Abstract
Based on the results of a randomized controlled trial, we examined a model of the mechanisms of efficacy of culturally adapted cognitive‐behavior therapy (CBT) for Cambodian refugees with pharmacology‐resistant posttraumatic stress disorder (PTSD) and comordid orthostatic panic attacks (PAs). Twelve patients were in the initial treatment condition, 12 in the delayed treatment condition. The patients randomized to CBT had much greater improvement than patients in the waitlist condition on all psychometric measures and on one physiological measure—the systolic blood pressure response to orthostasis (d = 1.31)—as evaluated by repeated‐measures MANOVA and planned contrasts. After receiving CBT, the Delayed Treatment Group improved on all measures, including the systolic blood pressure response to orthostasis. The CBT treatment's reduction of PTSD severity was significantly mediated by improvement in orthostatic panic and emotion regulation ability. The current study supports our model of the generation of PTSD in the Cambodian population, and suggests a key role of decreased vagal tone in the generation of orthostatic panic and PTSD in this population. It also suggests that vagal tone is involved in emotion regulation, and that both vagal tone and emotion regulation improve across treatment.
Keywords: Cambodian refugees, Cognitive‐behavior therapy, Emotion regulation, Orthostatic intolerance, Panic attacks, Posttraumatic stress disorder
Introduction
During Khmer Rouge rule (1975–1979), approximately 1.7 million of Cambodia's 7.9 million people died, and those that survived endured starvation, slave labor, untreated illness, torture, and constant threat of death [1]. Owing to severe trauma, Cambodian refugees at psychiatric clinics in the United States have elevated rates of posttraumatic stress disorder (PTSD; 56%) [2] and panic disorder (60%) [3], and they have unique panic‐attack (PA) subtypes: frequent orthostatic panic, that is, a panic attack triggered by standing up [4]. In a study of Cambodian patients attending a psychiatric clinic, 45% had current (i.e., at least one episode in the last month) orthostasis‐triggered panic, which was highly associated with having PTSD [5] (also see [6]).
There is evidence that PTSD may predispose to an impaired blood pressure response to orthostasis. A study using the Finapres (which provides a second‐by‐second assessment of blood pressure) of Gulf War veterans with PTSD and chronic fatigue syndrome demonstrated an impaired SBP response to orthostasis, with the degree of impairment significantly correlated to PTSD severity [7] (see too [4, 8]). The orthostatic blood response is of particular interest in PTSD as it is a measure of vagal control of the heart, which is linked to CNS vagal tone; the ability to maintain blood pressure upon standing up is largely determined by the ability to withdraw the “vagal break” at the heart [4]. Another measure of vagal control of the heart, and of parasympathetic nervous system (vagal) functioning, is baroreceptor sensitivity, the ability to increase blood pressure upon there being a blood pressure drop at detectors at and near the heart. Baroreceptor sensitivity is also decreased in PTSD [9]. More generally, studies indicate that parasympathetic activity is decreased in PTSD, and that this explains observed deficits in emotion regulation [10, 11], a key deficit in PTSD [12].
Our previous studies of Cambodian refugees indicate that orthostatic panic is strongly associated with PTSD, and that orthostatic panic is produced by several processes: impaired blood pressure response to standing up as well as catastrophic cognitions about and trauma associations to orthostasis‐induced sensations [5]. In addition, a previous study revealed cognitive‐behavior therapy's (CBT's) improvement of PTSD severity in a Cambodian population was partially mediated by improvement in orthostatic severity [5]. Based on these studies, conjoined with the literature reviewed previously, of the key role of vagal control of the in heart in the orthostatic blood pressure response and of the importance of emotional regulation in PTSD, we have developed a model of how PTSD is generated in Cambodian refugees (see Fig. 1).
Figure 1.
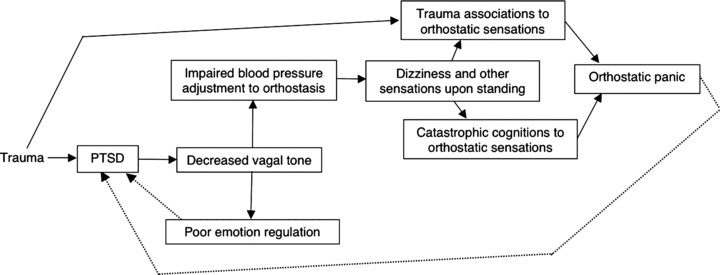
A model of the generation of PTSD among Cambodian refugees.
According to this model, initially trauma results in PTSD, and next PTSD decreases vagal tone (as indicated at the level of the heart by decreased heart rate variability). Poor vagal tone then impairs emotion regulation—this is because the person will be less able to dampen and tolerate anger, fear, shame, and other emotions (e.g., owing to decreased ability to disengage and shift attentional focus; see the Discussion section)—and in a feedback loop, poor emotion regulation worsens PTSD. And too, poor vagal tone worsens orthostatic adjustment, and hence may result in dizziness and other symptoms upon standing. These orthostatic sensations may then activate two types fear networks: catastrophic cognitions and trauma memories.
In respect to catastrophic cognitions, the patient may fear that the orthostatic sensations indicate a serious disorder of physiology: dizziness may be attributed to a wind‐like substance called khyâl, which as it rises upward in the body is thought to possibly cause various disasters—upon reaching the chest, asphyxia; upon reaching the heart, heart arrest; upon reaching the neck, neck vessel rupture; and upon reaching the head, fatal syncope. Or the orthostatic sensations may trigger recall: dizziness may recall various Pol Pot period traumas, such as a blow to the head, or collapse while doing slave labor without food. Owing to the activation of these two types of fear networks, orthostatic panic may well occur, and then the panic and trauma recall may increase PTSD severity.
This model (Fig. 1) has clear treatment implications, and based on it, we have created a model of how CBT brings about improvement in PTSD (see Fig. 2). As indicated in the model (Fig. 2), any technique that increases emotion regulation capacity will decrease PTSD, and decreased PTSD will increase vagal tone; and too, because the vagal system is tightly coupled to the emotion regulation system, increased emotion regulation ability directly increases vagal tone [13, 14, 15, 16]. In these two ways (through decrease of PTSD and direct effects on vagal tone), improved emotion regulation should improve vagal tone and orthostatic tolerance. And CBT—through such techniques as interoceptive exposure to somatic sensations, for example, head rolling; modification of catastrophic cognitions; elicitation of trauma associations—will decrease the activation of fear networks by sensations experienced upon standing up, and this decreased activation of fears networks by orthostatic sensations will decrease orthostatic panic, and PTSD; in turn, decreased PTSD severity should increase vagal tone.
Figure 2.
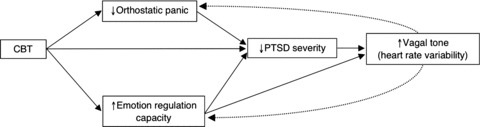
A model of how CBT improves PTSD among Cambodian refugees.
In the current study we examine the effect of a culturally sensitive CBT for traumatized Cambodian refugees with PTSD and comorbid orthostatic PAs [17, 18]. Our treatment focuses on increasing emotional regulation capacity and decreasing PAs, including orthostatic panic (see Procedure section for a description of the treatment). We hypothesized that our CBT intervention would impact all variables in our model of how CBT decreases panic and PTSD (see Fig. 2), namely, PTSD, orthostatic panic, orthostatic‐panic‐associated catastrophic cognitions, and trauma associations, and systolic BP response to orthostasis. Second, we also expected, as indicated by our treatment model (see Fig. 2), that improvement in PTSD severity would be mediated by improvement in emotion regulation capacity and orthostatic panic. And third, we hypothesized that the change in systolic BP across treatment (a proxy measure of vagal tone) would be correlated to change in a measure of the ability to emotionally regulate, given the literature demonstrating the relationship of SBP adjustment to vagal tone, and of vagal tone to emotion regulation capacity.
Method
Participants
Participants attended a community‐based outpatient clinic that provides specialized services to Cambodian refugees. We offered CBT treatment to Cambodian patients who were considered to have pharmacology‐resistant PTSD with comorbid orthostatic panic. All patients were on a maximally tolerated dosage of an SSRI. Inclusion criteria were (1) having passed through the Cambodian genocide (1975–1979); (2) having been at least 6 years old at the beginning of the genocide; (3) having pharmacology‐resistant PTSD as defined by continued presence of PTSD (as assessed by the SCID module for PTSD [19]) despite receiving supportive counseling and an adequate trial of a selective serotonin reuptake inhibitor at the maximally tolerated dose for a minimum of 6 months; and (4) having current (in the last month) orthostatic panic, as determined by the Orthostatic PA Interview (see subsequently). Exclusion criteria included (1) inability to give informed consent; (2) organic mental disorder, psychotic spectrum disorder, bipolar disorder, or active substance abuse or dependence; (3) serious suicide ideation currently or in the last 6 months; and (4) pregnancy. Patients were randomly assigned to either the Immediate Treatment or the Delayed Treatment Group. See Table 1 for a comparison of the immediate and Delayed Treatment Groups in terms of gender, age, years of education, years living in the United States, language facility, and religion. The study was approved by the Institutional Internal Review Board. The patients gave informed consent after a full explanation of the procedures. Two eligible patients declined to participate because of time and transportation constraints. All 24 randomized patients completed the study, and there were no missing data.
Table 1.
Demographic and acculturation variables as a function of treatment condition
| Variable | IT patients (n= 12) | DT patients (n= 12) | χ2(1) or t(22) |
|---|---|---|---|
| Female gender (%) | 60% | 60% | 0.00 |
| Age | 49.92 (9.23) | 49.08 (7.56) | 0.81 |
| Years of education | 3.68 (1.54) | 3.25 (1.95) | 1.03 |
| Years in the United States | 16.22 (3.60) | 15.50 (3.53) | 0.29 |
| Fluency in written English (%) | 0% | 0% | 0.00 |
| Fluency in spoken English (%) | 0% | 0% | 0.00 |
| Active Buddhist (%) | 100% | 100% | 0.00 |
Chi‐square test used for variables involving percentage; t test for all other variables. IT = immediate treatment; DT = delayed treatment. Years of education = time studying in Cambodian, in refugee camps, and when a monk (Cambodians males often become a monk for a few years and study at that time). Fluency in spoken English = an ability to communicate in a medication session without need of translation. Fluency in written English = an ability to read an English newspaper. An active Buddhist = someone who self‐identified as Buddhist who also visits the temple at least once a year to attend or participate in a ceremony. *P < 0.05.
Measures
All measures were translated to Khmer and then translated back to English for confirmation of accuracy, as per standard procedure [20].
Clinician‐Administered PTSD Scale (CAPS)
The CAPS rates, on 0–4 Likert‐type scales, the frequency and intensity of each of the 17 DSM–IV‐based PTSD symptoms [21]. Scores range from 0 to 136. The translated CAPS demonstrates good interrater (r = 0.92) and test–retest (r = 0.84; at 1 week) reliability [2].
Emotion Regulation Scale
The ability to distance from negative affects is one of the most important component skills in emotion regulation. We have created a 10‐item Likert‐type scale that assesses the patient's ability to distance from a number of dysphoric affects, including worry, anxiety, and depression, and the ability to distance from dysphoric emotions induced by particular situations: trauma recall or someone saying something hurtful. If the patient has not recently experienced the affect, we instruct the patient to answer how they would react if the emotion or emotion‐inducing situation were encountered. Each item is rated on a 0–4 Likert‐type scale, rating the ability to distance from affects, ranging from “not at all” to “very much so.” Most of the questions have the following form, “When you become X, are you able to distance yourself from that emotion?” where “X” is the emotion in question. In the Cambodian population, the scale has a high internal consistency (Cronbach's α= 0.79), and good test–retest reliability at 1 week (r = 0.86), as assessed with 30 patients.
Orthostatic PA Interview
We used a structured interviewed to assess for the presence of OP. As the initial probe question, we asked the following: “In the last four weeks, upon standing up, did you suddenly feel anxious, dizzy, or ill?” If the patient answered affirmatively, the patient was queried about the most recent episode, making sure that it (1) constituted orthostatically induced dysphoria, (2) met PA criteria (as per the Structured Clinical Interview for DSM–IV; [19]), and (3) was not preceded just before standing by a PA. The Orthostatic PA Interview has good interrater reliability (between the first author and a Cambodian speaker; κ= 1; N = 30).
Orthostatic‐PA Severity Scale (O‐PASS)
We assessed severity of orthostatic PAs on three dimensions [22], each scored on a 0–4 Likert‐type scale: (1) frequency, (2) length, and (3) amount of distress. The O‐PASS has good interrater (Pearson r = 0.97) and test–retest (at 1 week; r = 0.88) reliability (N = 20).
Orthostatic‐PA Flashback Severity Scale (O‐FSS)
We assessed the severity of flashbacks associated with orthostatic‐induced PAs (cf. [23]) on the following three dimensions, each rated on a 0–4 Likert‐type scale: (1) degree of dissociation, (2) length, and (3) amount of distress. To assess degree of dissociation, the O‐FSS includes the 0–4 Likert‐type scale of the CAPS's Flashback Intensity Scale [21]. The O‐FSS has good interrater (r = 0.95) and test–retest (at 1 week; r = 0.92) reliability (N = 20).
Orthostatic‐PA Catastrophic Cognition Severity Scale (O‐CCSS)
The patient was asked about the degree of fear during orthostatic PAs of five culturally specific catastrophic cognitions. As described in the Introduction section, there is the culturally belief that upon standing, “khyâl” and blood may rush upward in the body and into the head, an event called “khyâl overload,” causing various catastrophes. And also, standing up is thought to possibly cause a disruption of the flow of khyâl and blood to the arms and legs, resulting “death of the limbs,” and a surge of those substances up in the body. The five assessed catastrophic cognitions are as follows: (1) “When you stood up, did you fear ‘khyâl overload’?,” (2) “When you stood up, did you fear you would be unable to breath?,” (3) “When you stood up, did you fear heart arrest?,” (4) “When you stood up, did you fear neck vessel rupture?,” and (5) “When you stood up, did you fear the ‘death’ of your arms and legs?” Responses to these items were rated on 0–4 Likert‐type scales and comprise the Orthostasis Catastrophic Cognition Inventory (OCCI). If the patient had not experienced any PAs in the previous 4 weeks, the patient was asked about the presence and severity of the five catastrophic cognitions upon standing up during the previous 4 weeks. The OCCI has good interrater reliability [4].
Orthostatic Change Scores
We assessed systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) change scores upon rising from a sitting position—that is, the standing value subtracted from the sitting value (averaged over the previous 30 sec)—sampling at 5‐sec intervals up until 60 sec. SBP and DBP reach a nadir at about 15 sec after standing, then rising back to values comparable to those in the sitting position [7, 24]. For a comparison of the two groups in physiological response, for each patient, we selected the time point with the SBP nadir [7, 24].
Procedure
We used a repeated‐measures design, in which one group of patients was immediately started on the CBT treatment (Immediate Treatment Group) while the other group was given treatment as usual (Delayed Treatment Group). After the Initial Treatment Group finished CBT, the Delayed Treatment Group was given CBT.
Eligible patients who agreed to participate were stratified by gender, with random allocation to either initial or delayed treatment decided by a coin toss. All patients continued supportive psychotherapy, which consisted of a meeting with a social worker every 2 weeks, and medications, which consisted in all cases of a combination of an SSRI (in most cases, paroxetine) and the benzodiazepine, clonazepam. The treating psychiatrist was instructed to try to maintain the level of medication at the same level, but to adjust levels if deemed clinically necessary. Studies indicate that SSRIs [25] and benzodiazapines [26] do not adversely affect the BP response to orthostasis.
All participants completed three assessments of the CAPS: Emotion Regulation Scale, O‐PASS, O‐FSS, and O‐CCSS: (1) pretreatment (first assessment); (2) after the Immediate Treatment Group had undergone 12 weekly sessions of CBT (second assessment); and (3) after the Delayed Treatment Group had undergone 12 weekly sessions of CBT (third assessment).
We also assessed the physiological adjustment to orthostasis at the three time points. SBP, DBP, and HR were measured using a Finapres (Model 2300, Ohmeda, Louisville, CO, USA). The Finapres cuff was wrapped around the index finger of the left hand; and the hand was supported so as to remain at the level of the heart at all times. Once the monitoring equipment was attached, the patient was asked to sit quietly for 5 min. Next, the patient stood up, and remained standing for 1 min. Physiologic measures were sampled every 5 sec from 30 sec prior to standing until 1 min after standing.
Blind to treatment condition, all assessments were made by a Cambodian bicultural worker with over 2 years of mental health experience. The assessor of orthostatic PA severity was blind to all other measures, and the assessor of the other psychometric assessments was blind to both the severity of orthostatic PAs and physiological change scores.
Treatment
The first author (DH), who is fluent in Cambodian, conducted or co‐led the CBT sessions, utilizing a manual‐based protocol developed by our team [17, 18, 27, 28]. CBT was offered across 12 weekly sessions and emphasized information about a cognitive‐behavioral model of PTSD and panic disorder, muscle relaxation and diaphragmatic breathing, guided imagery and mindfulness training (mindfulness training emphasized multi‐sensorial attention to the present moment, each session emphasizing a particular sensory modality), yoga‐like stretching linked to self‐images of flexibility (this involved stretching new muscle groups in each session, with an emphasis on the neck, back, and leg musculature), cognitive restructuring, various exercises used to teach emotional distancing and switching, and interoceptive exposure. Orthostasis‐associated flashbacks and catastrophic cognitions were specifically addressed. The therapy emphasized various techniques to promote emotional regulation, for example, applied muscle relaxation, applied stretching, mindfulness, and various exercises aiming to increase psychological and emotional flexibility and promote emotional distancing.
Results
For both groups, neither psychometric nor physiological scores differed significantly at baseline (no Ps < 0.05). In order to illustrate the magnitude of treatment benefits relative to other studies, we computed between‐group effect sizes, comparing the Immediate Treatment (IT) and Delayed Treatment (DT) groups at the second assessment, using Cohen's d: d= MeanDT Group– MeanIT Group/SDpooled. Table 2 provides symptom scores for the three assessments and between‐group effect sizes.
Table 2.
The CAPS, emotion regulation scale, orthostatic panic severity, orthostasis‐associated flashbacks, orthostasis‐associated catastrophic cognitions, and physiological change score as a function of treatment and time of assessment (N = 24)
| First assessment M (SD) | Second assessment M (SD) | Third assessment M (SD) | Between‐group effect size, based on second assessment, Cohen's d | |
|---|---|---|---|---|
| CAPS | ||||
| Immediate treatment | 75.41 (13.47) | 46.83 (17.17) | 44.75 (14.85) | 1.98 |
| Delayed treatment | 77.25 (11.47) | 74.25 (9.43)* | 45.83 (8.45) | |
| Emotion regulation scale | ||||
| Immediate treatment | 0.9 (0.6) | 2.5 (0.40) | 2.7 (0.60) | 2.53 |
| Delayed treatment | 0.8 (0.5) | 0.9 (0.80) | 2.4 (0.50) | |
| O‐PASS | ||||
| Immediate treatment | 8.04 (2.40) | 2.25 (2.05) | 2.41 (1.98) | 2.84 |
| Delayed treatment | 7.71 (1.47) | 7.25 (1.42)* | 1.67 (2.14) | |
| O‐FSS | ||||
| Immediate treatment | 9.08 (4.33) | 3.50 (3.29) | 1.83 (2.37) | 1.18 |
| Delayed treatment | 8.25 (4.50) | 7.75 (3.93)* | 1.58 (2.02) | |
| O‐CCSS | ||||
| Immediate treatment | 2.98 (0.65) | 1.71 (0.64) | 1.87 (0.49) | 2.79 |
| Delayed treatment | 3.24 (0.34) | 3.12 (0.32)* | 1.79 (0.42) | |
| Systolic BP Δ score | ||||
| Immediate treatment | −17.01 (8.41) | −7.42 (4.87) | −7.16 (5.13) | 1.31 |
| Delayed treatment | −16.33 (7.30) | −15.50 (7.18)* | −7.50 (5.47) | |
| Diastolic BP Δ score | ||||
| Immediate treatment | −6.00 (6.98) | −3.83 (3.15) | −4.00 (4.35) | 0.30 |
| Delayed treatment | −4.33 (3.39) | −5.01 (4.35) | −3.25 (3.27) | |
| Heart rate Δ score | ||||
| Immediate treatment | 7.17 (3.61) | 6.62 (2.82) | 7.25 (3.91) | 0.10 |
| Delayed treatment | 8.75 (3.96) | 7.91 (3.07) | 7.58 (3.42) | |
Note. N = 24; CAPS = clinician‐administered PTSD severity scale; O‐PASS = orthostatic‐PA severity scale; O‐FSS = orthostatic‐PA flashback severity scale; O‐CCSS = orthostatic‐PA catastrophic cognitions scale. Systolic BP Δ change score = the SBP change score, which was calculated by subtracting the post‐orthostasis SBP nadir from the sitting value; diastolic BP Δ score = the DBP change score, utilizing the DBP value at the SBP nadir; HR Δ score = the HR change score, utilizing the HR value at the SBP nadir.
*P < 01, in respect to the difference between the two groups at that time point.
In order to evaluate the effects of treatment on psychometric outcome measures, we conducted a 2 (GROUP: initial vs. delayed treatment) by three (TIME: first, second, and third assessment) repeated‐measures multivariate analysis of variance (MANOVA) with the CAPS, Emotion Regulation Scale, O‐PASS, O‐FSS, and O‐CCSS as the dependent variables. Similar procedures were used for the physiologic outcomes, with the change in scores between sitting and standing serving as the dependent variable.
For the psychometric outcomes, analyses indicated a significant Group effect, F(5, 18) = 3.02, P < 0.05; Time effect, F(16, 13) = 34.15, P < 0.001; and Group by Time interaction effect, F(10, 13) = 46.16, P < 0.001. The Mauchley Sphericity test, a measure of homogeneity of covariance, was nonsignificant for any variable; this indicates the sphericity was not violated. In follow‐up analyses, repeated‐measures analysis of variances (ANOVAs) showed, for all dependent variables, a significant Group effect (Fs > 8.10, Ps < 0.002), Time effect (Fs > 50.38, Ps < 0.001), and Time by Group interaction effect (Fs > 9.01, Ps < 0.001). In order to examine group differences, we conducted unpaired t tests. The results showed significant group differences for all variables at the second assessment (ts > 2.81, Ps < 0.01), but not for any variable at either the first (ts < 1.2, Ps > 0.05) or third (ts < 0.23, Ps > 0.05) assessment. In addition, we examined time effect as a polynomial, testing for a quadratic trend by group interaction. As hypothesized, the quadratic, but not linear, trends were significant for all psychometric variables (quadratic: Fs > 21.61, Ps < 0.01; linear: Fs < 1, Ps = ns).
For the three physiologic measures—SBP, DBP, and HR change scores from sitting to standing—analyses showed a nonsignificant Group effect, F(3, 20) = 1.97, P= 0.15, but a significant Time effect, F(6, 17) = 6.12, P < 0.01, and Group by Time interaction effect, F(6, 17) = 4.95, P < 0.01. The Mauchley Sphericity test was nonsignificant for each of the three variables. Follow‐up analyses of repeated‐measures ANOVAs showed a nonsignificant Group effect for all three variables (Fs < 2, Ps > 0.05); a significant Time effect for the SBP change score, F(2, 20) = 22.37, P < 0.001, but not for DBP or HR change scores (Fs < 2.55, Ps > 0.05); and a significant Time by Group interaction effect for the SBP change score, F(2, 20) = 5.86, P < 0.01, but not for the DBP or the HR change score (Fs < 2.24, Ps > 0.05). In order to examine group differences in SBP scores, we conducted unpaired t tests. The results showed significant group differences at the second assessment for the SBP score, t(22) = 3.23, P < 0.01, but not for DBP and HR scores (ts < 0.92, P > 0.05). No group differences were observed on any of the outcome variables at the first (ts < 0.84, Ps > 0.05) and third assessment (ts < 1.54, Ps > 0.05). In addition, we examined time effect as a polynomial, testing for a quadratic trend by group interaction for SBP score. As hypothesized, the quadratic, but not linear, trend was significant (quadratic: F= 14.9, P < 0.01; linear: F < 1, Ps = ns).
As predicted by the model (see Fig. 1), the correlation of the residualized SBP change score to residualized emotion regulation change score was high: r = 0.72, P < 0.01. (We used residualized change scores for all variables to more accurately reflect outcome [29].) To explore the treatment model (see Fig. 2), we then used a Baron and Kenny analysis to examine whether improvement in emotion regulation capacity and orthostatic panic severity mediated the effect of treatment on PTSD severity (for a similar analytic approach, see [29]).
First, we determined whether treatment condition (waitlist to treatment) explained variance in the residualized CAPS change score. The results suggested that 64% of the variance in the residualized CAPS change score was explained by the treatment condition, r = 0.80, P < 0.01. Second, we investigated the correlation of treatment condition to the residualized emotion regulation scale change score and the orthostatic panic change score, which were 0.75 and 0.69, respectively (Ps < 0.01). Next, we determined the correlation of both the residualized emotion regulation scale change score and that of the residualized orthostatic panic severity scale change score to the residualized CAPS change score, which were 0.71 and 0.61, respectively (Ps < 0.01). Finally, we performed a regression analysis with all three predictor variables with the residualized CAPS change score as the dependent variable. In that regression, when all three predictors were entered, β of the treatment predictor variable was nonsignificant, whereas that of the residualized emotion regulation change score and that of the residualized orthostatic panic change score were significant, β= 0.61 and β= 0.51, respectively (Ps < 0.01). That model explained 78% of the variance in PTSD severity, F(3, 18) = 20.7, P < 0.001. These results support the mediation model.
Discussion
The current article demonstrated CBT's improvement of multiple psychometric variables—including emotion regulation ability and orthostatic panic severity—and one physiological variable, namely, orthostatic blood pressure adjustment. The study supported our model of how PTSD and orthostatic panic is generated (Fig. 1), and how CBT brings about improvement in the model variables (Fig. 2). And as hypothesized, improvement in SBP adjustment (our proxy measure of vagal tone) was highly correlated to the ability to successfully regulate negative affect.
As indicated in the Introduction section, we hypothesize that the CBT intervention improved systolic blood pressure response to adjustment by the following means (see Fig. 2). CBT decreased PTSD, for example, through decreasing orthostatic panic and increasing emotion regulation capacity; then the decrease in PTSD resulted in increased vagal tone, which improved orthostatic blood pressure response. And we hypothesize that when CBT improved emotion regulation capacity, it directly increased vagal tone (in addition to doing so through decreasing PTSD), because emotion regulation and vagal tone are based in the same neurocognitive and biological system (as reviewed previously) and have complex links.
How might vagal tone serve as a key aspect of emotional regulation? It seems to play a key role in the control of attention, allowing the disengaging from attentional object and the choosing of another [15]. It would seem to have as its main role the disengagement from total attention on one object, from a fixed mindset; only through disengagement from total absorption in an attentional object, a given mindset, are other attentional objects, and mindsets, an option. Disengagement allows more action‐behavioral flexibility, allows the regulation of affect by contemplating other attentional objects and by considering other mindsets. In the Cambodian case, low vagal tone delivers a double blow—decreased emotional regulation ability and worsened orthostatic panic.
We think our findings support the contention by a new wave of researchers that emotion regulation ability is a key cog in the production and maintenance of psychopathology, and a key dimension to be addressed in treatment [30, 31, 32]. Our study provides evidence that emotion regulation ability changes across treatment and is a key mediator of improvement, and that its improvement may be related to physiological variable, namely, vagal tone.
Of note, in the current study, we did not use other published scales of emotion regulation (e.g., [32]). We used the scale as described in the current study for several reasons. For one, we choose to create a scale that had clear face validity and that could be easily translated and used in non‐Western populations. Second, we consider the ability to emotionally distance from affects to be the key component of emotion regulation that underlies its other aspects (for descriptions of hypothesized aspects of emotion regulation, see [30, 31, 32]). (Of note, in Theravada Buddhism, based on the first author's discussions with Cambodian monks and patients and reading of Cambodian Buddhist texts, this emotion distancing capacity is considered to be the key aspect of emotion regulation; it is called in “ubekkha” in the Pali language, a term also commonly used in the Cambodian language.) And third, we considered the ability to distance from negative affects to be the aspect of emotion regulation that would be most likely to be related to vagal tone.
Several limitations of the present study should be mentioned. The current study was done with a very small sample, constituting a small pilot study. Replication with a larger sample is warranted. A future study should assess a measure of vagal tone, such as heart rate variability. In this way, all variables in the model can be explored. A study should be done to examine how a pharmacological intervention (such as a serotonin reuptake blocker) influences all the variables in the model. A future study should examine the variables identified in the current study at multiple time points in order to establish causality: that our CBT treatment improved emotion regulation ability that then decreased PTSD severity. We did not prevent medication changes, which create one other source of variability.
Conflict of Interest
Mark Pollack has been on advisory boards and has done consultation for AstraZeneca, Brain Cells Inc, Bristol Myers Squibb, Cephalon, Dov Pharmaceuticals, Forest Laboratories, GlaxoSmithKline, Janssen, Jazz Pharmaceuticals, Labopharm, Eli Lilly & Co, Medavante, Neurocrine, Neurogen, Novartis, Otsuka Pharmaceuticals, Pfizer, Predix, Roche, Laboratories, Sanofi, Sepracor, Solvay, Tikvah Therapeutics, Transcept Inc, UCB Pharma, Wyeth. He has received research grants from Astra‐Zeneca, Bristol Myers Squibb, Cephalon, Cyberonics, Forest Laboratories, GlaxoSmithKline, Janssen, Eli Lilly, NARSAD, NIDA, NIMH, Pfizer, Roche Laboratories, Sepracor, UCB Pharma, Wyeth. He has done presentations with support from Bristol Myers Squibb, Forest Laboratories, GlaxoSmithKline, Janssen, Lilly, Pfizer, Solvay, Wyeth. He has equity in Medavante, Mensante Corporation, Mindsite, Targia Pharmaceuticals and receives copyright royalties for the SIGH‐A, SAFER.
References
- 1. Kiernan B. The Pol Pot regime: Race, power, and genocide in Cambodia under the Khmer Rouge. New Haven , CT : Yale University Press, 2002;1975–1979. [Google Scholar]
- 2. Hinton DE, Chhean D, Pich V, Pollack MH, Orr SP, Pitman RK. Assessment of posttraumatic stress disorder in Cambodian refugees using the clinician‐administered PTSD scale: Psychometric properties and symptom severity. J Trauma Stress 2006;19:405–411. [DOI] [PubMed] [Google Scholar]
- 3. Hinton DE, Ba P, Peou S, Um K. Panic disorder among Cambodian refugees attending a psychiatric clinic: Prevalence and subtypes. Gen Hosp Psychiatry 2000;22:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hinton DE, Pich V, So V, Pollack MH, Pitman RK, Orr SP. The psychophysiology of orthostatic panic in Cambodian refugees attending a psychiatric clinic. J Psychopathol Behav Assess 2004;26:1–13. [Google Scholar]
- 5. Hinton DE, Hofmann SG, Pitman RK, Pollack MH, Barlow DH. The panic attack‐posttraumatic stress order model: Applicability to orthostatic panic among Cambodian refugees. Cogn Behav Ther 2008;37:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hinton DE, Pollack MH, Pich V, Fama JM, Barlow DH. Orthostatically induced panic attacks among Cambodian refugees: Flashbacks, catastrophic cognitions, and associated psychopathology. Cogn Behav Pract 2005;12:301–311. [Google Scholar]
- 7. Peckerman A, Dahl K, Chemitiganti R, LaManca JJ, Ottenweller JE, Natelson BH. Effects of posttraumatic stress disorder on cardiovascular stress responses in Gulf War veterans with fatiguing illness. Auton Neurosci 2003;108:63–72. [DOI] [PubMed] [Google Scholar]
- 8. Orr SP, Meyerhoff J, Edwards J, Pitman RK. Heart rate and blood pressure resting levels and responses to generic stressors in Vietnam veterans with posttraumatic stress disorder. J Trauma Stress 1988;11:155–164. [DOI] [PubMed] [Google Scholar]
- 9. Hughes JW, Dennis MF, Beckham JC. Baroreceptor sensitivity at rest and during stress in women with posttraumatic stress disorder or major depressive disorder. J Trauma Stress 2007;20:667–676. [DOI] [PubMed] [Google Scholar]
- 10. Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosom Med 2007;69:935–943. [DOI] [PubMed] [Google Scholar]
- 11. Sack M, Hopper JW, Lamprecht F. Low respiratory sinus arrhythmia and prolonged psychophysiological arousal in posttraumatic stress disorder: Heart rate dynamics and individual differences in arousal regulation. Biol Psychiatry 2004;55:284–290. [DOI] [PubMed] [Google Scholar]
- 12. Tull MT, Barrett HM, McMillan ES, Roemer L. A preliminary investigation of the relationship between emotion regulation and posttraumatic stress disorder. Behav Ther 2007;38:303–313. [DOI] [PubMed] [Google Scholar]
- 13. Cysarz D, Büssing A. Cardiorespiratory syncronization during Zen meditation. Eur J Appl Physiol 2005;95:88–95. [DOI] [PubMed] [Google Scholar]
- 14. Nickel C, Kettler C, Muehlbacher M, Lahmann C, Tritt K, Fartacek R, Bachler E, Rother N, Egger C, Rother WK, et al Effects of progessive muscle relaxation in adolescent female bronchial asthma patients: A randomized, double‐blind, controlled study. J Psychosom Res 2005;59:393–398. [DOI] [PubMed] [Google Scholar]
- 15. Porges S. The polyvagal theory: Phylogenetic contributions to social behavior. Physiol Behav 2003;79:503–513. [DOI] [PubMed] [Google Scholar]
- 16. Thayer JF, Lane R. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord 2000;61:201–216. [DOI] [PubMed] [Google Scholar]
- 17. Hinton DE, Chhean D, Pich V, Safren SA, Hofmann SG, Pollack MH. A randomized controlled trial of CBT for Cambodian refugees with treatment‐resistant PTSD and panic attacks: A cross‐over design. J Trauma Stress 2005;18:617–629. [DOI] [PubMed] [Google Scholar]
- 18. Otto MW, Hinton DE, Korbly NB, Chea A, Ba P, Gershuny BS, Pollack MH. Treatment of pharmacotherapy‐refractory posttraumatic stress disorder among Cambodian refugees: A pilot study of combination treatment with cognitive‐behavior therapy vs. sertraline alone. Behav Res Ther 2003;41:1271–1276. [DOI] [PubMed] [Google Scholar]
- 19. First MB, Spitzer RL, Gibbon M. Structured clinical interview for DSM–IV axis I disorders. New York : New York State Psychiatric Institute, 1995. [Google Scholar]
- 20. Mollica RF, Wyshak G, Marneffe D, Khuon F, Lavelle J. Indochinese versions of the Hopkins Symptom Checklist‐25: A screening instrument for the psychiatric care of refugees. Am J Psychiatry 1987;144:497–500. [DOI] [PubMed] [Google Scholar]
- 21. Weathers F, Keane T, Davidson J. Clinician‐administered PTSD scale: A review of the first ten years of research. Depress Anxiety 2001;13:132–156. [DOI] [PubMed] [Google Scholar]
- 22. Shear MK, Brown TA, Barlow DH, Money R, Sholomskas DE, Woods SW, Gorman JM, Papp LA. Multicenter collaborative panic disorder severity scale. Am J Psychiatry 1997;154:1571–1575. [DOI] [PubMed] [Google Scholar]
- 23. Hackmann A, Ehlers A, Speckens A, Clark DM. Characteristics and content of intrusive memories in PTSD and their changes with treatment. J Trauma Stress 2004;17:231–240. [DOI] [PubMed] [Google Scholar]
- 24. Coupland NJ, Wilson SJ, Potokar JP, Bell C, Nutt DJ. Increased sympathetic response to standing in panic disorder. Psychiatry Res 2003;118:69–79. [DOI] [PubMed] [Google Scholar]
- 25. Rodriguez de la Torre B, Dreher J, Malevany U, Bagli M, Kolbinger M, Omran H, Lüderitz B, Rao ML. Serum levels and cardiovascular effects of tricyclic antidepressants and selective serotonin reuptake inhibitors in depressed patients. Ther Drug Monit 2001;22:435–440. [DOI] [PubMed] [Google Scholar]
- 26. Van Den Berg F, Tulen J, Boomsma F, Noten J, Moleman P, Peepleinkhuizen I. Effects of alprazolam and lorazepam on catecholaminergic and cardiovascular activity during supine rest, mental load and orthostatic challenge. Psychopharmacology 1996;128:21–30. [DOI] [PubMed] [Google Scholar]
- 27. Hinton DE, Otto MW. Symptom presentation and symptom meaning among traumatized Cambodian refugees: Relevance to a somatically focused cognitive‐behavior therapy. Cogn Behav Pract 2006;13:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hinton DE, Pham T, Tran M, Safren SA, Otto MW, Pollack MH. CBT for Vietnamese with treatment‐resistant PTSD and panic attacks. J Trauma Stress 2004;17:429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Casey LM, Newcombe PA, Oei T. Cognitive mediation of panic severity: The role of catastrophic misinterpretation of bodily sensations and panic self‐efficacy. Cogn Ther Res 2005;29:187–200. [DOI] [PubMed] [Google Scholar]
- 30. Rottenberg J, Gross J. Emotion and emotion regulation: A map for psychotherapy researchers. Clin Psych: Science and Practice 2007;14:323–328. [Google Scholar]
- 31. Mennin D. Emotion regulation therapy: An integrative approach to treatment‐resistant disorder. J Contemp Psychother 2006;36:95–105. [Google Scholar]
- 32. Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. J Psychopath Behav Asessm 2004;26:41–54. [Google Scholar]


