Abstract
Atypical protein isoaspartyl residues arise spontaneously during the aging process from the deamidation of protein asparaginyl residues and the isomerization of protein aspartyl residues. These abnormal residues are modified in cells by a strongly conserved protein carboxyl methyltransferase (PCMT) as a first step in a repair pathway. Because a decline in cellular repair mechanisms is hypothesized to contribute to senescence, we determined whether increased PCMT activity was correlated with enhanced longevity. Two ubiquitous promoters were used with the binary GAL4-UAS system to drive PCMT overexpression in Drosophila melanogaster. Flies expressing PCMT activity under the regulation of either the hsp70 or actin5C promoter had enzyme activities that were 3- or 7-fold higher, respectively, than control flies at 29°C. Correlated with the observed increases in PCMT activities, such flies lived on average 32–39% longer than control flies. Lifespan extension was not observed at 25°C with either hsp70- or actin5C-driven expression, indicating a temperature-dependent effect on longevity. We conclude that protein repair is an important factor in the determination of lifespan under certain environmental conditions. PCMT activity may become limiting under mild stress conditions that accelerate rates of protein damage.
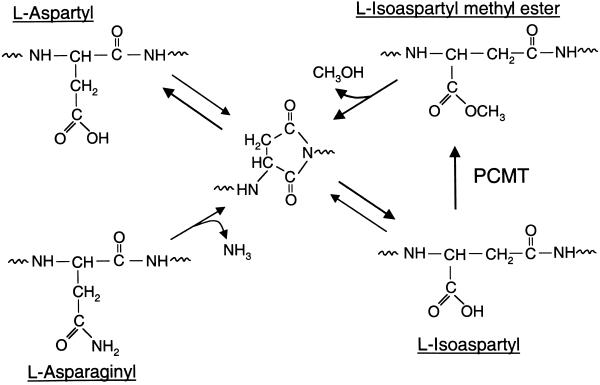
Cellular proteins are subject to spontaneous chemical modifications during the aging process that can negatively impact the normal function of a protein. One such change involves the generation of isoaspartyl residues (Fig. 1) from either aspartyl or asparaginyl residues via a mechanism involving the formation and cleavage of an internal succinimide ring. The production of an isoaspartyl residue is accompanied by the introduction of an additional carbon into the polypeptide backbone that can disrupt the local secondary structure and function of the protein (1). The accumulation of these potentially dysfunctional isoaspartyl proteins in cells is counteracted by a widely distributed protein carboxyl methyltransferase (PCMT, EC 2.1.1.77). In vitro, purified PCMT modifies isoaspartyl residues in a large number of protein and peptide substrates, resulting in the repair of the polypeptide backbone by the pathway outlined in Fig. 1 (1–3). Consistent with a repair function for PCMT, mice lacking PCMT activity accumulate isoaspartyl proteins with age and also suffer fatal epileptic seizures as young adults (4, 5).
Figure 1.
Repair of age-damaged proteins catalyzed by PCMT. Isoaspartyl residues arise spontaneously during the aging of proteins by deamidation of asparaginyl residues and the isomerization of aspartyl residues via a succinimidyl intermediate. Protein isoaspartyl residues serve as substrates for PCMT, which initiates the repair of the polypeptide backbone via reformation of the succinimidyl intermediate.
It is widely accepted that cellular repair processes decline with age and that this decline is a contributing factor to senescence (6). Because of its role in protein repair, we were interested in the possibility that PCMT might be involved with the regulation of lifespan. To test this hypothesis, we have overexpressed PCMT in Drosophila melanogaster and examined the consequences for longevity. Drosophila was chosen for these experiments because of the potential to genetically manipulate PCMT levels as well as the demonstrated utility of the fruit fly as a model for aging (7, 8). The primary sequence of Drosophila PCMT also is virtually identical to the mammalian sequences in regions required for binding isoaspartyl substrates and the methyl donor, S-adenosylmethionine (9, 10).
We have used the binary GAL4/UAS expression system to drive overexpression of PCMT in vivo. By using this system, overexpression is effected in the progeny of a cross between a “driver strain” expressing the yeast GAL4 transcriptional activator in a tissue-specific manner and a “responder strain” carrying an extra chromosomal copy of the Pcmt gene that is regulatable by GAL4 (11). A large number of driver strains have been generated previously and characterized by other investigators. Based on the expression pattern of the endogenous Pcmt gene, we used ubiquitous promoters to drive overexpression of PCMT. These studies demonstrate that the elevation of PCMT activity under certain conditions leads to a significant extension of the Drosophila lifespan.
Materials and Methods
Drosophila Strains and Culture Conditions.
Cultures were reared at 25°C on standard corn meal/agar medium. For longevity experiments, flies were maintained in vials in single-sex groups of 10 and transferred to new vials every 4 days. Stocks of flies carrying promoter-GAL4 transgenes were obtained from the Indiana University Stock Center (Bloomington, IN).
Germline Transformation of Drosophila Embryos.
Standard techniques were used for germline transformation (12). Briefly, white-eyed w1118/w1118 embryos were collected on grape-agar plates during the first half hour after deposition and used for microinjection. Embryos were microinjected with pUAST vector DNA (11) containing the full-length coding sequence of Drosophila Pcmt (9) inserted into its multiple cloning site. The Δ2-3 helper plasmid (13) was coinjected to provide transposase activity.
In Situ Hybridization of Embryos.
In situ hybridization of dechorionated stage-12 embryos (14) was performed as described (15) with slight modifications. Briefly, embryos were incubated for 24 h at 45°C in a mixture containing 5× SSC (1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7), 50% deionized formamide, 100 μg/ml denatured salmon sperm DNA, 50 μg/ml heparin, and 0.1% Tween 20 and 10 ng/ml digoxigenin-labeled riboprobe. Bound anti-digoxigenin antibody was detected by using the Genius nonradioactive detection reagents (Roche, Indianapolis, IN).
Enzymatic Assays.
Fly extracts were prepared by homogenizing flies in groups of 10 in 0.5-ml ice-cold 25 mM 2-(N-morphilino)ethanesulfonic acid, pH 6.5, 150 mM NaCl, 2 mM EDTA, and 2 mM phenylmethylsulfonyl fluoride with a sintered glass homogenizer. The extracts were clarified by centrifugation at 7,400 × g for 30 min at 4°C. The supernatants were removed and used immediately for methyltransferase assays. Reaction mixtures (25 μl) contained 3 μM S-adenosyl-L-[3H]methionine (15 Ci/mmol, 1 mCi = 37 MBq; Amersham Pharmacia), 20 mg/ml ovalbumin (Grade V, Sigma), and 12.5 μl of extract. Isotopic S-adenosyl-L-[3H]methionine was diluted with nonradioactive S-adenosylmethionine (Sigma) to a specific activity of 1 Ci/mmol for use in enzymatic assays. Reactions were incubated for 30 min at 37°C and then terminated by the addition of 1 ml of ice-cold 10% trichloroacetic acid. Protein methyl esters were quantified as described (16).
Results
In Situ Localization of Pcmt Transcripts in Embryos.
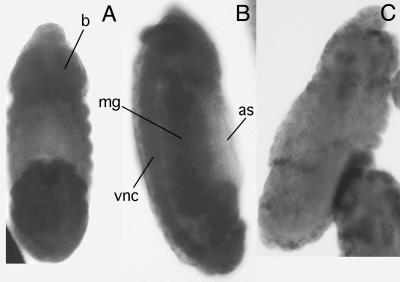
The expression pattern of a gene often mirrors the metabolic requirements for that particular gene product and therefore can provide valuable information to guide the selection of driver strains for overexpression experiments. The distribution of Pcmt transcripts in Drosophila embryos was analyzed by in situ hybridization (15). These studies showed that Pcmt transcripts are distributed uniformly in embryos before cellularization and in all tissues later in embryogenesis. In stage-12 embryos (Fig. 2), Pcmt transcripts were detected at particularly high levels in the embryonic nerve cord and brain, reminiscent of the elevated levels of PCMT activity observed in vertebrate brain tissue (17). Signal was observed also in the midgut, Malpighian tubules, and presumptive gonads but not in the largely acellular amnioserosa. Thus, Pcmt is expressed ubiquitously in Drosophila embryos.
Figure 2.
Ubiquitous expression of the Pcmt gene in Drosophila embryos. In situ hybridization of dechorionated stage-12 embryos was performed as described in Materials and Methods. Embryos are oriented with the anterior end of the embryo toward the top of the figure. The dorsal (A) and side (B) views of embryos hybridized with antisense probes are shown as well as the side view of an embryo hybridized with a sense probe (C). The positions of the embryonic brain (b), midgut (mg), ventral nerve cord (vnc), and amnioserosa (as) are indicated.
Construction of UAS-Pcmt Responder Strains.
Several responder strains carrying UAS-Pcmt transgenes were produced by using standard techniques for P element-mediated germline transformation (12). Multiple independent transformants were obtained, and the P element insertion sites were mapped to either the X, second, or third chromosomes by genetic segregation analysis. Southern analyses of genomic DNA from homozygous responder strains were consistent with the presence of a single Pcmt insertion in each strain. None of the insertions affected basal PCMT activity. In addition, there were no discernible effects of the insertions on the development or viability of the responder strains (data not shown).
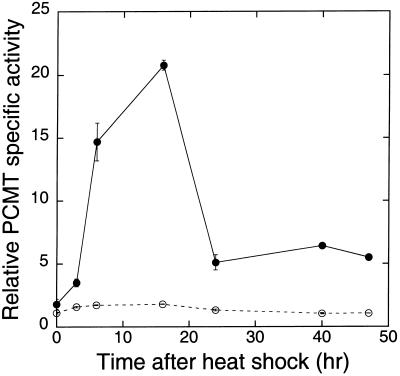
To establish the efficacy of the UAS-Pcmt transgene for ectopic overexpression, responder strains were mated to driver strains carrying an hsp70-GAL4 transgene. The hsp70-GAL4 driver strain was selected for these initial experiments, partly because its ubiquitous expression pattern (18) mirrors that of the Pcmt gene (Fig. 2). In addition, heat-inducible GAL4 expression allows one to dissociate the effects of PCMT overexpression on the aging process from potential developmental effects. Progeny carrying an hsp70-GAL4 driver and an X-linked UAS-Pcmt responder transgene (hsp-PCMT flies) were exposed to several 15-min heat shocks at 34°C. After heat treatment, PCMT activity reached levels ≈15–20 times higher than the basal value observed in identical genotypes maintained at 25°C (Fig. 3). Peak levels of expression were observed between 5 and 15 h after the heat shock. PCMT-specific activities returned to basal levels with a t1/2 of ≈4 h. During the same period, the endogenous PCMT activity of control flies lacking the hsp-GAL4 transgene increased only 50% after heat shock and then returned to basal levels with the same kinetics as the overexpressed enzyme (Fig. 3). Similarly, a small increase in PCMT activity was observed in Canton-S flies exposed to the same heat shock regimen (data not shown). This small effect on endogenous PCMT activity suggests that heat shock per se may produce a modest transcriptional activation of the Drosophila Pcmt gene, although there are no obvious heat-shock consensus elements within the 5′-flanking sequence.
Figure 3.
Overexpression of the UAS-Pcmt gene using an hsp70-GAL4 driver. Non-Cy female progeny of a cross between an hsp70-GAL4 driver strain (Bloomington stock 2077) and an X-chromosome UAS-Pcmt responder strain were exposed to five 15-min treatments at 34°C with the treatment separated by 15-min rest intervals at room temperature (●). Extracts were prepared from flies at various times after heat shock and analyzed for PCMT activity (8). PCMT-specific activities were measured in extracts of flies from the homozygous X-chromosome responder strain subjected to a similar heat-shock regimen (○).
Overexpression of PCMT Extends Lifespan at 29°C.
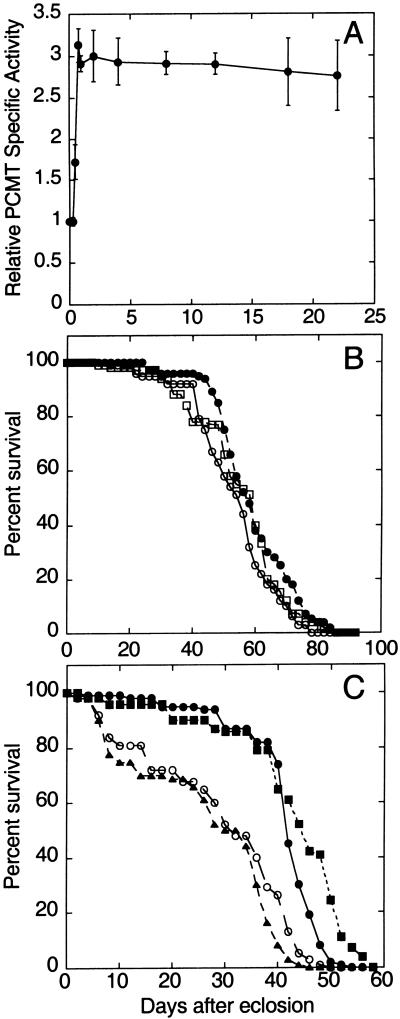
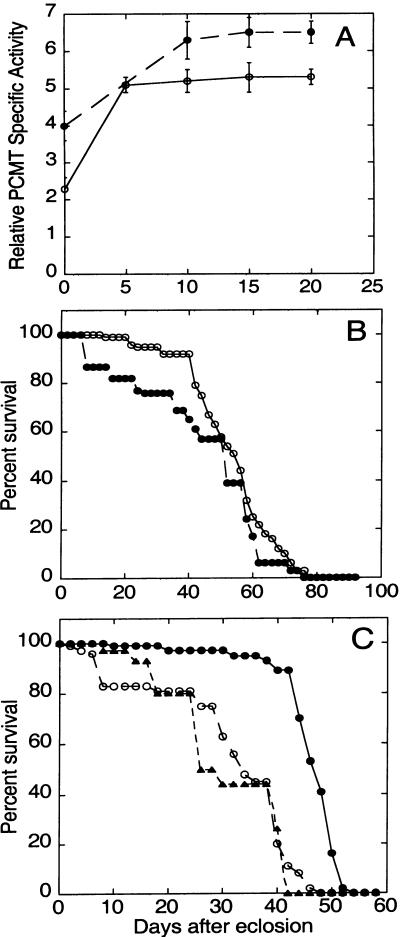
To test the effect of PCMT overexpression on adult lifespan, Drosophila populations were maintained at 29°C. This temperature is near the threshold value for activation of the temperature-sensitive hsp70 promoter (18). Although maximal activation of the hsp70 promoter occurs at higher temperatures, lifespan and other physiological parameters are affected adversely with increasing temperature (19). At 29°C, PCMT activity in hsp-PCMT flies reached a level approximately three times that of flies carrying only the UAS-Pcmt transgene within the first day of transfer to the elevated temperature (Fig. 4A), and PCMT activity then remained essentially constant for up to 3 weeks.
Figure 4.
(A) Expression of PCMT in hsp-Pcmt flies at 29°C. Progeny of a cross between the hsp70-GAL4 driver and UAS-Pcmt responder strains were transferred from 25 to 29°C within several hours after adult eclosion. PCMT activities were measured in extracts prepared from flies after various days of culture. Activities were normalized to PCMT levels measured in flies of the homozygous parental responder strain maintained at 29°C for the same period. (B and C) PCMT overexpression driven by the hsp70 promoter extends the lifespan of flies at 29°C. Female flies were maintained in vials in groups of 10 and transferred to new vials every 4 days. One hundred female flies were used for each lifespan measurement. The data points shown for X- or second-chromosome responder lines represent means of three or two independent experiments, respectively. (B) Survival at 25°C. ○, survival of homozygous responder flies carrying an insertion of the UAS-Pcmt transgene on the X. □, survival of homozygous responder flies carrying an insertion of the UAS-Pcmt transgene on the second chromosome. ●, survival of hsp-Pcmt (overexpressing) flies using an X-linked UAS-Pcmt transgene. (C) Survival at 29°C. The meainings of ● and ○ are the same as described in B. ■, survival of overexpressing hsp-Pcmt flies using a second-chromosome UAS-Pcmt transgene; ▴, survival of hsp70-GAL4 bearing progeny from an outcross between the driver strain and w1118/w1118 flies.
Dramatic differences in mean lifespan accompanied PCMT overexpression. The mean lifespan of flies overexpressing PCMT from an X-linked transgene was 41 days at 29°C (Fig. 4C), ≈35% longer than that of the parental responder strain or the outcrossed parental driver strain. (The hsp70-GAL4 driver strain was outcrossed against w1118 flies to eliminate the life-shortening effects associated with the CyO balancer chromosome.) The observed lifespan extension was not sex-specific, and similar results were obtained with either female or male flies (data not shown). Additional control experiments indicated that the lifespan extension associated with PCMT overexpression is not a secondary effect of Pcmt transgene insertion into an endogenous gene. To wit, similar effects on lifespan were observed by using UAS-Pcmt transgene insertions on the X or second chromosomes (Fig. 4C). In addition, no extension of lifespan was observed when hsp-Pcmt flies were maintained at 25°C (Fig. 4B), a temperature too low to induce PCMT overexpression.
In the early part of the lifespan, there are no other detectable phenotypic differences between PCMT-overexpressing and control flies. No differences in fecundity were observed between hsp-PCMT flies and individuals of the parental responder strain. Peak values of egg laying were observed for both control and hsp-PCMT flies at 4–6 days after eclosion. In the latter half of the lifespan, however, there was a notable difference in gross activity level between control and hsp-Pcmt flies. Flies overexpressing PCMT consistently exhibited increased locomotor activity (data not shown).
PCMT Overexpression Causes Temperature-Dependent Lifespan Extension.
To determine whether lifespan could be extended by using other GAL4 driver transgenes, we performed similar experiments with a noninducible actin 5C-GAL4 driver strain that expresses GAL4 protein at both 25 and 29°C. Flies carrying both actin 5C-GAL4 and UAS-Pcmt transgenes (actin-Pcmt flies) expressed 5- or 7–8-fold more PCMT activity than the parental responder strains at 25 or 29°C, respectively (Fig. 5A). Actin-Pcmt flies first eclosed on the same day as control strains, and they did not exhibit any obvious morphological abnormalities. Interestingly, we did not observe an extension of lifespan in actin-PCMT flies at 25°C, and indeed such flies exhibited slightly reduced viability early in the lifespan (Fig. 5B). Thus, overexpression of PCMT is not sufficient to effect lifespan extension under all physiological conditions.
Figure 5.
(A) Expression of PCMT in actin-Pcmt flies. Adult progeny of a cross between the actin 5C-GAL4 driver strain (Bloomington stock 4414) and the X-chromosome UAS-Pcmt responder strain were maintained at 25 (○) or 29°C (●). For the 29°C group, progeny were transferred from 25 to 29°C within several hours after adult eclosion. Activities were normalized to PCMT levels measured in flies of the homozygous parental responder strain maintained at the same temperatures for the same period. (B and C) PCMT overexpression driven by the actin5C promoter extends the lifespan of flies at 29°C. (B) Survival at 25°C. ●, survival of actin-Pcmt flies; ○, survival of the parental X-chromosome UAS-Pcmt responder strain. (C) Survival at 29°C. The meanings of ● and ○ are the same as described in B. ▴, survival of actin5C-GAL4 bearing progeny from an outcross between the driver strain and w1118/w1118 flies.
Quite different effects were observed at 29°C (Fig. 5C) with actin-Pcmt populations. At the higher temperature, significantly increased longevity was observed in actin-PCMT flies, and the effect was more dramatic than that seen with hsp-Pcmt populations. In these experiments, actin-Pcmt flies had a mean lifespan of 46 days. By comparison, flies carrying either the actin-GAL4 or UAS-Pcmt transgene (but not both) had mean lifespans of 28 or 34 days, respectively. As with hsp-Pcmt flies, actin-Pcmt flies were more vigorous late in life, and their numbers dropped precipitously late in the lifespan.
Discussion
Because of the unusual specificity of the PCMT for isoaspartyl residues, it was proposed originally that the enzyme initiates the repair of its age-damaged substrates (1). Subsequent experiments with purified PCMT have provided support for this hypothesis by demonstrating the successful conversion of isoaspartyl residues in model substrates to aspartyl residues albeit with less-than-full efficiency. The experiments reported here address the physiological significance of PCMT-catalyzed methylation to the aging process, which is characterized by a decline in cellular repair mechanisms. Our results demonstrate that PCMT overexpression has a dramatic positive effect on lifespan under certain environmental conditions.
The effects of PCMT overexpression on lifespan that we have noted depend on the ambient temperature. Extended lifespans were observed for both hsp-Pcmt and actin-Pcmt flies at 29°C but not at 25°C. Indeed, actin-Pcmt flies, when maintained at 25°C, had only normal lifespans even though their average PCMT activity was observed to be higher than that of hsp-Pcmt flies maintained at 29°C. We consider it unlikely that the extended lifespan observed at 29°C is simply caused by a higher turnover number of PCMT at elevated temperatures, because our enzymatic measurements show that the specific activity of purified Drosophila PCMT increases only 40% between 25 and 30°C (data not shown). By using this value to extrapolate specific PCMT activities in vivo, we predict that actin 5C-driven PCMT activity at 25°C is approximately equal to that of hsp70-driven activity at 29°C.
A number of different cellular mechanisms could underlie the temperature dependence of lifespan that we observe with PCMT overexpression. PCMT might act in concert with heat-inducible proteins to promote an extended longevity. A large number of heat-shock proteins have been described in Drosophila, some of which serve as molecular chaperones by associating with unfolded polypeptide chains (18). In this regard, it is of interest that the introduction of an isoaspartyl residue into a protein results in a perturbation of its native conformation (20), and it is possible that heat-shock proteins bind to these nonnative protein forms. Consistent with a potential role for heat-shock proteins in postponing senescence, the expression of several such proteins including hsp70 increases during aging (21), and the expression of heat-shock proteins has been shown to have a positive effect on longevity (22). Alternatively, it is possible that PCMT activities are not rate-limiting under normal growth conditions but become rate-limiting at higher temperatures because of the more rapid generation of protein isoaspartyl residues (23). This interpretation is supported by results obtained when PCMT function is deleted in bacteria. In Escherichia coli, the genetic elimination of PCMT activity has no effect under vegetative conditions, but null mutants show reduced viability under heat stress (24).
PCMT is one of very few proteins capable of extending lifespan in Drosophila when overexpressed in vivo. Several laboratories have described conditions under which the overexpression of superoxide dismutase extends lifespan, and this effect is presumably caused by a reduction in the concentration of reactive oxygen species. The effectiveness of superoxide dismutase overexpression is highly variable, however, and it has been found to depend on the specificity of GAL4 driver strains, genetic background, and coexpression of catalase in different experiments (25–27).
Several hypomorphic mutations have been characterized that extend Drosophila lifespan, and the proteins encoded by the relevant genes were identified by sequence homology to vertebrate counterparts. These proteins comprise a diverse group that includes the putative G protein-coupled receptor methuselah (28), an insulin receptor homolog (29), a potential insulin receptor substrate (30), and the Drosophila homolog of a mammalian dicarboxylate cotransporter (31). With the exception of methusaleh, these mutations are postulated to affect lifespan through caloric restriction.
Our results have identified a different mechanism correlated with longevity and indicate the importance of protein repair in the determination of lifespan. Future issues to be resolved include the identification of particularly critical substrates for PCMT as well as the identification of other proteins that may interact with PCMT to produce lifespan extension.
Acknowledgments
We thank Mary Roberts for assistance with the production of transgenic Drosophila and the Indiana University Stock Center (Bloomington, IN) for providing Drosophila strains. This work was supported by National Institutes of Health Grants AG08109 (to C.M.O.) and HL59873 (to F.R.J.).
Abbreviation
- PCMT
protein carboxyl methyltransferase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Clarke S. Annu Rev Biochem. 1985;54:479–506. doi: 10.1146/annurev.bi.54.070185.002403. [DOI] [PubMed] [Google Scholar]
- 2.Johnson B A, Murray E D, Jr, Clarke S, Glass D B, Aswad D W. J Biol Chem. 1987;262:5622–5629. [PubMed] [Google Scholar]
- 3.Johnson B A, Langmack E L, Aswad D W. J Biol Chem. 1987;262:12283–12287. [PubMed] [Google Scholar]
- 4.Kim E, Lowenson J D, MacLaren D C, Clarke S, Young S G. Proc Natl Acad Sci USA. 1997;94:6132–6137. doi: 10.1073/pnas.94.12.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto A, Takagi H, Kitamura D, Tatsuoka H, Nakano H, Kawano H, Kuroyanagi H, Yahagi Y, Kobayashi S, Koizumi K, et al. J Neurosci. 1998;18:2063–2074. doi: 10.1523/JNEUROSCI.18-06-02063.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knight J A. Adv Clin Chem. 2000;35:1–62. doi: 10.1016/s0065-2423(01)35014-x. [DOI] [PubMed] [Google Scholar]
- 7.Tower J. BioEssays. 1996;18:799–807. doi: 10.1002/bies.950181006. [DOI] [PubMed] [Google Scholar]
- 8.Kenyon C. Cell. 2001;105:165–168. doi: 10.1016/s0092-8674(01)00306-3. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor M B, Galus A, Hartenstine M, Magee M, Jackson F R, O'Connor C M. Insect Biochem Mol Biol. 1997;27:49–54. doi: 10.1016/s0965-1748(96)00071-9. [DOI] [PubMed] [Google Scholar]
- 10.Skinner M M, Puvathingal J M, Walter R L, Friedman A M. Structure (London) 2000;8:1189–1201. doi: 10.1016/s0969-2126(00)00522-0. [DOI] [PubMed] [Google Scholar]
- 11.Brand A H, Perrimon N. Development (Cambridge, UK) 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 12.Spradling A C, Rubin G M. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- 13.Robertson H M, Preston C R, Phillis R W, Johnson-Schlitz D M, Benz W K, Engels W R. Genetics. 1988;118:461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campos-Ortega J A, Hartenstein V. The Embryonic Development of Drosophila melanogaster. Berlin: Springer; 1985. [Google Scholar]
- 15.Tautz D, Pfeifle C. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- 16.O'Connor C M. J Biol Chem. 1987;262:10398–10403. [PubMed] [Google Scholar]
- 17.Diliberto E J, Jr, Axelrod J. J Neurochem. 1976;26:1159–1165. doi: 10.1111/j.1471-4159.1976.tb07001.x. [DOI] [PubMed] [Google Scholar]
- 18.Lindquist S. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 19.Ashburner M. Drosophila: A Laboratory Handbook. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 20.Capasso S, DiDonato A, Esposito L, Sica F, Sorrentino G, Vitagliano L, Zagari A, Mazzarella L. J Mol Biol. 1996;257:492–496. doi: 10.1006/jmbi.1996.0179. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler J C, Bieschke E T, Tower J. Proc Natl Acad Sci USA. 1998;92:10408–10412. doi: 10.1073/pnas.92.22.10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatar M, Khazaeli A A, Curtsinger J W. Nature (London) 1997;390:30. doi: 10.1038/36237. [DOI] [PubMed] [Google Scholar]
- 23.Geiger T, Clarke S. J Biol Chem. 1987;262:785–794. [PubMed] [Google Scholar]
- 24.Visick J E, Cai H, Clarke S. J Bacteriol. 1998;180:2623–2629. doi: 10.1128/jb.180.10.2623-2629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkes T L, Elia A J, Dickinson D, Hilliker A J, Phillips J P, Boulianne G L. Nat Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 26.Sun J, Tower J. Mol Cell Biol. 1999;19:216–228. doi: 10.1128/mcb.19.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orr W C, Sohal R S. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 28.Lin Y-J, Seroude L, Benzer S. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- 29.Tatar M, Kopelman A, Epstein D, Tu M-P, Yin C-M, Garofalo R S. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 30.Clancy D J, Gems D, Harshman L G, Oldham S, Stocker H, Hafen E, Leevers S J, Partridge L. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 31.Rogina B, Reenan R A, Nilsen S P, Helfand S L. Science. 2000;290:2137–2140. doi: 10.1126/science.290.5499.2137. [DOI] [PubMed] [Google Scholar]