Abstract
The adenosine triphosphate (ATP)-sensitive K+ (KATP) channels are hetero-octameric protein complexes comprising four pore-forming subunit Kir6.x subunits and four regulatory subunit sulfonylurea receptor SURx subunits. They are prominent in myocytes, pancreatic β cells and neurons, and link cellular metabolism with membrane excitability. Using genetically modified animals and genomic analysis in patients, recent studies have implicated certain KATP channel subtypes in physiological and pathological processes in a variety of cardiovascular diseases. In this review, we focus on the causal relationship between KATP channel activity and pathophysiology in the cardiovascular system, particularly from the perspective of genetic changes in human and animal models.
Journal Subject Terms: Animal Models of Human Disease, Basic Science Research, Clinical Studies, Ion Channels/Membrane Transport, Pathophysiology
Keywords: KATP, candidate genes, Kir6.1, arrhythmia, heart failure, ABCC9, ABCC8, KCNJ8, KCNJ9, Kir6.2, SUR1, SUR2A, SUR2B, Cantu syndrome
Introduction
It has been over 30 years since Noma first discovered adenosine triphosphate-sensitive potassium channels in cardiac muscle in 19831. They were subsequently found in skeletal myocytes2, pancreatic β cells3, vascular smooth muscle4, vascular endothelium5 and the central nervous system6. Although they may be the most densely expressed potassium channels in the heart7, KATP channels are closed under normal condition and play little or no role in cell excitability. However, when exposed to a severe metabolic stress, such as anoxia, metabolic inhibition, or ischemia, these channels can open and the consequent decrease in excitability and contractility is thought to be cardioprotective because of preservation of ATP8. In addition to preservation of ATP, KATP activation-dependent shortening of the action potential, as well as reduction of Ca2+ entry and inhibition of contractility, may in turn lead to arrhythmias and cardiac insufficiency9. If fully activated, KATP channel density in the heart can result in complete cessation of cardiac electrical activity and contractile failure7, 8. Therefore, the KATP channel may represent a ‘double-edged sword’ in regulating cardiac excitability. In vascular smooth muscle and endothelium, activation of KATP channels will lead to membrane hyperpolarization, resulting in decreased Ca2+ current and vasodilation10. Conversely, inhibition of KATP channels will cause membrane depolarization, increase in Ca2+ current and vasoconstriction10. Hence, KATP channels can also play a key role in regulating vessel tone and blood flow. Here, we give an overview of recent advances in understanding of the molecular structure and physiological function of KATP channel in heart and blood vessels, with specific focus on the relationship between genetic changes in KATP channels and cardiovascular function.
Molecular structure, distribution and regulation of KATP
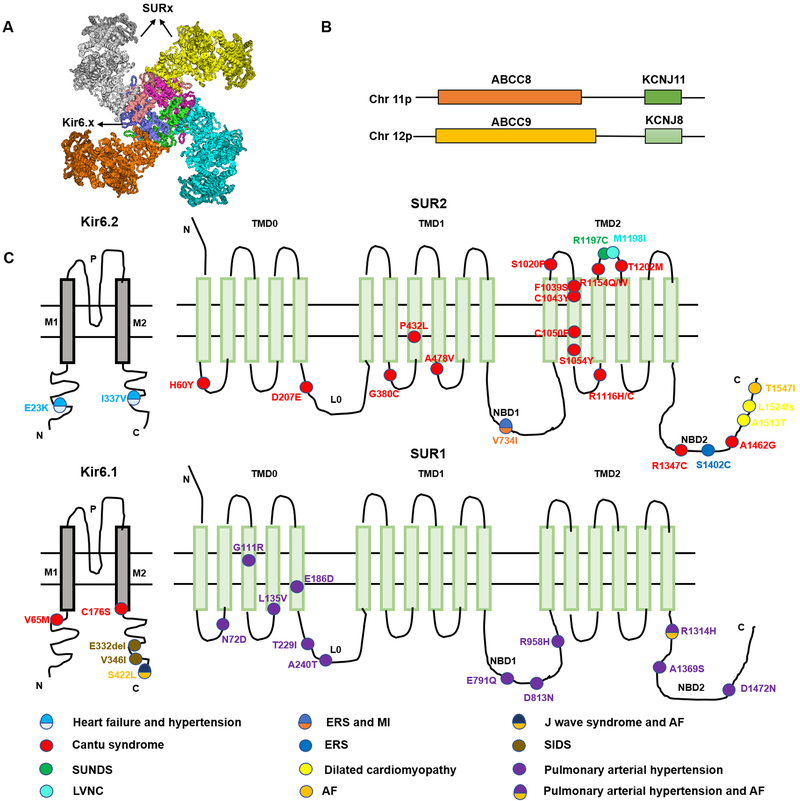
KATP channels are hetero-octamers composed of four pore-forming inward rectifier Kir6.X (Kir6.1 and Kir6.2, encoded by KCNJ8 and KCNJ11 respectively) subunits, each coupled with a regulatory subunit sulfonylurea receptor SURX (SUR1 or SUR2, encoded by SUR1 and SUR2 respectively, Figure 1A)11–13 . The SUR genes are large, each containing ~40 exons, and there are two recognized major spliced variants of SUR2, SUR2A and SUR2B, which result from alternative splicing of the terminal exon in ABCC914, 15. SUR2A and B consequently differ in the last 42 amino acids of the C terminus, resulting in distinct physiological and pharmacological properties. The obligate octameric arrangement may result from co-regulation of expression of Kir6 and SUR subunits: ABCC8 and KCNJ11 are immediately adjacent to each other on human chromosome 11p15.113, whereas ABCC9 and KCNJ8 are immediately adjacent to one another on human chromosome 12p12.1(Figure 1B)13, 14.
Figure 1:
KATP channel structure and KATP channel mutations associated with cardiovascular diseases. (A) KATP channels are octameric complexes of four Kir6 subunits and four SUR subunits. (B) Human SUR and Kir6 gene structures. ABCC8 and KCNJ11 are next to each other and located on human chromosome 11p15.1, ABCC9 and KCNJ8 are also adjacent to each other, located on human chromosome 12p12.1. (C) KATP channel subunit mutations associated with cardiovascular pathologies. P - p-helix, M1, M2 - transmembrane helices , TMD - transmembrane domain, L0 - intracellular linker domain, NBD1 - first nucleotide binding domain, NBD2 - second nucleotide binding domain.
Kir6.X subunits are typical Kir channel subunits, consisting of two transmembrane M1 and M2 helices connected by a pore-forming loop with a glycine-phenylalanine-glycine signature motif for K+ selectivity, and cytoplasmic N- and C-termini16. SUR subunits are members of the ABC protein superfamily, and consist of two six-helix transmembrane domains (TMD1 and TMD2), and an additional unique five-helix TMD0 at the N-terminus of SUR subunit joined to TMD1 by a cytoplasmic linker (L0) that provides a physical link between SUR function and Kir6 subunit gating and trafficking17. As in all ABC proteins, each of TMD1 and TMD2 are linked at the C-terminal ends to cytoplasmic nucleotide binding domains (NBD1 and NBD2, respectively)18. The structure and the sequence of NBDs are highly conserved. Both contain a conserved Walker A (WA) motif and a Walker B (WB) motif. These motifs contain Mg2+-adenosine nucleotide binding sites. At least NBD2 catalyzes ATP hydrolysis and is critical for Mg-nucleotide regulation of ABC protein functional activity (Figure 1C)19, 20.
KATP channels are expressed in various tissues, but the constitution differs significantly between and within different tissues. The current consensus is that KATP channels consist primarily of Kir6.2 and SUR2A in both normal human atrial and ventricular myocyte cells, although all four subunits are detected21. Under different physiological or pathological conditions, the KATP channel subunit constitution may change or there may be plasticity of SUR subunits which can lead to different subunits being functional in different conditions21. In mice, it is clear that SUR1 and Kir6.2 are the primary subunits in atrial KATP while SUR2 and Kir6.2 are the main subunits in the ventricle22, 23. KATP channels have also been identified throughout the cardiac pacemaker/conduction system, including the sino-atrial (SA) node24, atrio-ventricular (AV) node25 and Purkinje fibers26, and several studies have indicated Kir6.1, Kir6.2 and SUR2B are necessary for functional KATP in these tissues23, 25–28. Vascular smooth muscle KATP channels are primarily formed by Kir6.1 and SUR2B, whereas vascular endothelial KATP channel is suggested to be composed of Kir6.1, Kir6.2 and SUR2B29, 30.
In addition to the cell membrane, KATP channels have been reported to be present in mitochondria, and to be involved in regulation of oxidative phosphorylation, and protection from ischemia-reperfusion injury31–34. Recent studies have suggested that this proposed mitoKATP may contain Kir1.1 and/or SUR2A-5535–38, but direct evidence is lacking, and even the constitution of any mitoKATP remains unclear.
KATP channel regulation is complex, and involves metabolites, hormones and neurotransmitters as well as transcriptional mechanisms39. A hallmark feature of KATP channels is their sensitivity to metabolic changes in nucleotide levels. Micromolar ATP inhibits channels by direct interaction with Kir6 subunits, and since the cellular ATP concentration is relatively high (i.e. millimolar) in physiological conditions, ATP inhibition is usually sufficient to maintain channels in predominantly closed states (Figure 1D)40 . However, ATP inhibition is overridden by MgATP and MgADP interacting with SUR subunit NBFs7, and ATP sensitivity is reduced by membrane phosphoinositides, such as phosphatidylinositol-4,5-bisphosphate (PIP2), long-chain acyl-CoA esters (LC-CoAs), and metabolic derivatives of free fatty acids41. Both PIP2 and LC-CoAs act on Kir6.2 to antagonize ATP inhibition and increase channel open probability42. In addition, other metabolic factors including PH, nitric oxide (NO), eicosanoids, hydrogen sulfide (H2S) as well as hormones and neurotransmitters, can also affect KATP channel activity39, 43, 44.
KATP channels are uniquely endowed with sensitivity to a large number of pharmacological agents that interact with the SUR subunits. Multiple KATP channel openers (KCOs) (e.g., nicorandil, cromakalim, pinacidil and diazoxide) act on SURx subunits to activate the channel18. SUR1-containing channels can be strongly activated by diazoxide, but not by pinacidil or cromakalim23, 45, 46, whereas channels containing SUR2A respond potently to both pinacidil and cromakalim, but weakly to diazoxide46–48. Channels containing SUR2B are sensitive to diazoxide and cromakalim, as well as to pinacidil47–49. Because nucleotide binding and hydrolysis at NBDs are important for the binding and action of these KCOs, the different sensitivities of SUR1 and SUR2 subtypes to these KCOs may partially result from differences in nucleotide sensitivity45, 49–51. Pinacidil and cromakalim effectively act to decrease sensitivity to inhibitory ATP, leading to increased channel opening at a given level of cytosolic ATP, whereas diazoxide needs the presence of intracellular ADP for channel activity49. All SUR isoforms can be inhibited by sulfonylureas such as tolbutamide and glibenclamide, which are commonly used as KATP channel inhibitors52. SUR1-containing channels are more sensitive to sulfonylureas than SUR2-containing channels53–55, and are widely used to treat diabetes, where they act to trigger insulin secretion via interactions with SUR1-dependent KATP channels in pancreatic β-cells18.
KATP channels and cardiovascular diseases
There is now a significant literature reporting association of KATP gene mutations and variants with cardiovascular pathologies (Table 1). As discussed below, the evidence in support of causal association is weak in many cases, but some clear causal links have now been established.
Table 1.
: Cardiovascular pathologies associated with KATP channel variants
| Gene | Nucleotide change | Protein change | Mutation feature | Clinical Condition | References |
|---|---|---|---|---|---|
| KCNJ11 | c.67 G>A | E23K | GOF | Heart failure, hypertension, ventricular arrhythmias | 56–63 |
| c.570 C>T | A190A | GOF | Hypertension | 56, 58, 59 | |
| c.1009 G>A | I337V | GOF | Heart failure, hypertension | 56, 64 | |
| KCNJ8 | c.193 G>A | V65M | GOF | Cantu syndrome | 65 |
| c.526 T>A | C176S | GOF | Cantu syndrome | 66 | |
| c.del995–997 GAA | E332del | LOF | sudden infant death syndrome | 67 | |
| c.1036 G>A | V346I | LOF | sudden infant death syndrome | 67 | |
| c.1265C>T | S422L | GOF | J wave syndrome, atrial fibrillation | 68–71 | |
| ABCC8 | c.A214G | N72D | LOF | Pulmonary arterial hypertension, Atrial septal defect | 72 |
| c.G331A | G111R | LOF | Pulmonary arterial hypertension | 72 | |
| c.C403G | L135V | LOF | Pulmonary arterial hypertension, Heart block | 72 | |
| c.G558T | E186D | LOF | Pulmonary arterial hypertension | 72 | |
| c.C686T | T229I | LOF | Pulmonary arterial hypertension | 72 | |
| c.G718A | A240T | LOF | Pulmonary arterial hypertension | 72 | |
| c.G2371C | E791Q | LOF | Pulmonary arterial hypertension | 72 | |
| c.G2437A | E813N | LOF | Pulmonary arterial hypertension, Atrial fibrillation | 72 | |
| c.G2873A | R958H | LOF | Pulmonary arterial hypertension | 72 | |
| c.G3941A | R1314H | LOF | Pulmonary arterial hypertension, Ventricular septal defect, Atrial fibrillation | 72, 73 | |
| c.4105G>T | A1369S | LOF | Reduced risk of coronary heart disease | 74 | |
| c.G4414A | D1472N | LOF | Pulmonary arterial hypertension | 72 | |
| ABCC9 | c.178C>T | H60Y | GOF | Cantu syndrome | 75 |
| c.621C>A | D207E | GOF | Cantu syndrome | 75 | |
| c.1138G>T | G380C | GOF | Cantu syndrome | 75 | |
| c.1295C>T | P432L | GOF | Cantu syndrome | 75 | |
| c.1433C>T | A478V | GOF | Cantu syndrome | 76 | |
| c.2200G>A | V734I | GOF | myocardial infarction, Bradycardia, ICCD early repolarization syndrome, | 77–80 | |
| c.3058T>C | S1020P | GOF | Cantu syndrome | 75 | |
| c.3116T>C | F1039S | GOF | Cantu syndrome | 75 | |
| c.3128G>A | C1043Y | GOF | Cantu syndrome | 76 | |
| C1050F | GOF | Cantu syndrome | 81 | ||
| c.3161C>A | S1054Y | GOF | Cantu syndrome | 75 | |
| c.3347G>A | R1116H | GOF | Cantu syndrome | 75 | |
| c.3346C>T | R1116C | GOF | Cantu syndrome | 75 | |
| c.3460C>T | R1154W | GOF | Cantu syndrome | 75, 76, 82, 83 | |
| c.3461G>A | R1154Q | GOF | Cantu syndrome | 75, 76, 83 | |
| c.3589C>T | R1197C | Uncertain | SUNDS | 84 | |
| c.3594G>A | M1198I | Uncertain | LVNC | 85 | |
| c.3605C>T | T1202M | GOF | Cantu syndrome | 86 | |
| c.4039 C > T | R1347C | GOF | Cantu syndrome | 87 | |
| c.4205C>G | S1402C | GOF | early repolarization syndrome | 79 | |
| c.4385C>G | A1462G | GOF | Cantu syndrome | 88 | |
| c.4537G>A | A1513T | LOF | Dilated cardiomyopathy | 89 | |
| 4570–4572 delta InsAAAT | L1524fs | LOF | Dilated cardiomyopathy | 89 | |
| c.4640C>T | T1547I | LOF | Atrial fibrillation | 90 |
Bold font: Causality implied/confirmed by functional analyses
Normal font: Association unchallenged, but lacking functional analyses
Italic font: Association challenged by additional studies
Kir6.2
Kir6.2 is the primary pore-forming subunit of KATP channels in both cardiac myocytes and pancreatic β-cells91. Over 50 human mutations in KCNJ11–encoded Kir6.2 have been reported, and gain- and loss-of-function of Kir6.2 are were well known to cause neonatal diabetes and congenital hyperinsulinism, respectively92. In addition, the common Kir6.2 variant, E23K (encoded by c.67G>A, rs5219) in KCNJ11, has been well characterized as a type 2 diabetes-associated risk factor. It has also been reported to be overrepresented in human congestive heart failure60, and associated with adverse subclinical myocardial remodeling among subjects with hypertension in a cross-sectional community-based cohort study, as well as abnormal cardiopulmonary stress test results in heart failure patients, and also occurrence of ventricular arrhythmias (VAs) in dilated cardiomyopathy patients60, 61, 63. Other studies have also indicated an association of E23K, A190A (c.570C>T, rs5218) and I337V(c.1009G>A) variants in the KCNJ11 gene to hypertension susceptibility, especially in the Asian population56–59, 62. In one animal study, it was suggested that the E23K variant increases susceptibility to ventricular arrhythmia in response to ischemia in rats93. However, another study that aimed to evaluate the clinical impact of single-nucleotide polymorphisms in KCNJ11 found the SNPs - rs5215_GG, rs5218_CT, and rs5219_AA for KCNJ11 – did not affect susceptibility to ischemic heart disease (IHD) or coronary microvascular dysfunction94. More recently, the I337V and E23K variants were reported to be associated with left ventricular mass and left ventricular end-diastolic volume in heart failure patients64, but direct causation remains unconfirmed.
Animal models with transgenic expression of ATP-insensitive Kir6.2 subunits are strikingly insensitive to any potential overactivity95. Genetic ablation of the Kir6.2 subunit in mice (Kir6.2−/−) results in poor cardiac functional recovery after exercise96 or IR injury97, but does not alter cardiac function under basal aerobic conditions98. However, another study showed increased basal AMPK activity, fatty acid oxidation, and glycogen storage, as well as decreased glycolysis and reduced mitochondrial density in Kir6.2−/− hearts. This suggests that KATP channels may somehow regulate cardiac metabolism99. Further studies might consider whether genetic variations in KCNJ11 may help to provide biomarkers of relevance to various cardiac problems.
Kir6.1
Kir6.1 is the main channel forming subunit of KATP channels in smooth muscle. Disruption of KCNJ8 in mouse has been reported to cause ST segment elevation followed by atrioventricular block and early sudden cardiac death (SCD) because of coronary spasm100. Other studies do not report sudden death, but both Kir6.1−/− and mice with specific deletion Kir6.1 in smooth muscle do show elevated blood pressure101, 102. Two KCNJ8 mutations, an in-frame deletion (p.E332del, c.del995–997 GAA) and a missense mutation (p.V346I, c. 1036 G>A), both localized to the Kir6.1 C-terminus, were identified in sudden infant death syndrome (SIDS) patients, and demonstrated to be LOF mutations67.
Conversely, transgenic expression of gain-of-function Kir6.1 subunits in smooth muscle leads to hypotension102, consistent with a major role in BP control. Kir6.1 may also be expressed in the cardiac conduction system27. Transgenic mice expressing Kir6.1 subunits in cardiomyocytes revealed AV nodal conduction abnormalities and junctional rhythm103, and a recent study reported that mice with Kir6.1 specifically knocked out of conducting tissues display decreased heart rate and sinus arrest104. Several mutations in Kir6.1 subunits have been reported in human patients with rhythm disturbances. A missense variant in exon 3 (p. S422L, c.1265C>T) of the KCNJ8 gene was first reported in a patient with recurrent ventricular fibrillation secondary to early repolarization syndrome68. Subsequent studies indicated a higher KATP current in cells heterologously expressing Kir6.1/S422L+SUR2A channel in whole-cell patch-clamp studies, as well as reduced ATP sensitivity in inside-out patch clamp experiments69–71. However, causal association to the J-wave syndrome has been questioned by additional studies that (1) revealed the S422L variant to be a common occurrence in Ashkenazim105, (2) reported no effect on KATP channel activity or ATP-sensitivity66, and (3) show lack of any effects on the ECG of mice transgenically expressing the S422L variant in cardiac myocytes106. Most significantly, two novel KCNJ8 mutations have now been identified in patients with Cantu syndrome (see SUR2, below). A Cantu syndrome patient with the V65M (c. 193 G>A) variant in KCNJ8 had striking vascular abnormalities, including a dilated aortic root, very dilated and tortuous cerebral arteries and veins65, but no evidence of J-wave syndrome. Another Cantu patient with a missense mutation encoding Kir6.1[p.C176S, c.526 T>A], exhibited all clinical features of Cantu syndrome including cardiomegaly. Both of these two mutations were confirmed as gain of function mutations66, 107. ‘Cantu mice’, in which the Kir6.1[V65M] mutation was introduced to the endogenous gene locus using CRISPR/Cas9, also displayed the same phenotypes as Cantu patients, including dilated vessels, low blood pressure and cardiac hypertrophy108. These results, together with the findings of SUR2 association with Cantu syndrome (below) definitively tie this channel to a defined cardiovascular pathology.
SUR1
SUR1 is the predominant regulatory subunit of KATP channels in pancreatic β-cells as well as in mouse atria. Gain- and loss-of-function mutations in ABCC8 cause neonatal diabetes and congenital hyperinsulinism, respectively92. Recently several clinical studies have reported ABCC8 mutations to also be associated with cardiovascular diseases, including coronary heart disease, pulmonary arterial hypertension and atrial fibrillation72–74. The SUR1 (p. A1369S, c.4105G>T) missense variant, an inherited haplotype with the Kir6.2[E23K] variant (above), which is strongly associated with risk of type 2 diabetes, has been reported to be favorable for body fat distribution and reduced risk of coronary heart disease, based on analysis of data from the UK Biobank74. More recently, Bohnen et al72 reported twelve SUR1 coding variants (p.R958H, p.N72D, p.E186D, p.A240T, p.E791Q, c.T2694+2G, p.G111R, p.L135V, p.D813N, p.D1472N, p.T229I, p.R1314H) in a cohort study of pulmonary arterial hypertension. Patch-clamp analysis of recombinant channels revealed these to be consistently loss of function mutations, which could be pharmacologically rescued by the SUR1 activator diazoxide. Some of these variants have previously also been reported in association with hyperinsulinism, a disease that is definitively causally associated with loss of SUR1 or Kir6.2-dependent channel activity18. The N72D, L135V, D813N, R1314H variants were also associated with congenital heart disease, large atrial septal defect, first-degree heart block, atrial flutter and ventricular septal defect, respectively72. Coincidentally, a separate study reported the same SUR1 R1314H mutation in a cohort study of atrial fibrillation at almost the same time73, suggesting that SUR1 loss of function may also be related to atrial fibrillation. Given that KATP channels in both human heart and blood vessels are predominantly composed of SUR2, but not SUR1, the question then arises as to how these SUR1 variants are associated with cardiovascular diseases? Potentially, the precise subunit composition in any given cell type may, as suggested above, be more subtly variable, or more labile, than is currently perceived, making it critical to focus on precise subunit distributions. No basal cardiovascular problems have been reported to date in animal models with SUR1 deletion or mutation, although SUR1 knockout (SUR1−/−) mice exhibited reduced infarct size and preservation of left ventricular function in myocardial ischemia/reperfusion injury109. These results are not trivially consistent with the findings in Kir6.2 knockout (Kir6.2−/−) mice, which showed enhanced ischemic damage function in myocardial ischemia/reperfusion injury97, 110, which may imply that these cardiovascular outcomes may be dependent on SUR1 function in other tissues, emphasizing the need for investigation of cell- and tissue-specific elimination or expression of SUR1 subunits before and after ischemic events.
On the other hand, overexpression of SUR1 subunits in mouse heart does not result in overt cardiac phenotypes other than PR prolongation, unless Kir6.2 subunits are also overexpressed111. It should be noted that several SUR1 splice variants are expressed in the heart, but their contributions to cardiovascular function have not been explored112–114. A recent study described the presence of SUR1 in both atrial and ventricle, but although SUR1-containing KATP channels constitutively reach the cell surface in atrial myocytes, they are normally stalled in the Golgi of ventricular myocytes, until deployed to the cell surface under sustained β-adrenergic stimulation115. Such findings lend further support for the need to carefully define KATP channel subunit composition in specific cardiovascular cell types under different physiological and pathological conditions.
SUR2
SUR2 is the major regulatory sulfonylurea receptor of KATP channel in both hearts and vessels. There have been many isolated reports of mutations associated with human cardiovascular pathology. A heterozygous frameshift SUR2A mutation L1524fs(c.4570–4572 delta InsAAAT) and heterozygous missense SUR2A mutation A1513T(c.4537G>A) were identified in two patients in a cohort of 323 individuals with idiopathic dilated cardiomyopathy. Both individuals had severely dilated hearts with compromised contractile function and rhythm disturbances. Both mutations are located in exon 38 of ABCC9, which encodes the C-terminal domain of SUR2A and both were reported to reduce ATP hydrolytic activity, thus leading to loss-of-KATP channel function, and enhanced susceptibility to dilated cardiomyopathy89. Another ABCC9 missense mutation (c.4640C>T), also resulting in a coding mutation (T1547I) in the C-terminal domain of SUR2A, was shown to result in attenuated channel activation by MgADP and associated with predisposition to adrenergic AF originating from the vein of Marshall90. In addition, a missense mutation (p.Met1198Ile, c.3594G>A) in ABCC9 was detected in one Left Ventricular Non-Compaction Cardiomyopathy (LVNC) patient85. In a cohort study of 144 victims of sudden unexplained nocturnal death syndrome (SUNDS), a SUNDS victim with AF hosted a rare ABCC9 variant(p.Arg1197Cys, c.3589C>T)84. The functional characteristics of these two mutations have not been determined.
SUR2−/− mice exhibited similar phenotypes to Kir6.1 −/− mice, including repeated episodes of coronary artery vasospasm, elevated resting blood pressures and sudden death116. It was initially assumed that the presence of vasospasm and hypertension in SUR2−/− mice arose from the critical role of KATP channels in VSM cell function. However, subsequent studies provided conflicting results. In one117, SUR2 overexpression specifically in vascular smooth muscle cells, failed to rescue the SUR2 null phenotype, suggesting that spontaneous coronary vasospasm and sudden death in SUR2 null mice arose from a coronary artery vascular smooth muscle– extrinsic process. In another, overexpression of SUR2A generated a cardiac phenotype resistant to ischemia118. However, it was found that SUR2 null mice were also resistant to acute cardiovascular stress and exhibited reduced infarct size and improved cardiac function119. Clearly, further studies are required to fully explain SUR2 loss-of-function phenotypes.
The above mutations were all putative loss-of-function mutations, but a potential gain-of-function ABCC9 missense mutation, Val734Ile(c.2200G>A) in exon 17 which encodes a 13 amino acids peptide located in the first nucleotide binding fold (NBD1) of SUR2 was detected in one precocious myocardial infarction (MI) patient, with which the individuals have a 6.40-fold risk of suffering MI before the age of 60 years as compared to healthy controls77. This mutation was also identified in a further eleven patients diagnosed with acute myocardial infarction (AMI)78. In this study, the sensitivity to MgATP was assessed in cell lines expressing Kir6.2 and either SUR2x or SUR2x-V734I. It was found that mutant Kir6.2/SUR2B channels, but not Kir6.2/SUR2A or Kir6.1/SUR2B channels, had reduced sensitivity to MgATP inhibition, suggestive of KATP overactivity in the endothelial cell subunit combination. In addition, the V734I variant was reported as a gain-of-function mutation in four early repolarization syndrome (ERS) patients with bradycardia79 and in a patient with a permanent pacemaker who presented with isolated cardiac conduction disease80, perhaps consistent with KATP channels playing a unique role in pacemaker and conduction system cells.
In many of the above cases, phenotypes are subtle, or associations of specific phenotypes with the ABCC9 gene have not been replicated. However, this is not the case for Cantu syndrome, a multi-organ disease characterized by congenital hypertrichosis, distinctive facial features, osteochondrodysplasia and cardiac defects including cardiomegaly and dilated vessels. Cantu syndrome, was first reported in 1982 by Cantu120, and since the first genetic association of Cantu syndrome with ABCC9 in 201275, 76, more than 15 mutation sites in the gene have been reported from more than 100 patients75, 76, 81–83, 86–88, 121, 122. All identified mutations lead to GOF in KATP channel activity in recombinant cell experiments66, 107, 123. The mechanisms underlying these GOF mutations include decreased ATP inhibition and enhanced MgADP activation107, 123. A clear picture has emerged for mice carrying Cantu Syndrome SUR2 gain-of-function mutations introduced to the endogenous locus by CRISPR/Cas9 mutagenesis108. As with ‘V69M Cantu mice’ (above), introduction of the A478V mutation into the equivalent mouse SUR2 locus using CRISPR/Cas9, SUR2[A478V], results SUR2[A478V] ‘Cantu mice’ that display the same phenotypes as Cantu patients, including dilated vessels, low blood pressure and cardiac hypertrophy108108, definitively tying Kir6.1/SUR2-dependent KATP channels to a defined cardiovascular pathology. These ‘Cantu mice’ now make available appropriate models for mechanism study and treatment exploration in Cantu syndrome.
Summary
Over the last 30 years, much effort has been expended to investigate the role of KATP channels in cardiovascular tissues. Multiple lines of evidence, from detection of KATP channel variants in patients, and from animal models, indicate that the KATP channel is causally involved in cardiovascular pathologies, although a note of caution should be sounded regarding the relevance of all reported associations, and to caution against over-interpretation of human variants. The NIH Clinical Genome Resource Consortium (https://www.clinicalgenome.org/curation-activities/gene-disease-validity/educational-and-training-materials/standard-operating-procedures/) has developed specific guidelines for variant interpretation which currently conservatively only considers ABCC8 to be associated with hyperinsulinism, and ABCC9 to be associated with Cantu Syndrome. However, the evidence that ABCC8 is also associated with neonatal diabetes is very strong, and it is to be expected that additional associations will gradually be validated. In addition, the possibility that interaction of certain variants with other (seemingly benign) variants in other genes may contribute to disease progression, should be borne in mind. Even for variants within KATP channel genes, additional complexities may arise from the potentially complex subunit make-up of what should be considered a family of ion channels124, leading to distinct KATP channel properties and regulatory features in different organs and tissues, as well as potentially in subcellular organelles.
Although there is a rich available pharmacology of KATP channels, drug therapy as well as gene therapy for KATP channel mutant diseases remains unexplored. In future, animal models carrying different mutations identified in patients, as well as cell- and tissue-specific expression of KATP channel subunits, and isogenic human induced pluripotent stem cells should provide powerful tools with which to recapitulate and seek explanations for phenotypes observed in patients, and thereby advance our understanding of pathogenesis as well as pharmacotherapy for such diseases.
Sources of Funding:
Our own experimental work has been supported by NIH R35 grant HL140024 (to CGN)
Footnotes
Disclosures: None
References:
- 1.Noma A ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. [DOI] [PubMed] [Google Scholar]
- 2.Spruce AE, Standen NB, Stanfield PR. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985;316:736–738. [DOI] [PubMed] [Google Scholar]
- 3.Trube G, Rorsman P, Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986;407:493–499. [DOI] [PubMed] [Google Scholar]
- 4.Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–180. [DOI] [PubMed] [Google Scholar]
- 5.Janigro D, West GA, Gordon EL, Winn HR. ATP-sensitive K+ channels in rat aorta and brain microvascular endothelial cells. Am J Physiol. 1993;265:C812–821. [DOI] [PubMed] [Google Scholar]
- 6.Jonas P, Koh DS, Kampe K, Hermsteiner M, Vogel W. ATP-sensitive and Ca-activated K channels in vertebrate axons: novel links between metabolism and excitability. Pflugers Arch. 1991;418:68–73. [DOI] [PubMed] [Google Scholar]
- 7.Lederer WJ, Nichols CG. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol. 1989;419:193–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lederer WJ, Nichols CG, Smith GL. The mechanism of early contractile failure of isolated rat ventricular myocytes subjected to complete metabolic inhibition. J Physiol. 1989;413:329–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. [DOI] [PubMed] [Google Scholar]
- 10.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–822. [DOI] [PubMed] [Google Scholar]
- 11.Li N, Wu JX, Ding D, Cheng J, Gao N, Chen L. Structure of a Pancreatic ATP-Sensitive Potassium Channel. Cell. 2017;168:101–110 e110. [DOI] [PubMed] [Google Scholar]
- 12.Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. [DOI] [PubMed] [Google Scholar]
- 13.Inagaki N, Gonoi T, Clement JPt, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. [DOI] [PubMed] [Google Scholar]
- 14.Chutkow WA, Simon MC, Le Beau MM, Burant CF. Cloning, tissue expression, and chromosomal localization of SUR2, the putative drug-binding subunit of cardiac, skeletal muscle, and vascular KATP channels. Diabetes. 1996;45:1439–1445. [DOI] [PubMed] [Google Scholar]
- 15.Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem. 1996;271:24321–24324. [DOI] [PubMed] [Google Scholar]
- 16.Campbell JD, Sansom MS, Ashcroft FM. Potassium channel regulation. EMBO Rep. 2003;4:1038–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan KW, Zhang H, Logothetis DE. N-terminal transmembrane domain of the SUR controls trafficking and gating of Kir6 channel subunits. EMBO J. 2003;22:3833–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. [DOI] [PubMed] [Google Scholar]
- 19.Campbell JD, Proks P, Lippiat JD, Sansom MS, Ashcroft FM. Identification of a functionally important negatively charged residue within the second catalytic site of the SUR1 nucleotide-binding domains. Diabetes. 2004;53 Suppl 3:S123–127. [DOI] [PubMed] [Google Scholar]
- 20.Masia R, Nichols CG. Functional clustering of mutations in the dimer interface of the nucleotide binding folds of the sulfonylurea receptor. J Biol Chem. 2008;283:30322–30329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedorov VV, Glukhov AV, Ambrosi CM, Kostecki G, Chang R, Janks D, Schuessler RB, Moazami N, Nichols CG, Efimov IR. Effects of KATP channel openers diazoxide and pinacidil in coronary-perfused atria and ventricles from failing and non-failing human hearts. J Mol Cell Cardiol. 2011;51:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glukhov AV, Flagg TP, Fedorov VV, Efimov IR, Nichols CG. Differential K(ATP) channel pharmacology in intact mouse heart. J Mol Cell Cardiol. 2010;48:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flagg TP, Kurata HT, Masia R, Caputa G, Magnuson MA, Lefer DJ, Coetzee WA, Nichols CG. Differential structure of atrial and ventricular KATP: atrial KATP channels require SUR1. Circ Res. 2008;103:1458–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satoh H Effects of ATP-sensitive K+ channel openers on pacemaker activity in isolated single rabbit sino-atrial node cells. J Cardiovasc Pharmacol. 1993;22:863–868. [DOI] [PubMed] [Google Scholar]
- 25.Kakei M, Noma A. Adenosine-5’-triphosphate-sensitive single potassium channel in the atrioventricular node cell of the rabbit heart. J Physiol. 1984;352:265–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Light PE, Cordeiro JM, French RJ. Identification and properties of ATP-sensitive potassium channels in myocytes from rabbit Purkinje fibres. Cardiovasc Res. 1999;44:356–369. [DOI] [PubMed] [Google Scholar]
- 27.Bao L, Kefaloyianni E, Lader J, Hong M, Morley G, Fishman GI, Sobie EA, Coetzee WA. Unique properties of the ATP-sensitive K(+) channel in the mouse ventricular cardiac conduction system. Circ Arrhythm Electrophysiol. 2011;4:926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han X, Light PE, Giles WR, French RJ. Identification and properties of an ATP-sensitive K+ current in rabbit sino-atrial node pacemaker cells. J Physiol. 1996;490 ( Pt 2):337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mederos y Schnitzler M, Derst C, Daut J, Preisig-Muller R. ATP-sensitive potassium channels in capillaries isolated from guinea-pig heart. J Physiol. 2000;525 Pt 2:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida H, Feig JE, Morrissey A, Ghiu IA, Artman M, Coetzee WA. K ATP channels of primary human coronary artery endothelial cells consist of a heteromultimeric complex of Kir6.1, Kir6.2, and SUR2B subunits. J Mol Cell Cardiol. 2004;37:857–869. [DOI] [PubMed] [Google Scholar]
- 31.Pielen A, Kirsch M, Hofmann HD, Feuerstein TJ, Lagreze WA. Retinal ganglion cell survival is enhanced by gabapentin-lactam in vitro: evidence for involvement of mitochondrial KATP channels. Graefes Arch Clin Exp Ophthalmol. 2004;242:240–244. [DOI] [PubMed] [Google Scholar]
- 32.Garlid KD, Paucek P, Yarov-Yarovoy V, Sun X, Schindler PA. The mitochondrial KATP channel as a receptor for potassium channel openers. J Biol Chem. 1996;271:8796–8799. [DOI] [PubMed] [Google Scholar]
- 33.Garlid KD, Paucek P. Mitochondrial potassium transport: the K(+) cycle. Biochim Biophys Acta. 2003;1606:23–41. [DOI] [PubMed] [Google Scholar]
- 34.Vadzyuk OB, Kosterin SO. Mitochondria from rat uterine smooth muscle possess ATP-sensitive potassium channel. Saudi J Biol Sci. 2018;25:551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster DB, Ho AS, Rucker J, Garlid AO, Chen L, Sidor A, Garlid KD, O’Rourke B. Mitochondrial ROMK channel is a molecular component of mitoK(ATP). Circ Res. 2012;111:446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye B, Kroboth SL, Pu JL, Sims JJ, Aggarwal NT, McNally EM, Makielski JC, Shi NQ. Molecular identification and functional characterization of a mitochondrial sulfonylurea receptor 2 splice variant generated by intraexonic splicing. Circ Res. 2009;105:1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bednarczyk P, Kicinska A, Laskowski M, Kulawiak B, Kampa R, Walewska A, Krajewska M, Jarmuszkiewicz W, Szewczyk A. Evidence for a mitochondrial ATP-regulated potassium channel in human dermal fibroblasts. Biochim Biophys Acta Bioenerg. 2018;1859:309–318. [DOI] [PubMed] [Google Scholar]
- 38.Ramratnam M, Kenny B, Kyle JW, Wiedmeyer B, Hacker TA, Barefield DY, McNally EM, Makielski JC. Transgenic overexpression of the SUR2A-55 splice variant in mouse heart reduces infract size and promotes protective mitochondrial function. Heliyon. 2018;4:e00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi WW, Yang Y, Shi Y, Jiang C. K(ATP) channel action in vascular tone regulation: from genetics to diseases. Sheng Li Xue Bao. 2012;64:1–13. [PMC free article] [PubMed] [Google Scholar]
- 40.Randak CO, Welsh MJ. ADP inhibits function of the ABC transporter cystic fibrosis transmembrane conductance regulator via its adenylate kinase activity. Proc Natl Acad Sci U S A. 2005;102:2216–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin GM, Chen PC, Devaraneni P, Shyng SL. Pharmacological rescue of trafficking-impaired ATP-sensitive potassium channels. Front Physiol. 2013;4:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulze D, Rapedius M, Krauter T, Baukrowitz T. Long-chain acyl-CoA esters and phosphatidylinositol phosphates modulate ATP inhibition of KATP channels by the same mechanism. J Physiol. 2003;552:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tinker A, Aziz Q, Thomas A. The role of ATP-sensitive potassium channels in cellular function and protection in the cardiovascular system. Br J Pharmacol. 2014;171:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flagg TP, Enkvetchakul D, Koster JC, Nichols CG. Muscle KATP channels: recent insights to energy sensing and myoprotection. Physiol Rev. 2010;90:799–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gribble FM, Ashfield R, Ammala C, Ashcroft FM. Properties of cloned ATP-sensitive K+ currents expressed in Xenopus oocytes. J Physiol. 1997;498 ( Pt 1):87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gribble FM, Reimann F, Ashfield R, Ashcroft FM. Nucleotide modulation of pinacidil stimulation of the cloned K(ATP) channel Kir6.2/SUR2A. Mol Pharmacol. 2000;57:1256–1261. [PubMed] [Google Scholar]
- 47.Moreau C, Jacquet H, Prost AL, D’Hahan N, Vivaudou M. The molecular basis of the specificity of action of K(ATP) channel openers. EMBO J. 2000;19:6644–6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shindo T, Yamada M, Isomoto S, Horio Y, Kurachi Y. SUR2 subtype (A and B)-dependent differential activation of the cloned ATP-sensitive K+ channels by pinacidil and nicorandil. Br J Pharmacol. 1998;124:985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwanstecher M, Sieverding C, Dorschner H, Gross I, Aguilar-Bryan L, Schwanstecher C, Bryan J. Potassium channel openers require ATP to bind to and act through sulfonylurea receptors. EMBO J. 1998;17:5529–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuo M, Tanabe K, Kioka N, Amachi T, Ueda K. Different binding properties and affinities for ATP and ADP among sulfonylurea receptor subtypes, SUR1, SUR2A, and SUR2B. J Biol Chem. 2000;275:28757–28763. [DOI] [PubMed] [Google Scholar]
- 51.Shyng S, Ferrigni T, Nichols CG. Regulation of KATP channel activity by diazoxide and MgADP. Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J Gen Physiol. 1997;110:643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin GM, Kandasamy B, DiMaio F, Yoshioka C, Shyng SL. Anti-diabetic drug binding site in a mammalian KATP channel revealed by Cryo-EM. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gribble FM, Ashcroft FM. Differential sensitivity of beta-cell and extrapancreatic K(ATP) channels to gliclazide. Diabetologia. 1999;42:845–848. [DOI] [PubMed] [Google Scholar]
- 54.Raab-Graham KF, Cirilo LJ, Boettcher AA, Radeke CM, Vandenberg CA. Membrane topology of the amino-terminal region of the sulfonylurea receptor. J Biol Chem. 1999;274:29122–29129. [DOI] [PubMed] [Google Scholar]
- 55.Dorschner H, Brekardin E, Uhde I, Schwanstecher C, Schwanstecher M. Stoichiometry of sulfonylurea-induced ATP-sensitive potassium channel closure. Mol Pharmacol. 1999;55:1060–1066. [DOI] [PubMed] [Google Scholar]
- 56.Duan RF, Cui WY, Wang H. Association of the antihypertensive response of iptakalim with KCNJ11 (Kir6.2 gene) polymorphisms in Chinese Han hypertensive patients. Acta Pharmacol Sin. 2011;32:1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han YY, Wang LJ, Zhang L, Zhang WW, Ma KT, Li L, Si JQ. Association between potassium channel SNPs and essential hypertension in Xinjiang Kazak Chinese patients. Exp Ther Med. 2017;14:1999–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koo BK, Cho YM, Park BL, Cheong HS, Shin HD, Jang HC, Kim SY, Lee HK, Park KS. Polymorphisms of KCNJ11 (Kir6.2 gene) are associated with Type 2 diabetes and hypertension in the Korean population. Diabet Med. 2007;24:178–186. [DOI] [PubMed] [Google Scholar]
- 59.Zhuang L, Zhao Y, Zhao W, Li M, Yu M, Lu M, Zhang R, Ge X, Zheng T, Li C, Yin J, Yin J, Bao Y, Liu L, Jia W, Liu Y. The E23K and A190A variations of the KCNJ11 gene are associated with early-onset type 2 diabetes and blood pressure in the Chinese population. Mol Cell Biochem. 2015;404:133–141. [DOI] [PubMed] [Google Scholar]
- 60.Reyes S, Park S, Johnson BD, Terzic A, Olson TM. KATP channel Kir6.2 E23K variant overrepresented in human heart failure is associated with impaired exercise stress response. Hum Genet. 2009;126:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reyes S, Terzic A, Mahoney DW, Redfield MM, Rodeheffer RJ, Olson TM. K(ATP) channel polymorphism is associated with left ventricular size in hypertensive individuals: a large-scale community-based study. Hum Genet. 2008;123:665–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sakamoto Y, Inoue H, Keshavarz P, Miyawaki K, Yamaguchi Y, Moritani M, Kunika K, Nakamura N, Yoshikawa T, Yasui N, Shiota H, Tanahashi T, Itakura M. SNPs in the KCNJ11-ABCC8 gene locus are associated with type 2 diabetes and blood pressure levels in the Japanese population. J Hum Genet. 2007;52:781–793. [DOI] [PubMed] [Google Scholar]
- 63.Xi HL, Liu JF, Li L, Wan J. Relationship between dilated cardiomyopathy and the E23K and I337V polymorphisms in the Kir6.2 subunit of the KATP channel. Genet Mol Res. 2013;12:4383–4392. [DOI] [PubMed] [Google Scholar]
- 64.Strutynskyi RB, Voronkov LG, Nagibin VS, Mazur ID, Stroy D, Dosenko VE. Changes of the echocardiographic parameters in chronic heart failure patients with Ile337val, Glu23lys, and Ser1369ala polymorphisms of genes encoding the ATP-sensitive potassium channels subunits in the Ukrainian population. Ann Hum Genet. 2018;82:272–279. [DOI] [PubMed] [Google Scholar]
- 65.Brownstein CA, Towne MC, Luquette LJ, Harris DJ, Marinakis NS, Meinecke P, Kutsche K, Campeau PM, Yu TW, Margulies DM, Agrawal PB, Beggs AH. Mutation of KCNJ8 in a patient with Cantu syndrome with unique vascular abnormalities - support for the role of K(ATP) channels in this condition. Eur J Med Genet. 2013;56:678–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cooper PE, Reutter H, Woelfle J, Engels H, Grange DK, van Haaften G, van Bon BW, Hoischen A, Nichols CG. Cantu syndrome resulting from activating mutation in the KCNJ8 gene. Hum Mutat. 2014;35:809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tester DJ, Tan BH, Medeiros-Domingo A, Song C, Makielski JC, Ackerman MJ. Loss-of-function mutations in the KCNJ8-encoded Kir6.1 K(ATP) channel and sudden infant death syndrome. Circ Cardiovasc Genet. 2011;4:510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haissaguerre M, Chatel S, Sacher F, Weerasooriya R, Probst V, Loussouarn G, Horlitz M, Liersch R, Schulze-Bahr E, Wilde A, Kaab S, Koster J, Rudy Y, Le Marec H, Schott JJ. Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. J Cardiovasc Electrophysiol. 2009;20:93–98. [DOI] [PubMed] [Google Scholar]
- 69.Barajas-Martinez H, Hu D, Ferrer T, Onetti CG, Wu Y, Burashnikov E, Boyle M, Surman T, Urrutia J, Veltmann C, Schimpf R, Borggrefe M, Wolpert C, Ibrahim BB, Sanchez-Chapula JA, Winters S, Haissaguerre M, Antzelevitch C. Molecular genetic and functional association of Brugada and early repolarization syndromes with S422L missense mutation in KCNJ8. Heart Rhythm. 2012;9:548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Delaney JT, Muhammad R, Blair MA, Kor K, Fish FA, Roden DM, Darbar D. A KCNJ8 mutation associated with early repolarization and atrial fibrillation. Europace. 2012;14:1428–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Medeiros-Domingo A, Tan BH, Crotti L, Tester DJ, Eckhardt L, Cuoretti A, Kroboth SL, Song C, Zhou Q, Kopp D, Schwartz PJ, Makielski JC, Ackerman MJ. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac K(ATP) channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm. 2010;7:1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bohnen MS, Ma L, Zhu N, Qi H, McClenaghan C, Gonzaga-Jauregui C, Dewey FE, Overton JD, Reid JG, Shuldiner AR, Baras A, Sampson KJ, Bleda M, Hadinnapola C, Haimel M, Bogaard HJ, Church C, Coghlan G, Corris PA, Eyries M, Gibbs JSR, Girerd B, Houweling AC, Humbert M, Guignabert C, Kiely DG, Lawrie A, MacKenzie Ross RV, Martin JM, Montani D, Peacock AJ, Pepke-Zaba J, Soubrier F, Suntharalingam J, Toshner M, Treacy CM, Trembath RC, Vonk Noordegraaf A, Wharton J, Wilkins MR, Wort SJ, Yates K, Graf S, Morrell NW, Krishnan U, Rosenzweig EB, Shen Y, Nichols CG, Kass RS, Chung WK. Loss-of-Function ABCC8 Mutations in Pulmonary Arterial Hypertension. Circ Genom Precis Med. 2018;11:e002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Donate Puertas R, Millat G, Ernens I, Gache V, Chauveau S, Morel E, Christin E, Couturier N, Devaux Y, Chevalier P. Atrial Structural Remodeling Gene Variants in Patients with Atrial Fibrillation. Biomed Res Int. 2018;2018:4862480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Emdin CA, Klarin D, Natarajan P, Consortium CE, Florez JC, Kathiresan S, Khera AV. Genetic Variation at the Sulfonylurea Receptor, Type 2 Diabetes, and Coronary Heart Disease. Diabetes. 2017;66:2310–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harakalova M, van Harssel JJ, Terhal PA, van Lieshout S, Duran K, Renkens I, Amor DJ, Wilson LC, Kirk EP, Turner CL, Shears D, Garcia-Minaur S, Lees MM, Ross A, Venselaar H, Vriend G, Takanari H, Rook MB, van der Heyden MA, Asselbergs FW, Breur HM, Swinkels ME, Scurr IJ, Smithson SF, Knoers NV, van der Smagt JJ, Nijman IJ, Kloosterman WP, van Haelst MM, van Haaften G, Cuppen E. Dominant missense mutations in ABCC9 cause Cantu syndrome. Nat Genet. 2012;44:793–796. [DOI] [PubMed] [Google Scholar]
- 76.van Bon BW, Gilissen C, Grange DK, Hennekam RC, Kayserili H, Engels H, Reutter H, Ostergaard JR, Morava E, Tsiakas K, Isidor B, Le Merrer M, Eser M, Wieskamp N, de Vries P, Steehouwer M, Veltman JA, Robertson SP, Brunner HG, de Vries BB, Hoischen A. Cantu syndrome is caused by mutations in ABCC9. Am J Hum Genet. 2012;90:1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Minoretti P, Falcone C, Aldeghi A, Olivieri V, Mori F, Emanuele E, Calcagnino M, Geroldi D. A novel Val734Ile variant in the ABCC9 gene associated with myocardial infarction. Clin Chim Acta. 2006;370:124–128. [DOI] [PubMed] [Google Scholar]
- 78.Smith KJ, Chadburn AJ, Adomaviciene A, Minoretti P, Vignali L, Emanuele E, Tammaro P. Coronary spasm and acute myocardial infarction due to a mutation (V734I) in the nucleotide binding domain 1 of ABCC9. Int J Cardiol. 2013;168:3506–3513. [DOI] [PubMed] [Google Scholar]
- 79.Hu D, Barajas-Martinez H, Terzic A, Park S, Pfeiffer R, Burashnikov E, Wu Y, Borggrefe M, Veltmann C, Schimpf R, Cai JJ, Nam GB, Deshmukh P, Scheinman M, Preminger M, Steinberg J, Lopez-Izquierdo A, Ponce-Balbuena D, Wolpert C, Haissaguerre M, Sanchez-Chapula JA, Antzelevitch C. ABCC9 is a novel Brugada and early repolarization syndrome susceptibility gene. Int J Cardiol. 2014;171:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Celestino-Soper PB, Doytchinova A, Steiner HA, Uradu A, Lynnes TC, Groh WJ, Miller JM, Lin H, Gao H, Wang Z, Liu Y, Chen PS, Vatta M. Evaluation of the Genetic Basis of Familial Aggregation of Pacemaker Implantation by a Large Next Generation Sequencing Panel. PLoS One. 2015;10:e0143588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim H, Kim S, Jeon H, Kim J, Yoo J, Seong M, Park S. Clinical and Molecular Delineation of a Novel Cys1050Phe Missense Mutation in the ABCC9 Gene in a Korean Patient with Cantu Syndrome. Clin Lab. 2017;63:991–995. [DOI] [PubMed] [Google Scholar]
- 82.Afifi HH, Abdel-Hamid MS, Eid MM, Mostafa IS, Abdel-Salam GM. De Novo Mutation in ABCC9 Causes Hypertrichosis Acromegaloid Facial Features Disorder. Pediatr Dermatol. 2016;33:e109–113. [DOI] [PubMed] [Google Scholar]
- 83.Czeschik JC, Voigt C, Goecke TO, Ludecke HJ, Wagner N, Kuechler A, Wieczorek D. Wide clinical variability in conditions with coarse facial features and hypertrichosis caused by mutations in ABCC9. Am J Med Genet A. 2013;161A:295–300. [DOI] [PubMed] [Google Scholar]
- 84.Zhang L, Tester DJ, Lang D, Chen Y, Zheng J, Gao R, Corliss RF, Tang S, Kyle JW, Liu C, Ackerman MJ, Makielski JC, Cheng J. Does Sudden Unexplained Nocturnal Death Syndrome Remain the Autopsy-Negative Disorder: A Gross, Microscopic, and Molecular Autopsy Investigation in Southern China. Mayo Clin Proc. 2016;91:1503–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Waldmuller S, Schroeder C, Sturm M, Scheffold T, Imbrich K, Junker S, Frische C, Hofbeck M, Bauer P, Bonin M, Gawaz M, Gramlich M. Targeted 46-gene and clinical exome sequencing for mutations causing cardiomyopathies. Mol Cell Probes. 2015;29:308–314. [DOI] [PubMed] [Google Scholar]
- 86.Hiraki Y, Miyatake S, Hayashidani M, Nishimura Y, Matsuura H, Kamada M, Kawagoe T, Yunoki K, Okamoto N, Yofune H, Nakashima M, Tsurusaki Y, Satisu H, Murakami A, Miyake N, Nishimura G, Matsumoto N. Aortic aneurysm and craniosynostosis in a family with Cantu syndrome. Am J Med Genet A. 2014;164A:231–236. [DOI] [PubMed] [Google Scholar]
- 87.Marques P, Spencer R, Morrison PJ, Carr IM, Dang MN, Bonthron DT, Hunter S, Korbonits M. Cantu syndrome with coexisting familial pituitary adenoma. Endocrine. 2018;59:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park JY, Koo SH, Jung YJ, Lim YJ, Chung ML. A patient with Cantu syndrome associated with fatal bronchopulmonary dysplasia and pulmonary hypertension. Am J Med Genet A. 2014;164A:2118–2120. [DOI] [PubMed] [Google Scholar]
- 89.Bienengraeber M, Olson TM, Selivanov VA, Kathmann EC, O’Cochlain F, Gao F, Karger AB, Ballew JD, Hodgson DM, Zingman LV, Pang YP, Alekseev AE, Terzic A. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet. 2004;36:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Olson TM, Alekseev AE, Moreau C, Liu XK, Zingman LV, Miki T, Seino S, Asirvatham SJ, Jahangir A, Terzic A. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2007;4:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miki T, Seino S. Roles of KATP channels as metabolic sensors in acute metabolic changes. J Mol Cell Cardiol. 2005;38:917–925. [DOI] [PubMed] [Google Scholar]
- 92.Nichols CG, Koster JC, Remedi MS. beta-cell hyperexcitability: from hyperinsulinism to diabetes. Diabetes Obes Metab. 2007;9 Suppl 2:81–88. [DOI] [PubMed] [Google Scholar]
- 93.Feng Y, Liu J, Wang M, Liu M, Shi L, Yuan W, Ye J, Hu D, Wan J. The E23K variant of the Kir6.2 subunit of the ATP-sensitive potassium channel increases susceptibility to ventricular arrhythmia in response to ischemia in rats. Int J Cardiol. 2017;232:192–198. [DOI] [PubMed] [Google Scholar]
- 94.Fedele F, Mancone M, Chilian WM, Severino P, Canali E, Logan S, De Marchis ML, Volterrani M, Palmirotta R, Guadagni F. Role of genetic polymorphisms of ion channels in the pathophysiology of coronary microvascular dysfunction and ischemic heart disease. Basic Res Cardiol. 2013;108:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koster JC, Knopp A, Flagg TP, Markova KP, Sha Q, Enkvetchakul D, Betsuyaku T, Yamada KA, Nichols CG. Tolerance for ATP-insensitive K(ATP) channels in transgenic mice. Circulation research. 2001;89:1022–1029. [DOI] [PubMed] [Google Scholar]
- 96.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci U S A. 2002;99:13278–13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gumina RJ, O’Cochlain DF, Kurtz CE, Bast P, Pucar D, Mishra P, Miki T, Seino S, Macura S, Terzic A. KATP channel knockout worsens myocardial calcium stress load in vivo and impairs recovery in stunned heart. Am J Physiol Heart Circ Physiol. 2007;292:H1706–1713. [DOI] [PubMed] [Google Scholar]
- 98.Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Ogura T, Seino S, Marban E, Nakaya H. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res. 2001;88:570–577. [DOI] [PubMed] [Google Scholar]
- 99.Youssef N, Campbell S, Barr A, Gandhi M, Hunter B, Dolinsky V, Dyck JRB, Clanachan AS, Light PE. Hearts lacking plasma membrane KATP channels display changes in basal aerobic metabolic substrate preference and AMPK activity. Am J Physiol Heart Circ Physiol. 2017;313:H469–H478. [DOI] [PubMed] [Google Scholar]
- 100.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–472. [DOI] [PubMed] [Google Scholar]
- 101.Aziz Q, Thomas AM, Gomes J, Ang R, Sones WR, Li Y, Ng KE, Gee L, Tinker A. The ATP-sensitive potassium channel subunit, Kir6.1, in vascular smooth muscle plays a major role in blood pressure control. Hypertension. 2014;64:523–529. [DOI] [PubMed] [Google Scholar]
- 102.Li A, Knutsen RH, Zhang H, Osei-Owusu P, Moreno-Dominguez A, Harter TM, Uchida K, Remedi MS, Dietrich HH, Bernal-Mizrachi C, Blumer KJ, Mecham RP, Koster JC, Nichols CG. Hypotension due to Kir6.1 gain-of-function in vascular smooth muscle. J Am Heart Assoc. 2013;2:e000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Levin MD, Zhang H, Uchida K, Grange DK, Singh GK, Nichols CG. Electrophysiologic consequences of KATP gain of function in the heart: Conduction abnormalities in Cantu syndrome. Heart Rhythm. 2015;12:2316–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aziz Q, Finlay M, Montaigne D, Ojake L, Li Y, Anderson N, Ludwig A, Tinker A. ATP-sensitive potassium channels in the sinoatrial node contribute to heart rate control and adaptation to hypoxia. J Biol Chem. 2018;293:8912–8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Veeramah KR, Karafet TM, Wolf D, Samson RA, Hammer MF. The KCNJ8-S422L variant previously associated with J-wave syndromes is found at an increased frequency in Ashkenazi Jews. Eur J Hum Genet. 2014;22:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Watanabe Y, Matsumoto A, Miki T, Seino S, Anzai N, Nakaya H. Electrophysiological analyses of transgenic mice overexpressing KCNJ8 with S422L mutation in cardiomyocytes. J Pharmacol Sci. 2017;135:37–43. [DOI] [PubMed] [Google Scholar]
- 107.McClenaghan C, Hanson A, Sala-Rabanal M, Roessler HI, Josifova D, Grange DK, van Haaften G, Nichols CG. Cantu syndrome-associated SUR2 (ABCC9) mutations in distinct structural domains result in KATP channel gain-of-function by differential mechanisms. J Biol Chem. 2018;293:2041–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang Y, McClenaghan C, Harter TM, Hinman K, Halabi CM, Matkovich SJ, Zhang H, Brown GS, Mecham RP, England SK, Kovacs A, Remedi MS, Nichols CG. Cardiovascular consequences of KATP overactivity in Cantu syndrome. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Elrod JW, Harrell M, Flagg TP, Gundewar S, Magnuson MA, Nichols CG, Coetzee WA, Lefer DJ. Role of sulfonylurea receptor type 1 subunits of ATP-sensitive potassium channels in myocardial ischemia/reperfusion injury. Circulation. 2008;117:1405–1413. [DOI] [PubMed] [Google Scholar]
- 110.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Flagg TP, Patton B, Masia R, Mansfield C, Lopatin AN, Yamada KA, Nichols CG. Arrhythmia susceptibility and premature death in transgenic mice overexpressing both SUR1 and Kir6.2[DeltaN30,K185Q] in the heart. Am J Physiol Heart Circ Physiol. 2007;293:H836–845. [DOI] [PubMed] [Google Scholar]
- 112.Hambrock A, Preisig-Muller R, Russ U, Piehl A, Hanley PJ, Ray J, Daut J, Quast U, Derst C. Four novel splice variants of sulfonylurea receptor 1. Am J Physiol Cell Physiol. 2002;283:C587–598. [DOI] [PubMed] [Google Scholar]
- 113.Shi NQ, Ye B, Makielski JC. Function and distribution of the SUR isoforms and splice variants. J Mol Cell Cardiol. 2005;39:51–60. [DOI] [PubMed] [Google Scholar]
- 114.Philip-Couderc P, Tavares NI, Roatti A, Lerch R, Montessuit C, Baertschi AJ. Forkhead transcription factors coordinate expression of myocardial KATP channel subunits and energy metabolism. Circ Res. 2008;102:e20–35. [DOI] [PubMed] [Google Scholar]
- 115.Arakel EC, Brandenburg S, Uchida K, Zhang H, Lin YW, Kohl T, Schrul B, Sulkin MS, Efimov IR, Nichols CG, Lehnart SE, Schwappach B. Tuning the electrical properties of the heart by differential trafficking of KATP ion channel complexes. J Cell Sci. 2014;127:2106–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 K(ATP) channels. J Clin Invest. 2002;110:203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kakkar R, Ye B, Stoller DA, Smelley M, Shi NQ, Galles K, Hadhazy M, Makielski JC, McNally EM. Spontaneous coronary vasospasm in KATP mutant mice arises from a smooth muscle-extrinsic process. Circ Res. 2006;98:682–689. [DOI] [PubMed] [Google Scholar]
- 118.Du Q, Jovanovic S, Clelland A, Sukhodub A, Budas G, Phelan K, Murray-Tait V, Malone L, Jovanovic A. Overexpression of SUR2A generates a cardiac phenotype resistant to ischemia. FASEB J. 2006;20:1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stoller D, Kakkar R, Smelley M, Chalupsky K, Earley JU, Shi NQ, Makielski JC, McNally EM. Mice lacking sulfonylurea receptor 2 (SUR2) ATP-sensitive potassium channels are resistant to acute cardiovascular stress. J Mol Cell Cardiol. 2007;43:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cantu JM, Garcia-Cruz D, Sanchez-Corona J, Hernandez A, Nazara Z. A distinct osteochondrodysplasia with hypertrichosis- Individualization of a probable autosomal recessive entity. Hum Genet. 1982;60:36–41. [DOI] [PubMed] [Google Scholar]
- 121.Kirk EP, Scurr I, van Haaften G, van Haelst MM, Nichols CG, Williams M, Smithson SF, Grange DK. Clinical utility gene card for: Cantu syndrome. Eur J Hum Genet. 2017;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Grange DK, Nichols CG, Singh GK. Cantu Syndrome and Related Disorders In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, eds. GeneReviews((R)). Seattle (WA); 1993. [PubMed] [Google Scholar]
- 123.Cooper PE, Sala-Rabanal M, Lee SJ, Nichols CG. Differential mechanisms of Cantu syndrome-associated gain of function mutations in the ABCC9 (SUR2) subunit of the KATP channel. J Gen Physiol. 2015;146:527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Flagg TP, Nichols CG. “Cardiac KATP”: a family of ion channels. Circ Arrhythm Electrophysiol. 2011;4:796–798. [DOI] [PMC free article] [PubMed] [Google Scholar]