Abstract
Resveratrol is a phytoalexin structurally related to stilbenes, which is synthesized in considerable amounts in the skin of grapes, raspberries, mulberries, pistachios and peanuts, and by at least 72 medicinal and edible plant species in response to stress conditions. It was isolated in 1940 and did not maintain much interest for around five decades until its role in treatment of cardiovascular diseases was suggested. To date, resveratrol has been identified as an agent that may be useful to treat cancer, pain, inflammation, tissue injury, and other diseases. However, currently the attention is being focused in analyzing its properties against neurodegenerative diseases and as antiaging compound. It has been reported that resveratrol shows effects in in vitro models of epilepsy, Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis, and nerve injury. However, evidences in vivo as well as in human beings are still lacking. Thus, further investigations on the pharmacological effects of resveratrol in vivo are necessary before any conclusions on its effects on neurodegenerative diseases can be obtained.
Keywords: Brain injury, Clinical studies, Lifespan, Neurodegenerative diseases, Neuropathic pain, Pharmacokinetics, Resveratrol
Introduction
Flavonoids and stilbenes are biosynthesized through the shikimate pathway. They are important because they contribute to flavor and color of many fruits and plants, as well as normal growth, development, and defense [1]. Stilbenes are molecules of two phenolic rings connected by an ethene molecule, and can be classified according to the presence of different substituents on the aromatic rings and to the degree of ring saturation. Resveratrol is the main stilbene generated in considerable amounts in the skin of grapes, raspberries, mulberries, pistachios and peanuts, and by at least 72 medicinal and edible plants species such as Polygonum cuspidatum, Veratrum grandiflorum, Arachis hypogaea, Rhizoma polygoni cuspidate, Yucca Shidigera, Cassia quinquangulata, Rheum rhamponticum among others [2, 3]. It can be found as glucoside [4] forming oligomers [5], tetramers [6], trimers [7], and oxyresveratrol, which is also known as piceatannol [8].
Resveratrol (3,4′,5‐trihydroxystilbene) is a phytoalexin polyphenolic structurally related to stilbenes. It is found in two isomers, cis‐ (Z) and trans‐ (E) resveratrol. Initially, cis‐resveratrol was considered biologically inactive; however, several reports have shown that both isoforms possess biological activity, although trans‐resveratrol seems to be more potent than cis‐resveratrol in most of the comparative studies indicating certain stereo‐specific activity of the molecule [9]. Physically, it is a white powder with slight yellow cast, which is soluble in ethanol and dimethyl sulfoxide and very slightly soluble in warm water.
Resveratrol represents 5–10% of the biomass of grape skins and it is contained in concentrations ranging from 0.05 to 25 mg/L in most wines and grape juices [10] whereas its concentration in peanuts, pistachios, blueberries, and bilberries reach up to 5.1 μg/g. In addition, plants as R. rhamponticum contain around 3.9 mg/g of this compound [3]. The fact that resveratrol is contained in a wide variety of dietary resources gives a reason to think that regular consumption of resveratrol in the diet may be useful for treatment of multiple illnesses in which it has shown pharmacological activity. The present review attempts to integrate the established pharmacological properties of resveratrol on central nervous system function. Other actions such as those related with its cardiovascular [11] and cancer [12, 13] effects have been previously reviewed.
History
The ancient cultures already knew beneficial properties of red wine. In the medicinal book of Hindus titled Ayurveda is described a fermented red grape juice used as a cardiotonic compound [14]. Consequently, the Holy Bible described to Timothy, a Christian believer who was allowed to drink a little wine because of his stomach and frequent illnesses. In 1940, resveratrol was isolated and identified from V. grandiflorum[15] and later on from the roots of P. cuspidatum, an oriental plant used in traditional medicine to treat fungal infections, dermatitis and hyperlipidemic diseases [16]. At the beginning, resveratrol was described as a phytoalexin synthesized in the leaf epidermis and grape skin from Vitis vinifera in response to infection or injury [17].
This compound did not maintain much interest for about 50 years until 1992, when Siemann and Creasy suggested that the resveratrol was the active ingredient in wines causing reduction of serum lipids. After this paper, many authors focused on its cardiovascular properties and resveratrol was postulated as a candidate to explain the low incidence of heart diseases in France (French paradox), a country with high‐fat diet and moderate red wine consumption [18, 19].
The attention came back to resveratrol after 1997, when it was shown that it could have chemopreventive activity [20]. Since then, the number of articles published each year on resveratrol as well as its properties has been increasing in an exponential manner. To date, several reports have clearly established that resveratrol is an anti‐inflammatory, analgesic, antioxidant, and anti‐isquemic compound [3, 21, 22]. Currently, the new interest on resveratrol is in identifying its properties and mechanisms in neurodegenerative diseases and aging [23].
Pharmacology
Epilepsy
The term epilepsy refers to a neuronal disorder characterized by the periodic and unpredictable occurrence of seizures whereas the term seizure refers to a transient alteration of behavior due to the disordered, synchronous, and rhythmic firing of populations of brain neurons [24]. Resveratrol has shown anticonvulsant activity in the pentylentetrazole, ferric chloride, and kainic acid models. In the model of pentylentetrazole, a chemoconvulsant useful in identifying compounds that are effective against absence seizures in human beings, resveratrol reduced the incidence of generalized convulsions by an adenosinergic mechanism that did not involve A2 adenosine receptor [25]. On the other hand, the ferric chloride model simulates seizures produced after a post‐traumatic event where free radical formation seems to play a critical role in the neuronal disorder genesis. In this model, resveratrol delays the onset of epileptiform electroencephalographic changes and significantly reduces malondialdehyde levels, a marker of oxidative stress [26]. In addition, it attenuates the incidence of convulsions in the kainic acid model when this is administered as pre‐ and post‐treatment or in chronic administration protecting from the damage in the olfactory cortex and hippocampus [27, 28]. The main mechanism of action of classical antiseizure drugs, as carbamazepine, phenytoin, or valproate, is to promote the inactivated state of voltage‐activated Na+ channels. In this regard, resveratrol has shown to act through this mechanism in dorsal root ganglion cells [29].
Alzheimer's Disease
The pathological hallmarks of Alzheimer's disease are due to the loss of hippocampal and cortical neurons that clinically manifest a gradual impairment of cognitive abilities accompanied often of aphasia, disorientation, and desinhibition. The alterations are produced by the formation of neurofibrillary tangles and senile plaques of protein β‐amyloid which are the product of larger amyloid precursor proteins [30]. Epidemiological studies have shown that moderate red wine consumption can attenuate clinical dementia produced by Alzheimer's disease in humans [31]. Accordingly, red wine diminishes deterioration of Alzheimer's disease‐related spatial memory in mice [32] and it increases the cellular viability in cultures [33]. Furthermore, resveratrol has shown a memory improvement in different behavioral tests [34, 35].
Resveratrol treatment markedly inhibits polymerization of the β‐amyloid peptide [36] by a mechanism that does not involve β‐amyloid production because resveratrol has no effect on activity of β‐ and γ‐secretases but stimulates indirectly the proteosomal degradation of β‐amyloid peptides [37]. Han et al. [38] have reported that the protective effects of resveratrol on β‐amyloid protein‐induced toxicity in cultured rat hippocampal cells are specifically related to activation of protein kinase C. In addition, Miloso et al. [39] showed that resveratrol activates ERK1/2 kinases in neuroblastoma cells. Besides these described pathways, it has been postulated that resveratrol produces its neuroprotective activity against Alzheimer's disease through enhancing the intracellular free‐radical scavenger glutathione [40], decreasing malondialdehyde levels, suppressing acetylcholinesterase activity, and increasing Bax/BcL‐X(L) ratio [33, 34]. In addition, it ameliorates cellular damage reducing oxidized human low‐ and very low‐density lipoproteins [41, 42], and activating transthyretin, a protein that sequesters β‐amyloid protein and prevents β‐amyloid aggregation [43]. Chen et al. [44] have observed that the neurodegeneration induced by β‐amyloid peptides depends on contributions from surrounding glia where nuclear factor‐κB (NF‐κB) signaling activation plays an important role. Under this condition, resveratrol reduces NF‐κB signaling by sirtuin‐1 (SIRT1) activation. In support of these data, resveratrol strikingly reduces the expression of genes modulated by NF‐κB, such as inducible nitric oxide synthase, cyclooxygenase‐2, and cathepsin as well as nitric oxide and prostaglandin E2 metabolites [44, 45]. In addition, SIRT1 activation by resveratrol also deacetylates PGC‐1α and p53 proteins improving associate learning in p25 transgenic mice, an Alzheimer's disease‐like model characterized for decreased learning capability because p53 is a protein that undergoes phosphorylation by p25 enhancing neurodegenerative damage [Fig. 1; 35].
Figure 1.
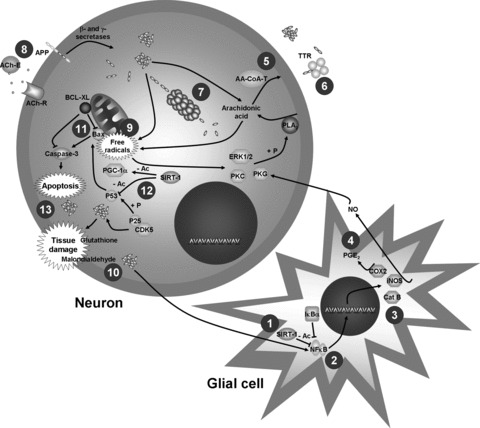
Mechanisms proposed of neuronal protection of resveratrol in Alzheimers disease. Glial cells play a critical role in the Aβ‐dependant neurodegeneration produced in this disease. Resveratrol reduces damage produced by glial cells activating sirtuin‐1 (SIRT‐1) [1], inhibiting transcription factors as nuclear factor κB (NFκB) [2] or reducing activity and expression of diverse enzymes [3] and the levels of their corresponding metabolites [4]. In addition, resveratrol improves stability of the plasmatic membrane increasing the arachidonic acid incorporation into phospholipids presumably by activation of arachidonyl‐coenzyme A transferase (AA‐CoA‐T) [5]. Likewise, it decreases the formation of senile plaques increasing activity of transthyretin (TTR) [6] and stimulating the degradation proteasomal of the amyloid peptide [7]. Resveratrol also inhibits the activity of acetylcholinesterase (ACh‐E), an enzyme that degrades acetylcholine [8] and it reduces the amount of free radicals directly or increasing free‐radical scanvenger systems as glutathione [9 and 10], likewise it increases the activity or expression of antiapoptotic factors and decreases expression or activity of pro‐apoptotic factors [11 and 12] improving the cell survival [13]. Other abbreviations: IκBα= Inhibitor κBα; Cat B = cathepsin B; iNOS = inducible nitric oxide synthase; COX2 = cyclooxygenase‐2; NO = nitric oxide; PGE2= prostaglandin E2; PKG = protein kinase G; PKC = protein kinase C; ERK1/2 = extracellular signal‐regulated kinases 1/2; PLA2= phospholipase A2; APP = amyloid precursor protein; ACh‐R = acetylcholine receptor; CDK5 = cyclin‐dependant kinase 5; Cas‐3 = caspase 3; PGC‐1α= peroxisome proliferator‐activated receptor gamma co‐activator 1 alpha; –Ac = deacetylation; +P = phosphorylation.
Notwithstanding all these data, there is no direct experimental evidence that the red wine (or specifically resveratrol) beneficially influence Alzheimer's disease. The role of resveratrol in several studies is not clear because the red wine used in that studies contains very low amounts of resveratrol (0.88 μM), which is 10‐fold lower than the minimal effective concentration shown to produce its in vitro effects (10–40 μM, Table 1; 32, 37].
Table 1.
Pharmacokinetic parameters of resveratrol and its main metabolites
| Dose | Treatment | Specie | Cmax | T max | t1/2 | AUC | CLT | References |
|---|---|---|---|---|---|---|---|---|
| Oral resveratrol | ||||||||
| Free resveratrol | ||||||||
| 25 mg | Single dose | Humans | <0.02 μM | 1.0 h | – | – | – | 96 |
| 25 mg | Single dose | Humans | ∼0.03 μM | 0.5 h | – | – | – | 92 |
| 500 mg | Single dose | Humans | 0.3 μM | 0.8 h | 2.9 h | 1.0 μM·h | 2.2 L/h | 97 |
| 5000 mg | Single dose | Humans | 2.4 μM | 1.5 h | 8.5 h | 5.9 μM·h | 4.9 L/h | 97 |
| 0.1 mg/kg | Single dose | Rats | 0.07 μM | 0.5 h | – | – | 98 | |
| 0.1 mg/kg | 15 days | Rats | 0.03 μM | – | – | – | – | 98 |
| 2 mg/kg | Single dose | Rats | 2.4 μM | 0.16 h | – | – | – | 100 |
| 5 mg/kg | Single dose | Rats | 0.1 μM | 1.5 h | – | – | – | 128 |
| 20 mg/kg | Single dose | Rabbits | 1.1 μM | 0.05 h | 0.24 h | – | – | 99 |
| 20 mg/kg | Single dose | Rats | 1.2 μM | 0.1 h | – | – | – | 99 |
| 20 mg/kg | Single dose | Mice | 2.6 μM | 0.5 h | – | – | – | 99 |
| 2.6 mg/kg | 10 days | Mice | 0.08 μM | – | – | – | – | 99 |
| 50 mg/kg | Single dose | Rats | 6.6 μM | 0.3 h | 1.5 h | 7.1 μM·h | 32.4 L/h/kg | 101 |
| 4000 mg | Single dose | Mice | 30.2 μM | 0.5 h | – | – | – | 102 |
| Resveratrol glucuronide | ||||||||
| 500 mg | Single dose | Humans | 1.8 μM | 2.0 h | 2.9 h | 8.6 μM·h | 282.7 L/h | 97 |
| 5000 mg | Single dose | Humans | 5.7 μM | 2.0 h | 7.9 h | 44.2 μM·h | 590.6 L/h | 97 |
| 50 mg/kg | Single dose | Rats | 105.2 μM | 0.4 h | 1.6 h | 324.7 μM·h | 0.7 L/h/kg | 101 |
| 4000 mg | Single dose | Mice | 534 μM | 1.0 h | – | – | – | 102 |
| Resveratrol sulphate | ||||||||
| 500 mg | Single dose | Humans | 5.1 μM | 1.5 h | 3.2 h | 18.0 μM·h | 131.2 L/h | 97 |
| 5000 mg | Single dose | Humans | 19.1 μM | 2.1 h | 7.7 h | 137.6 μM·h | 207.8 L/h | 97 |
| 4000 mg | Single dose | Mice | 386 μM | 1.0 h | – | – | – | 102 |
| Intravenous resveratrol | ||||||||
| Free resveratrol | ||||||||
| 15 mg/kg | Single dose | Rats | – | – | 1.3 h | 5.6 μM·h | 11.7 L/h/kg | 101 |
| Resveratrol glucuronide | ||||||||
| 15 mg/kg | Single dose | Rats | – | – | 1.5 h | 38.7 μM·h | 1.7 L/h/kg | 101 |
Parkinson's Disease
Parkinson's disease is a neurodegenerative disease characterized by a selective loss of dopaminergic neurons in the substantia nigra pars compacta that provide dopaminergic innervations to the striatum and its neuronal toxicity is related with the aggregates formation called Lowry bodies. Clinical syndrome includes resting tremor, muscle rigidity, bradykinesia, impairment of postural balance, and difficulty in walking [30].
Numerous theories have been described to explain the cell death of nigrostriatal dopaminergic neurons in Parkinson's disease. Dopamine theory establishes that this catecholamine can be oxidized spontaneously or by some enzymes as monoamine oxidase B or cyclooxygenase‐2 to generate free radicals [46, 47], then dopamine oxidation products can suffer polymerization to form a neurotoxin called neuromelanin [48]. In this regard, low doses of resveratrol (5 μM) markedly attenuate dopamine‐induced cell death in neuroblastoma cells by activation of the antiapoptotic factor Bcl‐2 and inhibition of caspase‐3 [49]. Another theory suggests that the nitric oxide is involved in the generation of superoxide radicals and lipid peroxidation, which leads to the release of arachidonic acid. Chalimoniuk et al. [50] demonstrated that resveratrol (0.1 mM) prevents nitric oxide‐ and cyclic GMP‐dependent inhibitory effect on arachidonic acid incorporation into phophatidylinositol possibly by nitric oxide synthase inhibition. Although this mechanism may be used to explain the neuroprotective effect of resveratrol in Alzheimer's and others neurodegenerative diseases, as resveratrol concentrations greater that 6 μM were used, it is likely that this mechanism has no clinical relevance. In addition, it has been postulated that the induction of heme‐oxygenase by antioxidants may protect against neuronal disorders [51].
Huntington's Disease
Huntington's disease is a fatal neurodegenerative disorder caused by the expansion of a cytosine adenine guanine (CAG) triplet‐repeat in the huntingtin gene, which is localized on chromosome 4p16.3 and that leads to an extended polyglutamine domain called PolyQ. The repeat length in normal subjects varies from 9 to 34 repeats but in patients with the illness the number of repeats is over 40. This neurodegenerative disorder is characterized by prominent loss of neurons in the striatum of the brain although other areas can be affected in a minor grade. Atrophy of these structures produces a gradual onset of ataxia, personality changes, and dementia in midlife [30]. At molecular level, mutant huntingtin polypeptides acquire an unusual conformation facilitating their aggregation into inclusion bodies, which impair vesicular and mitochondrial traffic and cause mitochondrial dysfunction leading to an increase in free radicals production and cell death [52].
Kumar et al. [53, 54] showed that resveratrol (5–10 mg/kg) reverses motor and cognitive impairment generated in rats by administration of 3‐nitropropionic acid or colchicine through its antioxidant activity. In another study, Solans et al. [52] developed a Huntington's disease‐like model in yeast cells where they found that the interactions of huntingtin polypeptides aggregate to the mitochondrial and actin networks cause alterations in the function of mitochondrial respiratory chain complex II and III activity leading to mitochondria‐dependent cell death. In this model, resveratrol (10–50 μM) significantly reduced the amount of free radicals produced in the mutant strain but it did not diminish the size of polyQ aggregates, mitochondrial fragmentation, or the rate of growth of the strains control and mutant suggesting that the resveratrol plays a role delaying but not inhibiting disease development. Others have demonstrated that resveratrol (10–100 μM) decreases cell death of neurons derived from 111Q knock‐in mice by sirtuin activation (Sir2) and not simply for its antioxidant properties [55]. Likewise, it rescues Caenorhabditis elegans worms carrying 128Q in a dependent‐manner of sir‐2.1 and daf‐16 being the latter a member of FOXO family. However, reduction of free radicals leading to a delay of Huntington's disease as well as sirtuin activation should be taken with caution as these mechanisms were observed after high resveratrol concentrations (10–100 μM).
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis is a neuromuscular disorder generated in motor neurons of the spinal, bulbar, and cortical regions. It is a progressive degenerative disease leading to muscle weakness and eventually paralysis with a fatality within 2–3 years of onset [30]. Only 2% of all cases are due to a mutation in the enzyme superoxide dismutase 1 [56]; however, the most cases are sporadic and their mechanisms are unknown.
It has been observed that resveratrol (50–500 nM) reduces mutant superoxide dismutase‐induced neurotoxicity in transfected neurons of mice by sirtuin‐1 protein activation [35]. Moreover, resveratrol and riluzole stimulate activity of large‐conductance Ca2+‐activated K+ channels in vascular endothelial and neuroendocrine cells, respectively. As riluzole is a drug used for the treatment of amyotrophic lateral sclerosis [57], data suggest that the resveratrol could be a promise of treatment in this disease.
Neuronal Injury
It is well established that the neurons are highly susceptible to oxidative injury due to its high consumption of oxygen. Several preclinical studies have suggested that resveratrol may be useful in protecting brain and spinal cord against traumatic neuronal injury following contusion or ischemia‐reperfusion injury because resveratrol modulates different oxidative stress neuronal markers in vivo at the injury site. In brain, resveratrol decreases lactate dehydrogenase, xanthine oxidase, metalloproteinase 9, heme‐oxygenase, and malondialdehyde levels, and it increases reduced glutathione levels induced by several types of injury such as cerebral artery occlusion, cerebral ischemia‐reperfusion, focal cerebral ischemia, and streptozotocin‐induced diabetes [58, 59, 60]. Tsai et al. [60] found that, after ischemia‐reperfusion injury, resveratrol produces a differential expression of nitric oxide synthases. In this study, inducible nitric oxide synthase was decreased, whereas endothelial nitric oxide synthase was increased and there was no change in neuronal nitric oxide synthase expression giving a possible explanation of controversial effects that produce resveratrol on nitric oxide. Moreover, resveratrol inhibits peroxisome proliferator‐activated receptors alpha (PPARs; 61] and decreases NFκB p65 expression [62] involved in inflammation after ischemia‐reperfusion injury. In cultures, resveratrol inhibits postsynaptic glutamate receptors, voltage‐activated K+ channels [63], and it increases heme‐oxygenase activity [64]. All these effects decrease anxiety, improve motor and cognitive skills, and attenuate edema and cellular death as well as glial cell activation [65, 66]. Moreover, there are suggestions that neuroprotective effects of resveratrol on memory improvement are mainly due to its antioxidant properties [53, 54].
The effects in spinal cord are similar to those described in brain [67, 68]. In addition, ionic microenvironment changes after spinal cord injury have been suggested as potent therapeutic targets. In support of this idea, resveratrol improved Na+, K+‐ATPase activity and augmented Ca2+‐ATPase, Mg2+‐ATPase and Ca2+, Mg2+‐ATPase activities protecting the spinal cord of ionic and energetic imbalance produced by ischemic injury or contusion [69]. Studies in cultures show that resveratrol not only has a protective role against reactive oxygen species but also modulates important glial functions, such as glutamate uptake activity, glutathione content and S100B secretion improving functional recovery, diminishing DNA fragmentation and apoptosis [70, 71].
Although in vivo experiments have shown that the resveratrol has beneficial effects on spinal cord injury in rodents [60, 65, 67, 69], some of the putative mechanisms of resveratrol have been obtained using in vitro preparations and concentrations greater (10–250 μM) than those reached in humans (6 μM) [61, 63, 71]. Thus, caution is mandatory when interpreting these mechanisms.
Pain
Neuropathic pain is a chronic pain state that affects up to 10% of people in United States and Europe. It is characterized by abnormal sensory processing resulting from lesions to nervous system where the conventional analgesics often have limited efficacy and undesirable side‐effects [72].
Resveratrol reduces tactile allodynia produced by neuropathic pain in some animal models as the L5/L6 spinal nerve ligation [73] and diabetic neuropathy [74, 75, 76]. Bermúdez‐Ocaña et al. [73] observed that the antiallodynic activity of spinal resveratrol can be attributed to the activation of the nitric oxide‐cyclic GMP‐protein kinase G‐large‐conductance Ca2+‐activated K+ channel pathway, whereas the antiallodynic effect observed in diabetic neuropathy was attributed to the reduction of oxidative stress produced in that pathology. The proposed mechanisms in this model include inhibition of reactive oxygen species (ONOO−), inhibition of the synthesis and release of pro‐inflammatory mediators (tumor necrosis factor‐alpha), and activation of endogenous antioxidant enzymes (catalase) that leads to a reduction in lipid peroxidation and DNA fragmentation. These effects also improve nerve blood flow and motor nerve conduction velocity deficits of diabetic rats attenuating the hyperalgesia and allodynia associated to oxidative stress [54, 74, 75, 76].
Recently, a study carried out by Kim et al. [29] showed that the resveratrol inhibits both tetrodotoxin‐sensitive and ‐resistant Na+ channels in rat dorsal root ganglion neurons causing a hyperpolarizing shift of the steady‐state inactivation and reducing the recovery from inactivation of both Na+ currents. All these activities point toward the potential of resveratrol in the treatment of neuropathic pain.
Lifespan
Calorie restriction is a dietary regimen in which an organism is provided with 30–50% fewer calories than it would naturally consume ad libitum[77]. It has been demonstrated that the calorie restriction increases lifespan in a wide diversity of organisms, such as yeast, fruit flies, nematodes, crustaceans, spiders, rodents, primates, and humans [78, 79]. Emerging data point out that sirtuins are responsible for extending lifespan in all these animals, they are NAD+‐dependent deacetylases and mono‐ADP‐ribosyl transferases that receive their names of silent information regulator (Sir2) protein discovered in Saccharomyces cerevisae, Sir2. Sirtuins are conserved proteins evolutionarily that have been associated to silencing of genes at the mating‐type loci and telomeres, as well as cell cycle progression and chromosome stability to promote survival and stress resistance under adverse conditions.
In 2003, Howitz et al. [80] screened over 20,000 molecules to identify those that enhance SIRT1 activity in vitro, turning out resveratrol to be (11–200 μM) the most potent SIRT1‐activating compound. Researchers found that resveratrol (5–10 μM) mimics calorie restriction by stimulating Sir2, extending yeast lifespan by 70%. In support of this data, resveratrol had no effect on lifespan under glucose restriction or in Sir2 null mutant yeast. A second study [81] showed that resveratrol (100 μM) could extend lifespan of Caenorhabidits elegans and Drosophila melanogaster up to 14 and 29%, respectively, but failed to extend lifespan in Sir2 null mutant animals or in animals with a restricted diet suggesting that the resveratrol extends lifespan through a mechanism related to calorie restriction. Likewise, resveratrol modestly increased egg production in fruit flies at the beginning of the adult life. Valenzano et al. [82] have reported similar results in Nothobranchius furzeri, a short‐lived seasonal fish. Supplementation with resveratrol resulted in a maximum extended lifespan of 59%. In addition, resveratrol delayed locomotor and cognitive aging and the offspring of resveratrol‐treated fishes developed into normal adults. Recently, Baur et al. [3] administered resveratrol to mice on a high‐caloric diet finding an increment of lifespan of 31% with improved motor skills. These mice had lower levels of markers that predict diabetes, decreased liver, pancreas and heart damage, and increased hepatic mitochondrial number suggesting that at the tested doses (12.5–50 μM) resveratrol not only extends lifespan but also improves general health. So far, it is difficult to assess the relevance of these mechanisms, as some of them are produced with concentrations greater than those reached in human beings. Thus, future studies should consider the concentration issue in order to get relevant conclusions.
Others
Besides its above‐mentioned pharmacological properties in several diseases, resveratrol protects against high‐caloric diet‐induced‐obesity [83, 84] and it increases athletic performance [84]. In addition, several studies have found that resveratrol inhibits the replication and shows activity against several viruses such as herpes simplex [85], human cytomegalovirus [86], influenza virus A [87], varicella‐zoster [88], and human immunodeficiency virus‐1 [89]. Moreover, resveratrol has protective effects on noise‐induced hearing loss [90] and it diminishes glucose in normal and diabetic rats [91].
Pharmacokinetics
Absorption
Resveratrol is well absorbed after oral administration, but it has a low bioavailability (around 2%) because it is metabolized rapidly in intestine and liver [92]. Two studies carried out in human intestinal epithelial cells show that resveratrol is absorbed via multidrug resistance‐associated proteins 2 and 3 with extensive sulphate conjugation [93, 94]. However, resveratrol sulphatation can be inhibited by different natural flavonoids or by some drugs [95], which have demonstrated to inhibit sulfotransferases, suggesting that the consumption of these polyphenols with resveratrol could increase the bioavailability of the free compound. However, another study disputed these results, as they were not able to detect free compound in humans [96]. Doses of 0.5 and 5 g showed blood peaks of 0.3 and 2.4 μM at 0.83 and 1.5 h, respectively [97].
In addition, the daily administration of 2‐mL of red wine containing 6.5 mg/L of total resveratrol for 15 days in rats showed an accumulation of this compound in heart, liver, and kidney but the concentrations were lower than those required for its pharmacological activity [98].
In animals, bioavailability of free resveratrol seems higher than humans, so the administration from 2 to 50 mg/kg in rats reaches blood concentrations from 1.2 to 6.6 μM in around 10 min [99, 100, 101]. A second peak is observed over the 4‐ to 8‐h time period when enterohepatic recirculation occurs [97, 101].
Interestingly, peak levels of resveratrol conjugates are up to 17 times higher than parent molecule [97, 101, 102] suggesting that some pharmacological activities could be due to resveratrol metabolites. In this regard, a study carried out in mice showed that the resveratrol‐glucuronide and resveratrol‐sulfate reached a maximum concentration of 534 and 386 μM, respectively. In contrast, free resveratrol reached only 30.2 μM in mice after a dose of 4 g/kg [102]. However, as conjugation is a process to inactivate xenobiotics rendering them water‐soluble to facilitate their elimination, it is unlikely that these conjugates may have the potency of resveratrol.
Distribution
After a single oral dose of 240 mg/kg, the higher peak concentrations and the greater area under tissue concentration of resveratrol were found in small intestine, heart, liver, and lung [103]. At 18 h, free resveratrol is the main form retained in liver, heart, lung and brain [104]. The binding of the compound to plasma proteins seems to occur particularly on albumin [105].
The plasma half‐life of free resveratrol is variable in humans and it is ranging from 2 to 14 h [96, 97], whereas in rats the half‐life has a value average of 1.4 h [101]. The rest of the pharmacokinetic parameters are summarized in Table 1.
Metabolism
It has been demonstrated in mice, rats, and humans that the resveratrol undergoes extensive first‐pass glucuronidation and enterohepatic recirculation, which contribute to the rapid plasmatic declination observed over the first 2 h. In addition, the uptake of resveratrol in hepatic cells occurs through a carrier‐mediated transport besides passive diffusion [93, 101].
Isoforms of human liver cytochrome P450 enzymes involved in the hepatic biotransformation of resveratrol include CYP1A1, CYP1A2, and CYP1B1 [106]. In turn, resveratrol can inhibit activity or expression of CYP1A1, CYP1A2, CYP1B1, CYP2A4, CYP2A5, CYP2B9, CYP3A4, CYP3A5, and CYP2E1 [107, 108, 109, 110, 111]. Recently, a study performed in HepG2 cells demonstrated that resveratrol autoinduces three phase II metabolizing enzymes, two isoforms of UDP‐glucuronosyltransferases (UGT1A1 and UGT2B7) and a sulfotransferase (ST1E1; 112]. These effects on drug metabolism suggest that the resveratrol acts to increase body detoxification, which may contribute to delaying the onset of neurodegenerative diseases.
Several metabolites have been identified in human and rats. At least, 11 of them have been well characterized [Fig. 2].
Figure 2.
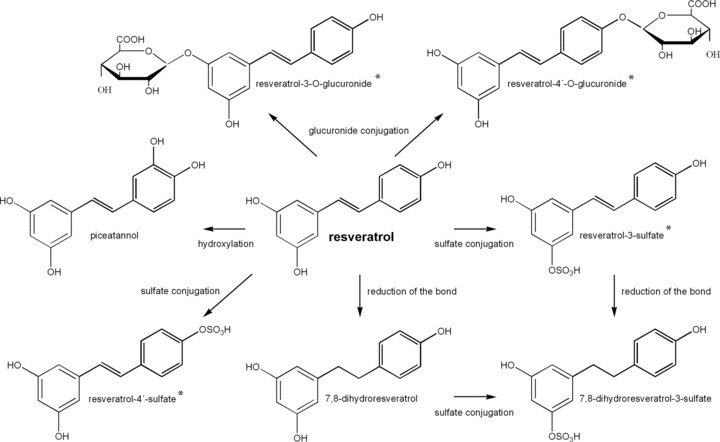
Proposed metabolic pathway of resveratrol. Metabolites were identified in rat or human urine and in human liver microsomes. The asterisk indicates the metabolites identified as cis‐ and trans‐isomers but were excluded for image clarity. Cis‐isomers are glucuronidated and sulfated faster than trans‐isomers. Data obtained by Piver et al. [106], Wang et al. [126], and Urpi‐Sarda et al. [127].
Excretion
Approximately 50–80% of the compound is excreted in urine and feces and only 0.04% of free resveratrol is excreted in the urine within 24 h post‐administration [96, 97].
Toxicology
While the therapeutic potential of resveratrol has been increasing year by year and its health benefits seem promising, there are only few studies about resveratrol toxicity. A study performed by Juan et al. [113] showed that the repeated oral administration of resveratrol (20 mg/kg/day) did not produce any hematological or biochemical change nor signs of toxicity in the organs. However, rats that received higher doses of resveratrol (3 g/kg/day for 4 weeks) showed a significant increase in the clinical signs of toxicity. These signs included reduced body weight and food consumption, as well as biochemical and hematological changes. In addition, histopathology revealed nephropathy and hyperplasia in the bladder [114]. In mice, resveratrol (2 and 4 g/kg/day) induced mortality associated with impaction and accumulation of the compound in the gastrointestinal tract. In this study, authors found increased liver weight and serum cholesterol and mild anemia at the highest dose, as well as hydronephrosis and epithelial hyperplasia in the urinary bladder [102].
In humans beings, the consumption of single doses of resveratrol (0.5, 1, 2.5, or 5 g) did not cause serious adverse effects [97]. However, more randomized clinical trials with protocols of chronic administration are necessary to establish a valid conclusion about the safety of resveratrol in human beings.
To investigate the estrogenicity of trans‐resveratrol during the development and its possible teratogenic effects, several studies have been performed in animals. In a study, adult mice received 3 mg/L of resveratrol in drinking water. After the offspring, animals were exposed to resveratrol during gestation, lactation, prepubertal period and pubertal period, up to adulthood. Mice parents did not show any alteration but the offspring presented a decreased kidney weight compared to parents. In addition, males and females in the first generation presented a decreased weight in seminal vesicles and ovaries, respectively [115]. In support of this study, Turner et al. [116] reported that resveratrol had no toxic effects in weanling rats of 3‐week old. In contrast, some authors have observed changes in adult and weanling rats treated with resveratrol, which include alterations in sociosexual behavior and in organs related to reproduction [117, 118, 119, 120]. In summary, resveratrol produces minor effects in adult animals and their offspring although more exhaustive studies are required before resveratrol can be recommended for pregnant mothers and children.
Finally, resveratrol is a polyphenol, which can be oxidized by polyphenol oxidases to quinonic compounds that, in turn, are capable to react with amino acids or proteins and to promote cancer initiation [121]. For instance, CYP3A converts resveratrol into p‐benzoquinone, a quinonic compound, which binds covalently to CYP3A4 and inactivates it [122].
Clinical Studies
The Food and Drug Administration authorized trans‐resveratrol as an investigational new compound on January 30, 2001. To date, it is only sold as a nutritional supplement and there are few studies on its bioavailability and pharmacological effects in humans. Goldberg et al. [123] investigated 24 healthy subjects who consumed red or white wine and two different grape juices during 4 weeks. They concluded that the favorable effects of wines observed in plasmatic lipids and lipoprotein concentrations are probably due to their alcohol content because these effects were not reproduced by grape juices. Accordingly, two studies showed an efficient absorption but very low bioavailability of unchanged resveratrol in plasma [92, 96] in 12 and 6 volunteers, respectively, demonstrating that orally administered resveratrol is insufficient to explain any possible biological activity in humans. The main problem of low availability of free resveratrol seems to be its extremely rapid conjugation by the intestine and liver. Considering this, a phase I study, carried out in forty volunteers who received high doses of resveratrol (until 5 g) found that maximal concentrations of free resveratrol at the highest dose was around 2.4 μM [97]. This value was below the resveratrol concentrations required in in vitro assays to produce diverse pharmacological effects such as inhibition of NF‐kB and AP‐1 or activation and inhibition of cytochrome P450 enzymes [124] but it could explain inhibition of adhesion molecules and phosphorylation of ERK1/2[39, 125].
So far, there are no clinical trials about resveratrol's effects on epilepsy, pain, depression, Huntington's disease, Parkinson's disease, and aging. Contrariwise, Orgogozo et al. [31] conducted a prospective study in 3,777 volunteers aged 65 and over to assess the effects of red wine, and indirectly resveratrol, on incidence of dementia and Alzheimer's disease. This study showed that people drinking three to four glasses of red wine per day had an 80% decreased incidence of dementia and Alzheimer's disease three years later, compared to those who drank less or did not drink at all. Larger follow‐up as well as possible side effects associated to red wine consumption are not reported in this study. Although this is a well‐conducted study, it does not support the claimed beneficial effect of resveratrol on Alzheimer's disease in humans because such effects could be due to other flavonoids present in red wine. Taken together, clinical data suggest that the resveratrol's effects, if any, still need to be demonstrated.
Concluding Remarks
In the recent years, there has been an extraordinary interest in resveratrol activities. Based on preliminary data, resveratrol could provide cellular resistance against insults that involve oxidative stress‐induced injury. However, more systematic preclinical work as well as well‐conducted clinical trials are necessary before it can be prescribed as a potential prophylactic compound in either acute or chronic conditions such as stroke, amyotrophic lateral sclerosis, Parkinson's disease, Alzheimer's disease, neuronal injury, neuropathic pain, and a variety of age‐related disorders.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
Authors greatly appreciate the bibliographic assistance of Héctor Vázquez. Mónica Ambriz‐Tututi and Héctor I. Rocha‐González are Conacyt fellows. The study has been partially supported by Conacyt, grant 59879 (VGS).
References
- 1. Dewick PM. Medicinal natural products: A biosynthetic approach, 2nd Edition New York , NY : John Wiley & Sons, Ltd., 2002;1–507. [Google Scholar]
- 2. Tokuşoglu O, Unal MK, Yemiş F. Determination of the phytoalexin resveratrol (3,5,4′‐ trihydroxystilbene) in peanuts and pistachios by high‐performance liquid chromatographic diode array (HPLC‐DAD) and gas chromatography‐mass spectrometry (GC‐MS). J Agric Food Chem 2005;53:5003–5009. [DOI] [PubMed] [Google Scholar]
- 3. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: The in vivo evidence. Nat Rev Drug Discov 2006;5:493–506. [DOI] [PubMed] [Google Scholar]
- 4. Orsini F, Pelizzoni F, Verotta L, Aburjai T, Rogers CB. Isolation, synthesis, and antiplatelet aggregation activity of resveratrol 3‐O‐beta‐D‐glucopyranoside and related compounds. J Nat Prod 1997;60:1082–1087. [DOI] [PubMed] [Google Scholar]
- 5. Lins AP, Ribeiro MN, Gottlieb OR, Gottlieb HE. Gnetins, resveratrol oligomers from Gnetum species . J Nat Prod 1982;45:754–761. [Google Scholar]
- 6. Atun S, Achmad SA, Niwa M, Arianingrum R, Aznam N. Oligostilbenoids from Hopea Mengarawan (Dipterocarpaceae) . Biochem Syst Ecol 2006;34:642–644. [Google Scholar]
- 7. Ohyama M, Tanaka T, Iinuma M, Goto K. Two novel resveratrol trimers, leachianols A and B, from Sophora leachiana . Chem Pharm Bull 1994;42:2117–2120. [Google Scholar]
- 8. Shin NH, Ryu SY, Choi EJ, Kang SH, Chang IM, Min KR, Kim Y. Oxyresveratrol as the potent inhibitor on dopa oxidase activity of mushroom tyrosinase. Biochem Biophys Res Commun 1998;243:801–803. [DOI] [PubMed] [Google Scholar]
- 9. Orallo F. Comparative studies of the antioxidant effects of cis‐ and trans‐resveratrol. Curr Med Chem 2006;13:87–98. [PubMed] [Google Scholar]
- 10. Ribeiro de Lima MT, Waffo‐Téguo P, Teissedre PL, Pujolas A, Vercauteren J, Cabanis JC, Mérillon JM. Determination of stilbenes (trans‐astringin, cis‐ and trans‐piceid, and cis‐ and trans‐ resveratrol) in Portuguese wines. J Agric Food Chem 1999;47:2666–2670. [DOI] [PubMed] [Google Scholar]
- 11. Das DK, Maulik N. Resveratrol in cardioprotection: A therapeutic promise of alternative medicine. Mol Interv 2006;6:36–47. [DOI] [PubMed] [Google Scholar]
- 12. Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol 2007;224:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: Molecular mechanisms and therapeutic potential. Front Biosci 2007;12:4839–4854. [DOI] [PubMed] [Google Scholar]
- 14. Paul B, Masih I, Deopujari J, Charpentier C. Occurrence of resveratrol and pterostilbene in age‐old darakchasava, an ayurvedic medicine from India. J Ethnopharmacol 1999;68:71–76. [DOI] [PubMed] [Google Scholar]
- 15. Takaoka MJ. Of the phenolic substances of white hellebore (Veratrum grandiflorum Loes. fil.). J Fac Sci Hokkaido Imperial Univ 1940;3:1–16. [Google Scholar]
- 16. Nonomura S, Kanagawa H, Makimoto A. Chemical constituents of polygonaceous plants. I. Studies on the components fo Ko‐jo‐kon (Polygonum cuspidatum Sieb. et Zucc.). Yakugaku Zasshi 1963;83:983–988. [PubMed] [Google Scholar]
- 17. Langcake P, Pryce RJ. The production of resveratrol by vitis vinifera and other members of the vitaceae as a response to infection or injury. Physiol Plant Pathol 1976;9:77–86. [Google Scholar]
- 18. Renaud S, De Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992;339:1523–1526. [DOI] [PubMed] [Google Scholar]
- 19. Goldberg DM, Hahn SE, Parkes JG. Beyond alcohol: Beverage consumption and cardiovascular mortality. Clin Chim Acta 1995;237:155–187. [DOI] [PubMed] [Google Scholar]
- 20. Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997;275:218–220. [DOI] [PubMed] [Google Scholar]
- 21. Frémont L. Biological effects of resveratrol. Life Sci 2000;66:663–673. [DOI] [PubMed] [Google Scholar]
- 22. Granados‐Soto V. Pleiotropic effects of resveratrol. Drug News Perspect 2003;16:299–307. [DOI] [PubMed] [Google Scholar]
- 23. Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: A review of their intracellular targets. Eur J Pharmacol 2006;545:51–64. [DOI] [PubMed] [Google Scholar]
- 24. Mc Namara JO. Pharmacotherapy of the epilepsies In: Brunton LL, Lazo JJ, Parker KL, eds. Goodman & Gilman's the pharmacological basis of therapeutics. New York : McGraw‐Hill, 2006;501–525. [Google Scholar]
- 25. Gupta YK, Chaudhary G, Srivastava AK. Protective effect of resveratrol against pentylenetetrazole‐induced seizures and its modulation by an adenosinergic system. Pharmacology 2002a;65:170–174. [DOI] [PubMed] [Google Scholar]
- 26. Gupta YK, Chaudhary G, Sinha K, Srivastava AK. Protective effect of resveratrol against intracortical FeCl3‐induced model of posttraumatic seizures in rats. Methods Find Exp Clin Pharmacol 2001;23:241–244. [DOI] [PubMed] [Google Scholar]
- 27. Virgili M, Contestabile A. Partial neuroprotection of in vivo excitotoxic brain damage by chronic administration of the red wine antioxidant agent, trans‐resveratrol in rats. Neurosci Lett 2000;281:123–126. [DOI] [PubMed] [Google Scholar]
- 28. Gupta YK, Briyal S, Chaudhary G. Protective effect of trans‐resveratrol against kainic acid‐induced seizures and oxidative stress in rats. Pharmacol Biochem Behav 2002b;71:245–249. [DOI] [PubMed] [Google Scholar]
- 29. Kim HI, Kim TH, Song JH. Resveratrol inhibits Na+ currents in rat dorsal root ganglion neurons. Brain Res 2005;1045:134–141. [DOI] [PubMed] [Google Scholar]
- 30. Standaert DG, Young AB. Treatment of central nervous system degenerative disorders In: Goodman & Gilman's the pharmacological basis of therapeutics. New York : McGraw‐Hill, 2006;527–545. [Google Scholar]
- 31. Orgogozo JM, Dartigues JF, Lafont S, Letenneur L, Commenges D, Salamon R, Renaud S, Breteler MB. Wine consumption and dementia in the elderly: A prospective community study in the Bordeaux area. Rev Neurol (Paris) 1997;153:185–192. [PubMed] [Google Scholar]
- 32. Wang J, Ho L, Zhao Z, Seror I, Humala N, Dickstein DL, Thiyagarajan M, Percival SS, Talcott ST, Pasinetti GM. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer's disease. FASEB J 2006;20:2313–2320. [DOI] [PubMed] [Google Scholar]
- 33. Jang JH, Surh YJ. Protective effect of resveratrol on beta‐amyloid‐induced oxidative PC12 cell death. Free Radic Biol Med 2003;34:1100–1110. [DOI] [PubMed] [Google Scholar]
- 34. Luo L, Huang YM. Effect of resveratrol on the cognitive ability of Alzheimeros mice. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2006;31:566–569. [PubMed] [Google Scholar]
- 35. Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J 2007;26:3169–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rivière C, Richard T, Quentin L, Krisa S, Mérillon JM, Monti JP. Inhibitory activity of stilbenes on Alzheimer's beta‐amyloid fibrils in vitro. Bioorg Med Chem 2007;15:1160–1167. [DOI] [PubMed] [Google Scholar]
- 37. Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer's disease amyloid‐beta peptides. J Biol Chem 2005;280:37377–37382. [DOI] [PubMed] [Google Scholar]
- 38. Han YS, Zheng WH, Bastianetto S, Chabot JG, Quirion R. Neuroprotective effects of resveratrol against beta‐amyloid‐induced neurotoxicity in rat hippocampal neurons: Involvement of protein kinase C. Br J Pharmacol 2004;141:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miloso M, Bertelli AA, Nicolini G, Tredici G. Resveratrol‐induced activation of the mitogen‐activated protein kinases, ERK1 and ERK2, in human neuroblastoma SH‐SY5Y cells. Neurosci Lett 1999;264:141–144. [DOI] [PubMed] [Google Scholar]
- 40. Savaskan E, Olivieri G, Meier F, Seifritz E, Wirz‐Justice A, Müller‐Spahn F. Red wine ingredient resveratrol protects from beta‐amyloid neurotoxicity. Gerontology 2003;49:380–383. [DOI] [PubMed] [Google Scholar]
- 41. Draczynska‐Lusiak B, Doung A, Sun AY. Oxidized lipoproteins may play a role in neuronal cell death in Alzheimer disease. Mol Chem Neuropathol 1998;33:139–148. [DOI] [PubMed] [Google Scholar]
- 42. Sun AY, Draczynska‐Lusiak B, Sun GY. Oxidized lipoproteins, beta amyloid peptides and Alzheimer's disease. Neurotox Res 2001;3:167–178. [DOI] [PubMed] [Google Scholar]
- 43. Bastianetto S, Brouillette J, Quirion R. Neuroprotective effects of natural products: Interaction with intracellular kinases, amyloid peptides and a possible role for transthyretin. Neurochem Res 2007;32:1720–1725. [DOI] [PubMed] [Google Scholar]
- 44. Chen J, Zhou Y, Mueller‐Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia‐dependent amyloid‐beta toxicity through inhibiting NF‐kappaB signaling. J Biol Chem 2005;280:40364–40374. [DOI] [PubMed] [Google Scholar]
- 45. Kim YA, Lim SY, Rhee SH, Park KY, Kim CH, Choi BT, Lee SJ, Park YM, Choi YH. Resveratrol inhibits inducible nitric oxide synthase and cyclooxygenase‐2 expression in beta‐amyloid‐treated C6 glioma cells. Int J Mol Med 2006;17:1069–1075. [PubMed] [Google Scholar]
- 46. Olanow CW. A rationale for monoamine oxidase inhibition as neuroprotective therapy for Parkinson's disease. Mov Disord 1993;8(Suppl. 1):S1–S7. [DOI] [PubMed] [Google Scholar]
- 47. Singh N, Pillay V, Choonara YE. Advances in the treatment of Parkinson's disease. Prog Neurobiol 2007;81:29–44. [DOI] [PubMed] [Google Scholar]
- 48. Jellinger K, Kienzl E, Rumpelmair G, Riederer P, Stachelberger H, Ben‐Shachar D, Youdim MB. Neuromelanin and nigrostriatal dopamine neuron degeneration. J Neurochem 1993;60:1976–1977. [DOI] [PubMed] [Google Scholar]
- 49. Lee MK, Kang SJ, Poncz M, Song KJ, Park KS. Resveratrol protects SH‐SY5Y neuroblastoma cells from apoptosis induced by dopamine. Exp Mol Med 2007;39:376–384. [DOI] [PubMed] [Google Scholar]
- 50. Chalimoniuk M, Glowacka J, Zabielna A, Eckert A, Strosznajder JB. Nitric oxide alters arachidonic acid turnover in brain cortex synaptoneurosomes. Neurochem Int 2006;48:1–8. [DOI] [PubMed] [Google Scholar]
- 51. Dore S. Unique properties of polyphenol stilbenes in the brain: More than direct antioxidant actions; gene/protein regulatory activity. Neurosignals 2005;14:61–70. [DOI] [PubMed] [Google Scholar]
- 52. Solans A, Zambrano A, Rodriguez M, Barrientos A. Cytotoxicity of a mutant huntingtin fragment in yeast involves early alterations in mitochondrial OXPHOS complexes II and III. Hum Mol Genet 2006;15:3063–3081. [DOI] [PubMed] [Google Scholar]
- 53. Kumar P, Padi SS, Naidu PS, Kumar A. Effect of resveratrol on 3‐nitropropionic acid‐induced biochemical and behavioural changes: Possible neuroprotective mechanisms. Behav Pharmacol 2006;17:485–492. [DOI] [PubMed] [Google Scholar]
- 54. Kumar A, Naidu PS, Seghal N, Padi SS. Neuroprotective effects of resveratrol against intracerebroventricular colchicine–induced cognitive impairment and oxidative stress in rats. Pharmacology 2007a;79:17–26. [DOI] [PubMed] [Google Scholar]
- 55. Parker AJ, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Neri C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet 2005;37:349–350. [DOI] [PubMed] [Google Scholar]
- 56. Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993;362:59–62. [DOI] [PubMed] [Google Scholar]
- 57. Wu SN. Large‐conductance Ca2+‐ activated K+ channels: Physiological role and pharmacology. Curr Med Chem 2003;10:649–661. [DOI] [PubMed] [Google Scholar]
- 58. Gao D, Zhang X, Jiang X, Peng Y, Huang W, Cheng G, Song L. Resveratrol reduces the elevated level of MMP‐9 induced by cerebral ischemia‐reperfusion in mice. Life Sci 2006;78:2564–2570. [DOI] [PubMed] [Google Scholar]
- 59. Ates O, Cayli S, Altinoz E, Gurses I, Yucel N, Sener M, Kocak A, Yologlu S. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol Cell Biochem 2007;294:137–144. [DOI] [PubMed] [Google Scholar]
- 60. Tsai SK, Hung LM, Fu YT, Cheng H, Nien MW, Liu HY, Zhang FB, Huang SS. Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. J Vasc Surg 2007;46:346–353. [DOI] [PubMed] [Google Scholar]
- 61. Inoue H, Jiang XF, Katayama T, Osada S, Umesono K, Namura S. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator‐activated receptor alpha in mice. Neurosci Lett 2003;352:203–206. [DOI] [PubMed] [Google Scholar]
- 62. Wang YJ, He F, Li XL. The neuroprotection of resveratrol in the experimental cerebral ischemia. Zhonghua Yi Xue Za Zhi 2003;83:534–536. [PubMed] [Google Scholar]
- 63. Gao ZB, Hu GY. Trans‐resveratrol, a red wine ingredient, inhibits voltage‐activated potassium currents in rat hippocampal neurons. Brain Res 2005;1056:68–75. [DOI] [PubMed] [Google Scholar]
- 64. Zhuang H, Kim YS, Koehler RC, Doré S. Potential mechanism by which resveratrol, a red wine constituent, protects neurons. Ann N Y Acad Sci 2003;993:276–288. [DOI] [PubMed] [Google Scholar]
- 65. Sinha K, Chaudhary G, Gupta YK. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci 2002;71:655–665. [DOI] [PubMed] [Google Scholar]
- 66. Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, Sun AY. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res 2002;958:439–447. [DOI] [PubMed] [Google Scholar]
- 67. Kiziltepe U, Turan NN, Han U, Ulus AT, Akar F. Resveratrol, a red wine polyphenol, protects spinal cord from ischemia‐reperfusion injury. J Vasc Surg 2004;40:138–145. [DOI] [PubMed] [Google Scholar]
- 68. Kaplan S, Bisleri G, Morgan JA, Cheema FH, Oz MC. Resveratrol, a natural red wine polyphenol, reduces ischemia‐reperfusion‐induced spinal cord injury. Ann Thorac Surg 2005;80:2242–2249. [DOI] [PubMed] [Google Scholar]
- 69. Yang YB, Piao YJ. Effects of resveratrol on secondary damages after acute spinal cord injury in rats. Acta Pharmacol Sin 2003;24:703–710. [PubMed] [Google Scholar]
- 70. Kutuk O, Adli M, Poli G, Basaga H. Resveratrol protects against 4‐HNE induced oxidative stress and apoptosis in Swiss 3T3 fibroblasts. Biofactors 2004;20:1–10. [DOI] [PubMed] [Google Scholar]
- 71. De Almeida LM, Piñeiro CC, Leite MC, Brolese G, Tramontina F, Feoli AM, Gottfried C, Gonçalves CA. Resveratrol increases glutamate uptake, glutathione content, and S100B secretion in cortical astrocyte cultures. Cell Mol Neurobiol 2007;27:661–668. [DOI] [PubMed] [Google Scholar]
- 72. McQuay HJ. Neuropathic pain: Evidence matters. Eur J Pain 2002;6(Suppl. A):11–18. [DOI] [PubMed] [Google Scholar]
- 73. Bermúdez‐Ocaña DY, Ambriz‐Tututi M, Pérez‐Severiano F, Granados‐Soto V. Pharmacological evidence for the participation of NO‐cyclic GMP‐PKG‐K+ channel pathway in the antiallodynic action of resveratrol. Pharmacol Biochem Behav 2006;84:535–542. [DOI] [PubMed] [Google Scholar]
- 74. Sharma S, Kulkarni SK, Chopra K. Effect of resveratrol, a polyphenolic phytoalexin, on thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Fundam Clin Pharmacol 2007a;21:89–94. [DOI] [PubMed] [Google Scholar]
- 75. Sharma S, Chopra K, Kulkarni SK. Effect of insulin and its combination with resveratrol or curcumin in attenuation of diabetic neuropathic pain: Participation of nitric oxide and TNF‐alpha. Phytother Res 2007b;21:278–283. [DOI] [PubMed] [Google Scholar]
- 76. Kumar A, Kaundal RK, Iyer S, Sharma SS. Effects of resveratrol on nerve functions, oxidative stress and DNA fragmentation in experimental diabetic neuropathy. Life Sci 2007b;80:1236–1244. [DOI] [PubMed] [Google Scholar]
- 77. Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, DeCabo R. Calorie restriction mimetics: An emerging research field. Aging Cell 2006;5:97–108. [DOI] [PubMed] [Google Scholar]
- 78. Walford RL, Mock D, MacCallum T, Laseter JL. Physiologic changes in humans subjected to severe, selective calorie restriction for two years in biosphere 2: Health, aging, and toxicological perspectives. Toxicol Sci 1999;52(2 Suppl.):61–65. [PubMed] [Google Scholar]
- 79. Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in biosphere 2: Alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2‐year period. J Gerontol A Biol Sci Med Sci 2002;57:B211–B224. [DOI] [PubMed] [Google Scholar]
- 80. Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003;425:191–196. [DOI] [PubMed] [Google Scholar]
- 81. Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004;430:686–689. [DOI] [PubMed] [Google Scholar]
- 82. Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age‐related markers in a short‐lived vertebrate. Curr Biol 2006;16:296–300. [DOI] [PubMed] [Google Scholar]
- 83. Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez‐Lluch G, Lewis K, et al Resveratrol improves health and survival of mice on a high‐calorie diet. Nature 2006;444:337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lagouge M, Argmann C, Gerhart‐Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC‐1alpha. Cell 2006;127:1109–1122. [DOI] [PubMed] [Google Scholar]
- 85. Faith SA, Sweet TJ, Bailey E, Booth T, Docherty JJ. Resveratrol suppresses nuclear factor‐kappaB in herpes simplex virus infected cells. Antiviral Res 2006;72:242–251. [DOI] [PubMed] [Google Scholar]
- 86. Evers DL, Wang X, Huong SM, Huang DY, Huang ES. 3,4',5‐trihydroxy‐trans‐stilbene (resveratrol) inhibits human cytomegalovirus replication and virus‐induced cellular signaling. Antiviral Res 2004;63:85–95. [DOI] [PubMed] [Google Scholar]
- 87. Palamara AT, Nencioni L, Aquilano K, De Chiara G, Hernandez L, Cozzolino F, Ciriolo MR, Garaci E. Inhibition of influenza A virus replication by resveratrol. J Infect Dis 2005;191:1719–1729. [DOI] [PubMed] [Google Scholar]
- 88. Docherty JJ, Sweet TJ, Bailey E, Faith SA, Booth T. Resveratrol inhibition of varicella‐zoster virus replication in vitro. Antiviral Res 2006;72:171–177. [DOI] [PubMed] [Google Scholar]
- 89. Heredia A, Davis C, Redfield R. Synergistic inhibition of HIV‐1 in activated and resting peripheral blood mononuclear cells, monocyte‐derived macrophages, and selected drug‐resistant isolates with nucleoside analogues combined with a natural product, resveratrol. J Acquir Immune Defic Syndr 2000;25:246–255. [DOI] [PubMed] [Google Scholar]
- 90. Seidman M, Babu S, Tang W, Naem E, Quirk WS. Effects of resveratrol on acoustic trauma. Otolaryngol Head Neck Surg 2003;129:463–470. [DOI] [PubMed] [Google Scholar]
- 91. Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin‐like effect in streptozotocin‐induced diabetic rats. Am J Physiol Endocrinol Metab 2006;290:E1339–E1346. [DOI] [PubMed] [Google Scholar]
- 92. Goldberg DM, Yan J, Soleas GJ. Absorption of three wine‐related polyphenols in three different matrices by healthy subjects. Clin Biochem 2003;36:79–87. [DOI] [PubMed] [Google Scholar]
- 93. De Santi C, Pietrabissa A, Spisni R, Mosca F, Pacifici GM. Sulphation of resveratrol, a natural product present in grapes and wine, in the human liver and duodenum. Xenobiotica 2000a;30:609–617. [DOI] [PubMed] [Google Scholar]
- 94. Kaldas MI, Walle UK, Walle T. Resveratrol transport and metabolism by human intestinal Caco‐2 cells. J Pharm Pharmacol 2003;55:307–312. [DOI] [PubMed] [Google Scholar]
- 95. De Santi C, Pietrabissa A, Spisni R, Mosca F, Pacifici GM. Sulphation of resveratrol, a natural compound present in wine, and its inhibition by natural flavonoids. Xenobiotica 2000b;30:857–866. [DOI] [PubMed] [Google Scholar]
- 96. Walle T, Hsieh F, DeLegge MH, Oatis JE Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos 2004;32:1377–1382. [DOI] [PubMed] [Google Scholar]
- 97. Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, et al Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev 2007;16:1246–1252. [DOI] [PubMed] [Google Scholar]
- 98. Bertelli AA, Giovannini L, Stradi R, Bertelli A, Tillement JP. Plasma, urine and tissue levels of trans‐ and cis‐resveratrol (3,4′,5‐trihydroxystilbene) after short‐term or prolonged administration of red wine to rats. Int J Tissue React 1996;18:67–71. [PubMed] [Google Scholar]
- 99. Asensi M, Medina I, Ortega A, Carretero J, Bano MC, Obrador E, Estrela JM. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med 2002;33:387–398. [DOI] [PubMed] [Google Scholar]
- 100. Juan ME, Buenafuente J, Casals I, Planas JM. Plasmatic levels of trans‐resveratrol in rats. Food Res Int 2002a;35:195–199. [Google Scholar]
- 101. Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP. Metabolism and disposition of resveratrol in rats: Extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked‐rat model. J Pharmacol Exp Ther 2002;302:369–373. [DOI] [PubMed] [Google Scholar]
- 102. Horn TL, Cwik MJ, Morrissey RL, Kapetanovic I, Crowell JA, Booth TD, McCormick DL. Oncogenicity evaluation of resveratrol in p53(+/−) (p53 knockout) mice. Food Chem Toxicol 2007;45:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sale S, Verschoyle RD, Boocock D, Jones DJ, Wilsher N, Ruparelia KC, Potter GA, Farmer PB, Steward WP, Gescher AJ. Pharmacokinetics in mice and growth‐inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3,4,5,4′‐tetramethoxystilbene. Br J Cancer 2004;90:736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Abd El‐Mohsen M, Bayele H, Kuhnle G, Gibson G, Debnam E, Kaila Srai S, Rice‐Evans C, Spencer JP. Distribution of [3H]trans‐resveratrol in rat tissues following oral administration. Br J Nutr 2006;96:62–70. [DOI] [PubMed] [Google Scholar]
- 105. Lancon A, Delma D, Osman H, Thenot JP, Jannin B, Latruffe N. Human hepatic cell uptake of resveratrol: Involvement of both passive diffusion and carrier‐mediated process. Biochem Biophys Res Commun 2004;316:1132–1137. [DOI] [PubMed] [Google Scholar]
- 106. Piver B, Fer M, Vitrac X, Merillon JM, Dreano Y, Berthou F, Lucas D. Involvement of cytochrome P450 1A2 in the biotransformation of trans‐resveratrol in human liver microsomes. Biochem Pharmacol 2004;68:773–782. [DOI] [PubMed] [Google Scholar]
- 107. Ciolino HP, Yeh GC. Inhibition of aryl hydrocarbon‐induced cytochrome P‐450 1A1 enzyme activity and CYP1A1 expression by resveratrol. Mol Pharmacol 1999;56:760–767. [PubMed] [Google Scholar]
- 108. Chan WK, Delucchi AB. Resveratrol, a red wine constituent, is a mechanism‐based inactivator of cytochrome P450 3A4. Life Sci 2000;67:3103–3112. [DOI] [PubMed] [Google Scholar]
- 109. Chang TK, Lee WB, Ko HH. Trans‐resveratrol modulates the catalytic activity and mRNA expression of the procarcinogen‐activating human cytochrome P450 1B1. Can J Physiol Pharmacol 2000;78:874–881. [PubMed] [Google Scholar]
- 110. Chang TK, Yeung RK. Effect of trans‐resveratrol on 7‐benzyloxy‐4‐trifluoromethylcoumarin O‐ dealkylation catalyzed by human recombinant CYP3A4 and CYP3A5. Can J Physiol Pharmacol 2001;79:220–226. [PubMed] [Google Scholar]
- 111. Piver B, Berthou F, Dreano Y, Lucas D. Inhibition of CYP3A, CYP1A and CYP2E1 activities by resveratrol and other non volatile red wine components. Toxicol Lett 2001;125:83–91. [DOI] [PubMed] [Google Scholar]
- 112. Lancon A, Hanet N, Jannin B, Delmas D, Heydel JM, Lizard G, Chagnon MC, Artur Y, Latruffe N. Resveratrol in human hepatoma HepG2 cells: Metabolism and inducibility of detoxifying enzymes. Drug Metab Dispos 2007;35:699–703. [DOI] [PubMed] [Google Scholar]
- 113. Juan ME, Vinardell MP, Planas JM. The daily oral administration of high doses of trans‐resveratrol to rats for 28 days is not harmful. J Nutr 2002b;132:257–260. [DOI] [PubMed] [Google Scholar]
- 114. Crowell JA, Korytko PJ, Morrissey RL, Booth TD, Levine BS. Resveratrol‐associated renal toxicity. Toxicol Sci 2004;82:614–619. [DOI] [PubMed] [Google Scholar]
- 115. Kyselova V, Peknicova J, Buckiova D, Boubelik M. Effects of p‐nonylphenol and resveratrol on body and organ weight and in vivo fertility of outbred CD‐1 mice. Reprod Biol Endocrinol 2003;1:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Turner RT, Evans GL, Zhang M, Maran A, Sibonga JD. Is resveratrol an estrogen agonist in growing rats? Endocrinology 1999;140:50–54. [DOI] [PubMed] [Google Scholar]
- 117. Henry LA, Witt DM. Resveratrol: Phytoestrogen effects on reproductive physiology and behavior in female rats. Horm Behav 2002;41:220–228. [DOI] [PubMed] [Google Scholar]
- 118. Liu Z, Yu B, Li W, Sun J. Estrogenicity of trans‐resveratrol in immature mice in vivo. Wei Sheng Yan Jiu 2002;31:188–190. [PubMed] [Google Scholar]
- 119. Nikaido Y, Yoshizawa K, Danbara N, Tsujita‐Kyutoku M, Yuri T, Uehara N, Tsubura A. Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD‐1 mouse offspring. Reprod Toxicol 2004;18:803–811. [DOI] [PubMed] [Google Scholar]
- 120. Henry LA, Witt DM. Effects of neonatal resveratrol exposure on adult male and female reproductive physiology and behavior. Dev Neurosci 2006;28:186–195. [DOI] [PubMed] [Google Scholar]
- 121. Bittner S. When quinones meet amino acids: Chemical, physical and biological consequences. Amino Acids 2006;30:205–224. [DOI] [PubMed] [Google Scholar]
- 122. Zhou S, Koh HL, Gao Y, Gong ZY, Lee EJ. Herbal bioactivation: The good, the bad and the ugly. Life Sci 2004;74:935–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Goldberg DM, Garovic‐Kocic V, Diamandis EP, Pace‐Asciak CR. Wine: Does the colour count? Clin Chim Acta 1996;246:183–193. [DOI] [PubMed] [Google Scholar]
- 124. Gescher AJ, Steward WP. Relationship between mechanisms, bioavailibility, and preclinical chemopreventive efficacy of resveratrol: A conundrum. Cancer Epidemiol Biomarkers Prev 2003;12:953–937. [PubMed] [Google Scholar]
- 125. Ferrero ME, Bertelli AE, Fulgenzi A, Pellegatta F, Corsi MM, Bonfrate M, Ferrara F, De Caterina R, Giovannini L, Bertelli A. Activity in vitro of resveratrol on granulocyte and monocyte adhesion to endothelium. Am J Clin Nutr 1998;68:1208–1214. [DOI] [PubMed] [Google Scholar]
- 126. Wang D, Hang T, Wu C, Liu W. Identification of the major metabolites of resveratrol in rat urine by HPLC‐MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 2005;829:97–106. [DOI] [PubMed] [Google Scholar]
- 127. Urpi‐Sarda M, Zamora‐Ros R, Lamuela‐Raventos R, Cherubini A, Jauregui O, De La Torre R, Covas MI, Estruch R, Jaeger W, Andres‐Lacueva C. HPLC‐tandem mass spectrometric method to characterize resveratrol metabolism in humans. Clin Chem 2007;53:292–299. [DOI] [PubMed] [Google Scholar]
- 128. Meng X, Maliakal P, Lu H, Lee MJ, Yang CS. Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. J Agric Food Chem 2004;52:935–942. [DOI] [PubMed] [Google Scholar]


