Abstract
We sought to determine whether maca, a Peruvian plant, is effective for selective‐serotonin reuptake inhibitor (SSRI)‐induced sexual dysfunction. We conducted a double‐blind, randomized, parallel group dose‐finding pilot study comparing a low‐dose (1.5 g/day) to a high‐dose (3.0 g/day) maca regimen in 20 remitted depressed outpatients (mean age 36 ± 13 years; 17 women) with SSRI‐induced sexual dysfunction. The Arizona Sexual Experience Scale (ASEX) and the Massachusetts General Hospital Sexual Function Questionnaire (MGH‐SFQ) were used to measure sexual dysfunction. Ten subjects completed the study, and 16 subjects (9 on 3.0 g/day; 7 on 1.5 g/day) were eligible for intent‐to‐treat (ITT) analyses on the basis of having had at least one postbaseline visit. ITT subjects on 3.0 g/day maca had a significant improvement in ASEX (from 22.8 ± 3.8 to 16.9 ± 6.2; z =−2.20, P= 0.028) and in MGH‐SFQ scores (from 24.1 ± 1.9 to 17.0 ± 5.7; z =−2.39, P= 0.017), but subjects on 1.5 g/day maca did not. Libido improved significantly (P < 0.05) for the ITT and completer groups based on ASEX item #1, but not by dosing groups. Maca was well tolerated. Maca root may alleviate SSRI‐induced sexual dysfunction, and there may be a dose‐related effect. Maca may also have a beneficial effect on libido.
Keywords: L. meyenii, Maca, Natural remedies, Sexual dysfunction, Side effects, SSRI
Introduction
Antidepressant‐induced sexual dysfunction remains a significant issue in the treatment of patients with depressive disorders, affecting up to 50% of patients who receive pharmacological treatment [1]. The emergence of sexual dysfunction in the context of antidepressant treatment may be so distressing to some patients that it may lead to treatment discontinuation, despite adequate efficacy [2].
Up to 50% of patients may not respond to the available treatments [3], or may find the cost of some of these treatments prohibitive. Consequently, antidepressant‐induced sexual dysfunction remains an obstacle to the effective treatment of depressed individuals, and many individuals have turned to complementary and natural treatments in the hopes of improving sexual functioning [4]. One such natural agent is maca root (Lepidium meyenii).
Maca, also known as “Peruvian Ginseng,” is a hardy perennial plant cultivated at high altitudes in the Andean Mountains [4], traditionally used for both nutritional and medicinal purposes. Its fertility‐enhancing properties were first reported in 1961, when Chacon [5] discovered that it increased the fertility of rats. It is unclear which components of maca are responsible for its putative sex‐enhancing properties. Though Chacon [5] identified four alkaloids responsible for sexual enhancing properties in animals, subsequent chemical analyses have not substantiated her results and some investigators have questioned the existence of these alkaloids [4].
While anecdotal evidence, especially from South America, supports the use of maca for treatment of sexual dysfunction, there are few peer‐reviewed English language journal reports to corroborate these claims in humans. However, several studies in mice and rats have examined the effects of maca on stress, prostate hyperplasia, spermatogenesis, testicular function, hormone levels, and sexual behavior [6, 7, 8, 9], concluding in general that maca improves spermatogenesis [10, 11, 12, 13].
Despite the robust body of animal studies (at least 23 studies with as many as 60 animals in one study), there are very few reports published in English that examine maca root administration in humans. Most studies with human subjects examine the effect of maca on sexual functioning in adult healthy men and have demonstrated a lack of effect of maca on serum gonadal hormone levels, finding no changes in serum levels of LH, FSH, PRL, 17‐alpha hydroxyprogesterone, TST, and 17‐beta estradiol in men treated with maca at either 1.5 g/day or 3.0 g/day or placebo [14, 15, 16].
Regarding clinical efficacy, there is one published 12‐week double‐blind placebo‐controlled randomized trial. Healthy adult men ages 21–56 received either placebo or maca at doses of 1.5 g/day or 3.0 g/day. The authors do not specify why they selected this particular dosing range. An improvement in sexual desire was observed with maca, compared to placebo, and it did not separate by dose by 8 weeks of treatment, in the absence of changes in serum testosterone and estradiol [16].
Though there have been no systematic human studies on the use of maca in antidepressant‐induced sexual dysfunction, the anecdotal evidence, as well as its relatively wide safety index, makes it a good candidate for controlled human studies. Given its benign side‐effect profile, maca can be used by the elderly and cardiac‐impaired populations taking oral nitrates who may not be eligible for sildenafil or other phophodiesterase inhibitors. Furthermore, maca is less costly than phophodiesterase inhibitors. Based on out‐of‐pocket costs and assuming daily sexual activity, maca costs about $150 per month; the cost for Viagra under these circumstances would be about $900 per month.
In view of the above, we sought to determine whether maca root was effective for the treatment of selective‐serotonin reuptake inhibitor (SSRI)‐induced sexual dysfunction and to further determine whether higher doses (3.0 g/day) of maca root were more effective than lower doses (1.5 g/day) in remitted depressed subjects with sexual dysfunction. An additional aim of the study was to document the safety and tolerability of maca root. We hypothesized that maca would reverse antidepressant‐induced sexual function in our study sample, and that the degree of improvement would be greater in the group receiving the higher dose of maca (3.0 g/day).
Methods
Twenty remitted depressed outpatients (mean age 36 ± 13; 17 women, 3 men) were recruited over 10 months (April, 2005 – February, 2006) via MGH IRB‐approved consenting procedures. Subjects with major depressive disorder (MDD) were diagnosed with the Structured Clinical Interview for DSM‐IV (SCID‐ I/P) [17] and were required to have a 17‐item Hamilton Rating Scale for Depression (HAM‐D‐17) [18, 19] score < 10 to indicate remission. Subjects were also required to be without significant anxiety symptoms as evidenced by a score of less than 10 on the Hamilton Rating Scale for Anxiety (HAM‐A<10) [20].
Subjects were required to have been taking an SSRI, venlafaxine, or tri/hetero cyclic antidepressant for the treatment of depression for at least 8 weeks, and be currently at a stable dose of the antidepressant for at least 4 weeks. Subjects were required to meet at least one of the following criteria for at least 4 weeks: (1) inability to have an orgasm (anorgasmia) during sexual activity; (2) clinically significant orgasm delay with masturbation or intercourse that, according to self‐report, representing a meaningful delay and interfering with sexual function compared with the subject's usual time to achieve orgasm prior to antidepressant medication; (3) inability to attain or maintain until completion of sexual activity an adequate erection or lubrication swelling response of sexual excitement that, according to self‐report, interfered with sexual function compared to prior to antidepressant medication; (4) decreased libido according to self‐report.
Subjects must have had no sexual dysfunction prior to taking an antidepressant (by self‐report) and there had to be a clear temporal relationship between the sexual dysfunction and the antidepressant treatment. Sexual dysfunction secondary to the depressive illness itself was exclusionary.
Subjects were required to be having some form of regular sexual activity with or without a partner at least twice monthly prior to the antidepressant treatment and had to be willing to continue efforts at sexual activity at least once weekly for the duration of the study. Subjects were required to be in good general physical health and be able to understand and communicate in English, and give informed consent to participate in the study.
Exclusion criteria included: primary or prior diagnosis of a sexual disorder (other than the side effect of the antidepressant drug or symptom of major depression); sexual dysfunction secondary to general underlying medical condition; no other current primary psychiatric disorder; alcohol or substance abuse or dependence within the past 6 months; recent major sexual relationship changes, disruption, or turmoil ongoing or anticipated which are unrelated to their sexual dysfunction, HAM‐D‐17 or HAM‐A score (either) >10; current use of other drugs for antidepressant‐induced sexual dysfunction or other therapies or medications to treat sexual dysfunction; hormone replacement therapy, unless patient had been on stable dose of hormone therapy for at least 3 months prior to the antidepressant treatment, had no sexual dysfunction while on the same hormone therapy regimen, and there was no change in the hormone replacement therapy during the study; pregnancy, lactation, or plans to become pregnant during the study; any clinically significant abnormality of the screening physical examination; any medical or psychological condition or social circumstances that would impair subject's ability to participate reliably in the study, or that may have increased the risk to subjects or others as a result of participating in the study; testosterone implant during 6 months prior to screening; receiving psychosexual or other therapy for sexual dysfunction and not willing to discontinue that treatment at screening; subjects for whom sexual activity was inadvisable; changes in antidepressant agent and/or dose of prescribed antidepressant agent.
A double‐blind, randomized parallel group dose‐finding design was used for the study. Subjects were stratified by gender prior to randomization, to minimize potential confounding of data. Ten subjects were randomized to low‐dose (1.5 g/day) maca, and 10 were randomized to high‐dose (3.0 g/day) maca. The upper dose was vetted by our IRB as the highest allowable dose for which safety data were available. The 3.0 g/day group received 6 capsules of 500 mg maca daily; the 1.5 g/day group received 6 capsules of 250 mg daily. Maca was purchased from the company A Healthy Alternative (ahealthya.com) in Long Island.
Assessments of sexual function were obtained as part of routine clinical appointments every 2 weeks over 12 weeks of treatment, using the Arizona Sexual Experience Scale (ASEX) [21] and the Massachusetts General Hospital Sexual Function Questionnaire (MGH‐SFQ) [22]. In its validation study, the MGH scale items were stratified as normal (score of ≤2) or dysfunctional (score >2) [22]. In the validation study for the ASEX, a total ASEX score of ≥19, any one item with a score of ≥5, or any three items with a score of ≥4 was considered sexual dysfunction [21]. Depressive symptoms were monitored with the HAM‐D‐17 at each visit. Patients were also asked to fill out the Symptoms Questionnaire (SQ) [23]. Additional instruments that were administered biweekly include the Clinical Global Impression of Severity (CGI‐S) and Improvement Scales (CGI‐I) [24] and the HAM‐A.
Patients were asked to track all of their sexual attempts in a personal diary, and to record when they took or forgot to take the study medication (starting at the baseline visit when they began the maca root). Endpoints were defined as the final study visit at the end of the 12 weeks, or the termination/final visit for patients who chose to (or had to) end participation in the study prematurely. The primary test of outcome was the analysis of the degree of improvement in ASEX or MGH Sexual Function Questionnaire scores compared to baseline.
Two analyses were carried out: (1) a “completer” analysis of all patients finishing the trial; (2) an “intent‐to‐treat” (ITT) analysis examining all patients randomized to the trial, as long as they had at least one follow up visit postbaseline (evaluable sample). Nonparametric tests were used because the assumptions about the population distribution strictly necessary for parametric methods to apply were inappropriate for this small sample. The Wilcoxon Paired Signed Ranks Test [25] was used to examine whether the degrees of improvement in MGH and ASEX scores were significant for each of the two groups (low‐dose and high‐dose maca). A two‐tailed alpha level of 0.05 was set for all statistical tests.
Exploratory regression analysis was carried out to investigate any differences in response observed with age or partner status (independent variables). The MGH‐SFQ and ASEX scores were set as the dependent variables in these analyses.
Statistical analyses were carried out using Statview software (SAS Product).
Results
Subjects in the study used a variety of antidepressants and augmenting agents (Table 1). Eleven subjects were on antidepressant monotherapy; the other nine subjects were on regimens consisting of at least two and no more than four psychotropic drugs.
Table 1.
Primary antidepressant and augmenting agents used by subjects in the study
| Primary SSRI antidepressants | Secondary/augmenting agents |
|---|---|
| Escitalopram (n = 5) | Bupropion (n = 5) |
| Citalopram (n = 4) | Thyroid hormone (n = 3) |
| Sertraline (n = 3) | Mirtazapine (n = 1) |
| Venlafaxine (n = 3) | Olanzapine (n = 1) |
| Fluoxetine (n = 2) | Zolpidem (n = 1) |
| Paroxetine (n = 1) | Adderall (amphetamine salts) (n = 1) |
| Duloxetine (n = 1) | Gabapentin (n = 1) |
| Fluvoxamine (n = 1) | Pramipexole (n = 1) |
In the three primary areas of inquiry regarding sexual dysfunction, 19 (95%) subjects endorsed delayed orgasm as a complaint; 14 (70%) endorsed difficulty with arousal; and 19 (95%) endorsed lack of libido. Fifteen (75%) reported a history of sexual dysfunction from past antidepressant trials. Among the 17 women, two were postmenopausal. Eighteen subjects described themselves as heterosexual, one as homosexual, and one as bisexual. Nine were married or living with someone as if married, 10 were never married, and one was divorced.
Four subjects left the study after the screen visit. Ten subjects completed the study. Sixteen subjects (14 women, 2 men; 9 on 3.0 g/day maca, and 7 on 1.5 g/day) met criteria for ITT analysis. Any subject who completed at least one study visit after starting medication was included in the ITT sample.
The reasons for discontinuation include: relationship change; inability to make scheduled appointments; change in antidepressant regimen; displeasing smell of maca; personal reasons and several subjects simply lost to follow‐up. To the best of our knowledge, no subject discontinued due to tolerability issues.
Efficacy of Maca on Sexual Function and Libido
Using the Wilcoxon test, we found statistically significant improvement in the mean total ASEX score for all ITT subjects (low‐ and high‐dose maca pooled together) at the completion of the study or final visit (from 23.9 ± 4.1 to 17.3 ± 5.5; z =−2.87, P= 0.004) (Table 2). Improvement on the MGH‐SFQ scale also reached significance, with a similar pattern to the ASEX (from 23.8 ± 4.4 to 17.9 ± 5.7; z =−2.42; P= 0.016) (Table 2).
Table 2.
Improvement in sexual function based on ASEX and MGH‐SFQ scores before and after treatment with maca
| ASEX | MGH‐SFQ | |
|---|---|---|
| (combined low and high dose maca groups) | ||
| ITT | N = 16 | N = 16 |
| Baseline visit | 23.9 (SD = 4.1) | 23.8 (SD = 4.4) |
| Final visit | 17.3 (SD = 5.7) | 17.9 (SD = 5.7) |
| Significance | z =−2.87; P= 0.004* | z =−2.42; P= 0.016* |
| COMPLETERS | n = 10 | n = 10 |
| Baseline visit | 24.8 (SD = 4.0) | 23.0 (SD = 5.6) |
| Final visit | 17.2 (SD = 5.9) | 17.6 (SD = 6.1) |
| Significance | z =−2.30; P= 0.022* | z =−1.48; P= 0.139 |
| (comparison between maca dosing groups) | ||||
|---|---|---|---|---|
| ASEX | MGH‐SFQ | |||
| LOW (1.5 g/d) | HIGH (3.0 g/d) | LOW (1.5 g/d) | HIGH (3.0 g/d) | |
| ITT | n = 7 | n = 9 | n = 7 | n = 9 |
| Baseline visit | 25.4 (SD = 4.2) | 22.8 (SD = 3.8) | 23.4 (SD = 6.4) | 24.1 (SD = 1.9) |
| Final visit | 17.7 (SD = 5.4) | 16.9 (SD = 6.2) | 19.0 (SD = 5.8) | 17.0 (SD = 5.7) |
| Significance | z =−1.89; P= 0.059 | z =−2.20; P= 0.028* | z =−1.15; P= 0.249 | z =−2.39; P= 0.017* |
| COMPLETERS | n = 6 | n = 4 | n = 6 | n = 4 |
| Baseline visit | 25.5 (SD = 4.6) | 23.8 (SD = 3.1) | 22.8 (SD = 6.8) | 23.3 (SD = 3.2) |
| Final visit | 16.5 (SD = 4.8) | 18.3 (SD = 7.9) | 17.7 (SD = 5.1) | 17.5 (SD = 8.2) |
| Significance | z =−1.89; P= 0.059 | z =−1.29; P= 0.198 | z =−1.15; P= 0.249 | z =−1.069; P= 0.285 |
*Statistically significant (P < 0.05).
ITT subjects on high maca dose had a statistically significant improvement in the ASEX score (from 22.8 ± 3.8 to 16.9 ± 6.2; z =−2.20, P= 0.028), whereas ITT subjects on low‐dose maca improved but just missed significance (from 25.4 ± 4.2 to 17.7 ± 5.4; z =−1.89; P= 0.059) (Table 2). Mean MGH‐SFQ scores showed a similar trend, with statistically significant improvement for ITT subjects on high maca dose (from 24.1 ± 1.9 to 17.0 ± 5.7; z =−2.39, P= 0.017) and nonsignificant improvement on low dose (from 23.4 ± 6.4 to 19.0 ± 5.8; z =−1.15; P= 0.249) (Table 2, Fig. 1).
Figure 1.
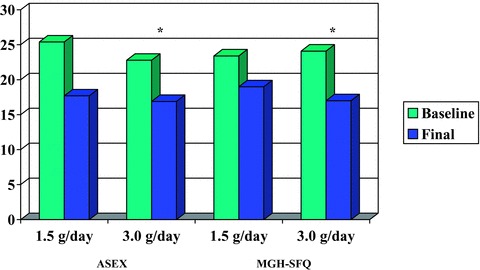
Change in ASEX and MGH‐SFQ scores over 12 weeks (pre‐/post‐treatment) in intent‐to‐treat sample. *Change in score was significant (P < 0.05).
For all completers, there was a statistically significant improvement in mean total ASEX score (from 24.8 ± 4.0 to 17.2 ± 5.9; z =−2.30; P= 0.022), and nonsignificant improvement in MGH‐SFQ score (Table 2). Among completers receiving low‐dose (n = 6) or high‐dose maca (n = 4), we found a similar pattern of improvement for both ASEX and MGH‐SFQ scores, but these results did not reach significance (Table 2).
Libido was measured by the score on the first items of the ASEX and the MGH‐SFQ scales (ASEX #1 and MGH‐SFQ a, respectively). In the ITT sample as a whole (low‐ and high‐dose maca), we found a significant improvement in the ASEX libido item (from 4.9 ± 1.1 to 3.6 ± 1.6; z =−2.19, P= 0.028), but the improvement in MGH‐SFQ libido item just missed significance (Table 3). The same pattern was observed for all completers, with a significant improvement in the ASEX libido item (from 5.1 ± 1.0 to 3.5 ± 1.7; z =−1.97; P= 0.049) and nonsignificant improvement in the MGH‐SFQ libido item (Table 3). When we examined these items by dosing groups, we found improvement in each dosing group, both for completers and for the ITT group, but none reached statistical significance (Table 3).
Table 3.
Improvement in libido based on ASEX #1 and MGH‐SFQ item a before and after treatment with maca
| Libido‐ASEX #1 | Libido‐MGH‐SFQ a | |
|---|---|---|
| (combined low and high dose maca groups) | ||
| ITT | N = 16 | N = 16 |
| Baseline visit | 4.9 (SD = 1.1) | 4.6 (SD = 1.2) |
| Final visit | 3.6 (SD = 1.6) | 3.4 (SD = 1.5) |
| Significance | z =−2.19; P= 0.028* | z =−1.90; P= 0.058 |
| COMPLETERS | n = 10 | n = 10 |
| Baseline visit | 5.1 (SD = 1.0) | 4.8 (SD = 1.5) |
| Final visit | 3.5 (SD = 1.7) | 3.3 (SD = 1.5) |
| Significance | z =−1.97; P= 0.049 | z =−1.53; P= 0.127 |
| (comparison between maca dosing groups) | ||||
|---|---|---|---|---|
| Libido‐ASEX #1 | Libido‐MGH‐SFQ a | |||
| LOW (1.5 g/d) | HIGH (3.0 g/d) | LOW (1.5 g/d) | HIGH (3.0 g/d) | |
| ITT | n = 7 | n = 9 | n = 7 | n = 9 |
| Baseline visit | 5.1 (SD = 1.2) | 4.7 (SD = 1.1) | 4.4 (SD = 1.5) | 4.8 (SD = 1.0) |
| Final visit | 3.4 (SD = 1.7) | 3.7 (SD = 1.7) | 3.4 (SD = 1.7) | 3.3 (SD = 1.3) |
| Significance | z =−1.63; P= 0.104 | z =−1.42; P= 0.156 | z =−0.95; P= 0.340 | z =−1.86; P= 0.063 |
| COMPLETERS | n = 6 | n = 4 | n = 6 | n = 4 |
| Baseline visit | 5.0 (SD = 1.3) | 5.3 (SD = 0.5) | 4.3 (SD = 1.6) | 5.7 (SD = 0.6) |
| Final visit | 3.0 (SD = 1.4) | 4.3 (SD = 2.1) | 3.0 (SD = 1.4) | 3.8 (SD = 1.7) |
| Significance | z =−1.63; P= 0.104 | z =−1.07; P= 0.285 | z =−1.22; P= 0.223 | Z =−1.41; P= 0.157 |
*Statistically significant (P < 0.05).
As an exploratory analysis, we examined whether subject age or having a regular sexual partner had a significant impact on response to maca, based on total ASEX and MGH‐SFQ scores. Logistic regression analysis yielded no significant relationship between degree of improvement with maca (dependent variable) and having a steady partner (independent variable) both in the ITT sample and in completers (P > 0.05 for all). Linear regression yielded no significant relationship between age and degree of improvement with maca, both in the ITT sample and in completers (P > 0.05 for all). The two male patients included in the ITT sample showed similar degree of improvement to the population as a whole, though the smallness of this group does not allow for significant comparisons.
Attempts at Sexual Activity, and Satisfaction Measures
We examined the frequency of attempts at sexual activity made by study subjects in each 2‐week period, whether they considered their sexual attempts enjoyable, and whether they were able to achieve orgasm. In the ITT sample as a whole, modest improvement overall was seen in the number of biweekly sexual attempts made, number of sexual experiences considered enjoyable, and number of orgasms attained (Table 4). Examination by dosing arms revealed a more notable improvement in the ITT subjects receiving higher maca doses, with statistically significant improvement reached in the number of biweekly sexual attempts (from 3.1 ± 2.4 to 4.6 ± 3.7; z =−1.98, P= 0.048) and in number of enjoyable experiences for the 3.0 g/day maca group (from 1.2 ± 1.2 to 3.4 ± 2.9; z =−2.34, P= 0.019) (Table 4). The ITT group receiving 1.5 g/day maca did not reach significant improvement in any of these parameters, nor did the pooled sample of high and low maca groups. No significant increase in number of orgasms was found in any group. Completer analysis for each dosing group found no statistically significant improvement in any of these parameters.
Table 4.
Sexual attempts, satisfaction, and ability to achieve orgasm over a 2‐week period
| ITT‐ALL SUBJECTS (N = 16) | # Sexual attempts | # Enjoyable | # Orgasms |
|---|---|---|---|
| Baseline visit | 3.5 (SD = 2.8) | 1.7 (SD = 1.6) | 1.3(SD = 1.5) |
| Final visit | 4.0 (SD = 3.0) | 3.0 (SD = 2.4) | 1.8 (SD = 2.8) |
| Significance | z =−0.88; P= 0.38 | z =−1.83; P= 0.07 | z =−0.81; P= 0.42 |
| ITT‐LOW DOSE (1.5 g/day) (n = 7) | |||
| Baseline visit | 4.0 (SD = 3.4) | 2.3 (SD = 1.9) | 1.3 (SD = 1.6) |
| Final visit | 3.3 (SD = 1.7) | 2.6 (SD = 1.8) | 1.0 (SD = 1.3) |
| Significance | z =−0.42; P= 0.67 | z =−0.17; P= 0.86 | z =−0.41; P= 0.68 |
| ITT‐HIGH DOSE (3.0 g/day) (n = 9) | |||
| Baseline visit | 3.1 (SD = 2.4) | 1.2 (SD = 1.2) | 1.3 (SD = 1.5) |
| Final visit | 4.6 (SD = 3.7) | 3.4 (SD = 2.9) | 2.5 (SD = 3.6) |
| Significance | z =−1.98; P= 0.048* | z =−2.34; P= 0.019* | z =−1.40; P= 0.16 |
| COMPLETERS‐ALL (n = 10) | # Sexual Attempts | # Enjoyable | # Orgasms |
|---|---|---|---|
| Baseline visit | 3.1 (SD = 3.1) | 1.5 (SD = 1.6) | 0.8 (SD = 0.9) |
| Final visit | 2.6 (SD = 1.3) | 2.0 (SD = 1.4) | 1.2 (SD = 13) |
| Significance | z =−0.24; P= 0.81 | z =−0.62; P= 0.54 | z =−0.88; P= 0.38 |
| COMPLETERS‐LOW DOSE (1.5 g/day) (n = 6) | |||
| Baseline visit | 4.0 (SD = 3.7) | 2.0 (SD = 1.9) | 0.8 (SD = 1.2) |
| Final visit | 2.8 (SD = 1.3) | 2.2 (SD = 1.6) | 1.2 (SD = 1.3) |
| Significance | z =−0.68; P= 0.50 | z =−0.11; P= 0.92 | z =−0.38 P= 0.71 |
| COMPLETERS‐HIGH DOSE (3.0 g/day) (n = 4) | |||
| Baseline visit | 1.8 (SD = 1.0) | 0.8 (SD = 0.5) | 0.8 (SD = 0.5) |
| Final visit | 2.3 (SD = 1.3) | 1.8 (SD = 1.3) | 1.3 (SD = 1.5) |
| Significance | z =−1.00; P= 0.32 | z =−1.30; P= 0.19 | z =−0.82; P= 0.41 |
*Statistically significant (P < 0.05).
Course of Depression and Anxiety Symptoms During Maca Treatment
Subjects as a whole remained stable with regard to their depressive and anxious symptoms throughout the course of the study. For the ITT population as a whole, we found no significant changes in mean HAM‐D and HAM‐A scores following treatment. However, examination of the ITT sample by dosing groups revealed a small but significant decrease in mean HAM‐D score for the high maca group only (from 4.2 ± 1.6 to 3.2 ± 1.9; z =−1.98, P= 0.047) (Table 5). No significant changes in HAM‐D or HAM‐A scores were found among completers, both when pooled and when broken down by dosing arms (Table 5).
Table 5.
Hamilton D‐17 and Hamilton A Scores
| ITT | All subjects (N = 16) | Low‐dose maca (1.5 g/day) (n = 7) | High‐dose maca (3.0 g/day) (n = 9) |
|---|---|---|---|
| HAM‐D‐17‐BL | 4.2 (SD = 2.0) | 4.1 (SD = 2.6) | 4.2 (SD = 1.6) |
| HAM‐D‐17‐ final | 4.3 (SD = 4.1) | 5.7 (SD = 7.2) | 3.2 (SD = 1.9) |
| Significance | z =−1.32; P= 0.19 | z =−0.14; P= 0.89 | z =−1.98; P= 0.047* |
| HAM‐A‐ BL | 2.0 (SD = 1.9) | 0.8 (SD = 0.8) | 2.8 (SD = 2.1) |
| HAM‐A‐final | 2.8 (SD = 2.4) | 3.0 (SD = 3.5) | 2.6(SD = 1.4) |
| Significance | z =−0.31; P= 0.76 | z =−0.96; P= 0.34 | z =−0.85; P= 0.40 |
| COMPLETERS | All Completers (n = 10) | Low Maca (n = 6) | High Maca (n = 4) |
|---|---|---|---|
| HAM‐D‐17‐BL | 3.8 (SD = 2.0) | 3.5 (SD = 2.2) | 4.2 (SD = 2.1) |
| HAM‐D‐17‐ final | 3.2 (SD = 2.4) | 3.2 (SD = 2.6) | 3.3 (SD = 2.5) |
| Significance | z =−1.30; P= 0.19 | z =−0.74; P= 0.46 | z =−1.63; P= 0.10 |
| HAM‐A‐ BL | 1.4 (SD = 1.8) | 0.5 (SD = 0.6) | 2.7 (SD = 2.3) |
| HAM‐A‐final | 2.1 (SD = 1.7) | 1.8 (SD = 1.7) | 2.5 (SD = 1.9) |
| Significance | z =−0.54; P= 0.59 | z =−0.38; P= 0.71 | (not enough observations to compute) |
*Statistically significant (P < 0.05).
Tolerability of Maca
The maca treatment was well tolerated overall. Eleven of the 16 ITT patients reported at least one adverse effect during the study. These included GI upset (n = 5), headache (n = 2), irritability (n = 2), panic attack (n = 1), urinary frequency (n = 1), blurry vision (n = 1), sleep disruption (n = 1), increased sweating (n = 1), increased dreaming (n = 1), thicker menstrual discharge (n = 1), and fibromyalgia exacerbation (n = 1). These events were consistently transient, and none led to subject discontinuation. In most cases, it was difficult to establish a direct relationship with maca administration.
Discussion
Sexual dysfunction remains a significant obstacle to successful treatment with antidepressants. The current armamentarium for sexual dysfunction includes the phosphodiesterase‐5 (PDE‐5) inhibitors, such as sildenafil, effective in the treatment of antidepressant‐induced erectile dysfunction and anorgasmia in men [26, 27, 28, 29], but benefits to women are less clear. To our knowledge, this represents the first report of a prospective study of maca root as a potential treatment for SSRI‐induced sexual dysfunction.
Our sample showed significant improvement in sexual function based on responses to both the ASEX and MGH‐SFQ questionnaires, and the degree of improvement appeared to be dose related. Although all subject groups showed a notable mean improvement in sexual function, the most robust and statistically significant improvement was observed for subjects receiving 3.0 g/day of maca, as opposed to 1.5 g/day.
Maca treatment resulted in a significant improvement in libido in the ITT sample, and this improvement may be more notable in the high‐dose group. The high‐dose group made more attempts at sexual activity—perhaps a reflection of improved libido—and also found their sexual experiences more enjoyable. The improvement in sexual desire would appear to reflect previous findings in animal studies and in the one published human study [20]. Furthermore, given that the vast majority (85%) of the patients in this study were women, maca may represent a significant step in the management of antidepressant‐induced sexual dysfunction in women.
Unexpectedly, we found a modest but significant improvement in Hamilton‐D‐17 scores for the ITT recipients of high‐dose maca. Given the remitted status of all subjects, and the low baseline HAM‐D‐17 scores, the clinical significance of this remains unclear. The improvement in depressive symptoms could be a direct effect of maca or perhaps a reflection of increased sexual satisfaction. Maca appeared to be well tolerated but this must be said with caution, in view of the high dropout rate, which may signify tolerability problems.
Our study is limited by a number of factors. Seventeen of the 20 participants were women, which would restrict generalizability of the results to primarily one gender. However, because Gonzales et al's positive study [16] consisted of a male sample, it would appear that both genders may benefit from maca administration. Almost half of our subjects were taking at least one other psychotropic drug in addition to their primary antidepressant, which could perhaps affect responsiveness to maca, depending on the type of drug and its potential impact on sexual function.
Other limitations of our study include the lack of a placebo arm and a small sample size. The number of completers was also relatively small, limiting the information obtained from the completer analysis, because they may represent a rarified subject group. However, our dose‐finding design yielded an encouraging dose‐response pattern in many dimensions of sexual dysfunction for both for completers and for the ITT sample, suggesting that the observed response to maca is a real phenomenon. However, expectation effect cannot be discounted in the absence of a placebo group. A larger scale replication study with a placebo arm and with a more even gender distribution will be necessary to further clarify the efficacy of maca.
We suspect that given the high consumption of maca in the South American diet, it would be perfectly safe to examine doses of maca higher than those reported here for antidepressant‐induced sexual dysfunction. Additional investigation will be necessary to establish the optimal dose of maca for antidepressant‐induced sexual dysfunction in humans.
As a final observation, the authors were pleasantly surprised that the entire cohort of subjects was recruited more than twice as quickly as originally anticipated. This suggests that a natural remedy such as maca may be far more acceptable and desirable to patients suffering from medication‐related sexual side effects. Given its high acceptability and tolerability, as well as its greater affordability compared to the PDE‐5 inhibitors, maca could become a valuable addition to the current armamentarium for the treatment of antidepressant‐induced sexual dysfunction.
In summary, maca root may alleviate SSRI‐induced sexual dysfunction including having a beneficial effects on libido, and there may be a dose‐related effect. A replication study with a larger sample and higher maca doses is currently under development.
Conflict of Interest
Christina Dording, MD
Research Support: Abbott Laboratories, Alkermes, Aspect Medical Systems, Astra‐Zeneca, Bristol‐Myers Squibb Company, Cephalon, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmithkline, J& J Pharmaceuticals, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Novartis, Organon Inc., PamLab, LLC, Pfizer Inc, Pharmavite, Roche, Sanofi/Synthelabo, Solvay Pharmaceuticals, Inc., Wyeth‐Ayerst Laboratories
Speakers' Honoraria: Wyeth
Maurizio Fava, MD
Advisory/Consulting: Aspect Medical Systems, Astra‐Zeneca, Bayer AG, Biovail Pharmaceuticals, Inc., BrainCells, Inc. Bristol‐Myers Squibb Company, Cephalon, Compellis, Cypress Pharmaceuticals, Dov Pharmaceuticals, Eli Lilly & Company, EPIX Pharmaceuticals, Fabre‐Kramer Pharmaceuticals, Inc., Forest Pharmaceuticals Inc., GlaxoSmithkline, Grunenthal GmBH, Janssen Pharmaceutica, Jazz Pharmaceuticals, J & J Pharmaceuticals, Knoll Pharmaceutical Company, Lundbeck, MedAvante, Inc., Neuronetics, Novartis, Nutrition 21, Organon Inc., PamLab, LLC, Pfizer Inc, PharmaStar, Pharmavite, Roche, Sanofi/Synthelabo, Sepracor, Solvay Pharmaceuticals, Inc., Somaxon, Somerset Pharmaceuticals, Wyeth‐Ayerst Laboratories
Speaking: Astra‐Zeneca, Boehringer‐Ingelheim, Bristol‐Myers Squibb Company, Cephalon, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmithkline, Novartis, Organon Inc., Pfizer Inc, PharmaStar, Wyeth‐Ayerst Laboratories
Equity Holdings: Compellis, MedAvante
Royalty/patent, other income: none
David Mischoulon, MD, PhD
Research Support: Lichtwer Pharma GmbH, Bristol‐Myers Squibb Company, Cederroth, Laxdale (Amarin), Nordic Naturals, SwissMedica
Advisory/Consulting: None
Speaking: Bristol‐Meyers Squibb Company, Pamlab LLC, Nordic Naturals, Virbac, Pfizer, Wyeth, AstraZeneca, Cephalon, Janssen, Lilly
Equity Holdings: None
Royalty/patent, other income: PMS Escape (patent co‐holder)
Andrew A. Nierenberg, MD
Grant/Research Support: Bristol‐Myers Squibb, Cederroth, Cyberonics, Forest Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, Lichtwer Pharma, Eli Lilly, NARSAD, NIMH, Pfizer, Stanley Foundation, Wyeth‐Ayerst
Advisory/Consulting: Bristol‐Myers Squibb, Genaissance, GlaxoSmithKline, Innapharma, Janssen Pharmaceutica, Eli Lilly, Novartis, Pfizer, Sepracor, Shire, Somerset
Equity Holdings: None
Speaking/Honoraria: Bristol‐Myers Squibb, Cyberonics, Forest Pharmaceuticals, GlaxoSmithKline, Eli Lilly, Wyeth‐Ayerst
George I. Papakostas, MD
Research Support: Bristol‐Myers Squibb Company, Pamlab LLC, Pfizer Inc.
Advisory/Consulting: Aphios Corporation, Evotec Ltd., GlaxoSmithKline, Inflabloc Pharmaceuticals, Inc, Jazz Pharmaceuticals, Pamlab, LLC
Honoraria: Evotec Ltd., GlaxoSmithKline, Inflabloc Pharmaceuticals, INC., Jazz Pharmaeuticals,
Pamlab, LLC, Pfizer, Inc., Titan Pharmaceuticals
Equity Holdings: none
Royalty/patent, other income: none
Shamsah Sonawalla, M.D.
Research Support: Abbott Laboratories, Alkermes, Aspect Medical Systems, Astra‐Zeneca, Bristol‐Myers Squibb Company, Cephalon, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmithkline, J& J Pharmaceuticals, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Novartis, Organon Inc., PamLab,LLC, Pfizer Inc, Pharmavite, Roche, Sanofi/Synthelabo, Solvay Pharmaceuticals, Inc., Wyeth‐Ayerst Laboratories
All other authors report no conflicts of interest.
Acknowledgment
Dr. Dording was supported by the Loan Repayment Program of the National Institutes of Health.
References
- 1. Landen M, Hogberg P, Thase ME. Incidence of sexual side effects in refractory depression during treatment with citalopram or paroxetine. J Clin Psychiatry 2005;661:100–106. [DOI] [PubMed] [Google Scholar]
- 2. Cassano P, Fava M. Tolerability issues during long‐term treatment with antidepressants. Ann Clin Psychiatry 2004;16(1):115–125. [DOI] [PubMed] [Google Scholar]
- 3. Sperling H, Lummen G, Schneider T, Rubben H. New treatment options for erectile dysfunction. Pharmacologic and nonpharmacologic options. [Article in German] Herz. 2003;28(4):314–324. [DOI] [PubMed] [Google Scholar]
- 4. Mischoulon D. Polypharmacy and side effects management with natural psychotropic medications In: Mischoulon D, Rosenbaum J, eds. Natural medications for psychiatric disorders:Considering the alternatives Philadelphia , Lippincott, Williams & Wilkins, 2002; pp. 207–215. [Google Scholar]
- 5. Chacon RC. A study of the chemical composition of Lepidium meyenii. Dissertation, Univ., Nac. Mayo de San Marcos, Peru , 1961.
- 6. Cicero AF, Bandieri W, Arletti R. Lepidium meyenii Walp. Improves sexual behavior in male rats independently from its action on spontaneous locomotor activity. J Ethnopharmacol 2001;75(2–3):225–229. [DOI] [PubMed] [Google Scholar]
- 7. Zheng BL, He K, Kim CH, Rogers L, Shao Y, Huang ZY, Lu Y, Yan SJ, Qien LC, Zheng QY. Effect of a lipidic extract from lepidium meyenii on sexual behavior in mice and rats. Urology 2000;55(4):598–602. [DOI] [PubMed] [Google Scholar]
- 8. Oshima M, Gu Y, Tsukada S. Effects of Lepidium meyenii Walp and Jatropha macrantha on blood levels of estradio‐17 beta, progesterone, testosterone and the rate of embryo implantation in mice. J Vet Med Sci 2003;65(10):1145–1146. [DOI] [PubMed] [Google Scholar]
- 9. Bogani P, Simonini F, Iriti M, Rossoni M, Faoro F, Poletti A, Visioli F. Lepidium Meyenii (Maca) does not exert direct androgenic activities. J Ethnopharmacol 2006;104(3):415–417. [DOI] [PubMed] [Google Scholar]
- 10. Gonzales GF, Ruiz A, Gonzales C, Villegas L, Cordova A. Effect of lepidium meyenii (maca) roots on spermatogenesis of males rats. Asian J Androl 2001;33:231–233. [PubMed] [Google Scholar]
- 11. Bustos‐Obregon JE, Yucra S, Gonzales GF. Lepidium meyenii (Maca) reduces spermatogenic damage induced by a single dose of malathion in mice. Asian J Androl 2005;7(1):71–76. [DOI] [PubMed] [Google Scholar]
- 12. Gonzales GF, Gasco M, Cordova A, Chung A, Rubio J, Villegas L. Effect of Lepidium meyenii (Maca) on spermatogenesis in male rats exposed to high altitude (4340m). J Endocrinol 2004;180(1):87–95. [DOI] [PubMed] [Google Scholar]
- 13. Rubio J, Riqueros MI, Gasco M, Yucra S, Miranda S, Gonzales GF. Lepidium Meyenii (Maca) reversed the lead acetate induced‐damage on reproductive function in male rats. Food Chem Toxiol 2006;44(7):1114–1122. [DOI] [PubMed] [Google Scholar]
- 14. Gonzales GF, Cordova A, Gonzales C, Chung A, Vega K, Villena A. Lepidium meyenii (Maca) improved semen parameters in adult men. Asian J Androl 2001;3(4):301–303. [PubMed] [Google Scholar]
- 15. Gonzales GF, Cordova A, Vega K, Chung A, Villena A, Gonez D. Effect of Lepidium meyenii (Maca), a root with aphrodisiac and fertility‐enhancing properties, on serum reproductive hormone levels in adult healthy men. J Endocrinol 2003;176(1):163–168. [DOI] [PubMed] [Google Scholar]
- 16. Gonzales GF, Cordova A, Vega K, Chung A, Villena A, Gonez C, Castillo S. Effect of Lepidium meyenii (MACA) on sexual desire and its absent relationship with serum testosterone levels in adult healthy men. Andrologia 2002;34(6):367–372. [DOI] [PubMed] [Google Scholar]
- 17. Spitzer RL, Williams JBW, Gibbon MB, First M. Structured Clinical Interview for DSM‐III‐R—Patient Version (SCID‐P, 9/1/1989 version New York , NY , Biometrics Research Department, New York State Psychiatric Institute, 1989. [Google Scholar]
- 18. Hamilton, M . Development of a rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamilton M. Development of a rating scale for primary depressive illness. Br J Social Clin Psychology 1967;6(4):278–296. [DOI] [PubMed] [Google Scholar]
- 20. Hamilton, M . The assessment of anxiety states by rating. Br J Med Psychol 1959;32(1):50–55. [DOI] [PubMed] [Google Scholar]
- 21. McGahuey CA, Gelenberg AJ, Laukes CA, Moreno FA, Delgado PL, McKnight KM, Manber R. The Arizona Sexual Experience Scale (ASEX): Reliability and validity. J Sex Marital Ther 2000;26(1):25–40. [DOI] [PubMed] [Google Scholar]
- 22. Labbate LA, Lare SB. Sexual dysfunction in male psychiatric outpatients: Validity of the Massachusetts General Hospital Sexual Functioning Questionnaire. Psychother Psychosom 2001;70(4):221–225. [DOI] [PubMed] [Google Scholar]
- 23. Kellner R. A Symptom Questionnaire. J Clin Psychiatry 1987;48:268–274. [PubMed] [Google Scholar]
- 24. Guy W. (ed). ECDEU Assessment Manual for Psychopharmacology, revised. DHEW Pub. No. (ADM)76‐338. National Institute of Mental Health, Rockville , MD , 1976. [Google Scholar]
- 25. Leon AC. Descriptive and inferential statistics In: Bellack AS, Hersen M, eds. Comprehensive clinical psychology. Oxford , Pergamon, 1998; pp. 243–285. [Google Scholar]
- 26. Fava M, Rankin MA, Alpert JE, Nierenberg AA, Worthington JJ. An open trial of oral sildenafilin antidepressant‐induced sexual dysfunction. Psychother Psychosom 1998;67(6):328–331. [DOI] [PubMed] [Google Scholar]
- 27. Reznik I, Zemishlany Z, Kotler M, Spivak B, Weizman A, Mester R. Sildenafil citrate for the sexual dysfunction in antidepressant‐treated male patients with posttraumatic stress disorder. A preliminary pilot open‐label study. Psychother Psychosom 2002;71(3):173–176. [DOI] [PubMed] [Google Scholar]
- 28. Nurnberg HG, Hensley PL, Gelenberg AJ, Fava M, Lauriello J, Paine S. Treatment of antidepressant‐associated sexual dysfunction with sildenafil: a randomized controlled trial. JAMA 2003;289(1):56–64. [DOI] [PubMed] [Google Scholar]
- 29. Fava M, Nurnberg HG, Seidman SN, Holloway W, Nicholas S, Tseng LJ, Stecher VJ. Efficacy and safety of sildenafil in men with serotonergic antidepressant‐associated erectile dysfunction: Results from a randomized, double‐blind, placebo‐controlled trial. J Clin Psychiatry 2006;67(2):240–246. [DOI] [PubMed] [Google Scholar]


