Abstract
Panic disorder with or without agoraphobia is a common, often chronic and refractory anxiety disorder. Although a number of pharmacotherapies are now indicated for panic disorder, many patients do not respond to available interventions. We hypothesized that duloxetine, a serotonin‐norepinephrine reuptake inhibitor (SNRI) that has greater initial noradrenergic effects than venlafaxine, would have broad efficacy for individuals with panic disorder. Fifteen individuals with panic disorder with or without agoraphobia received 8 weeks of open label duloxetine flexibly dosed from 60 to 120 mg per day. Duloxetine treatment resulted in significant anxiolysis as measured by the primary outcome measure, the Panic Disorder Severity Scale (PDSS) (paired t(df) = 4.02(14), P= 0.0013), as well as measures of generalized anxiety, depression and quality of life (all P < 0.05). Although definitive conclusions are limited due to its small open‐label nature, this first prospective study provides preliminary support for the efficacy of duloxetine for panic disorder and suggests larger randomized controlled study is warranted.
Keywords: Anxiety, antidepressant, duloxetine, panic, pharmacotherapy, treatment
Introduction
Panic disorder with or without agoraphobia is a common, often chronic and refractory anxiety disorder, occurring in 4.7% of the population [1]. Treatment of panic disorder is focused on the reduction of panic attacks, avoidance behavior, and anticipatory anxiety, as well as the resolution of comorbid symptoms and conditions to allow improvement in overall quality of life [2]. There have been a growing number of treatments with reported efficacy for panic disorder in clinical trials and practice, including pharmacotherapies such as serotonin selective reuptake inhibitor (SSRI) antidepressants, tricyclic antidepressants, and benzodiazepines, as well as psychotherapy such as cognitive behavioral therapy in recent years. Acute and longitudinal follow‐up studies suggest, however, that many individuals remain symptomatic with treatment [1, 3, 4, 5]. Thus additional treatment options for panic disorder are still needed.
Duloxetine is a serotonin‐norepinephrine reuptake inhibitor (SNRI) that has been shown to have significant noradrenergic effects even at low doses in preclinical binding studies [6], while available studies show mixed support for clinical noradrenergic effects in humans starting at 60–80 mg/day of duloxetine [7]. Although data from placebo controlled fixed dose studies in panic disorder suggested generally comparable efficacy for venlafaxine extended release (75, 150, and 225 mg/day) and paroxetine 40 mg/day [8], venlafaxine at 225 mg/day (a dose at which noradrenergic effects are likely to be relevant) was more efficacious on a number of panic measures than the SSRI [9]. Although published studies examining duloxetine prospectively for panic disorder are lacking, these indirect data combined with initial clinical experience suggested duloxetine may be effective for panic disorder. In this study, we provide initial open‐label, prospective safety and efficacy data for duloxetine for primary DSM‐IV panic disorder. We hypothesized that duloxetine would have efficacy for panic disorder and associated symptoms.
Methods
Outpatients 18–75 years of age with a current DSM‐IV diagnosis of panic disorder with or without agoraphobia were recruited through hospital and media advertisement between August 2006 and April 2008 for participation in an 8‐week open‐label, flexible dose prospective trial of duloxetine. Participants were diagnosed by a study psychiatrist using the Structured Clinical Interview for DSM‐IV (SCID) and were required for entry to be at least moderate in severity as defined by an MGH Anchored Panic CGI Severity Rating (Panic CGI‐S) greater than or equal to 4 (“moderate”). All patients signed informed consent and study procedures were approved by the Institutional Review Board at Massachusetts General Hospital.
Comorbid social anxiety, generalized anxiety, specific phobia, and unipolar depressive disorders were allowed as long as panic disorder was the primary disorder identified by the patient and clinician as causing the most distress and disability, and the Montgomery‐Asberg Depression Rating Scale (MADRS) score was below 20 points. Lifetime posttraumatic stress disorder, obsessive compulsive disorder, bipolar disorder, and psychosis were exclusionary. Exclusion criteria also included current suicidality, a history of alcohol or substance abuse or dependence within the last 6 months, or a positive toxicology screen consistent with substance abuse at baseline. Pregnant or lactating women, patients with significant unstable medical illness or with ongoing psychotherapy directed toward the treatment of panic disorder or agoraphobia were not permitted. In addition, those with prior intolerance of duloxetine or more than four previous treatment failures with panic pharmacotherapy were also excluded. Subjects were required to be free of all psychiatric medications except for benzodiazepines initiated at least 2 weeks prior to study initiation and with the dose held stable during the trial.
Duloxetine was initiated at 30 mg/day at the baseline visit, flexibly increased to 60 mg/day after 1 week, then flexibly titrated up to a maximum of 120 mg/day over the next 4 weeks based on response and tolerability with a minimum dose of 60 mg by week 4 required in order for the patient to remain in the study. Participants were seen weekly for the first 3 weeks of the study, and then at 2‐week intervals for the remainder of the trial for a total of 8 weeks. Ratings were conducted by trained study psychiatrists.
Psychometric Measurements
The primary outcome measure was the Panic Disorder Severity Scale (PDSS). The PDSS contains seven items assessing multiple dimensions of panic disorder severity, including (a) frequency of panic attacks, (b) distress during panic attacks, (c) anticipatory anxiety, (d) agoraphobic fear and avoidance, (e) interoceptive fear and avoidance, (f) impairment of work functioning, and (g) impairment of social functioning. Shear et al. [10] established interrater reliability to be 0.87. Secondary panic outcome measures included panic attack frequency as measured by the Panic Attack Scale (PAS, and the Clinical Global Impression of Severity scale (CGI‐S). The CGI‐S is a clinician‐rated instrument used to assess global severity of symptoms [11]. The CGI‐S ranges from 1 (normal, not at all ill) to 7 (among the most extremely ill). Remission was defined strictly as a CGI‐S score of 1 or 2 (not at all ill or borderline ill) and zero panic attacks at endpoint.
Additional symptom measures included the clinician rated Montgomery Asberg Depression Rating Scale (MADRS), which has demonstrated good reliability and specificity for depressive compared to anxiety symptomatology [12], and the patient rated Beck Anxiety Inventory (BAI). The Quality of Life Enjoyment and Satisfaction Questionnaire (Q‐LES‐Q: higher scores indicate better quality of life: [13], the Sheehan Disability Scale (SDS), and the Longitudinal Interval Follow‐up Evaluation Range of Impaired Functioning Tool (LIFE‐RIFT) were used to assess quality of life and functional impairment [14].
Statistical Methods
All analyses were performed examining baseline to endpoint change with a last observation carried forward method in an intent‐to‐treat (ITT) sample of all patients with at least one assessment on duloxetine. A normal distribution of the primary outcome data was confirmed with a Skewness/Kurtosis test. Statistical significance was examined with two‐tailed paired t‐tests, utilizing an alpha level of 0.05.
Results
Of the 17 individuals with panic disorder initially enrolled, two individuals completed the screening procedure but did not initiate pharmacotherapy (one decided against pharmacotherapy, one lost to follow up) and are thus not included in analyses. Fifteen individuals, including 6 (40%) with agoraphobia, had at least one assessment on duloxetine and are included in analyses. Patient demographics and baseline characteristics are described in Table 1. Six individuals (40%) had a co‐morbid anxiety disorder, major depression, dysthymia, or a combination of the three. The majority of participants (53%) reported they had not responded to at least one prior pharmacotherapy trial, with a mean ± SD total of 1.2 ± 1.3 medication trials (range 0 to 3) prior to duloxetine. Four patients were taking concomitant benzodiazepines. Subjects presented with clinically significant symptoms as assessed by the PDSS (14.2 ± 4.28), CGI‐S (mean ± SD = 4.80 ± 0.67, i.e., “Moderately Ill” to “Markedly Ill”), and PAS (3.90 ± 2.38 attacks per week): see Table 2.
Table 1.
Patient demographics and disorder characteristics
| Subject Characteristics, N = 15 | |
|---|---|
| Age (years), mean (SD) | 41.1 (15.0) |
| Gender, % female (n) | 60.0 (9) |
| Race, % caucasian (n) | 93.3 (14) |
| Educational status, % college graduate (n) | 53.3 (8) |
| Duration of Illness, mean years (SD) | 14.7 (14.8) |
| Current Comorbidity, % (n) | |
| MDD | 13.3 (2) |
| Dysthymia | 20.0 (3) |
| Agoraphobia | 40.0 (6) |
| SAD | 13.3 (2) |
| Specific Phobia | 6.67 (1) |
| GAD | 20.0 (3) |
| Concomitant Medications, % (n) | |
| Benzodiazepine | 26.7 (4) |
GAD = generalized anxiety disorder; SAD = social anxiety disorder; MDD = major depressive disorder.
Table 2.
Treatment response with Duloxetine for panic disorder (n= 15)
| Instrument | Baseline Mean ± SD | Endpoint Mean ± SD | t(df) | Significance P |
|---|---|---|---|---|
| PDSS | 14.2 ± 4.28 | 9.13 ± 5.26 | 4.02 (14) | 0.0013 |
| PAS attacks/week | 3.90 ± 2.38 | 0.90 ± 1.44 | 6.06 (14) | 0.00001 |
| MADRS | 15.1 ± 7.08 | 9.07 ± 6.68 | 4.30 (14) | 0.0007 |
| CGI‐S | 4.80 ± 0.67 | 3.47 ± 1.30 | 4.64 (14) | 0.0004 |
| BAI | 28.1 ± 10.6 | 14.7 ± 13.7 | 3.83 (12) | 0.0024 |
| QLESQ | 41.3 ± 8.28 | 49.3 ± 12.8 | −3.03 (14) | 0.0091 |
| SDS | 17.4 ± 5.57 | 9.40 ± 8.61 | 3.07 (14) | 0.0083 |
| LIFE‐RIFT | 11.7 ± 2.23 | 9.33 ± 3.63 | 2.65 (11) | 0.0228 |
BAI = beck anxiety inventory; CGI‐S = clinical global impressions‐severity of illness scale; LIFE‐RIFT = longitudinal interval follow‐up evaluation range of impaired functioning tool; MADRS = montgomery‐Asberg depression rating scale, PAS = panic attack scale, PDSS = panic disorder severity scale, QLESQ = qualtiy of life and enjoyment and satisfaction questionnaire, SDS = Sheehan disability scale.
Efficacy
The mean ± SD duloxetine dose at endpoint was 94 ± 25 mg/day. Twelve subjects (80%) completed the 9‐week trial, with 3 discontinuations (n= 1 lost to follow‐up, n= 1 insomnia and loss of appetite first 5 days, n= 1 due to worsening depression). Duloxetine was associated with a significant improvement in anxiety and depressive symptoms (all primary and secondary measures P < 0.01: See Table 2 and Figure 1). Quality of life and functional measures were also all significantly improved (QLESQ, SDS, LIFE‐RIFT, all Ps < 0.03; see Table 2). At endpoint, eight (53%) patients achieved a PAS score of 0, indicating zero full panic attacks in the past 2 weeks, and 4 (26.7%) patients achieved full remission, defined as CGI‐S of 1 or 2 and zero panic attacks. There was no significant difference in reduction in PDSS score with duloxetine for those with at least one comorbid mood or anxiety disorder compared with those without comorbidity (6.0 ± 5.8 vs. 4.4 ± 4.4: t(df) = 0.59(13), P > 0.6). Stable concomitant benzodiazepine use was associated with a smaller but not significantly different reduction in PDSS score than those without benzodiazepine use (2.5 ± 1.0 vs. 6.0 ± 5.4, t(df) =−1.2(13), P > 0.2), although power was quite limited for these post hoc analyses.
Figure 1.
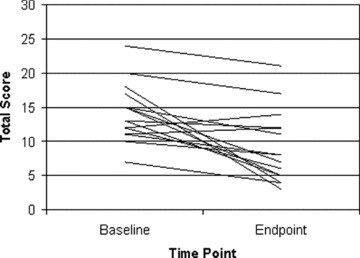
Baseline and Endpoint PDSS total scores for individual subjects (n= 15).
Safety
Duloxetine was generally well tolerated, with only two study discontinuations (13%) due to side effects (one due to insomnia and loss of appetite, one with reported worsened depression both in first week on medication) and one loss to follow up (week 2). Side effects experienced by at least 10% of the subjects included: constipation (2), dry mouth (2), nausea (3), headache (2), jitteriness (2), sexual dysfunction (3), urinary hesitation (2), insomnia (2), and sedation (4).
Discussion
This 8‐week open‐label study of the SNRI duloxetine flexibly dosed from 60 to 120 mg per day for individuals with panic disorder found preliminary support for broad efficacy across panic, anxiety and depressive symptom domains, and was associated with significant improvement in quality of life and function. Duloxetine was well tolerated with minimal study discontinuation. In post hoc analyses, we did not find evidence that duloxetine's effects on panic were accounted for by the presence of comorbid depression and anxiety disorders, or by concomitant benzodiazepine use. While conclusions are limited by the relatively small sample size and the short term, open nature of the trial, these data provide preliminary support that duloxetine may be effective and well tolerated for panic disorder, and suggest randomized placebo controlled study is indicated. In addition, follow‐up research is needed to examine potential biological predictors of response including genetic predictors that might help clarify which type of pharmacotherapy may be optimal for a given patient. For example, polymorphisms of the serotonin transporter gene have been associated with differential response to SSRIs (e.g., see [15]). Thus future studies of duloxetine should examine potential differences in both serotonin and norepinephrine related genes that might be specifically associated with treatment response to the SNRI.
Conflict of Interest
Naomi M. Simon: Research Support: Astra Zeneca, Bristol‐Myers Squibb, Cephalon, Forest Laboratories, Glaxo SmithKline, Janssen, Lilly, NARSAD, NIMH, Pfizer, UCB‐Pharma, Sepracor. Advisory/Consulting: American Foundation for Suicide Prevention, Paramount Biosciences, Pfizer, Sepracor, Solvay. Speaking: Forest Laboratories, MGH Psychiatry Academy, Janssen, Lilly, Pfizer, UCB‐Pharma. Equity Holdings: None Royalty/patent. Other income: None.
Rebecca Kaufman, Nannette N. Herlands, Maryann E. Owens. Authors declare no conflict of interest.
Elizabeth A. Hoge: Research Support: Clinical Investigator Training Program: Harvard‐MIT Health Sciences and Technology/Beth Israel Deaconess Medical Center, in collaboration with Pfizer Inc. and Merck and Company, Inc.; and Eli Lilly, Inc. Advisory/Consulting: None. Speaking: None. Equity Holdings: None. Royalty/patent, other income: None.
John J. Worthington: Research Support: Abbott Laboratories, Alkermes, Aspect Medical Systems, Astra‐Zeneca, Bristol‐Myers Squibb Company, Cephalon, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmithkline, J & J Pharmaceuticals, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Novartis, Organon Inc., PamLab, LLC, Pfizer Inc, Pharmavite, Roche, Sanofi‐Aventis, Sepracor, Solvay Pharmaceuticals, Inc., UCB Pharma and Wyeth‐Ayerst Laboratories. Speaking: Bristol‐Myers Squibb Company, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmithKline, Pfizer Inc., Sanofi‐Aventis and Wyeth‐Ayerst Laboratories.
Mark H. Pollack: Research Support: Astra‐Zeneca, Bristol Myers Squibb, Cephalon, Cyberonics, Forest Laboratories, GlaxoSmithKline, Janssen, Eli Lilly, NARSAD, NIDA, NIMH, Pfizer, Roche Laboratories, Sepracor, UCB Pharma, Wyeth.
Advisory/Consulting: AstraZeneca, Brain Cells Inc, Bristol Myers Squibb, Cephalon, Dov Pharmaceuticals, Forest Laboratories, GlaxoSmithKline, Janssen, Jazz Pharmaceuticals, Eli Lilly & Co, Medavante, Neurocrine, Neurogen, Novartis, Otsuka Pharmaceuticals, Pfizer, Predix, Roche, Laboratories, Sanofi, Sepracor, Solvay, Tikvah Therapeutics, Transcept Inc, UCB Pharma, Wyeth. Speaking: Bristol Myers Squibb, Forest Laboratories, GlaxoSmithKline, Janssen, Lilly, Pfizer, Solvay, Wyeth.
Equity Holdings: Medavante, Mensante Corporation. Royalty/patent, other income: Copyright royalties for the SIGH‐A, SAFER.
Acknowledgments
This trial was funded by an investigator initiated study grant from Lilly. Lilly was not involved in study design, data collection, data analysis, and interpretation, manuscript preparation or publication decisions, although they were given the opportunity to offer comments for author consideration prior to submission.
References
- 1. Kessler RC, et al The epidemiology of panic attacks, panic disorder, and agoraphobia in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2006;63(4):415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pollack MH. The pharmacotherapy of panic disorder. J Clin Psychiatry 2005;66( Suppl 4):23–27. [PubMed] [Google Scholar]
- 3. Pollack MH, et al Cognitive behavior therapy for treatment‐refractory panic disorder. J Clin Psychiatry 1994;55(5):200–205. [PubMed] [Google Scholar]
- 4. Roy‐Byrne PP, Craske MG, Stein MB. Panic disorder. Lancet 2006;368(9540):1023–1032. [DOI] [PubMed] [Google Scholar]
- 5. Katon WJ. Clinical practice. Panic disorder. N Engl J Med 2006;354(22):2360–237. [DOI] [PubMed] [Google Scholar]
- 6. Bymaster FP, et al Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology 2001;25(6):871–80. [DOI] [PubMed] [Google Scholar]
- 7. Trivedi MH, et al Clinical evidence for serotonin and norepinephrine reuptake inhibition of duloxetine. Int Clin Psychopharmacol 2008;23(3):161–169. [DOI] [PubMed] [Google Scholar]
- 8. Pollack MH, et al A double‐blind study of the efficacy of venlafaxine extended‐release, paroxetine, and placebo in the treatment of panic disorder. Depress Anxiety 2007;24(1):1–14. [DOI] [PubMed] [Google Scholar]
- 9. Pollack M, et al A randomized controlled trial of venlafaxine ER and paroxetine in the treatment of outpatients with panic disorder. Psychopharmacology (Berl) 2007;194(2):233–242. [DOI] [PubMed] [Google Scholar]
- 10. Shear MK, et al Multicenter collaborative panic disorder severity scale. Am J Psychiatry 1997;154(11):1571–1575. [DOI] [PubMed] [Google Scholar]
- 11. Guy W. Assessment manual for psychopharmacology. US Government Printing Office, 1976. [Google Scholar]
- 12. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 13. Endicott J, et al Quality of Life Enjoyment and Satisfaction Questionnaire: A new measure. Psychopharmacol Bull 1993;29(2):321–326. [PubMed] [Google Scholar]
- 14. Leon AC, et al The Range of Impaired Functioning Tool (LIFE‐RIFT): A brief measure of functional impairment. Psychol Med 1999;29(4):869–878. [DOI] [PubMed] [Google Scholar]
- 15. Perna G, et al Antipanic efficacy of paroxetine and polymorphism within the promoter of the serotonin transporter gene. Neuropsychopharmacology 2005;30(12):2230–2235. [DOI] [PubMed] [Google Scholar]


