Abstract
Carbapenem-resistant Enterobacteriaceae (CRE) are multidrug-resistant organisms with few treatment options that cause infections associated with substantial morbidity and mortality. CRE outbreaks have been increasingly reported worldwide and are mainly due to the emergence and spread of strains that produce carbapenemases. In the United States, transmission of CRE is primarily driven by the spread of organisms carrying the Klebsiella pneumoniae carbapenemase enzyme, but other carbapenemase enzymes, such as the New-Delhi metallo-β-lactamase, have also emerged. Currently recommended control strategies for healthcare facilities include the detection of patients infected or colonized with CRE and implementation of measures to prevent further spread. In addition to efforts in individual facilities, effective CRE control requires coordination across all healthcare facilities in a region. This review describes the current epidemiology and surveillance of CRE in the United States and the recommended approach to prevention.
Keywords: carbapenem-resistant Enterobacteriaceae, infection prevention, Klebsiella pneumoniae carbapenemase, multidrug-resistant organism, New Delhi metallo-β-lactamase
In recent years, carbapenem-resistant Enterobacteriaceae (CRE) have been increasingly recognized as a cause of healthcare-associated infections in many parts of the world. Outbreaks of disease have been reported from several countries including the USA [1–6]. Although non-susceptibility to carbapenems among Enterobacteriaceae can be acquired through different mechanisms, including the combination of porin mutations that decrease carbapenem penetration with production of certain types of β-lactamases (i.e., AmpC β-lactamases or extended-spectrum β-lactamases [ESBL] [7–9]), much of the increase in CRE is due to the emergence and spread of organisms producing β-lactamases effective against the carbapenem class of antibiotics (i.e., carbapenemases). These β-lactamases are frequently encoded by transmissible genetic elements that can facilitate their spread among bacterial species. In the USA, the early expansion of CRE was largely driven by transmission of a single strain of Klebsiella pneumoniae (multilocus sequence type 258) producing the K. pneumoniae carbapenemase (KPC) that has subsequently been identified in other parts of the world [6,10–12]. To date, numerous KPC alleles have been identified; hereafter, we will refer to this class of carbapenemase as ‘KPC’.
Experience investigating CRE clusters has resulted in a better understanding of effective infection prevention strategies and the development of tools and resources for healthcare facilities as well as state and local health departments. In this review, we will summarize the epidemiology of CRE in the USA, focusing primarily on carbapenemase producers, describe the surveillance and detection of CRE and discuss strategies to prevent CRE transmission at both the facility and regional level.
Epidemiology of CRE in the USA
Overview of carbapenemase-producing CRE
Data on the incidence and epidemiology of CRE in the USA are available from several surveillance systems. The Surveillance Net-work Database USA, which is a nationally representative repository of antimicrobial susceptibility results from approximately 300 laboratories in the USA, first identified resistance to imipenem among K. pneumoniae in 2004 and demonstrated a gradual increase; 4.3% of all K. pneumoniae were imipenem resistant by 2010 [13]. Larger increases in the percent of Enterobacteriaceae non-susceptible to a carbapenem (i.e., imipenem meropenem or doripenem) have been reported from the CDC surveillance system, which includes the National Healthcare Safety Network (NHSN) and its precursor, the National Nosocomial Infection Surveillance System [14]. Collectively, approximately 1.2% of the most common Enterobacteriaceae reported to Nosocomial Infection Surveillance System in 2001 were non-susceptible to at least one of the three carbapenems listed above. However, by 2011, the percentage of Enterobacteriaceae reported to the NHSN that were non-susceptible to at least one of the three carbapenems had risen to 4.2%, with the greatest increase observed among K. pneumoniae (from 1.6 to 10.4%). In addition, although the sensitivity and specificity of discharge coding data for CRE is unknown, a recent report using such data suggests that, beginning in 2006, CRE emerged as an important cause of urinary tract infections associated with hospitalizations, reaching an annual rate of 0.51 cases per 1000 hospitalizations in 2009 [15].
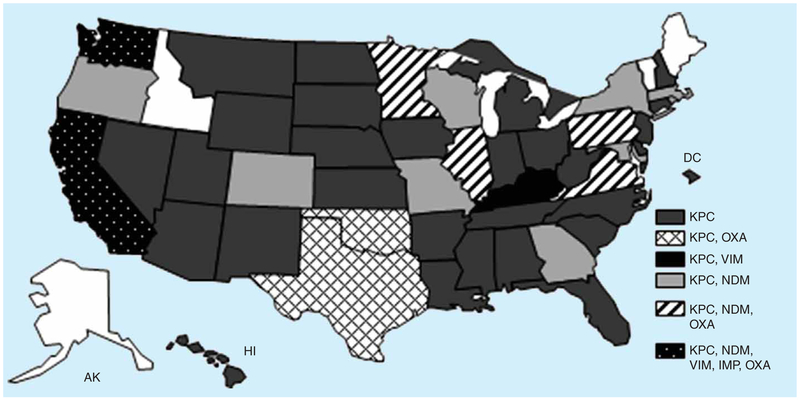
As alluded to above, much of the increasing incidence of CRE in the USA is due to the emergence and spread of KPC-producing Enterobacteriaceae. KPC was first identified in a K. pneumoniae isolated from a patient in North Carolina in 1996 as part of a project evaluating antimicrobial resistance in intensive care units (ICU), but was reported in 2001 [16]. The initial spread of KPC-producing strains was concentrated in the eastern USA, particularly in parts of New York and New Jersey [17–19], but over the last 5 years, KPC-producing Enterobacteriaceae have been reported from across the country and throughout the world [6,20]. As of November 2013, at least one KPC-producing CRE isolate has been reported from 46 states (FIGURE 1). Among CRE isolates reported to CDC for reference testing, KPC has been primarily found in K. pneumoniae, Escherichia coli and Enterobacter spp. As previously noted, the majority of the US KPC-producing K. pneumoniae isolates belong to a common strain type, ST258 [11]. Despite the expansion of KPC-producing strains across the USA, they still remain heterogeneously distributed within most states.
Figure 1. Geographical distribution of carbepenemase-producing Enterobacteriaceae in the USA, November 2013.
IMP: Active on imipenem; KPC: Klebsiella pneumoniae carbapenemase; MBL: Metallo-β-lactamases; NDM: New Delhi metallo-β-lactamase; OXA: Oxacillinases; VIM: Verona integron-encoded.
While KPC remains the predominant carbapenemase among Enterobacteriaceae in the USA, other carbapenemases that are more common in other parts of the world have also been identified (FIGURE 1). Although still rare in the USA, the most frequently reported among these is the New Delhi metallo-β-lactamase (NDM). Of note, several NDM alleles have been identified to date; hereafter, we will refer to this class of carbapenemase as ‘NDM’. The first NDM-producing isolate was recovered in 2008 from a patient in Sweden who had previously received medical care in India [21]. NDM was subsequently identified in multiple species of Enterobacteriaceae from patients in the UK, many of whom had previous hospitalizations in India and Pakistan, and from patients from various areas within the Indian subcontinent [22]. By 2010, NDM-producing CRE were being described worldwide [23–28], including the first report of a US isolate in 2009 [29]. Consistent with initial reports in the UK and other parts of the world [22,30], many of the early cases in the USA were in patients who had received prior medical care in countries where these organisms are more common, including the Indian subcontinent [29,31]. The majority of these early cases were either not associated with further transmission or associated with transmission only to a single patient. However, beginning in 2012, there has been a sharp rise in the number of NDM-producing CRE reported to CDC. Among the 91 US NDM-producing isolates identified as of 1 December 2013, 80 (88%) were identified since the beginning of 2012. Furthermore, the epidemiology of these organisms also appears to be changing with increasing numbers of NDM-producing CRE isolated from patients who had not traveled outside the country, suggesting local acquisition.
Three US outbreaks of NDM-producing CRE have been published to date. The first outbreak involved transmission between a hospitalized patient in Rhode Island who had recently received medical care in Vietnam and a second patient on the same hospital ward, who was identified through surveillance cultures of epidemiologically linked contacts of the initial patient [32]. No additional NDM-producing CRE were identified among other patients housed on the same ward. The second outbreak occurred in Colorado and involved eight patients with NDM-producing K. pneumoniae isolates that were highly related by pulsed-field gel electrophoresis (PFGE) [33]. Three of these patients had clinical infection, and five were found to be asymptomatically colonized. One of the patients had previously been hospitalized in the Philippines; none of the other patients had traveled outside of the USA. An investigation identified several hospital units that were likely transmission sites, but an index patient was never identified. The third NDM outbreak occurred in northeastern Illinois and was associated with a contaminated duodenoscope used for endoscopic retrograde cholangiopancreatography (ERCP) that resulted in transmission of NDM-producing E. coli to at least 29 patients [34]. Both an NDM-producing E. coli and KPC-producing K. pneumoniae were cultured from the distal part of the duodenoscope (around the elevator riser) after it had been reprocessed. All E. coli isolates recovered from the patients and duodenoscope were highly related by PFGE. No breaches in the recommended procedures for reprocessing of ERCP endoscopes were identified during the investigation.
In addition to NDM, Enterobacteriaceae producing other metallo-β-lactamases (MBL) have been identified in the USA. Between November 2009 and July 2013, nine patients with Enterobacteriaceae isolates producing active on imipenem (IMP) or Verona integron-encoded MBL (VIM) enzymes were confirmed at the CDC. Only three of the eight patients, for whom detailed epidemiology was available (two VIM, one IMP), had received recent medical care outside the USA.
Another group of carbapenemases found in CRE are the oxacillinases (OXA), which comprise a heterogeneous group of class D β-lactamases and have increasingly been reported among Enterobacteriaceae [5]. Of particular concern is the OXA-48 family (hereafter ‘OXA-48’), which has recently emerged as one of the predominant carbapenemases in the Middle East, North Africa and Europe [35]. The first published description of OXA-48-producing CRE in the USA was of two isolates that were collected in 2009 as part of a worldwide laboratory-based surveillance of carbapenemase-producing K. pneumoniae isolates from intra-abdominal infections [36]. Since then, additional OXA-48-producing CRE have been identified, including a recent report of two patients with OXA-48-producing K. pneumoniae recovered from perirectal swabs who were hospitalized within a 4-month period at the same facility [37]. Both of these patients had previous healthcare exposures outside the USA. Including these two patients, from January 2011 to July 2013, 14 patients with OXA-48-producing CRE have been confirmed by CDC. Of note, two of the OXA-48-producing K. pneumoniae isolates also produced NDM.
Risk factors & outcomes associated with CRE
To evaluate factors associated with CRE-positive cultures, CDC piloted a laboratory-initiated, population-based surveillance program, known as the Multi-site Gram-negative Surveillance Initiative (MuGSI), in three US metropolitan areas beginning in August 2011 [38]. During the 5-month pilot in 2011, 72 CRE (includes both carbapenemase-producing and non-carbapenemase-producing isolates) were identified from 64 patients, with the vast majority isolated from urine specimens (89%). The majority of CRE-positive cultures (65%) were collected outside of short-stay acute care hospitals; however, they were mostly from patients with previous hospitalization or other healthcare exposures, such as admission to long-term care facilities, current maintenance dialysis or presence of indwelling medical devices. Six of these (13%) community-onset isolates were recovered from patients who did not have any healthcare exposure identified in the preceding year after thorough review of their medical records.
Several studies have evaluated the exposures that put patients at risk for colonization or infection with CRE (primarily KPC producers). Identified risk factors include prolonged hospitalization, presence of invasive devices, severity of underlying disease, low functional status, increasing colonization pressure and exposures to antimicrobials including, but not limited to, carbapenems [18,39–42]. In one study, the odds of acquiring CRE during a single hospitalization increased by 4% per day of antimicrobial therapy and by 15% for every 1% increase in the colonization pressure (defined as the percentage of patients on the unit who were CRE-positive) to which a patient was exposed [39]. Recent admission to post-acute care settings (long-term care settings), including long-term acute care hospitals, has also been strongly associated with CRE acquisition [43,44].
Another potential risk factor for CRE is endoscopy procedures [45–47]. Transmission of multidrug-resistant (MDR) bacteria following endoscopy procedures has been previously reported [48]. In addition to the NDM outbreak described above, at least three CRE outbreaks (two KPC, one OXA-48) associated with endoscopy have been reported; each of these three outbreaks resulted from inadequately reprocessed endoscopes used for gastrointestinal procedures [49–52]. The first outbreak occurred in the USA and involved a contaminated ERCP endoscope that resulted in transmission of KPC-producing K. pneumoniae to at least 10 patients [49]. Bacterial cultures from the implicated duodenoscope grew carbapenemase-producing Enterobacteriaceae. The second outbreak was reported in France and resulted from exposure to a contaminated duodenosope that had previously been used on a patient colonized with KPC-producing K. pneumoniae who was transferred from a hospital in Greece [50,51]. KPC-producing K. pneumoniae was recovered from 7 of 17 potentially exposed patients as well as from the duodenoscope; all recovered isolates were indistinguishable by PFGE. In the third CRE outbreak associated with duodenoscopy, which occurred in Germany, 10 patients became infected with OXA-48-producing K. pneumoniae, and 5 were found to be colonized with the organism following their exposure [52]. The implicated duodenoscope most probably had a defect that impacted its ability to be properly disinfected. In addition, NDM transmission has been linked to the endoscopic camera head used for urologic procedures, where camera sheathing was not routinely used, although the camera head was regularly cleaned with detergent wipes [53]. A second endoscopy-associated outbreak of OXA-48-producing K. pneumoniae was reported in Germany, which involved a contaminated bronchoscope from which bacteria were recovered [52]. The true extent of transmission of MDR organisms from contaminated endoscopes is unknown.
The percentage of patients colonized with CRE who subsequently develop a positive clinical culture has ranged from 8.8 to 47% [44,54,55], with most (86%) representing a true infection [54]. Predictors for infection among CRE carriers include admission to the ICU, having a central venous catheter, exposure to antibiotics, previous invasive surgery and diabetes mellitus [44,54]. Mortality rates associated with invasive infections caused by CRE, such as bloodstream infections, often exceed 40% and are higher than those associated with carbapenem-susceptible Enterobacteriaceae [56–58]. However, as evident in the MuGSI surveillance, the overall in-hospital mortality rate may be substantially lower (4%) when including isolates from clinical cultures of non-sterile sites, such as the genitourinary tract [38].
Spread of CRE in post-acute care settings
Certain post-acute care settings, particularly long-term acute care hospitals (LTACHs), are increasingly being recognized as a reservoir for patients colonized with carbapenemase-producing CRE, in which transmission can often go undetected [59–64]. In the USA, prevalence of CRE-colonized patients in post-acute care settings during outbreak investigations has ranged from 9 to 48% [60,62,63]. In one study that screened patients admitted to four Chicago-area hospitals, the prevalence of KPC carriage among patients admitted from post-acute care settings was 8.3%, compared with a prevalence of 0% among patients from the community [65]. Prevalence of CRE also varied by the type of post-acute care setting, with sevenfold greater odds of colonization among patients admitted from LTACHs and skilled nursing facilities (SNFs) with ventilator units, compared with patients from an SNF without ventilator care [65]. During 2010–2011, point prevalence surveys for KPC-producing CRE in the Chicago region revealed a prevalence of 30.4% among LTACH patients, compared with 3.3% among ICU patients in short-stay hospitals [66].
CRE incidence in short-stay acute care hospitals compared with LTACHs has also been evaluated using NHSN. During the first half of 2012, 3.9% of all short-stay acute care hospitals participating in NHSN surveillance for central-line-associated bloodstream infections or catheter-associated urinary tract infections reported one or more infections with CRE [14]; however, the percentage of LTACHs reporting at least one CRE infection was substantially higher (17.8%).
LTACHs can also play an important role in the regional emergence of CRE [64]. By serving as a point of convergence for patients at high risk for CRE colonization, LTACHs may facilitate the amplification and dissemination of CRE as colonized patients are transferred to surrounding facilities providing higher and lower levels of care [63,64]. This process was described in a report of a multistate outbreak of KPC affecting 26 healthcare facilities; 60% of 40 cases were linked to one LTACH [64]. In this and other regional outbreaks, lack of knowledge about CRE among facility staff early in the outbreak period and lack of communication between facilities during patient transfers contributed to the spread of CRE [60,63,64].
Clinical & epidemiologic importance of CRE
Slowing the spread of CRE, particularly carbapenemase-producing strains, has become an important public health goal in the USA for several reasons. First, invasive infections caused by CRE are associated with high mortality rates [56–58]. Second, CRE often carry other resistance genes, thereby reducing the number of effective antimicrobials and substantially limiting treatment options. Pan-resistant CRE strains have been reported [67], and it may be years before new antimicrobial agents are available that have activity against these organisms. Third, as with any MDR organism, CRE have spread from patient to patient through healthcare systems as colonized or infected patients move across the continuum of care. In addition, because of the mobile nature of the plasmids that harbor these resistance genes, resistance can be transmitted between different species of Enterobacteriaceae. Finally, in the USA, CRE are primarily identified from patients with exposure to healthcare, but Enterobacteriaceae are also a common cause of infections in the community. It follows that potential exists for CRE to become a more common cause of community infections. Spread outside of healthcare has already been described for NDM-producing CRE in other countries, both as a source of community-acquired infection [22,68] and from the community environment in both India (drinking and seepage water) [69] and Vietnam (seepage water) [70].
CRE surveillance & laboratory detection
The first step to CRE control is to understand how commonly these organisms are encountered at the facility and regional level. For healthcare facilities, this may include a retrospective review of microbiology records to determine the frequency with which CRE are identified from clinical cultures over the past 6–12 months. At a regional level, surveillance efforts might consist of surveys of local laboratories or Infection Preventionists from all facilities within the region.
In general, Enterobacteriaceae that are non-susceptible to a carbapenem represent multidrug-resistant organisms (MDROs) and should be managed accordingly [71]. Of particular concern are CRE strains that produce carbapenemases; these organisms appear to have been responsible for much of the spread of CRE in the USA since 2001. However, surveillance for carbapenemase-producing CRE is complicated by the fact that current guidance for detection of CRE in clinical specimens does not recommend routine testing for the mechanism of resistance; resistance mechanism testing is suggested only for special epidemiologic studies [72]. Furthermore, only one mechanism-specific test, the modified Hodge test (MHT), is widely used in the US clinical laboratories. The MHT was developed and evaluated during a time when carbapenemases other than KPC were exceedingly rare in the USA, and although it demonstrated good sensitivity for carbapenemase detection, even then it was known to have poor specificity among Enterobacteriaceae producing AmpC or ESBL enzymes combined with porin loss [73–76]. Since that time, as additional carbapenemase enzymes have been detected in the USA, sensitivity of the MHT has been called into question, especially for detection of NDM. In addition to the MHT, several other methods have been developed to detect carbapenemases (e.g., Carba NP test, matrix-associated laser desorption ionization-time-of-flight mass spectrometry) [77–80]. Although these methods are currently used in other parts of the world, they are not yet in widespread use in the USA.
Developing a phenotypic definition that predicts carbapenemase production has been difficult because non-carbapenemase-producing CRE can exhibit an antimicrobial susceptibility pattern that can be very similar to CRE that produce carbapenemase. In an attempt to increase specificity for CRE that produce carbapenemases (i.e., KPC, NDM), CDC has utilized the following CRE surveillance definition: nonsusceptibility to imipenem, meropenem or doripenem (using current Clinical and Laboratory Standards Institute interpretive criteria) [81], and resistant to all the third-generation cephalosporins that were tested (because many plasmid-mediated carbapenemases also inactivate β-lactam antimicrobial agents) [82]. However, based on CDC reference testing for CRE, even when these more stringent criteria are applied, specificity for carbapenemase-producing strains can remain low in regions with low CRE prevalence and for certain Enterobacteriaceae. For example, among 114 CRE isolates submitted to CDC from the six US states or metropolitan areas between December 2011 and August 2013 that met this surveillance definition, 54 (47%) were carbapenemase-producing strains (specifically KPC). The majority of KPC-producing strains (>74%) submitted from five of the sites were K. pneumoniae, whereas 53% of the KPC-producing strains from Minnesota were Enterobacter cloacae. In addition, this surveillance definition has the potential to exclude some carbapenemase-producing CRE, including those that can be susceptible to the third-generation cephalosporins (e.g., OXA-48-producing CRE).
Preventing CRE transmission
Preventing CRE transmission in healthcare settings can be challenging, but is critical to delaying the further emergence of these organisms. A number of complex issues need to be considered when designing facility-specific CRE control interventions, including the extended periods that CRE-positive patients remain colonized and the inherent differences between short-stay acute care hospitals and long-term care settings which necessitate different approaches to implementation. Although much of the effort to control MDROs like CRE has been done at the facility level, the interconnectedness of the healthcare system also underscores the importance of working ‘regionally’ across facilities that share patients to prevent transmission. The next two sections will describe interventions for controlling CRE transmission at both facility and regional levels.
Several resources containing recommendations for the prevention of CRE transmission have been developed. In 2009, CDC released CRE-specific recommendations for the US acute care facilities [83] based on strategies outlined in the 2006 Guidelines for the Management of MDROs [71]. These recommendations were updated in 2012 with the release of the CDC CRE Toolkit [82]; this document expands upon the 2009 guidance by including facility-level interventions for both acute and long-term care settings. In addition, the CRE Toolkit provides regional prevention strategies for state and local health department implementation. Several state health departments have also developed state-specific resources and tools to guide facilities in their CRE prevention efforts [84–88].
Facility level CRE prevention
Current CDC recommendations for preventing CRE transmission in healthcare facilities are organized into core measures and supplemental interventions (BOX 1). Core prevention measures are well-supported by evidence and should be utilized by all facilities regardless of the prevalence of CRE in the facility or region. These are based on Standard Precautions as well as Contact Precautions that apply to any MDRO. Supplemental interventions are either less well-supported by evidence or more difficult to implement. These can be used by facilities when the prevalence or incidence of CRE has not decreased, despite the use of core strategies or as part of a more aggressive initial approach when the first case or an outbreak has been identified within a facility or unit. For the purpose of this review, the next section will focus on selected core and supplemental interventions, which may include aspects that are less familiar to facilities and public health professionals or that pose implementation challenges. While the focus of the following discussion is on carbapenemase-producing CRE, many of the interventions (e.g., Contact Precautions) described below also apply to non-carbapenemase-producing CRE.
Box 1. Core and supplemental carbapenem-resistant Enterobacteriaceae prevention activities for acute and long-term care facilities in the USA.
Core measures
Enhance hand hygiene
Promote and improve hand hygiene as part of routine uptake of Standard Precautions
Monitor hand hygiene adherence and provide feedback
Ensure access to hand hygiene stations and supplies
Implement CP
Develop protocols for notifying appropriate staff when a patient with CRE is identified
In short-stay acute care hospitals and long-term acute care hospitals, place CRE-colonized or -infected patients on CP
In lower-acuity long-term care facilities (e.g., skilled nursing facilities, nursing homes), place CRE-colonized or -infected residents that are high-risk for transmission on CP; for residents at lower risk for transmission use Standard Precautions for most situations
Preemptive CP might be used for patients transferred from high-risk settings
Educate healthcare personnel about CP
Monitor CP adherence and provide feedback
No recommendation can be made for discontinuation of CP
Promote patient and staff cohorting
Whenever possible, cohort CRE-colonized or –infected patients with designated staffing even if patients are housed in single rooms
If the number of single patient rooms is limited, reserve these rooms for patients at highest risk for transmission
Educate healthcare personnel about CRE
Minimize use of invasive devices and dedicate noncritical or disposable devices to individual patient use
Promote antimicrobial stewardship
Screen CRE among epidemiologically-linked contacts
Screen current and prior roommates of CRE-colonized or -infected patients
Screening may also include patients who have shared the same healthcare personnel or those located on the same ward or unit (i.e., point prevalence surveys) as CRE-colonized or-infected patients
Perform inter-facility communication
When transferring patients, facilities should notify accepting facilities of the patient’s CRE status, type and duration of any invasive devices, and duration of any ongoing antimicrobial therapy
Supplemental interventions
Conduct active surveillance testing for CRE
Screen high-risk patients at admission or at admission and periodically during their facility stay; preemptive CP can be used while results of admission surveillance testing are pending
Consider admission screening of patients transferred from facilities known to have CRE
Implement chlorhexidine bathing
Bathe all patients in targeted unit or ward daily with 2% chlorhexidine
CP: Contact precautions; CRE: Carbapenem-resistant enterobacteriaceae.
Data taken from [82].
Contact precautions
The intent of Contact Precautions is to prevent transmission of epidemiologically important organisms, such as CRE, by minimizing the contamination of healthcare personnel when they are interacting with colonized or infected patients [71]. In order to be effective, adherence to Contact Precautions requires the appropriate use of gown and gloves by healthcare personnel for all interactions that may involve contact with the patient or the patient’s environment. In general, gowns and gloves should be discarded before leaving the patient-care environment and should not be reused between patients.
CDC recommends that patients colonized or infected with CRE who are in short-stay acute care hospitals or LTACHs should be placed on Contact Precautions. The use of Contact Precautions for residents in lower-acuity long-term care settings (e.g., skilled nursing facilities, nursing homes) is more complex and must include consideration of the potential impact of these interventions on their wellbeing and rehabilitation potential as well as the overall risk that they pose as a source for additional transmission based on their functional and clinical status [71,83]. For example, use of Contact Precautions should be prioritized for residents who are colonized or infected with CRE who are ventilator-dependent, incontinent of stool that is difficult to contain, have draining secretions or wounds that cannot be controlled or are completely dependent on healthcare personnel for all activities of daily living. For more functional residents who are able to perform hand hygiene and are able to contain stool and secretions, the use of strict Contact Precautions might be relaxed by allowing them to attend common gatherings in the facility (e.g., meals). However, healthcare personnel should continue using Standard Precautions when interacting with these residents, including strict adherence to hand hygiene and gown and glove use for any anticipated exposures that might contaminate their hands or clothes.
To facilitate prompt implementation of Contact Precautions, both acute and long-term care facilities should have systems in place to identify patients with a history of CRE colonization or infection when they are readmitted. In addition, facility protocols should be developed that ensure prompt notification of appropriate staff by laboratory personnel when CRE are identified from clinical or surveillance cultures.
At present, CDC does not have recommendations for identifying patients for whom Contact Precautions might be discontinued; however, several factors are important to consider when making decisions about when this might be acceptable. First, the duration of CRE colonization can be prolonged. Zimmerman et al. found that the rate of CRE carriage declined over time following the initial positive culture for hospitalized CRE patients; however, the mean time from the initial positive CRE culture to the first negative culture without a subsequent positive was 387 days [89]. Second, certain exposures might increase the risk of prolonged carriage. The same authors also found that having multiple repeat hospitalizations and clinical disease due to CRE were both significantly associated with persistent carriage [89]. Schechner et al. also assessed factors associated with persistent carriage and found that patients with rectal cultures positive for CRE were 50% more likely to be positive again at their next hospital encounter if they had prior antimicrobial use (particularly fluoroquinolones), admission from another healthcare facility or duration of 3 months or less since their first positive CRE test [90]. If none of these factors was present, the risk of being CRE positive at the next admission was 14%. In another study, Feldman et al. followed known CRE carriers monthly with serial rectal cultures for 3–6 months after discharge from a short-stay acute care hospital [91]. They found that the presence of an invasive device was significantly associated with persistent CRE carriage. Other risk factors for persistent carriage included low functional status and long-term care facility residence among patients with recent CRE acquisition (within preceding 4 months) and high co-morbidity index (Charlson’s score) among patients with remote CRE acquisition (4 months or longer). Consistent with the other two studies, Feldman et al. found that the percentage of patients with positive CRE rectal cultures declined over time, from a 74% positivity rate when testing within 30 days of initial CRE detection to <30% when testing after 6 months. Importantly, only 67% of CRE carriers in this study with at least one negative rectal surveillance culture for CRE remained negative on subsequent cultures [91], suggesting that a single negative rectal culture might be inadequate to rule out ongoing CRE colonization.
Patient & staff cohorting
In addition to placing CRE-colonized or -infected patients in single-patient rooms, acute care hospitals and long-term care facilities should consider cohorting CRE patients together in the same ward or unit. If feasible, there should be designated staffing to care exclusively for patients with CRE to minimize the risk of transmission to other patients. In several outbreak investigations where multiple interventions were combined in a step-wise fashion to halt transmission, the use of patient and staff cohorting with spatial separation from other patients was found to be one of the most beneficial interventions in decreasing CRE transmission in the affected unit or facility [12,63,92–95]. For example, during a CRE outbreak involving an ICU, where a two-phase intervention was employed, cohorting of patients and staff during the second phase was shortly followed by a decrease in the number of new cases [12].
Antimicrobial stewardship
Hospitals that have established antimicrobial stewardship programs have shown reductions in rates of infections caused by certain MDROs, such as Clostridium difficile and MDR Enterobacteriaceae following the implementation of these programs [96,97]. However, the direct impact of antimicrobial stewardship on limiting the emergence of carbapenem resistance among epidemiologically important Gram-negative pathogens has not been widely studied [98]. Of the few studies available, most have focused on Pseudomonas aeruginosa and have found that the restriction of certain antimicrobials, such as carbapenems or fluoroquinolones, was associated with a lower incidence of carbapenem-resistant P. aeruginosa [99,100]. In one of the few studies that assessed other MDR Gram-negative pathogens, including Enterobacteriaceae, a comprehensive antimicrobial stewardship program implemented in two ICU that included protocols for therapeutic antibiotics and surgical prophylaxis and quarterly rotation of antibiotic classes demonstrated a significant decrease in the proportion of healthcare-associated infections caused by MDR Gram-negative pathogens during the study period (37.4 to 8.5%) [101]. In a study at a tertiary care oncology hospital in India, a reduction in the prevalence of CRE was observed following restriction of certain antimicrobial agents, including carbapenems, colistin and tigecycline, along with enforcement of infection control measures [102].
Several elements that comprise successful hospital antimicrobial stewardship programs have been described, including commitment from facility leadership to support antimicrobial stewardship efforts, designation of personnel to lead stewardship programs and implementation of policies and interventions to support optimal antimicrobial use (e.g., ‘antibiotic time out’ after 48 h) [103–106]. Additional components might include having a system in place to monitor and regularly report information on antibiotic use and antimicrobial resistance patterns to relevant staff as well as providing education on optimal prescribing practices [103–106]. CDC has developed a checklist that hospitals can use to assess key elements and actions to ensure optimal antibiotic prescribing and limit overuse and misuse of antibiotics [107].
CRE screening
Patients colonized with CRE are frequently not detected by diagnostic cultures obtained during the course of routine clinical care. One study found that only 31% of CRE-colonized patients had a clinical culture positive for CRE [108]. Unrecognized CRE-colonized patients can serve as a potential source for transmission of CRE to other patients. Given that clinical cultures are likely to identify only a minority of patients colonized with CRE, surveillance cultures have been used to detect colonization. Samples for surveillance cultures are generally collected from stool, the rectum or the peri-rectal area, although one study found that rectal cultures were more sensitive than perirectal cultures [108]. Intact skin, including the inguinal and axillary sites, can also be colonized with CRE, and one small study found that adding inguinal cultures to stool/rectal cultures increased sensitivity for detecting CRE [109]. Screening cultures for CRE can be labor intensive and costly and may not be readily available in all clinical laboratories. CDC has recommended a protocol for screening for carbapenem-resistant Klebsiella spp. and E. coli from rectal swabs [110]. In brief, the protocol recommends inoculating trypticase soy broth that contains a 10 mg ertapenem or meropenem disc with a rectal culture swab. After incubation, the specimen is vortexed and plated on MacConkey agar. Lactose-fermenting colonies are then screened for carbapenemase or tested for susceptibility to carbapenems. Although complicated and time-intensive, this protocol should be implementable in most clinical laboratories. Other screening tests that require less time (e.g., use of chromogenic agars) [111] or that can directly determine the resistance mechanism (e.g., direct PCR) [112] are not yet widely adopted in the USA, and none are approved by the US FDA for detection of CRE from surveillance specimens. How well these screening methods perform relative to each other warrants further evaluation.
Screening of epidemiologically linked contacts
Identification of CRE from a culture of a patient or resident of the facility should generally prompt screening of epidemiologically linked contacts to assess for unrecognized transmission that may have occurred. The decision to screen might be influenced by several factors including whether or not the patient had been on Contact Precautions, how common CRE are in the facility or region or how long the patient has been in the facility. Typically, screening includes current and prior roommates of the index patient who are still hospitalized and might also include patients who have shared the same healthcare personnel or patients located on the same unit or ward (i.e., point prevalence survey). This approach has been used for the control of outbreaks of other MDROs [71] and has also effectively identified unrecognized CRE transmission in several investigations [33,60,63]. Point prevalence surveys may also be used on a regular basis (e.g., monthly) to assess for ongoing transmission and to evaluate the effectiveness of CRE control interventions.
Although healthcare facilities should have a low threshold for screening epidemiologically linked contacts of patients with CRE, the risk of transmission to roommates and other contacts might depend, at least in part, on the duration of exposure. In an NDM-producing K. pneumoniae outbreak in Canada, roommates of NDM cases who subsequently tested positive for NDM had significantly longer mean duration of exposure to the index case compared with roommates who did not test positive (26.5 vs 6.5 days) [113]. Similarly, in a study assessing transmission of ESBL-producing Enterobacteriaceae from colonized patients to roommates during the interval between collection of screening cultures at admission and available test result (mean exposure time 4.4 days), only 2 (1.5%) of 133 roommates had evidence of transmission of PFGE-matched ESBL strains; in both of these instances, the exposure time was longer than the mean (9 and 10 days) [114].
Active surveillance testing
This form of CRE screening is considered a supplemental measure in the 2012 CDC Toolkit. This intervention differs slightly from screening epidemiologically linked contacts and consists of systematic screening, usually at admission, of patients who are not necessarily known to be linked to CRE patients. Facilities that employ this approach often target patients admitted to high-risk units (e.g., ICU) or those who meet certain pre-specified criteria that may place them at higher risk of CRE colonization (e.g., those admitted from LTACHs). In one study assessing the use of active surveillance cultures on patients admitted to the ICU, 37% of all patients with carbapenem-resistant K. pneumoniae were first identified through active surveillance testing [115]. The authors estimated that earlier detection and implementation of Contact Precautions may have prevented approximately 1400 days of unprotected exposure to carbapenem-resistant K. pneumoniae. This intervention has been used effectively as part of a package of interventions during CRE outbreaks [62,63,94]. Despite these findings, the use of active surveillance testing for prevention of MDROs, including CRE, remains controversial for the following reasons. First, because active surveillance testing is often implemented together with other infection control measures, the specific contribution of this intervention in reducing MDRO transmission is difficult to determine. Second, most studies of active surveillance testing have been observational in nature. One of the few randomized controlled studies to assess the use of active surveillance testing found that it did not significantly reduce transmission of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci [116]. However, during the study, the turnaround time for reporting a positive surveillance result was often prolonged, and adherence to Contact Precautions and hand hygiene was suboptimal, potentially contributing to the lack of impact. The effect on transmission of other MDROs, including CRE, was not assessed in this study.
Chlorhexidine bathing
Use of chlorhexidine bathing has been demonstrated to successfully reduce bloodstream infections and colonization with methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci primarily in ICU settings [117,118], but its role in reducing CRE transmission is less clear. Limited evidence exists for its use as part of a multifaceted strategy to control CRE outbreaks [62,119]. Some CRE might have reduced susceptibility to chlorhexidine, as recently described with some clinical isolates of the epidemic ST258 strain of KPC-producing K. pneumoniae [120]. If used as a supplemental measure, chlorhexidine bathing should be applied to all patients in the targeted unit or ward, regardless of their CRE colonization status, and be performed daily to ensure inhibitory concentrations of chlorhexidine remain on the skin [121].
Environmental cleaning
The role of the environment in CRE outbreaks is not clear. Although environmental cleaning is not one of the interventions outlined in the 2012 CDC CRE Toolkit, several healthcare facilities have included modifications to environmental cleaning in response to CRE outbreaks [12,62,63,93]. CRE have been cultured from the environment during outbreaks [63,122,123]. However, a study performed in LTACHs with large reservoirs of CRE-colonized patients found that the environmental burden of these organisms was low [109], with CRE detected in only 2 (0.5%) of 371 environmental specimens. In instances where CRE have been detected in the healthcare environment, contamination was highest on surfaces in the immediate vicinity of the colonized patient [109,124]. Therefore, if enhanced environmental cleaning is used to supplement other CRE prevention interventions, cleaning and disinfection efforts should focus on high-touch surfaces located in areas around the patient during regular daily and terminal cleaning.
Regional approach to CRE prevention
The US healthcare system is composed of an intricate network of inpatient, outpatient and residential facilities. Patients might be cared for in several different facilities, including ambulatory, acute and long-term care facilities, during one episode of an illness. This complex movement of patients between different levels of care can facilitate the transmission of MDROs from one healthcare facility to another [125–127]. Several multifacility and regional outbreaks of MDROs [128], including CRE [60,63,64], have resulted from the flow of colonized or infected patients across facilities. In one of the largest documented outbreaks of CRE, extensive sharing of patients between facilities in the Chicago area facilitated the dissemination of CRE regionally [64].
Inter-facility communications
Given the extent of inter-facility patient sharing, an effective infection control strategy against MDROs like CRE will require engagement of healthcare facilities across the region. To minimize inter-facility spread of CRE, a healthcare facility that is discharging or transferring patients colonized or infected with CRE should notify any receiving facility of the patient’s CRE status. This is critically important in assuring that appropriate precautions are implemented upon the patient’s arrival. For example, lack of communications between facilities likely contributed to several multifacility outbreaks of CRE [60,63,64]. Additional information to communicate during patient transfers should include type and duration of invasive devices present as well as reasons for and recommended duration of ongoing antimicrobial use. Communication of this important information should be routinely performed as part of the patient transfer process and is an essential component of regional approaches to CRE prevention.
Regional CRE surveillance
In the USA, state and local health departments could be in a unique position to help facilitate regional MDRO control efforts by providing updates to facilities regarding the regional prevalence of CRE and promoting implementation of recommended prevention measures. One important part of regional CRE prevention is developing an understanding of how common these organisms are at the regional level. Several state health departments have surveyed facilities within their jurisdictions using either the CDC-designed survey tool (available in the CDC CRE Toolkit) or a laboratory-based survey to determine the regional frequency of CRE detection [129–131]. Alternatively, regional surveillance for CRE can be performed through mandatory reporting of CRE isolates to state health departments by facilities or clinical laboratories. As of December 2013, 15 US state health departments have established some kind of CRE reporting requirement within their state [132]. One example of a state-wide effort to improve CRE surveillance and inter-facility communications was the creation of a web-based CRE registry, known as the extensively drug-resistant organism registry (XDRO registry), by the Illinois Department of Public Health in partnership with the Chicago CDC Prevention EpiCenter [133]. Starting in November 2013, all healthcare facilities and laboratories within Illinois were required to report to the XDRO registry any CRE isolate that met the state’s surveillance definition for a carbapenemase-producing organism; only the first CRE-positive culture from a patient is reportable. Healthcare facilities can query the registry to determine if a patient has been previously reported as CRE-positive so that appropriate precautions can be promptly implemented. The XDRO registry currently requires manual entry, but future updates may include automated uploading of patient data and electronic notification.
Coordinated regional control
‘Collaborative’ approaches that seek to engage all the facilities in a region to work together to develop and implement control interventions have been successful in preventing healthcare-associated infections [134,135]. By working closely to standardize and enhance uptake of infection prevention practices, healthcare facilities in two large regional prevention collaboratives reduced the rate of bloodstream infections in ICU by almost 70% [134,135]. The implementation of a regional approach has also been successful in the control of MDROs. Under public health guidance, acute and long-term care facilities in the Siouxland region of Iowa, Nebraska and South Dakota collaborated on the development and implementation of an infection-control strategy that led to a significant decrease in the regional prevalence of vancomycin-resistant enterococci [136]. The interventions included screening of patients on admission and in the ICU, use of Contact Precautions, dedicated use of non-critical medical equipment and education of healthcare personnel, patients and visitors. The collaboration among facilities in the Siouxland region also improved communication and facilitated the transfer of patients colonized with vancomycin-resistant enterococci between facilities. A nationwide outbreak of CRE in Israel was also successfully managed following the introduction of a coordinated national prevention strategy that included dissemination of guidelines to all facilities (i.e., strict adherence to contact isolation, cohorting of CRE patients with dedicated staffing) and the establishment of a national task force charged with overseeing facility adherence to recommended practices [95].
As CRE prevention has gained more attention in the USA, some state and local health departments have established dedicated programs to coordinate regional CRE prevention efforts [137,138]. Components of these regional initiatives vary, but have generally included improved CRE surveillance, dissemination of prevention recommendations, laboratory support for confirmatory susceptibility testing and mechanism detection and expert consultation about prevention when cases are identified. One example of a state-led CRE prevention initiative is Oregon’s Drug-Resistant Organism Prevention and Coordinated Regional Epidemiology (DROP-CRE) Network [138]. CRE are less common in Oregon compared with other parts of the USA, with only four carbapenemase-producing CRE isolates identified statewide as of November 2013. In an aggressive effort to prevent emergence and spread of CRE in Oregon, the state health department collaborated with leading healthcare institutions within the state and CDC to form the DROP-CRE Network. Starting in December 2011, all CRE isolates that met the state’s surveillance definition were reportable to the state health department. In response to reports of CRE, the health department provides real-time outbreak assistance to facilities. The Oregon State Public Health Laboratory has also expanded its capacity for carbapenem resistance mechanism testing to facilitate response efforts. In addition, a statewide database was created for tracking movement of CRE cases between facilities and capturing pertinent epidemiologic information that are reported monthly on a dedicated website. Other components of the program included a statewide education campaign on CRE and the development of a state-specific CRE Toolkit for all Oregon facilities to implement.
Expert commentary & five-year view
The emergence and spread of CRE, particularly those that produce a carbapenemase, pose a major clinical and public health challenge worldwide. Although KPC is the predominant carbapenemase found among Enterobacteriaceae in the USA, other carbapenemases, such as NDM, have increasingly been identified and have the potential to add to the overall burden of CRE. Currently recommended CRE prevention strategies are founded on basic infection control measures such as hand hygiene and standard approaches to the control of MDROs (e.g., Contact Precautions, patient and staff cohorting). Specific strategies include increased detection of patients infected or colonized with CRE. These efforts have been shown to control CRE transmission at a facility-level but they can be labor-intensive, and some interventions, such as surveillance cultures, can involve added costs. Universal adherence to recommended measures among healthcare personnel can also be challenging. In addition, efforts in individual facilities need to be complemented with coordinated regional approaches involving all the healthcare facilities in the area for maximum effect. However, more work is needed to better define the requirement of regional CRE prevention efforts and to promote their widespread implementation.
Future improvements in CRE prevention will require improved detection of carbapenemase-producing strains, including screening tests that are more sensitive and less labor intensive than the currently available culture-based techniques. Readily and rapidly available CRE resistance mechanism testing is also needed to help target prevention. Limiting transmission of CRE will also require the optimization of existing interventions. A greater understanding of how best to operationalize many of the current interventions, including Contact Precautions and CRE screening, in various types of healthcare settings is needed. Current methods of inter-facility communication about MDROs have been suboptimal and poor communication has led to CRE transmission. Future efforts in this area may include the enhancement of communications protocols, with a standardized transfer form for use among regional facilities, or creation of state-based CRE registries similar to the XDRO registry in Illinois [133]. As new antimicrobial agents to treat CRE may not be available for years, efforts to develop and expand effective antimicrobial stewardship programs across facilities will increasingly become a focus for CRE prevention.
In addition to the currently available interventions, novel interventions such as CRE decolonization warrant more thorough evaluation, possibly in concert with even more innovative interventions [139]. For example, one promising area involves harnessing the colonization resistance afforded by an intact microbiome to prevent, decrease or eliminate colonization and, thereby, transmission [140]. As noted, antibiotic exposure that is not limited to carbapenems is an important risk factor for CRE colonization in settings where transmission is likely. This risk is mediated by the disruption of the lower intestinal microbiome caused by a large number of different antibiotics, thereby leading to the loss of colonization resistance to CRE. Just as human fecal transplantation is being utilized to restore the intestinal microbiome and break the cycle of recurrent C. difficile infection (and subsequent eradication of colonization) [141], models for manipulating the microbiome to eradicate colonization caused by other MDR enteric organisms are already under development [142].
In conclusion, CRE represents an emerging MDRO of global concern. In the USA, although CRE have increased over the last decade, they remain relatively uncommon in many parts of the USA, suggesting that time is now to act aggressively to prevent their further emergence. Limiting the spread of these organisms will require a continued commitment to implement control strategies in both individual facilities and across regions.
Key issues.
The percent of Enterobacteriaceae that are non-susceptible to carbapenems continues to increase in the USA, likely due to the spread of carbapenem-resistant Enterobacteriaceae (CRE) strains that produce carbapenemases.
Since Klebsiella pneumoniae carbapenemase first emerged, it has remained the predominant carbapenemase in the USA; however, Enterobacteriaceae producing other carbapenemases, such as the New Delhi metallo-b-lactamase, are increasingly being identified.
Invasive infections (e.g., bloodstream infections) caused by CRE are associated with limited treatment options and high mortality rates.
Long-term acute care hospitals may have a high prevalence of patients colonized with CRE that can play an important role in the spread of CRE across a region as patients move across the continuum of care.
A basic element in any CRE prevention program is to understand how commonly these organisms are encountered at the facility and regional level through regular surveillance.
Current CRE prevention strategies for individual healthcare facilities include increased detection of patients infected or colonized with CRE and implementation of interventions to prevent transmission to other patients (i.e., hand hygiene, Contact Precautions and patient and staff cohorting).
Given the extent of inter-facility patient sharing among the US healthcare facilities, successful control of CRE will require a coordinated approach that engages all healthcare facilities that share patients in a region. State and local health departments are well-positioned to facilitate regional control efforts.
Future research for CRE control should include the development of better laboratory methods for CRE screening and mechanism testing as well as a greater understanding of how to operationalize current prevention interventions and identification of novel CRE prevention interventions (e.g., manipulating intestinal microbiome).
Footnotes
Disclaimer
The findings and conclusions in the report are those of the authors and do not necessarily represent the official position of the CDC.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Leavitt A, Navon-Venezia S, Chmelnitsky I, et al. Emergence of KPC-2 and KPC-3 in carbapenem-resistant Klebsiella pneumoniae strains in an Israeli hospital. Antimicrob Agents Chemother 2007;51(8):3026–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villegas MV, Lolans K, Correa A, et al. First detection of the plasmid-mediated class A carbapenemase KPC-2 in clinical isolates of Klebsiella pneumoniae from South America. Antimicrob Agents Chemother 2006;50(8): 2880–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuzon G, Naas T, Demachy MC, Nordmann P. Nosocomial outbreak of Klebsiella pneumoniae harbouring bla (KPC-3) in France subsequent to patient transfer from Italy. Int J Antimicrob Agents 2012;39(5):448–9 [DOI] [PubMed] [Google Scholar]
- 4.Papadimitriou M, Voulgari E, Ranellou K, et al. Emergence of VIM-1 metallo-β-lactamase-producing Escherichia coli in a neonatal intensive care unit. Microb Drug Resist 2011;17(1): 105–8 [DOI] [PubMed] [Google Scholar]
- 5.Patel G, Bonomo RA “Stormy waters ahead”: global emergence of carbapenemases. Front Microbiol 2013;4:48–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 2011;53(1):60–7 [DOI] [PubMed] [Google Scholar]
- 7.Bradford PA, Urban C, Mariano N, et al. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC beta-lactamase, and the foss of an outer membrane protein. Antimicrob Agents Chemother 1997;41(3):563–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow JW, Shlaes DM. Imipenem resistance associated with the loss of a 40 kDa outer membrane protein in Enterobacter aerogenes. J Antimicrob Chemother 1991; 28(4):499–504 [DOI] [PubMed] [Google Scholar]
- 9.MacKenzie FM, Forbes KJ, Dorai-John T, et al. Emergence of a carbapenem-resistant Klebsiella pneumoniae. Lancet 1997; 350(9080):783. [DOI] [PubMed] [Google Scholar]
- 10.Baraniak A, Grabowska A, Izdebski R, et al. Molecular characteristics of KPC-producing Enterobacteriaceae at the early stage of their dissemination in Poland, 2008-2009. Antimicrob Agents Chemother 2011;55(12): 5493–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitchel B, Rasheed JK, Patel JB, et al. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Anitmicrob Agents Chemother 2009;53(8):3365–70• This study describes the finding of a dominant strain of Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae, sequence type 258, which could represent either a strain that has successfully disseminated across the USA or a strain that has more readily acquired or maintained this resistance mechanism.
- 12.Agodi A, Voulgari E, Barchitta M, et al. Containment of an outbreak of KPC-3-producing Klebsiella pneumoniae in Italy. J Clin Microbiol 2011;49(11): 3986–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez GV, Master RN, Clark RB, et al. Klebsiella pneumoniae antimicrobial drug resistance, United States, 1998-2013. Emerg Infect Dis 2013;19(1):133–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep 2013;62(9):165–70•• This review describes recent changes in the epidemiology and incidence of CRE in the USA.
- 15.Zilberberg MD, Shorr AF. Secular trends in gram-negative resistance among urinary tract infection hospitalizations in the United States, 2000-2009. Infect Control Hosp Epidemiol 2013;34(9):940–6 [DOI] [PubMed] [Google Scholar]
- 16.Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001;45(4): 1151–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodford N, Tierno PM Jr, Young K, et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York Medical Center. Antimicrob Agents Chemother 2004;48(12):4793–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bratu S, Landman D, Haag R, et al. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch Intern Med 2005;165(12):1430–5 [DOI] [PubMed] [Google Scholar]
- 19.Chiang T, Mariano N, Urban C, et al. Identification of carbapenem-resistant Klebsiella pneumoniae harboring KPC enzymes in New Jersey. Microb Drug Resist 2007;13(4):235–9 [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Tracking CRE. Available from: www.cdc.gov/hai/organisms/cre/TrackingCRE.html [Last accessed 15 November 2013]
- 21.Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla (NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 2009;53(12):5046–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 2010;10(9):597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bogaerts P, Verroken A, Jans B, et al. Global spread of New Delhi metallo-β-lactamase 1. Lancet Infect Dis 2010;10(12): 831–2 [DOI] [PubMed] [Google Scholar]
- 24.D’Andrea MM, Venturelli C, Giani T, et al. Persistent carriage and infection by multidrug-resistant Escherichia coli ST405 producing NDM-1 carbapenemase: report on the first Italian cases. J Clin Microbiol 2011;49(7):2755–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huo TI. The first case of multidrug-resistant NDM-1-harboring Enterobacteriaceae in Taiwan: here comes the superbacteria!. J Chin Med Assoc 2010;73(11):557–8 [DOI] [PubMed] [Google Scholar]
- 26.Mulvey MR, Grant JM, Plewes K, et al. New Delhi metallo-β-lactamase in Klebsiella pneumoniae and Escherichia coli, Canada. Emerg Infect Dis 2011;17(1):103–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denis C, Poirel L, Carricajo A, et al. Nosocomial transmission of NDM-1-producing Escherichia coli within a non-endemic area in France. Clin Microbiol Infect 2012;18(5):E128–30 [DOI] [PubMed] [Google Scholar]
- 28.Brink AJ, Coetzee J, Clay CG, et al. Emergence of New Delhi metallo-beta-lactamase (NDM-1) and Klebsiella pneumoniae carbapenemase (KPC-2) in South Africa. J Clin Microbiol 2012;50(2): 525–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasheed JK, Kitchel B, Zhu W, et al. New Delhi metallo-β-lactamase-producing Enterobacteriaceae, United States. Emerg Infect Dis 2013;19(6):870–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nordmann P, Poirel L, Walsh TR, Livermore DM. The emerging NDM carbapenemases. Trends Microbiol 2011; 19(12):588–95 [DOI] [PubMed] [Google Scholar]
- 31.Kallen A, Rasheed JK, Lonsway D, et al. Evolving epidemiology of New Delhi metallo-ß-lactamase-producing Enterobacteriaceae reported in the United States. Presented at: ID Week 2013; 2 – 6 October 2013; San Francisco, CA, USA [Google Scholar]
- 32.Centers for Disease Control and Prevention. Carbapenem-resistant Enterobacteriaceae containing New Delhi metallo-beta-lactamase in two patients - Rhode Island, March 2012. MMWR Morb Mortal Wkly Rep 2012;61(24):446–8 [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Notes from the field: hospital outbreak of carbapenem-resistant Klebsiella pneumoniae producing New Delhi metallo-beta-lactamase – Denver, Colorado, 2012. MMWR Morb Mortal Wkly Rep 2013; 62(6):108. [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Notes from the Field: New Delhi Metallo-β-Lactamase-Producing Escherichia coli Associated with Endoscopic Retrograde Cholangiopancreatography - Illinois, 2013. MMWR Morb Mortal Wkly Rep 2014; 62(51):1051. [PMC free article] [PubMed] [Google Scholar]
- 35.Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 2012; 67(7):1597–606 [DOI] [PubMed] [Google Scholar]
- 36.Lascols C, Peirano G, Hackel M, et al. Surveillance and molecular epidemiology of Klebsiella pneumoniae isolates that produce carbapenemases: first report of OXA-48-like enzymes in North America. Antimicrob Agents Chemother 2013;57(1): 130–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathers AJ, Hazen KC, Carroll J, et al. First clinical cases of OXA-48-producing carbapenem-resistant Klebsiella pneumoniae in the United States: the “menace” arrives in the new world. J Clin Microbiol 2013; 51(2):680–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kallen A, Bulens S, Jacob J, et al. Characteristics of episodes of positive cultures for carbapenem non-susceptible gram-negative bacilli from three communities. Presented at: ID Week 2012; 17 – 21 October 2012; San Diego, CA, USA [Google Scholar]
- 39.Swaminathan M, Sharma S, Polianksy Blash S, et al. Prevalence and risk factors for acquisition of carbapenem-resistant Enterobacteriaceae in the setting of endemicity. Infect Control Hosp Epidemiol 2013;34(8):809–17 [DOI] [PubMed] [Google Scholar]
- 40.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis 2009;9(4):228–36 [DOI] [PubMed] [Google Scholar]
- 41.Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, et al. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother 2008;52(3): 1028–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gasink LB, Edelstein PH, Lautenbach E, et al. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect Control Hosp Epidemiol 2009;30(12): 1180–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marchaim D, Chopra T, Bogan C, et al. The burden of multidrug-resistant organisms on tertiary hospitals posed by patients with recent stays in long-term acute care facilities. Am J Infect Control 2012; 40(8):760–5 [DOI] [PubMed] [Google Scholar]
- 44.Borer A, Saidel-Odes L, Eskira S, et al. Risk factors for developing clinical infection with carbapenem-resistant Klebsiella pneumoniae in hospital patients initially only colonized with carbapenem-resistant K pneumoniae. Am J Infect Control 2012; 40(5):421–5 [DOI] [PubMed] [Google Scholar]
- 45.Orsi GB, García-Fernández A, Giordano A, et al. Risk factors and clinical significance of ertapenem-resistant Klebsiella pneumoniae in hospitalised patients. J Hosp Infect 2011; 78(1):54–8 [DOI] [PubMed] [Google Scholar]
- 46.Orsi GB, Bencardino A, Vena A, et al. Patient risk factors for outer membrane permeability and KPC-producing carbapenem-resistant Klebsiella pneumoniae isolation: results of a double case-control study. Infection 2013;41(1):61–7 [DOI] [PubMed] [Google Scholar]
- 47.Orsi GB, Venditti M. Carbapenem-resistant Klebsiella pneumoniae transmission associated to endoscopy. Am J Infect Control 2013;41(9):849–50 [DOI] [PubMed] [Google Scholar]
- 48.Aumeran C, Poincloux L, Souweine B, et al. Multidrug-resistant Klebsiella pneumoniae outbreak after endoscopic retrograde cholangiopancreatography. Endoscopy 2010;42(11):895–9 [DOI] [PubMed] [Google Scholar]
- 49.Alrabaa SF, Nguyen P, Sanderson R, et al. Early identification and control of carbapenemase-producing Klebsiella pneumoniae, originating from contaminated endoscopic equipment. Am J Infect Control 2013;41(6):562–4 [DOI] [PubMed] [Google Scholar]
- 50.Carbonne A, Thiolet JM, Fournier S, et al. Control of a multi-hospital outbreak of KPC-producing Klebsiella pneumoniae type 2 in France, September to October 2009. Euro Surveill 2010;15:48. [DOI] [PubMed] [Google Scholar]
- 51.Naas T, Cuzon G, Babics A, et al. Endoscopy-associated transmission of carbapenem-resistant Klebsiella pneumoniae producing KPC-2 beta-lactamase. J Antimicrob Chemother 2010;65(6): 1305–6 [DOI] [PubMed] [Google Scholar]
- 52.Gastmeier P, Vonberg RP. Klebsiella spp. in endoscopy-associated infections: we may only be seeing the tip of the iceberg. Infection 2014;42(1):15–21 [DOI] [PubMed] [Google Scholar]
- 53.Koo VS, O’Neill P, Elves A. Multidrug-resistant NDM-1 Klebsiella outbreak and infection control in endoscopic urology. BJU Int 2012; 110(11 Pt C):E922–6 [DOI] [PubMed] [Google Scholar]
- 54.Schechner V, Kotlovsky T, Kazma M, et al. Asymptomatic rectal carriage of bla(KPC) producing carbapenem-resistant Enterobacteriaceae: who is prone to become clinically infected? Clin Microbiol Infect 2013;19(5):451–6• This is a matched case–control study that assessed for independent predictors of subsequent clinical specimen with CRE among newly identified CRE rectal carriers.
- 55.Debby BD, Ganor O, Yasmin M, et al. Epidemiology of carbapenem resistant Klebsiella pneumoniae colonization in an intensive care unit. Eur J Clin Microbiol Infect Dis 2012;31(8):1811–17 [DOI] [PubMed] [Google Scholar]
- 56.Patel G, Huprikar S, Factor SH, et al. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008; 29(12):1099–106• This is one of the first studies in the USA to describe the mortality of invasive carbapenem-resistant K. pneumoniae infections and associated risk factors.
- 57.Hussein K, Raz-Pasteur A, Finkelstein R, et al. Impact of carbapenem resistance on the outcome of patients’ hospital-acquired bacteraemia caused by Klebsiella pneumoniae. J Hosp Infect 2013;83(4): 307–13 [DOI] [PubMed] [Google Scholar]
- 58.Ben-David D, Kordevani R, Keller N, et al. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect 2012;18(1):54–60 [DOI] [PubMed] [Google Scholar]
- 59.Urban C, Bradford PA, Tuckman M, et al. Carbapenem-resistant Escherichia coli harboring Klebsiella pneumoniae carbapenemase beta-lactamases associated with long-term care facilities. Clin Infect Dis 2008;46(11):e127–30 [DOI] [PubMed] [Google Scholar]
- 60.Centers for Disease Control and Prevention. Carbapenem-resistant Klebsiella pneumoniae associated with a long-term – care facility – West Virginia, 2009-2011. MMWR Morb Mortal Wkly Rep 2011;60(41):1418–20 [PubMed] [Google Scholar]
- 61.Endimiani A, Depasquale JM, Forero S, et al. Emergence of blaKPC-containing Klebsiella pneumoniae in a long-term acute care hospital: a new challenge to our healthcare system. J Antimicrob Chemother 2009;64(5):1102–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Munoz-Price LS, Hayden MK, Lolans K, et al. Successful control of an outbreak of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae at a long-term acute care hospital. Infect Control Hosp Epidemiol 2010;31(4):341–7 [DOI] [PubMed] [Google Scholar]
- 63.Chitnis AS, Caruthers PS, Rao AK, et al. Outbreak of carbapenem-resistant Enterobacteriaceae at a long-term acute care hospital: sustained reductions in transmission through active surveillance and targeted interventions. Infect Control Hosp Epidemiol 2012;33(10):984–92• This report describes the successful control of a large CRE outbreak at a long-term acute care hospital with spread to regional hospitals that utilized a stepwise approach to implementation of multiple infection prevention interventions over a 1-year period.
- 64.Won SY, Munoz-Price LS, Lolans K, et al. Emergence and rapid regional spread of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis 2011;53(6):532–40•• This report describes one of the largest regional outbreaks of CRE using exposure network analysis and highlights the importance of a coordinated regional control effort.
- 65.Prabaker K, Lin MY, McNally M, et al. Transfer from high-acuity long-term care facilities is associated with carriage of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae: a multihospital study. Infect Control Hosp Epidemiol 2012;33(12):1193–9•• This study consisted of a microbiologic survey to determine the relative prevalence of KPC-producing CRE carriage among patients admitted to short-term acute care hospitals from long-term care facilities (LTCF) or from the community. It also included a nested case–control study that showed CRE prevalence among LTCF patients differed by facility type.
- 66.Lin MY, Lyles-Banks RD, Lolans K, et al. The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis 2013;57(9):1246–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elemam A, Rahimian J, Mandell W. Infection with panresistant Klebsiella pneumoniae: a report of 2 cases and a brief review of the literature. Clin Infect Dis 2009;49(2):271–4 [DOI] [PubMed] [Google Scholar]
- 68.Nordmann P, Couard JP, Sansot D, Poirel L. Emergence of an autochthonous and community-acquired NDM-1-producing Klebsiella pneumoniae in Europe. Clin Infect Dis 2012;54(1):150–1 [DOI] [PubMed] [Google Scholar]
- 69.Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis 2011;11(5): 355–62• This is one of the first studies describing the presence of NDM-producing carbapenem-resistant Enterobacteriaceae (CRE) in environmental samples (water and seepage samples in India), highlighting the spread of CRE outside of healthcare.
- 70.Isozumi R, Yoshimatsu K, Yamashiro T, et al. bla(NDM-1)-positive Klebsiella pneumoniae from environment, Vietnam. Emerg Infect Dis 2012;18(8):1383–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siegel JD, Rhinehart E, Jackson M, Chiarello L; the Healthcare Infection Control Practices Advisory Committee. Management of multidrug-resistant organisms in healthcare settings, 2006. Available from: www.cdc.gov/hicpac/pdf/MDRO/MDROGuideline2006.pdf [Last accessed 21 November 2013] [DOI] [PubMed]
- 72.Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; Twentieth informational supplement (June 2010 update). CLSI document M100-S20-U. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2010 [Google Scholar]
- 73.Anderson KF, Lonsway DR, Rasheed JK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 2007; 45(8):2723–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carvalhaes CG, Picão RC, Nicoletti AG, et al. Cloverleaf test (modified Hodge test) for detecting carbapenemase production in Klebsiella pneumoniae: be aware of false positive results. J Antimicrob Chemother 2010;65(2):249–51 [DOI] [PubMed] [Google Scholar]
- 75.Endimiani A, Perez F, Bajaksouzian S, et al. Evaluation of updated interpretative criteria for categorizing Klebsiella pneumoniae with reduced carbapenem susceptibility. J Clin Microbiol 2010;48(12):4417–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lonsway DR, Wong BK, Anderson KF, Patel JB. Evaluation of testing schemes for the detection of KPC-producing Enterobacteriaceae. Presented at: American Society for Microbiology General Meeting 2010; 23 – 27 May 2010; San Diego, CA, USA [Google Scholar]
- 77.Nordmann P, Poirel L, Dortet L. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 2012; 18(9):1503–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsakris A, Kristo I, Poulou A, et al. Evaluation of boronic acid disk tests for differentiating KPC-possessing Klebsiella pneumoniae isolates in the clinical laboratory. J Clin Microbiol 2009;47(20): 362–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsakris A, Poulou A, Pournaras S, et al. A simple phenotypic method for the differentiation of metallo-beta-lactamases and class A KPC carbapenemases in Enterobacteriaceae clinical isolates. J Antimicrob Chemother 2010;65(8): 1664–71 [DOI] [PubMed] [Google Scholar]
- 80.Hrabák J, Studentová V, Walková R, et al. Detection of NDM-1, VIM-1, KPC, OXA-48, and OXA-162 carbapenemases by matrix-assisted laser desorption ionizationtime of flight mass spectrometry. J Clin Microbiol 2012;50(7):2441–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; Twenty-second informational supplement (January 2012). CLSI document M100-S22. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2012 [Google Scholar]
- 82.Centers for Disease Control and Prevention. 2012 CRE toolkit - guidance for control of carbapenem-resistant Enterobacteriaceae (CRE). Available from: www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf [Last accessed 21 November 2013]
- 83.Centers for Disease Control and Prevention. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR Morb Mortal Wkly Rep 2009;58(10):256–60 [PubMed] [Google Scholar]
- 84.Oregon Health Authority. Guidance for control of carbapenem-resistant Enterobacteriaceae (CRE), 2012 Oregon toolkit. Available from: http://public.health.oregon.gov/DiseasesConditions/DiseasesAZ/CRE/Documents/cre_toolkit.pdf [Last accessed 21 November 2013]
- 85.Wisconsin Division of Public Health. Guidance for preventing transmission of carbapenem-resistant Enterobacteriaceae (CRE) in acute care and long-term care hospitals. Available from: www.dhs.wisconsin.gov/publications/P0/p00532A.pdf [Last accessed 21 November 2013]
- 86.Wisconsin Division of Public Health. Guidance for preventing transmission of carbapenem-resistant Enterobacteriaceae (CRE) in skilled nursing facilities. Available from: www.dhs.wisconsin.gov/publications/P0/p00532.pdf [Last accessed 21 November 2013]
- 87.Minnesota Department of Health. Recommendations for the management of carbapenem-resistant Enterobacteriaceae (CRE) in acute and long-term acute care hospitals. Available from: www.health.state.mn.us/divs/idepc/dtopics/cre/acuterecs.pdf [Last accessed 21 November 2013]
- 88.Minnesota Department of Health. Recommendations for the management of carbapenem-resistant Enterobacteriaceae (CRE) in long-term care facilities. Available from: www.health.state.mn.us/divs/idepc/dtopics/cre/rec.pdf [Last accessed 21 November 2013]
- 89.Zimmerman FS, Assous MV, Bdolah-Abram T, et al. Duration of carriage of carbapenem-resistant Enterobacteriaceae following hospital discharge. Am J Infect Control 2013;41(3):190–4 [DOI] [PubMed] [Google Scholar]
- 90.Schechner V, Kotlovsky T, Tarabeia J, et al. Predictors of rectal carriage of carbapenem-resistant Enterobacteriaceae (CRE) among patients with known CRE carriage at their next hospital encounter. Infect Control Hosp Epidemiol 2011;32(5):497–503 [DOI] [PubMed] [Google Scholar]
- 91.Feldman N, Adler A, Molshatzki N, et al. Gastrointestinal colonization by KPC-producing Klebsiella pneumoniae following hospital discharge: duration of carriage and risk factors for persistent carriage. Clin Microbiol Infect 2013;19(4):E190–6• This study examined the duration of KPC-producing K. pneumoniae carriage following hospital discharge, assessed for risk factors for persistent carriage among patients with remote versus recent acquisition of KPC-producing Klebsiella pneumoniae, and examined rates of resolution of carriage using varying number of negative rectal tests as criteria.
- 92.Gregory CJ, Llata E, Stine N, et al. Outbreak of carbapenem-resistant Klebsiella pneumoniae in Puerto Rico associated with a novel carbapenemase variant. Infect Control Hosp Epidemiol 2010;31(5): 476–84 [DOI] [PubMed] [Google Scholar]
- 93.Poulou A, Voulgari E, Vrioni G, et al. Imported Klebsiella pneumoniae carbapenemase-producing K. pneumoniae clones in a Greek hospital: impact of infection control measures for restraining their dissemination. J Clin Microbiol 2012; 50(8):2618–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Munoz-Price LS, Quinn JP. Deconstructing the infection control bundles for the containment of carbapenem-resistant Enterobacteriaceae. Curr Opin Infect Dis 2013;26(4):378–87 [DOI] [PubMed] [Google Scholar]
- 95.Schwaber MJ, Lev B, Israeli A, et al. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin Infect Dis 2011;52(7):848–55•• This study describes the implementation of a centrally coordinated intervention with national oversight that resulted in the control of a nationwide outbreak of CRE in Israel.
- 96.Valiquette L, Cossette B, Garant MP, et al. Impact of a reduction in the use of high-risk antibiotics on the course of an epidemic of Clostridium difficile-associated disease caused by the hypervirulent NAP1/027 strain. Clin Infect Dis 2007; 45(Suppl 2):S112–21 [DOI] [PubMed] [Google Scholar]
- 97.Carling P, Fung T, Killion A, et al. Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect Control Hosp Epidemiol 2003;24(9):699–706 [DOI] [PubMed] [Google Scholar]
- 98.Bogan C, Marchaim D. The role of antimicrobial stewardship in curbing carbapenem resistance. Future Microbiol 2013;8(8):979–91 [DOI] [PubMed] [Google Scholar]
- 99.Lima AL, Oliveira PR, Paula AP, et al. Carbapenem stewardship: positive impact on hospital ecology. Braz J Infect Dis 2011; 15(1):1–5 [DOI] [PubMed] [Google Scholar]
- 100.Lewis GJ, Fang X, Gooch M, Cook PP. Decreased resistance of Pseudomonas aeruginosa with restriction of ciprofloxacin in a large teaching hospital’s intensive care and intermediate care units. Infect Control Hosp Epidemiol 2012;33(4):368–73 [DOI] [PubMed] [Google Scholar]
- 101.Dortch MJ, Fleming SB, Kauffmann RM, et al. Infection reduction strategies including antibiotic stewardship protocols in surgical and trauma intensive care units are associated with reduced resistant gram-negative healthcare-associated infections. Surg Infect 2011;12(1):15–25 [DOI] [PubMed] [Google Scholar]
- 102.Ghafur A, Nagvekar V, Thilakavathy S, et al. “Save Antibiotics, Save lives”: an Indian success story of infection control through persuasive diplomacy. Antimicrob Resist Infect Control 2012;1(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Centers for Disease Control and Prevention. Core Elements of Hospital Antibiotic Stewardship Programs. US Department of Health and Human Services, CDC, Atlanta, GA, USA; 2014. Available from: www.cdc.gov/getsmart/healthcare/implementation/core-elements.html [Google Scholar]
- 104.Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007;44(2):159–77 [DOI] [PubMed] [Google Scholar]
- 105.The Joint Commission. Antimicrobial Stewardship Toolkit. Available from: http://store.jcrinc.com/antimicrobial-stewardship-toolkit/ [Last accessed 27 February 2014]
- 106.American Society of Health-System Pharmacists. Implementing antimicrobial stewardship programs in health systems: an interprofessional team approach. Available from: www.leadstewardship.org [Last accessed 27 February 2014]
- 107.Centers for Disease Control and Prevention. Get Smart for Healthcare, Implementation Resources. Checklist for Core Elements of Hospital Antibiotic Stewardship Programs. Available from: www.cdc.gov/getsmart/healthcare/implementation.html [Last accessed 4 March 2014]
- 108.Wiener-Well Y, Rudensky B, Yinnon AM, et al. Carriage rate of carbapenem-resistant Klebsiella pneumoniae in hospitalised patients during a national outbreak. J Hosp Infect 2010;74(4):344–9 [DOI] [PubMed] [Google Scholar]
- 109.Thurlow CJ, Prabaker K, Lin MY, et al. Anatomic sites of patient colonization and environmental contamination with Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae at long-term acute care hospitals. Infect Control Hosp Epidemiol 2013;34(1):56–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Centers for Disease Control and Prevention. Available from: www.cdc.gov/HAI/pdfs/labSettings/Klebsiella_or_Ecoli.pdf
- 111.Vrioni G, Daniil I, Voulgari E, et al. Comparative evaluation of a prototype chromogenic medium (ChromID CARBA) for detecting carbapenemase-producing Enterobacteriaceae in surveillance rectal swabs. J Clin Microbiol 2012;50(6):1841–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nijhuis R, Samuelsen O, Savelkoul P, van Zwet A. Evaluation of a new real-time PCR assay (Check-Direct CPE) for rapid detection of KPC, OXA-48, VIM, and NDM carbapenemases using spiked rectal swabs. Diagn Microbiol Infect Dis 2013; 77(4):316–20 [DOI] [PubMed] [Google Scholar]
- 113.Lowe CF, Kus JV, Salt N, et al. Nosocomial transmission of New Delhi metallo-β-lactamase-1-producing Klebsiella pneumoniae in Toronto, Canada. Infect Control Hosp Epidemiol 2013;34(1):49–55 [DOI] [PubMed] [Google Scholar]
- 114.Tschudin-Sutter S, Frei R, Dangel M, et al. Rate of transmission of extended-spectrum beta-lactamase-producing Enterobacteriaceae without contact isolation. Clin Infect Dis 2012;55(11):1505–11 [DOI] [PubMed] [Google Scholar]
- 115.Calfee D, Jenkins SG. Use of active surveillance cultures to detect asymptomatic colonization with carbapenem-resistant Klebsiella pneumoniae in intensive care unit patients. Infect Control Hosp Epidemiol 2008;29(10):966–8• This is one of the first studies in the USA that describes the use of active surveillance testing to enhance facility-level prevention of carbapenem-resistant K. pneumoniae.
- 116.Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med 2011;364(15):1407–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Derde LP, Dautzenberg MJ, Bonten MJ. Chlorhexidine body washing to control antimicrobial-resistant bacteria in intensive care units: a systematic review. Intensive Care Med 2012;38(6):931–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: expanding the armamentarium for infection control and prevention. Clin Infect Dis 2008;46(2): 274–81 [DOI] [PubMed] [Google Scholar]
- 119.Palmore TN, Henderson DK. Managing transmission of carbapenem-resistant enterobacteriaceae in healthcare settings: a view from the trenches. Clin Infect Dis 2013;57(11):1593–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Naparstek L, Carmeli Y, Chmelnitsky I, et al. Reduced susceptibility to chlorhexidine among extremely-drug-resistant strains of Klebsiella pneumoniae. J Hosp Infect 2012; 81(1):15–19 [DOI] [PubMed] [Google Scholar]
- 121.Popovich KJ, Lyles R, Hayes R, et al. Relationship between chlorhexidine gluconate skin concentration and microbial density on the skin of critically ill patients bathed daily with chlorhexidine gluconate. Infect Control Hosp Epidemiol 2012;33(9): 889–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kotsanas D, Wijesooriya WR, Korman TM, et al. “Down the drain”: carbapenem-resistant bacteria in intensive care unit patients and handwashing sinks. Med J Aust 2013;198(5):267–9 [DOI] [PubMed] [Google Scholar]
- 123.Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med 2012;4(148):148ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lerner A, Adler A, Abu-Hanna J, et al. Environmental contamination by carbapenem-resistant Enterobacteriaceae. J Clin Microbiol 2013;51(1):177–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smith DL, Dushoff J, Perencevich EN, et al. Persistent colonization and the spread of antibiotic resistance in nosocomial pathogens: resistance is a regional problem. Proc Natl Acad Sci USA 2004;101(10): 3709–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Evans RS, Lloyd JF, Abouzelof RH, et al. System-wide surveillance for clinical encounters by patients previously identified with MRSA and VRE. Stud Health Technol Inform 2004;107(Pt 1):212–16 [PubMed] [Google Scholar]
- 127.Marquez P, Terashita D, Dassey D, Mascola L. Population-based incidence of carbapenem-resistant Klebsiella pneumoniae along the continuum of care, Los Angeles County. Infect Control Hosp Epidemiol 2013;34(2):144–50 [DOI] [PubMed] [Google Scholar]
- 128.Lolans K, Rice TW, Munoz-Price LS, Quinn JP. Multicity outbreak of carbapenem-resistant Acinetobacter baumannii isolates producing the carbapenemase OXA-40. Antimicrob Agents Chemother 2006;50(9):2941–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guh AY, McDonald LC, Sinkowitz-Cochran R. Assessment of public health perspectives on responding to an emerging pathogen: carbapenem-resistant enterobacteriaceae. J Public Health Manag Pract 2013;19(4):E27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thibodeau E, Duncan R, Snydman DR, et al. Carbapenem-resistant enterobacteriaceae: a statewide survey of detection in Massachusetts hospitals. Infect Control Hosp Epidemiol 2012;33(9):954–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vermont Department of Health Agency of Human Services, Health Advisory, Carbapenem-resistant Enterobacteriaceae. Available from: http://healthvermont.gov/advisory/2011/071211_Enterobacteriaceae.aspx [Last accessed 25 November 2013]
- 132.The Association for Professionals in Infection Control and Epidemiology. Summary of State CRE Reporting Requirements. Available from: www.apic.org/Resource_/TinyMceFileManager/Advocacy-PDFs/CRE_ReportingRequirements_Final.pdf [Last accessed 25 November 2013]
- 133.XDRO Registry. Available from: www.xdro.org/index.html [Last accessed 25 November 2013]
- 134.Centers for Disease Control and Prevention. Reduction in central line-associated bloodstream infections among patients in intensive care units – Pennsylvania, April 2001-March 2005. MMWR Morb Mortal Wkly Rep 2005;54(40):1013–16 [PubMed] [Google Scholar]
- 135.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2005;355(26): 2725–32 [DOI] [PubMed] [Google Scholar]
- 136.Ostrowsky BE, Trick WE, Sohn AH, et al. Control of vancomycin-resistant enterococcus in health care facilities in a region. N Engl J Med 2001;344(19): 1427–33 [DOI] [PubMed] [Google Scholar]
- 137.Wisconsin Department of Health Services. Carbapenem-resistant Enterobacteriaceae. Available from: www.dhs.wisconsin.gov/communicable/ARO/CRE.htm [Last accessed 25 November 2013]
- 138.Oregon Health Authority, Oregon Public Health Division. CD Summary, “Drop everything, the CRE are coming!”. Available from: http://public.health.oregon.gov/DiseasesConditions/CommunicableDisease/CDSummaryNewsletter/Documents/2013/ohd6209.pdf [Last accessed 25 November 2013]
- 139.Oren I, Sprecher H, Finkelstein R, et al. Eradication of carbapenem-resistant Enterobacteriaceae gastrointestinal colonization with nonabsorbable oral antibiotic treatment: a prospective controlled trial. Am J Infect Control 2013;41(12): 1167–72 [DOI] [PubMed] [Google Scholar]
- 140.Tosh PK, McDonald LC. Infection control in the multidrug-resistant era: tending the human microbiome. Clin Infect Dis 2012; 54(5):707–13 [DOI] [PubMed] [Google Scholar]
- 141.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013;368(5):407–15 [DOI] [PubMed] [Google Scholar]
- 142.Ubeda C, Bucci V, Caballero S, et al. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infect Immun 2013;81(3):965–73 [DOI] [PMC free article] [PubMed] [Google Scholar]