Abstract
Background
The shift toward value-based care in the United States emphasizes the role of quality measures in payment models. Many diseases, such as prostate cancer, have a proliferation of quality measures, resulting in resource burden and physician burnout. This study aimed to identify and summarize proposed prostate cancer quality measures and describe their frequency and use in peer-reviewed literature.
Methods
The PubMed database was used to identify quality measures relevant to prostate cancer care, and included articles in English through April 2018. A gray literature search for other documents was also conducted. After the selection process of the pertinent articles, measure characteristics were abstracted, and uses were summarized for the 10 most frequently utilized measures in the literature.
Results
A total of 26 articles were identified for review. Of the 71 proposed prostate cancer quality measures, only 47 were used, and less than 10% of these were endorsed by the National Quality Forum. Process measures were most frequently reported (84.5%). Only 6 outcome measures (8.5%) were proposed—none of which were among the most frequently utilized.
Conclusion
Although a high number of proposed prostate cancer quality measures are reported in the literature, few were assessed, and the majority of these were non-endorsed process measures. Process measures were most commonly assessed; outcome measures were rarely evaluated. In a step to close the quality chasm, a “top 5” core set of quality measures for prostate cancer care, including structure, process, and outcomes measures, is suggested. Future studies should consider this comprehensive set of quality measures.
Variations in prostate cancer care are well documented.1 Treatment varies by vulnerable populations, geographical regions, hospitals, and individual clinicians.2 Quality metrics address concerns about treatment and outcome variations and include measures on the structures, processes, and outcomes of care to address the Donabedian framework of quality measurement.3 Many prostate cancer quality measures have been proposed over the past decade, including an early, comprehensive set of candidate measures for early-stage prostate cancer developed by RAND.4
The number of quality measures proposed, endorsed, and/or directed to health care providers in the United States has increased rapidly in the past decade.5–7 For prostate cancer care, several quality measures are either used or endorsed by regulatory agencies, such as the Centers for Medicare & Medicaid Services and the Quality Oncology Practice Initiative.8–10 As the number of proposed quality measures continues to grow, care communities have difficulty knowing which measures to focus on and often monitor and report on many quality measures, causing a substantial burden, which in turn may increase clinician burnout.11,12 Often measures are developed and proposed based on what data are available rather than their association with actual “quality” of care. Furthermore, measures are often slightly modified to suit specific organizational needs, which limits the ability to compare results across health care settings. This suggests a need to identify a set of core quality measures for focused, comparable, and comprehensive reporting.
As the health care system in the United States transforms to a value-based care system, organizational focus is on improving the quality and efficiency of health care delivery. Quality measures are crucial to improving efficiency because the delivery system cannot improve what cannot be measured.13 Appropriate quality measurement can contribute evidence to understand and prevent the overuse, misuse, and underuse of health care resources. This shift to value-based care also emphasizes the need for a comprehensive and parsimonious set of quality measures that should address all the steps of health care, from structure through process and, in the end, outcomes. In fact, according to Donabedian’s health care quality model, improvements in the structure of care should lead to improvements in clinical processes that should in turn, in the end, improve patient-oriented outcomes.3,14 Here, we focused on prostate cancer, which is the most common malignant cancer diagnosis and second leading cause of death in men.15 The past 20 years witnessed a more than 50% decrease in mortality and a steep increase (up to 80%) in survival for prostate cancer because of better and earlier diagnosis and treatment.16 It is hence of paramount importance to have a core set of indicators that can appropriately measure structure, processes, and outcomes of care in this particular population.
Given the aforementioned reasons, this study focused on identifying all prostate cancer quality metrics proposed by the scientific community or measurement initiatives and determining their utilization for quality improvement and scientific research purposes. This project had three goals: (1) develop a comprehensive list of all quality measures available to assess prostate cancer care, classified according to Donabedian’s model of structure, process, and outcome measures; (2) identify the most widely used prostate quality measures reported in the literature; and (3) understand how these measures were used for actual quality improvement vs. research purposes.
METHODS
Data Sources
A search of all the possible literature was conducted in February 2017 through the MEDLINE database, using the following search terms combined into a search string: ((Prostate cancer OR Prostate OR tumor OR tumors OR tumour)) AND (“Quality metrics” OR “Quality measures” OR “Quality care”). The search string was built up combining the terms related to prostate cancer and quality indicators, using PubMed free-text terms and MeSH terms, and combining them using Boolean operators to optimize specificity. A follow-up search was carried out on April 30, 2018.
Inclusion and Exclusion Criteria
All published articles in English were included. No selection based on type of publication was adopted, and there was no time period restriction. The last search date was April 30, 2018. Any type of primary research article or quality improvement report was included in the selection process and in the final assessment. Other types of primary articles (for example, letter to the editor, commentaries) or secondary/review articles were not included. Reference lists of selected reviews, original articles, and textbooks were scanned to find additional articles. A gray literature search for other documents and hand searches of journals, professional organizations’ websites, and guideline clearinghouses was carried out. Snowball technique was applied to the search strategy.17
Data Selection
Two authors [D.G., R.D.] independently screened all identified articles by scanning abstracts or portions of the text to determine if they met the inclusion criteria. Any disagreements were resolved through discussion and/or reaching consensus with the aid of a third reviewer [T.H-B.] who decided for the final adjudication.
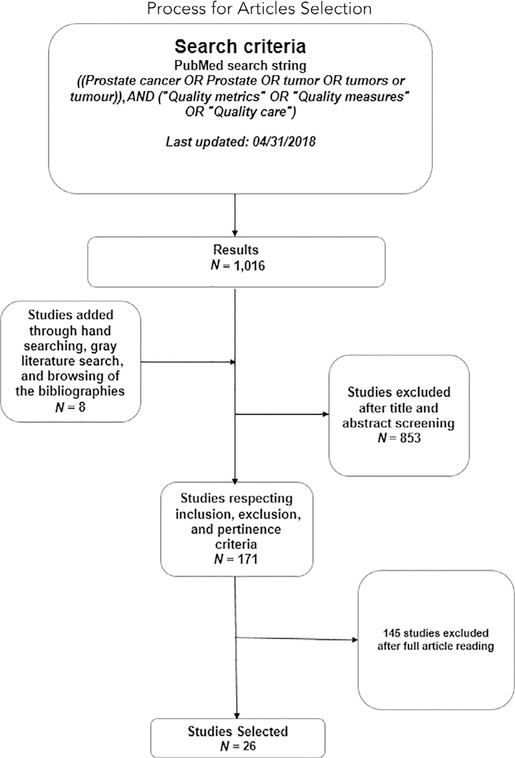
In the first screening stage, only the articles related to quality of care for prostate cancer were included. All other articles that discussed the quality of care for the diseases other than prostate cancer care were excluded. In the final screening stage, only those articles that assessed quality measures/metrics related to prostate cancer care were selected. More precisely, only the articles that assessed one of the proposed quality measures/metrics for research and/or for actual quality improvement with a particular sample of patients were considered for the final sample. This was carried out with a thorough review of the abstracts and/or the full articles. Figure 1 shows the flowchart for the article inclusion/exclusion and selection process.
Figure 1:
This flow diagram provides the phases of article identification and selection. From the 1,016 articles that were initially found through the search, 26 studies were identified after a two-step selection screening and were deemed eligible for inclusion. Prepared in accordance with Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012.
Data Extraction
Three authors [D.G., R.D., T.H-B.] independently extracted the data from included articles. Key information was gathered systematically using a standardized form to capture descriptions of the data sources and institutions that used or proposed the quality measure. The same team also adjudicated categorization of each measure to one of the three constructs of Donabedian’s model of “structure,” “process,” and “outcome” measures/concepts.3 The 71 proposed prostate cancer quality measurements were ranked based on (1) how many studies assessed the measures in a patient population and (2) the sample sizes in which they were implemented and/or tested. Two authors [D.G., R.D.] independently classified studies as “quality improvement” if it was specified in their purpose that they directly targeted the quality of care enhancement. On the other hand, “research studies” were those that were not clearly associated with direct care quality improvement. Each measure was compared to a list we constructed of National Quality Forum (NQF)–endorsed measures from the NQF online database (accessed December 10, 2016).7 Any disagreement by the two authors was resolved through discussion.
Outcomes of Interest
Finally, we created a general table of the 10 most cited and used measures/concepts, and also a “top 5” table. The “top 5” measures were selected via research team consensus. This selection took into account several factors: (1) a set of measures that contained all three constructs of the Donabedian model, (2) measures that provide the most insight into high-quality health care delivery, and (3) measures that were frequently reported in the literature.
RESULTS
Search Results
The initial search yielded 1,016 articles. Hand searching and a gray literature search added 8 pertinent articles. The first selection was carried out through titles and abstract screening, and 171 articles were eligible for reading in extenso. After this second step, 26 articles18–43 were eligible for data extraction.
Study and Quality Measures Characteristics
Appendix 1 (available in online article) summarizes the descriptive characteristics of the prostate cancer care quality measures assessed. A total of 71 proposed quality measures were identified from these articles and/or from websites of the proposing institutions/initiatives. Process measures accounted for 84.5% of all the measures proposed. Only 6 outcome measures and 5 structure measures were proposed, each contributing less than 10% of the total measures assessed. Of the 71 quality measures identified, only 7 (avoidance of overuse measure—bone scan for staging low-risk patients, treatment summary communication; radical prostatectomy pathology reporting, androgen deprivation therapy (ADT)/adjuvant hormonal therapy for high-risk prostate cancer patients, following up with the patient at least twice during the first year after treatment, constipation assessed at time of narcotic prescription or following visit, and patient emotional well-being assessed by the second office visit) were endorsed by NQF.
RAND was the leading source for proposed prostate cancer quality measures with 23 (32.4% of all measures assessed). Other proposing institutions/initiatives were the Quality Ontology Practice Initiative under the American Society of Clinical Oncology, the American Urological Association Quality Registry, the College of American Pathologists, the Physician Consortium for Performance Improvement, the American Medical Association, and the Physician Quality Reporting Initiative.
Three of the 5 structure measures were used in the literature. One (proportion of patients treated by a high-volume provider) appeared in six studies, another (board certification of urologists and radiation oncologists) was used in two studies, and the third (availability of radiation oncology facilities and psychological counseling), appeared in only one study (Table 1a). Of the 38 process measures that were used in the literature, 15 (39.5%) appeared in only one study each (Table 1b). All 6 outcome measures identified in the literature were used exclusively in scientific studies; however, only 2 of those appeared in more than one study—patient’s assessment of urinary, sexual, and bowel functioning after treatment, and patient’s satisfaction with treatment choice, continence, and potency were each used in three studies as quality measures (Table 1c).
Table 1a.
Prostate Cancer Structure Measures Used in the Literature*
| Measures | Used By | Data Source | Type of Institution That Performed the Study |
|---|---|---|---|
| The proportion of patients treated by a high-volume (upper tertile) provider (surgeon or radiation oncologist) | Shroeck FR, et al. (2014)33 | Survey | Academic, nonprofit, for-profit |
| Herrel LA, et al. (2017)23 | Administrative | Academic, nonprofit, for-profit | |
| Schroeck FR, et al. (2015)32 | EHRs | Academic, nonprofit, for-profit | |
| Schroeck FR, et al. (2014 April)34 | EHRs | Academic, nonprofit, for-profit | |
| Schroeck FR, et al. (2014 Nov)35 | EHRs | Academic, nonprofit, for-profit | |
| Miller DC, et al. (2007)28 | EHRs | Teaching, nonprofit | |
| Availability of radiation oncology facilities and psychological counseling for patients | Miller DC, et al. (2007)28 | EHRs | Teaching, nonprofit |
| Board certification of urologists and radiation oncologists | Bekelman JE, et al. (2007)18 | EHRs and Administrative | Teaching, for-profit, nonprofit |
| Miller DC, et al. (2007)28 | EHRs | Teaching, nonprofit |
References can be found at the end of this article.
EHR, electronic health record.
Table 1b.
Prostate Cancer Process Measures Used in the Literature
| Measures | Studies (N) | Data Source | Type of Institution That Performed the Study |
|---|---|---|---|
| Androgen deprivation therapy (ADT)/adjuvant hormonal therapy for high-risk prostate cancer patients | 15 | Survey, EHRs, Administrative | Academic, nonprofit, for-profit, federal |
| Clinical T stage documented | 10 | Survey, EHRs, Administrative | Academic, nonprofit, for-profit |
| Avoidance of overuse measure—bone scan for staging low-risk patients | 10 | Survey, EHRs, Administrative | Academic, for-profit, federal |
| PSA level documented | 9 | EHRs, Survey, Administrative | Academic, nonprofit, for-profit |
| Gleason score reported to patient | 9 | Survey, EHRs, Administrative | Academic, for-profit, federal |
| Treatment options counseling (multidisciplinary) | 9 | Survey, EHRs, Administrative | Academic, for-profit, nonprofit |
| Following up with the patient at least twice during the first year after treatment | 7 | Survey, EHRs, Administrative | Academic, for-profit, nonprofit |
| Comorbidity assessment | 6 | Survey, EHRs, Administrative | Academic, for-profit |
| Radical prostatectomy pathology reporting | 4 | Survey, EHRs, Administrative | Academic, for-profit, nonprofit |
| Assessing the stage of the disease before the treatment/pretreatment clinical staging with DRE, total PSA, Gleason grade | 3 | Survey, EHRs, Administrative | Academic, for-profit, federal (VA) |
| Assessment of the family history of prostate cancer | 3 | Survey, EHRs | Teaching, nonprofit |
| Bone scan done in high-risk patients | 3 | EHRs, Administrative | Academic, nonprofit, for-profit |
| Avoidance of overuse measure—CT scan for staging low-risk patients | 3 | Survey, EHRs, Administrative | Academic, nonprofit, for profit |
| Central axis doses of at least 75 Gy for radiotherapy | 3 | EHRs, Administrative | Federal, teaching, nonprofit |
| DRE | 2 | EHRs | Academic, nonprofit, for-profit |
| CT scan done in high-risk patients | 2 | EHRs, Administrative | Academic, nonprofit, for-profit |
| A clear description of the risk of treatment complications | 2 | Survey, EHRs, Administrative | Academic, nonprofit, for profit |
| Patient’s assessment of urinary, sexual, and bowel functioning before treatment | 2 | EHRs | Academic, nonprofit, for-profit |
| Conformal radiation utilized for patients receiving XRT | 2 | EHRs | Academic, nonprofit, for-profit |
| Immobilizing the patient during treatment while protecting rectal mucosa | 2 | EHRs and Administrative | Academic, for-profit, nonprofit |
| Docetaxel-based chemotherapy for castration-resistant, metastatic prostate cancer | 2 | EHRs, Administrative | Federal (VA ) |
| Use of high-energy photon (> 10 MV) photons | 2 | EHRs, Administrative | Academic, for-profit, nonprofit |
| Plan of care for moderate/severe pain documented | 2 | Survey | Nonprofit |
| At least 10 core-needle samples taken at time of prostate biopsy | 1 | EHRs | Federal (VA hospitals) |
| Using computerized tomography to plan treatment for radiation therapy | 1 | EHRs | Teaching, nonprofit |
| Received radiation therapy (RT) within 30 days of death | 1 | EHRs | Academic |
| Surgery performed under epidural anesthesia after retropubic prostatectomy | 1 | Survey | Academic |
| Received chemotherapy in the last 14 days of life | 1 | Administrative | Academic, for-profit, nonprofit |
| 3-D CRT or IMRT for prostate cancer patients treated with EBRT | 1 | EHRs, Administrative | Federal (VA hospitals) |
| Prescription of narcotic pain medication for advanced cancer patients in pain | 1 | EHRs and Administrative | Federal (VA hospitals) |
| Communicating with the patient’s primary care physician to ensure continuing care | 1 | EHRs | Teaching, nonprofit |
| Pain assessed by second office visit | 1 | Survey | Nonprofit |
| Constipation assessed at the time of narcotic prescription or following visit | 1 | Survey | Nonprofit |
| Patient emotional well-being assessed by the second office visit | 1 | Survey | Nonprofit |
| PSA monitoring after treatment | 1 | EHRs and Administrative | Federal |
| DRE monitoring in active surveillance | 1 | EHRs and Administrative | Federal |
| Repeat biopsy in 18 months in active surveillance | 1 | EHRs and Administrative | Federal |
| Use of active surveillance, watchful waiting for low-risk prostate cancer | 1 | Administrative | Academic, for-profit, nonprofit |
EHR, electronic health record; PSA, prostate-specific antigen; DRE, digital rectal examination; VA, US Department of Veterans Affairs; CT, computed tomography; XRT, radiation therapy; 3D-CRT, three-dimensional conformal radiation therapy; IMRT, intensity modulated radiation therapy; EBRT, external beam radiation therapy.
Table 1c.
Prostate Cancer Outcome Measures Used in the Literature*
| Measures | Used By | Data | Type of Institution That Performed the Study |
|---|---|---|---|
| Hospitalization or medical or surgical treatment for a variety of serious complications | Worwag E, et al. (1998)42 | Survey | Academic hospital, nonprofit |
| Avoided multiple hospital admissions in the last 30 days of life | Herrel LA, et al. (2017)23 | Administrative | Academic, for-profit, nonprofit |
| Hospice admissions for cancer in the final days of life | O’Connor NR, et al. (2014)29 | EHRs | Nonprofit (hospice) |
| T stage stratified surgical margin status | Webster TM, et al. (2012)40 | EHRs, Administrative | Nonprofit (community) |
| Patient’s assessment of urinary, sexual, and bowel functioning after treatment | Hernandez-Boussard T, et al. (2016)22 | EHRs | Academic hospital |
| Shroeck FR, et al. (2015)32 | EHRs | Academic, for-profit, nonprofit | |
| Wei JT, et al. (2002)41 | Survey, Administrative | Teaching hospital | |
| Patients’ satisfaction with treatment choice, continence, and potency | Kamal AH, et al. (2013)25 | Survey | Nonprofit (community based) |
| Worwag E, et al. (1998)42 | Survey | Academic hospital, nonprofit | |
| Wright JD, et al. (2015)43 | Survey | Academic, for-profit, nonprofit |
References can be found at the end of this article.
EHR, electronic health record.
Table 2 lists the 10 most frequently used quality measures found in the literature, the majority of which were process measures. Androgen deprivation therapy (ADT)/adjuvant hormonal therapy for high-risk patients ranked first, with 15 studies citing it as a quality measure. Research was the primary intent of most of these 15 studies, with only 1 focused on assessing quality improvement (Figure 2).
Table 2.
The 10 Most Utilized Quality Measures Found in the Literature
| Rank | Measures/Concepts | No. of Studies |
|---|---|---|
| 1 | Androgen deprivation (ADT)/adjuvant hormonal therapy for high-risk prostate cancer patients | 15 |
| 2 | Clinical T stage documented | 10 |
| 2 | Avoidance of overuse measure—bone scan for staging low-risk patients | 10 |
| 3 | PSA level documented | 9 |
| 3 | Gleason score reported to patient | 9 |
| 3 | Treatment options counseling (multidisciplinary) | 9 |
| 4 | Following up with the patient at least twice during the first year after treatment | 7 |
| 5 | Comorbidity assessment | 6 |
| 5 | The proportion of patients treated by a high-volume (upper tertile) provider (surgeon or radiation oncologist) | 6 |
| 6 | Radical prostatectomy pathology reporting | 4 |
Figure 2:
This bar chart shows the prevalences of articles addressed to quality improvement or scientific research purposes in the top 10 measures assessed. From the graph it emerges that articles written for research purposes account for the absolute majority of articles.
Two quality measures (clinical T stage documented and avoidance of overuse measure—bone scan for staging low-risk patients) ranked second, as they were each cited by 10 studies. For these two measures, quality improvement was addressed in only 5 of 20 (25.0%) studies (Figure 2). Appearing in 9 studies each were Gleason score reported to patient, PSA level documented, and counseling regarding treatment options. The final five measures on the list are follow up with the patient at least twice during the first year after primary treatment (7 studies), comorbidity assessment and the proportion of patients treated by a high-volume provider (6 studies each), and radical prostatectomy pathology reporting (4 studies).
Overall, the intent of the articles (research vs. quality improvement) was skewed heavily in favor of research, with only 22 of 63 (34.9%) articles focusing on quality improvement. For these measures, the ratio of quality improvement to research studies ranged from 2:7 down to 1:1 (Figure 2). The majority of the measures reported in the literature were focused on quality improvement.
Final Results
Generally speaking, research was the mostly prevalent purpose of each of the 10 most frequently utilized quality measures (Figure 2). The study of quality improvement is directly related to enhancing the quality of care in itself. For example, Flynn et al. (2010) surveyed primary care physicians practicing in two northeastern US states. Assessment of family history as a quality measure was based on the extent of family history taken and ascertaining age at cancer diagnosis for affected family members.21 Research studies, on the other hand, may not be directly associated with quality improvement. For example, Bekelman et al. (2007) conducted a study to evaluate the variations in adherence to quality measures of external beam radiotherapy (EBRT) for localized prostate cancer care. They assessed adherence to five EBRT quality measures, including the assessment of completion of two follow-up visits with a radiation oncologist in the year following therapy using Surveillance, Epidemiology, and End Results (SEER) data.18
DISCUSSION
A systematic review of the literature identified 71 proposed prostate cancer quality measures. The most frequently reported quality measures were associated with care processes (process quality measures), and among the least studied measures were those related to important patient outcomes, such as assessment of sexual function and urinary incontinence. Among all of the quality metrics identified, only 3 were reported in 10 or more studies, and all of these were process measures. Given the movement to value-based care and quality measure–associated payment modifiers, identification of a manageable, comprehensive set of measures that are important to both the clinician and patient could help guide both quality improvement efforts and resource allocation.
Overall, less than 10% of the total proposed prostate cancer quality measures were endorsed by NQF. For the endorsement of a quality measure, NQF requires an extensive literature review and gathers input from stakeholders across the health care system. In general, only those quality measures endorsed get preference for potential use in federal public reporting and performance-based payment programs.44 Given the paucity of endorsed measures for prostate cancer, the prostate care community has limited metric choices for federal payment modifiers, and therefore selection may result in measures that may not truly reflect the quality of care they provide to their patients. Furthermore, the limited degree of reporting on meaningful quality measures means that patients have little data to help them select a provider for their prostate cancer care, as most quality initiatives (for example, Hospital Compare) highlight only endorsed measures, which are largely process focused. Measures focusing on patient outcomes, in particular those related to urinary and sexual function, need to be identified, as these measures are of high importance for both providers and patients. These outcome measures could have the greatest impact in improving care if targeted for value-based payment services.
Among the 10 most commonly used quality measures identified in the literature, 9 were process quality measures that focused on whether pretreatment/treatment assessment was performed and/or documented by the care team (for example, digital rectal exam documentation or pretreatment sexual function assessed). These process-centered measures are not unique to prostate cancer care. Other investigators have shown that most of the measures used in government programs (for example, Medicare and Medicaid) have focused on process measures, many of which were not clinically meaningful.45,46 It is likely that the abundance of process measures is partly due to their being generally easier to measure and implement. However, other measures, such as prostate cancer–specific patient-centered outcomes, are likely to be of higher value for patients, providers, and payers.
It is notable that most studies utilizing these process quality measures were research focused, with only a limited number reporting application of process measures for quality improvement. Although concepts and implementation of quality improvement measures are relatively recent, there appears to be a gap between academic research and actual clinical practice in improving performance on these quality measures, at least in terms of what is available in the medical literature. Accurate measurement is critical to effecting changes, but attempts to integrate research and clinical practice for quality improvement could be implemented in the near term to test whether they will improve patient care across settings, rather than in a limited number of academic hospitals.
We found that only a small fraction of proposed measures were patient outcome based, and that they are rarely reported in the literature, either for research or quality improvement. Recently, Jha and Pronovost also emphasized the need for a shift in quality measures to focus on meaningful outcomes, eliminating unnecessary process quality measures from government programs (for example, pay for performance).45 In addition, failure to develop and use outcome measures that matter to the patient and clinician makes it difficult for government programs to engage stakeholders in quality improvement. As noted recently from NQF, it is crucial to get feedback from patients and their families in implementing any measure.47
Our study not only identifies a set of the 10 most commonly reported quality indicators but also has suggested 5 core indicators that together cover the three classifications of quality measures theorized by Donabedian, have clinical face validity of quality improvement, and are well represented in the literature (Table 3). The first of these measures reflects structure—the proportion of patients treated by a high-volume provider. Volume is often considered a proxy for health outcomes, and recent literature highlights its importance in oncology, including better health outcomes and survival for prostate cancer in both European48 and US practice settings.49,50 The second and third most frequently used measures were process focused: the use of androgen deprivation therapy (ADT)/adjuvant hormonal therapy for high-risk patients, and documentation of clinical T stage. ADT agents have demonstrated benefits achieved for effective palliation51 as well as survival,52 while the assessment and documentation of the clinical T stage is fundamental to treatment selection.53 The fourth and fifth measures represent outcomes of care and include assessment and documentation of urinary, sexual, and intestinal functions of the patient after treatment; and the patient’s satisfaction with the choice of treatment and with his function in regard to continence and sexual potency. Urinary incontinence and erectile dysfunction have been highlighted as the most common side effects of radical prostatectomy (the most common intervention for prostate cancer)54,55 as well as radiation therapy. In radiation therapy, bowel dysfunction is another important outcome measure. Together, urinary, sexual, and bowel function measures most influence postintervention quality of life.56,57 Collection of these outcomes also can be very useful in measurement efforts that “give voice” to the patient.58 Implementing one or more of the 5 suggested core measures would allow direct comparison across studies and practice settings. By agreeing on and focusing on these 5 measures, studies can be better compared for outcomes for both process and outcome measures that are meaningful to patients and health care providers.
Table 3.
Proposed Set of Comprehensive Quality Measures
| Measures | Type |
|---|---|
| The proportion of patients treated by a high-volume provider | Structure |
| Androgen deprivation therapy (ADT)/Adjuvant hormonal therapy for high-risk patients | Process |
| Clinical T stage documented | Process |
| Patient’s assessment of urinary, sexual, and bowel functioning after treatment | Outcome |
| Patients’ satisfaction with treatment choice, continence, and potency | Outcome |
This study has several limitations. The search terms and methods may not have captured all studies that report prostate cancer quality measures. Also, studies focused on quality measurements for clinical care vs. research are not always easily distinguishable. Future studies that include systematic reviews and meta-analyses can improve the robustness of the findings and should also investigate the association of popular quality measures with clinically meaningful outcome measures and patient satisfaction. Finally, we found that a breadth of studies touched on quality measures only indirectly and were excluded from our review. Therefore, the number of studies assessed was relatively low and confounded by the fact that study designs and types varied greatly. However, we have synthesized the available information on prostate cancer quality assessment as a foundation for developing a core set of quality measures that could be used in both research and quality improvement efforts.
CONCLUSION
A systematic review identified a substantial number of proposed prostate quality measures in the literature; however, less than 10% of these were NQF endorsed. The diversity of nonaccredited quality measures limits the prostate cancer care community’s ability to have appropriate value-based payment modifiers and prioritize quality improvement efforts. The absence of outcome credentialed measures for prostate cancer care shows a disconnect between the measures that are reported in the literature and the true quality measures that matter for both providers and patients. From this review, five core indicators were identified that are commonly used in the literature and most likely to be clinically impactful. These measures can serve as the basis for further studies in prostate cancer care and process.
Supplementary Material
Acknowledgments
Funding and Disclaimer. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R01CA183962. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest. All authors report no conflicts of interest.
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.jcjq.2018.06.004.
Contributor Information
Davide Gori, Research Fellow, Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy..
Rajendra Dulal, Postdoctoral Fellow, Department of Medicine, Stanford University, Stanford, California, is Assistant Professor, Department of Economics, Tulane University, New Orleans..
Douglas W. Blayney, Professor of Medicine (Oncology), Stanford Cancer Center, Stanford University..
James D. Brooks, Professor, Department of Urology, Stanford University School of Medicine..
Maria P. Fantini, Professor, Department of Biomedical and Neuromotor Sciences, University of Bologna..
Kathryn M. McDonald, Executive Director and Senior Scholar, Center for Health Policy / Center for Primary Care and Outcomes Research, Stanford University School of Medicine..
Tina Hernandez-Boussard, Associate Professor, Departments of Medicine, Surgery, and Biomedical Data Science, Stanford University..
REFERENCES
- 1.Ghani KR, Miller DC. Variation in prostate cancer care. JAMA. 2015. May 26;313:2066–2067. [DOI] [PubMed] [Google Scholar]
- 2.Harlan L, et al. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13:93–100. [DOI] [PubMed] [Google Scholar]
- 3.Donabedian A The quality of care: how can it be assessed? JAMA. 1988. September 23–30;260:1743–1748. [DOI] [PubMed] [Google Scholar]
- 4.RAND Corporation. Prostate Cancer Patient Outcomes and Choice of Providers: Development of an Infrastructure for Quality Assessment. 2000. Litwin MS, et al. Accessed Aug 13, 2018 https://www.rand.org/content/dam/rand/pubs/monograph_reports/2005/MR1227.pdf.
- 5.Blumenthal D, McGinnis JM. Measuring Vital Signs: an IOM report on core metrics for health and health care progress. JAMA. 2015. May 19;313:1901–1902. [DOI] [PubMed] [Google Scholar]
- 6.Agency for Healthcare Research and Quality. Guidelines and Measures. 2018. Accessed Aug 13, 2018 https://www.qualitymeasures.ahrq.gov.
- 7.National Quality Forum. Quality Positioning System. 2018. Accessed August 13, 2018 https://www.qualityforum.org/QPS/QPSTool.aspx.
- 8.Centers for Medicare & Medicaid Services. Hospital Compare. 2018. Accessed Aug 13, 2018 https://www.medicare.gov/hospitalcompare.
- 9.Panzer RJ, et al. Increasing demands for quality measurement. JAMA. 2013. November 13;310:1971–1980. [DOI] [PubMed] [Google Scholar]
- 10.Chien AT, Rosenthal MB. Medicare’s physician value-based payment modifier—will the tectonic shift create waves? N Engl J Med. 2013. November 28;369:2076–2078. [DOI] [PubMed] [Google Scholar]
- 11.Nowels D, Kamerow DB. New “Core Quality Measures”: only a beginning. J Am Board Fam Med. 2017;30:4–7. [DOI] [PubMed] [Google Scholar]
- 12.Casalino LP, et al. US physician practices spend more than $15.4 billion annually to report quality measures. Health Aff (Millwood). 2016;35:401–406. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine. To Err Is Human: Building a Safer Health System, Washington, DC: National Academies Press, 2000. Accessed Aug 13, 2018 http://nap.edu/9728. [PubMed] [Google Scholar]
- 14.Agency for Healthcare Research and Quality. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies, vol. 7: Care Coordination. McDonald KM, et al. Technical Review No. 9, AHRQ Publication No. 04(07)-0051–7. Jun 2007. Accessed Aug 13, 2018 https://www.ahrq.gov/downloads/pub/evidence/pdf/caregap/caregap.pdf. [PubMed]
- 15.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 16.Etzioni R, et al. The prostate cancer conundrum revisited: treatment changes and prostate cancer mortality declines. Cancer. 2012. December 1;118:5955–5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ. 2005. November 5;331:1064–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bekelman JE, et al. Variation in adherence to external beam radiotherapy quality measures among elderly men with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007. December 1;69:1456–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brundage M, et al. A criterion-based audit of the technical quality of external beam radiotherapy for prostate cancer. Radiother Oncol. 2013;107:339–345. [DOI] [PubMed] [Google Scholar]
- 20.D’Avolio LW, et al. Facilitating clinical outcomes assessment through the automated identification of quality measures for prostate cancer surgery. J Am Med Inform Assoc. 2008;15:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flynn BS, et al. Primary care physicians’ use of family history for cancer risk assessment. BMC Fam Pract. 2010. June 3;11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez-Boussard T, et al. New paradigms for patient–centered outcomes research in electronic medical records: an example of detecting urinary incontinence following prostatectomy. EGEMS (Wash DC). 2016. May 12;4:1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrel LA, et al. Health care integration and quality among men with prostate cancer. J Urol. 2017;197:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmes JA, et al. Quality of care received and patient-reported regret in prostate cancer: analysis of a population-based prospective cohort. Cancer. 2017. January 1;123: 138–143. [DOI] [PubMed] [Google Scholar]
- 25.Kamal AH, et al. Conformance with supportive care quality measures is associated with better quality of life in patients with cancer receiving palliative care. J Oncol Pract. 2013;9:e73–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee DJ, et al. Association of healthcare barriers with prostate-specific antigen screening among African-American and Afro-Caribbean men. Urology. 2012;80:556–563. [DOI] [PubMed] [Google Scholar]
- 27.Makarov DV, et al. Appropriate and inappropriate imaging rates for prostate cancer go hand in hand by region, as if set by thermostat. Health Aff (Millwood). 2012;31: 730–740. [DOI] [PubMed] [Google Scholar]
- 28.Miller DC, et al. Treatment choice and quality of care for men with localized prostate cancer. Med Care. 2007;45:401–409. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor NR, et al. Hospice admissions for cancer in the final days of life: independent predictors and implications for quality measures. J Clin Oncol. 2014. October 1;32:3184–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ranpura V, et al. Improving documentation of pain management at MedStar Washington Cancer Institute. J Oncol Pract. 2015;11:155–157. [DOI] [PubMed] [Google Scholar]
- 31.Samuel CA, et al. Racial disparities in cancer care in the Veterans Affairs health care system and the role of site of care. Am J Public Health. 2014;104:S562–S571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schroeck FR, et al. Receipt of best care according to current quality of care measures and outcomes in men with prostate cancer. J Urol. 2015;193:500–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schroeck FR, et al. Adherence to performance measures and outcomes among men treated for prostate cancer. J Urol. 2014;192:743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schroeck FR, et al. Regional variation in quality of prostate cancer care. J Urol. 2014;191:957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroeck FR, et al. Technology diffusion and prostate cancer quality of care. Urology. 2014;84:1066–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toole M, Lutz S, Johnstone PA. Radiation oncology quality: aggressiveness of cancer care near the end of life. J Am Coll Radiol. 2012;9:199–202. [DOI] [PubMed] [Google Scholar]
- 37.Shelton JB, et al. Validating electronic cancer quality measures at Veterans Health Administration. Am J Manag Care. 2014;20:1041–1047. [PubMed] [Google Scholar]
- 38.Skolarus TA, et al. Quality of prostate cancer care among rural men in the Veterans Health Administration. Cancer. 2013. October 15;119:3629–3635. [DOI] [PubMed] [Google Scholar]
- 39.Sohn W, et al. Impact of adherence to quality measures for localized prostate cancer on patient-reported health-related quality of life outcomes, patient satisfaction, and treatment-related complications. Med Care. 2016;54:738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webster TM, et al. Cancer Care Ontario guidelines for radical prostatectomy: striving for continuous quality improvement in community practice. Can Urol Assoc J. 2012;6:442–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei JT, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002. January 15;20:557–566. [DOI] [PubMed] [Google Scholar]
- 42.Worwag E, Chodak GW. Overnight hospitalization after radical prostatectomy: the impact of two clinical pathways on patient satisfaction, length of hospitalization, and morbidity. Anesth Analg. 1998;87:62–67. [DOI] [PubMed] [Google Scholar]
- 43.Wright JD, et al. Relationship between surgical oncologic outcomes and publically reported hospital quality and satisfaction measures. J Natl Cancer Inst. 2015. March 1;107:dju435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldwein JW, Rose CM. QOPI, EHRs, and quality measures. J Oncol Pract. 2006;2:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jha A, Pronovost P. Toward a safer health care system: the critical need to improve measurement. JAMA. 2016. May 3;315:1831–1832. [DOI] [PubMed] [Google Scholar]
- 46.Jha AK. Time to get serious about pay for performance. JAMA. 2013. January 23;309:347–348. [DOI] [PubMed] [Google Scholar]
- 47.National Quality Forum. Patient Reported Outcomes (PROs) in Performance Measurement. Jan 10, 2013. Accessed Aug 13, 2018 https://www.qualityforum.org/WorkArea/linkit.aspx?LinkIdentifier=id&ItemID=72537.
- 48.Amato L, et al. Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data. Epidemiol Prev. 2017;41(5–6 Suppl 2):1–128. [DOI] [PubMed] [Google Scholar]
- 49.Clarke CA, et al. Public reporting of hospital-level cancer surgical volumes in California: an opportunity to inform decision making and improve quality. J Oncol Pract. 2016;12:e944–e948. [DOI] [PubMed] [Google Scholar]
- 50.Leow JJ, et al. Systematic review of the volume-outcome relationship for radical prostatectomy. Eur Urol Focus. 2017. April 6 Epub. [DOI] [PubMed] [Google Scholar]
- 51.Agency for Healthcare Research and Quality. Therapies for Clinically Localized Prostate Cancer: Update of a 2008 Systematic Review. Sun F, et al. Comparative Effectiveness Review No. 146. AHRQ Publication No. 15-EHC004-EF. Dec 2014. Accessed Aug 13, 2018 https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/prostate-cancer-therapies-update_research.pdf. [PubMed] [Google Scholar]
- 52.Wilson HC, et al. Contemporary hormone therapy with LHRH agonists for prostate cancer: avoiding osteoporosis and fracture. Cent European J Urol. 2015;68:165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamada S, et al. Comparative effectiveness of radical prostatectomy and curative radiotherapy in localized prostate cancer: long-term follow-up. J Radiat Res. 2017. July 1;58:552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barocas DA, et al. Association between radiation therapy, surgery, or observation for localized prostate cancer and patient-reported outcomes after 3 years. JAMA. 2017. March 21;317:1126–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donovan JL, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016. October 13;375:1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013. June 11;13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Penson DF, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer out-comes study. J Urol. 2005;173:1701–1705. [DOI] [PubMed] [Google Scholar]
- 58.Basch E The missing voice of patients in drug-safety reporting. N Engl J Med. 2010. March 11;362:865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.