Abstract
The relative efficacy has not been adequately established for the two catechol‐O‐methyltransferase (COMT) inhibitors that are currently available for adjunctive therapy in Parkinson's disease; tolcapone and entacapone. A recent Cochrane meta‐analysis of 14 studies in 2566 patients, conducted to assess the efficacy and safety of tolcapone and entacapone, found both to be statistically superior to placebo in increasing ON time and decreasing OFF time. The meta‐analysis also showed that the weighted mean difference from baseline to endpoint in tolcapone‐treated patients was twice that in entacapone‐treated patients for both placebo‐corrected ON time and OFF time. Withdrawal rates were generally lower for tolcapone. Two additional studies have examined the switch between tolcapone and entacapone. In 40 Parkinson's disease patients with fluctuations who were switched from tolcapone to entacapone, improvements in ON time and reductions in OFF time were approximately twice the magnitude for tolcapone than for entacapone. In a second study examining the switch from entacapone to tolcapone, the results for several exploratory variables also suggested that tolcapone has greater efficacy than entacapone. These findings indicate that tolcapone should be considered in all patients with entacapone‐refractory motor fluctuations.
Keywords: Catechol‐O‐methyltransferase, Entacapone, Meta‐analysis, Motor fluctuations Parkinson's disease, Tolcapone
Introduction
Catechol‐O‐methyltransferase (COMT) inhibitors are an important class of medications for the management of Parkinson's disease. They enhance the efficacy of levodopa by reducing its peripheral catabolism and improving its effective delivery to the brain (Forsberg et al. 2003; Jorga et al. 1998a; Jorga et al. 1998b; Ruottinen and Rinne 1996). For patients with the “wearing off” phenomenon who are receiving optimal doses of levodopa, the addition of a COMT inhibitor can increase ON time (period of time experiencing symptom relief) and reduce OFF time (duration of time experiencing little to no therapeutic benefit) (Adler et al. 1998; Baas et al. 1997; Brooks and Sagar 2003; Kurth et al. 1997; Parkinson Study Group 1997; Poewe et al. 2002; Rajput et al. 1997; Rinne et al. 1998). Currently, two COMT inhibitors—tolcapone and entacapone—are available for use in clinical practice. A crucial question left largely unaddressed in the published literature is whether one of these agents is more efficacious than the other.
The general view held by movement disorder specialists is that entacapone is a useful adjuvant medication but not a highly effective one; for example, a recent naturalistic study of entacapone usage showed that approximately half of the patients had discontinued it within 6 months (Parashos et al. 2004). The most common reason for drug discontinuation over the entire 3‐year follow‐up period was lack of efficacy (46%); discontinuation because of common adverse events associated with entacapone, such as diarrhea and nausea, which occurred in 9% and 11% of patients, respectively. This large subset of patients who experience poor clinical outcomes on entacapone could potentially benefit from a more effective COMT inhibitor. It is, therefore, important to clarify the question of the efficacy of tolcapone relative to that of entacapone.
Three deaths due to fulminant hepatitis were reported in 1998 among tolcapone‐treated patients. Research suggested that tolcapone induces uncoupling of mitochondrial oxidative phosphorylation and, therefore, reduces the cell's capacity to generate ATP. Although the concentrations of tolcapone required to create this effect are significantly higher than those needed to inhibit COMT (Borges 2005), the deaths prompted the issuance of a black box warning in the United States, along with stricter liver monitoring guidelines (Olanow and Tasmar Advisory Panel 2000). Two years after institution of these guidelines, a postmarketing surveillance analysis found no deaths among 1725 patients treated with tolcapone for up to 2 years or longer (Lew and Kricorian 2007). In addition, transaminase elevations were rare and transient (Lew and Kricorian 2007). Such findings have improved the safety profile of tolcapone and led the U.S. Food and Drug Administration (FDA) to relax the hepatic enzyme monitoring guidelines in February 2006 for patients receiving this medication (Table 1) (Tasmar Prescribing Information 2005, 2006). Monitoring requirements in the European Union remain unchanged (Tasmar Product Characteristics 2006). Entacapone has not been shown to cause liver toxicity and has no liver monitoring restrictions (Gordin et al. 2004).
Table 1.
Summary of liver monitoring requirements with tolcapone under prior and revised labeling
| Prior label | Current label |
|---|---|
| (Tasmar prescribing information, 2005) | (Tasmar prescribing information, 2006) |
| (1999–2005) | (February 2006 and forward) |
| • ALT/AST levels should be determined | • ALT/AST levels should be determined |
 At baseline At baseline |
 At baseline At baseline |
 Every 2 weeks for the first year Every 2 weeks for the first year |
 Every 2–4 weeks for first 6 months Every 2–4 weeks for first 6 months |
 Every 4 weeks for the next 6 months Every 4 weeks for the next 6 months |
 At intervals deemed clinically relevant thereafter At intervals deemed clinically relevant thereafter |
 Every 8 weeks thereafter Every 8 weeks thereafter |
• Discontinue tolcapone if AST or ALT exceeds 2 times the upper limit of normal |
| • Discontinue tolcapone if AST or ALT exceeds the upper limit of normal | |
Tolcapone and entacapone (Fig. 1) both inhibit human COMT activity after oral administration in healthy volunteers (Comtan Package Insert 2000; Tasmar Package Insert 2006). Both are indicated as adjunctive therapy with levodopa and carbidopa for the treatment of the signs and symptoms of idiopathic Parkinson's disease. The entacapone label further stipulates that patients must be experiencing the signs and symptoms of end‐of‐dose “wearing‐off” (Comtan Package Insert 2000).
Figure 1.
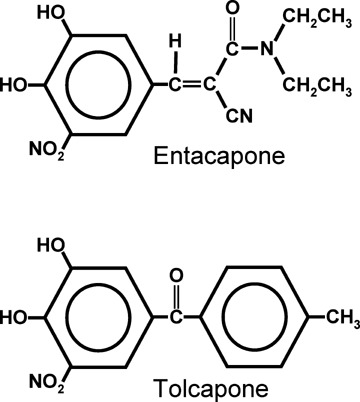
The chemical structures of entacapone and tolcapone.
The pharmacokinetics of adjunctive tolcapone over a 50 to 400 mg dose range of levodopa/carbidopa are linear and independent of coadministration. The elimination half‐life (t1/2) of tolcapone is 2 to 3 h with no significant accumulation, and the maximum concentrations (Cmax) are approximately 3 and 6 μg/mL with three‐times‐daily dosing of 100 and 200 mg, respectively. Tolcapone is absorbed rapidly and has a time to maximum concentrations (Tmax) of approximately 2 h. The absolute bioavailability is approximately 65% following oral administration. Ingestion of food within 1 h before and 2 h after tolcapone dosing decreases the relative bioavailability by 10% to 20% (Tasmar Package Insert 2006). The pharmacokinetics of entacapone are also linear over the dose range of 5 to 800 mg and independent of levodopa/carbidopa coadministration (Comtan Package Insert, 2000). The elimination of entacapone is biphasic, with a t1/2 of 0.3 to 0.4 h for the α‐phase and 1.6 to 3.4 h for the β‐phase (Kaakkola, 2000). After a single 200‐mg dose of entacapone, the Cmax is approximately 1.2 μg/mL. Like tolcapone, entacapone is rapidly absorbed and has a Tmax of approximately 1 h. The absolute bioavailability following oral administration of entacapone is 35%. Unlike tolcapone, food does not affect the pharmacokinetics of entacapone.
The differences in the pharmacokinetic and pharmacodynamic profiles of the COMT inhibitors may affect their potential for clinical efficacy. With respect to COMT inhibition itself, a 200 mg oral dose of tolcapone inhibits erythrocyte COMT activity by >80%, and at trough levels with t.i.d. administration, inhibition of 30–45% is still present (Tasmar Prescribing Information 2006). Entacapone 200 mg, in contrast, produces a maximal COMT inhibition of 65%, with full recovery of COMT activity at 8 h post‐dose (Kaakkola, 2000). More importantly, the effect on the pharmacokinetics of concomitantly administered levodopa is more pronounced with tolcapone, resulting in a 60% to 90% increase in levodopa area under the concentration‐time curve (Tasmar Prescribing Information, 2006), compared with approximately 35% for entacapone (Kaakkola, 2000). Another difference between the two drugs is that tolcapone has been demonstrated, at least in animal models, to penetrate the blood‐brain barrier and reach potentially therapeutic concentrations in the striatum and other brain regions (Forsberg et al. 2003). However, the clinical relevance of this is unclear.
Whether these differences translate into superior clinical efficacy for tolcapone is an important question. No head‐to‐head clinical trials have been conducted, but useful information can also be obtained from a properly conducted meta‐analysis of available randomized, double‐blind, placebo‐controlled trials for each of these drugs. The Cochrane Collaboration, which maintains the most extensive collection of quality, up‐to‐date meta‐analyses in the world, has carried out a meta‐analysis of both of these COMT inhibitors (Deane et al. 2004) using standard Cochrane methodology (Cochrane Handbook 2005). The primary purpose of that analysis was to assess the efficacy and safety of COMT inhibitors as a class rather than to compare one drug to the other.
In this article, the results of the Cochrane meta‐analysis of the primary efficacy outcome measures were used to specifically compare tolcapone with entacapone. In addition, further indirect evidence for a difference in efficacy was provided by two switch studies—a randomized, controlled trial examining a switch from entacapone to tolcapone (The Entacapone to Tolcapone Switch Study Investigators 2007) and a prospective case series examining the reverse (Onofrj et al. 2001). The results of these studies are summarized and related to the meta‐analytic findings. Finally, a brief history of the safety of tolcapone, some of which has been well publicized, is described herein.
Methods
Overview of the Cochrane Meta‐Analysis
The Cochrane review included all randomized trials that compared adjunctive use of tolcapone or entacapone therapy (vs. placebo) in patients having a clinical diagnosis of Parkinson's disease and who were experiencing long‐term motor complications such as dyskinesia or end‐of‐dose deterioration, or both. The primary efficacy measures in almost all of the studies were a change in ON time and OFF time; other efficacy outcomes examined included changes in United Parkinson's Disease Rating Scale (UPDRS) scores, reduction in levodopa dose, and withdrawals due to lack of efficacy.
Table 2 summarizes the standard Cochrane methodology that was used for the meta‐analysis (Cochrane Collaboration 2007; Cochrane Handbook 2005). The manufacturers of entacapone (Orion, Espoo, Finland) and tolcapone (Valeant Pharmaceuticals, Aliso Viejo, CA, USA) also provided additional assistance. Study quality was rated on the basis of numerous factors, including the method of randomization, the method of analysis, and the number of participants lost to follow‐up. A total of 14 studies in 4566 patients were included in the analysis (entacapone, 8; tolcapone, 6). Table 3 summarizes each of the trials included in the meta‐analysis (Adler et al. 1998; Baas et al. 1997; Brooks and Sagar 2003; Dupont et al. 1997; Fénelon et al. 2003; Im et al. 2002; Kurth et al. 1997; Myllyla et al. 1997; Myllyla et al. 2001; Parkinson Study Group 1997; Poewe et al. 2002; Rajput et al. 1997; Rinne et al. 1998; Ruottinen and Rinne 1996).
Table 2.
Cochrane meta‐analysis methods
| Inclusion criteria |
 All randomized, placebo‐controlled trials of adjunctive tolcapone or entacapone therapy All randomized, placebo‐controlled trials of adjunctive tolcapone or entacapone therapy |
 Trials enrolled patients having a clinical diagnosis of Parkinson's disease with long‐term motor complications such as dyskinesia and/or end‐of‐dose deterioration Trials enrolled patients having a clinical diagnosis of Parkinson's disease with long‐term motor complications such as dyskinesia and/or end‐of‐dose deterioration |
| Trial and data identification methods |
 Computerized searches of MEDLINE, EMBASE, the Cochrane Controlled Trials Register Computerized searches of MEDLINE, EMBASE, the Cochrane Controlled Trials Register |
 Hand searches of relevant neurology journals and the reference lists of located trials Hand searches of relevant neurology journals and the reference lists of located trials |
 Contacting manufacturers of entacapone (Orion) and tolcapone (Valeant) Contacting manufacturers of entacapone (Orion) and tolcapone (Valeant) |
| Outcomes of interest |
 ON time and OFF time ON time and OFF time |
 Changes in United Parkinson's Disease Rating Scale scores Changes in United Parkinson's Disease Rating Scale scores |
 Reduction in levodopa dose Reduction in levodopa dose |
 Frequency of adverse events Frequency of adverse events |
 All‐cause withdrawals and withdrawal due to lack of efficacy All‐cause withdrawals and withdrawal due to lack of efficacy |
| Data extraction |
 Independently, each author used a standardized form to record outcomes of interest from each trial Independently, each author used a standardized form to record outcomes of interest from each trial |
 Forms were checked for agreement/accuracy Forms were checked for agreement/accuracy |
 For continuous variables, the weighted mean difference across all the trials with each drug was calculated, and 95% confidence intervals were determined For continuous variables, the weighted mean difference across all the trials with each drug was calculated, and 95% confidence intervals were determined |
 A similar method was used for dichotomous variables (withdrawal, adverse events; Peto odds ratio) A similar method was used for dichotomous variables (withdrawal, adverse events; Peto odds ratio) |
From Higgins JPT, et al. (Cochrane Handbook, 2005).
Table 3.
Randomized, controlled trials of tolcapone and entacapone in the Cochrane meta‐analysis
| Trial | Duration | Treatment | Patient baseline characteristics | |||
|---|---|---|---|---|---|---|
| Age (y) | Disease duration (y) | Levodopa dose (mg/day) | ||||
| Entacapone trials* (n= 1560) | ||||||
| Brooks and Sagar 2003 | 24 wk | Placebo | n= 97 | 65.3 | 9.4 | 697.0 |
| 200 mg | n= 203 | |||||
| Fénelon et al. 2003 | 52 wk | Placebo | n= 63 | 64.2 | (Not available) | (Not available) |
| 200 mg | n= 99 | |||||
| Im et al. 2002 | 8 wk | Placebo | n= 99 | (Not available) | (Not available) | (Not available) |
| (entacapone dose not given) | n= 98 | |||||
| Myllyla et al. 2001 | 52 wk | Placebo | n= 108 | 62.2 | 6.1 | 634.0 |
| 200 mg | n= 218 | |||||
| Parkinson Study Group. 1997 | 24 wk | Placebo | n= 102 | 63.3 | 11.1 | 772.0 |
| 200 mg | n= 103 | |||||
| Poewe et al. 2002 | 24 wk | Placebo | n= 104 (88 fluct.) | 61.0 | 8.9 | 571.0 |
| 200 mg | n= 197 (172 fluct.) | |||||
| Rinne et al. 1998 | 24 wk | Placebo | n= 86 | 62.7 | 10.8 | 703.0 |
| 200 mg | n= 85 | |||||
| Ruottinen and Rinne 1996 ‡ | 8 wk | Placebo | n= 22 | 61.3 | 14.0 | 905.0 |
| 200 mg | n= 23 | |||||
| Tolcapone trials (n = 1006) | ||||||
| Adler et al. 1998 | 6 wk | Placebo | n= 72 | 62.0 | 10.5 | (Not available) |
| 100 mg t.i.d. | n= 69 | |||||
| 200 mg t.i.d. | n= 74 | |||||
| Baas et al. 1997 | 3 mo | Placebo | n= 58 | 63.0 | 9.8 | 668.0 |
| 100 mg t.i.d. | n= 60 | |||||
| 200 mg t.i.d. | n= 59 | |||||
| Dupont et al. 1997 ‡† | 6 wk | Placebo | n= 33 | 66.0 | (Not available) | 662.0 |
| 200 mg t.i.d. | n= 32 | |||||
| Kurth et al. 1997 † | 6 wk | Placebo | n= 42 | 64.5 | 9.2 | (Not available) |
| 200 mg t.i.d. | n= 40 | |||||
| Myllyla et al. 1997 † | 6 wk | Placebo | n= 42 | 63.0 | 11.0 | 734.0 |
| 200 mg t.i.d. | n= 38 | |||||
| Rajput et al. 1997 | 24 wk | Placebo | n= 66 | 64.0 | 10.9 | 867.4 |
| 100 mg t.i.d. | n= 69 | |||||
| 200 mg t.i.d. | n= 67 | |||||
*For all entacapone trials, dosing with either placebo or entacapone occurred with each dose of levodopa, up to 10 times daily.
†Trial also included groups that received nonapproved doses of tolcapone (50 or 400 mg t.i.d.).
‡Crossover study; all others shown used parallel‐group designs.
Entacapone Trials
The eight entacapone trials involved 1560 patients. The trials ranged in duration from 8–52 weeks. In all of the studies in which the dose was described, patients received entacapone 200 mg or placebo with each levodopa dose (up to 10 doses per day). One of the trials (Brooks and Sagar 2003) enrolled some patients who did not have motor fluctuations; for the purposes of the meta‐analysis, only data from the 260 patients who did have fluctuations were included. Only one investigation (Ruottinen and Rinne 1996) did not allow levodopa/carbidopa dose reduction.
Of the eight entacapone studies included in the meta‐analysis, four provided data for analysis of both ON time and OFF time (Brooks and Sagar 2003; Parkinson Study Group 1997; Poewe et al. 2002; Rinne et al. 1998). The published report by Poewe and colleagues (Poewe et al. 2002) provided sufficient data for the meta‐analysis. For the other three studies, the authors of the Cochrane meta‐analysis obtained additional data from the manufacturer. The total number of patients treated with entacapone in these four trials who were included in the analysis of ON or OFF time was 389 (n= 221 for placebo). All trials were 6 months in duration.
Tolcapone Trials
The six tolcapone trials involved 1006 patients, all with motor fluctuations. All studies were parallel‐group, randomized, placebo‐controlled investigations. Four of the six trials were 6‐week, short‐term investigations (Adler et al. 1998; Dupont et al. 1997; Kurth et al. 1997; Myllyla et al. 1997). One trial (Baas et al. 1997) was 12 weeks in duration, and the remaining trial (Rajput et al. 1997) had a defined primary end point at 3 months, although patients could opt to continue double‐blind treatment for up to a total of 12 months. Tolcapone dosages ranged between 50 mg and 400 mg t.i.d.; the most commonly examined doses were 100 mg and 200 mg t.i.d. Patients were instructed to take the test medication every 6 h while awake. Levodopa/carbidopa dose reduction was permitted in all six tolcapone trials.
Of the six tolcapone trials, four, Adler et al. 1998; Baas et al. 1997; Kurth et al. 1997; Myllyla et al. 1997) provided sufficient data for analysis of both ON time and OFF time. These trials involved a total of 333 patients treated with tolcapone (n= 339 for placebo). Participants received tolcapone 100 mg or 200 mg t.i.d. for either 6 weeks (Adler et al. 1998; Kurth et al. 1997; Myllyla et al. 1997) or 12 weeks (Baas et al. 1997).
The mean age, duration of disease, and baseline levodopa dose for subjects in the trials included in the ON and OFF time analyses were very similar between the entacapone and tolcapone trials (Table 3, shaded studies).
Assessments
In all of the studies included in the meta‐analysis, ON and OFF times were determined by patient‐completed diaries in which the patients rated their mobility at baseline, on designated days during the trial, and upon trial completion. Mobility was rated as ON, OFF, or asleep; in some trials, an intermediate option was possible. Ratings were usually done at 30‐min intervals throughout the daytime hours. Although changes in ON and OFF times were generally reciprocal, they do not exactly mirror each other because other categories, such as Intermediate or Dyskinetic, were used in some studies, and because the recording of time can be imperfect.
A meta‐analysis is more than just a pooling of data from multiple studies and involves a process that first selects only those studies sufficiently similar to include in the meta‐analysis and then evaluates the quality of each of these studies according to a variety of parameters. Each study is subsequently “weighted” based on its quality rating, allowing the final conclusion to be predominantly determined by the higher‐quality studies (Cochrane Handbook 2005).
Results
ON Time
Entacapone Versus Placebo
The weighted, baseline‐to‐end point placebo‐corrected mean increase in ON time for entacapone was 1.01 h (P < 0.00001 vs. placebo; Table 4).
Table 4.
Weighted* mean of baseline‐to‐end point placebo‐corrected mean differences in ON and OFF time for all studies in the meta‐analysis
| Drug | No. of studies | No. of patients | Weighted mean | ||
|---|---|---|---|---|---|
| Placebo | Drug | difference, h | 95% CI | ||
| ON Time | |||||
| Entacapone | 4 | 301 | 389 | 1.02 | 0.62–1.39 |
| Tolcapone† | 4 | 209 | 333 | 1.86 | 1.29–2.42 |
| OFF Time | |||||
| Entacapone | 4 | 221 | 389 | 0.68 | 0.22–1.13 |
| Tolcapone† | 4 | 209 | 333 | 1.60 | 1.10–2.10 |
*Weighted, in this context, refers to a meta‐analytic procedure that assigns a multiplier to each study based on its quality rating, such that the results from higher‐quality studies are accorded more weight in determining the overall mean.
†Includes 100 mg t.i.d. and 200 mg t.i.d. arms of the studies by Adler et al. (Adler et al. 1998) and Bass et al. (Baas et al. 1997).
The smallest placebo‐corrected mean difference in ON time for entacapone was 0.7 h (Parkinson Study Group 1997), and the greatest was 1.6 h.(Rinne et al. 1998). Interestingly, in two of the studies (Poewe et al. 2002; Brooks and Sagar 2003), the lower limit of the 95% confidence interval (CI) was <0, in a third it was just 0.02 (Parkinson Study Group 1997), and in only one (Rinne et al. 1998) was it clearly >0 (Fig. 2) (Deane et al. 2004).
Figure 2.
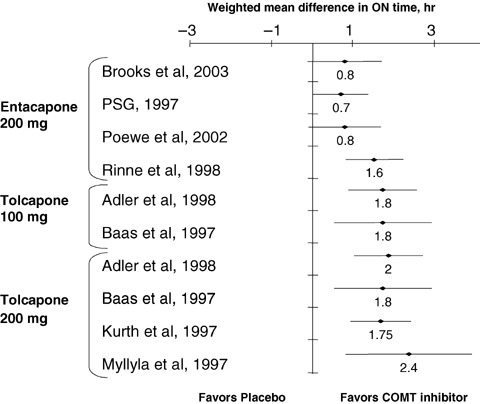
Baseline‐to‐end point placebo‐ corrected, weighted mean differences (± 95% CI) in ON time for entacapone and tolcapone (Adler et al. 1998; Baas et al. 1997; Brooks and Sagar 2003; Kurth et al. 1997; Myllyla et al. 1997; Parkinson Study Group, 1997; Poewe et al. 2002; Rinne et al. 1998). PSG, Parkinson Study Group. (Data from Deane KH (Deane et al. 2004)).
Tolcapone Versus Placebo
The weighted, baseline‐to‐end point placebo‐corrected mean difference in ON time for tolcapone was 1.86 h (P < 0.00001 vs. placebo; Table 4).
The smallest baseline‐to‐end point placebo‐corrected mean difference in ON time for tolcapone was 1.75 h (tolcapone 200 mg t.i.d.) (Kurth et al. 1997), and the greatest was 2.4 h (tolcapone 200 mg t.i.d.) (Myllyla et al. 1997). The lowest value of the lower limit of the 95% CI for the baseline‐to‐end point placebo‐corrected mean difference in ON time for tolcapone was 0.60 h (Fig. 2) (Deane et al. 2004).
Summary Comparison
Table 4 displays the final results for ON time, showing that the weighted mean difference in baseline‐to‐end point placebo‐corrected ON time was almost twice as much in the tolcapone‐treated patients as in the entacapone‐treated patients. Furthermore, the upper limit of the 95% CI for entacapone was just 0.1 h greater than the lower limit of the 95% CI for tolcapone, which means that there was practically no overlap at all in the distribution of means.
OFF Time
Entacapone Versus Placebo
The weighted, baseline‐to‐end point placebo‐corrected mean difference in OFF time for entacapone was 0.68 h (P= 0.004 vs. placebo; Table 4).
The smallest placebo‐corrected mean difference in OFF time for entacapone was 0.5 h (Brooks and Sagar 2003), and the greatest was 1.2 h (Rinne et al. 1998). Even more remarkable than the ON time data, in all four of these studies, the lower limit of the 95% CI was ≤0.02 h (Fig. 3) (Deane et al. 2004).
Figure 3.
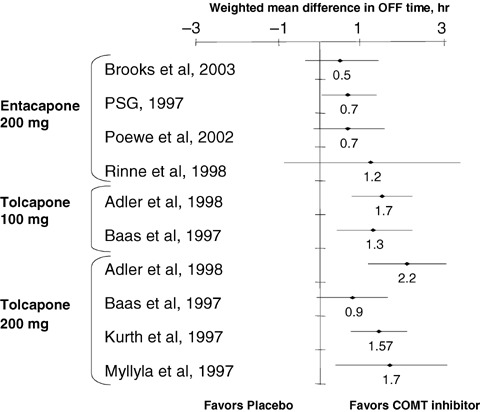
Baseline‐to‐end point placebo‐ corrected, weighted mean differences (± 95% CI) in OFF time for entacapone and tolcapone. *The large 95% CI reflects the few placebo subjects in this analysis (n= 5) (Adler et al. 1998; Baas et al. 1997; Brooks and Sagar 2003; Kurth et al. 1997; Myllyla et al. 1997; Parkinson Study Group, 1997; Poewe et al. 2002; Rinne et al. 1998). PSG, Parkinson Study Group. (Data from Deane KH (Deane et al. 2004)).
Tolcapone Versus Placebo
The weighted, baseline‐to‐end point placebo‐corrected mean difference in OFF time for tolcapone was 1.6 h (P < 0.0001 vs. placebo; Table 4). The smallest baseline‐to‐end point placebo‐corrected mean difference in OFF time for tolcapone was 0.9 h (tolcapone 100 mg t.i.d.) (Baas et al. 1997), and the greatest was 2.2 h (tolcapone 200 mg t.i.d.) (Adler et al. 1998). In only one of the six studies included in the meta‐analysis was the lower limit of the 95% CI for the baseline‐to‐end point placebo‐corrected mean difference in OFF time ≤ 0 h (Fig. 3).
Summary Comparison
Table 4 displays the final results for OFF time, showing that the weighted mean difference in baseline‐to‐end point placebo‐corrected OFF time was more than twice as much in the tolcapone‐treated as in the entacapone‐treated patients. Furthermore, the upper limit of the 95% CI for entacapone was just 0.03 h greater than the lower limit of the 95% CI for tolcapone, indicating virtually no overlap whatsoever in the distribution of means.
All‐Cause Withdrawal Rates
The clinical usefulness of a medication depends on both its efficacy and tolerability. The all‐cause discontinuation rate is, therefore, a good surrogate measure of effectiveness because it incorporates the patient's assessment of both efficacy and tolerability.
Five of the entacapone trials provided data concerning all‐cause discontinuation rates (Brooks and Sagar 2003; Myllyla et al. 2001; Parkinson Study Group 1997; Poewe et al. 2002; Rinne et al. 1998). In the individual studies, withdrawal rates in the entacapone group ranged from a low of 9.4% to a high of 42.6%. Overall, patients treated with entacapone were approximately 40% more likely to withdraw for any reason than those given placebo (Table 5) (Deane et al. 2004; Berlin et al. 1989).
Table 5.
Summary of all‐cause withdrawal rates, Peto odds ratios,* and 95% CIs in analyzable trials
| Drug | Number of studies | Withdrawals n/N (%) | Peto | ||
|---|---|---|---|---|---|
| Placebo | COMT | odds ratio | 95% CI | ||
| Entacapone | 5 | 67/441 | 152/693 | 1.40 | 1.02–1.93 |
| (15.2%) | (21.9%) | ||||
| Tolcapone | 2 | 8/105 | 10/175 | 0.75 | 0.09–2.74 |
| (7.62%) | (5.71%) | ||||
Data from Deane KH (Deane et al. 2004).
*A specific statistical method for obtaining odds ratios in meta‐analyses (Berlin et al. 1989).
Only two of the tolcapone trials provided high‐quality data concerning all‐cause discontinuation rates (Adler et al. 1998; Dupont et al. 1997). In the study conducted by Adler and colleagues (Adler et al. 1998) patients were given either tolcapone 100 mg or tolcapone 200 mg t.i.d. (vs. placebo) for 6 weeks; the study reported by Dupont and colleagues (Dupont et al. 1997) was limited to patients receiving tolcapone 200 mg t.i.d. Among patients treated with the 100 mg dose in the Adler study (Adler et al. 1998), just 2.89% (2 of 69) withdrew prematurely compared with 8.33% (6 of 72) in the placebo group. In the investigation from Dupont and colleagues (Dupont et al. 1997), 12.50% (4 of 32) patients given tolcapone 200 mg t.i.d. for up to 6 weeks withdrew prematurely, and 6.06% (2 of 33) withdrew in the placebo group. Overall, tolcapone‐treated patients were less likely to withdraw from these studies than were placebo‐treated patients (Table 5). However, because of the smaller number of tolcapone treated patients included in the analysis of all‐cause discontinuations, the 95% CI for the tolcapone value was much larger, extending above the upper limit of the entacapone 95% CI.
The Cochrane meta‐analysis suggests that the lower rate of all‐cause discontinuation in the tolcapone group compared with the entacapone group was attributable, at least in part, to lower rates of withdrawal due to an adverse event with tolcapone. Compared with withdrawals in placebo‐treated patients, withdrawals because of adverse events occurred at a statistically higher rate in entacapone‐treated patients (P= 0.02, vs. placebo) but not in tolcapone‐treated patients (P= 0.2 vs. placebo). These data suggest that, for patients receiving tolcapone, adverse events were not particularly bothersome or, alternatively, that the clinical benefits outweighed the discomfort.
Changes in liver function parameters had a minimal impact on withdrawal in all trials of both entacapone and tolcapone. Clinically significant elevations of liver enzymes (defined as ≥ 3 times the upper reference limit) were found in two patients in the study of entacapone reported by Myllyla and coworkers (Myllyla et al. 2001), but neither incidence was considered to be related to entacapone treatment. In the study from Poewe and associates (Poewe et al. 2002), two patients (one patient each in the entacapone and placebo groups) exhibited clinically significant transaminase elevations that were limited to a single study visit and did not result in study discontinuation. Similarly, clinically significant transaminase increases occurred in only two of the included tolcapone trials. Rajput and colleagues (Rajput et al. 1997) observed transaminase elevations in three patients at 100 mg t.i.d. and two patients at 200 mg t.i.d., one of whom (200 mg group) was asymptomatic but consequently discontinued from the trial (no follow‐up information was given). In the study from Baas and associates (Baas et al. 1997), three patients (one patient at 100 mg t.i.d. and two patients at 200 mg t.i.d.) had persistently elevated transaminase levels. One of these patients (200 mg group) withdrew on study day 113 (transaminase levels were normal at follow‐up on day 169). These data are consistent with the recently published safety data from a large European randomized, controlled trial of tolcapone 100 mg t.i.d. in early‐stage Parkinson's disease patients (n= 677) that was abruptly discontinued in 1998 upon withdrawal of tolcapone from the European market (Lees et al. 2006). In this trial, elevations ≥3 times the upper limit of normal occurred in just 1.2% and 1.8% of placebo and tolcapone patients, respectively (P= 0.5).
Switch Studies
The results of two switch studies are also important to consider in assessing the differential efficacy of entacapone and tolcapone. One published randomized, prospective trial evaluated the switch from entacapone to tolcapone (The Entacapone to Tolcapone Switch Study Investigators 2007), and one uncontrolled case series evaluated the switch from tolcapone to entacapone (Onofrj et al. 2001). The case series was based on observations in 40 Parkinson's disease patients with fluctuations who were discontinued from tolcapone after 3 to 7 months when tolcapone was withdrawn from the market in Europe and who were switched to entacapone therapy. During tolcapone therapy, improvements in ON time (15% increase) and OFF time (16% decrease) were approximately twice the magnitude of those seen later with entacapone (8% increase and 7% decrease, respectively).
The controlled study of the switch from entacapone to tolcapone (The Entacapone to Tolcapone Switch Study Investigators 2007) was conducted at the request of the European Agency for the Evaluation of Medicinal Products to determine whether reauthorization of tolcapone would be justified. This study involved Parkinson's disease patients taking levodopa who were experiencing poor clinical response to adjunctive entacapone (≥3 h/day OFF time). After a run‐in period of up to 2 weeks, during which the levodopa dose was optimized, patients were randomly assigned to entacapone 200 mg given with each levodopa dose or tolcapone 100 mg t.i.d. (n= 75 for both groups) for 3 weeks of double‐blind treatment.
The proportion of patients achieving the defined primary end point (ON time increased by ≥1 h/day) was not significantly different between the entacapone group (43%) and the tolcapone group (53%; P= 0.19; intent‐to‐treat population). However, the results for several exploratory variables strongly favored tolcapone. In the intent‐to‐treat analysis, the mean increase in ON time among patients switched to tolcapone was 1.34 h, whereas it was less than half that amount, 0.65 h, for those who remained on entacapone. In addition, the proportion of patients exhibiting an increase in ON time that was ≥3 h/day was 25% in the tolcapone group versus 13% in the entacapone group. With restriction of the exploratory analysis to just the perprotocol population, these differences were even greater (1.63 vs. 0.77 h for the mean increase in ON time, and 29% vs. 12% for the increase in ON time ≥3 h/day, for tolcapone vs. entacapone).
Safety of Tolcapone
Within 1 year of the initial launch of tolcapone in Europe, four cases of severe hepatocellular injury, including three deaths, occurred. This led to the removal of tolcapone from the European market in 1998, as well as to numerous changes to the U.S. drug label, including a black box warning, a requirement for written informed consent, and the recommendation of a strict hepatic enzyme monitoring program.
In 2000, a panel of neurologists convened to reconsider the safety profile of tolcapone (Olanow and Tasmar Advisory Panel 2000). A review of the four cases found that liver monitoring had not been performed or had not followed the schedule required in the original product labeling. Moreover, in two of the cases, tolcapone therapy was continued after development of clinical signs of liver dysfunction. The panel also reviewed the safety data from controlled clinical trials of tolcapone in more than 4000 patients. When liver monitoring was conducted as per the protocol, no cases of serious liver dysfunction occurred (Olanow and Tasmar Advisory Panel 2000). In these trials, elevations of hepatic enzymes to >3 times the upper limit of normal were observed in approximately 1% of patients receiving 100 mg t.i.d. and 3% receiving 200 mg t.i.d., resulting in drug discontinuation in 0.3% and 0.7% of patients, respectively (Tasmar prescribing information 2006). More recently, postmarketing surveillance reports and other liver safety‐monitoring programs showed that serious hepatotoxicity with tolcapone was rare. Between 1999 and 2004 in the United States, after introduction of the FDA‐mandated liver‐monitoring guidelines, an estimated 60,000 patient‐years of tolcapone exposure was accrued and resulted in no hepatotoxicity‐related deaths attributable to the drug and only one case of serious liver injury (Keating and Lyseng‐Williamson 2005; Watts and Kricorian 2005).
Largely as a result of these data, the FDA determined that the liver‐monitoring schedule for tolcapone could be revised without sacrificing patient safety. The modified monitoring recommendations took effect from February 2006.
Discussion
Available evidence indicates that tolcapone is a more efficacious adjunctive therapy for wearing‐off effects than entacapone. The Cochrane meta‐analysis of randomized, double‐blind, placebo‐controlled trials of tolcapone and entacapone showed that tolcapone, when given in the recommended dose range, improved OFF and ON times to a greater extent than entacapone. With respect to both reduction in OFF time and increase in ON time, the tolcapone group had, on average, a 2‐fold larger improvement than the entacapone group, and there was almost no overlap in the statistical distribution of the means. In the randomized, controlled switch study, the mean increase in ON time for patients switched to tolcapone from entacapone was 1.63 h, almost identical to the placebo‐corrected increase in ON time found in the meta‐analysis (1.60 h). These efficacy data support the consistent recommendation in recently developed Parkinson's disease clinical practice guidelines that tolcapone may be considered an effective alternative to entacapone in patients for whom the latter agent is not effective or is not tolerated (National Institute for Health and Clinical Excellence, 2006; Pahwa et al. 2006). These results are also consistent with findings from two long‐term open‐label extension studies of tolcapone and entacapone, which found that tolcapone was more effective than entacapone in lowering scores on the UPDRS Motor and Complication subscales, duration of OFF time, and levodopa doses for up to 3 years; both treatments showed similar tolerability (Factor et al. 2001).
Patients treated with tolcapone in the recommended dose range also showed greater medication persistence, relative to placebo patients, than did the patients treated with entacapone. The data reviewed here show that patients receiving tolcapone are less likely to withdraw than were patients receiving placebo, whereas the entacapone‐treated patients withdrew at a rate 40% higher than placebo‐treated patients. Although this observation is based on a relatively smaller population of tolcapone‐ than entacapone‐treated patients, and hence a broad overlap of the confidence intervals for the Peto odds ratio for study discontinuation, it nevertheless indicates that tolerability concerns do not compromise the potential benefits of greater efficacy for tolcapone. It is also notable that the elevated rate of discontinuation for entacapone seen in the meta‐analysis was entirely consistent with the results shown from the retrospective chart analysis published by Parashos and colleagues (Parashos et al. 2004) of Parkinson's disease patients prescribed entacapone in routine clinical practice. In that study, 124 of 222 patients (55.9%) discontinued entacapone during a 3‐year follow‐up period, the majority of them (approximately 46%) within the first year.
It should be noted that a possible explanation for the apparent greater efficacy of tolcapone versus entacapone may be related to the use of the clinically recommended doses of the two COMT inhibitors. The differences in observed efficacy may be related to tolcapone having greater potency than entacapone at similar doses. This could cause a perception of both better efficacy and increased safety concerns. Indeed, at least one publication asserts that no firm evidence exists that tolcapone is effective in patients who do not respond to entacapone (Prescrire Editorial Staff 2006).
In conclusion, the results of the meta‐analysis of placebo‐controlled clinical trials of entacapone and tolcapone, the placebo‐controlled entacapone‐to‐tolcapone switch study, and the several uncontrolled switch studies are all consistent with one another and indicate a favorable efficacy profile for tolcapone. This body of evidence suggests that patients experiencing Parkinson's disease with motor fluctuations who fail to benefit from an adequate trial of adjunctive entacapone, or who are no longer obtaining much benefit from its use, warrant a therapeutic trial of tolcapone with recommended hepatic monitoring.
Conflict of Interest
A.J. Lees has received honoraria from Roche, Valeant, Orion and Novartis for lectures and has sat on tolcapone and entcapone advisory boards for Roche, Valeant and Orion. A.J. Lees has no shares or direct financial interests in either entacapone or tolcapone.
Acknowledgment
The author wishes to acknowledge John Kincaid, M.D., and Bill Kadish, M.D., for their editorial assistance during the preparation of this article.
References
- Adler CH, Singer C, O'Brien C, Hauser RA, Lew MF, Marek KL, Dorflinger E, Pedder S, Deptula D, Yoo K (1998) Randomized, placebo‐controlled study of tolcapone in patients with fluctuating Parkinson disease treated with levodopa‐carbidopa. Arch Neurol 55:1089–1095. [DOI] [PubMed] [Google Scholar]
- Baas H, Beiske AG, Ghika J, Jackson M, Oertel WH, Poewe W, Ransmayr G (1997) Catechol‐O‐methyltransferase inhibition with tolcapone reduces the "wearing off" phenomenon and levodopa requirements in fluctuating Parkinsonian patients. J Neurol Neurosurg Psychiatry 63:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin JA, Laird NM, Sacks HS, Chalmers TC (1989) A comparison of statistical methods for combining event rates from clinical trials. Stat Med 8:141–151. [DOI] [PubMed] [Google Scholar]
- Borges N (2005) Tolcapone in Parkinson's disease: Liver toxicity and clinical efficacy. Expert Opin Drug Saf 4:69–73. [DOI] [PubMed] [Google Scholar]
- Brooks DJ, Sagar H (2003) Entacapone is beneficial in both fluctuating and non‐fluctuating patients with Parkinson's disease: A randomised, placebo controlled, double blind, six month study. J Neurol Neurosurg Psychiatry 74:1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane Collaboration (2007) http://www.cochrane.org. Accessed: Aug. 10, 2007.
- Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 (2005) In: The Cochrane library. Chichester , UK , John Wiley & Sons. [Google Scholar]
- Comtan® (entacapone) tablets [package insert] (2000) East Hanover , NJ , USA , Novartis Pharmaceuticals Corporation .
- Deane KHO, Spieker S, Clarke CE (2004) Catechol‐O‐methyltransferase inhibitors for levodopa‐induced complications in Parkinson's disease. Cochrane Database Syst Rev Issue 4, Art. No.: CD004554. DOI: DOI: 10.1002/14651858.CD004554.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont E, Burgunder JM, Findley LJ, Olsson JE, Dorflinger E (1997) Tolcapone added to levodopa in stable parkinsonian patients: A double‐blind placebo‐controlled study. Tolcapone in Parkinson's disease study group II (TIPS II). Mov Disord 12:928–934. [DOI] [PubMed] [Google Scholar]
- Factor SA, Molho ES, Feustel PJ, Brown DL, Evans SM (2001) Long‐term comparative experience with tolcapone and entacapone in advanced Parkinson's disease. Clin Neuropharmacol 24:295–299. [DOI] [PubMed] [Google Scholar]
- Fénelon G, Giménez‐Roldán S, Montastruc JL, Bermejo F, Durif F, Bourdeix I, Péré JJ, Galiano L, Schadrack J (2003) Efficacy and tolerability of entacapone in patients with Parkinson's disease treated with levodopa plus a dopamine agonist and experiencing wearing‐off motor fluctuations. A randomized, double‐blind, multicentre study. J Neural Transm 110:239–251. [DOI] [PubMed] [Google Scholar]
- Forsberg M, Lehtonen M, Heikkinen M, Savolainen J, Jarvinen T, Mannisto PT (2003) Pharmacokinetics and pharmacodynamics of entacapone and tolcapone after acute and repeated administration: A comparative study in the rat. J Pharmacol Exp Ther 304:498–506. [DOI] [PubMed] [Google Scholar]
- Gordin A, Kaakkola S, Teravainen H (2004) Clinical advantages of COMT inhibition with entacapone – a review. J Neural Transm 111:1343–1363. [DOI] [PubMed] [Google Scholar]
- Im JH, Jeon BS, Lee MS, Lee WY, Kim JW, Lee MC (2002) A multicentre, double‐blind, placebo‐controlled study to assess efficacy and safety of entacapone in Korean patients with Parkinson's disease experiencing end‐of‐dose deterioration [abstract P83]. Mov Disord 17:S40. [Google Scholar]
- Jorga K, Fotteler B, Sedek G, Nielsen T, Aitken J (1998a) The effect of tolcapone on levodopa pharmacokinetics is independent of levodopa/carbidopa formulation. J Neurol 245:223–230. [DOI] [PubMed] [Google Scholar]
- Jorga KM, Fotteler B, Heizmann P, Zurcher G (1998b) Pharmacokinetics and pharmacodynamics after oral and intravenous administration of tolcapone, a novel adjunct to Parkinson's disease therapy. Eur J Clin Pharmacol 54:443–447. [DOI] [PubMed] [Google Scholar]
- Kaakkola S (2000) Clinical pharmacology, therapeutic use and potential of COMT inhibitors in Parkinson's disease. Drugs 59:1233–1250. [DOI] [PubMed] [Google Scholar]
- Keating GM, Lyseng‐Williamson KA (2005) Tolcapone: A review of its use in the management of Parkinson's disease. CNS Drugs 19:165–184. [DOI] [PubMed] [Google Scholar]
- Kurth MC, Adler CH, Hilaire MS, Singer C, Waters C, LeWitt P, Chernik DA, Dorflinger EE, Yoo K (1997) Tolcapone improves motor function and reduces levodopa requirement in patients with Parkinson's disease experiencing motor fluctuations: A multicenter, double‐blind, randomized, placebo‐controlled trial. Neurology 48:81–87. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Ratziu V, Tolosa E, Oertel WH (2007) Safety and tolerability of adjunctive tolcapone therapy in early Parkinson's disease patients. J Neurol Neurosurg Psychiatry 78:944–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew MF, Kricorian G (2007) Results from a 2‐year centralized tolcapone liver enzyme monitoring program. Clin Neuropharmacol 30:281–286. [DOI] [PubMed] [Google Scholar]
- Myllyla VV, Jackson M, Larsen JP, Baas H (1997) Efficacy and safety of tolcapone in levodopa‐treated Parkinson's disease patients with "wearing‐off" phenomenon: A multicentre, double‐blind, randomised, placebo‐controlled trial. Eur J Neurol 4:333–341. [Google Scholar]
- Myllyla VV, Kultalahti ER, Haapaniemi H, Leinonen M (2001) Twelve‐month safety of entacapone in patients with Parkinson's disease. Eur J Neurol 8:53–60. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence (2006) Parkinson's disease: Diagnosis and management in primary and secondary care In: NICE clinical guideline 35. London , UK : National Collaborating Centre for Chronic Conditions. [Google Scholar]
- Olanow CW, Tasmar Advisory Panel (2000) Tolcapone and hepatotoxic effects. Arch Neurol 57:263–267. [DOI] [PubMed] [Google Scholar]
- Onofrj M, Thomas A, Iacono D, Di Iorio A, Bonanni L (2001) Switch‐over from tolcapone to entacapone in severe Parkinson's disease patients. Eur Neurol 46:11–16. [DOI] [PubMed] [Google Scholar]
- Pahwa R, Factor SA, Lyons KE, Ondo WG, Gronseth G, Bronte‐Stewart H, Hallett M, Miyasaki J, Stevens J, Weiner WJ (2006) Practice parameter: Treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence‐based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 66:983–995. [DOI] [PubMed] [Google Scholar]
- Parashos SA, Wielinski CL, Kern JA (2004) Frequency, reasons, and risk factors of entacapone discontinuation in Parkinson disease. Clin Neuropharmacol 27:119–123. [DOI] [PubMed] [Google Scholar]
- Parkinson Study Group (1997) Entacapone improves motor fluctuations in levodopa‐treated Parkinson's disease patients. Ann Neurol 42:747–755. [DOI] [PubMed] [Google Scholar]
- Poewe WH, Deuschl G, Gordin A, Kultalahti ER, Leinonen M (2002) Efficacy and safety of entacapone in Parkinson's disease patients with suboptimal levodopa response: A 6‐month randomized placebo‐controlled double‐blind study in Germany and Austria (Celomen study). Acta Neurol Scand 105:245–255. [DOI] [PubMed] [Google Scholar]
- Prescrire Editorial Staff (2006) Tolcapone: New drug. In Parkinson's disease: Unacceptable risk of severe hepatitis. Prescrire Int 15:54–57. [PubMed] [Google Scholar]
- Rajput AH, Martin W, Saint‐Hilaire MH, Dorflinger E, Pedder S (1997) Tolcapone improves motor function in parkinsonian patients with the "wearing‐off" phenomenon: A double‐blind, placebo‐controlled, multicenter trial. Neurology 49:1066–1071. [DOI] [PubMed] [Google Scholar]
- Rinne UK, Larsen JP, Siden A, Worm‐Petersen J (1998) Entacapone enhances the response to levodopa in parkinsonian patients with motor fluctuations. Neurology 51:1309–1314. [DOI] [PubMed] [Google Scholar]
- Ruottinen HM, Rinne UK (1996) A double‐blind pharmacokinetic and clinical dose‐response study of entacapone as an adjuvant to levodopa therapy in advanced Parkinson's disease. Clin Neuropharmacol 19:283–296. [DOI] [PubMed] [Google Scholar]
- Tasmar® (tolcapone) Prescribing Information (2005) In: Physicians' desk reference. Montvale , NJ : Thompson PDR, 59th ed. [Google Scholar]
- Tasmar® (tolcapone) Prescribing Information (2006) In: Physicians' desk reference. Montvale , NJ : Thompson PDR, 60th ed. [Google Scholar]
- Tasmar® (tolcapone) tablets [package insert] (2006) Aliso Viejo , CA , USA , Valeant Pharmaceuticals International .
- Tasmar® Summary of Product Characteristics (2006) http://www.emea.eu.int/humandocs/PDFs/EPAR/tasmar/H‐132‐PI‐en.pdf. Accessed: Oct. 10, 2007.
- The Entacapone to Tolcapone Switch Study Investigators (2007) Entacapone to tolcapone switch: Multicenter double‐blind, randomized, active‐controlled trial in advanced Parkinson's disease. Mov Disord 22:14–19. [DOI] [PubMed] [Google Scholar]
- Watts R, Kricorian G (2005) Evaluation of liver‐related adverse events with tolcapone: A review of 7 years of worldwide safety data [abstract P400]. Mov Disord 20 Suppl 10:S118. [Google Scholar]


