Abstract
Lysergic acid diethylamide (LSD) was synthesized in 1938 and its psychoactive effects discovered in 1943. It was used during the 1950s and 1960s as an experimental drug in psychiatric research for producing so‐called “experimental psychosis” by altering neurotransmitter system and in psychotherapeutic procedures (“psycholytic” and “psychedelic” therapy). From the mid 1960s, it became an illegal drug of abuse with widespread use that continues today. With the entry of new methods of research and better study oversight, scientific interest in LSD has resumed for brain research and experimental treatments. Due to the lack of any comprehensive review since the 1950s and the widely dispersed experimental literature, the present review focuses on all aspects of the pharmacology and psychopharmacology of LSD. A thorough search of the experimental literature regarding the pharmacology of LSD was performed and the extracted results are given in this review. (Psycho‐) pharmacological research on LSD was extensive and produced nearly 10,000 scientific papers. The pharmacology of LSD is complex and its mechanisms of action are still not completely understood. LSD is physiologically well tolerated and psychological reactions can be controlled in a medically supervised setting, but complications may easily result from uncontrolled use by layman. Actually there is new interest in LSD as an experimental tool for elucidating neural mechanisms of (states of) consciousness and there are recently discovered treatment options with LSD in cluster headache and with the terminally ill.
Keywords: LSD, Psychopharmacology, Pharmacology, Pharmacokinetics, Mechanism of action, Hallucinogen, Psychedelic
Introduction
Lysergic acid diethylamide (LSD) is a semisynthetic product of lysergic acid, a natural substance from the parasitic rye fungus Claviceps purpurea. Albert Hofmann, a natural products chemist at the Sandoz AG Pharmaceutical Company (Basel, Switzerland) synthesized it in 1938 while searching for pharmacologically active derivatives of lysergic acid. He accidentally discovered its dramatic psychological effects in 1943. Though he synthesized many lysergic acid derivatives, none had LSD's unique spectrum of psychological effects. During the 1950s LSD (Delysid© Sandoz) was introduced to the medical community as an experimental tool to induce temporary psychotic‐like states in normals (“model‐psychosis”) and later to enhance psychotherapeutic treatments (“psycholytic” or “psychedelic” therapy) [1, 2].
Toward the end of the 1960s, people began using LSD for recreational and spiritual purposes, [3] leading to the formation of a “psychedelic movement” during the international student protests of that era [4, 5]. Though the protest movement declined, the use of LSD continued. It is still a major hallucinogen, illegally used worldwide. The National Survey on Drug Use and Health[6] has, for example, reported LSD as a major drug of abuse in every annual survey since the 1970s.
Despite LSD's successful and safe use as a psychotherapeutic adjunct and experimental tool (cf. Ref. [7] and the retrospective surveys of Cohen [8] and Malleson [9]), almost no legal clinical research with LSD has occurred since the 1970s. Exceptions include the continued use in psychotherapy by Hanscarl Leuner at Göttingen University (Germany) and by a limited number of psychotherapists in Switzerland from 1988 to 1993 [10, 11]. Today, interest is increasing for using LSD in brain research, treatment of cluster headache [12], and as an aid in the psychotherapeutic treatment of the terminally ill [13, 14].
Though no physical damage results from the use of LSD, many psychiatric complications have been reported, with a peak occurring at the end of the 1960s [15, 16]. Although the dosage appears mostly unchanged since the 1970s [16], the number of complications has probably declined since the late 1960s and early 1970s because today there may be better‐informed users, better mental preparation and attention to surrounding conditions, and reduction in dosage weight (although one report claims LSD dosage has remained fairly constant since the 1970 [16]).
Due to the widely dispersed (across time and languages) experimental literature concerning the pharmacological properties of LSD, old and new data are together reviewed here. It should be noted that the characterization of the complex effects on the human psyche are not the focus of this review [17, 18, 19].
Chemistry
LSD is a semisynthetic substance derived from lysergic acid as found in the parasitic rye fungus C. purpurea. The molecule consists of an indole system with a tetracyclic ring (C20H25ON3) (see Figure 1).
Figure 1.
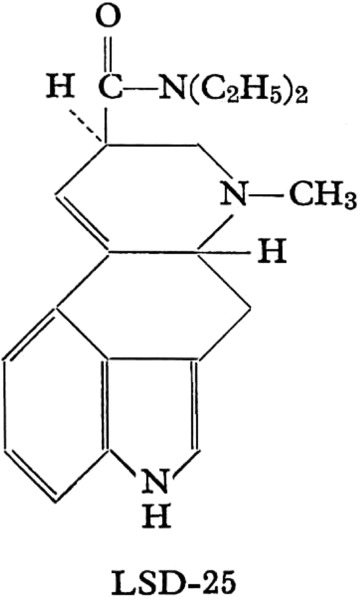
Lysergic acid diethylamide
Carbons 5 and 8 are asymmetric: therefore, four isomeric, optically‐active LSD isomers are possible and known. These are d‐ and l‐LSD and d‐ and l‐isolysergic acid diethylamide. Only the d‐LSD isomer has psychoactive properties. D‐LSD crystallizes from benzene in pointed prisms. It is water‐soluble and its melting point is 83°C. LSD is usually stabilized in solution as its tartrate salt. The molar mass is 323.42 g/mol.
A great number of homologs and analogs of LSD has been studied [20, 21, 22, 23]. These derivatives consist of variations of substituents on the amide group, sometimes accompanied by substituents on the indolic pyrrole ring. Except for derivates substituted at the N‐6 [24], no other derivate has shown a potency comparable to that of LSD [25].
Pharmacology of LSD
Psychological Effects
A moderate dose (75–150 μg p.o.) of LSD will significantly alter state of consciousness. This alteration is characterized by a stimulation of affect (mostly experienced as euphoria), enhanced capacity for introspection, and altered psychological functioning in the direction of Freudian primary processes, known otherwise as hypnagogic experience and dreams [26]. Especially noteworthy are perceptual changes such as illusions, pseudohallucinations, synesthesias, and alterations of thinking and time experience. Changes of body‐image and ego‐function also often occur [27, 28].
The acute psychological effects of LSD last between 6 and 10 h, depending on the dose applied.
The minimal recognizable dose of LSD in humans is about 25 μg p.o. [29, 30]. The “optimum” dosage for a typical fully unfolded LSD reaction is estimated to be in the range of 100–200 μg [18, 29, 31].
Traumatic experiences (called “bad trips”) can have long‐lasting effects on LSD users, including mood swings and rarely flashback phenomena [15]. It should be noted, however, that these generally take place in uncontrolled conditions. Conversely, it has been shown that under controlled and supportive conditions, the LSD experience may have lasting positive effects on attitude and personality [32].
Acute Neurocognitive Effects
One problem with acute cognitive testing is that after a clinical dose of LSD (100μg or more) is given, subjects become too impaired to cooperate due to the intensity of perceptual and physical changes. Lower doses may not capture the real cognitive effects LSD may provoke. Nevertheless, many tests have been given and the most representative studies are cited.
Psychomotor functions (coordination and reaction time) are frequently impaired after LSD [33, 34, 35]. LSD also decreases performance on tests of attention and concentration [36, 37]. Jarvik et al. [38] found 100 μg LSD to impair recognition and recall of various stimuli. Aronson and Watermann [39] showed learning processes to be unaffected by 75–150 μg LSD. Jarvik et al. [40] found that 100 μg LSD significantly impaired performance on arithmetic while 50 μg had no such effect. Memory was also affected by LSD as was illustrated with the Wechsler Bellevue Scale [41]. Impairment of visual memory was shown in the Bender–Gestalt test [34]. Thinking processes are more resistant but can be also affected when higher doses of LSD are given [42, 43]. Under the influence of LSD, subjects will overestimate time intervals [44]. Lienert [45, 46, 47, 48, 49] showed in several intelligence tests, that intellectual functions are impaired under LSD. He interpreted his results as a regression of intellectual functions to an ontogenetically younger state of development.
See Hintzen [50] for a complete review of neurocognitive studies with LSD.
Turning to chronic neurocognitive after‐effects from LSD exposure, Halpern and Pope's [51] review indicated no evidence for lasting impairments in performance.
Toxicological Data
The LD50 of LSD varies from species to species. The most sensitive species is the rabbit, with an LD50 of 0.3 mg/kg i.v. [52]. The LD50 for rats (16.5 mg/kg i.v.) is much higher [52, 53], though mice tolerate doses of 46–60 mg/kg i.v. [52, 54]. These animals expired by paralysis and respiratory failure. Monkeys (Macaca mulatta) have been injected with doses as high as 1 mg/kg i.v. without any lasting somatic effects [55].
There have been no documented human deaths from an LSD overdose. Eight individuals who accidentally consumed a very high dose of LSD intranasally (mistaking it for cocaine) had plasma levels of 1000–7000 μg per 100 mL blood plasma and suffered from comatose states, hyperthermia, vomiting, light gastric bleeding, and respiratory problems. However, all survived with hospital treatment and without residual effects [56].
In 1967, a report gave evidence for LSD‐induced chromosomal damage [57]. This report could not stand up to meticulous scientific examination and was disproved by later studies (for example, Dishotsky [58] and for complete review Grof [31]). Empirical studies showed no evidence of teratogenic or mutagenic effects from use of LSD in man [59, 60, 61]. Teratogenic effects in animals (mice, rats, and hamsters) were found only with extraordinarily high doses (up to 500 μg/kg s.c.) [62]. The most vulnerable period in mice was the first 7 days of pregnancy [63]. LSD has no carcinogenic potential [31].
Somatic Effects
The threshold dose for measurable sympathomimetic effects in humans is 0.5–1.0 μg/kg LSD p.o. [64]. A moderate dose of LSD for humans is estimated as 75–150 μg LSD p.o. [18, 31]. Dosing of animals (rats and cats) with very high doses of LSD (up to 100 μg/kg i.v.) leads to mild autonomic changes of mydriasis, tachycardia, tachypnea, hyperthermia, hypertonia, and hyperglycemia [65]. These changes may be the result of an excitatory syndrome caused by central stimulation of the sympathetic system. Lowering of blood pressure and bradycardia was found in the affected animals, and it was concluded that the sympathomimetic effects of LSD require the activation of higher cortical centers [66].
Autonomic changes reflect a stimulation of both branches of the autonomic nervous system. Sympathetic stimulation is evidenced, in most subjects, by a pupillary dilation and light to moderate increases in heart rate and blood pressure (see Table 2) [67, 68]; other more inconsistent signs are slight blood‐sugar elevation [69, 70] and, rarely, some increase in body temperature. Respiration remains generally unchanged (see Table 3). Other symptoms point to parasympathetic stimulation: diaphoresis and salivation are frequent, nausea may occur, emesis is exceptional, and flushing of the face is more frequent than paleness (see Table 4). Sympathicotonia usually predominates, but there are great individual variations and a marked parasympathicotonia with bradycardia and hypotension are observed in some subjects [18, 67]. Temporary headache and near‐syncope have sometimes been reported [35, 71].
Table 2.
Blood pressure and heart rate changes during acute effects of moderate doses of LSD
| Parameter | Sokoloff et al. [75], (n = 13, 120 μg i.v.) | DiMascio et al. [67], (n = 6, 1μg/kg p.o.) | Kornetsky [35], (n = 10, 100 μg p.o.) |
|---|---|---|---|
| Arterial blood pressure (mmHg) | +5, SD 2.9 | +12% (syst.) | +13 (SD unknown) |
| +10% (diast.) | |||
| Heart rate (beats per min) | +15 SD 7 | +18% | +19 (SD unknown) |
SD = standard deviation.
Table 3.
Measurements of somatic parameters during acute effects of a medium dose of LSD (n = 13, 120 μg LSD i.v.) from Sokoloff et al. [75]
| Physiological parameters | Changes during LSD |
|---|---|
| Respiration rate per minute | NS |
| Oral temperature | NS |
| Blood oxygen saturation (%) | NS |
| Blood CO2 tension (mmHg) | NS |
| Blood pH | NS |
| Blood glucose concentration in mg (%) | NS |
| Hemoconcentration | +0.57 g (%); SD 0.34 |
NS = not significant compared to controls.
Table 4.
Somatic symptoms as experienced subjectively by healthy subjects (n = 14, double blind, 100–225 μg LSD p.o.) [71]
| Symptom | Percentage of yes‐answers |
|---|---|
| Initial nausea | 30 |
| Decreased appetite | 25 |
| Temporary mild headache | 20 |
| Feeling dizzy | 45 |
| Limbs feeling light | 30 |
| Inner trembling | 45 |
There is no evidence that LSD alters liver function [18, 72, 73].
Reports of changes in adrenaline levels due to LSD are contradictory, [70, 74] which may reflect individual variations of sympathicotonia induced by individually different experiences on a psychological level.
The most consistent neurological effect is an exaggeration of the patellar (and other deep tendon) reflexes [42]. More unusual signs include slight unsteadiness of gait to full ataxia, positive Romberg's sign, and mild tremor [18, 31]. Other physiological measures are unaffected.
Beyond objectively measurable somatic changes, there are other somatic symptoms experienced by some subjects (cf. Table 1).
Table 1.
Typical sensory and psychological effects under the influence of a medium dose of LSD (100–200 μg p.o.)
| Sensory alterations (visual, auditory, taste, olfactory, kinaesthetic) |
| Illusion |
| Pseudo‐hallucination |
| Intensification of color perception |
| Metamorphosis‐like change in objects and faces |
| Intense (kaleidoscopic or scenic) visual imagery with transforming |
| content |
| Alterations of affectivity |
| Intensification of emotional experience: euphoria, dysphoria, anxiety, |
| mood swing |
| Alterations of thinking |
| Less abstract and more imaginative thought |
| Broader and unusual association |
| Attention span shortened |
| Alterations of body perceptions |
| Change in body image |
| Unusual inner perception of bodily processes |
| Metamorphic alteration of body contours |
| Memory changes |
| Reexperiencing significant biographical memories |
| Hypermnesia |
| Age‐regression |
| Mystical‐type experiences |
Sokoloff et al. [75] elicited only mild pulse rate and blood pressure changes (as well as slight hemoconcentration) in normal subjects. It remains undetermined whether the hemoconcentration represents an absolute increase in circulating hemoglobin mobilized from stored pools of red cells or is the result of a relative increase of hemoglobin concentration because of a loss of plasma volume. Sokoloff et al. [75: 475–476] suspect that “the elevated blood pressure and the hemoconcentration could both be explained by the increased motor activity” of their LSD subjects. Most somatic effects ascribed to LSD, and reported mainly in less methodologically sophisticated studies, may be secondary effects caused by the psychological reaction to the drug (i.e., the physiological and CNS response to the psychological experiences) [35].
The effects of LSD on blood pressure are probably complex, because of its in situ action on blood vessels, cardiac and other muscular systems, lungs, and respiration, as well as its effects on the central nervous system and carotid sinuses.
Biochemical Changes
LSD given to normals (0.5 to 1 μg/kg p.o.) reduced the excretion of inorganic phosphate (as found also with the other hallucinogens mescaline and psilocybin), suggesting that LSD may act on enzymatic systems to facilitate the binding of phosphate [76]. Although this decrease is consistently observed, its significance in regard to the action of LSD is unclear, and it may just be a simple nonspecific manifestation of psychological stress [77].
Messiha and Grof [78] studied the effects LSD on biogenic amine excretion (n = 7, 200–300 μg p.o.). LSD significantly reduced urinary dopamine excretion (to 476 μg per 24 h), but excretion of norepinephrine, serotonin, homovanillic acid, vanillylmandelic acid, and 5‐hydroxyindoleacetic acid were not affected.
LSD induces a slight decrease in creatinine clearance, but no change in calcium clearance and serum calcium levels [77]. No changes were documented for serum creatinine, plasma urea, plasma sodium, chloride, serum cholesterol, total lipids, and osmolality. Transaminase levels were essentially unchanged as were all other hepatic tests applied. Examination of urinary constituents has also failed to reveal any abnormality (data summary in Hollister [69]).
Another finding (consistent with the presence of psychological stress) is the mobilization of free fatty acids after ingestion of LSD (1–1.5 μg/kg p.o.) [79].
Changes in Sleep‐Waking Cycle and Dreaming
Low doses of LSD (n = 12, 6–40 μg p.o.) immediately applied before or 1 h after sleep onset lead (in a dose‐dependent manner) to a prolongation of the first or second rapid eye movement (REM) periods by 30–240% and a shortening of following periods. Eye movements during these periods are less numerous. Total REM sleep is prolonged. No qualitative changes in sleep as measured on EEG have been found [80]. Torda [81] infused LSD (n = 2, 5 μg i.v./hour) after the start of the third REM period and found that the beginning of the fourth REM period began after 10–15 min, instead of the usual 40–60 min. Theta activity was decreased in this study. Sleep deprivation prior to LSD application leads to more intense psychological reactions [82, 83].
Endocrinological Changes
LSD significantly lowers resting plasma prolactin levels in male rats (0.05 and 0.2 mg/kg) [84]. No changes were found for luteinizing hormone (LH) and follicle‐stimulating hormone (FSH) (100 or 500 μg/kg LSD i.p.), even with long time regimes [85].
In humans, LSD increases serum growth hormone with a peak at 120 min. but does not alter serum prolactin levels [86]. Rinkel et al. [87] found a significant increase of 17‐ketosteroid excretion (single‐blind, n = 100, 0.5 μg/kg LSD p.o.).
Pharmacokinetics
Resorption
After p.o. ingestion, LSD is completely absorbed in the digestive tract [22, 52]. After 100–250 μg LSD p.o., psychological and sympathomimetic effects persist for 30–45 min, reaching their peak after 1.5–2.5 h (see Figure 2) [18, 88].
Figure 2.
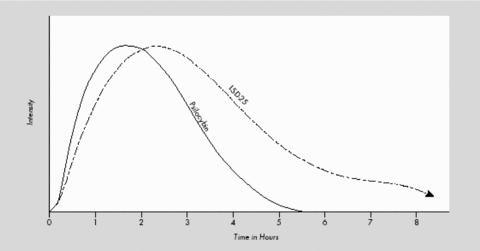
Course of clinical effects of LSD p.o. compared to the hallucinogen psilocybin (modified from Leuner [61]; identical with results of Hoch [88]).
Upshall and Wailling [89] demonstrated that with a large meal, plasma concentrations of orally ingested LSD were half as much as on an empty stomach. When a smaller meal was eaten, plasma levels were somewhere between. It was concluded that the amount of the meal, as well as the pH of the stomach and duodenum, will influence the absorption of LSD.
Clinical data about different modes of application are shown in Table 5.
Table 5.
Clinical pharmacokinetics of LSD with different modes of application (data from Hoch [88]) (number of subjects not reported)
| Mode of application | Dose (μg) | Onset of symptoms (min) | Peak effect (h)* | Total duration (h)* |
|---|---|---|---|---|
| Per os | 100–250 | 30–45 | 1.0–2.5 | 9–12 |
| Intramuscular | 100–250 | 15–20 | 1.0 | 9–10 |
| Intravenous | 40–180 | 3–5 | 1.0 | 9–10 |
| Intraspinal | 20–60 | <1 | 1.0 | 9–10 |
*Hours after application of LSD.
Hoch [88] found no qualitative differences regarding psychological LSD effects, regardless of the route of administration. Differences were chiefly of a quantitative nature and in rapidity of onset of effects. Sokoloff et al. [75] found identical questionnaire results comparing orally dosed subjects (n = 14, 100–225 μg p.o.) of Abramson et al. [71] with their intravenously dosed subjects (n = 13, 120 μg i.v.). Both studies employed the Abramson‐questionnaire, designed to evaluate psychological LSD effects.
Distribution in the Organism
The distribution of LSD across tissue and organ systems is yet to be quantified for the human organism.
In mice, [14C]‐LSD (50 μg i.v.) disappeared in a few minutes from blood and was found within 10 min in nearly all organs [90]. In the duodenum, the activity reached a maximum (with 50% of radioactivity) at the 2 h mark. [14C]‐LSD is then transported in the chyme through the digestive tract and reaches a maximum in the colon after approximately 3 h (see Figure 3) [91]. The digestive tract contains 70–80% of the radioactivity 3–12 h after ingestion [92].
Figure 3.
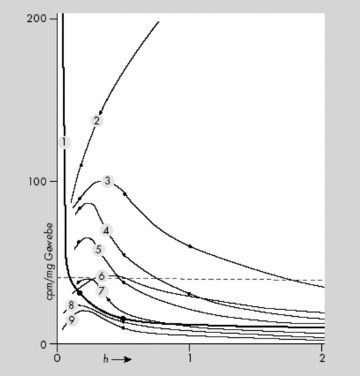
Distribution and excretion of 14C‐LSD in mice: 1 = blood; 2 = duodenum; 3 = liver; 4 = kidney and adrenal glands; 5 = lung, Spleen, and Pancreas; 6 = viscera; 7 = heart; 8 = muscle, skin; and 9 = brain (from Stoll et al. [91])
The largest quantity of [14C]‐LSD was found in the liver, where it slowly disappeared during the first 12 h, which points to a significant enterohepatic circle [92, 93].
In rat brain, a much lower LSD concentration is found compared to blood plasma levels. [14C]‐LSD disappears from rat brain much more rapidly than from blood plasma [94]. Other researchers found high amounts of radioactive LSD in the hypophysis of rats (500μg/kg i.v.) [95] as well as monkeys (0.5–2 mg/kg i.v.) [96].
In cats (1 mg/kg i.v. LSD), the highest concentrations were detected in the gallbladder and blood plasma. Lower concentrations were found in the lungs, liver, brain, digestive tract, spleen, and muscle, with the lowest concentrations found in fat tissue [93, 97].
The presence of considerable amounts of the drug in the brain and cerebrospinal fluid (CSF) of rats and cats indicates that LSD may easily pass the blood–brain barrier [97].
Hoff and Arnold [98] demonstrated that [14C]‐LSD passes the blood‐brain barrier in mice. It was suggested that the choroid plexus may be central for this passage [99].
Axelrod [97] studied liver and blood levels of LSD in monkeys (M. mulatta) after 0.2 mg LSD/kg i.v. The maximum LSD level in CSF was reached within 10 min and subsequently fell during the next hours. The amount of LSD in CSF was about the same as the unbound form in blood plasma. This data suggest as well that LSD easily passes the blood‐brain barrier (Fig. 5).
Figure 5.
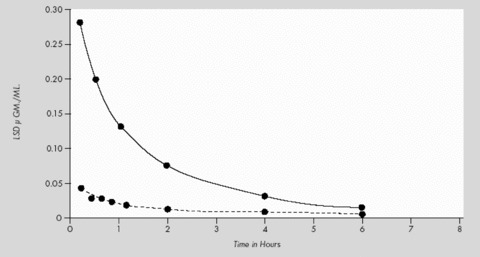
LSD‐levels in plasma (———) and liver (‐ ‐ ‐ ‐ ‐) after 0.2 mg/kg LSD i.v. in monkeys (Macaca mulatta) (from Axelrod et al. [97]).
Two studies [100, 101], which evaluated a two‐compartment model, concluded that the relation between (neuropsychological) LSD effects and LSD tissue concentration could be linear, logarithmic‐linear, or neither.
Plasma Protein Binding
No data about the binding of LSD to human plasma proteins are available. At plasma concentrations of 0.1 and 20 mg/L, in vitro experimentation on guinea pigs showed that 65–90% of LSD is bound to nondiffusible plasma constituents [97].
Course of Plasma Levels
It has been calculated that LSD exerts its psychological effects in man (given at 1 μg/kg p.o.) at a concentration of 0.0005 μg/g of brain tissue [97].
The only study about the course of plasma levels after administration of LSD was done by Aghajanian and Bing [102]. When humans were given doses of 2 μg/kg i.v., the plasma level was 6–7 ng/mL in about 30 min. Over the course of the next 8 h, plasma levels gradually fell until only a small amount of LSD was present (cf. Fig. 6).
Figure 6.
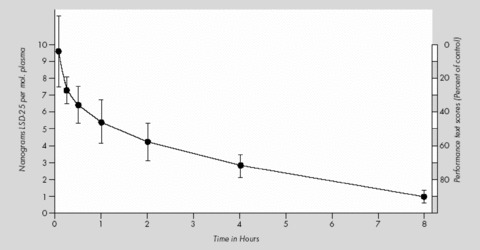
Course of plasma levels of LSD after 2 μg/kg i.v. in humans (modified from Aghajanian and Bing [102]).
Metabolism and Excretion
Species vary greatly in their LSD metabolism rate. The half‐life in mice (2 mg/kg i.p.) is 7 min, 130 min in cats (0.2 mg/kg i.v.), and 100 min in monkeys (M. mulatta) (0.2 mg/kg i.v.) [97]. The half‐life of LSD in humans was found to be 175 min [89, 102].
The metabolism of [14C]‐LSD has been investigated in rats (1 mg/kg i.p.), guinea pigs (1 mg/kg i.p.), and rhesus monkeys (0.15 mg/kg i.m.) by Siddik et al. [103]. [14C]‐LSD is almost completely metabolized by all three species, and only very little of the unchanged drug is excreted. The metabolites identified were 13‐ and 14‐hydroxy‐LSD and their glucuronic acid conjugates, 2‐oxo‐LSD, nor‐LSD, as well as a not further specified naphthostyril derivative. However, important differences in the nature and amounts of the various metabolites occur in different species. The major metabolites in rats and guinea pigs (found in urine and bile) were glucuronic acid conjugates of 13‐ and 14‐hydroxy‐LSD. Guinea pigs excrete significant amounts of 2‐oxo‐LSD in urine and bile. Lysergic acid ethylamide (LAE) was a minor urinary metabolite in both species. The metabolic fate of LSD also appears to be unique in rhesus monkeys. Their urine contains at least nine metabolites. Four of them were identified as: 13‐ and 14‐hydroxy‐LSD (as glucuronic acid conjugates), LAE, and a (not exactly defined) naphthostyril derivative of LSD. Glucuronic acid conjugates of 13‐ and 14‐hydroxy‐LSD were present only in small amounts, setting rhesus monkeys apart from rats and guinea pigs.
In humans, LSD is metabolized rapidly into some structurally similar metabolites (see Figure 4). It was first established through in vitro studies that LSD is metabolized in humans by some NADH‐dependent microsomal liver enzymes to the inactive 2‐oxy‐LSD [97, 104] and 2‐oxo‐3‐hydroxy LSD. Metabolites were first detected in urine with infrared spectroscopy [93]. In a later study, Niwaguchi et al. [105] identified LAE (which originates from enzymatic N‐dealkylation of the diethylamide radical at side chain position 8) and nor‐LSD, an N‐de‐methylated degradation product of LSD. Another metabolite was identified as di‐hydroxy‐LSD [106]. Klette et al. [106] and Canezin et al. [107] found the following LSD metabolites in human urine: nor‐LSD, LAE, 2‐oxo‐LSD, 2‐oxy‐3‐hydroxy‐LSD, 13‐ and 14‐hydroxy‐LSD as glucoronides, lysergic acid ethyl‐2‐hydroxyethylamide (LEO), and trioxylated LSD. The major metabolite in urine is 2‐oxy‐3‐hydroxy‐LSD (which could not be detected in blood plasma).
Figure 4.
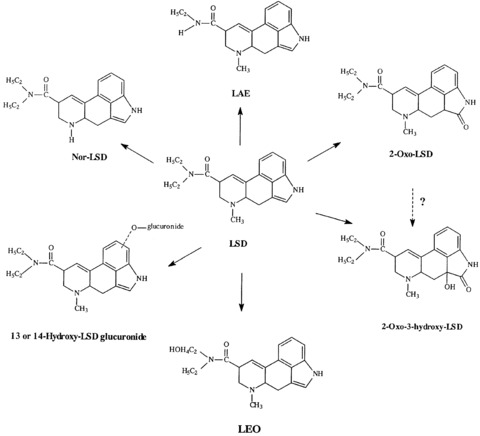
The metabolites of LSD (Canezin et al. [107]).
Urine was collected for 24 h and feces for 48 h from monkeys (M. mulatta) (0.2 mg/kg LSD i.v.). Less than 1% of the administered LSD was found in urine or feces. This observation suggests that LSD underwent almost complete metabolic change in monkeys [97].
The elimination of [14C]‐LSD in the rat, guinea pig, and rhesus monkey over a 96‐h period has been investigated by Siddik et al. [103]. Rats (1 mg/kg i.p.) excreted 73% of the 14C in feces, 16% in urine, and 3.4% in the expired air as 14CO2. Guinea pigs (1 mg/kg i.p.) excreted 40% in feces, 28% in urine, and 18% as expired 14CO2. Rhesus monkeys (0.15 mg/kg i.m.) eliminated 23% in the feces and 39% in the urine. Extensive binary excretion of [14C]‐LSD occurred in both the rat and guinea pig [103].
Determination of urinary concentrations of LSD following a single dose of the drug (200 μg p.o.) in humans shows that the rate of excretion of LSD reaches a maximum approximately 4–6 h after administration [108]. The elimination half‐life for LSD is 3.6 h. LSD and its metabolites are reported to be detectable in the urine for as long as 4 days after ingestion [108]. Using a radioimmunoassay (RIA) screening test (cut‐off at 0.1 ng/mL) the detection limit for 100 μg LSD p.o. is usually around 30 h. Each doubling of the initial amount will add about 5 h [109]. LSD or its cross‐reactive metabolites were detectable for periods of 34–120 h at concentrations of 2–28 μg/L in urine (n = 7, 300 μg LSD p.o.) [110].
Detection of LSD in Body Fluids
Since LSD is ingested in quite small amounts, the LSD to be detected in biological samples is likewise very small. How long LSD can be detected in the body varies by (1) the test being used, (2) the detection limit placed on the test, (3) the point of collection, (4) the type of sample fluid, (5) the amount of LSD that was ingested, and (6) the specific individual organism. A moderate dose of LSD (100–200 μg p.o.) within a few hours after ingestion results in plasma and urine concentrations at the sub‐ng/mL level [111]. LSD content of body fluids may be detected by RIA and enzyme immunoassay. Laboratory tests have shown that RIA results are accurate down to at least 0.5 ng/mL [112]. A new indirect enzyme‐linked immunosorbent assay (ELISA) was used to detect as little as 1 pg of total drug in 25 μL blood [113].
Routine forensic methods for confirmatory and quantitative testing for LSD employ high‐performance thin layer chromatography (HPTLC) and different forms of gas chromatography/mass spectrometry (GC/MS) with detection limits set to approximately 0.4 μg/L [109, 114]. The practical (forensic) detection limits are as low as 0.1 and 0.25 ng/mL for LSD and N‐desmethyl‐LSD, respectively.
The average time for determination of LSD in blood specimens is estimated to be 6–12 h and 2–4 days in urine specimens [109, 111, 115]. In most LSD‐positive urine samples the metabolite, 2‐oxo‐3‐hydroxy‐LSD, is present at higher concentrations than LSD and can be detected after LSD ingestion for a longer time than LSD itself [116]. Determination of LSD in hair specimens is now available even for low and single time dosing but not for LSD metabolites [117, 118].
Pharmacodynamics
In 1966, LSD was placed into the most restrictive drug control schedule, and since that time there have been no human studies about the effects of LSD on the human brain. Until 1966, many in vitro and in vivo studies were done but with older and less refined methods.
Regional Distribution in Brain Tissue
Arnold et al. [119] studied mice with extraordinarily high doses (8.12 mg/kg i.p.) of [14C]‐LSD to elucidate its distribution in the brain. They demonstrated that cellular structures contained more LSD than all other brain matter. The highest concentration was found in the hippocampus and, in decreasing order, in the basal ganglia, periventricular gray matter, and the frontoparietal cortex.
Snyder and Reivich [96] studied the regional distribution of LSD in squirrel monkey (Saimiri sciurcus) brains (0.5–2 mg/kg i.v., n = 4). These animals were sacrificed 30 min after LSD infusion. LSD was found unequally distributed in different areas of the brain. The highest concentrations were found in the pituitary and pineal glands with concentrations seven to eight times higher as in the cortex. Structures of the limbic system (hippocampus, amygdala, fornix, and septal region) contained two to three times more LSD than cortical structures. LSD compared to cortical regions was two to five times more concentrated in the visual and auditory areas, hypothalamus, extrapyramidal system, and thalamus. The brain stem contained LSD concentrations similar to the cortex. LSD was equally distributed between white and gray matter.
Effects on Cerebral Circulation
Cerebral circulation and metabolism have been investigated in humans only by Sokoloff et al [75]. At the height of LSD‐effects (n = 13, 120 μg i.v.), the general cerebral blood flow (measured with the nitrous oxide method), cerebral vascular resistance, cerebral oxygen consumption, and glucose utilization were not significantly changed. Sokoloff et al. [74] summed their results critically in this way: “It is possible that the action of lysergic acid is associated with changes in cerebral circulation or metabolism, but in areas representing so small a fraction of the total brain that the effects are obscured in measurements in the brain as a whole. Alternatively, it may be that in a heterogeneous organ like the brain, many of those parts are functionally inversely or reciprocally related, changes in the net metabolic rate of the brain remains unchanged” (p. 475).
Neurophysiological Actions
Forrer and Goldner [68] and Hertle et al. [120] described a dose‐dependent hyperreflexia and a mild ataxia as the major neurological effects of LSD.
The EEG shows mild and little specific signs of activation after LSD ingestion. Most common is an increase in α mean frequency [121, 122, 123]. Other researchers describe a progressive desynchronization due to a quantitative decrement of the slow component after LSD [124, 125]. Goldstein et al. [126] reported a decrease of EEG variability of 33% after LSD (0.3–1.0 μg/kg p.o.). Goldstein and Stoltzfus [127] analyzed human EEG amplitude levels in right and left occipital areas and found that in most subjects the normal pattern of lateralization was reversed by LSD.
Neurometabolic Effects
No studies about the neurometabolic actions of LSD have been completed. However, there are neurometabolic studies published for related hallucinogens like psilocybin [129, 130], dimethyltryptamine (DMT) [131], and mescaline [132]. There are many incongruencies regarding the results of the different studies, which limit the plausibility of hypotheses developed to explain neurofunctional alterations during hallucinogen effects [133]. Only the major and congruent results will be mentioned here. The major hallucinogens appear to activate the right hemisphere, influence thalamic functioning, and increase metabolism in paralimbic structures and in the frontal cortex. Because most of these metabolic changes are also found in persons during psychological stress [134, 135], it is not easy to distinguish which alterations are primary substance‐induced and which are due to secondary (compensatory) psychophysical processes induced by general psychosocial stress during hallucinogen intoxication under experimental conditions. In regard to global brain metabolism, some investigators found an increased metabolism [130, 132], but others found no change [129, 136].
Interactions with Receptors
The complex receptor interactions of LSD are a significant topic of experimental work and speculation about LSD's working mechanisms. The predominant hypothesis on how indole hallucinogens affect serotonin (5‐HT) is summarized as follows: LSD acts to preferentially inhibit serotonergic cell firing while sparing postsynaptic serotonergic receptors from upregulation/downregulation. This preference is shared in a somewhat limited fashion by non‐indole hallucinogens. Nonhallucinogenic analogs of LSD show no such preference.
Serotonin (5‐hydroxytryptamine; 5‐HT) is produced by a small number of neurons (1000s) that each innervate as many as 500,000 other neurons. For the most part, these neurons originate in the raphe nuclei (RN) of the midbrain. One major target of these is the locus coeruleus (LC), which controls the release of norepinephrine, which regulates the sympathetic nervous system. The LC also has neurons that extend into the cerebellum, thalamus, hypothalamus, cerebral cortex, and hippocampus. The RN extends its projections into the brainstem and up into the brain. It has been suggested that neurons in this brain region may inhibit sensation, thus protecting the brain from sensory overload. The fact that the LC and the RN innervate virtually every part of the brain shows that serotonin can activate large portions of the brain from a relatively small area of origination [146].
In general, 5‐HT may be seen as a mainly inhibitory transmitter; thus, when its activity is decreased, the next neuron in the chain is freed from inhibition and becomes more active. This view is limited by the fact that a few 5‐HT receptors are excitatory ion channels (5‐HT3) and some subtypes may have excitatory effects depending upon the G protein coupling within specific neurons. Since serotonergic systems appear to be intimately involved in the control of sensation, sleep, attention, and mood, it may be possible to explain the actions of LSD and other hallucinogens by their disinhibition of these critical systems [146].
LSD acts as a 5‐HT autoreceptor agonist on 5‐HT1A receptors in the LC, the RN, and the cortex. It inhibits firing and serotonin release of these cells. It also acts as a partial agonist on the postsynaptic 5‐HT1A site. LSD has high affinity for other 5‐HT1 subtypes 5‐HT1B, 5‐HT1D, and 5‐HT1E. Effects of LSD on 5‐HT2C, 5‐HT5A, 5‐HT6, and 5‐HT7 receptors [e.g., 147, 148, 149] are described, but their significance remains uncertain. However, the hallucinogenic effect of LSD has been linked to its affinity for the 5‐HT2 receptor where it acts as a 5‐HT2 agonist, as this property is shared by hallucinogens of the phenethylamine group (mescaline, 2,5‐dimethoxy‐4‐iodoamphetamine, etc.) and the indolamine group (psilocybin, DMT). A strong correlation was described between psychoactive doses of these hallucinogens and their respective potency at the 5‐HT2 receptor [150, 151]. Most data indicate a specific 5‐HT2A mechanism, although a 5‐HT2C effect cannot be ruled out.
LSD is probably best called a mixed 5‐HT2/5‐HT1 receptor partial agonist. Today it is believed that LSD is a partial agonist at 5‐HT2A receptors [e.g.,152, 153], especially those expressed on neocortical pyramidal cells. Activation of 5‐HT2A also leads to increased cortical glutamate levels [154, 155] probably mediated by thalamic afferents [25]. However, this increase in glutamate release can lead to an alteration in corticocortical and corticosubcortical transmission. LSD's dual effect on 5‐HT2 (stimulatory) and 5‐HT1 (inhibitory) can explain how it may appear as an antagonist because it can modulate its own effect.
In a recent study, Gonzalez‐Maeso et al. [156] compared 2‐HT2A agonists with and without hallucinogenic activity in mice. It was found that these types of agonists differ in regard to the G‐protein activiation induced, especially those of the pertussis toxin‐sensitive heterotrimeric Gi/o and Ga/11 proteins and their coactivation. Using mice modified to genetically express 5‐HT2A receptors only in the cortex, it was shown that these receptors were sufficient to produce hallucinogenic effects (as indicated by hallucinogen‐specific head twitch response) with identical firing rates of pyramidal neurons as without this manipulation. This may imply that the hallucinogenic effects are mainly mediated by cortico‐cortical neural circuits rather than by thalamo‐cortical circuits as proposed earlier by some scientists [133].
Nichols and Sanders‐Bush [157] first described an LSD‐mediated increase in gene expression, which Nichols et al. [158] found to be due to activation of 5‐HT2A receptors.
There is also evidence that LSD interacts with dopaminergic systems. In comparison to other hallucinogens, LSD interacts agonistically and antagonistically with central dopamine D1 und D2‐receptors [159, 160]. It is not established how these changes are involved in psychoactive effects of LSD, but studies with the related, but more selective 5‐HT2A hallucinogen psilocybin demonstrated an increased release of dopamine, as evidenced by a 20% decrease of [11C]raclopride binding after psilocybin in human subjects [161].
Marona‐Lewicka et al. [162] discovered receptor activation by LSD to be time‐dependent. LSD in rats 15–30 min prior to testing in a discriminative stimulus task leads to 5‐HT2A activation, while after 90 min D2‐receptors may mediate major parts of LSD reactions. These data suggest an interaction between dopamine and serotonin receptors and might be a possible explanation for the enormous range of effects LSD engenders in humans.
Tolerance
Tolerance is defined as a decrease in responsiveness to a drug after repeated administration. Tolerance to the effects of LSD occurs in humans and animals. Tolerance to autonomic and psychological effects of LSD occurs in humans after a few moderate daily doses of LSD [42, 163, 164]. Abramson et al. [163] gave 5–100 μg LSD p.o. for 3–6 days to healthy volunteers. After 2–3 days, a solid tolerance developed as demonstrated in psychological and physiological tests. After tolerance to LSD is achieved and placebo instead of LSD is given for the next 3 days, the typical LSD effects will finally reoccur on the fourth day [42].
A recent animal experiment with rats (130 μg/kg LSD i.v. for 5 consecutive days), who were previously trained to discriminate LSD from saline, indicated a decrease in 5‐HT2A receptor signaling caused by a reduction of 5‐HT2A receptor density [165]. This reduction in receptor density may point to a possible mechanism for the development of acute tolerance to LSD.
Pretreatment with BOL‐148, a nonhallucinogenic congener of LSD with serotonin antagonist properties like LSD, [166] will not block the effects of LSD [167, 168]. But other derivates of LSD, such as UML‐491 and MLD‐41, are able to induce cross‐tolerance if applied in the days prior to LSD [167].
There is partial cross‐tolerance (depending on whether LSD is given first or second) among LSD, mescaline, and psilocybin [168, 169, 170]. The most complete cross‐tolerance is to mescaline in LSD‐tolerant subjects. One study suggested that one‐way cross‐tolerance from LSD to DMT does not occur [171]. Studies with Δ‐9‐tetrahydrocannabiol (THC) in subjects tolerant to LSD did not demonstrate a cross‐tolerance between these drugs [172, 173]. There is no cross‐tolerance between LSD and amphetamine [169]. See Wyatt et al. [174] and Hintzen [50] for a complete review of tolerance and cross‐tolerance studies with LSD.
Interactions of LSD with Other Substances
Various studies have evaluated drug–drug interactions with LSD. Early clinical studies focussed primarily on LSD interactions with neuroleptics, especially chlorpromazine (CPZ). CPZ has proven to be an incomplete antagonist of LSD. When CPZ is given simultaneously with LSD to humans in small doses (below 0.4 mg/kg), it produces no changes in LSD's effects [175]. At higher doses (0.7 mg/kg) of CPZ, LSD‐induced side effects, such as nausea, vomiting, dizziness, reduction in motor activity, and/or anxiety, have been reported to diminish or disappear [176]. CPZ did not appreciably alter the production of hallucinations or delusions, but associated unpleasant feelings were reduced or eliminated [177].
As mentioned, sedative‐hypnotics like diazepam (5mg p.o./i.m.) are often used in the emergency room setting for acute presentations of LSD intoxication to help reduce panic and anxiety [178, 179]. Chronic administration of selective serotonin reuptake inhibitors (SSRIs) as well as monoamine oxidase inhibitor (MAOI) antidepressants are reported to diminish LSD effects [180]. An explanation may be that chronic application of antidepressants decrease 5‐HT2‐receptor expression in several brain regions [181]. In turn, one could predict that reabsorption of 5‐HT2A receptors will not be complete after single predosing with an SSRI or MAOIs; in such circumstances, the risk for serotonin syndrome may be heightened. Lithium and some tricyclic antidepressants have also been reported to increase the effects of LSD [180]. It has to be mentioned that LSD in combination with lithium drastically increases LSD reactions and can lead to temporary comatose states as suggested by anecdotal medical reports [182].
Psychiatric Complications
Many reports exist about psychiatric complications following LSD ingestion outside the research setting. The most common unpleasant reaction is an episode of anxiety or panic (with severe, terrifying thoughts and feelings, fear of losing control, fear of insanity or death, and despair)—the “bad trip”[15]. Other complicated reactions may include temporary paranoid ideation and, as after‐effects in the days following a LSD experience, temporary depressive mood swings and/or increase of psychic instability [17, 61].
Crucially, there is a lack of evidence that other complications will routinely occur or persist in healthy persons taking LSD in a familiar surrounding. Cohen [8], Malleson [9], and Gasser [11] observed approximately 10,000 patients safely treated with LSD as a psycholytic agent. Indeed, past clinical studies with LSD were completed reporting very few if any complications (cf. Table 1).
An extensive number of individuals participated in LSD research, with Passie [2] estimating some 10,000 patients participating in research of the 1950s and 1960s. The incidence of psychotic reactions, suicide attempts, and suicides during treatment with LSD, as noted in Table 1, appears comparable to the rate of complications during conventional psychotherapy.
“Flashbacks” are characterized in the WHO International Classification of Diseases, Version 10 (ICD‐10) as of an episodic nature with a very short duration (seconds or minutes) and by their replication of elements of previous drug‐related experiences. These reexperiences of previous drug experiences occur mainly following intense negative experiences with hallucinogens, but can sometimes also be self‐induced by will for positive reexperiences and are in this case sometimes referred to as “free trips” (for complete review see Holland [198].). The Diagnostic and Statistical Manual of Mental Disorders, Version IV (DSM‐IV) defines clinically significant flashbacks as “Hallucinogen Persisting Perception Disorder”(HPPD), which appears to be particularly associated with LSD. Halpern and Pope [199] reviewed 20 quantitative studies from 1955 to 2001 and concluded that the occurrence of HPPD is very rare, but, when it occurs, it typically will have a limited course of months to a year, but can, in some even rarer cases, last for years with considerable morbidity.
Conclusion
The pharmacology of LSD is indeed quite complex, which may, in part, explain why its mechanisms of action remain unclear. LSD is physiologically well tolerated and there is no evidence for long‐lasting effects on brain and other parts of the human organism. The above review of pharmacology, psychopharmacology, related preclinical research, as well as basic studies with human subjects are gleaned from research that was for the most part conducted in the 1950s and 1960s during an era that held great promise for LSD and related hallucinogens. Hope was placed in these substances for new treatments for psychiatric conditions and discoveries that would “unlock the mysteries” of the mind. And hallucinogen research did indeed lead to the discovery of serotonin, brain second‐messenger systems, and a variety of other research techniques such as prepulse inhibition and the use of animals for detection of activation of specific subreceptors. The research of LSD faded after these advancements and also because the clinical promises failed to be realized while illicit use of hallucinogens pressured governments into taking police action against such use. Government funding of research dried up, as well, and a generation of scientists moved on to other topics. Today, LSD and other hallucinogens are once again being evaluated for specific purposes, such as for treatment of cluster headache and as tools in therapy for working with those suffering from anxiety provoking end‐of‐life issues and for posttraumatic stress disorder. As these new studies move forward, it is hoped that this present paper will be a roadmap for also securing the data missing from our knowledge of the pharmacology of LSD.
Conflict of Interest
The authors have no conflict of interest.
References
- 1. Abramson HA. The use of psychotherapy and alcoholism. Indianapolis, New York, Kansas City : Bobbs Merrill, 1967. [Google Scholar]
- 2. Passie T. Psycholytic and psychedelic therapy research: A complete international bibliography 1931–1995. Hannover : Laurentius Publishers, 1997. [Google Scholar]
- 3. Lee MA, Shlain B. Acid dreams, the CIA, LSD, and the sixties rebellion. New York : Grove Press, 1985. [Google Scholar]
- 4. Boskin J, Rosenstone RA. Protest in the sixties. Ann Am Acad Pol Soc Sci 1969;382:1–219. [Google Scholar]
- 5. Hunter R. The storming of the mind. Toronto , Montreal: Doubleday , 1971. [Google Scholar]
- 6. SAMHSA . Available at: http://www.oas.samhsa.gov/ecstasy.htm, 2006. Accessed 13 July 2008.
- 7. McGlothlin WH, Arnold DO. LSD revisited: A ten‐year follow‐up of medical LSD use. Arch Gen Psychiatry 1971;24:35–49. [DOI] [PubMed] [Google Scholar]
- 8. Cohen S. Lysergic acid diethylamide: Side effects and complications. J Nerv Ment Dis 1960;130:30–40. [DOI] [PubMed] [Google Scholar]
- 9. Malleson N. Acute adverse reactions to LSD in clinical and experimental use in the United Kingdom. Br J Psychiatry 1971;118:229–230. [DOI] [PubMed] [Google Scholar]
- 10. Leuner H. Preface In: Passie T. Psycholytic and psychedelic therapy research 1931–1995: A complete international bibliography. Hannover : Laurentius Publishers, 1997, pp. 5–7. [Google Scholar]
- 11. Gasser P. Die psycholytische Psychotherapie in der Schweiz von 1988–1993 Eine katamnestische Erhebung. Schweiz Arch Neurol Psychiatr 1997;147:59–65. [Google Scholar]
- 12. Sewell RA, Halpern JH, Pope HG Jr. Response of cluster headache to psilocybin and LSD. Neurology 2006;66:1920–1922. [DOI] [PubMed] [Google Scholar]
- 13. Gasser P. LSD‐assisted psychotherapy in persons suffering from anxiety associated with advanced‐stage life threatening diseases. A phase‐II, double‐blind, placebo‐controlled dose‐response pilot study. Solothurn (Switzerland): Unpublished research protocol 2007. Available at: http://www.maps.org/research/lsd/swisslsd/LDA1010707.pdf. Accessed 13 July 2008.
- 14. Winkelman MJ, Roberts TB. Psychedelic medicine: New evidence for hallucinogenic substances as treatments. Westport , CT : Praeger, Greenwood, 2007. [Google Scholar]
- 15. Strassman RJ. Adverse reactions to psychedelic drugs: A review of the literature. J Nerv Ment Dis 1984;172:577–595. [DOI] [PubMed] [Google Scholar]
- 16. Henderson LA, Glass WJ. LSD: Still with us after all these years. New York : Macmillan, 1994. [Google Scholar]
- 17. Grof S. Realms of the human unconscious: Observations from LSD research. New York : Viking, 1975. [Google Scholar]
- 18. Leuner H. Die experimentelle Psychose. Berlin , Göttingen , Heidelberg: Springer , 1962. [Google Scholar]
- 19. Masters REL, Houston J. The varieties of psychedelic experience. New York : Holt, 1966. [Google Scholar]
- 20. Bradley RJ, Smythies JR. Structure‐activity studies of lysergates and their interaction with biological molecules In: Sankar DVS, ed. LSD–A total study. New York : PJD Publications, 1975, pp. 141–196. [Google Scholar]
- 21. Isbell H, Miner EJ, Logan CR. Relationships of psychotomimetic to anti‐serotonin potencies of congeners of lysergic acid diethylamide (LSD‐25). Psychopharmacol 1959;l:20–28. [DOI] [PubMed] [Google Scholar]
- 22. Rothlin E. Lysergic acid diethylamide and related substances. Ann NY Acad Sci 1957;66:668–676. [DOI] [PubMed] [Google Scholar]
- 23. Watts VJ, Lawler CP, Fox DR, Neve KA, Nichols DE, Mailman RB. LSD and structural analogs: Pharmacological evaluation at D1 dopamine receptors. Psychopharmacol 1995;118:401–409. [DOI] [PubMed] [Google Scholar]
- 24. Hoffman AJ, Nichols DE. Synthesis and LSD‐like discriminative stimulus properties in a series of N(6)‐Alkyl norlysergic acid N,N‐diethylamide derivatives. J Med Chem 1985;28:1252–1255. [DOI] [PubMed] [Google Scholar]
- 25. Nichols DE. Hallucinogens. Pharmacol Ther 2004;101:131–181. [DOI] [PubMed] [Google Scholar]
- 26. Farthing GW. The Psychology of Consciousness. Englewood Cliffs : Prentice Hall, 1992. [Google Scholar]
- 27. Katz MM, Waskow IE, Olsson J. Characterizing the psychological state produced by LSD. J Abnorm Psychol 1968;73:1–14. [DOI] [PubMed] [Google Scholar]
- 28. Savage C. Variations in ego feeling induced by D‐lysergic acid diethylamide (LSD‐25). Psychoanal Rev 1955;1:1–16. [PubMed] [Google Scholar]
- 29. Hoffer A. LSD: A review of its present status. Clin Pharmacol Ther 1965;6:183–255. [DOI] [PubMed] [Google Scholar]
- 30. Stoll WA. Lysergsäurediäthylamid, ein Phantastikum aus der Mutterkorngruppe. Schweiz Arch Neurol Psychiatr 1947;60:279–323. [Google Scholar]
- 31. Grof S. The effects of LSD on chromosomes, genetic mutation, fetal development and malignancy In: Grof S. LSD psychotherapy. Pomoma , CA : Hunter House, 1980, pp. 320–347. [Google Scholar]
- 32. McGlothlin WH, Cohen S, McGlothlin MS. Long lasting effects of LSD on normals. Arch Gen Psychiatry 1967;17:521–532. [DOI] [PubMed] [Google Scholar]
- 33. Abramson HA, Jarvik ME, Kaufman MR, Kornetsky C, Levine A, Wagner M. Lysergic acid diethylamide (LSD‐25): I. Physiological and perceptual response. J Psychol 1955;39:3–60. [Google Scholar]
- 34. Abramson HA, Waxenberg SE, Levine A, Kaufman MR, Kornetsky C. Lysergic acid diethylamide (LSD‐25): XIII Effect on Bender‐Gestalt test performance. J Psychol 1955;40:341–349. [Google Scholar]
- 35. Kornetsky C. Relation of physiological and psychological effects of lysergic acid diethylamide. AMA Arch Neurol Psychiat 1957;77:657–658. [DOI] [PubMed] [Google Scholar]
- 36. Jarvik ME, Abramson AH, Hirsch MW. Lysergic acid diethylamide: Effects on attention and concentration. J Psychol 1955;39:373–383. [Google Scholar]
- 37. Wapner S, Krus DM. Effects of lysergic acid diethylamide, and differences between normals and schizophrenics on the Stroop Color‐Word Test. J Neuropsychiatry 1960;2:76–81. [PubMed] [Google Scholar]
- 38. Jarvik ME, Abramson AH, Hirsch MW. Lysergic acid diethylamide: Effects upon recall and recognition of various stimuli. J Psychol 1955;39:443–454. [Google Scholar]
- 39. Aronson H, Watermann CE. The effect of D‐lysergic acid diethylamide (LSD‐25) on learning and retention. J Clin Exp Psychopath 1962;23:17–23. [PubMed] [Google Scholar]
- 40. Jarvik ME, Abramson AH, Hirsch MW, Ewals AT. Lysergic acid diethylamide: Effects on arithmetic test performance. J Psychol 1955;39:465–473. [Google Scholar]
- 41. Sloane B, Lovett Doust JW. Psychophysiological investigations in experimental psychoses; results of the exhibition of d‐lysergic acid diethylamide to psychiatric patients. J Ment Sci 1954;100:129–144. [DOI] [PubMed] [Google Scholar]
- 42. Isbell H, Belleville RE, Fraser HF, Wikler A, Logan CR. Studies on lysergic acid diethylamide: Effects in former morphine addicts and development of tolerance during chronic intoxication. AMA Arch Neurol Psychiatry 1956;76:468–478. [PubMed] [Google Scholar]
- 43. Silverstein AB, Klee GD. Effects of lysergic acid diethylamide (LSD‐25) on intellectual functions. AMA Arch Neurol Psychiatry 1958;80:477–480. [DOI] [PubMed] [Google Scholar]
- 44. Aronson H, Silverstein AB, Klee MD. Influence of LSD‐25 on subjective time. Arch Gen Psychiatry 1959;1:469–472. [DOI] [PubMed] [Google Scholar]
- 45. Benda P, Orsini F. Étude expérimentale de l'estimation du temps sous LSD‐25. Ann Med Psychol 1959;117:525–533. [PubMed] [Google Scholar]
- 46. Lienert GA. Pharmakopsychologische Untersuchungen über den Abbau der geistigen Leistungsfähigkeit (Bericht über den 20 Kongress der Deutschen Gesellschaft für Psychologie), Berlin, Sept 26–29. 1955, Verlag für Psychologie, 1956;144–147.
- 47. Lienert GA. Changes in the factor structure of intelligence tests produced by d‐lysergic acid diethylamide (LSD) In: Bradley PB, ed. Neuro‐Psychopharmacology. Amsterdam : Elsevier, 1959, pp. 461–465. [Google Scholar]
- 48. Lienert GA. Über die Regression psychischer Funktionen als Folge pharmakologischer Belastung durch LSD und Alkohol. Med Exp Int J Exp Med 1961;5:203–208. [PubMed] [Google Scholar]
- 49. Lienert GA. Mental age regression induced by lysergic acid diethylamide. J Psychol 1966;63:3–11. [DOI] [PubMed] [Google Scholar]
- 50. Hintzen A. Die (Psycho)‐Pharmakologie von Lysergsäurediäthylamid. Hannover : Hannover Medical School Dissertation, 2006. [Google Scholar]
- 51. Halpern JH, Pope HG Jr. Do hallucinogens cause residual neuropsychological toxicity? Drug Alcohol Depend 1999;53:247–256. [DOI] [PubMed] [Google Scholar]
- 52. Rothlin E, Cerletti A. Pharmacology of LSD‐25 In: Cholden L, ed. Lysergic acid diethylamide and mescaline in experimental psychiatry. New York : Grune and Stratton, 1956, pp. 1–7. [Google Scholar]
- 53. Freedman DX. The psychopharmacology of hallucinogenic agents. Ann Rev Med 1969;20:409–418. [DOI] [PubMed] [Google Scholar]
- 54. Rothlin E. Metabolism of lysergic acid diethylamide. Nature 1956;178:1400–1401. [DOI] [PubMed] [Google Scholar]
- 55. Evarts EV. Some effects of bufotenine and LSD on the monkey. AMA Arch Neurol Psychiatry 1956;75:49–53. [DOI] [PubMed] [Google Scholar]
- 56. Klock JC, Boerner U, Becker CE. Coma, hyperthermia and bleeding associated with massive LSD overdose. A report of eight cases. West J Med 1974;120:183–188. [PMC free article] [PubMed] [Google Scholar]
- 57. Cohen MM, Marinello MJ, Back N. Chromosomal damage in human leukocytes induced by lysergic acid diethylamide. Science 1967;155:1417–1419. [DOI] [PubMed] [Google Scholar]
- 58. Dishotsky NI, Loughman WD, Mogar RE, Lipscomb WR. LSD and genetic damage. Science 1971;172:431–447. [DOI] [PubMed] [Google Scholar]
- 59. Robinson JT, Chitham RG, Greenwood RM, Taylor JW. Chromosome aberrations and LSD. Br J Psychiatry 1974;125: 238–244. [DOI] [PubMed] [Google Scholar]
- 60. Smart RG, Bateman K. The chromosomal and teratogenic effects of lysergic acid diethylamide: A review of the current literature. Can Med Assoc J 1968;99:805–810. [PMC free article] [PubMed] [Google Scholar]
- 61. Leuner H. Halluzinogene. Psychische Grenzzustände in Forschung und Psychotherapie. Bern , Stuttgart , Wien : Huber, 1981. [Google Scholar]
- 62. Idanpään‐Heikkilä JE, Schoolar JC. 14C‐lysergide in early pregnancy. Lancet 1969;2:221. [DOI] [PubMed] [Google Scholar]
- 63. Auerbach R, Rugowski JA. LSD: Effect on embryos. Science 1968;157:1325–1326. [DOI] [PubMed] [Google Scholar]
- 64. Greiner TH, Burch NR, Edelberg R. Psychopathology and psychophysiology of minimal LSD‐25 dosage. AMA Arch Neurol Psychiatry 1958;79:208–210. [DOI] [PubMed] [Google Scholar]
- 65. Salmoiraghi GC, McCubbin JW, Page IH. Effects of d‐lysergic acid diethylamide and its brom derivative on cardiovascular responses to serotonin and on arterial pressure. J Pharmacol Exp Ther 1957;19:240–247. [PubMed] [Google Scholar]
- 66. Tauberger G, Klimmer OR. Effects of high doses of d‐lysergic acid diethylamide on the respiration, blood circulation and central sympathicus tone of cats. Arzneimittelforschung 1968;18:1489–1491. [PubMed] [Google Scholar]
- 67. DiMascio A, Greenblatt M, Hyde RW. A study of the effects of LSD: Physiologic and psychological changes and their interrelations. Am J Psychiatry 1957;114:309–317. [DOI] [PubMed] [Google Scholar]
- 68. Forrer G, Goldner R. Experimental physiological studies with LSD. AMA Arch Neurol Psychiatry 1951;65:581–588. [DOI] [PubMed] [Google Scholar]
- 69. Hollister LE. Pharmacology of LSD in man In: Radouco‐Thomas S, Villeneuve A, Radouco‐Thomas C, eds. Pharmacology, toxicology and abuse of psychotominetics. Quebec : Les Presses de l'Université Laval, 1974, pp. 173–183. [Google Scholar]
- 70. Liddell DW, Weil‐Malherbe H. The effects of methedrine and of lysergic acid diethylamide on mental processes and on the blood adrenaline level. J Neurochem 1953;16:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Abramson HA, Jarvik ME, Hirsch MW. Lysergic acid diethylamide (LSD‐25): X. Effects on reaction time to auditory and visual stimuli. J Psychol 1955;40:39–46. [Google Scholar]
- 72. Belsanti R. Nuove ricerche in psichiatria sperimentale con la dietilamide dell'acido lisergico. Acta Neurol 1955;10:460. [Google Scholar]
- 73. Fischer R, Georgi F, Weber R. Psychophysical correlations VIII. Experimental tests in schizophrenia: Lysergic acid diethylamide and mescaline. Schweiz Med Wochenschr 1951;81:817–819. [PubMed] [Google Scholar]
- 74. Manger WM, Schwarz BE, Barrs CW. Plasma and cerebrospinal fluid concentration of epinephrine and norepinephrine in certain psychiatric conditions. In: abstract of communication, 12th Inter Physiol Congress. Brussels , 1956, 610.
- 75. Sokoloff L, Perlin S, Kornetsky C, Kety SS. The effects of d‐lysergic acid diethylamide on cerebral circulation and overall metabolism. Ann NY Acad Sci 1957;66:468–477. [DOI] [PubMed] [Google Scholar]
- 76. Pincus G, Hoagland H. Adrenal cortical responses to stress in normal and psychotic subjects. Am J Psychiatry 1950;106:651–659. [DOI] [PubMed] [Google Scholar]
- 77. Hollister LE, Moore FF. Increased plasma free fatty acids following psychotomimetic drugs. J Psychiat Res 1965;3:199–203. [DOI] [PubMed] [Google Scholar]
- 78. Messiha FS, Grof S. D‐lysergic acid diethylamide (LSD)‐effect on biogenic amines excretion in man. Biochem Pharmacol 1973;22:2352–2354. [DOI] [PubMed] [Google Scholar]
- 79. Hollister LE, Hartman AM. Mescaline, lysergic acid diethylamide and psilocybin comparison of clinical syndromes, effects on color perception and biochemical measures. Compr Psychiatry 1962;3:235–242. [DOI] [PubMed] [Google Scholar]
- 80. Muzio JN, Roffwarg HP, Kaufman E. Alterations in the nocturnal sleep cycle resulting from LSD. Electroencephalogr Clin Neurophysiol 1966;21:313–324. [DOI] [PubMed] [Google Scholar]
- 81. Torda C. Contribution to serotonin theory of dreaming (LSD infusion). NY State J Med 1968;68:1135–1138. [PubMed] [Google Scholar]
- 82. Bliss EL, Clark LD, West CD. Studies of sleep deprivation—Relationship to schizophrenia. AMA Arch Neur Psychiatry 1959;81:348–359. [DOI] [PubMed] [Google Scholar]
- 83. Safer DJ. The effect of LSD on sleep‐deprived men. Psychopharmacol 1970;17:414–424. [DOI] [PubMed] [Google Scholar]
- 84. Meltzer HY, Fessler RG, Simonovic M, Doherty J, Fang VS. LSD: Evidence for stimulation of pituritary dopamine receptors. Psychopharmacol 1977;54:39–44. [DOI] [PubMed] [Google Scholar]
- 85. Collu R, Letarte J, Leboeuf G, Ducharme JR. Endocrine effects of chronic administration of psychoactive drugs to prepubertal male rats II: LSD. Can J Physiol Pharmacol 1975;53:1023–1026. [DOI] [PubMed] [Google Scholar]
- 86. Meltzer HY, Simonovic M, Fang VS, Goode DJ. Neuroendocrine effects of psychotomimetic drugs. McLean Hosp J 1981;6:115–137. [Google Scholar]
- 87. Rinkel M, Hyde RW, Solomon HC, Hoagland H. Experimental psychiatry. II. Clinical and physio‐chemical observations in experimental psychosis. Am J Psychiatry 1955;111:881–895. [DOI] [PubMed] [Google Scholar]
- 88. Hoch PH. Studies in routes of administration and counteracting drugs In: Cholden L, ed. Lysergic acid diethylamide and mescaline in experimental psychiatry. New York : Grune and Stratton, 1956, pp. 8–12. [Google Scholar]
- 89. Upshall DG, Wailling DG. The determination of LSD in human plasma following oral administration. Clin Chim Act 1972;36:67–73. [DOI] [PubMed] [Google Scholar]
- 90. Stoll A, Rutschmann J, Hofmann A. Über die Synthese von 14C‐Diäthylamin und 14C‐Lysergsäure‐diäthylamid. Helv Chim Acta 1954;37:820–824. [Google Scholar]
- 91. Stoll A, Rothlin E, Rutschmann J, Schalch WR. Distribution and fate of 14C‐labeled lysergic acid diethylamide (LSD 25) in the animal body. Experientia 1955;11:396–397. [DOI] [PubMed] [Google Scholar]
- 92. Boyd ES, Rothlin E, Bonner JF, Slater JH, Hodge HC. Preliminary studies of the metabolism of LSD using radioactive carbonmarked molecules. J Nerv Ment Dis 1955;122:470–471. [DOI] [PubMed] [Google Scholar]
- 93. Boyd ES. The metabolism of LSD. Arch Int Pharmacodyn Ther 1959;120:292–311. [PubMed] [Google Scholar]
- 94. Lanz U, Cerletti A, Rothlin E. Distribution of lysergic acid diethylamide in the organism. Helv Physiol Pharmacol Acta 1955;13:207–216. [PubMed] [Google Scholar]
- 95. Freedman DX, Coquet CA. Regional and subcellular distribution of LSD and effects on 5‐HT levels. Pharmacologist 1965;7:183. [Google Scholar]
- 96. Snyder SH, Reivich M. Regional localization of LSD in monkey brain. Nature 1966;209:1093–1095. [DOI] [PubMed] [Google Scholar]
- 97. Axelrod J, Brady RO, Witkop B, Evarts EV. The distribution and metabolism of LSD. Ann NY Acad Sci 1957;66:435–444. [DOI] [PubMed] [Google Scholar]
- 98. Hoff H, Arnold OH. Allgemeine Gesichtspunkte zur Pharmakopsychiatrie In: Bradley PB, ed. Neuro‐Psychopharmacology. Amsterdam : Elsevier, 1959, pp. 326–337. [Google Scholar]
- 99. Diab IM, Freedman DX, Roth LJ. [3H] lysergic acid diethylamide: Cellular autoradiographic localization in rat brain. Science 1971;173:1022–1024. [DOI] [PubMed] [Google Scholar]
- 100. Wagner JG, Aghajanian GK, Bing OHL. Correlation of performance test scores with „tissue concentration” of lysergic acid diethylamide in human subjects. Clin Pharmacol Ther 1968;9:635–638. [DOI] [PubMed] [Google Scholar]
- 101. CM Metzler. A mathematical model for the pharmacokinetics of LSD effect. Clin Pharmacol Ther 1969;10:737–740. [DOI] [PubMed] [Google Scholar]
- 102. Aghajanian GK, Bing OH. Persistence of lysergic acid diethylamide in the plasma of human subjects. Clin Pharmacol Ther 1964;5:611–614. [DOI] [PubMed] [Google Scholar]
- 103. Siddik ZH, Barnes RD, Dring LG, Smith RL, Williams RT. The fate of lysergic acid di[14C]ethylamide ([14C]LSD) in the rat, guinea pig and rhesus monkey and of [14C]iso‐LSD in rat. Biochem Pharmacol 1979;28:3093–3101. [DOI] [PubMed] [Google Scholar]
- 104. Freter K, Axelrod J, Witkop B. Biochemical and pharmacological studies with d‐ and l‐5‐hydroxytryptophan. J Am Chem Soc 1957;79:3111–3117. [DOI] [PubMed] [Google Scholar]
- 105. Niwaguchi T, Inoue T, Sakai T. Studies on enzymatic dealkylation of d‐lysergic acid diethylamide (LSD). Biochem Pharmacol 1974;23:1073–1078. [DOI] [PubMed] [Google Scholar]
- 106. Klette KL, Anderson CJ, Poch GK, Nimrod AC, ElSohly MA. Metabolism of lysergic acid diethylamide (LSD) to 2‐oxo‐3‐hydroxy LSD (O‐H‐LSD) in human liver microsomes and cryopreserved human hepatocytes. J Anal Toxicol 2000;24:550–556. [DOI] [PubMed] [Google Scholar]
- 107. Canezin J, Cailleux A, Turcant A, Le Bouil A, Harry P, Allain P. Determination of LSD and its metabolites in human biological fluids by high‐performance liquid chromatography with electrospray tandem mass spectrometry. J Chromatogr B Biomed Sci Appl 2001;765:15–27. [DOI] [PubMed] [Google Scholar]
- 108. Faed EM, McLeod WR. A urine screening test of lysergide. J Chromatogr Sci 1973;11:4–6. [Google Scholar]
- 109. Papac DI, Foltz RL. Measurement of lysergic acid diethylamide (LSD) in human plasma by gas chromatography/negative ion chemical ionization mass spectrometry. J Anal Toxicol 1990;14:189–190. [DOI] [PubMed] [Google Scholar]
- 110. Peel HW, Boynton AL. Analysis of LSD in urine using radioimmunoassay—Excretion and storage effects. Can Soc Forensic Sci J 1980;13:23–28. [Google Scholar]
- 111. Van Bocxlaer JF, Clauwaert KM, Lambert WE, Deforce DL, Van Den Eeckhout EG, De Leenheer AP. Liquid chromatography‐mass spectrometry in forensic toxicology. Mass Spectrom Rev 2000;19:165–214. [DOI] [PubMed] [Google Scholar]
- 112. McCarron MM, Walberg CB, Baselt RC. Confirmation of LSD intoxication by analysis of serum and urine. J Anal Toxicol 1990;14:165–167. [DOI] [PubMed] [Google Scholar]
- 113. Kerrigan S, Brooks DE. Immunochemical extraction and detection of LSD in whole blood. J Immunol Methods 1999;224:11–18. [DOI] [PubMed] [Google Scholar]
- 114. Paul BD, Mitchell JM, Burbage R, Moy M, Sroka R. Gas chromatographic‐electron‐impact mass fragmentometric determination of lysergic acid diethylamide in urine. J Chromatogr 1990;529:103–112. [DOI] [PubMed] [Google Scholar]
- 115. Taunton‐Rigby A, Sher SE, Kelley PR. Lysergic acid diethylamide: Radioimmunoassay. Science 1973;181:165–166. [DOI] [PubMed] [Google Scholar]
- 116. Reuschel SA, Eades D, Foltz RL. Recent advances in chromatographic and mass spectrometric methods for determination of LSD and its metabolites in physiological specimens. J Chromatogr B Biomed Sci Appl 1999;733:145–159. [DOI] [PubMed] [Google Scholar]
- 117. Rohrich J, Zorntlein S, Becker J. Analysis of LSD in human body fluids and hair samples applying ImmunElute columns. Forensic Sci Int 2000;107:181–190. [DOI] [PubMed] [Google Scholar]
- 118. Nakahara Y, Kikura R, Takahashi K, Foltz RL, Mieczkowski T. Detection of LSD and metabolite in rat hair and human hair. J Anal Toxicol 1996;20:323–329. [DOI] [PubMed] [Google Scholar]
- 119. Arnold OH, Hofmann G, Leupold‐Lowenthal H. Untersuchungen zum Schizophrenieproblem IV Mitteilung: Die Verteilung des C14‐radioaktiven Lysergsäurediäthylamid (C14‐LSD‐25) im tierischen Organismus. Wien Z Nervenheilk 1958;15:15–27. [PubMed] [Google Scholar]
- 120. Hertle F, Zipf KE, Broghammer H. Beobachtungen über Kreislaufverhalten, Muskeltonus und einige Kreislaufgrößen beim Menschen unter LSD‐25‐Einwirkung. Arch Psychiatr Nervenkr 1962;202:569–591. [DOI] [PubMed] [Google Scholar]
- 121. Anderson EW, Rawnsley K. Clinical studies of lysergic acid diethylamide. Monatsschr Psychiatr Neurol 1954;128:38–55. [DOI] [PubMed] [Google Scholar]
- 122. Bradley PB, Elkes C, Elkes J. On some effects of LSD in normal volunteers. J Physiol 1953;121:50. [PubMed] [Google Scholar]
- 123. Elkes J, Elkes C, Bradley PB. The effect of some drugs on the electrical activity of the brain and on behaviour. J Ment Sci 1954;100:125–128. [DOI] [PubMed] [Google Scholar]
- 124. Itil TM. Quantitative EEG and behavior changes after LSD and Ditran In: Karczmar AG, Koella WP, eds. Neurophysiological and behavioral aspects of psychotropic drugs. Springfield : Thomas, CC, III, 1969, pp. 72–87. [Google Scholar]
- 125. Were PF. Electroencephalographic effects of LSD, and some psychiatric implications. J Neuropsychiatr 1964;5:516–524. [PubMed] [Google Scholar]
- 126. Goldstein L, Murphree HB, Sugerman AA, Pfeiffer CC, Jenney EH. Quantitative electroencephalographic analysis of naturally occuring (schizophrenic) and drug‐induced psychotic states in human males. Clin Pharmacol Ther 1963;4:10–21. [DOI] [PubMed] [Google Scholar]
- 127. Goldstein L, Stoltzfus NW. Psychoactive drug‐induced changes of interhemispheric EEG amplitude relationships. Agents Actions 1973;3:124–132. [DOI] [PubMed] [Google Scholar]
- 128. Fink M, Itil T. Neurophysiology of phantastica: EEG and behavioral relations in man In: Efron D, ed. Psychopharmacology: A review of progress 1957–1967. Washington , DC : US Government Printing Office, 1968, pp. 1231–1239. [Google Scholar]
- 129. Gouzoulis‐Mayfrank E, Schreckenberger M, Sabri O, Arning C, Thelen B, Spitzer M, Kovar K A, Hermle L, Bull U, et al Neurometabolic effects of psilocybin, 3,4‐methylenedioxyethylamphetamine (MDE) and d‐methamphetamine in healthy volunteers. A double‐blind, placebo‐controlled PET study with [18F]‐FDG. Neuropsychopharmacol 1999;20:565–581. [DOI] [PubMed] [Google Scholar]
- 130. Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacol 1997;16:357–372. [DOI] [PubMed] [Google Scholar]
- 131. Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ. Human pharmacology of ayahuasca: Subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics. J Pharmacol Exp Ther 2003;306:73–83. [DOI] [PubMed] [Google Scholar]
- 132. Hermle L, Funfgeld M, Oepen G, Botsch H, Borchardt D, Gouzoulis E, Fehrenbach RA, Spitzer M. Mescaline‐induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: Experimental psychosis as a tool for psychiatric research. Biol Psychiatry 1992;32:976–991. [DOI] [PubMed] [Google Scholar]
- 133. Vollenweider FX, Geyer MA. A systems model of altered consciousness: Integrating natural and drug‐induced psychoses. Brain Res Bull 2001;56:495–507. [DOI] [PubMed] [Google Scholar]
- 134. Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci USA 2005;102:17804–17809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sinha R, Lacadie C, Skudlarski P, Wexler BE. Neural circuits underlying emotional distress in humans. Ann NY Acad Sci 2004;1032:254–257. [DOI] [PubMed] [Google Scholar]
- 136. Riba J, Romero S, Grasa E, Mena E, Carrio I, Barbanoj MJ. Increased frontal and paralimbic activation following ayahuasca, the pan‐Amazonian inebriant. Psychopharmacology 2006;186:93–98. [DOI] [PubMed] [Google Scholar]
- 137. Nichols DE, Frescas S, Marona‐Lewicka D, Kurrasch‐Orbaugh DM. Lysergamides of isomeric 2,4‐dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N‐diethyllysergamide (LSD). J Med Chem 2002;45:4344–4349. [DOI] [PubMed] [Google Scholar]
- 138. Peroutka SJ, Sleight AJ, McCarthy BG, Pierce PA, Schmidt AW, Hekmatpanah CR. The clinical utility of pharmacological agents that act at serotonin receptors. J Neuropsychiatry Clin Neurosci 1989;1:253–262. [DOI] [PubMed] [Google Scholar]
- 139. Egan CT, Herrick‐Davis K, Miller K, Glennon RA, Teitler M. Agonist activity of LSD and lisuride at cloned 5HT2A and 5HT2C receptors. Psychopharmacol 1998;136:409–414. [DOI] [PubMed] [Google Scholar]
- 140. Milburn CM, Peroutka SJ. Characterization of [3H]quipazine binding to 5‐hydroxytryptamine3 receptors in rat brain membranes. J Neurochem 1989;52:1787–1792. [DOI] [PubMed] [Google Scholar]
- 141. Gerald C, Adham N, Kao HT, Olsen MA, Laz TM, Schechter LE, Bard JA, Vaysse PJ, Hartig PR, Branchek TA, et al The 5‐HT4 receptor: Molecular cloning and pharmacological characterization of two splice variants. EMBO J 1995;14:2806–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Boess FG, Martin IL. Molecular biology of 5‐HT receptors. Neuropharmacol 1994;33:275–317. [DOI] [PubMed] [Google Scholar]
- 143. Hirst WD, Abrahamsen B, Blaney FE, Calver AR, Aloj L, Price GW, Medhurst AD. Differences in the central nervous system distribution and pharmacology of the mouse 5‐hydroxytryptamine‐6 receptor compared with rat and human receptors investigated by radioligand binding, site‐directed mutagenesis, and molecular modelling. Mol Pharmacol 2003;64:1295–1308. [DOI] [PubMed] [Google Scholar]
- 144. Eglen RM, Jasper JR, Chang DJ, Martin GR. The 5‐HT7 receptor: Orphan found. Trends Pharmacol Sci 1997;18:104–107. [DOI] [PubMed] [Google Scholar]
- 145. U'prichard DC, Greenberg DA, Snyder SH. Binding characteristics of a radiolabelled agonist and antagonist at central nervous system alpha noradrenergic receptors. Mol Pharmacol 1977;13:454–473. [PubMed] [Google Scholar]
- 146. Hüther G, Rüther E. Das serotonerge system. Bremen : Uni‐Med Verlag, 2000. [Google Scholar]
- 147. Erlander MG, Lovenberg TW, Baron BM, De Lecea L, Danielson PE, Racke M, Slone AL, Siegel BW, Foye PE, Cannon K. Two members of a distinct subfamily of 5‐hydroxytryptamine receptors differentially expressed in rat brain. Proc Natl Acad Sci USA 1993;90:3452–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lovenberg TW, Erlander MG, Baron BM, Racke M, Slone AL, Siegel BW, Craft CM, Burns JE, Danielson PE, Sutcliffe JG. Molecular cloning and functional expression of 5‐HT1E‐like rat and human 5‐hydroxytryptamine receptor genes. Proc Natl Acad Sci USA 1993;90:2184–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Monsma FJ Jr, Shen Y, Ward RP, Hamblin MW, Sibley DR. Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol Pharmacol 1993;43:320–327. [PubMed] [Google Scholar]
- 150. Titeler M, Lyon RA, Glennon RA. Radioligand binding evidence implicates the brain 5‐HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacol 1988;94:213–216. [DOI] [PubMed] [Google Scholar]
- 151. Glennon RA, Titeler M, McKenney JD. Evidence for 5‐HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci 1984;35:2505–2511. [DOI] [PubMed] [Google Scholar]
- 152. Sanders‐Bush E, Burris KD, Knoth K. Lysergic acid diethylamide and 2,5‐dimethoxy‐4‐methylamphetamine and partial agonists at serotonin receptors linked to phosphoinositide hydrolysis. J Pharm Exp Ther 1988;246:924–928. [PubMed] [Google Scholar]
- 153. Pierce PA, Peroutka SJ. Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex. Psychopharmacol 1989;97:118–122. [DOI] [PubMed] [Google Scholar]
- 154. Martin‐Ruiz R, Puig MV Celada P, Shapiro DA, Roth BL, Mengod G, Artigas F. Control of serotonergic function in medial prefrontal cortex by serotonin2A receptors through a glutamate‐dependent mechanism. J Neurosci 2001;21:9856–9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Winter JC, Eckler JR, Rabin RA. Serotonergic/glutamatergic interactions: The effects of mGlu2/3 receptor ligands in rats trained with LSD and PCP as discriminative stimuli. Psychopharmacol 2004;172:233–240. [DOI] [PubMed] [Google Scholar]
- 156. Gonzalez‐Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley‐Moore M, Ge Y, Zhou Q, et al Hallucinogens recruit specific cortical 5‐HT(2A) receptor‐mediated signaling pathways to affect behaviour. Neuron 2007;53:439–452. [DOI] [PubMed] [Google Scholar]
- 157. Nichols CD, Sanders‐Bush EA. Single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacol 2002;26:634–642. [DOI] [PubMed] [Google Scholar]
- 158. Nichols CD, Garcia EE, Sanders‐Bush E. Dynamic changes in prefrontal cortex gene expression following lysergic acid diethylamide administration. Brain Res Mol Brain Res 2003;111:182–188. [DOI] [PubMed] [Google Scholar]
- 159. Von Hungen K, Roberts S, Hill DF. LSD as an agonist and antagonist at central dopamine receptors. Nature 1974;252:588–589. [DOI] [PubMed] [Google Scholar]
- 160. Watts VJ, Lawler CP, Fox DR, Neve KA, Nichols DE, Mailman RB. LSD and structural analogs: Pharmacological evaluation at D1 dopamine receptors. Psychopharmacol 1995;118:401–409. [DOI] [PubMed] [Google Scholar]
- 161. Vollenweider FX, Vontobel P, Hell D, Leenders KL. 5‐HT modulation of dopamine release in basal ganglia in psilocybin‐induced psychosis in man–A PET study with [11C]raclopride. Neuropsychopharmacol 1999;20:424–433. [DOI] [PubMed] [Google Scholar]
- 162. Marona‐Lewicka D, Kurrasch‐Orbaugh DM, Selken JR, Cumbay MG, Lisnicchia JG, Nichols DE. Re‐evaluation of lisuride pharmacology: 5‐HT1a receptor‐mediated behavioral effects overlap its other properties in rats. Psychopharmacol 2002;164:93–107. [DOI] [PubMed] [Google Scholar]
- 163. Abramson HA, Jarvik ME, Govin MH, Hirsch MW. Lysergic acid diethylamide (LSD‐25): XVIII. Tolerance development and is relationship to a theory of psychosis. J Psychol 1956;41:81–105. [Google Scholar]
- 164. Cholden LS, Kurland A, Savage CU. Clinical reactions and tolerance to LSD in chronic schizophrenia. J Nerv Ment Dis 1955;122:211–221. [DOI] [PubMed] [Google Scholar]
- 165. Gresch PJ, Smith RL, Barrett RJ, Sanders‐Bush E. Behavioral tolerance to lysergic acid diethylamide is associated with reduced serotonin‐2A receptor signaling in rat cortex. Neuropsychopharmacol 2005;30:1693–1702. [DOI] [PubMed] [Google Scholar]
- 166. Cerletti A, Rothlin E. Role of 5‐HT in mental diseases and its antagonism to lysergic acid derivates. Nature 1955;176:785–786. [DOI] [PubMed] [Google Scholar]
- 167. Abramson HA, Sklarofsky B, Baron MO, Freemont‐Smith F. Lysergic acid diethylamide antagonists: II. Development of tolerance in man to LSD by prior administration of MLD. Arch Neurol Psychiatry 1958;79:201–207. [PubMed] [Google Scholar]
- 168. Balestrieri A, Fontanari D. Acquired and cross‐tolerance to mescaline, LSD and BOL. Arch Gen Psychiatry 1959;1:279–282. [DOI] [PubMed] [Google Scholar]
- 169. Isbell H, Wolbach AB, Wikler A, Miner EJ. Cross tolerance between LSD and psilocybin. Psychopharmacologia 1961;2:147–159. [DOI] [PubMed] [Google Scholar]
- 170. Balestrieri A. Studies on cross tolerance with LSD‐25, UML‐491 and JB‐336. Psychopharmacol 1960;1:257–259. [DOI] [PubMed] [Google Scholar]
- 171. Rosenberg DE, Isbell H, Miner EJ, Logan CR. The effect of N,N‐dimethyltryptamine in human subjects tolerant to lysergic acid diethylamide. Psychopharmacologia 1964;13:217–227. [DOI] [PubMed] [Google Scholar]
- 172. Isbell H, Gorodetsky W, Jasinski D, Claussen U, Spulak FV, Korte F. Effects of delta‐trans‐tetrahydrocannabinol in man. Psychopharmacologia. 1967;11:187–188. [DOI] [PubMed] [Google Scholar]
- 173. Isbell H, Jasinski DR. A comparison of LSD‐25 with (‐)‐delta‐trans‐tetrahydrocannabinol (THC) and attempted cross‐tolerance between LSD and THC. Psychopharmacologia 1969;14:115–123. [DOI] [PubMed] [Google Scholar]
- 174. Wyatt RJ, Cannon EH, Stoff DM, Gillin JC. Interactions of hallucinogens at the clinical level In: Vesell ES, Braude MC, eds. Interactions of drugs of abuse. New York : NY Acad Sci, 1976, pp. 456–486. [DOI] [PubMed] [Google Scholar]
- 175. Murphree HB. Quantitative studies in humans on the antagonism of lysergic acid diethylamide by chlorpromazine and phenoxybenzamine. Clin Pharmacol Ther 1962;3:314–320. [DOI] [PubMed] [Google Scholar]
- 176. Hoch PH. Comments. Experimental psychiatry. Am J Psychiatry 1955;111:787–790. [DOI] [PubMed] [Google Scholar]
- 177. Giberti F, Gregoretti L. Prime esperienze di antagonismo psicofarmacologico. Sist Nerv 1955;4:301–310. [PubMed] [Google Scholar]
- 178. Barnett BE. Diazepam treatment for LSD intoxication. Lancet 1971;2:270. [DOI] [PubMed] [Google Scholar]
- 179. Levy RM. Diazepam for LSD intoxication. Lancet 1971;1:1297. [DOI] [PubMed] [Google Scholar]
- 180. Bonson KR, Murphy DL. Alterations in response to LSD in humans associated with chronic administration of tricyclic antidepressants, monoamine oxidase inhibitors or lithium. Behav Brain Res 1996;73:229–233. [DOI] [PubMed] [Google Scholar]
- 181. Heninger GR, Charney DS. Mechanism of action of antidepressant treatments: Implications for the etiology and treatment of depressive disorders In: Meltzer HY, ed. Psychopharmacology: The third generation of progress. New York : Raven Press, 1987;pp. 535–545. [Google Scholar]
- 182. Middendorf W. Personal communication. 2003.
- 183. Isbell H, Logan CR. Studies on the diethylamide of lysergic acid (LSD‐25) II: Effects of chlorpromazine, azacyclonol and reserpine on the intensity of the LSD‐reaction. AMA Arch Neurol Psychiatry 1957;77:350–358. [DOI] [PubMed] [Google Scholar]
- 184. Resnick O, Krus DM, Raskin M. LSD‐25 action in normal subjects treated with a monoamine oxidase inhibitor. Life Sci 1964;35:1207–1214. [DOI] [PubMed] [Google Scholar]
- 185. Marecek P, Bakalar E, Zeman K. Attempt of blocking LSD intoxication with tranylcypromine. Act Nerv Super 1968;10:276–277. [PubMed] [Google Scholar]
- 186. Grof S, Dytrych Z. Blocking of LSD reaction by premedication with Niamid. Act Nerv Super 1965;7:306. [PubMed] [Google Scholar]
- 187. Bonson KR, Buckholtz JW, Murphy DL. Chronic administration of serotonergic antidepressants attenuates the subjective effects of LSD in humans. Neuropsychopharmacol 1996;14:425–436. [DOI] [PubMed] [Google Scholar]
- 188. Miller AI, Williams HL, Murphree HB. Niacin, niacinamide or atropine versus LSD model psychoses in human volunteers. J Pharmacol Exp Ther 1957;119:169–170. [Google Scholar]
- 189. Clark LD, Bliss EL. Psychopharmacological studies of LSD intoxication—Effects of premedication with BOL, mescaline, atropine, amobarbital and chlorpromazine. AMA Arch Neurol Psychiatry 1957;78:653–655. [DOI] [PubMed] [Google Scholar]
- 190. Isbell H, Logan CR, Miner EJ. Studies on LSD III: Attempts to attenuate the LSD reaction in man by pretreatment with neuronal blocking agents. AMA Arch Neurol Psychiatry 1959;81:20–27. [PubMed] [Google Scholar]
- 191. Abramson HA, Sklarofsk YB. Lysergic acid diethylamide (LSD‐25) antagonists III: Modification of syndrome by prior administration of prednisone. Arch Gen Psychiatry 1960;2:89–93. [DOI] [PubMed] [Google Scholar]
- 192. Clark LD, Clark LS. The effects of cortisone on LSD intoxication in schizophrenic patients. J Nerv Ment Dis 1956;123:561–562. [DOI] [PubMed] [Google Scholar]
- 193. Krus DM, Wapner S, Bergen J, Freeman H. The influence of progesterone on behavioral changes induced by lysergic acid diethylamide (LSD‐25) in normal males. Psychopharmacologia 1961;2:177–84. [DOI] [PubMed] [Google Scholar]
- 194. Balestrieri A. Amphetamine shock during LSD and mescaline psychosis In: Garattini S, Ghetti V, eds. Psychotropic drugs. New York : Elsevier, 1957, p. 582 ff. [Google Scholar]
- 195. Kristensen K. LSD‐behandling kombineret med parenteral Ritalinbehandling. Nord Psykiatr Tidsskr 1962;16:111–116. [DOI] [PubMed] [Google Scholar]
- 196. Marecek P, Bakalar E. Attempt to affect the course of LSD intoxication with ethyl alcohol. Act Nerv Super 1967;9:379–380. [PubMed] [Google Scholar]
- 197. Vojtechovsky M, Skala J, Safratova V. Clinical picture of LSD psychosis following premedication with l‐tyrosine and l‐DOPA in chronic alcoholics. Act Nerv Super 1968;10:275–276. [PubMed] [Google Scholar]
- 198. Holland D. Flashback‐Phänomene als Nachwirkung von Halluzinogeneinnahme. Hannover : Hannover Medical School Dissertation, 2001. [Google Scholar]
- 199. Halpern JH, Pope HG Jr. Hallucinogen persisting perception disorder: What do we know after 50 years? Drug Alcohol Depend 2003;69:109–119. [DOI] [PubMed] [Google Scholar]


