7, and Nav1.8α‐subunits were heterologously expressed in oocytes of X. laevis and the corresponding sodium currents were measured with the two‐electrode‐voltage‐clamp method, utilizing agarose cushion electrodes (Schreibmayer et al. 1994). Cumulative application of both tolperisone and lidocaine resulted in marked differences in IC50s between both drugs, as well as between the different isoforms tested: For tolperisone, the lowest IC50 (49 μmole/L) was measured for Nav1.8, a voltage‐dependent sodium channel isoform known to be important in peripheral nerve. In comparison, the highest IC50 was more than one order of magnitude larger for Nav1.3 (802 μmole/L; a subunit that plays important roles in the central nervous system). Most sensitive to lidocaine was not the Nav1.8 subunit (IC50 of 128 μmole/L), as was the case for tolperisone, but instead the Nav1.2 subunit with an IC50 value of 68 μmole/L. When the effect of both drugs on the conformational transitions of the different voltage‐dependent sodium channel isoforms was tested, it turned out that tolperisone exerted pharmacokinetic properties similar to the general action of local anesthetics, that is a shift of the steady‐state availability to more negative potential, accompanied by a considerable prolongation of channel refractoriness. Kocsis et al. tested the blocking efficiency of tolperisone on sodium currents of medium‐sized dorsal root ganglia, isolated from rat pups (Kocsis et al. 2005). A shift of the steady‐state availability curve of voltage‐dependent sodium currents to more negative potentials, increasing with the tolperisone concentration, was observed. Furthermore two tolperisone‐related compounds, that is silperisone and eperisone, were also effective on sodium currents from this preparation. Local anesthetic compounds such as lidocaine exert a pronounced effect on the refractoriness especially of the Nav1.5 isoform, that is prevalent in cardiac muscle (Bean et al. 1983). The cardiac side effects that possibly result, appear indeed to belong to the major obstacles for more frequent systemic application of lidocaine and congenitors for neuropathic pain relief (Mao and Chen 2000). In direct comparison, however, marked qualitative differences in the mode of action between lidocaine and tolperisone were found: In the study by Hofer et al. a 35× prolongation of the time constant for recovery from inactivation was observed in the presence of lidocaine (from 23.1 ms to 0.80 s), whereas tolperisone did not alter the time constant of the fast recovery process at all (Hofer et al. 2006; see Fig. 3). The structural basis for the differing behavior of the two related substances remains unclear, but the finding indicates that differentiation of the antiarrhytmic action from the systemic pain reliever properties may be possible within the class of so‐called “local anesthetics” and encourages research on the further development of the tolperisone structure.
Figure 3.
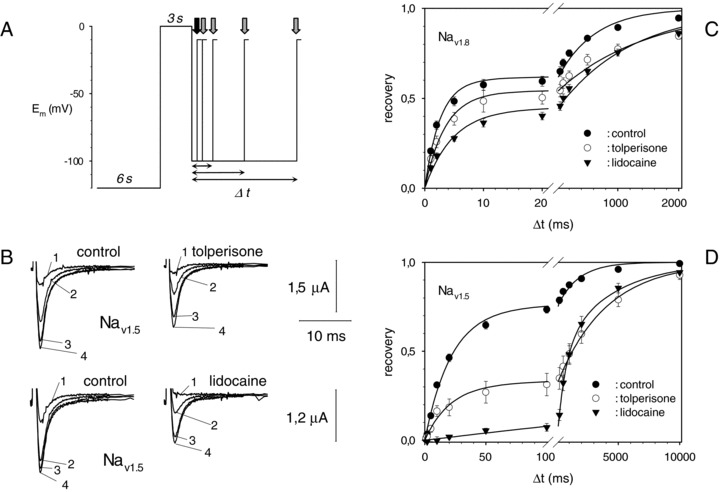
Effects of tolperisone and lidocaine on recovery kinetics of sodium channels (Reprinted from Hofer et al. (2006) with permission.). (A) Voltage jump protocol that was used to elicit sodium currents: The oocyte membrane was held at −120 mV for 6 s to allow for recovery, then steady‐state inactivation was achieved using a first suprathreshold pulse. Recovery was assessed using a second suprathreshold pulse after a variable time interval at rest (arrows). (B) Original current recordings from Nav1.5 under control conditions (left) and under the influence of 100 μmol/L tolperisone (right, upper) as well as under 300 μmol/L lidocaine (right, lower). Recovery time intervals were, (1) 10 ms; (2) 500 ms; (3) 5 s, and (4) 50 s, respectively. (C) Average recovery of Nav1.8 for control conditions ( •), 100 μmol/L tolperisone (○), and 100 μmol/L lidocaine ( ). Bars represent S.E.M. Solid lines represent a biexponential fit. Time axis was split in two regions of different scaling to better visualize the domains of the fast and the slow recovery process. (D) As in C, but for Nav1.5.
). Bars represent S.E.M. Solid lines represent a biexponential fit. Time axis was split in two regions of different scaling to better visualize the domains of the fast and the slow recovery process. (D) As in C, but for Nav1.5.
Investigations of the action of eperisone, which is structurally closely related to tolperisone, on the basilar artery of the guinea pig revealed that eperisone possesses potent vasodilating action mediated by a direct effect on smooth muscle cell excitability. Since K+ induced contractions (in the presence of external Ca2+ ions) were potently inhibited by increasing concentrations of eperisone, starting at 10 μmole/L, the authors concluded that eperisone was acting as a classical Ca2+ channel antagonist. Since eperisone acted also in the absence of extracellular Ca2+, additional, intracellular, drug targets were also suggested (Fujioka and Kuriyama 1985). Similarly, Inoue et al. found that eperisone antagonized contractions of dog saphenous arteries and veins induced by norepinephrine, serotonin, acetylcholine, K+ or Ba2+, in accordance with Ca2+ antagonistic action. (Inoue et al. 1989). Again, however, actions other than Ca2+ antagonistic ones were observed, that is potentiation of prostaglandin F2α induced contractions of the blood vessels, indicating that eperisone might have additional effects on prostaglandin synthesis. A first direct assessment of the Ca2+ antagonistic actions of eperisone, but also of tolperisone and inaperisone, was performed in a study by Novalesli et al. in 1989. These drugs blocked snail neuron Ca2+ currents in a frequency‐dependent manner, accompanied by a shift of steady‐state availability of Ca2+ channels to more negative potentials (Novalesli et al. 1989). Later on, Kocsis et al. observed direct inhibition of Ca2+ currents in dorsal root ganglia cells also from mammals (Kocsis et al. 2005). Hence, both the Na+ channel and the Ca2+ channel antagonistic actions of tolperisone and some related compounds are well established, the Ca2+ antagonistic actions occurring generally at higher concentrations, when compared to the action on Na+ channels. Additional principles of action, as those cited above (K+ channel antagonistic, antagonism to intracellular Ca2+ release, and interference with prostaglandin biosynthesis), have also been described indicating that tolperisone, like many other drugs, may exert numerous less‐specific side effects. Nevertheless, these side effects may contribute to the unique profile of action of tolperisone, when compared to other closely related structures. In this context it is worth mentioning that Slusher et al., in searching for possible cocaine antagonists, found low affinity, but clearly detectable binding of tolperisone to the plasmalemmal, presynaptic, dopamine transporter, accompanied by the inhibition of dopamine reuptake (Slusher et al. 1997). This finding is not surprising, if one considers that tolperisone was developed, starting initially from the cocaine structure.
Although of enormous importance for the further development of tolperisone‐like structures, little is known about the tolperisone receptor site on the actual target protein(s). In a molecular modeling study (Fels 1996), Fels concluded that tolperisone may well act via the so‐called local anesthetic receptor site on voltage‐dependent sodium channels (Ragsdale et al. 1994, 1996). So far, no data on direct competition between tolperisone and local anesthetics like lidocaine exist, although the synthesis of 3H‐labelled tolperisone has been described (Dietrich and Fels 1999).
Clearly, the local anesthetics receptor site on voltage‐dependent sodium channels turns out to be the most important principle of action of tolperisone and related drugs. Regarding the local anesthetics receptor site, however, several qualitative differences to the action of lidocaine exist: the effect on the fast refractoriness of the cardiac isoform and significant differences in the isoform specificity of tolperisone, when compared to lidocaine. Besides action on voltage‐dependent sodium channels, also blockage of voltage‐dependent calcium channels and event action on other drug targets, mentioned above, may contribute to the pharmacological profile of tolperisone and related compounds.
A still unresolved enigma of the action of tolperisone is the long time course of several weeks until the drug exerts relief of neuropathic pain in humans, which is in sharp contrast to the rapid metabolism of the substance within the human body. Neuronal plasticity, induced by blockage of sodium channel populations by tolperisone, or maybe even by more stable metabolites, could be responsible. In contrast to tolperisone, the onset of pain relief has been reported to occur within minutes after systemic lidocaine application. The therapeutic effect of lidocaine, however, lasts up to several weeks after single, systemic administration indicating that also in this case the exact mechanism of action is not fully understood (Mao and Chen 2000).
Toxicology and Side Effects
In general tolperisone was well tolerated in clinical use and little side effects or none are reported in the clinical studies. However, some adverse effects like muscle pain, generalized body weakness, fatigue, and dizziness were recorded in patients taking the drug but all were minor and self‐limited, none requiring discontinuation of treatment. Nevertheless 13 cases of more severe side effects like allergic reactions to tolperisone have been documented in the literature. The first case was described in 1974 in a Russian journal but did not attract much attention (Aleksandrov 1974). More recently, two other case reports of severe anaphylaxis have been published. The case of anaphylactic shock due to tolperisone administration was presented in a 49‐year‐old woman who suffered from spinal osteoarthritis. She was treated with NSAIDs and tolperisone for many years. Six weeks before the admission to hospital the first anaphylactic shock was developed with loss of consciousness after oral administration of tolperisone. Percutaneous test with tolperisone was performed and it caused anaphylactic shock (Kwasniewski 2003). Four patients with anaphylaxis attributed to the intake of the centrally acting muscle relaxant tolperisone hydrochloride were observed at the Emergency Department of the Geneva University Hospital between November 2001 and March 2003. All patients were middle‐aged women who took tolperisone for chronic muscular pain. All reactions occurred within an hour after oral intake of this drug frequently prescribed in Switzerland. The severity of anaphylaxis ranged from urticarial reactions to shock with arterial hypotension (Ribi et al. 2003). Anaphylactic reactions to this drug are also mentioned in the WHO drug reaction database (http://www.who‐umc.org/). Together, these findings suggest that anaphylaxis to tolperisone is not uncommon and should be known to physicians.
Use on Humans
Experimental Conditions
A number of experimental conditions have been investigated with variable dosage of tolperisone. These studies were aimed to determine the site of action (centrally, spinal, or on the muscle itself) of the substance in a clinical setting. First, the sedative effects of single and repeated doses of 50 mg and 150 mg tolperisone hydrochloride (Mydocalm) were evaluated in a placebo‐controlled double‐blind clinical trial. A total of 72 healthy young adults were randomized to receive 50 mg or 150 mg tolperisone hydrochloride or placebo t.i.d. for a period of 8 days. Control examinations were performed in the mornings of days 1 and 8 before the intake of the morning dose and at 1.5, 4, and 6 h postdose. The psychomotoric test battery used in this trial revealed no sedative effects of tolperisone hydrochloride in the given doses at any control examination. The lack of differences in sedative potentials of tolperisone hydrochloride and placebo was confirmed. The study substantiates clinical experience and previous clinical trials demonstrating that tolperisone hydrochloride, although being a centrally active muscle relaxant, does not cause any sedation and does not impair reaction times (Dulin et al. 1998). Another randomized, double‐blind, placebo‐controlled three‐way crossover study was performed to investigate the effect of the muscle relaxants tolperisone hydrochloride on experimental jaw‐muscle pain and jaw‐stretch reflexes. Fifteen healthy men participated in three randomized sessions separated by at least 1 week. In each session 300 mg tolperisone or placebo was administered orally as a single dose. One hour after drug administration 0.3 mL hypertonic saline (5.8%) was injected into the right masseter to produce muscle pain. Subjects continuously rated their perceived pain intensity on an electronic 10‐cm visual analogue scale (VAS). The pressure pain threshold (PPT) was measured and short‐latency reflex responses were evoked in the precontracted masseter and temporalis muscles by a standardized stretch device before, 1 h after medication, during ongoing experimental muscle pain, and 15 min after pain had vanished. Analysis of variance demonstrated significantly lower VAS peak pain scores after administration of tolperisone hydrochloride compared with placebo. In conclusion, tolperisone hydrochloride provided a small, albeit significant reduction in the perceived intensity of experimental jaw‐muscle pain whereas the present dose had no effect on the short‐latency jaw‐stretch reflex (Svensson et al. 2003). A third study investigated the role of tolperisone hydrochloride, the primarily centrally acting muscle relaxant in relieving painful muscle spasm. The study hypothesizes that the prophylactic use of tolperisone hydrochloride may effectively relieve postexercise muscle soreness, based on the spasm theory of exercise pain. This study was of special interest, since no information about the clinical effects of tolperisone on the muscle itself was known. Twenty male volunteers participated in 10 sessions in which they received oral treatment with placebo or tolperisone hydrochloride (150 mg) three times daily for 8 days, in randomized crossover double‐blind design. Time course assessments were made for PPT, Likert's pain score (0–5), pain areas, range of abduction, isometric force, and electromyography (EMG) root mean square (RMS) during maximum voluntary isometric force on days 1 and 6, immediately after an eccentric exercise of first dorsal interosseous muscle, and 24 and 48 h after the exercise. Treatment with placebo or tolperisone hydrochloride was initiated immediately after the assessments on the first day baseline assessments. On the sixth day baseline investigations were repeated and then the subjects performed six bouts of standardized intense eccentric exercise of first dorsal interosseous muscle for provocation of postexercise muscle soreness. Perceived intensity of warmth, tiredness, soreness, and pain during the exercise bouts were recorded on a 10‐cm visual analogue pain scale. VAS scores and PPTs did not differ between tolperisone and placebo treatment. All VAS scores increased during the exercise bouts 2, 3, 4, 5, and 6 as compared to bout 1. Increased pain scores and pain areas were reported immediately after, and 24 and 48 h after exercise. PPTs were reduced at 24 and 48 h after the exercise in the exercised hand. The EMG RMS amplitude was also reduced immediately after the exercise, but was increased at 24 and 48 h. Isometric force was reduced immediately after the exercise as compared to days 1 and 6, and the 24 and 48 h postexercise assessments with a greater reduction following the tolperisone hydrochloride treatment and the reduction was more in tolperisone group as compared to the placebo group. These results suggested that the prophylactic intake of tolperisone hydrochloride provides no relief to pain in course of postexercise muscle soreness but results in reduction in isometric force (Bajaj et al. 2003).
Clinical Trials
First reports about the clinical use of tolperisone (Mydocalm) appear in the early seventies describing the effect of the substance on spastic muscle, myotonia, and in peripheral arterial disease (Dobi 1961; Lehoczky 1961; Molnar 1962; Solti 1961; for overview see Table 1). Most publications are case reports that have been published in Hungarian journals since the substance was developed in Hungary. Since then more than 130 publications concerning the substance can be found on the internet. Most papers describe clinical applications of the substance in different clinical settings and diseases. However, only three randomized, double‐blind, placebo‐controlled studies in diseases have been published in international reviewed journals (Pain, Ethiop J. Med., and Eur. J. Neurol.). A total of 330 patients have been included in these three studies. The most recent study evaluated the effects of tolperisone (300–900 mg) during 12 weeks, on the degree of spasticity following a stroke (Stamenova et al. 2005). About 78.3% of the patients on tolperisone versus 45% of the placebo patients experienced a reduction by at least 1 point on the Ashworth spasticity Scale (P < 0.0001). Functional and overall assessments of efficacy confirmed superior efficacy of tolperisone. Adverse events occurred less often on active treatment (n = 19) than on placebo (n = 26) and were mostly of mild‐to‐moderate intensity. The findings of this study demonstrate the efficacy and excellent tolerance of tolperisone in the treatment of spastic hypertonia following cerebral stroke. The authors suggest that an individual dose titration that may exceed the recommended maximum dose of 450 mg daily results in an optimized therapeutic benefit. Another clinical trail describes the symptomatic treatment of neurolathyrism with tolperisone HCl (Mydocalm; Melka et al. 1997). The efficacy and safety of oral Tolperisone was evaluated in a double‐blind, placebo‐controlled, randomized trial in 72 patients with neurolathyrism. Taken orally daily for 12 weeks, tolperisone in a dose of 150 mg twice daily significantly improved subjective complaints such as muscle cramps, heaviness of the legs, startle attacks, flexor spasms, and repeated falls. An overall subjective improvement was observed in 75% of the patients on tolperisone HCl and 39% of the placebo group (P= 0.002). When objectively assessed spastic muscle tone was significantly reduced in tolperisone HCl group. Walking ability and speed of walking was also significantly improved. Some adverse effects such as muscle pain, generalized body weakness, and dizziness were recorded in patients taking the drug but all were minor and self‐limited, none requiring discontinuation of treatment. The efficacy and tolerance of repeated oral dose of tolperisone was investigated in the treatment of painful reflex muscle spasm (Pratzel et al. 1996). In this prospective, randomized, double‐blind, placebo‐controlled trial a total of 138 patients, aged between 20 and 75 years, with painful reflex muscle spasm associated with diseases of the spinal column or proximal joints were included. Patients were randomized to receive either 300 mg tolperisone hydrochloride or placebo for a period of 21 days. Both treatment groups recovered during the 3 weeks rehabilitation program. However, tolperisone hydrochloride proved to be significantly superior to placebo: the change score of the PPT as the primary target parameter significantly increased during therapy with tolperisone hydrochloride (P= 0.03, valid‐case‐analysis) compared to the results obtained on placebo treatment. The overall assessment of efficacy by the patient also demonstrated significant differences in favor of tolperisone hydrochloride. Adverse events, biochemical and hematological laboratory parameters, demonstrated no differences between tolperisone hydrochloride and placebo. As a conclusion tolperisone hydrochloride represents an effective and safe treatment of central post stroke spasticity, neurolathyrism, and painful reflex muscle spasm without the typical side effects of centrally active muscle relaxants. Beside the above‐mentioned clinical trials in post stroke pain, treatment of neurolathyrism and painful reflex muscle spasm, the use of tolperisone has been described in other clinical conditions such as central spinal pain, neuropathic pain, peripheral vascular disease, multiple sclerosis, tension headache, and myotonias. Altogether, these clinical conditions are quite frequent. An additional pharmacological tool to relieve pain in these conditions would be more than welcome. However, the poor quality (no randomization, not blinded, no cross over, low number of study subjects, case reports, personal observations) of the studies in this field makes it difficult to give a general recommendation about the use of tolperisone in these various clinical conditions. Taken the results from the preclinical studies one would expect to see a clinical meaningful benefit of tolperisone in chronic pain conditions (low‐back pain, cervical pain, fibromyalgia), neuropathic pain as well as myotonias. One can hypothesize that in clinical routine in Europe, similar to the use of the topical application of the lidocaine patch in various clinical conditions (e.g. low back pain) in the US, tolperisone will already be used in the above‐mentioned conditions, without clear evidence from high‐standard clinical trials.
Table 1.
Clinical conditions and diseases were tolperisone was studied.
| Clinical condition or disease | Quality of the study | Tolperisone dosage | Reference |
|---|---|---|---|
| Low back pain | B | 150–400 mg per day | (Chernysheva and Bagirova 2005)a |
| (Vorob'eva and Kozlova 2006) | |||
| Post cerebral stroke spasticity | A | 300–900 mg per day | (Stamenova et al. 2005) |
| (Stamenova et al. 2006) | |||
| Spinal pain | C | 300 mg | (Parfenov and Batysheva 2003) |
| Neuropathic diabetic foot syndrome | B | 150–400 mg | (Briskin et al. 2000a) |
| Painful reflex muscle spasm (including cervical and low back pain) | A | 300 mg per day | (Pratzel et al. 1996) |
| Peripheral vascular disease | C | Unknown | (Liubishchev 1967) |
| (Sztankay 1970) | |||
| (Abranyi 1988) | |||
| (Briskin et al. 2000b) | |||
| Multiple sclerosis | C | Unknown | (Lashch and Avakian 2000) |
| Neurolathyrism | A | 300 mg per day | (Melka et al. 1997) |
| Myotonias | C | Unknown | (Abranyi 1988) |
| Tension headache | C | 150–900 mg | (Csanyi 1989)b |
| (Solozhenkin 1999) | |||
| (Solovieva et al. 2005) |
A: High standard clinical trial, placebo‐controlled, randomized, double blind.
B: Medium standard clinical trial, to some extend placebo‐controlled and randomized.
C: Low standard clinical trial, no placebo‐controlled, not randomized, not blinded, case studies
aNo abstract available.
bReview.
A general recommendation of the optimal dosage of tolperisone (Mydocalm) in clinical practice is difficult to give since it will depend on the clinical condition and disease that is to be influenced by the drug. Not only is there a wide range of dosages used in the clinical trials (150–900 mg per day) but also considerable interindividual variation in the pharmacokinetics of tolperisone HCl that have been reported. This was investigated in one clinical trial that was aimed to determine the pharmacokinetic profiles of oral tolperisone hydrochloride in healthy volunteers. After the oral administration of tolperisone hydrochloride, the plasma concentrations of tolperisone were measured. The tolperisone concentration was determined using high‐performance liquid chromatography. Very large interindividual differences in the area under the curve (AUC) and the maximum concentration of a drug in the body after dosing (Cmax) were detected after oral tolperisone HCl. These results suggest that the pharmacological effect of oral tolperisone HCl varies between individuals, and the oral tolperisone HCl dose might need to be individualized (Bae et al. 2007).
Future Perspectives
There is a need for additional placebo‐controlled high‐standard clinical trials that are able to demonstrate the effectiveness of tolperisone in chronic pain conditions such as chronic low‐back pain, fibromyalgia, and neuropathic pain. Although voltage‐dependent sodium channels emerge as the principal molecular target of tolperisone action, the exact mechanism of tolperisone action still remains obscured, especially when the rapid decay that is in apparent contradiction with the rather slow onset of therapeutic action is considered.
Structural and mechanistical similarities between tolperisone and other “lidocaine‐like” drugs exist, but the exact mode of action of tolperisone and the isoform‐specific profile of action encourage further development of the tolperisone structure.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
The authors are grateful to Dr. T. DeVaney (Graz) for improving the English language of the manuscript. Research of the authors is supported by the Austrian National bank (OENB12575; WS).
References
- Abranyi I (1988) Study of the effect of 150 mg Mydocalm coated tablets in peripheral vascular diseases and in myotonias of various origin. Ther Hung 36:56–61. [PubMed] [Google Scholar]
- Aleksandrov ISDLN (1974) Case of an allergic reaction to mydocalm. Klin Med (Mosk) 52:142. [PubMed] [Google Scholar]
- Armstrong DW, Chang CD, Lee SH (1991) (R)‐naphthylethylcarbamate‐substituted and (S)‐naphthylethylcarbamate‐substituted beta‐cyclo‐dextrin bonded stationary phases for the reversed‐phase liquid‐chromatographic separation of enantiomers. J Chromatogr 539:83–90. [Google Scholar]
- Bae JW, Kim MJ, Park YS, Myung CS, Jang CG, Lee SY (2007) Considerable interindividual variation in the pharmacokinetics of tolperisone HCl. Int J Clin Pharmacol Therapeutics 45:110–113. [DOI] [PubMed] [Google Scholar]
- Bae JW, Park YS, Sohn UD, Myung CS, Ryu BK, Jang CG, Lee SY (2006) HPLC determination of tolperisone in human plasma. Arch Pharmacal Res 29:339–342. [DOI] [PubMed] [Google Scholar]
- Bajaj P, Rendt‐Nielsen L, Madeleine P, Svensson P (2003) Prophylactic tolperisone for post‐exercise muscle soreness causes reduced isometric force—a double‐blind randomized crossover control study. Euro J Pain 7:407–418. [DOI] [PubMed] [Google Scholar]
- Balint J, Egri G, Markovits I, Czugler M, Marthi K, Demeter A, Temesvari‐Takacs K, Fogassy E (2002) Synthesis of some tolperisone metabolites in racemic and optically active form. Tetrahedron-Asymmetry 12:3417–3422. [Google Scholar]
- Balint J, Hell Z, Markovits I, Parkanyi L, Fogassy E (2000) Synthesis and resolution of a Tolperisone metabolite. Tetrahedron-Asymmetry 11:1323–1329. [Google Scholar]
- Balint J, Markovits I, Egri G, Tuza Z, Parkanyi L, Fogassy E (2001) Synthesis, resolution and absolute configuration of a tolperisone metabolite. Tetrahedron-Asymmetry 12:719–724. [Google Scholar]
- Bean BP, Cohen CJ, Tsien RW (1983) Lidocaine block of cardiac sodium channels. J Gen Physiol 81:613–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohuslavizki KH, Kneip A, Koppenhofer E (1994) State‐of‐the‐art potential clamp device for myelinated nerve‐fibers using a new versatile input probe. Gen Physiol Biophys 13:357–376. [PubMed] [Google Scholar]
- Briskin BS, Sakunova TI, Iakobishvili II (2000a) Role of midocalm in combined treatment of neuropathic diabetic foot syndrome. Khirurgiia (Mosk) 5:34–37. [PubMed] [Google Scholar]
- Briskin BS, Sakunova TI, Iakobishvili II (2000b) Treatment of chronic arterial insufficiency of legs with midocalm in elderly. Khirurgiia (Mosk) 4:52–54. [PubMed] [Google Scholar]
- Cappiello A, Mangani F, Palma P, Sisti E, Bruner F (1990) Sub Ppb level determination of eperison in human plasma by Gc/Ms. Chromatographia 30:357–360. [Google Scholar]
- Chernysheva TV, Bagirova GG (2005) Midocalm in complex therapy of chronic low back pain syndrome. Klin Med (Mosk) 83:45–49. [PubMed] [Google Scholar]
- Csanyi L (1989) High‐dose Mydocalm therapy in certain myogenous headaches. Ther Hung 37:115–118. [PubMed] [Google Scholar]
- Dalmadi B, Leibinger J, Borbas T, Szeberenyi S, Tihanyi K (2003a) Kinetic characterization of tolperisone hydroxylation by human microsomal enzymes. Drug Metabol Rev 35:118. [DOI] [PubMed] [Google Scholar]
- Dalmadi B, Leibinger J, Szeberenyi S, Borbas T, Farkas S, Szombathelyi Z, Tihanyi K (2003b) Identification of metabolic pathways involved in the biotransformation of tolperisone by human microsomal enzymes. Drug Metabol Dispos 31:631–636. [DOI] [PubMed] [Google Scholar]
- Davies J (1989) Effects of tizanidine, eperisone and afloqualone on feline dorsal horn neuronal responses to peripheral cutaneous noxious and innocuous stimuli. Neuropharmacology 28:1357–1362. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Fels G (1999) Synthesis of H‐3‐tolperisone. J Label Comp Radiopharmaceut 42:1125–1134. [Google Scholar]
- Ding L, Wei X, Zhang SQ, Sheng JP, Zhang YD (2004) Rapid and sensitive liquid chromatography‐electrospray ionization‐mass spectrometry method for the determination of eperisone in human plasma: Method and clinical applications. J Chromatogr Sci 42:254–258. [DOI] [PubMed] [Google Scholar]
- Dobi S (1961) Mydocalm and muscle tone. Ther Hung 9:15–18. [PubMed] [Google Scholar]
- Dulin J, Kovacs L, Ramm S, Horvath F, Ebeling L, Kohnen R (1998) Evaluation of sedative effects of single and repeated doses of 50 mg and 150 mg tolperisone hydrochloride. Results of a prospective, randomized, double‐blind, placebo‐controlled trial. Pharmacopsychiatry 31:137–142. [DOI] [PubMed] [Google Scholar]
- Farkas S (2006) Silperisone: A centrally acting muscle relaxant. CNS Drug Rev 12:218–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas S, Tarnawa I, Berzsenyi P (1989) Effects of some centrally acting muscle‐relaxants on spinal root potentials—a comparative‐study. Neuropharmacology 28:161–173. [DOI] [PubMed] [Google Scholar]
- Fels G (1996) Tolperisone: Evaluation of the lidocaine‐like activity by molecular modeling. Arch Pharm 329:171–178. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Kuriyama H (1985) Eperisone, an antispastic agent, possesses vasodilating actions on the guinea‐pig basilar artery. J Pharmacol Exp Therapeutics 235:757–763. [PubMed] [Google Scholar]
- Funakoshi M, Kawamura S (1986) Effects of tolperisone‐Hcl on the silent period of the masseter. J Dental Res 65:592. [PubMed] [Google Scholar]
- Funakoshi M, Nakashima M (1982) Effects of tolperisone hydrochloride on the jaw muscle activities in the rat. J Dental Res 61:596. [Google Scholar]
- Furuta Y, Ishii Y (1977) Effect of Tolperisone on Insitu blood‐perfused dog trachea. Jap J Pharmacol 27:71. 864884 [Google Scholar]
- Furuta Y, Yoshikawa A (1976) Reversible adrenergic alpha‐receptor blocking action of 2,4′‐Dimethyl‐3‐Piperidino‐propiophenone (Tolperisone). Jap J Pharmacol 26:543–550. [DOI] [PubMed] [Google Scholar]
- Gazdag M, Szepesi G, Nyiredy S (1978) Determination of tolperisone in pharmaceutical formulations by G. 1. C. Pharmazie 33:538–539. [PubMed] [Google Scholar]
- Haginaka J, Kagawa C, Matsunaga H (1999) Separation of enantiomers on a chiral stationary phase based on ovoglycoprotein—VII. Comparison of chiral recognition ability of ovoglycoprotein from chicken and Japanese quail egg whites. J Chromatogr A 858:155–165. [DOI] [PubMed] [Google Scholar]
- Hinck D, Koppenhofer E (1997) Effects of tolperisone on the excitation process in myelinated axons. Pflugers Archiv-Euro J Physiol 433:503. [Google Scholar]
- Hinck D, Koppenhofer E (2001) Tolperisone—a novel modulator of ionic currents in myelinated axons. Gen Physiol Biophys 20:413–429. [PubMed] [Google Scholar]
- Hofer D, Lohberger B, Steinecker B, Schmidt K, Quasthoff S, Schreibmayer W (2006) A comparative study of the action of tolperisone on seven different voltage dependent sodium channel isoforms. Euro J Pharmacol 538:5–14. [DOI] [PubMed] [Google Scholar]
- Inoue S, Bian K, Okamura T, Okunishi H, Toda N (1989) Mechanisms of action of eperisone on isolated dog saphenous arteries and veins. Jap J Pharmacol 50:271–282. [DOI] [PubMed] [Google Scholar]
- Ishizuki M, Yanagisawa M (1992) Antinociceptive effects of tizanidine, diazepam and eperisone in isolated spinal‐cord tail preparations of newborn rat. Pain 48:101–106. [DOI] [PubMed] [Google Scholar]
- Kato M, Yang YH (1990) Effects of eperisone on Renshaw Cells of cats. Neurosci Lett 112:246–250. [DOI] [PubMed] [Google Scholar]
- Kocsis P, Farkas S, Fodor L, Bielik N, Than M, Kolok S, Gere A, Csejtei M, Tarnawa I (2005) Tolperisone‐type drugs inhibit spinal reflexes via blockade of voltage‐gated sodium and calcium channels. J Pharmacol Exp Therapeutics 315:1237–1246. [DOI] [PubMed] [Google Scholar]
- Kwasniewski AK‐GBMS (2003) Mydocalm causing anaphylaxis. Pneumonol Alergol Pol 71:250–252. [PubMed] [Google Scholar]
- Lashch NI, Avakian GN (2000) The use of midokalm in patients with multiple sclerosis. Zh Nevrol Psikhiatr Im S S Korsakova 100:24–28. [PubMed] [Google Scholar]
- Lehoczky T (1961) Mydocalm, a new drug to relax spastic muscles in neurological diseases. Ther Hung 10–13. [PubMed] [Google Scholar]
- Liawruangrath S, Liawruangrath B (1999) High performance thin layer chromatographic determination of tolperisone hydrochloride. J Pharmac Biomed Anal 20:401–404. [DOI] [PubMed] [Google Scholar]
- Liawruangrath S, Liawruangrath B, Pibool P (2001) Simultaneous determination of tolperisone and lidocaine by high performance liquid chromatography. J Pharmac Biomed Anal 26:865–872. [DOI] [PubMed] [Google Scholar]
- Liubishchev SA (1967) The use of mydocalm in the treatment of vascular diseases of the extremities. Klin Med (Moscow) 45:100–102. [PubMed] [Google Scholar]
- Mao JR, Chen LL (2000) Systemic lidocaine for neuropathic pain relief. Pain 87:7–17. [DOI] [PubMed] [Google Scholar]
- Matsunaga H, Sadakane Y, Haginaka J (2003) Separation of basic drug enantiomers by capillary electrophoresis using chicken alpha(1)‐acid glycoprotein: Insight into chiral recognition mechanism. Electrophoresis 24:2442–2447. [DOI] [PubMed] [Google Scholar]
- Melka A, TekleHaimanot R, Lambien F (1997) Symptomatic treatment of neurolathyrism with Tolperizone HCL (Mydocalm): A randomized double blind and placebo controlled drug trial. Ethiop Med J 35:77–91. [PubMed] [Google Scholar]
- Mihara K, Matsumura M, Yoshioka E, Hanada K, Nakasa H, Ohmori S, Kitada M, Ogata H (2001) Intestinal first‐pass metabolism of eperisone in the rat. Pharmac Res 18:1131–1137. [DOI] [PubMed] [Google Scholar]
- Miskolczi P, Vereczkey L, Frenkl R (1987) Gas‐liquid‐chromatographic method for the determination of tolperisone in human‐plasma—pharmacokinetic and comparative bioavailability studies. J Pharmac Biomed Anal 5:695–700. [DOI] [PubMed] [Google Scholar]
- Miyazaki H, Ishibashi M, Izawa T, Takayama H, Idzu G (1975) Mass fragmentographic determination of 1‐piperidino‐2,4′‐dimethyl‐propiophenone(mydocalm) by use of 1/1 mixture technique. Chem Pharmac Bull 23:837–843. [DOI] [PubMed] [Google Scholar]
- Molnar L (1962) Successful use of mydocalm in peripheral arterial disease. Ther Hung 10:10–12. [PubMed] [Google Scholar]
- Morikawa K, Oshita M, Yamazaki M, Ohara N, Mizutani F, Kato H, Ito Y, Kontani H, Koshiura R (1987) Pharmacological studies of the new centrally acting muscle‐relaxant 4′‐Ethyl‐2‐Methyl‐3‐Pyrrolidinopropiophenone hydrochloride 616. Arzneimittel-Forschung/Drug Res 37:331–336. [PubMed] [Google Scholar]
- Nakajima Y, Wada N (1989) Effects of eperisone‐Hcl on the stretch reflex in anesthetized cats. Jap J Pharmacol 51:572–578. [DOI] [PubMed] [Google Scholar]
- Novalesli P, Sun XP, Takeuchi H (1989) Suppression of calcium current in a snail neuron by eperisone and its analogs. Eur J Pharmacol 168:299–305. [DOI] [PubMed] [Google Scholar]
- Okada H, Honda M, Ono H (2001) Method for recording spinal reflexes in mice: Effects of thyrotropin‐releasing hormone, DOI, tolperisone and baclofen on monosynaptic spinal reflex potentials. Jap J Pharmacol 86:134–136. [DOI] [PubMed] [Google Scholar]
- Ono H, Fukuda H, Kudo Y (1984) Mechanisms of depressant action of muscle‐relaxants on spinal reflexes—participation of membrane stabilizing action 616. J Pharmacobio-Dyn 7:171–176. [DOI] [PubMed] [Google Scholar]
- Parfenov VA, Batysheva TT (2003) Spinal pain and its treatment with midocalm. Ter Arkh 75:82–83. [PubMed] [Google Scholar]
- Porszasz J, Barankay T, Nador K, Gibiszer K (1961) Pharmakologie einer neuen interneuron‐lahmenden substanz 1‐Piperidino‐2‐Methyl‐3‐(P‐Tolyl)‐Propan‐3‐on. Arzneimittel-Forschung-Drug Res 11:257–260. [Google Scholar]
- Pratzel HG, Alken RG, Ramm S (1996) Efficacy and tolerance of repeated oral doses of tolperisone hydrochloride in the treatment of painful reflex muscle spasm: Results of a prospective placebo‐controlled double‐blind trial. Pain 67:417–425. [DOI] [PubMed] [Google Scholar]
- Quasthoff S, Pojer C, Mori A, Hofer D, Liebmann P, Kieseier BC, Schreibmayer W (2003) No blocking effects of the pentapeptide QYNAD on Na+ channel subtypes expressed in Xenopus oocytes or action potential conduction in isolated rat sural nerve. Neurosci Lett 352:93–96. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA (1994) Molecular determinants of state‐dependent block of Na+ channels by local anesthetics. Science 265:1724–1728. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA (1996) Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage‐gated Na+ channels. Proc Natl Acad Sci U.S.A. 93:9270–9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribi C, Vermeulen C, Hauser C (2003) Anaphylactic reactions to tolperisone (Mydocalm (R)). Swiss Medical Weekly 133:369–371. [DOI] [PubMed] [Google Scholar]
- Saegusa K, Furukawa Y, Akahane K, Haniuda M, Chiba S (1991) Anti‐nicotinic and anti‐muscarinic actions of eperisone in the isolated canine atrium. Jap J Pharmacol 56:187–193. [DOI] [PubMed] [Google Scholar]
- Sakaue A, Honda M, Tanabe M, Ono H (2004) Antinociceptive effects of sodium channel‐blocking agents on acute pain in mice. J Pharmacol Sci 95:181–188. [DOI] [PubMed] [Google Scholar]
- Sakitama K (1993) The effects of centrally acting muscle‐relaxants on the intrathecal noradrenaline‐induced facilitation of the flexor reflex mediated by group‐Ii afferent‐fibers in rats. Jap J Pharmacol 63:369–376. [DOI] [PubMed] [Google Scholar]
- Sakitama K, Ozawa Y, Aoto N, Nakamura K, Ishikawa M (1995) Pharmacological properties of Nk433: A new centrally acting muscle‐relaxant. Euro J Pharmacol 273:47–56. [DOI] [PubMed] [Google Scholar]
- Sakitama K, Ozawa Y, Aoto N, Tomita H, Ishikawa M (1997) Effects of a new centrally acting muscle relaxant, NK433 (lanperisone hydrochloride) on spinal reflexes. Euro J Pharmacol 337:175–187. [DOI] [PubMed] [Google Scholar]
- Schreibmayer W, Lester HA, Dascal N (1994) Voltage clamping of xenopus‐laevis oocytes utilizing agarose‐cushion electrodes. Pflugers Archiv-Euro J Physiol 426:453–458. [DOI] [PubMed] [Google Scholar]
- Slusher BS, Tiffany CW, Olkowski JL, Jackson PF (1997) Use of identical assay conditions for cocaine analog binding and dopamine uptake to identify potential cocaine antagonists. Drug Alcohol Depen 48:43–50. [DOI] [PubMed] [Google Scholar]
- Solovieva AD, Akarachkova ES, Gordeev SA (2005) A study of mydocalm efficiency in the treatment of chronic headache of tension. Zh Nevrol Psikhiatr Im S S Korsakova 105:13–17. [PubMed] [Google Scholar]
- Solozhenkin V (1999) Cavinton and mydocalm in combined therapy of anxiety disorders and tension headaches. Zh Nevrol Psikhiatr Im S S Korsakova 99:46. [PubMed] [Google Scholar]
- Solti F (1961) Use of Mydocalm in influencing the effect of muscle tremor on the ECG. Ther Hung 9:31–32. [PubMed] [Google Scholar]
- Stamenova P, Koytchev R, Kuhn K, Hansen C, Horvath F, Ramm S, Pongratz D (2005) A randomized, double‐blind, placebo‐controlled study of the efficacy and safety of tolperisone in spasticity following cerebral stroke. Euro J Neurol 12:453–461. [DOI] [PubMed] [Google Scholar]
- Stamenova P, Koytchev R, Kuhn K, Hansen C, Horvath F, Ramm S, Pongratz D (2006) A randomized, double blind, placebo‐controlled study of the efficacy and safety of tolperisone in spasticity following cerebral stroke. Zh Nevrol Psikhiatr Im S S Korsakova 106:34–42. [PubMed] [Google Scholar]
- Svensson P, Wang KL, Rendt‐Nielsen L (2003) Effect of muscle relaxants on experimental jaw‐muscle pain and jaw‐stretch reflexes: A double‐blind and placebo‐controlled trial. Euro J Pain 7:449–456. [DOI] [PubMed] [Google Scholar]
- Sztankay C (1970) Treatment of vascular diseases with Mydocalm. Ther Hung 18:121–124. [PubMed] [Google Scholar]
- Tsukamoto T, Ushio T, Haginaka J (1997) Separation of basic drug enantiomers by capillary electrophoresis with new glycosaminoglycan. Chem Lett 589–590. [Google Scholar]
- Tsukamoto T, Ushio T, Haginaka J (1999) Chiral resolution of basic drugs by capillary electrophoresis with new glycosaminoglycans. J Chromatogr A 864:163–171. [DOI] [PubMed] [Google Scholar]
- Velmurugan T, Ching CB, Ng SC, Bai ZW, Ong TT (2002) Optimization of the reversed‐phase high‐performance liquid chromatographic separation of the enantiomers of a cationic chiral drug (tolperisone) on a heptakis(6‐azido‐6‐deoxy) perphenylcarbamated beta‐cyclodextrin column. Chromatographia 56:229–232. [Google Scholar]
- Vorob'eva OV, Kozlova IM (2006) Comparative efficiency of bakloferon and tolperizon central muscle relaxants in complex therapy of back pain. Voen Med Zh 327:52–55. [PubMed] [Google Scholar]
- Welch CJ, Szczerba T, Perrin SR (1997) Some recent high‐performance liquid chromatography separations of the enantiomers of pharmaceuticals and other compounds using the Whelk‐O 1 chiral stationary phase. J Chromatogr A 758:93–98. [Google Scholar]
- Yang SI, Park HY, Lee SH, Lee SJ, Han OY, Lim SC, Jang CG, Lee WS, Shin YH, Kim JJ, et al. (2004) Transdermal eperisone elicits more potent and longer‐lasting muscle relaxation than oral eperisone. Pharmacology 71:150–156. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Fukuda K, Mori S, Ogawa M, Nagasawa K (1992) Determination of tolperisone enantiomers in plasma and their disposition in rats. Chem Pharmac Bull 40:272–274. [DOI] [PubMed] [Google Scholar]
- Youngvises N, Liawruangrath B, Liawruangrath S (2003) Simultaneous micellar LC determination of lidocaine and tolperisone. J Pharmac Biomed Anal 31:629–638. [DOI] [PubMed] [Google Scholar]


