Summary
Astrocytes are emerging as important players in synaptic function, and, consequently, on brain function and animal behavior. According to the Tripartite Synapse concept, astrocytes are integral elements involved in synaptic function. They establish bidirectional communication with neurons, whereby they respond to synaptically-released neurotransmitters and, in turn, release gliotransmitters that influence neuronal and synaptic activity. Accumulating evidence is reveling that the mechanisms and functional consequences of astrocyte-neuron signaling is more complex than originally thought. Furthermore, astrocyte-neuron signaling is not based on broad, unspecific interaction; rather, it is a synapse-, cell- and circuit-specific phenomenon that presents a high degree of complexity. This diversity and complexity of astrocyte-synapse interactions greatly enhances the degrees of freedom of the neural circuits and the consequent computational power of the neural systems.
Introduction
Astrocytes are recognized as key supportive elements in neuronal function, providing structural and metabolic support for neurons, and controlling brain homeostasis mechanisms. Historically, they were ignored as being active players in cellular processes underlying brain function. This was likely because nervous system function at the time was known to rely on electrical signals, but astrocytes, unlike neurons, are not electrically excitable cells. However, the advent of calcium imaging techniques in the 1990s, which allowed for monitoring changes in cytosolic calcium, challenged the longheld view that neurons are the only active players in brain communication by demonstrating that astrocytes display a form of excitability based on intracellular calcium variations (Cornell-Bell et al., 1990, Charles et al., 1991, Dani et al., 1992, Porter and McCarthy, 1996, Pasti et al., 1997). This cellular excitability can be manifested as intrinsic spontaneous events, and more importantly, as physiological responses to neurotransmitters, neuromodulators, and extracellular changes in environmental conditions. The seminal studies using cultured astrocytes have been expanded in the last two decades in more intact preparations and in vivo to show that calcium-based excitability of astrocytes is a ubiquitous phenomenon in the nervous system (Araque et al., 2014, Rusakov et al., 2014, Volterra et al., 2014, Khakh and Sofroniew, 2015).
Since those early discoveries, researchers have investigated the many types of stimuli that activate astrocytes, the nature of the resultant calcium changes, and the consequence of such astrocyte activation on neuronal and synaptic activity. The fundamental properties and consequences of neuron-astrocyte communication have been extensively described and discussed in a large number of reviews (Hamilton and Attwell, 2010, Min et al., 2012, Araque et al., 2014, Rusakov et al., 2014, Volterra et al., 2014, Khakh and Sofroniew, 2015, De Pitta et al., 2016, Shigetomi et al., 2016, Guerra-Gomes et al., 2017, Savtchouk and Volterra, 2018). In this review, we will focus on paradigmatic examples that reveal a synapse-, cell-, and circuit-specific diversity of the properties and consequences of neuron-astrocyte signaling.
Diversity of astrocyte responses to synaptic transmission
Astrocytes express a plethora of channels, transporters, and receptors that provide a variety of homeostatic and synaptic functions. Notably, astrocytes can express many of the same neurotransmitter receptors expressed by neurons. Consequently, it is not surprising that astrocytes have been shown to be able to respond to a wide variety of neurotransmitters. Most of the original studies using cultured cells and pharmacological assays to test the astrocyte responsiveness to different neurotransmitters and neuromodulators found that astrocytes can respond to most, if not all, neurotransmitters. Because astrocytes are known to undergo strong phenotypic changes in culture, the actual existence and physiological relevance of the responses might be compromised. However, a large amount of subsequent evidence obtained in more intact preparations, like acute brain slices and in vivo, has shown that astrocytes in many brain areas indeed respond to many synaptically-released neurotransmitters.
Most of these responses are via G protein-coupled receptors (GPCR) that, upon stimulation by synaptically-released neurotransmitters, trigger different intracellular signaling cascades, including calcium mobilization from internal stores. Additional mechanisms of calcium elevations have also been described, such as transmembrane calcium flux through activation of TRP channels (Shigetomi et al., 2011, Shigetomi et al., 2013), and have been reviewed elsewhere (Zorec et al., 2012, Rusakov et al., 2014, Volterra et al., 2014, Bazargani and Attwell, 2016, Shigetomi et al., 2016). By measuring changes in calcium levels, astrocytes have been found to respond to glutamate (Porter and McCarthy, 1996, Perea and Araque, 2005, Wang et al., 2006, Di Castro et al., 2011, Panatier et al., 2011), acetylcholine (Perea and Araque, 2005, Takata et al., 2011, Chen et al., 2012), ATP (Bowser and Khakh, 2004, Perea and Araque, 2007), GABA (Kang et al., 1998, Meier et al., 2008, Mariotti et al., 2016, Perea et al., 2016, Covelo and Araque, 2018, Mariotti et al., 2018), and endocannabinoids (Navarrete and Araque, 2008, 2010, Min and Nevian, 2012, Gomez-Gonzalo et al., 2014, Martin et al., 2015, Martin-Fernandez et al., 2017, Robin et al., 2018). While these studies have shown that astrocytes can be activated by the most abundant neurotransmitters in the brain, it is noteworthy that evidence is still scarce regarding the astrocyte responsiveness to other neurotransmitter systems, like dopamine, histamine, serotonin, and opioids (Eriksson et al., 1993, Stiene-Martin et al., 1998, Shelton and McCarthy, 2000, Schipke et al., 2011, Agulhon et al., 2013, Jennings et al., 2017, Nam et al., 2018), which have important roles in brain function.
As stated above, astrocytes can respond to most neurotransmitters, which raises the question of whether signal integration occurs or, not a mutually exclusive possibility, if subsets of astrocytes respond to different neurotransmitters. For example, in the hippocampus, astrocyte populations have been shown to respond to a large array of neurotransmitters, including glutamate, GABA, ACh, ATP and endocannabinoids (Pasti et al., 1997, Kang et al., 1998, Guthrie et al., 1999, Navarrete and Araque, 2008, 2010). What is less known, however, is the responsiveness of single astrocytes to different stimuli in the same preparation. One fundamental property of neurons is their ability to integrate multiple synaptic inputs from the activity of incoming synaptic inputs. Likewise, there is evidence that single astrocytes integrate incoming synaptic information as measured through calcium dynamics (Perea and Araque, 2005, Shigetomi et al., 2008). For example, in single hippocampal astrocytes, the simultaneous activation of glutamatergic and cholinergic inputs that activate mGluR and muscarinic receptors, respectively, evoked a relatively diminished calcium elevation response as compared to the linear summation of the responses induced by the independent activation of both pathways. This phenomenon results in the control of the spatial propagation of the intracellular calcium signal within the astrocyte (Perea and Araque, 2005), which may have important functional consequences, as it may influence the spatial extent of the gliotransmitter release and the consequent synaptic regulation.
Notably, the non-linear integration of synaptic inputs found by co-activation of glutamate and ACh receptors was absent when receptors for Glu and GABA or ACh and GABA were activated, indicating that the phenomenon may be different depending on the neurotransmitters involved. Moreover, while metabotropic glutamatergic and cholinergic receptors are coupled to Gq/11 GPCRs, GABAB receptors are coupled to Gi/o GPCRs, which suggests that the mechanisms underlying astrocyte signaling integration occurs through interaction of intracellular pathways.
A more recent study (Mariotti et al., 2018) examining GABA signaling in astrocytes reported that astrocyte responsiveness to neurotransmitters is plastic and cell-type specific. Mariotti and colleagues stimulated either parvalbumin- or (PV) somatostatinpositive (SST) interneurons in the neocortex in vivo and observed differential astrocyte calcium responsiveness to individual stimulation. While repetitive SST stimulation resulted in potentiated calcium responses, repetitive PV stimulation resulted in decreased responses. The specificity of the calcium responsiveness was dependent upon co-activation of both GABAB and neuropeptide SST receptors on astrocytes. These results indicate that astrocyte responsiveness to neurotransmitters may show plasticity. They also further show that astrocyte responsiveness is cell-type specific.
Diversity of mechanisms underlying gliotransmitter release
Astrocytes are known to influence synaptic transmission via a variety of mechanisms. Historically, astrocytes were thought to only play a passive, homeostatic role in influencing synaptic transmission, through, for example, potassium buffering and transmitter clearance (Hertz, 1965, Chaudhry et al., 1995, Higashi et al., 2001, Lehre and Rusakov, 2002, Witcher et al., 2007, Xin and Bonci, 2018). Within the last few decades, however, astrocytes have been shown to play a more active role via the release of gliotransmitters. Though some reports have raised doubt about the mechanisms and physiological role of gliotransmission (reviewed in (Fiacco and McCarthy, 2018, Savtchouk and Volterra, 2018)), there is a large wealth of evidence demonstrating that astrocytes release neuroactive substances that have a variety of effects on synaptic activity (Min et al., 2012, Zorec et al., 2012, Araque et al., 2014, De Pitta et al., 2016, Guerra-Gomes et al., 2017). The multiple potential regulatory effects of astrocytes add to the diversity of astrocyte-neuron signaling.
One important aspect of the consequences of astrocyte activation by different neurotransmitters is that not all of them might be equally efficient in stimulating the release of gliotransmitters. Shigetomi et al. (2008) showed that activation of two different receptors – Protease-activated receptor 1 (PAR1) and the purinergic receptor P2Y1– in hippocampal astrocytes similarly elevated astrocyte calcium levels, with PAR1 receptor stimulation producing longer-lasting calcium events (Shigetomi et al., 2008). However, only PAR1 receptor activation resulted in gliotransmitter release, as detected by the enhancement in the number of slow inward currents (SICs) detected in nearby neurons, a well-known phenomenon that is known to be mediated by activation of neuronal NMDARs by glutamate released from astrocytes (Araque et al., 1998, Fellin et al., 2004, Perea and Araque, 2005).
The cellular and molecular mechanism responsible for gliotransmitter release is largely debated, and several mechanisms, probably not mutually exclusive, have been postulated (for detailed reviews on the topic, see (Hamilton and Attwell, 2010, Rusakov et al., 2014, Volterra et al., 2014, Shigetomi et al., 2016, Guerra-Gomes et al., 2017). One of the general mechanisms used by eukaryotic cells, from yeast to mammalian neurons, to export cargo to the extracellular space is through regulated vesicular release dependent on SNARE proteins. Consequently, a large body of evidence has shown that gliotransmitter release from astrocytes is a calcium- and SNARE protein-dependent mechanism (Araque et al., 2000, Bezzi et al., 2004, Perea and Araque, 2005, Bohmbach et al., 2018); for a recent review see (Savtchouk and Volterra, 2018); but see (Hamilton and Attwell, 2010, Fiacco and McCarthy, 2018). A recent study (Schwarz et al., 2017) has shown that, even with a similar SNARE protein-dependent process, there is diversity in the mechanisms of gliotransmitter release. Schwarz and colleagues demonstrated two separate vesicular SNARE-dependent release pathways in astrocytes. Astrocytes were found to express either synaptopbreven II (sybII) on glutamatergic-containing vesicles or cellubrevin (ceb) on NPY-containing vesicles. Stimulation of astrocytes with glutamate induced the exocytosis of NPY-containing vesicles (that also contained ATP) that was dependent on mGluR5 activation and ceb expression. Loss of ceb expression in autaptic hippocampal neuron cultures increased the amplitude of evoked EPSCs. In contrast, stimulation of astrocytes with the mGluR1 agonist DHPG induced the release of glutamate-containing vesicles that was dependent on sybII expression. Loss of sybII expression had the effect of decreasing the frequency of mEPSCs. When expression of both v-SNAREs was absent, synaptic signaling was comparable to control, suggesting that these two distinct forms of gliotransmission have opposing effects on glutamatergic transmission.
Diversity of synaptic effects of gliotransmitters
Numerous studies have found that astrocytes can release a variety of signaling molecules, such as glutamate, D-serine, ATP/adenosine, and GABA (Min et al., 2012, Araque et al., 2014, De Pitta et al., 2016, Guerra-Gomes et al., 2017, Savtchouk and Volterra, 2018). The effect of each gliotransmitter on synaptic communication depends on the specific neuronal environment. For instance, the release of just one gliotransmitter (e.g. glutamate) can have both inhibitory (Andersson et al., 2007) or excitatory (Jourdain et al., 2007, Santello et al., 2011, Martin et al., 2015) effects on either a short (Navarrete and Araque, 2010, Perea et al., 2014, Perea et al., 2016, Gomez-Gonzalo et al., 2017) or long (Han et al., 2012, Min and Nevian, 2012, Navarrete et al., 2012, Adamsky et al., 2018) timescale. The diversity of such gliotransmitter effects is in part dependent on the location of gliotransmitter binding and, more specifically, the specific type of receptor it binds. For example, the release of astrocytic glutamate has been found to enhance hippocampal glutamatergic synaptic transmission via presynaptic NMDAR-independent mechanisms (Araque et al., 1998, Jourdain et al., 2007). Astrocytic glutamate release, however, can also act at postsynaptic NMDARs in the hippocampus to enhance neuronal excitability (Angulo et al., 2004, Fellin et al., 2004, Perea and Araque, 2005). Lastly, astrocytic glutamate has also been shown to bind presynaptic Group II-III metabotropic glutamate receptors to mediate heterosynaptic depression (Andersson et al., 2007). Thus, the effect of glutamate gliotransmission is varied, as it depends on the specific receptor profile of the pre- and postsynaptic components of the Tripartite Synapse.
These studies show that astrocytes are capable of releasing gliotransmitters that have a wide array of effects depending on the gliotransmitter and the neuronal receptors activated. Moreover, we have recently shown that a single gliotransmitter may simultaneously act at specific synaptic terminals, exerting differential control of excitatory and inhibitory neurotransmission in the central amygdala (CeM) (Martin-Fernandez et al., 2017). In this study, we found that the release of ATP/adenosine from the same astrocyte could both excite and depress synaptic transmission depending on the neuronal circuit under investigation. For example, astrocytic ATP/adenosine release depressed excitatory transmission via activation of presynaptic A1 receptors at synaptic inputs from the basolateral amygdala, whereas the same gliotransmitter enhanced inhibitory transmission via activation of presynaptic A2A receptors at synaptic inputs from the central lateral amygdala. The combined effect of this gliotransmission resulted in decreased output from the CeM, which consequently reduced fear expression, suggesting that astrocytes are integral components of the amygdala fear circuit. This study further indicates that the astrocyte-neuron communication is not broad and unspecific; rather, the signaling between astrocytes and neurons is a circuit- and synapse-specific phenomenon. The diversity and complexity of the phenomenon has important functional implications, given that the coordinated neuronal activity and astrocyte synaptic effects lead to significant behavioral consequences.
The circuit- and synapse-specific astrocyte-mediated synaptic regulation adds further diversity to the astrocyte-neuron signaling. Similar to the amygdala results described above, the functional interaction between astrocytes and synapses in the dorsal striatum has been shown to be highly specific. In this brain area, Martin et al. (2015) demonstrated the existence of two different populations of astrocytes that selectively interact with medium spiny neurons (MSN) and synapses belonging to the two main corticostriatal circuits – the direct and indirect pathways (Martin et al., 2015). Subsets of astrocytes specifically respond to the activity of either direct or indirect pathway MSNs, and, in turn, selectively modulate corticostriatal synapses belonging to either direct or indirect pathways, respectively. The functional consequences of this circuitspecific astrocyte-neuron signaling has not yet been explored, but it can be envisaged that astrocytes may play a role in the coordinated activity of these two circuits in basal ganglia, which is critical to the function and dysfunction of the striatum.
As describe above, astrocytes are known to be able to release different gliotransmitters with different functional consequences on synaptic physiology. However, one outstanding question is whether a single astrocyte can release different gliotransmitters, and, if so, what are the circumstances that govern the phenomenon. We have recently addressed these questions studying the effects of different forms of neuronal stimulation on single astrocytes and the downstream effects of gliotransmission on single glutamatergic synapses in the hippocampus (Covelo and Araque, 2018). We found that low levels of stimulation (e.g. short duration or low frequency) of GABAergic interneurons activated astrocytes through GABAB receptors, which resulted in a transient increase in synaptic efficacy mediated by glutamate release from astrocytes and activation of presynaptic type 1 mGluRs. In contrast, higher levels of stimulation (e.g. long duration or high frequency) resulted in a biphasic response: an initial transient increase and a subsequent longer-lasting decrease in synaptic efficacy, which depended on mGluR1 and A1 adenosine receptors activated by glutamate and ATP/adenosine, respectively, released from a single astrocyte. Taken together, it suggests that a single astrocyte can decode neuronal input and integrate this information into specific gliotransmitter release that can have either an excitatory or inhibitory effect on synaptic transmission. These results add to the complexity of neuron-astrocyte networks; not only can one gliotransmitter from one astrocyte have disparate effects on synaptic communication, but a single astrocyte can also release two or more gliotransmitters that exert their effects with different temporal dynamics. Altogether, these findings further reveal the complexity of astrocyte-neuron signaling and its potential diverse effects.
Concluding remarks
As discussed above, astrocyte-neuron signaling is a complex phenomenon that encompasses great diversity in the mechanisms and functional consequences on network function (Figure 1). There is diversity in the types of input astrocytes receive, such as the pattern of neuronal input and the type of neurotransmitter. The resultant change in cytosolic calcium after transmitter binding is also diverse, as it depends on the various forms of intracellular signaling cascades and integration of signals. Astrocytes do not simply follow synaptic activity; they process and integrate synaptic information into diverse and plastic responses. Downstream of this integration, astrocytes can release different gliotransmitters through diverse mechanisms. The effect of these gliotransmitters on neuronal activity and communication is synapse-, cell-, and circuitspecific. Thus, different forms of gliotransmission are appropriately coordinated with the surrounding neuronal environment. Though there is much work to be done, we are beginning to shed more light on the seemingly complex input-output relationship of bidirectional communication between neurons and astrocytes. This diversity and complexity of astrocyte-neuron signaling provide a high degree of freedom to the networks underlying brain function.
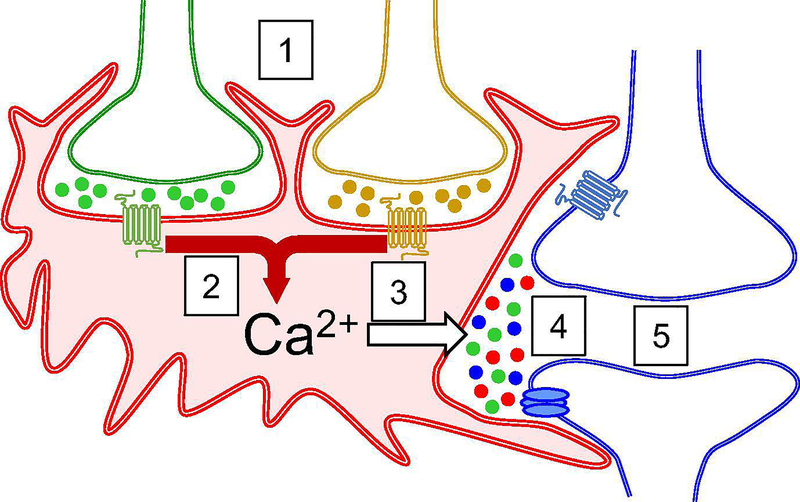
Fig. 1.
Sources of diversity in astrocyte-neuron signaling. Scheme of the key steps in bidirectional astrocyte-neuron signaling. Sources of diversity are marked by numbers, as follows: (1) Astrocytes sense synaptic activity by responding with calcium elevations to many different neurotransmitters and secretory mole-cules, such as glutamate or GABA. (2) The neurotransmitter-evoked downstream calcium signal is a result of specific integration of the input via intracellular signaling cascades that may or may not interact depending of the neurotransmitter receptors activated. The synaptic integration controls the spatial extent of the calcium signal, and can produce a non-linear input-output relationship, which is indicative of synaptic information processing by astrocytes. (3) Diverse, not mutually exclusive, mechanisms for gliotransmitter release down-stream of astrocyte activation may exist. Thus, different gliotransmit-ters may be released by different mechanisms. For example, two different SNARE proteins are responsible for the release of vesicles containing two different gliotransmitters. (4) Astrocytes can release different types of gliotransmitters. The type of gliotransmitter released may depend on the synaptic activity pattern that stimulates astro-cytes. (5) There is large diversity in the effects of gliotransmitters. It depends on the pre- or postsynaptic location of binding and type of neuronal receptors activated, as well as the neuronal cell type, which results in the synapse-, cell-, and pathway-specificity of astrocyte neuron-signaling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T, Refaeli R, Horn H, et al. (2018) Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell. [DOI] [PubMed]
- Agulhon C, Boyt KM, Xie AX, Friocourt F, Roth BL, McCarthy KD (2013) Modulation of the autonomic nervous system and behaviour by acute glial cell Gq protein-coupled receptor activation in vivo. The Journal of physiology 591:5599–5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M, Blomstrand F, Hanse E (2007) Astrocytes play a critical role in transient heterosynaptic depression in the rat hippocampal CA1 region. J Physiol 585:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, Audinat E (2004) Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience 24:6920–6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A (2014) Gliotransmitters travel in time and space. Neuron 81:728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Li N, Doyle RT, Haydon PG (2000) SNARE protein-dependent glutamate release from astrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience 20:666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG (1998) Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. The European journal of neuroscience 10:2129–2142. [DOI] [PubMed] [Google Scholar]
- Bazargani N, Attwell D (2016) Astrocyte calcium signaling: the third wave. Nat Neurosci 19:182–189. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A (2004) Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nature neuroscience 7:613–620. [DOI] [PubMed] [Google Scholar]
- Bohmbach K, Schwarz MK, Schoch S, Henneberger C (2018) The structural and functional evidence for vesicular release from astrocytes in situ. Brain Res Bull 136:65–75. [DOI] [PubMed] [Google Scholar]
- Bowser DN, Khakh BS (2004) ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. The Journal of neuroscience : the official journal of the Society for Neuroscience 24:8606–8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AC, Merrill JE, Dirksen ER, Sanderson MJ (1991) Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron 6:983–992. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J (1995) Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron 15:711–720. [DOI] [PubMed] [Google Scholar]
- Chen N, Sugihara H, Sharma J, Perea G, Petravicz J, Le C, Sur M (2012) Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proceedings of the National Academy of Sciences of the United States of America 109:E2832–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ (1990) Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247:470–473. [DOI] [PubMed] [Google Scholar]
- Covelo A, Araque A (2018) Neuronal activity determines distinct gliotransmitter release from a single astrocyte. eLife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JW, Chernjavsky A, Smith SJ (1992) Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron 8:429–440. [DOI] [PubMed] [Google Scholar]
- De Pitta M, Brunel N, Volterra A (2016) Astrocytes: Orchestrating synaptic plasticity? Neuroscience 323:43–61. [DOI] [PubMed] [Google Scholar]
- Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, Volterra A (2011) Local Ca2+ detection and modulation of synaptic release by astrocytes. Nature neuroscience 14:1276–1284. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Nilsson M, Wagberg M, Hansson E, Ronnback L (1993) Kappa-opioid receptors on astrocytes stimulate L-type Ca2+ channels. Neuroscience 54:401–407. [DOI] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G (2004) Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43:729–743. [DOI] [PubMed] [Google Scholar]
- Fiacco TA, McCarthy KD (2018) Multiple Lines of Evidence Indicate That Gliotransmission Does Not Occur under Physiological Conditions. The Journal of neuroscience : the official journal of the Society for Neuroscience 38:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gonzalo M, Martin-Fernandez M, Martinez-Murillo R, Mederos S, Hernandez-Vivanco A, Jamison S, Fernandez AP, Serrano J, et al. (2017) Neuron-astrocyte signaling is preserved in the aging brain. Glia 65:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gonzalo M, Navarrete M, Perea G, Covelo A, Martin-Fernandez M, Shigemoto R, Lujan R, Araque A (2014) Endocannabinoids Induce Lateral Long-Term Potentiation of Transmitter Release by Stimulation of Gliotransmission. Cerebral cortex. [DOI] [PubMed] [Google Scholar]
- Guerra-Gomes S, Sousa N, Pinto L, Oliveira JF (2017) Functional Roles of Astrocyte Calcium Elevations: From Synapses to Behavior. Front Cell Neurosci 11:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB (1999) ATP released from astrocytes mediates glial calcium waves. The Journal of neuroscience : the official journal of the Society for Neuroscience 19:520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D (2010) Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci 11:227–238. [DOI] [PubMed] [Google Scholar]
- Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, Koehl M, Abrous DN, et al. (2012) Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148:1039–1050. [DOI] [PubMed] [Google Scholar]
- Hertz L (1965) Possible role of neuroglia: a potassium-mediated neuronal--neuroglial--neuronal impulse transmission system. Nature 206:1091–1094. [DOI] [PubMed] [Google Scholar]
- Higashi K, Fujita A, Inanobe A, Tanemoto M, Doi K, Kubo T, Kurachi Y (2001) An inwardly rectifying K(+) channel, Kir4.1, expressed in astrocytes surrounds synapses and blood vessels in brain. Am J Physiol Cell Physiol 281:C922–931. [DOI] [PubMed] [Google Scholar]
- Jennings A, Tyurikova O, Bard L, Zheng K, Semyanov A, Henneberger C, Rusakov DA (2017) Dopamine elevates and lowers astroglial Ca(2+) through distinct pathways depending on local synaptic circuitry. Glia 65:447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, et al. (2007) Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci 10:331–339. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M (1998) Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nature neuroscience 1:683–692. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Sofroniew MV (2015) Diversity of astrocyte functions and phenotypes in neural circuits. Nature neuroscience 18:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Rusakov DA (2002) Asymmetry of glia near central synapses favors presynaptically directed glutamate escape. Biophys J 83:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti L, Losi G, Lia A, Melone M, Chiavegato A, Gomez-Gonzalo M, Sessolo M, Bovetti S, et al. (2018) Interneuron-specific signaling evokes distinctive somatostatin-mediated responses in adult cortical astrocytes. Nature communications 9:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti L, Losi G, Sessolo M, Marcon I, Carmignoto G (2016) The inhibitory neurotransmitter GABA evokes long-lasting Ca(2+) oscillations in cortical astrocytes. Glia 64:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fernandez M, Jamison S, Robin LM, Zhao Z, Martin ED, Aguilar J, Benneyworth MA, Marsicano G, et al. (2017) Synapse-specific astrocyte gating of amygdala-related behavior. Nature neuroscience 20:1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Bajo-Graneras R, Moratalla R, Perea G, Araque A (2015) Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349:730–734. [DOI] [PubMed] [Google Scholar]
- Meier SD, Kafitz KW, Rose CR (2008) Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. Glia 56:1127–1137. [DOI] [PubMed] [Google Scholar]
- Min R, Nevian T (2012) Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nature neuroscience 15:746–753. [DOI] [PubMed] [Google Scholar]
- Min R, Santello M, Nevian T (2012) The computational power of astrocyte mediated synaptic plasticity. Frontiers in computational neuroscience 6:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam MH, Han KS, Lee J, Bae JY, An H, Park S, Oh SJ, Kim E, et al. (2018) Expression of micro-Opioid Receptor in CA1 Hippocampal Astrocytes. Exp Neurobiol 27:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Araque A (2008) Endocannabinoids mediate neuron-astrocyte communication. Neuron 57:883–893. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Araque A (2010) Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68:113–126. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Perea G, Fernandez de Sevilla D, Gomez-Gonzalo M, Nunez A, Martin ED, Araque A (2012) Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS biology 10:e1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Vallee J, Haber M, Murai KK, Lacaille JC, Robitaille R (2011) Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146:785–798. [DOI] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G (1997) Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. The Journal of neuroscience : the official journal of the Society for Neuroscience 17:7817–7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A (2005) Glial calcium signaling and neuron-glia communication. Cell calcium 38:375–382. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A (2007) Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317:1083–1086. [DOI] [PubMed] [Google Scholar]
- Perea G, Gomez R, Mederos S, Covelo A, Ballesteros JJ, Schlosser L, Hernandez-Vivanco A, Martin-Fernandez M, et al. (2016) Activity-dependent switch of GABAergic inhibition into glutamatergic excitation in astrocyte-neuron networks. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Yang A, Boyden ES, Sur M (2014) Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo. Nature communications 5:3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD (1996) Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. The Journal of neuroscience : the official journal of the Society for Neuroscience 16:5073–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin LM, Oliveira da Cruz JF, Langlais VC, Martin-Fernandez M, Metna-Laurent M, Busquets-Garcia A, Bellocchio L, Soria-Gomez E, et al. (2018) Astroglial CB1 Receptors Determine Synaptic D-Serine Availability to Enable Recognition Memory. Neuron 98:935–944 e935. [DOI] [PubMed] [Google Scholar]
- Rusakov DA, Bard L, Stewart MG, Henneberger C (2014) Diversity of astroglial functions alludes to subcellular specialisation. Trends Neurosci 37:228–242. [DOI] [PubMed] [Google Scholar]
- Santello M, Bezzi P, Volterra A (2011) TNFalpha controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 69:988–1001. [DOI] [PubMed] [Google Scholar]
- Savtchouk I, Volterra A (2018) Gliotransmission: Beyond Black-and-White. The Journal of neuroscience : the official journal of the Society for Neuroscience 38:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipke CG, Heuser I, Peters O (2011) Antidepressants act on glial cells: SSRIs and serotonin elicit astrocyte calcium signaling in the mouse prefrontal cortex. J Psychiatr Res 45:242–248. [DOI] [PubMed] [Google Scholar]
- Schwarz Y, Zhao N, Kirchhoff F, Bruns D (2017) Astrocytes control synaptic strength by two distinct v-SNARE-dependent release pathways. Nat Neurosci 20:1529–1539. [DOI] [PubMed] [Google Scholar]
- Shelton MK, McCarthy KD (2000) Hippocampal astrocytes exhibit Ca2+-elevating muscarinic cholinergic and histaminergic receptors in situ. J Neurochem 74:555–563. [DOI] [PubMed] [Google Scholar]
- Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS (2008) Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 28:6659–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Jackson-Weaver O, Huckstepp RT, O’Dell TJ, Khakh BS (2013) TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. The Journal of neuroscience : the official journal of the Society for Neuroscience 33:10143–10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Patel S, Khakh BS (2016) Probing the Complexities of Astrocyte Calcium Signaling. Trends in cell biology 26:300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS (2011) TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci 15:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiene-Martin A, Zhou R, Hauser KF (1998) Regional, developmental, and cell cycledependent differences in mu, delta, and kappa-opioid receptor expression among cultured mouse astrocytes. Glia 22:249–259. [PMC free article] [PubMed] [Google Scholar]
- Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K, Hirase H (2011) Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience 31:18155–18165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A, Liaudet N, Savtchouk I (2014) Astrocyte Ca(2)(+) signalling: an unexpected complexity. Nature reviews Neuroscience 15:327–335. [DOI] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, et al. (2006) Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci 9:816–823. [DOI] [PubMed] [Google Scholar]
- Witcher MR, Kirov SA, Harris KM (2007) Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia 55:13–23. [DOI] [PubMed] [Google Scholar]
- Xin W, Bonci A (2018) Functional Astrocyte Heterogeneity and Implications for Their Role in Shaping Neurotransmission. Front Cell Neurosci 12:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V (2012) Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN Neuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]