Abstract
The membrane dopamine transporter (DAT) has a pivotal role in the regulation of dopamine (DA) neurotransmission involved in a number of physiological functions and brain disorders. Molecular imaging techniques, such as positron emission tomography (PET) and single photon emission computerized tomography (SPECT), are relevant tools to explore the DAT, and we developed the cocaine derivative N‐(3‐iodopro‐2E‐enyl)‐2β‐carbomethoxy‐3β‐(4′‐methylphenyl) nortropane (PE2I) that has proved to be a very potent radiopharmaceutical to image the DAT by these techniques.
Several methods are available to obtain PE2I labeled with iodine‐123 or ‐125, carbon‐11 and tritium. The pharmacological properties of PE2I have demonstrated that it has good affinity for the DAT (4 nM) and is one of the most selective DAT ligands. [125I]PE2I characterized postmortem in human brains has revealed very intense and selective binding in the basal ganglia. Ex vivo autoradiography in rats has shown that high level of [125I]PE2I accumulates in the striatum and also in the substantia nigra and ventral tegmental area. [125I]PE2I accumulation in the rat striatum is rapid, high, and selective, providing a maximum striatum/cerebellum ratio of 10 during the first 30 min post injection. Using SPECT or PET, rapid, high, and selective accumulation of PE2I was found in the caudate nucleus and putamen in monkeys, whereas rapid wash out from the cerebellum was observed. In vivo investigations in healthy humans have demonstrated that PE2I has high striatal uptake, low nonspecific binding, low radiation exposure, and a fairly short scanning time.
A number of findings in various animal models of Parkinson's disease in rats and monkeys have demonstrated the high efficacy of PE2I for detection of reduction in the density of DAT, thus showing the potential value of PE2I for early diagnosis and evaluation of treatment of this disease. The excellent properties of PE2I are basis for the development of new DAT tracers for use in future PET explorations using fluor‐18.
Keywords: PE21, Brain, Dopamine, Dopamine transporter, DAT, Imaging, Parkinson's disease, PET, SPECT
Introduction
Dopamine (DA) is a major central nervous system (CNS) neurotransmitter involved in the mediation and/or control of a variety of physiological functions. DA, which does not cross the blood‐brain barrier, is synthesized from tyrosine in presynaptic neurons by a hydroxylation step to provide L‐DOPA and then a decarboxylation step to provide DA. It is then concentrated in vesicles for later Ca+‐dependent release in the synaptic cleft. The released DA binds to pre‐ and post‐synaptic receptors, involved in a series of biological events. These receptors of D1‐like (D1, D5 subtypes) or D2‐like (D2, D3, and D4 subtypes) type belong to the G protein‐coupled receptor family. Two main processes regulate the life of DA in the synapse and thus the intensity and duration of dopaminergic neurotransmission. The first involves monoamine oxidases and catecholamine O‐methyl transferases, which metabolize DA. The second involves the dopamine transporter (DAT), a presynaptic transmembrane protein that accumulates DA into presynaptic neurons. These processes are therefore important regulators of dopamine action on physiological functions such as locomotion, cognition, affect, and neuroendocrine functions (Vizi 2000). It could thus be hypothesized that the regulation of DA neurotransmission is altered in pathological contexts involving DA neurotransmission such as schizophrenia, bipolar disorder, Parkinson's disease, Attention Deficit Hyperactivity Disorder (ADHD), Tourette syndrome, Rett syndrome, and Lesch‐Nyhan syndrome, and also in patients chronically abusing dopaminergic drugs such as cocaine or methamphetamine. In view of its pivotal role in the regulation of DA transmission, the DAT can be used as a reliable molecular target to evaluate neuronal activity and in the diagnosis and monitoring of the evolution and severity of brain disorders involving dopaminergic systems.
Molecular imaging techniques are highly relevant tools to explore the DA neurotransmission through the DAT as they can be used to visualize and quantify molecular targets in vivo. Positron Emission Tomography (PET) and Single Photon Emission Computerized Tomography (SPECT) are noninvasive imaging methods using different types of radionuclide and different modes of radiation detection. These techniques use radiopharmaceuticals labeled with either β+ (PET) or γ (SPECT) radionuclides, which can target molecular entities such as proteins, membrane cells, hormones, and antibodies. These imaging techniques are thus able to provide information at a molecular level regarding biological functions such as a signalization, transmission, and migration processes. Exploration of the DAT by PET or SPECT has been proposed to evaluate dopaminergic nerve terminals during the normal aging process and also in pathological contexts (in particular Parkinson's disease) in order to increase the range of clinical probes for the early diagnosis and treatment follow‐up of disease.
Many studies have been undertaken to develop radiopharmaceuticals for in vivo exploration of the DAT. Because cocaine belonging to the tropane structure has been found to be an inhibitor of dopamine, serotonin (SERT), and norepinephrine (NET) transporters, several attempts have been made to label cocaine with a radioisotope and to chemically modulate the cocaine skeleton to direct the selectivity of the tropane structure to DAT (Fig. 1).
Figure 1.
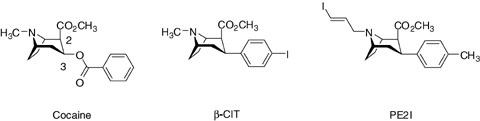
Cocaine derivatives.
Among these studies, changes at position 3 and at the bridging nitrogen atom of the tropane structure were found to be the most effective to obtain potent and selective DAT compounds for use in vivo. First, the substitution of the benzoyloxy group of cocaine for a para‐iodophenyl group, directly linked at the 3β position, led to 2β‐carbomethoxy‐3β‐(4′‐iodophenyl) tropane (β‐CIT) (Boja et al. 1991), which in vitro displays markedly improved DAT affinity when compared to cocaine (Ki= 1.6 vs. 221 nM). Although β‐CIT can be radioiodinated and has been widely used in human DAT imaging (Asenbaum et al. 1997; Innis et al. 1993; Seibyl et al. 1995), this compound also binds with a high affinity to the SERT (Ki= 3.78 vs. 207 nM for cocaine) (Boja et al. 1992). Moreover, its in vivo kinetics reveal an equilibrium state necessitating performing quantification 24 hours post injection and this limits routine clinical use (Brucke et al. 1993; Laruelle et al. 1994). In addition to results obtained in SAR studies, focusing on evaluation of the effects of the nature of the 3 substituent on DAT affinity, these findings have shown that a substituted phenyl ring directly linked at the 3β position guarantees a high DAT affinity.
On the basis of these results, we decided to modulate the β‐CIT structure (in particular at the bridgehead nitrogen and at the 4′‐position of the phenyl ring) in order to obtain a more DAT‐selective ligand. Among the series of compounds that we synthesized, N‐(3‐iodopro‐2E‐enyl)‐2β‐carbomethoxy‐3β‐(4′‐methylphenyl)nortropane (PE2I) was selected as a very potent radiopharmaceutical to image the DAT in vivo by PET and SPECT in view of its pharmacological profile. We summarize here all the pharmacological results.
Chemistry and Radiochemistry
PE2I and its radiolabeling precursors are prepared from cocaine as starting material, going through nortropane 1 , which is N‐alkylated with 3‐(tributyltin)‐prop‐(2E)‐enyl chloride to provide the tin derivative 2 (Fig. 2). This tin compound is then used to obtain either cold or radioiodinated PE2I. The labeling of PE2I with C‐11 or tritium at the carbomethoxy function requires the use of the carboxylic acid precursor 3, which is obtained by hydrolysis of PE2I in a mixture of dioxane/water at reflux for 2 days. The acid precursor 3 is then purified by HPLC to avoid the presence of cold PE2I in the precursor mixture for labeling (Stepanov et al. 2007).
Figure 2.
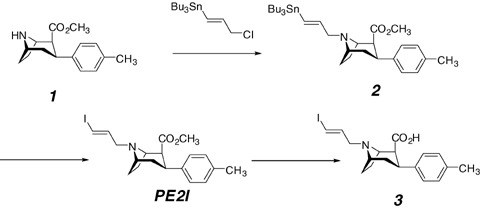
Synthesis of precursor for radiolabeling with iodine‐123/125 (compound 2) and tritium or carbon‐11 (compound 3).
Iodine Radiolabeling
Radioiodinated PE2I is obtained by iododestannylation of the corresponding tin precursor 2 (Fig. 3). Typically, the labeling procedure involves [125/123I] NaI in the presence of an oxidizing agent to provide more than 50% yield of iodination.
Figure 3.
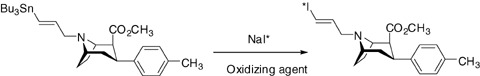
Radiolabeling of PE2I with iodine‐123/125.
In the first attempt, 50 μg of the tin precursor in 50 μg of EtOH and 3% w/v of hydrogen peroxide were used to oxidize NaI (125 or 123), and, after a purification step on reverse phase HPLC, pure radioiodinated PE2I was obtained with more than 50% radiochemical yield, more than 95% radiochemical purity and specific activity (no‐carrier‐added) of 75 and 185 TBq/mmol for [125I]PE2I and [123I]PE2I, respectively (Guilloteau et al. 1998). Using choramine‐T as oxidizing agent was reported to label PE2I with I‐123 (Kuikka et al. 1998). After a sterilization step, the radiochemical yield in these conditions was 70—85%, with radiochemical purity of more than 98% and an estimated specific activity of 8.7 TBq/μmol. Iodogen was also essayed as oxidizing agent and required the reaction vial be previously coated with iodogen (Berthommier et al. 2002). After proceeding at room temperature for 15 min, the oxidation reaction step was stopped by removing the reaction mixture from the vial. Comparison of radiolabeling yields using hydrogen peroxide or iodogen showed that the use of iodogen resulted in higher yields (> 80%). Moreover, the use of iodogen resulted in the smoothest reaction conditions, allowing a simplified purification step by a single solid phase extraction on a Sep Pack® C‐18 column. After loading the Sep Pack® C‐18 with the crude reaction mixture, followed by elution of an ethanol/water mixture by 1 mL fractions (70/30), pure [123I]PE2I was collected in the fourth fraction.
Reasonably good radiochemical yields of [125/123I]PE2I labeling can be obtained whatever the radiolabeling method used, and the tracer can be formulated for in vitro, ex vivo, or in vivo use, including use in humans.
Carbon Radiolabeling
PE2I can also be labeled with carbon‐11 (half‐life = 20.4 min) at the methyl ester function. Such labeling involves the acid precursor as a free base and [11C]methyl triflate in acetone. [11C]methyl triflate is prepared from [11C]methyl iodide (Fig. 4) using silver triflate (Jewett 1992). [11C]methyl iodide is obtained from [11C]carbon dioxide using a two step, one pot synthesis. [11C]CO2 is first trapped and reduced to [11C]methanol by lithium aluminum hydride, followed by iodination in aqueous hydrogen iodide. This synthetic sequence provides [11C]methyl triflate in 80% decay corrected yield 7 to 8 min after the end of the bombardment (Dollé et al. 2000).
Figure 4.
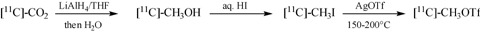
Preparation of methyl triflate for carbon‐11 labeling.
Reaction of the acid precursor 3 (400–600 μg as the free base) with [11C]methyl triflate in acetone containing NaOH in water yields 49–74%[11C]PE2I after decay correction (Fig. 5). This radiosynthesis is achieved within 25 min including the HPLC purification step and leads to [11C]PE2I (typically 7.4–11 GBq) with specific radioactivity ranging from 29.6 to 44.4 GBq/μmol.
Figure 5.
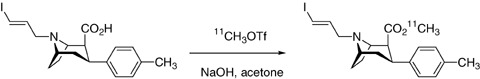
Radiolabeling of PE2I with carbon‐11.
The high efficiency of this radiolabeling strategy provides large amounts of [11C]PE2I for animal or human use.
Tritium Radiolabeling
Labeling of PE2I with tritium involves the acid precursor 3 (900 μg), [3H]methyl iodide, and tetrabutylammonium hydroxide in dimethylformamide/toluene mixture (Fig. 6). After 5 min at 80°C in a sealed vial, almost complete incorporation of tritium into the labeled compound (> 99%) is achieved, as confirmed by HPLC analysis (Stepanov et al. 2007).
Figure 6.
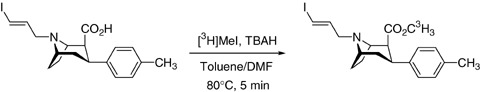
Radiolabeling of PE2I with tritium.
By this procedure and after HPLC purification, [3H]PE2I is obtained with a specific activity of 73.4 Ci/mmol. To confirm the absence of unlabeled PE2I in the starting material (acid precursor 3) Stepanov et al. performed carbon‐11 labeling using the acid precursor used for the tritium labeling. Measurement of high specific activity for [11C]PE2I (8200 Ci/mmol) confirmed the absence of PE2I in the starting material.
Pharmacological Properties
Pharmacological properties were determined to establish whether PE2I could be used as a radiopharmaceutical to explore the DAT in vivo by PET or SPECT, and the results are described below.
In vitro Studies
Competition Studies
The pharmacological properties of PE2I were first evaluated in vitro by competition studies using crude membrane fractions of homogenates of rat striatum for DAT assays and frontoparietal cerebral cortex for SERT and NET assays (Emond et al. 1997). These competition studies were performed using tritiated 1‐[2‐(diphenylmethoxy)ethyl]‐4‐(3‐phenylpropyl)piperazine (GBR‐12935), paroxetine, or nisoxetine as selective ligands for the DAT, SERT, and NET, respectively. When compared to β‐CIT, PE2I showed a slightly higher binding affinity for the DAT (17 vs. 30 nM). However, SERT and NET affinity of PE2I were found to be markedly reduced compared to β‐CIT. These results made this compound one of the most selective DAT ligands in vitro (Table 1).
Table 1.
In vitro competitive binding assays.a
| Compound | Affinity (Ki, nM) | ||
|---|---|---|---|
| DAT | SERT | NET | |
| β‐CIT | 27 ± 2 | 3 ± 0.2 | 80 ± 28 |
| PE2I | 17 ± 7 | 500 ± 30 | > 1000 |
aData from Emond et al. 1997; results are expressed as means ± SEM; n= 3 independent experiment for each value.
Because of the high degree of structure homology shared by the three monoamine transporters, the first cocaine derivatives such as β‐CIT developed as radiopharmaceuticals lacked DAT selectivity. The gain in DAT selectivity obtained with PE2I was attributed to the combination of the phenyl ring substitution (4‐methyl) with the N‐3‐iodoprop‐2‐enyl group. When compared to N‐alkyl groups such as fluoroethyl or fluoropropyl, an E‐alkenyl group was found to enhance DAT selectivity compared to that of SERT or NET.
Competition studies using [125I]PE2I on rat striatal membranes were also performed to evaluate the potency of several drugs to inhibit PE2I binding (Chalon et al. 1999b; Guilloteau et al. 1998).
As shown in Table 2, ligands of the dopamine D1 and/or D2 receptor (haloperidol, sulpiride, and (R)‐(+)‐8‐Chloro‐2,3,4,5‐tetrahydro‐3‐methyl‐5‐phenyl‐1H‐3‐benzazepin‐7‐ol or SCH‐23390), which are present in high amounts in the striatum, had no effect on the [125I]PE2I binding. By contrast, PE2I binding was inhibited by several drugs, with efficiency closely corresponding to the pharmacological profile of a DAT ligand. β‐CIT and 1‐[2‐[Bis(4‐fluorophenyl)methoxy]ethyl]‐4‐(3‐phenylpropyl)piperazine (GBR‐12909) highly inhibited [125I]PE2I binding, whereas inhibition by paroxetine and nisoxetine was weak. The poor inhibitory potency of cocaine is in agreement with findings published with other tropane derivatives such as (−)‐2‐β‐Carbomethoxy‐3‐β‐(4‐fluorophenyl)tropane (WIN‐35,428) (Boja et al. 1990), β‐CIT (Boja et al. 1992) and N‐(3′‐iodopropen‐2′‐yl)‐2β‐carbomethoxy‐3β‐(4‐chlorophenyl) tropane (IPT) (Kung et al. 1995) and can be explained by the enhancement of the DAT affinity obtained with these cocaine‐like derivatives. These results also confirm that PE2I binds to the DAT in vitro with high affinity and selectivity.
Table 2.
Inhibition of specific [125I]PE2I binding on rat striatal membranes.a
| Drug | Ki (nM) | Drug | Ki (nM) |
|---|---|---|---|
| GBR‐12909 | 1.3 ± 0.3 | Nisoxetine | 450 ± 50 |
| β‐CIT | 1.4 ± 0.8 | Haloperidol | > 1000 |
| Cocaine | 480 ± 28 | Sulpiride | > 1000 |
| Paroxetine | 416 ± 76 | SCH‐23390 | > 1000 |
aData from Chalon et al. 1999b; results are expressed as means ± SEM; n= 3 independent experiment for each value.
Because cocaine is an inhibitor of the DAT, competition experiments with PE2I on the uptake of [3H]dopamine have been performed on rat striatal synaptosomes and compared to GBR‐12909, β‐CIT, and cocaine (Chalon et al. 1999b). The results showed that PE2I, GBR‐12909, and β‐CIT displayed the same inhibitory effect. The inhibitory potency of these three compounds was ∼100 times greater than that of cocaine.
These experiments have also been performed on rat DAT expressed in cell line from African green monkey kidney (COS) cells (Page et al. 2002). These transfected cells offer an easier in vitro protocol for the evaluation of DAT ligands. In this system, PE2I was found to be a potent inhibitor of DA uptake, with an EC50 value of 6.0 ± 1.4 nM, similar to that observed in rat striatal synaptosomes. Interestingly, the potency of PE2I in inhibiting DA uptake in transfected COS cells was 25 times greater than that of GBR‐12935, whereas in rat synaptosomes PE2I had the same potency as GBR‐12909. This difference in the potency of GBR derivatives between native and cloned DAT seems to be characteristic of this class of compound because similar potencies have been found in both systems for cocaine derivatives (Eshleman et al. 1995; Madras et al. 1989).
All these results confirmed that PE2I binds selectively to the DAT with high affinity and is also a potent DAT inhibitor, both in native and recombinant in vitro systems. Labeled with iodine‐125 or tritium, PE2I could thus easily be used in competition studies to evaluate the binding and inhibitory potency of new DAT ligands.
Saturation Studies
Saturation studies have been performed on rat striatal membranes to determine the affinity and density of specific [125I]PE2I binding sites and also the influence of the NaCl concentration after Scatchard analysis (Chalon et al. 1999b). PE2I bound to the DAT according to a one site model in two buffer systems (Tris‐HCl and phosphate) with a Kd value of ∼4 nM and a Bmax∼12 pmol/mg of protein (Tris‐HCl buffer), and a Kd value of 4.9 nM and a Bmax∼12 pmol/mg of protein (phosphate buffer). This binding was found to be Na+ dependent (Table 3), as already observed for other DAT inhibitors such as GBR (Janowsky et al. 1986) and β‐CIT (Wall et al. 1993).
Table 3.
Effects of Na+ concentration on PE2I binding to rat striatal membranes.a
| [NaCl] mMol | Kd (nM) | Bmax (pmol/mg protein) |
|---|---|---|
| 30 | 13.3 ± 5.7 | 30.6 ± 7.9 |
| 120 | 3.9 ± 0.8 | 11.8 ± 2.6 |
| 300 | 4.4 ± 0.8 | 11.2 ± 3.5 |
aData from Chalon et al. 1999b; results are expressed as means ± SEM; n= 3 independent experiment for each value.
The presence of DAT has been demonstrated in the frontal cortex with immunohistochemical methods (Ciliax et al. 1995). Because the mesocortical dopaminergic system has major regulatory roles, exploration of the DAT in this low DAT density brain area should provide information on these regulatory processes. Assays on rat cortical membranes with [125I]PE2I confirmed the much lower DAT concentration in this brain area, as 12 times greater tissue concentrations were needed than in the striatum. In these conditions, a Kd value close to that obtained in the striatum (∼5 nM) and a 10 times lower Bmax value were obtained. Moreover, competition studies in the presence of GBR‐12909 or paroxetine using cortical rat membranes showed that the PE2I binding was exclusively related to the DAT (IC50= 6.4 ± 2.5 and >1000 nM for GBR and paroxetine, respectively). From these binding experiments, it could be assumed that PE2I selectively recognizes the DAT even in low DAT density brain regions such as the cortex.
[3H]PE2I has also recently been characterized on mouse striatal membranes (Stepanov and Jarv 2006). In this study, experiments in the equilibrium state and the kinetics of PE2I binding were investigated to explain further the molecular mechanism of drug recognition and distinction by the DAT. The PE2I–DAT interaction was found to be reversible with at least two kinetically distinguishable steps because of the dependence of the apparent association rate constant values upon radioligand concentration. This two‐step interaction starts with rapid bimolecular binding of PE2I to DAT, followed by slow monomolecular equilibrium of the "isomerization" complex. Such an isomerization step has been identified in the case of antagonists of G‐protein‐coupled receptors (Strickland et al. 1975). This isomerization step probably consists of a conformational transition state of the DAT induced by PE2I. Characterization of this mechanism opens up the possibility of further investigations into the mechanisms of the DAT interaction with drugs and also characterization of the DAT function in disease contexts.
Autoradiography Studies
Two types of autoradiography studies can be carried out. The first (ex vivo autoradiography) consists of the exposure of rat brain sections after injection of the tracer (Fig. 7). The general cerebral tracer biodistribution can thus be assessed. Using this technique, high levels of [125I]PE2I were found to accumulate in the striatum, accumbens nuclei, and olfactory tubercles, which are brain areas with the highest DAT density. [125I]PE2I binding was also found in the substantia nigra and ventral tegmental area, corresponding to DAT localized on dopaminergic cell bodies (Guilloteau et al. 1998).
Figure 7.
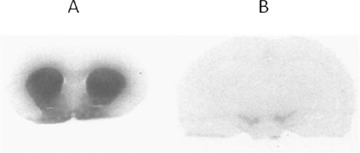
Cerebral biodistribution of [125I]PE2I in rats according to autoradiography studies. High accumulation was observed in the striatum, accumbens nuclei and olfactory tubercles (A), and also in the substantia nigra (B). Adapted from Guilloteau et al. 1998.
The second type (in vitro autoradiography) consists of the incubation of the tracer with brain sections. Semiquantitative data are obtained by the measurement of regional optical densities with an image analyzer after identification of anatomical regions.
As shown in Table 4, the highest binding was observed in the striatum, with a preference for the lateral part compared to the medial part, and PE2I was also highly localized in the nucleus accumbens and olfactory tubercle. This radioactivity distribution corresponds to that obtained for DAT characterization using immunohistochemical probes (Ciliax et al. 1995). The selectivity of such binding was assayed by competition studies using GBR‐12909, paroxetine, and nisoxetine. The strong displacing effect of GBR‐12909 compared to the inability of paroxetine and nisoxetine to displace the PE2I binding demonstrated the selective binding of PE2I to DAT in these experimental conditions.
Table 4.
Autoradiographic analysis of [125I]PE2I binding to several areas of rat coronal brain sections.a
| Cerebral area | Total Binding | GBR‐12909 (1 μM) | Paroxetine (20 nM) | Nisoxetine (20 nM) |
|---|---|---|---|---|
| Medial striatum | 31.4 ± 2.6 | 8.3 ± 0.9** | 26.3 ± 1.4 | 25.7 ± 2.0 |
| Lateral striatum | 37.8 ±1.6 | 11.1 ± 0.9** | 33.5 ± 1.6 | 34.2 ± 2.2 |
| Nucleus accumbens | 29.9 ± 2.6 | 8.7 ± 1.0** | 26.6 ± 2.0 | 27.3 ± 2.2 |
| Olfactory tubercle | 20.2 ± 3.3 | 4.3 ± 1.0** | 16.2 ± 2.2 | 16.6 ± 1.0 |
aData from Chalon et al. 1999b; results are expressed as means ± SEM; n= 3 independent experiment for each value.*P < 0.05, **P < 0.001 compared to the total binding.
[125I]PE2I has also been characterized postmortem in normal human brains using whole hemisphere autoradiography (Hall et al. 1999). Autoradiograms showed very intense binding of [125I]PE2I in the basal ganglia (putamen, caudate, nucleus accumbens) (Fig. 8). By contrast, only weak binding was observed in neocortical areas and the thalamus and no binding was observed in the raphe nuclei where the SERT is abundant.
Figure 8.
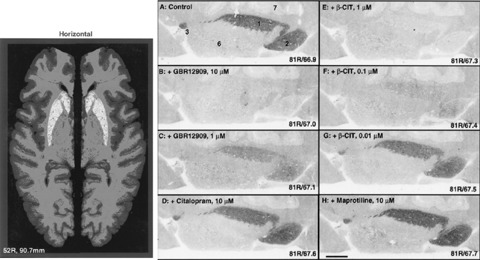
In vitro binding of PE2I to whole hemisphere human brain sections at the level of the caudate‐putamen. Right: blocking of [125I]PE2I binding by GBR‐12909 and β‐CIT at different concentrations, whereas citalopram (SERT inhibitor) and maprotiline (NET inhibitor) had no effect. Adapted from Hall et al. 1999.
Similarly, no binding was observed in regions where the NET is located (e.g., locus coeruleus). The selective SERT and NET inhibitors (citalopram and maprotiline, respectively) did not reduce the binding of [125I]PE2I in the striatum. By contrast, GBR‐12909 and β‐CIT induced markedly reduce binding of PE2I in a concentration‐dependent manner. These findings demonstrated that in vitro binding of PE2I in the human brain is also highly selective of the DAT. In addition, this study showed that DAT distribution in many aspects parallels the distribution of the DA receptor, especially the D2‐type DA receptor. The main localization of DAT was in the basal ganglia, with lower density in the substantia nigra. Very low specific binding was observed in the cortex where the density of the D1‐receptor is relatively high. The low density of DAT found in many extrastriatal regions in this study suggests that inactivation of the DA released in those areas is more dependent on enzymatic degradation than on uptake. This study also showed that the selectivity of PE2I makes this compound a valuable tool to study postmortem the dopamine neurons of the brains of patients with neurologic and psychiatric diseases.
Ex Vivo Studies in Rats in Physiological Conditions
Ex vivo experiments were first performed in rats to evaluate [125I]PE2I as a radiopharmaceutical to explore the DAT (Guilloteau et al. 1998). For kinetics studies, rats received an i.v. injection of [125I]PE2I and were sacrificed 30 min, 1, 2, or 4 h post injection. For blocking studies, three groups of animals received an i.v. injection of saline, GBR‐12909 (5 mg/kg), or paroxetine (5 mg/kg) before [125I]PE2I and were sacrificed 2 h post injection. Samples of cerebral regions (cerebellum, striatum, and frontal cortex) were removed and weighed, and their radioactivity was measured. The time course of [125I]PE2I accumulation (percentage of injected dose per gram of tissue) in different brain regions is shown in Figure 9. The highest accumulation was observed in the striatum compared to the frontal cortex and the cerebellum. Radioactivity in the striatum was 1.06 ± 0.13 ID/g tissue at 30 min and was decreased by 49%, 74%, and 93% at 1, 2, and 4 h, respectively.
Figure 9.
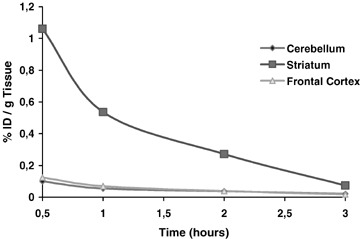
Time‐course of PE2I accumulation in rat brain regions. Adapted from Guilloteau et al. 1998.
In contrast, the frontal cortex and cerebellum displayed rapid wash out, providing a very weak degree of radioactivity from 1 h post injection. This study showed that PE2I accumulates rapidly in the rat brain, with predominant concentration in the striatum.
We also showed in blocking studies that preinjection of GBR‐12909 induced strong inhibition of the [125I]PE2I accumulation in the striatum (−74%) whereas it had no effect on the tracer binding in the cerebellum and frontal cortex. Moreover, preinjection of paroxetine had no effect on [125I]PE2I accumulation, whatever the brain region. These results showed that [125I]PE2I accumulation in the rat striatum is rapid and high whereas low accumulation is found in brain areas rich in SERT (such as the frontal cortex) providing a maximum striatum/cerebellum ratio of 10 during the first 30 min post injection. In addition, competition studies demonstrated the high in vivo specificity of [125I]PE2I binding to the DAT.
A metabolite study has recently been performed in rats using [11C]PE2I to assess further DAT quantification in vivo using this tracer (Shetty et al. 2007). The first result was that using small animal PET imaging, the kinetics of [11C]PE2I brain accumulation was similar to that obtained with [125I]PE2I, with a high degree of radioactivity in the striatum compared to other brain regions such as the frontal cortex and cerebellum.
HPLC analysis of the plasma compartment showed two radiometabolites coming from [11C]PE2I, both being less lipophilic than PE2I. They were expected to have originated from peripheral metabolism as PE2I was found to be stable in brain homogenates. Radioactivity analysis of brain areas revealed that 30 min post injection, [11C]PE2I concentrations were 92.5% and 67.1% in the striatum and cerebellum, respectively. The two metabolites were also found in all brain areas and represented 6% and 3% of the total radioactivity found in the striatum. These findings also showed that metabolite 2 (Fig. 10) displayed homogenous brain distribution, which could be attributed to nonspecific binding. By contrast, and even at the low degree of accumulation, the main accumulation of metabolite 1 in the striatum could be attributed to specific DAT binding. To identify these radiometabolites, rat experiments were performed using cold PE2I, with or without [11C]PE2I, in sufficient concentrations to induce a high degree of metabolism and thus to generate significant amounts of metabolites for LC‐MS detection. The coinjection of cold and radiolabeled PE2I made possible the identification of radiometabolites and also cold metabolites by LC‐MS. These experiments were performed on brain and urine samples and led to the proposition of a metabolism figure described below.
Figure 10.
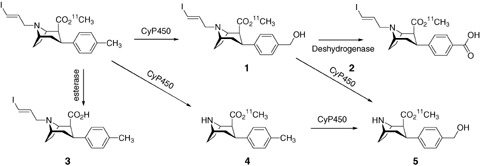
Metabolism pathway of PE2I in rats. Adapted from Shetty et al. 2007.
Metabolites 1 and 2 were found in the brain and urine whereas metabolites 3, 4, and 5 were found only in urine. Metabolites 3–5 are thus generated peripherally and do not interfere with brain radioactivity. On the other hand, metabolite 1 is likely to cross the blood‐brain barrier but not metabolite 2, which might originate from conversion of 1 inside the brain. It should be noted that no cold metabolites were localized in the brain, as observed for the PE2I analog FE‐CNT (Zoghbi et al. 2006) for which the nor‐CCT (nortropane analog) concentration was twice that of the parent tracer. Although nor‐CCT is generated from the tracer dose injected, competition between this metabolite (which is known to have a DAT affinity component) and the tracer could occur at the DAT binding site, especially when the brain DAT concentration declines, as observed in neurodegenerative diseases. For PE2I, only a small amount of metabolite 1 (3% of the striatal accumulation), which may bind to the DAT, was found. It should also be borne in mind that the metabolism, kinetics, and neuroreceptor binding may be affected by the type of anesthetic used for animal experiments. In fact isoflurane, which was used in the metabolism study reported above (Shetty et al. 2007), has been shown to reduce the number of available DAT reuptake sites to less than 50% under 1% inhalation (Elfving et al. 2003) as measured with the DAT ligand [18F]FECNT in the monkey brain. However, to explain further how this metabolite may interfere in DAT quantification using [11C]PE2I, separate PET studies are needed to examine the distribution of this radiolabeled metabolite in the brain.
In vivo Evaluation in the Monkey in Physiological Conditions
In the first preliminary experiment, injection of [123I]PE2I was performed in Cynomolgus monkeys (Guilloteau et al., 1998). Rapid uptake in the striatum was observed with high contrast compared to cortical areas, and the striatal accumulation showed a plateau between 30 and 80 min post injection. These results were confirmed using [11C]PE2I in Papio Anubis baboons (Dollé et al. 2000). Rapid and high accumulation was found in the caudate nucleus and putamen, reaching a plateau 10 min post injection. By contrast, rapid wash‐out from the cerebellum and thalamus was observed, leading to an increase in the caudate nucleus and putamen to cerebellum ratios from 2 at 10 min p.i. to around 5 at 40 min p.i.. Moreover, injection of unlabeled PE2I at 40 min p.i. induced rapid wash‐out of the radioactivity in the caudate nucleus and putamen. These preliminary results made PE2I a good candidate to explore the DAT in vivo when labeled with iodine‐123 or carbon‐11, as high accumulation displaceable by unlabeled PE2I was found in DAT brain areas within a short time after injection. To obtain better understanding of the in vivo pharmacology of this tracer, the specificity and selectivity of binding was examined by displacement and pretreatment experiments in Cynomolgus monkeys using [11C]PE2I (Halldin et al., 2003). In addition, radioligand metabolism was measured in plasma using HPLC. Four minutes post injection, 3.8% of the injected dose was present in the monkey brain and this provided a good signal for analysis of DAT binding. The lowest accumulation was found in the cerebellum. Because no effect on the cerebellum to blood ratio was observed for any compound used in the displacement and pretreatment measurements, the cerebellum was used to estimate the time curve for free and nonspecifically bound radioligand in the brain.
The selectivity of [11C]PE2I binding was examined in pretreatment measurements using maprotiline and citalopram as NET and SERT antagonist, respectively. The absence of effect on the regional time curve radioactivity in these pretreatment challenges indicated that [11C]PE2I bound selectively to the DAT in vivo, thus representing an advantage when compared to other tracers such as [11C]β‐CIT‐FP (known as DaTSCAN when labeled with iodine‐123) (Lundkvist et al. 1997). Moreover, the high striatum to cerebellum ratio of about 10 should be an advantage for subregional analysis of [11C]PE2I binding in brain disorders. These results clearly demonstrated the affinity, specificity, and selectivity of PE2I binding in the monkey. Moreover, the rapid peak equilibrium and the high signal to noise ratio in the striatum, and also in the substantia nigra and in the thalamic region, make this radiotracer a useful tool to explore the DAT in animal models of neuropsychiatric and neurodegenerative diseases.
In vivo Evaluation of PE2I in Humans
[123I]PE2I
The first study in humans was performed to evaluate the pharmacokinetics, SPECT imaging quality and radiation dosimetry in the living brain (Kuikka et al. 1998). The tracer dose used in this study was 140–215 MBq with a specific activity of 8.7 TBq/μmol. The percentage of unchanged plasma [123I]PE2I from blood samples collected at 2, 5, 10, 30, 60, 120, 160, and 220 min after injection determined by HPLC was found to decrease rapidly with time to 13% at 30 min and 7% at 60 and 120 min.
[123I]PE2I was rapidly transformed into a main polar metabolite, which was unlikely to enter brain tissue prior to the scan time of 60 and 100 min. Regional time activity curves of [123I]PE2I in the striatum and cerebellum showed a maximum specific uptake between 64–84 min p.i., and imaging (Siemens MultiSPECT 3 gamma camera, Siemens Medical Systems Inc., Hoffman Estates, IL, USA) at 70 min p.i. revealed a marked accumulation of the tracer in the striatum. From these findings, and using the reference region compartment model (Acton et al. 1999), a striatal volume of distribution (VD) of 94 ± 24 mL/mL and a potential binding of 0.89 ± 0.02 were calculated. Although the VD of PE2I was smaller than that of β‐CIT as a consequence of different binding kinetics, PE2I had a high striatum to background ratio of 9 at 70 min p.i. Whole‐body imaging study revealed that the most intense uptake was found in the bladder, liver, and intestinal tract, with predominant clearance through the urinary tract. An estimated effective dose of 0.022 ± 0.004 mSv/MBq was found in this study that was significantly less than that of β‐CIT. One hour p.i., the brain uptake represented 3.1% of the injected dose and the striatal accumulation at the peak uptake (64 min) represented 0.34% of the injected dose. All these results (high striatal uptake, low nonspecific binding, low radiation exposure, scan time) make [123I]PE2I a good candidate for clinical practice in daily routine.
Based on these preliminary results, the same group evaluated the influence of age on PE2I binding in healthy volunteers. Sixteen right‐handed male volunteers (23–75 years of age) were studied after injection of 140–215 MBq of [123I]PE2I (specific activity of 8.7 TBq/μmol). Eight scans per subject were performed within 100 min after injection. Data analysis was performed using the reference region model to estimate the binding potential of PE2I to DAT, and the Logan‐Patlak graphical technique was used to estimate the striatal volume of distribution (VD). When analyzing the dependence of DAT on age in healthy males, a difference in the striatal VD was found between male subjects > 40 years of age compared to those <40 years of age. More precisely, although no significant striatal side‐to‐side difference was observed, a clear decrease in DAT binding was found (4% per decade; r=−0.72). This reduction in PE2I–DAT binding reflected the loss of DAT with age and highlighted the necessity of age‐matched controls in patient studies when assessing or comparing striatal DAT VD. In order to describe a method for accurate quantification of binding after injection of [123I]PE2I, Pinborg et al. (2002) evaluated different methods of data analysis such as compartment analysis, Area Under Curve (AUC) analysis, and Logan analysis with plasma. Of these different approaches, Logan analysis after bolus injection was recommended, with a total scanning time of 120 min. Moreover, introduction of a reference region (occipital cortex) to approximate the input function was found to be useable to avoid blood sampling and radiometabolite correction. However, this method needs the occipital cortex to be healthy to avoid changes in the determination of input function. To overcome limitations of the transient and peak equilibrium approaches related to non steady‐state conditions in the brain and plasma, administration of [123I]PE2I as a bolus followed by a constant infusion was assayed (Pinborg et al. 2005). This technique has the advantage of providing a tracer steady state in the brain and plasma. The net flux of the tracer between compartments thus becomes zero and the sizes of compartments become constant. This state simplifies the calculation of binding parameters as simple ratios and offers several advantages such as insensitivity of binding parameters to cerebral blood flow. Venous blood sampling could be used if no brain reference area is available to assess the input function to replace arterial blood sampling. This study was performed with five healthy subjects who underwent SPECT twice. The first was performed after a bolus injection of [123I]PE2I and the second after a bolus plus a constant infusion of [123I]PE2I. These experiments conducted on the same subject allowed direct comparison of the injection protocols as variation in DAT density due to genetic or environmental variability between subjects was eliminated. Although it may be considered that constant infusion of the tracer could be a limitation for clinical use, the advantages of the bolus/infusion (B/I) approach used in this study (e.g., accuracy and precision of data quantification) made this protocol attractive. Moreover, the B/I approach was found to be less sensitive to individual pharmacokinetic variability compared to the bolus approach, and this is particularly important when considering disease conditions. The B/I protocol (B/I ratio of 2.7 h) was further evaluated to test the reproducibility and reliability of obtaining binding potential (BP) values as outcome parameters (Ziebell et al. 2007). At constant tracer levels, quantification of DAT density is achieved through the calculation of outcome parameters obtained as ratios of tracer concentrations in tissue to venous blood (BP1) or, if a suitable reference brain area exists, as tissue to reference tissue ratios (BP2). In practice, the tracer concentration in a region of interest (ROI) or in the reference region is computed by SPECT ROIs (counts/mL) from SPECT acquisitions. Methods for ROI delineation could thus markedly influence variations in BP determination. In this study, two different methods for ROI delineation (manual and probability map‐based automatic delineation with MRI coregistration) were used and compared. Firstly, the B/I protocol was confirmed to provide stable brain activity from 120 to 180 min post injection. Moreover, [123I]PE2I SPECT with the B/I protocol yielded highly reproducible and reliable values for striatal DAT binding.
As shown in Table 5, measurement of BP2 was highly reproducible using the B/I protocol. Interestingly, although the manual delineation method normally provides less precise and less reproducible volume assessment, it provided better reproducibility compared to the map‐based method. However, it is likely that the probability map‐based method would be better in patients with striatal DAT disorders as the striatal tracer concentration may be close to the nonspecific tracer concentration, thereby hampering correct adjustment of the striatum delineation. Whatever the delineation method used, the B/I protocol yielded highly reproducible BP2 quantification in healthy subjects as variability was considerably lower than that reported in similar studies using other DAT ligands such as FP‐CIT (5–16%) or β‐CIT (7–16%) (Booij et al. 1998; Morrish 2003; Pirker et al. 2002; Seibyl et al. 1997). [123I]PE2I provides highly reproducible measurement of striatal binding and allows the study of at least three subjects within a normal working day.
Table 5.
Variability in binding potential (BP) depending on ROI delineation.a
| Delineation method | BP1 variability | BP2 variability |
|---|---|---|
| Manual | 13.9% (1.8–35.7) | 4.1% (0.5–9.7) |
| Probability map‐based | 17.2% (4.3–40.5) | 5.2% (0.1–10.9) |
aData from Ziebell et al. 2007.
[11C]PE2I
The first study of [11C]PE2I in humans showed rapid uptake of the tracer in the striatum, reaching a maximum level within 15 min post injection (Halldin et al. 2003). Specific binding represented by the striatum and the substantia nigra to cerebellum ratios were 10 and 1.8, respectively, at peak equilibrium. These ratios were higher than those obtained for other DAT ligands such as [11C]FE‐CIT (Farde et al. 2000; Halldin et al. 1996). The rapid peak equilibrium, added to the high specific binding not only in the striatum but also in the thalamic region, may be of advantage for subregional analysis of the striatum in neuropsychiatric disorders. Three further studies have been reported on in vivo evaluation of this tracer in humans (Jucaite et al. 2006; Leroy et al. 2007; Ribeiro et al. 2007).
The first focus on the quantification of PE2I binding in the human brain and comparison of quantitative methods related to suitability for applied clinical studies (Jucaite et al. 2006). [11C]PE2I binding could be described by a four‐compartment model, simplified to a three‐ or two‐compartment model. Two approaches can be used to obtain the binding potential (BP) values in brain areas and also other kinetics rates. The first applies kinetic compartment analysis, which uses the metabolite‐corrected arterial plasma curve as an input function, and the second applies the reference tissue approach, which uses the cerebellum as a reference brain area representing the free fraction of PE2I and the nonspecific radioligand binding. This study first showed that [11C]PE2I provided high contrast images in the striatum and intermediate contrast images in the midbrain, whereas low contrast images were obtained in the cerebellum and neocortex. This in vivo distribution was consistent with the binding pattern observed by autoradiography in the human brain using [125I]PE2I, and with distinct DAT expression (demonstrated by in situ hybridization) in the striatum, substantia nigra, and ventral tegmental area (Hall et al. 1999; Ciliax et al. 1995). Moreover, [11C]PE2I displayed favorable distribution with high brain penetration (6% ID 6 min post injection), suggesting that high imaging quality could be obtained at a relatively low radiation exposure, especially when using several radiopharmaceuticals in the same subject and in studies on children.
To quantify the DAT in striatal areas, the kinetic compartment and the reference tissue approaches were evaluated and compared. Kinetic compartment analysis showed high inter‐individual variability (threefold range) of the rate constants, which could be explained by unreliable estimation of the plasma concentration of [11C]PE2I and its metabolites, high variability in subject metabolism, and/or variability in DAT concentration. Whatever the explanation, clinical PET studies are required to avoid invasive technique such as arterial blood sampling. The reference tissue approach using the cerebellum may therefore be more applicable, especially in disease contexts. However, this approach showed that BP values are time‐dependent throughout the time of measurement (60 min). A statistical comparison indicates that PE2I binding in the cerebellum is better characterized by a three instead of two compartments model. This observation of a possible additional compartment calls for the identification of labeled metabolites and their cerebral distribution. As shown by Shetty et al. (2007), a polar metabolite may enter the brain and interfere in rat with the PE2I nonspecific binding especially in the cerebellum. The use of prolonged data acquisition would be useful for clinical applications as found for SPECT studies using [123I]PE2I showing that striatum measurements require data acquisition times of up to 90 min (Pinborg et al., 2002). Whatever, the use of the occipital cortex as a reference region may be an alternative to evaluate the nonspecific binding as it is already done for FP‐CIT (DaTSCAN).
This reference tissue model using cerebellum time activity curves with prolonged data acquisition times and high specific activity could be used to evaluate [11C]PE2I binding in living humans. Finally, in addition to quantification of the DAT in the striatum, the possibility of quantitative DAT exploration in the midbrain makes this compound attractive to examine dopaminergic cell bodies during the pathogenesis of several neurodegenerative disorders.
[11C]PE2I was also evaluated for its whole body distribution and radiation dosimetry in healthy subjects (Ribeiro et al., 2007). Ten minutes post injection, whole‐body images showed high activity in the kidneys, intestines, liver, stomach, salivary glands, vertebral bodies, and brain. Thereafter, a high concentration was observed in brain, mainly in the striatum. On later images (55–112 min), most of the radioactivity was found in the bladder but a relatively high radioactive concentration remained in the striatum.
Maximum brain extraction was observed between 10 and 20 min post injection and striatal activity was found to be stable from 10 to 60 min. As for [123I]PE2I, the critical organ for dosimetry was the bladder wall. However, dosimetric evaluation of [11C]PE2I (6.4 μSv/MBq) remained within the range of doses acceptable for use in clinical studies and could be used in multi‐PET study protocols. When using 222 MBq per injection (estimated dose for adequate cerebral DAT PET imaging), the average effective dose represented 33% of that delivered during a typical [18F]FDG cerebral study (1.4 mSv for [11C]PE2I vs. 4.5 mSv for [18F]‐FDG). The required dose of [11C]PE2I for DAT PET imaging in humans resulted in a favorable biodistribution and in an acceptable effective dose providing a level of risk that can be considered as minor.
Another study investigated the impact of the gain in spatial resolution of a PET scanner on the determination of dopaminergic transmission by measurement of [11C]PE2I binding to the DAT (Leroy et al. 2007). Using two 3D PET scanners, the first that has been used routinely for cerebral imaging for 10 years, was the whole body ECAT EXACT HR+ scanner (Siemens Medical Solutions, Knoxville, TN, USA). The HR+ scanner has a radial intrinsic resolution of > 5.7 mm. The second was a dedicated brain imaging system with an isotropic spatial resolution of <3 mm in all three directions (High Resolution Research Tomograph: HRRT by Siemens Medical Solutions). The aim of this study was to evaluate the benefit of a >6‐fold improvement in intrinsic volumetric resolution to obtain greater accuracy in the measurement of small volumes of radioactivity concentration. Similar protocols were used for both scanners, with dynamic acquisition starting at the bolus injection of [11C]PE2I and lasting for 60 min. PET images were analyzed using a compartment model approach by quantifying [11C]PE2I‐BP using the Simplified Reference Tissue Model (SRTM). This model approximates the tissue of interest as a single tissue compartment and assumes that both the tissue of interest and the reference region have the same level of nonspecific radioligand binding. Moreover, to compare the HRRT and the HR+ data at matched partial volume effect, smoothed HRRT images, which have the same amount of partial volume effect in the brain as the HR+ images, were generated from native HRRT images. The mean values of the caudate, putamen, and midbrain from HR+ and smoothed HRRT images were comparable. Moreover, similar magnitudes of BP values and intersubject variability were observed in this study compared to the results of Jucaite et al. For both scanners and both spatial resolutions, the DAT binding evaluation confirmed the well‐known DAT binding decline of 6.6–9% per decade. Conversely, no age dependence on the DAT binding was observed for the midbrain. One of the main results of this study was that a reduced partial volume consistently improves the quantification of DAT binding, and the improvement was greater for small brain structures, which are largely affected by the partial volume effect. For example, the higher resolution of HRRT allowed recovery of higher BP values of [11C]PE2I binding to the DAT (native HRRT) (30% in the striatum and 90% in the midbrain) compared to BP values obtained with HR+. Moreover, this increase in BP values was associated with reduced relative interindividual variability of measurements as compared to those obtained with the HR+ scanner (11.5% vs. 28.0%). In the striatum, where a 30% improvement in BP value was found, the comparison of TACs obtained from native HRRT and HR+ showed that this higher BP value was related to higher radioactivity levels measured in the striatum and not from lower levels measured in the cerebellum. This increased accuracy in radioactivity measurement using HRRT provides the opportunity to assess small variations in [11C]PE2I DAT binding. This can be of particular value to explore small brain areas or small amplitude variations in DAT density in addictive and psychiatric disorders. Moreover, in Parkinson's disease, characterized by early degeneration of nigrostriatal dopaminergic neurons, the more accurate measurement of [11C]PE2I binding to the DAT would be valuable for early individual diagnosis, follow‐up of the disease and evaluation of treatment efficacy.
Value of PE2I in Disease Conditions
The DAT is intrinsically involved in the physiopathological mechanisms of several neurodegenerative and neurodevelopmental disorders such as Parkinson's disease (Uhl et al. 1994) and Attention‐Deficit/Hyperactivity Disorder (Dougherty et al. 1999). Due to its localization, the DAT can be considered as an index of the density of dopaminergic cell bodies and endings. In vivo exploration of the DAT by scintigraphic methods could thus be a valuable way to study the physiopathological mechanisms of neurologic disorders that involve dopaminergic neurotransmission, achieve early detection of the loss of dopaminergic neurons, assess the progression of the disease, and validate the efficacy of treatments. In this field, a number of DAT PET and SPECT ligands have been used in animal models and clinical situations involving an established or supposed role of the DAT. Due to its pharmacological characteristics in terms of affinity, specificity and in vivo kinetics for the DAT, PE2I can be proposed as a valuable tool in disease situations.
Animal Models of Dopaminergic Disorders
Several experiments using rat models of PD have demonstrated the high efficacy of PE2I for the detection of the reduction in the number of DAT reflecting the loss of dopaminergic neurons, thus showing the potential value of PE2I for early diagnosis of Parkinson's disease. We studied the time course of the loss in DAT in a rat model obtained by an anterograde lesion of the nigrostriatal dopaminergic pathway using 6‐hydroxydopamine (6‐OHDA) (Chalon et al. 1999a) and showed that a significant decrease in [125I]PE2I binding was observed as early as 24 h after lesion. The decrease reached 50% at 48 h post lesion, and PE2I binding had almost totally disappeared between 3 and 14 days post lesion (Fig. 11)
Figure 11.
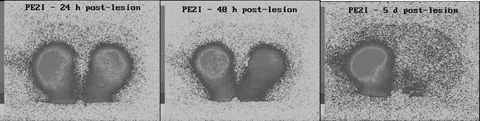
In vitro autoradiographic study with [125I]PE2I in coronal rat brain sections at 24 h, 48 h, and 5 days after 6‐hydroxydopamine injection in the right striatum. Adapted from Chalon et al. 1999a.
In an experimental model obtained by intrastriatal injection of the neurotoxin MPP+ in the rat, we showed that, compared to different in vitro presynaptic markers, the binding of [125I]PE2I in membrane homogenates was the most relevant index of the degenerative state of the nigrostriatal pathway (Barc et al. 2002).
PE2I has also been used in rat models as a relevant index of the density of dopaminergic striatal endings in vivo, as was shown using several doses of 6‐OHDA (Inaji et al. 2005a). In this model, in vivo binding of [11C]PE2I was dose‐dependently decreased in response to the intensity of the lesion.
The above findings all demonstrate the potency of PE2I for detecting the integrity of the nigrostriatal dopaminergic pathway in animal models. With the development of SPECT and PET molecular imaging methods in small animals such as rodents, it can be assumed that PE2I will be used as a valuable tool for the follow‐up of brain lesions in the same animal.
Several other animal experiments have also demonstrated that PE2I can be used for testing therapeutic approaches aimed at PD. We tested the effects of the grafting of microspheres containing the neurotrophic factor GDNF in a 6‐OHDA lesion rat model. The effects were assessed by quantitative autoradiography using PE2I that showed an increase in striatal DAT density (+17%) at 3 and 6 weeks after grafting, suggesting a neuroprotective action of GDNF delivered from microspheres (Gouhier et al. 2002).
More recently, we demonstrated that the transplantation of mesenchymal stem cells (MCS) from bone‐marrow 2 weeks after a 6‐OHDA striatal lesion allowed partial recovery of the dopaminergic nigrostriatal function, assessed in particular by a parallel decrease in induced rotational behavior, and in PE2I binding to DAT both in the striatum and substantia nigra (Fig. 12; Bouchez et al. 2007).
Figure 12.
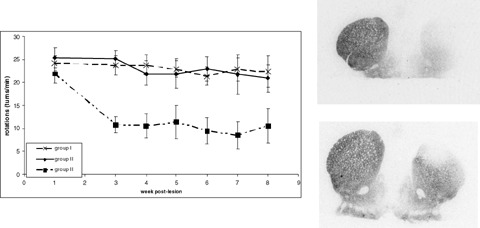
Left: induced rotational behavior in lesioned rats and nongrafted (group I) or sham‐grafted (group II) and in lesioned rats grafted with MCS 2 weeks after lesion (group III). Right: in vitro binding experiments with [125I]PE2I 8 weeks post lesion; top, sham‐grafted (group II), bottom, MSC grafted (group III). Bouchez et al., unpublished results.
In another attempt to restore the dopaminergic nigrostriatal pathway, this time using fetal mesencephalic cells, it was also shown that PET experiments with [11C]PE2I were able to provide an evaluation of the restorative effects that correlated with other methods such as behavioral tests, striatal content of dopamine and tyrosine hydroxylase‐positive cells (Inaji et al. 2005b).
The potency of PE2I in animal models has also been demonstrated in the monkey. The first study using MPTP‐induced dopaminergic degeneration in the baboon showed that the DAT density measured in vivo with [11C]PE2I was considerably decreased in treated animals compared to controls (Poyot et al. 2001). In addition, this decrease was correlated with the in vivo[18F]Dopa Ki value and the number of nigral dopaminergic neurons evaluated in vitro by TH‐immunohistochemistry. In another model of PD obtained by progressive intoxication with MPTP in the macaque, we first studied the time course of the neurodegenerative process using in vitro autoradiography with [125I]PE2I (Bézard et al. 2001a; Bézard et al. 2001b; Meissner et al. 2003) and then investigated the appearance of clinical signs and DAT density in vivo with SPECT and [123I]PE2I in the same model. This study demonstrated that a significant decrease in in vivo PE2I binding was detected 12 days after the beginning of intoxication whereas the clinical signs appeared on the 15th day, thus showing the relevance of PE2I for early detection of neuron loss (Prunier et al. 2003a).
PE2I in Human Disorders
PE2I has been used in several disorders in humans, both in vitro on postmortem brain sections and in vivo using SPECT or PET.
The in vitro method of whole hemisphere autoradiography extensively used by the Karolinska Institute group (Hall et al. 1998) combined with PE2I allowed the detection of modifications of DAT density in the striatum (the highest DAT density area) in alcoholic subjects (Tupala et al. 2001, 2003) and also in cortical regions (Tupala et al. 2006). Although these modifications remain to be explained fully, they are in agreement with dysfunction of dopaminergic systems in this brain disorder.
Using in vivo SPECT exploration with [123I]PE2I we showed that in Parkinsonian subjects the distribution volume ratio in the putamen was significantly decreased and inversely correlated with disease duration and Hoenh and Yahr stage (Prunier et al. 2003b). This first in vivo human study in this field showed PE2I to be a valuable tool for accurate evaluation of the nigrostriatal dopaminergic pathway, and thus potentially for the preclinical diagnosis of PD, and to follow the progression of the disease. The closely related analog of PE2I, that is, FP‐CIT, that is commercially available as DaTSCAN, is currently being used in a number of clinical protocols (Benamer et al. 2000; Catafau and Tolosa 2004). However, it is regrettable that the use of PE2I is much lower compared to the latter tracer, as a number of experiments indicate better selectivity of PE2I for the DAT than FP‐CIT.
A number of neuropsychiatric disorders are known or believed to involve the dopaminergic systems, including ADHD. This disorder mainly affects boys, is characterized by increased impulsivity and hyperactivity (Castellanos 1997), and may include abnormalities in parameters of dopaminergic neurotransmission, especially the DAT. This hypothesis was initially based on the fact that pharmacological agents that inhibit the DAT can be effective in the treatment of ADHD (Seeman and Madras 1998; Solanto 1984). In agreement with this, several genetic findings have indicated that specific alleles of the DAT gene seem to be associated with ADHD (Cook et al. 1995; Gill et al. 1997; Swanson et al. 2000). More recently, SPECT neuroimaging studies in adults suffering from ADHD have suggested that DAT density can be enhanced in the striatum (Dresel et al. 2000; Krause et al. 2000). However, this increase has not been observed in other SPECT (Van Dick et al. 2002) or PET (Jucaite et al. 2005) studies. The latter study, using [11C]PE2I in a fairly small sample (12 adolescent suffering from ADHD vs. 10 young adults as controls), showed that although the tracer binding was similar in both groups in the striatum, it was significantly reduced in the midbrain of ADHD subjects. In addition, a significant correlation was found between the levels of PE2I binding in the striatum and the degree of hyperactivity in ADHD subjects. PE2I appears therefore to be a marker that is sufficiently sensitive to allow detection of modifications of the DAT in small extrastriatal brain areas and the correlation between the striatal DAT level and locomotor hyperactivity in ADHD.
The excellent properties of PE2I detailed above are to date the basis for the development of new DAT tracers like LBT‐999 to be used in future PET explorations using fluorine‐18 (Chalon et al. 2006; Dollé et al. 2006; Saba et al. 2007).
Conflict of Interest
The authors have no conflict of interest.
References
- Acton PD, Kushner SA, Kung MP, Mozley PD, Plossl K, Kung HF (1999) Simplified reference region model for the kinetic analysis of [99mTc]TRODAT‐1 binding to dopamine transporters in nonhuman primates using single‐photon emission tomography. Eur J Nucl Med 26:518–526. [DOI] [PubMed] [Google Scholar]
- Asenbaum S, Brucke T, Pirker W, Podreka I, Angelberger P, Wenger S, Wober C, Muller C, Deecke L (1997) Imaging of dopamine transporters with iodine‐123‐beta‐CIT and SPECT in Parkinson's disease. J Nucl Med 381:1–6. [PubMed] [Google Scholar]
- Barc S, Page G, Barrier L, Garreau L, Guilloteau D, Fauconneau B, Chalon S (2002) Relevance of different striatal markers in assessment of the MPP+‐induced dopaminergic nigrostriatal injury in rat. J Neurochem 80:365–374. [DOI] [PubMed] [Google Scholar]
- Benamer TS, Patterson J, Grosset DG, Booij J, De Bruin K, Van Royen E, Speelman JD, Horstink MH, Sips HJ, Dierckx RA, et al. (2000) Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]‐FP‐CIT SPECT imaging: The [123I]‐FP‐CIT study group. Mov Disord 15:503–510. [PubMed] [Google Scholar]
- Berthommier E, Loc'h C, Chalon S, Olivier C, Emond P, Dao Boulanger H, Lelait MA, Mauclaire L (2002) New preparation of [123I]PE2I: Investigation of the oxidation and purification steps. J Lab Compd Radiopharm 45:1019–1028. [Google Scholar]
- Bézard E, Boraud T, Chalon S, Brotchie JM, Guilloteau D, Gross CE (2001a) Pallidal border cells: An anatomical and electrophysiological study in the 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine‐treated monkey. Neuroscience 103:117–123. [DOI] [PubMed] [Google Scholar]
- Bézard E, Dovero S, Prunier C, Ravenscroft P, Chalon S, Guilloteau D, Crossman AR, Bioulac B, Brotchie JM, Gross CE (2001b) Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine‐lesioned macaque model of Parkinson's disease. J Neurosci 21:6853–6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boja JW, Carroll FI, Rahman MA, Philip A, Lewin AH, Kuhar MJ (1990) New, potent cocaine analogs: Ligand binding and transport studies in rat striatum. Eur J Pharmacol 184:329–332. [DOI] [PubMed] [Google Scholar]
- Boja JW, Patel A, Carroll FI, Rahman MA, Philip A, Lewin AH, Kopajtic TA, Kuhar MJ (1991) [125I]RTI‐55: A potent ligand for dopamine transporters. Eur J Pharmacol 194:133–134. [DOI] [PubMed] [Google Scholar]
- Boja JW, Mitchell WM, Patel A, Kopajtic TA, Carroll FI, Lewin AH, Abraham P, Kuhar MJ (1992) High‐affinity binding of [125I]RTI‐55 to dopamine and serotonin transporters in rat brain. Synapse 12:27–36. [DOI] [PubMed] [Google Scholar]
- Booij J, Habraken JB, Bergmans P, Tissingh G, Winogrodzka A, Wolters EC, Janssen AG, Stoof JC, Van Royen EA (1998) Imaging of dopamine transporters with iodine‐123‐FP‐CIT SPECT in healthy controls and patients with Parkinson's disease. J Nucl Med 39:1879–1884. [PubMed] [Google Scholar]
- Bouchez G, Sensebé L, Garreau L, Besnard JC, Guilloteau D, Charbord P, Chalon S (2007) Graft of adult mesenchymal stem cells cultures in a standard or neuronal differentiation medium in a rat model of Parkinson's disease 5th Forum of European Neuroscience, abstract. [Google Scholar]
- Brucke T, Kornhuber J, Angelberger P, Asenbaum S, Frassine H, Podreka I (1993) SPECT imaging of dopamine and serotonin transporters with [123I]beta‐CIT. Binding kinetics in the human brain. J Neural Transm Gen Sect 94:137–146. [DOI] [PubMed] [Google Scholar]
- Castellanos FX (1997) Toward a pathophysiology of attention‐deficit/hyperactivity disorder. Clin Pediatr (Phila) 36:381–393. [DOI] [PubMed] [Google Scholar]
- Catafau AM, Tolosa E, DaTSCAN Clinically Uncertain Parkinsonian Syndromes Study Group (2004) Impact of dopamine transporter SPECT using 123I‐Ioflupane on diagnosis and management of patients with clinically uncertain Parkinsonian syndromes. Mov Disord 19:1175–1182. [DOI] [PubMed] [Google Scholar]
- Chalon S, Emond P, Bodard S, Vilar MP, Thiercelin C, Besnard JC, Guilloteau D (1999a) Time course of changes in striatal dopamine transporters and D2 receptors with specific iodinated markers in a rat model of Parkinson's disease. Synapse 31:134–139. [DOI] [PubMed] [Google Scholar]
- Chalon S, Garreau L, Emond P, Zimmer L, Vilar MP, Besnard JC, Guilloteau D (1999b) Pharmacological characterization of (E)‐N‐(3‐iodoprop‐2‐enyl)‐2β‐carbomethoxy‐3β‐(4′‐methylphenyl)nortropane as a selective and potent inhibitor of the neuronal dopamine transporter. J Pharmacol Exp Ther 291:648–654. [PubMed] [Google Scholar]
- Chalon S, Hall H, Saba W, Garreau L, Dollé F, Halldin C, Emond P, Bottlaender M, Deloye J‐B, Helfenbein J, et al. (2006) Pharmacological characterization of (E)‐N‐(4‐fluorobut‐2‐enyl)‐2β‐carbomethoxy‐3β‐(4′‐tolyl) nortropane (LBT‐999) as a highly promising fluorinated ligand for the dopamine transporter. J Pharmacol Exp Ther 317:147–152. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB, Levey AI (1995) The dopamine transporter: Immunochemical characterization and localization in brain. J Neurosci 15:1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EH, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, Leventhal BL (1995) Association of attention‐deficit disorder and the dopamine transporter gene. Am J Hum Genet 56:993–998. [PMC free article] [PubMed] [Google Scholar]
- Dollé F, Bottlaender M, Demphel S, Emond P, Fuseau C, Coulon C, Ottaviani M, Valette H, Loc'h C, Halldin C, et al. (2000) Highly efficient synthesis of [11C]PE2I, a selective radioligand for the quantification of the dopamine transporter using PET. J Lab Compounds Radiopharm 43:997–1004. [Google Scholar]
- Dollé F, Emond P, Mavel S, Demphel S, Hinnen F, Mincheva Z, Saba W, Valette H, Chalon S, Halldin C, et al. (2006) Synthesis, radiosynthesis and in vivo preliminary evaluation of [(11)]LBT‐999, a selective radioligand for the visualisation of the dopamine transporter with PET. Bioorg Med Chem 14:1115–1125. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Bonab AA, Spencer TJ, Rauch SL, Madras BK, Fischman AJ (1999) Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet 354:2132–2133. [DOI] [PubMed] [Google Scholar]
- Dresel S, Krause J, Krause KH, LaFougere C, Brinkbaumer K, Kung HF, Hahn K, Tatsch K (2000) Attention deficit hyperactivity disorder: Binding of [99mTc]TRODAT‐1 to the dopamine transporter before and after methylphenidate treatment. Eur J Nucl Med 27:1518–1524. [DOI] [PubMed] [Google Scholar]
- Elfving B, Bjornholm B, Knudsen GM (2003) Interference of anaesthetics with radioligand binding in neuroreceptor studies. Eur J Nucl Med Mol Imaging 30:912–915. [DOI] [PubMed] [Google Scholar]
- Emond P, Garreau L, Chalon S, Boazi M, Caillet M, Bricard J, Frangin Y, Mauclaire L, Besnard JC, Guilloteau D (1997) Synthesis and ligand binding of nortropane derivatives: N‐substituted 2β‐carbomethoxy‐3β‐(4′‐iodophenyl) nortropane and N‐(3‐iodoprop‐(2E)‐enyl)‐2β‐carbomethoxy‐3β‐(3′,4′‐disubstituted phenyl)nortropane. New high‐affinity and selective compounds for the dopamine transporter. J Med Chem 40:1366–1372. [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Neve RL, Janowsky A, Neve KA (1995) Characterization of a recombinant human dopamine transporter in multiple cell lines. J Pharmacol Exp Ther 274:276–283. [PubMed] [Google Scholar]
- Farde L, Ginovart N, Halldin C, Chou YH, Olsson H, Swahn CG (2000) A PET study of [11C]‐β‐CIT‐FE, binding to the dopamine transporter in the monkey and human brain. Int J Neuropsychopharmacol 3:203–214. [DOI] [PubMed] [Google Scholar]
- Gill M, Daly G, Heron S, Hawi Z, Fitzgerald M (1997) Confirmation of association between attention deficit hyperactivity disorder and a dopamine transporter polymorphism. Mol Psychiatry 2:311–313. [DOI] [PubMed] [Google Scholar]
- Gouhier C, Chalon S, Aubert‐Pouessel A, Venier‐Julienne MC, Jollivet C, Benoit JP, Guilloteau D (2002) Protection of dopaminergic nigrostriatal afferents by GDNF delivered by microspheres in a rodent model of Parkinson's disease. Synapse 44:124–131. [DOI] [PubMed] [Google Scholar]
- Guilloteau D, Emond P, Baulieu JL, Garreau L, Frangin Y, Pourcelot L, Mauclaire L, Besnard JC, Chalon S (1998) Exploration of the dopamine transporter: in vitro and in vivo characterization of a high‐affinity and high‐specificity iodinated tropane derivative (E)‐N‐(3‐iodoprop‐2‐enyl)‐2β‐carbomethoxy‐3β‐(4′‐methylphenyl)nortropane (PE2I). Nucl Med Biol 25:331–337. [DOI] [PubMed] [Google Scholar]
- Hall H, Halldin C, Farde L, Sedvall G (1998) Whole hemisphere autoradiography of the postmortem human brain. Nucl Med Biol 25:715–719. [DOI] [PubMed] [Google Scholar]
- Hall H, Halldin C, Guilloteau D, Chalon S, Emond P, Besnard J, Farde L, Sedvall G (1999) Visualization of the dopamine transporter in the human brain postmortem with the new selective ligand [125I]PE2I. Neuroimage 9:108–116. [DOI] [PubMed] [Google Scholar]
- Halldin C, Erixon‐Lindroth N, Pauli S, Chou YH, Okubo Y, Karlsson P, Lundkvist C, Olsson H, Guilloteau D, Emond P, Farde L (2003) [(11)C]PE2I: A highly selective radioligand for PET examination of the dopamine transporter in monkey and human brain. Eur J Nucl Med Mol Imaging 30:1220–1230. [DOI] [PubMed] [Google Scholar]
- Halldin C, Farde L, Lundkvist C, Ginovart N, Nakashima Y, Karlsson P, Swahn CG (1996) [11C]β‐CIT‐FE, a radioligand for quantitation of the dopamine transporter in the living brain using positron emission tomography. Synapse 22:386–390. [DOI] [PubMed] [Google Scholar]
- Inaji M, Okauchi T, Ando K, Maeda J, Nagai Y, Yoshizaki T, Okano H, Nariai T, Ohno K, Obayashi S, et al. (2005a) Correlation between quantitative imaging and behavior in unilaterally 6‐OHDA‐lesioned rats. Brain Res 1064:136–145. [DOI] [PubMed] [Google Scholar]
- Inaji M, Yoshizaki T, Okauchi T, Maeda J, Nagai Y, Nariai T, Ohno K, Ando K, Okano H, Obayashi S, et al. (2005b) In vivo PET measurements with [11C]PE2I to evaluate fetal mesencephalic transplantations to unilateral 6‐OHDA‐lesioned rats. Cell Transplant 14:655–663. [DOI] [PubMed] [Google Scholar]
- Innis RB, Seibyl JP, Scanley BE, Laruelle M, Abi‐Dargham A, Wallace E, Baldwin RM, Zea‐Ponce Y, Zoghbi S, Wang S, et al. (1993) Single photon emission computed tomographic imaging demonstrates loss of striatal dopamine transporters in Parkinson disease. Proc Natl Acad Sci USA 90:11965–11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky A, Berger P, Vocci F, Labarca R, Skolnick P, Paul S (1986) Characterization of sodium‐dependent [3H]GBR‐12935 binding in brain: A radioligand for selective labelling of the dopamine transport complex. J Neurochem 46:1272–1276. [DOI] [PubMed] [Google Scholar]
- Jewett DM (1992) A simple synthesis of [11C]methyl triflate. Int J Rad Appl Instrum [A 43:1383–1385. [DOI] [PubMed] [Google Scholar]
- Jucaite A, Fernell E, Halldin C, Forssberg H, Farde L (2005) Reduced midbrain dopamine transporter binding in male adolescents with attention‐deficit/hyperactivity disorder: Association between striatal dopamine markers and motor hyperactivity. Biol Psychiatry 57:229–238. [DOI] [PubMed] [Google Scholar]
- Jucaite A, Odano I, Olsson H, Pauli S, Halldin C, Farde L (2006) Quantitative analyses of regional [11C]PE2I binding to the dopamine transporter in the human brain: A PET study. Eur J Nucl Med Mol Imaging 33:657–668. [DOI] [PubMed] [Google Scholar]
- Krause KH, Dresel SH, Krause J, Kung HF, Tatsch K (2000) Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder: Effects of methylphenidate as measured by single photon emission computed tomography. Neurosci Lett 285:107–110. [DOI] [PubMed] [Google Scholar]
- Kuikka JT, Baulieu JL, Hiltunen J, Halldin C, Bergstrom KA, Farde L, Emond P, Chalon S, Yu M, Nikula T, et al. (1998) Pharmacokinetics and dosimetry of iodine‐123 labelled PE2I in humans, a radioligand for dopamine transporter imaging. Eur J Nucl Med 25:531–534. [DOI] [PubMed] [Google Scholar]
- Kung MP, Essman WD, Frederick D, Meegalla S, Goodman M, Mu M, Lucki I, Kung HF (1995) IPT: A novel iodinated ligand for the CNS dopamine transporter. Synapse 20:316–324. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Wallace E, Seibyl JP, Baldwin RM, Zea‐Ponce Y, Zoghbi SS, Neumeyer JL, Charney DS, Hoffer PB, Innis RB (1994) Graphical, kinetic, and equilibrium analyses of in vivo[123I] beta‐CIT binding to dopamine transporters in healthy human subjects. J Cereb Blood Flow Metab 14:982–994. [DOI] [PubMed] [Google Scholar]
- Leroy C, Comtat C, Trebossen R, Syrota A, Martinot JL, Ribeiro MJ (2007) Assessment of 11C‐PE2I binding to the neuronal dopamine transporter in humans with the high‐spatial‐resolution PET scanner HRRT. J Nucl Med 48:538–546. [DOI] [PubMed] [Google Scholar]
- Lundkvist C, Halldin C, Ginovart N, Swahn C‐G, Farde L (1997) [18F][beta]‐CIT‐FP is superior to [11C][beta]‐CIT‐FP for quantitation of the dopamine transporter. Nucl Med Biol 24:621–627. [DOI] [PubMed] [Google Scholar]
- Madras BK, Spealman RD, Fahey MA, Neumeyer JL, Saha JK, Milius RA (1989) Cocaine receptors labeled by [3H]2 beta‐carbomethoxy‐3 beta‐(4‐fluorophenyl)tropane. Mol Pharmacol 36:518–524. [PubMed] [Google Scholar]
- Meissner W, Prunier C, Guilloteau D, Chalon S, Gross CE, Bezard E (2003) Time‐course of nigrostriatal degeneration in a progressive MPTP‐lesioned macaque model of Parkinson's disease. Mol Neurobiol 28:209–218. [DOI] [PubMed] [Google Scholar]
- Morrish PK (2003) How valid is dopamine transporter imaging as a surrogate marker in research trials in Parkinson's disease? Mov Disord 18:S63–S70. [DOI] [PubMed] [Google Scholar]
- Page G, Chalon S, Emond P, Maloteaux JM, Hermans E (2002) Pharmacological characterisation of (E)‐N‐(3‐iodoprop‐2‐enyl)‐2β‐carbomethoxy‐3β‐(4′‐methylphenyl)nortropane (PE2I) binding to the rat neuronal dopamine transporter expressed in COS cells. Neurochem Int 40:105–113. [DOI] [PubMed] [Google Scholar]
- Pinborg LH, Videbaek C, Svarer C, Yndgaard S, Paulson OB, Knudsen GM (2002) Quantification of [(123)I]PE2I binding to dopamine transporters with SPECT. Eur J Nucl Med Mol Imaging 29:623–631. [DOI] [PubMed] [Google Scholar]
- Pinborg LH, Ziebell M, Frokjaer VG, De Nijs R, Svarer C, Haugbol S, Yndgaard S, Knudsen GM (2005) Quantification of 123I‐PE2I binding to dopamine transporter with SPECT after bolus and bolus/infusion. J Nucl Med 46:1119–1127. [PubMed] [Google Scholar]
- Pirker W, Djamshidian S, Asenbaum S, Gerschlager W, Tribl G, Hoffmann M, Brucke T (2002) Progression of dopaminergic degeneration in Parkinson's disease and atypical parkinsonism: A longitudinal β‐CIT SPECT study. Mov Disord 17:45–53. [DOI] [PubMed] [Google Scholar]
- Poyot T, Conde F, Gregoire MC, Frouin V, Coulon C, Fuseau C, Hinnen F, Dolle F, Hantraye P, Bottlaender M (2001) Anatomic and biochemical correlates of the dopamine transporter ligand 11C‐PE2I in normal and parkinsonian primates: Comparison with 6‐[18F]fluoro‐L‐dopa. J Cereb Blood Flow Metab 21:782–792. [DOI] [PubMed] [Google Scholar]
- Prunier C, Bezard E, Montharu J, Mantzarides M, Besnard JC, Baulieu JL, Gross C, Guilloteau D, Chalon S (2003a) Presymptomatic diagnosis of experimental Parkinsonism with 123I‐PE2I SPECT. Neuroimage 19:810–816. [DOI] [PubMed] [Google Scholar]
- Prunier C, Payoux P, Guilloteau D, Chalon S, Giraudeau B, Majorel C, Tafani M, Bezard E, Esquerre JP, Baulieu JL (2003b) Quantification of dopamine transporter by 123I‐PE2I SPECT and the noninvasive Logan graphical method in Parkinson's disease. J Nucl Med 44:663–670. [PubMed] [Google Scholar]
- Ribeiro MJ, Ricard M, Lievre MA, Bourgeois S, Emond P, Gervais P, Dolle F, Syrota A (2007) Whole‐body distribution and radiation dosimetry of the dopamine transporter radioligand [(11)C]PE2I in healthy volunteers. Nucl Med Biol 34:465–470. [DOI] [PubMed] [Google Scholar]
- Saba W, Valette H, Schöllhorn‐Peyronneau M‐A, Coulon C, Ottaviani M, Chalon S, Dollé F, Emond P, Halldin C, Helfenbein J, et al. (2007) [11C]LBT‐999: A suitable radioligand for investigation of extra‐striatal dopamine transporter with PET. Synapse 61:17–23. [DOI] [PubMed] [Google Scholar]
- Seeman P, Madras BK (1998) Antihyperactivity medication: Methylphenidate and amphetamine. Mol Psychiatry 3:386–396. [DOI] [PubMed] [Google Scholar]
- Seibyl JP, Marek KL, Quinlan D, Sheff K, Zoghbi S, Zea‐Ponce Y, Baldwin RM, Fussell B, Smith EO, Charney DS, et al. (1995) Decreased single‐photon emission computed tomographic [123I]β‐CIT striatal uptake correlates with symptom severity in Parkinson's disease. Ann Neurol 38:589–598. [DOI] [PubMed] [Google Scholar]
- Seibyl JP, Marek K, Sheff K, Baldwin RM, Zoghbi S, Zea‐Ponce Y, Charney DS, Van Dyck CH, Hoffer PB, Innis RB (1997) Test/retest reproducibility of iodine‐123‐βCIT SPECT brain measurement of dopamine transporters in Parkinson's patients. J Nucl Med 38:1453–1459. [PubMed] [Google Scholar]
- Shetty HU, Zoghbi SS, Liow JS, Ichise M, Hong J, Musachio JL, Halldin C, Seidel J, Innis RB, Pike VW (2007) Identification and regional distribution in rat brain of radiometabolites of the dopamine transporter PET radioligand [(11)C]PE2I. Eur J Nucl Med Mol Imaging 34:667–678. [DOI] [PubMed] [Google Scholar]
- Solanto MV (1984) Neuropharmacological basis of stimulant drug action in attention deficit disorder with hyperactivity: A review and synthesis. Psychol Bull 95:387–409. [PubMed] [Google Scholar]
- Stepanov V, Jarv J (2006) Slow isomerization step in the interaction between mouse dopamine transporter and dopamine re‐uptake inhibitor N‐(3‐iodoprop‐2E‐enyl)‐2β‐carbo‐[3H]methoxy‐3β‐(4′‐methylphenyl)nortropane. Neurosci Lett 410:218–221. [DOI] [PubMed] [Google Scholar]
- Stepanov V, Schou M, Jarv J, Halldin C (2007) Synthesis of 3H‐labeled N‐(3‐iodoprop‐2E‐enyl)‐2β‐carbomethoxy‐3β‐(4‐methylphenyl)nortropane (PE2I) and its interaction with mice striatal membrane fragments. Appl Radiat Isot 65:293–300. [DOI] [PubMed] [Google Scholar]
- Strickland S, Palmer G, Massey V (1975) Determination of dissociation constants and specific rate constants of enzyme‐substrate (or protein‐ligand) interactions from rapid reaction kinetic data. J Biol Chem 250:4048–4052. [PubMed] [Google Scholar]
- Swanson JM, Flodman P, Kennedy J, Spence MA, Moyzis R, Schuck S, Murias M, Moriarity J, Barr C, Smith M, et al. (2000) Dopamine genes and ADHD. Neurosci Biobehav Rev 24:21–25. [DOI] [PubMed] [Google Scholar]
- Tupala E, Kuikka JT, Hall H, Bergstrom K, Sarkioja T, Rasanen P, Mantere T, Hiltunen J, Vepsalainen J, Tiihonen J (2001) Measurement of the striatal dopamine transporter density and heterogeneity in type I alcoholics using human whole hemisphere autoradiography. Neuroimage 14:87–94. [DOI] [PubMed] [Google Scholar]
- Tupala E, Hall H, Bergstrom K, Mantere T, Rasanen P, Sarkioja T, Hiltunen J, Tiihonen J (2003) Different affect of age on dopamine transporters in the dorsal and ventral striatum of controls and alcoholics. Synapse 48:205–211. [DOI] [PubMed] [Google Scholar]
- Tupala E, Halonen P, Tiihonen J (2006) Visualization of the cortical dopamine transporter in type I and 2 alcoholics with human whole hemisphere autoradiography. Eur Neuropsychopharmacol 16:552–560. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Walther D, Mash D, Faucheux B, Javoy‐Agid F (1994) Dopamine transporter messenger RNA in Parkinson's disease and control substantia nigra neurons. Ann Neurol 35:494–498. [DOI] [PubMed] [Google Scholar]
- Van Dick CH, Quinlan DM, Cretella LM, Staley JK, Malison RT, Baldwin RM, Seibyl JP, Innis RB (2002) Unaltered dopamine transporter availability in adult attention deficit hyperactivity disorder. Am J Psychiatry 159:309–312. [DOI] [PubMed] [Google Scholar]
- Vizi ES (2000) Role of high‐affinity receptors and membrane transporters in nonsynaptic communication and drug action in the central nervous system. Pharmacol Rev 52:63–89. [PubMed] [Google Scholar]
- Wall SC, Innis RB, Rudnick G (1993) Binding of the cocaine analog 2β‐carbomethoxy‐3β‐(4‐[125I]iodophenyl)tropane to serotonin and dopamine transporters: Different ionic requirements for substrate and 2β‐carbomethoxy‐3β‐(4‐ [125I]iodophenyl)tropane binding. Mol Pharmacol 43:264–270. [PubMed] [Google Scholar]
- Ziebell M, Thomsen G, Knudsen GM, De Nijs R, Svarer C, Wagner A, Pinborg LH (2007) Reproducibility of [(123)I]PE2I binding to dopamine transporters with SPECT. Eur J Nucl Med Mol Imaging 34:101–109. [DOI] [PubMed] [Google Scholar]
- Zoghbi SS, Shetty HU, Ichise M, Fujita M, Imaizumi M, Liow JS, Shah J, Musachio JL, Pike VW, Innis RB (2006) PET imaging of the dopamine transporter with 18F‐FECNT: A polar radiometabolite confounds brain radioligand measurements. J Nucl Med 47:520–527. [PubMed] [Google Scholar]


